Soluble PD-1 but Not PD-L1 Levels Predict Poor Outcome in Patients with High-Risk Diffuse Large B-Cell Lymphoma
Abstract
:Simple Summary
Abstract
1. Introduction
2. Results
2.1. Clinical Characteristics
2.2. sPD-1 and sPD-L1 Levels During Therapy
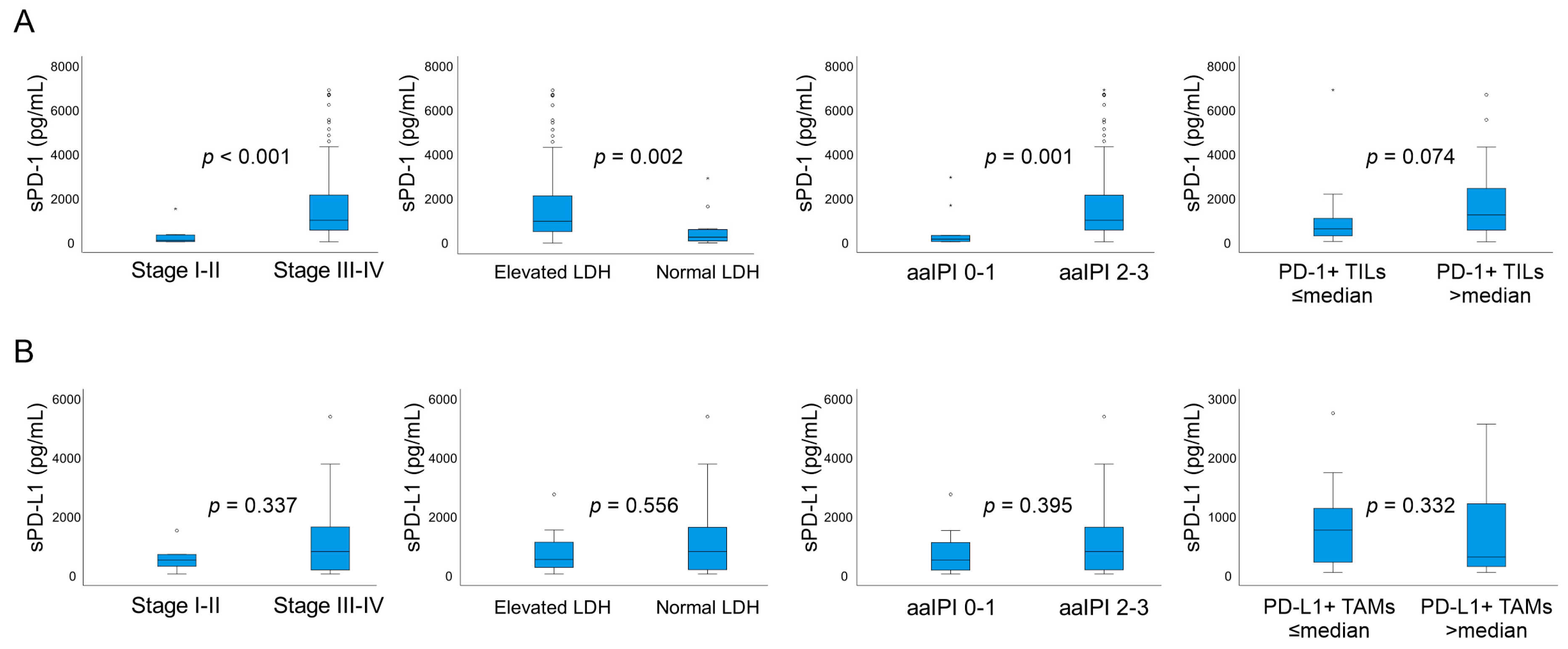
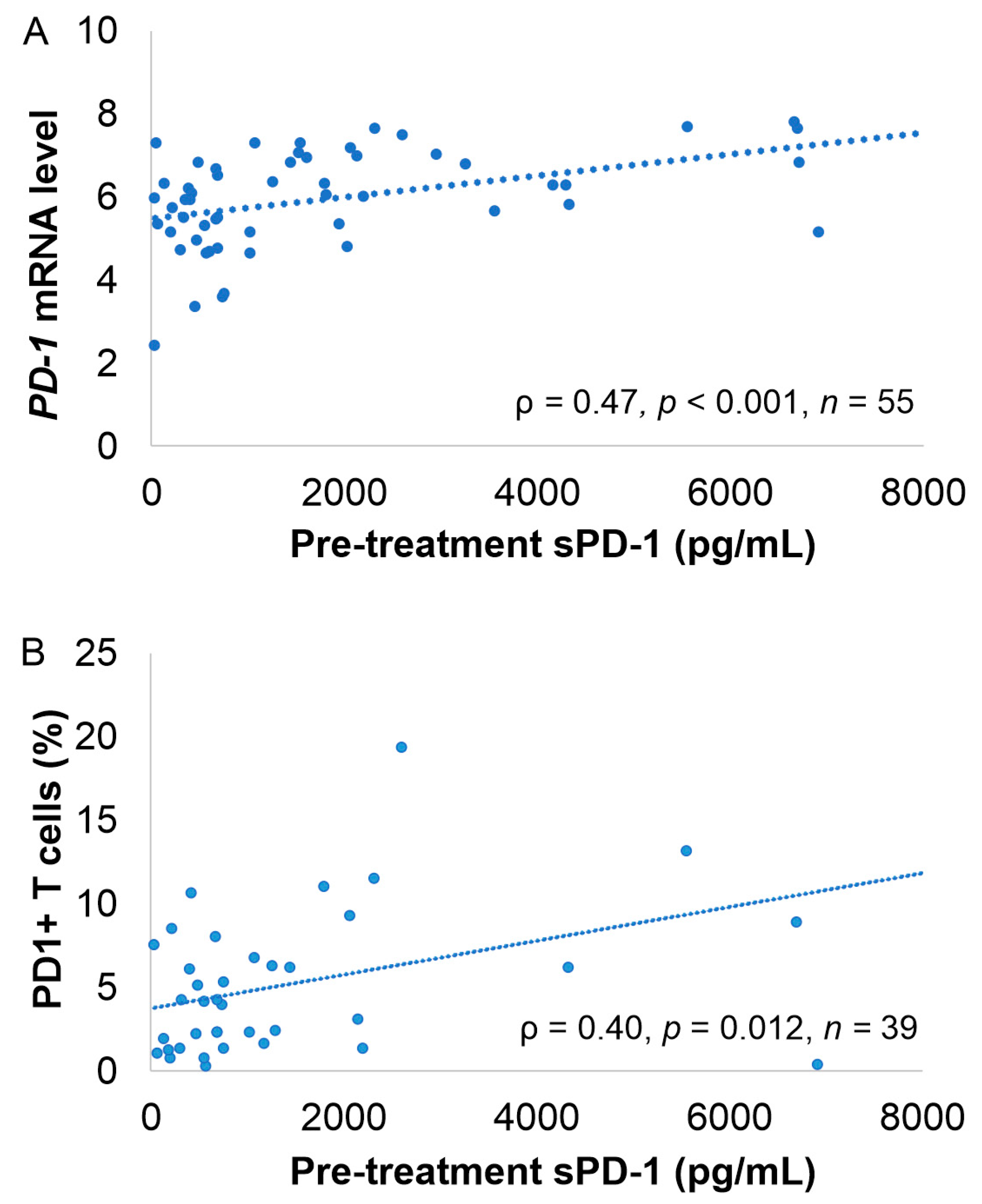
2.3. Correlation of sPD-1 and sPD-L1 Levels with Clinical Parameters, Gene and Protein Expression and Lymphocyte Count
2.4. Association of sPD-1 and sPD-L1 Levels with Survival
3. Discussion
4. Materials and Methods
4.1. Patients
4.2. Measurement of sPD-1 and sPD-L1 Levels
4.3. PD-1 and PD-L1 Gene Expression in the Tumor Tissue, Multiplex Immunohistochemistry, and Blood Lymphocyte Counts
4.4. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Salles, G.; Barrett, M.; Foà, R.; Maurer, J.; O’Brien, S.; Valente, N.; Wenger, M.; Maloney, D. Rituximab in B-cell hematologic malignancies: A review of 20 years of clinical experience. Adv. Ther. 2017, 34, 2232–2273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chapuy, B.; Stewart, C.; Dunford, A.J.; Kim, J.; Kamburov, A.; Redd, R.A.; Lawrence, M.S.; Roemer, M.G.M.; Li, A.J.; Ziepert, M.; et al. Molecular subtypes of diffuse large B cell lymphoma are associated with distinct pathogenic mechanisms and outcomes. Nat. Med. 2018, 24, 679–690. [Google Scholar] [CrossRef] [PubMed]
- Reddy, A.; Zhang, J.; Davis, N.S.; Moffitt, A.B.; Love, C.L.; Waldrop, A.; Leppa, S.; Pasanen, A.; Meriranta, L.; Karjalainen-Lindsberg, M.; et al. Genetic and functional drivers of diffuse large B cell lymphoma. Cell 2017, 171, 481–494.e15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schmitz, R.; Wright, G.W.; Huang, D.W.; Johnson, C.A.; Phelan, J.D.; Wang, J.Q.; Roulland, S.; Kasbekar, M.; Young, R.M.; Shaffer, A.L.; et al. Genetics and pathogenesis of diffuse large B-cell lymphoma. N. Engl. J. Med. 2018, 378, 1396–1407. [Google Scholar] [CrossRef] [PubMed]
- Ciavarella, S.; Vegliante, M.C.; Fabbri, M.; De Summa, S.; Melle, F.; Motta, G.; De Iuliis, V.; Opinto, G.; Enjuanes, A.; Rega, S.; et al. Dissection of DLBCL microenvironment provides a gene expression-based predictor of survival applicable to formalin-fixed paraffin-embedded tissue. Ann. Oncol. 2018, 29, 2363–2370. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leivonen, S.; Pollari, M.; Brück, O.; Pellinen, T.; Autio, M.; Karjalainen-Lindsberg, M.; Mannisto, S.; Kellokumpu-Lehtinen, P.; Kallioniemi, O.; Mustjoki, S.; et al. T-cell inflamed tumor microenvironment predicts favorable prognosis in primary testicular lymphoma. Haematologica 2019, 104, 338–346. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Keane, C.; Gill, D.; Vari, F.; Cross, D.; Griffiths, L.; Gandhi, M. CD4+ Tumor infiltrating lymphocytes are prognostic and independent of R-IPI in patients with DLBCL receiving R-CHOP chemo-immunotherapy. Am. J. Hematol. 2013, 88, 273–276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pardoll, D.M. The blockade of immune checkpoints in cancer immunotherapy. Nat. Rev. Cancer 2012, 12, 252–264. [Google Scholar] [CrossRef] [Green Version]
- Abril-Rodriguez, G.; Ribas, A. SnapShot: Immune checkpoint inhibitors. Cancer Cell. 2017, 31, 848.e1. [Google Scholar] [CrossRef]
- Sun, C.; Mezzadra, R.; Schumacher, T.N. Regulation and Function of the PD-L1 Checkpoint. Immunity 2018, 48, 434–452. [Google Scholar] [CrossRef] [Green Version]
- Bardhan, K.; Anagnostou, T.; Boussiotis, V.A. The PD1:PD-L1/2 pathway from discovery to clinical implementation. Front Immunol. 2016, 7, 550. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu-Monette, Z.Y.; Zhou, J.; Young, K.H. PD-1 expression and clinical PD-1 blockade in B-cell lymphomas. Blood 2018, 131, 68–83. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, W.; Bai, J.; Zuo, M.; Cao, X.; Chen, M.; Zhang, Y.; Han, X.; Zhong, D.; Zhou, D. PD-1 expression on the surface of peripheral blood CD4+ T cell and its association with the prognosis of patients with diffuse large B-cell lymphoma. Cancer Med. 2016, 5, 3077–3084. [Google Scholar] [CrossRef] [PubMed]
- Xu-Monette, Z.Y.; Xiao, M.; Au, Q.; Padmanabhan, R.; Xu, B.; Hoe, N.; Rodríguez-Perales, S.; Torres-Ruiz, R.; Manyam, G.C.; Visco, C.; et al. Immune profiling and quantitative analysis decipher the clinical role of immune-checkpoint expression in the tumor immune microenvironment of DLBCL. Cancer Immunol. Res. 2019, 7, 644–657. [Google Scholar] [CrossRef] [PubMed]
- Autio, M.; Leivonen, S.; Brück, O.; Mustjoki, S.; Jørgensen, J.M.; Karjalainen-Lindsberg, M.; Beiske, K.; Holte, H.; Pellinen, T.; Leppä, S. Immune cell constitution in the tumor microenvironment predicts the outcome in diffuse large B-cell lymphoma. Haematologica 2020. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kiyasu, J.; Miyoshi, H.; Hirata, A.; Arakawa, F.; Ichikawa, A.; Niino, D.; Sugita, Y.; Yufu, Y.; Choi, I.; Abe, Y.; et al. Expression of programmed cell death ligand 1 is associated with poor overall survival in patients with diffuse large B-cell lymphoma. Blood 2015, 126, 2193–2201. [Google Scholar] [CrossRef] [PubMed]
- Godfrey, J.; Tumuluru, S.; Bao, R.; Leukam, M.; Venkataraman, G.; Phillip, J.; Fitzpatrick, C.; McElherne, J.; MacNabb, B.W.; Orlowski, R.; et al. PD-L1 gene alterations identify a subset of diffuse large B-cell lymphoma harboring a T-cell–inflamed phenotype. Blood 2019, 133, 2279–2290. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Sun, R.; Miao, Y.; Tran, T.; Adams, L.; Roscoe, N.; Xu, B.; Manyam, G.C.; Tan, X.; Zhang, H.; et al. PD-1/PD-L1 expression and interaction by automated quantitative immunofluorescent analysis show adverse prognostic impact in patients with diffuse large B-cell lymphoma having T-cell infiltration: A study from the international DLBCL consortium program. Mod. Pathol. 2019, 32, 741–754. [Google Scholar] [CrossRef]
- Rossille, D.; Azzaoui, I.; Feldman, A.L.; Maurer, M.J.; Labouré, G.; Parrens, M.; Pangault, C.; Habermann, T.M.; Ansell, S.M.; Link, B.K.; et al. Soluble programmed death-ligand 1 as a prognostic biomarker for overall survival in patients with diffuse large B-cell lymphoma: A replication study and combined analysis of 508 patients. Leukemia 2017, 31, 988–991. [Google Scholar] [CrossRef] [Green Version]
- Nielsen, C.; Ohm-Laursen, L.; Barington, T.; Husby, S.; Lillevang, S.T. Alternative splice variants of the human PD-1 gene. Cell Immunol. 2005, 235, 109–116. [Google Scholar] [CrossRef]
- Mahoney, K.M.; Shukla, S.A.; Patsoukis, N.; Chaudhri, A.; Browne, E.P.; Arazi, A.; Eisenhaure, T.M.; Pendergraft, W.F.; Hua, P.; Pham, H.C.; et al. A secreted PD-L1 splice variant that covalently dimerizes and mediates immunosuppression. Cancer Immunol. Immunother. 2019, 68, 421–432. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hassounah, N.B.; Malladi, V.S.; Huang, Y.; Freeman, S.S.; Beauchamp, E.M.; Koyama, S.; Souders, N.; Martin, S.; Dranoff, G.; Wong, K.; et al. Identification and characterization of an alternative cancer-derived PD-L1 splice variant. Cancer Immunol. Immunother. 2019, 68, 407–420. [Google Scholar] [CrossRef] [PubMed]
- Wan, B.; Nie, H.; Liu, A.; Feng, G.; He, D.; Xu, R.; Zhang, Q.; Dong, C.; Zhang, J.Z. Aberrant regulation of synovial T cell activation by soluble costimulatory molecules in rheumatoid arthritis. J. Immunol. 2006, 177, 8844–8850. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, M.; Park, S.; Nam, H.J.; Choi, D.; Sung, Y. Enhancement of vaccine-induced primary and memory CD8+ T-cell responses by soluble PD-1. J. Immunother. 2011, 34, 297–306. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Hu, K.; Feng, L.; Su, R.; Lai, N.; Yang, Z.; Kang, S. Senescent cells re-engineered to express soluble programmed death receptor-1 for inhibiting programmed death receptor-1/programmed death ligand-1 as a vaccination approach against breast cancer. Cancer Sci. 2018, 109, 1753–1763. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Wang, Q.; Shi, B.; Xu, P.; Hu, Z.; Bai, L.; Zhang, X. Development of a sandwich ELISA for evaluating soluble PD-L1 (CD274) in human sera of different ages as well as supernatants of PD-L1+ cell lines. Cytokine 2011, 56, 231–238. [Google Scholar] [CrossRef]
- Ng, K.W.; Attig, J.; Young, G.R.; Ottina, E.; Papamichos, S.I.; Kotsianidis, I.; Kassiotis, G. Soluble PD-L1 generated by endogenous retroelement exaptation is a receptor antagonist. eLife 2019, 8, e50256. [Google Scholar] [CrossRef]
- Kruger, S.; Legenstein, M.; Rösgen, V.; Haas, M.; Modest, D.P.; Westphalen, C.B.; Ormanns, S.; Kirchner, T.; Heinemann, V.; Holdenrieder, S.; et al. Serum levels of soluble programmed death protein 1 (sPD-1) and soluble programmed death ligand 1 (sPD-L1) in advanced pancreatic cancer. OncoImmunology 2017, 6, e1310358. [Google Scholar] [CrossRef] [PubMed]
- Chang, B.; Huang, T.; Wei, H.; Shen, L.; Zhu, D.; He, W.; Chen, Q.; Zhang, H.; Li, Y.; Huang, R.; et al. The correlation and prognostic value of serum levels of soluble programmed death protein 1 (sPD-1) and soluble programmed death-ligand 1 (sPD-L1) in patients with hepatocellular carcinoma. Cancer Immunol. Immunother. 2019, 68, 353–363. [Google Scholar] [CrossRef] [Green Version]
- Silva, P.B.; Real, J.M.; Ferreira, L.R.P.; Esteves, G.H.; Brito, F.d.N.; Baiocchi, O.C.G. Soluble PD-1 and PD-L1 as potential biomarkers for classical Hodgkin lymphoma. Hematol. Oncol. 2018, 36, 709–712. [Google Scholar] [CrossRef]
- Rossille, D.; Gressier, M.; Damotte, D.; Maucort-Boulch, D.; Pangault, C.; Semana, G.; Le Gouill, S.; Haioun, C.; Tarte, K.; Lamy, T.; et al. Groupe Ouest-Est des Leucemies et Autres, Maladies du, Groupe Ouest-Est des Leucemies et Autres, Maladies du. High level of soluble programmed cell death ligand 1 in blood impacts overall survival in aggressive diffuse large B-Cell lymphoma: Results from a French multicenter clinical trial. Leukemia 2014, 28, 2367–2375. [Google Scholar] [CrossRef] [PubMed]
- Cho, I.; Lee, H.; Yoon, S.E.; Ryu, K.J.; Ko, Y.H.; Kim, W.S.; Kim, S.J. Serum levels of soluble programmed death-ligand 1 (sPD-L1) in patients with primary central nervous system diffuse large B-cell lymphoma. BMC Cancer 2020, 20, 120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El-Ghammaz, A.; Gadallah, H.A.; Kamal, G.; Maher, M.M.; Mohamad, M.A. Impact of serum soluble programed death ligand 1 on end of treatment metabolic response of diffuse large B cell lymphoma patients. Clin. Exp. Med. 2018, 18, 505–512. [Google Scholar] [CrossRef] [PubMed]
- Mortensen, J.B.; Hansen, I.M.; Clausen, M.R.; Bjerre, M.; d’Amore, F. Elevated pre-therapeutic serum levels of soluble programmed death 1 protein (sPD-1) identify DLBCL patients with adverse prognostic features. Blood 2017, 130 (Suppl. 1), 4148. [Google Scholar] [CrossRef]
- Leppä, S.; Jørgensen, J.; Tierens, A.; Meriranta, L.; Østlie, I.; de Nully Brown, P.; Fagerli, U.; Larsen, T.S.; Mannisto, S.; Munksgaard, L.; et al. Patients with high-risk DLBCL benefit from dose-dense immunochemotherapy combined with early systemic CNS prophylaxis. Blood Adv. 2020, 4, 1906–1915. [Google Scholar] [CrossRef] [PubMed]
- Keane, C.; Vari, F.; Hertzberg, M.; Cao, K.L.; Green, M.R.; Han, E.; Seymour, J.F.; Hicks, R.J.; Gill, D.; Crooks, P.; et al. Ratios of T-cell immune effectors and checkpoint molecules as prognostic biomarkers in diffuse large B-cell lymphoma: A population-based study. Lancet Haematol. 2015, 2, e445–e455. [Google Scholar] [CrossRef] [Green Version]
- Cheng, X.; Veverka, V.; Radhakrishnan, A.; Waters, L.C.; Muskett, F.W.; Morgan, S.H.; Huo, J.; Yu, C.; Evans, E.J.; Leslie, A.J.; et al. Structure and interactions of the human programmed cell death 1 receptor. J. Biol. Chem. 2013, 288, 11771–11785. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Liang, Z.; Tian, Y.; Cai, W.; Weng, Z.; Chen, L.; Zhang, H.; Bao, Y.; Zheng, H.; Zeng, S.; et al. High-affinity PD-1 molecules deliver improved interaction with PD-L1 and PD-L2. Cancer Sci. 2018, 109, 2435–2445. [Google Scholar] [CrossRef] [Green Version]
- Ansell, S.M.; Minnema, M.C.; Johnson, P.; Timmerman, J.M.; Armand, P.; Shipp, M.A.; Rodig, S.J.; Ligon, A.H.; Roemer, M.G.M.; Reddy, N.; et al. Nivolumab for relapsed/refractory diffuse large B-cell lymphoma in patients ineligible for or having failed autologous transplantation: A single-arm, phase II study. J. Clin. Oncol. 2019, 37, 481–489. [Google Scholar] [CrossRef]
- Kline, J.; Godfrey, J.; Ansell, S.M. The immune landscape and response to immune checkpoint blockade therapy in lymphoma. Blood 2020, 135, 523–533. [Google Scholar] [CrossRef]
- Greisen, S.R.; Rasmussen, T.K.; Stengaard-Pedersen, K.; Hetland, M.L.; Hørslev-Petersen, K.; Hvid, M.; Deleuran, B. Increased soluble programmed death-1 (sPD-1) is associated with disease activity and radiographic progression in early rheumatoid arthritis. Scand. J. Rheumatol. 2014, 43, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Autio, M.; Leivonen, S.; Pellinen, T.; Leppä, S. Clinical impact of tumor-associated macrophage and T-cell contents in diffuse large B-cell lymphoma. Blood 2020, 136 (Suppl. 1), 33. [Google Scholar] [CrossRef]


| Characteristic | All Patients, n (%) | Low Pretreatment sPD-1, n (%) | High Pretreatment sPD-1, n (%) | p Value | Low Pretreatment sPD-L1, n (%) | High Pretreatment sPD-L1, n (%) | p Value |
|---|---|---|---|---|---|---|---|
| Total | 121 (100) | 80 (66) | 41 (34) | 40 (50) | 40 (50) | ||
| Median age (range) | 56 (21–65) | 54 (21–65) | 57 (30–65) | 54 (21–65) | 54 (22–65) | ||
| Age | |||||||
| <60 years | 82 (68) | 54 (68) | 28 (68) | 1.000 | 30 (75) | 29 (73) | 0.799 |
| 60–65 years | 39 (32) | 26 (32) | 13 (32) | 10 (25) | 11 (27) | ||
| Gender | |||||||
| Male | 75 (62) | 46 (58) | 29 (71) | 0.172 | 25 (63) | 26 (65) | 0.816 |
| Female | 46 (38) | 34 (42) | 12 (29) | 15 (37) | 14 (35) | ||
| Entity | |||||||
| DLBCL NOS | |||||||
| GCB | 53 (44) | 41 (51) | 12 (29) | 0.055 1 | 18 (45) | 17 (42) | 0.788 1 |
| Non-GCB | 36 (30) | 21 (26) | 15 (37) | 11 (28) | 12 (30) | ||
| ND | 16 (13) | 10 (13) | 6 (15) | 2 (5) | 5 (13) | ||
| Other | 16 (13) | 8 (10) | 8 (19) | 9 (22) | 6 (15) | ||
| ECOG PS | |||||||
| 0–1 | 86 (71) | 60 (75) | 26 (63) | 0.208 | 32 (80) | 20 (50) | 0.005 |
| 2–3 | 35 (29) | 20 (25) | 15 (37) | 8 (20) | 20 (50) | ||
| Stage | |||||||
| 1–2 | 8 (7) | 8 (10) | 0 (0) | 0.050 | 5 (13) | 1 (3) | 0.090 |
| 3–4 | 113 (93) | 72 (90) | 41 (100) | 35 (87) | 39 (98) | ||
| aaIPI score | |||||||
| 0 | 4 (3) | 4 (5) | 0 (0) | 0.341 | 4 (10) | 0 (0) | 0.016 |
| 1 | 6 (5) | 4 (5) | 2 (5) | 2 (5) | 2 (5) | ||
| 2 | 74 (61) | 51 (64) | 23 (56) | 25 (63) | 18 (45) | ||
| 3 | 37 (31) | 21 (26) | 16 (39) | 9 (22) | 20 (50) |
| Variable | OS | PFS | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p Value | HR | 95% CI | p Value | |
| sPD-1, low vs. high | 4.06 | 1.00–16.50 | 0.050 | 4.10 | 1.21–13.89 | 0.024 |
| Age | 1.03 | 0.95–1.12 | 0.446 | 1.06 | 0.98–1.14 | 0.165 |
| ECOG PS (0, 1, 2, 3) | 1.59 | 0.77–3.25 | 0.209 | 1.30 | 0.70–2.40 | 0.403 |
| Stage (1, 2, 3, 4) | 0.81 | 0.27–2.40 | 0.700 | 1.02 | 0.35–2.93 | 0.975 |
| LDH, low vs. high | 1.27 | 0.14–11.51 | 0.830 | 0.99 | 0.12–8.43 | 0.995 |
| Subtype, GCB vs. non-GCB | 1.27 | 0.37–4.34 | 0.706 | 1.07 | 0.36–3.17 | 0.899 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vajavaara, H.; Mortensen, J.B.; Leivonen, S.-K.; Hansen, I.M.; Ludvigsen, M.; Holte, H.; Jørgensen, J.; Bjerre, M.; d’Amore, F.; Leppä, S. Soluble PD-1 but Not PD-L1 Levels Predict Poor Outcome in Patients with High-Risk Diffuse Large B-Cell Lymphoma. Cancers 2021, 13, 398. https://doi.org/10.3390/cancers13030398
Vajavaara H, Mortensen JB, Leivonen S-K, Hansen IM, Ludvigsen M, Holte H, Jørgensen J, Bjerre M, d’Amore F, Leppä S. Soluble PD-1 but Not PD-L1 Levels Predict Poor Outcome in Patients with High-Risk Diffuse Large B-Cell Lymphoma. Cancers. 2021; 13(3):398. https://doi.org/10.3390/cancers13030398
Chicago/Turabian StyleVajavaara, Heli, Julie Bondgaard Mortensen, Suvi-Katri Leivonen, Ida Monrad Hansen, Maja Ludvigsen, Harald Holte, Judit Jørgensen, Mette Bjerre, Francesco d’Amore, and Sirpa Leppä. 2021. "Soluble PD-1 but Not PD-L1 Levels Predict Poor Outcome in Patients with High-Risk Diffuse Large B-Cell Lymphoma" Cancers 13, no. 3: 398. https://doi.org/10.3390/cancers13030398






