Learned Immobility Produces Enduring Impairment of the HPA Axis Reactivity in Mice without Replicating the Broad Spectrum of Depressive-Like Phenotype
Abstract
1. Introduction
2. Results
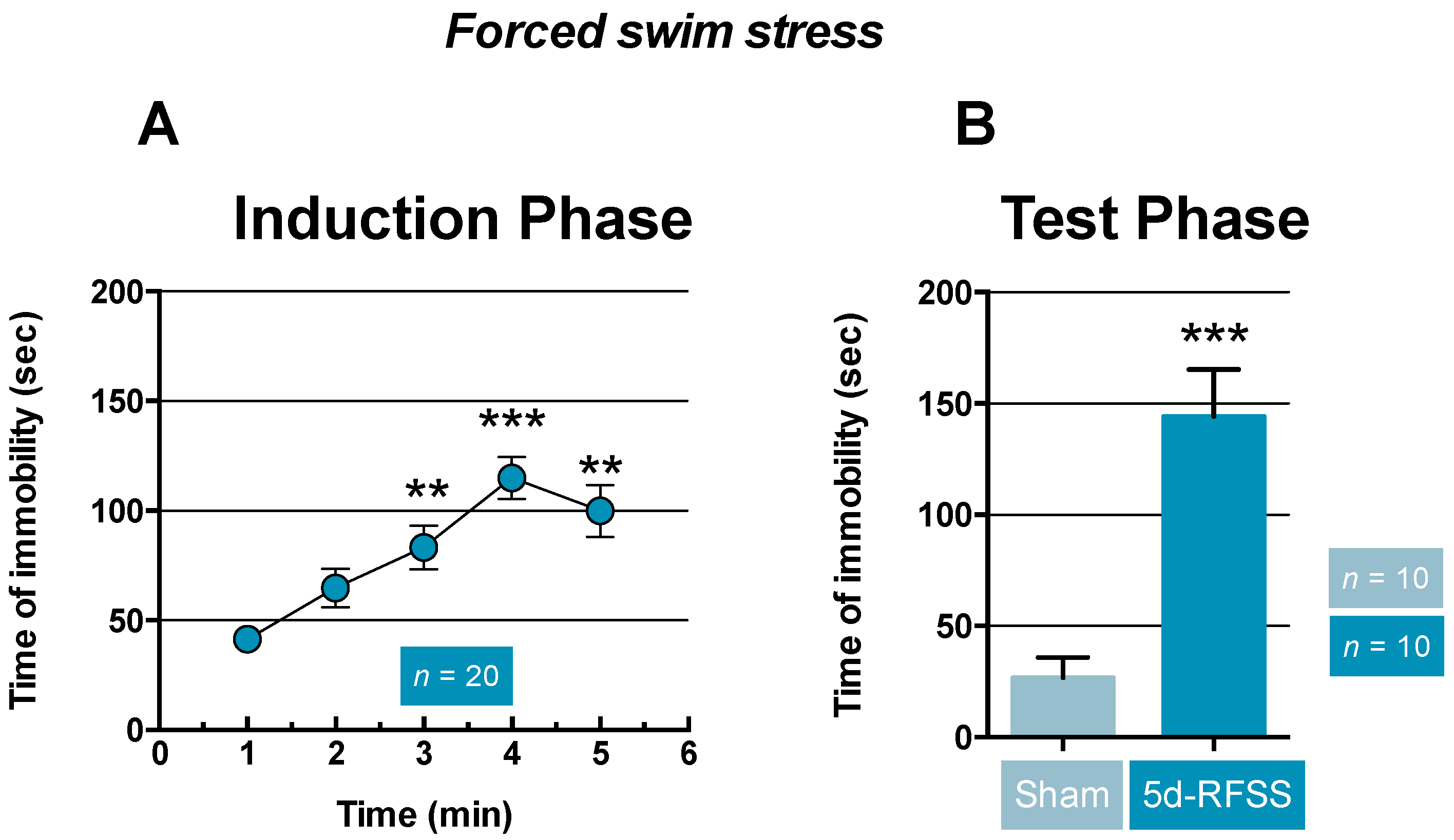
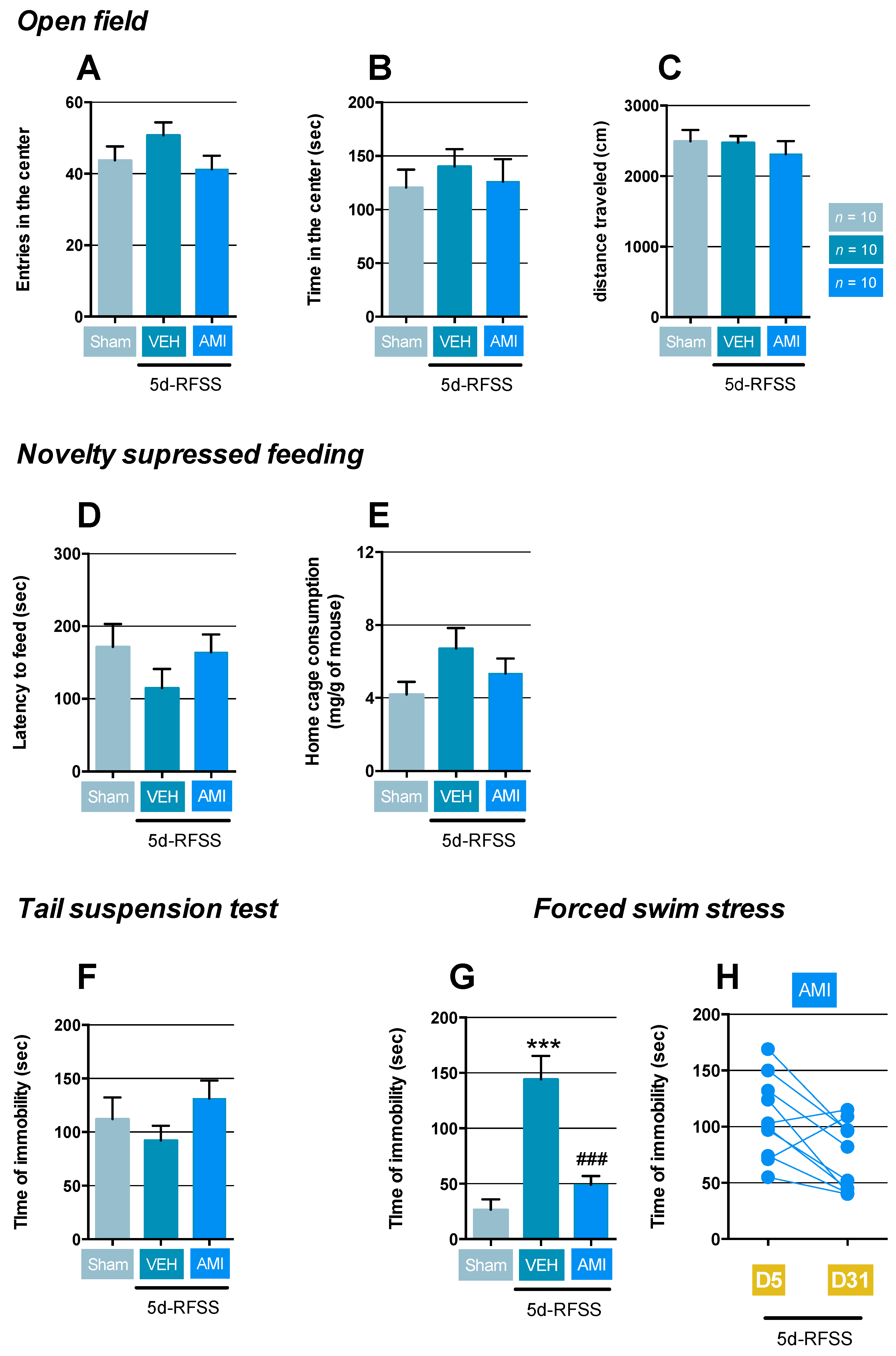
2.1. Effects of 5d-RFSS on Depressive-Like Behavior in C57BL/6J Mice
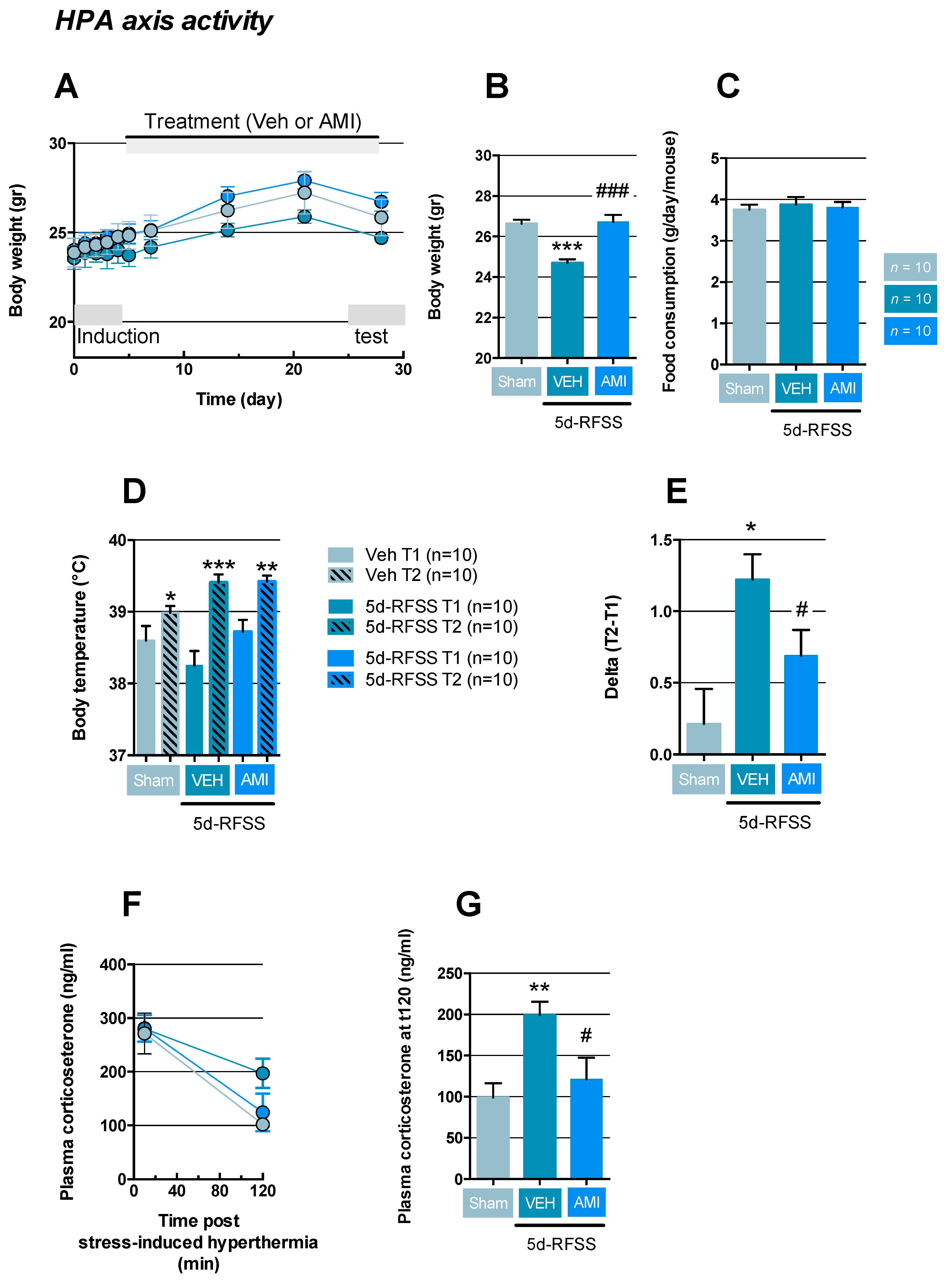
2.2. Effects of 5d-RFSS on HPA Axis Reactivity in C57BL/6J Mice
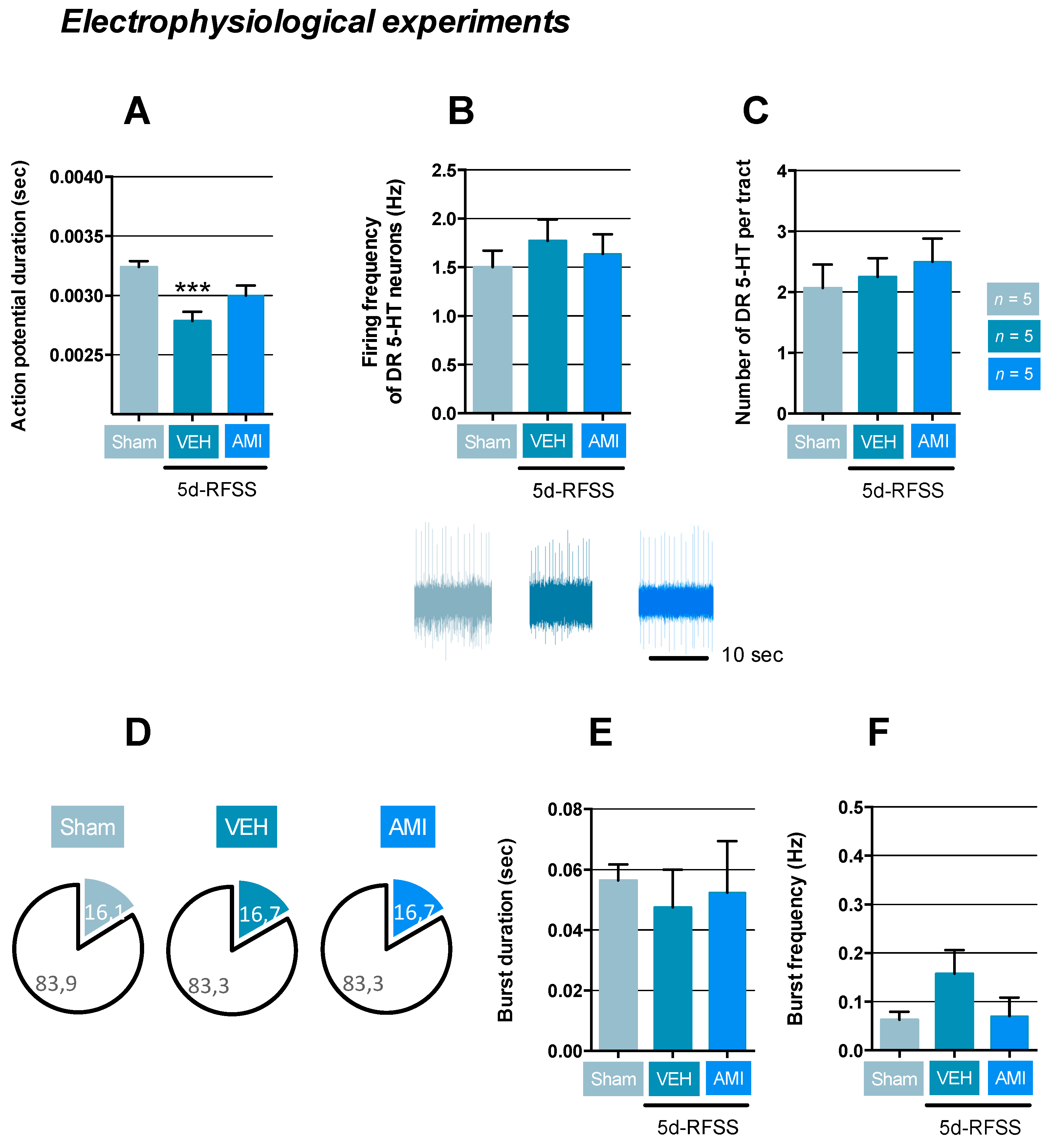
2.3. Effects of 5d-RFSS on Serotonergic Neurotransmission in C57BL/6J Mice
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Drugs
4.3. The Five Days Repeated-Forced Swim Stress and Experimental Protocol
4.4. Behavioral Paradigms
4.4.1. The Open-Field
4.4.2. The Novelty-Suppressed Feeding
4.4.3. The Tail Suspension Test
4.4.4. The Forced-Swim Stress
4.4.5. Body Weight and Food Consumption Measurements
4.4.6. The Stress-Induced Hyperthermia Test
4.5. Plasma Corticosterone Levels
4.6. In Vivo Single unit Recordings of 5-HT Neurons in the Dorsal Raphe
4.7. In Vivo Intracerebral Microdialysis in the Hippocampus of Freely Moving Mice
4.8. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 5d-RFSS | five-day repeated forced-swim stress |
| AMI | amitriptyline |
| CSDS | chronic social defeat stress |
| CORT | corticosterone |
| DR | dorsal raphe |
| FST | forced swim stress |
| HPA | hypothalamic-pituitary-adrenal axis |
| MD | major depression |
| NSF | novelty suppressed feeding |
| OF | open-field |
| SIH | stress-induced hyperthermia |
| UCMS | unpredictable chronic mild stress |
| Veh | vehicle |
References
- Krishnan, V.; Nestler, E.J. The molecular neurobiology of depression. Nature 2008, 455, 894–902. [Google Scholar] [CrossRef] [PubMed]
- Lynch, C.J.; Gunning, F.M.; Liston, C. Causes and Consequences of Diagnostic Heterogeneity in Depression: Paths to Discovering Novel Biological Depression Subtypes. Biol. Psychiatry 2020, 88, 83–94. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Timberlake, M.A., 2nd; Prall, K.; Dwivedi, Y. The recent progress in animal models of depression. Prog. Neuropsy-chopharmacol. Biol. Psychiatry 2017, 77, 99–109. [Google Scholar] [CrossRef] [PubMed]
- Wong, M.L.; Kling, M.A.; Munson, P.J.; Listwak, S.; Licinio, J.; Prolo, P.; Karp, B.; McCutcheon, I.E.; Geracioti, T.D., Jr.; DeBellis, M.D.; et al. Pronounced and sustained central hypernor-adrenergic function in major depression with melancholic features: Relation to hypercortisolism and corticotropin-releasing hormone. Proc. Natl. Acad. Sci. USA 2000, 97, 325–330. [Google Scholar] [CrossRef] [PubMed]
- David, D.J.; Samuels, B.A.; Rainer, Q.; Wang, J.-W.; Marsteller, D.; Mendez, I.; Drew, M.R.; Craig, D.A.; Guiard, B.P.; Guilloux, J.-P.; et al. Neurogenesis-Dependent and -Independent Effects of Fluoxetine in an Animal Model of Anxiety/Depression. Neuron 2009, 62, 479–493. [Google Scholar] [CrossRef]
- Orrico-Sanchez, A.; Chausset-Boissarie, L.; Alves de Sousa, R.; Coutens, B.; Rezai Amin, S.; Vialou, V.; Louis, F.; Hessani, A.; Dansette, P.M.; Zornoza, T.; et al. Antidepressant efficacy of a se-lective organic cation transporter blocker in a mouse model of depression. Mol. Psychiatry 2020, 25, 1245–1259. [Google Scholar] [CrossRef]
- Darcet, F.; Mendez-David, I.; Tritschler, L.; Gardier, A.M.; Guilloux, J.P.; David, D.J. Learning and memory impairments in a neuro-endocrine mouse model of anxiety/depression. Front. Behav. Neurosci. 2014, 8, 136. [Google Scholar] [CrossRef]
- Le Dantec, Y.; Hache, G.; Guilloux, J.P.; Guiard, B.P.; David, D.J.; Adrien, J.; Escourrou, P. NREM sleep hypersomnia and reduced sleep/wake continuity in a neuroendocrine mouse model of anxiety/depression based on chronic corticosterone administration. Neuroscience 2014, 274, 357–368. [Google Scholar] [CrossRef]
- Hache, G.; Guiard, B.P.; Le Dantec, Y.; Orvoën, S.; David, D.J.; Gardier, A.M.; Coudoré, F. Antinociceptive effects of fluoxetine in a mouse model of anxiety/depression. NeuroReport 2012, 23, 525–529. [Google Scholar] [CrossRef]
- Quesseveur, G.; David, D.J.; Gaillard, M.C.; Pla, P.; Wu, M.V.; Nguyen, H.T.; Nicolas, V.; Auregan, G.; David, I.; Dranovsky, A.; et al. BDNF overexpression in mouse hippocampal astrocytes promotes local neuro-genesis and elicits anxiolytic-like activities. Transl. Psychiatry 2013, 3, e253. [Google Scholar] [CrossRef]
- Rainer, Q.; Nguyen, H.T.; Quesseveur, G.; Gardier, A.M.; David, D.J.; Guiard, B.P. Functional Status of Somatodendritic Serotonin 1A Autoreceptor after Long-Term Treatment with Fluoxetine in a Mouse Model of Anxiety/Depression Based on Repeated Corticosterone Administration. Mol. Pharmacol. 2011, 81, 106–112. [Google Scholar] [CrossRef] [PubMed]
- Mendez-David, I.; Guilloux, J.P.; Papp, M.; Tritschler, L.; Mocaer, E.; Gardier, A.M.; Bretin, S.; David, D.J. S 47445 Produces Antide-pressant- and Anxiolytic-Like Effects through Neurogenesis Dependent and Independent Mechanisms. Front. Pharmacol. 2017, 8, 462. [Google Scholar] [CrossRef] [PubMed]
- Mekiri, M.; Gardier, A.M.; David, D.J.; Guilloux, J.-P. Chronic corticosterone administration effects on behavioral emotionality in female c57bl6 mice. Exp. Clin. Psychopharmacol. 2017, 25, 94–104. [Google Scholar] [CrossRef] [PubMed]
- Albert, P.R. Why is depression more prevalent in women? J. Psychiatry Neurosci. 2015, 40, 219–221. [Google Scholar] [CrossRef]
- Nestler, E.J.; Gould, E.; Manji, H.; Buncan, M.; Duman, R.S.; Greshenfeld, H.K.; Hen, R.; Koester, S.; Lederhendler, I.; Meaney, M.; et al. Preclinical models: Status of basic research in depression. Biol. Psychiatry 2002, 52, 503–528. [Google Scholar] [CrossRef]
- Faye, C.; McGowan, J.C.; Denny, C.A.; David, D.J. Neurobiological Mechanisms of Stress Resilience and Implications for the Aged Population. Curr. Neuropharmacol. 2018, 16, 234–270. [Google Scholar] [CrossRef]
- Molendijk, M.L.; De Kloet, E.R. Immobility in the forced swim test is adaptive and does not reflect depression. Psychoneuroendocrinology 2015, 62, 389–391. [Google Scholar] [CrossRef]
- Mul, J.D.; Zheng, J.; Goodyear, L.J. Validity Assessment of 5 Day Repeated Forced-Swim Stress to Model Human Depression in Young-Adult C57BL/6J and BALB/cJ Mice. Eneuro 2016, 3. [Google Scholar] [CrossRef]
- Sun, P.; Wang, F.; Wang, L.; Zhang, Y.; Yamamoto, R.; Sugai, T.; Zhang, Q.; Wang, Z.; Kato, N. Increase in Cortical Pyramidal Cell Excitability Accompanies Depression-Like Behavior in Mice: A Transcranial Magnetic Stimulation Study. J. Neurosci. 2011, 31, 16464–16472. [Google Scholar] [CrossRef] [PubMed]
- Delcourte, S.; Dkhissi-Benyahya, O.; Cooper, H.; Haddjeri, N. Stress Models of Depression: A Question of Bad Timing. Eneuro 2017, 4. [Google Scholar] [CrossRef]
- Olivier, B.; Zethof, T.; Pattij, T.; Van Boogaert, M.; Van Oorschot, R.; Leahy, C.; Oosting, R.; Bouwknecht, A.; Veening, J.; Van Der Gugten, J.; et al. Stress-induced hyperthermia and anxiety: Pharmacological validation. Eur. J. Pharmacol. 2003, 463, 117–132. [Google Scholar] [CrossRef]
- Lanfumey, L.; Mongeau, R.; Cohen-Salmon, C.; Hamon, M. Corticosteroid-serotonin interactions in the neurobiological mecha-nisms of stress-related disorders. Neurosci. Biobehav. Rev. 2008, 32, 1174–1184. [Google Scholar] [CrossRef]
- Guiard, B.P.; El Mansari, M.; Murphy, D.L.; Blier, P. Altered response to the selective serotonin reuptake inhibitor escitalopram in mice heterozygous for the serotonin transporter: An electrophysiological and neurochemical study. Int. J. Neuropsychopharmacol. 2011, 15, 349–361. [Google Scholar] [CrossRef] [PubMed]
- Karatsoreos, I.N. Links between Circadian Rhythms and Psychiatric Disease. Front. Behav. Neurosci. 2014, 8. [Google Scholar] [CrossRef] [PubMed]
- Griesauer, I.; Diao, W.; Ronovsky, M.; Elbau, I.; Sartori, S.; Singewald, N.; Pollak, D.D. Circadian abnormalities in a mouse model of high trait anxiety and depression. Ann. Med. 2014, 46, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Anyan, J.; Verwey, M.; Amir, S. Individual differences in circadian locomotor parameters correlate with anxiety- and depres-sion-like behavior. PLoS ONE 2017, 12, e0181375. [Google Scholar] [CrossRef]
- Serchov, T.; Clement, H.W.; Schwarz, M.K.; Iasevoli, F.; Tosh, D.K.; Idzko, M.; Jacobson, K.A.; de Bartolomeis, A.; Normann, C.; Biber, K.; et al. Increased Signaling via Adenosine A1 Receptors, Sleep Deprivation, Imipramine, and Ketamine Inhibit Depres-sive-like Behavior via Induction of Homer1a. Neuron 2015, 87, 549–562. [Google Scholar] [CrossRef]
- Redrobe, J.P.; Pinot, P.; Bourin, M. The effect of the potassium channel activator, cromakalim, on antidepressant drugs in the forced swimming test in mice. Fundam. Clin. Pharmacol. 1996, 10, 524–528. [Google Scholar] [CrossRef]
- Caldarone, B.J.; Karthigeyan, K.; Harrist, A.; Hunsberger, J.G.; Wittmack, E.; King, S.L.; Jatlow, P.; Picciotto, M.R. Sex differences in response to oral amitriptyline in three animal models of depression in C57BL/6J mice. Psychopharmacology 2003, 170, 94–101. [Google Scholar] [CrossRef]
- Pehrson, A.L.; Leiser, S.C.; Gulinello, M.; Dale, E.; Li, Y.; Waller, J.A.; Sanchez, C. Treatment of cognitive dysfunction in major depressive disorder—a review of the preclinical evidence for efficacy of selective serotonin reuptake inhibitors, serotonin–norepinephrine reuptake inhibitors and the multimodal-acting antidepressant vortioxetine. Eur. J. Pharmacol. 2015, 753, 19–31. [Google Scholar] [CrossRef]
- Parra, A.; Vinader-Caerols, C.; Ferrer-Añó, A.; Urquiza, A.; Monleón, S. The effect of amitriptyline on inhibitory avoidance in mice is dose-dependent. Psicothema 2009, 21, 528–530. [Google Scholar] [PubMed]
- Goldman, P.; Scranton, T.; Messer, W.S. Interaction of amitriptyline with muscarinic receptor subtypes in the rat brain. Neurochem. Int. 1989, 14, 447–454. [Google Scholar] [CrossRef]
- Lamping, N.L.; Spring, B.; Gelenberg, A.J. Effects of two antidepressants on memory performance in depressed outpatients: A double-blind study. Psychopharmacology 1984, 84, 254–261. [Google Scholar] [CrossRef] [PubMed]
- Privitera, G.J.; Misenheimer, M.L.; Doraiswamy, P.M. From weight loss to weight gain: Appetite changes in major depressive disorder as a mirror into brain-environment interactions. Front. Psychol. 2013, 4. [Google Scholar] [CrossRef] [PubMed]
- Surget, A.; Wang, Y.; Leman, S.; Ibarguen-Vargas, Y.; Edgar, N.; Griebel, G.; Belzung, C.; Sibille, E. Corticolimbic Transcriptome Changes are State-Dependent and Region-Specific in a Rodent Model of Depression and of Antidepressant Reversal. Neuropsychopharmacology 2008, 34, 1363–1380. [Google Scholar] [CrossRef]
- Iio, W.; Takagi, H.; Ogawa, Y.; Tsukahara, T.; Chohnan, S.; Toyoda, A. Effects of chronic social defeat stress on peripheral leptin and its hypothalamic actions. BMC Neurosci. 2014, 15, 72. [Google Scholar] [CrossRef]
- Allard, J.S.; Tizabi, Y.; Shaffery, J.P.; Trouth, C.O.; Manaye, K. Stereological analysis of the hypothalamic hypocretin/orexin neurons in an animal model of depression. Neuropeptides 2004, 38, 311–315. [Google Scholar] [CrossRef]
- Nollet, M.; Gaillard, P.; Tanti, A.; Girault, V.; Belzung, C.; Leman, S. Neurogenesis-Independent Antidepressant-Like Effects on Behavior and Stress Axis Response of a Dual Orexin Receptor Antagonist in a Rodent Model of Depression. Neuropsychopharmacology 2012, 37, 2210–2221. [Google Scholar] [CrossRef]
- Nakamura, K. Neural circuit for psychological stress-induced hyperthermia. Temperature 2015, 2, 352–361. [Google Scholar] [CrossRef]
- Veening, J.G.; Bouwknecht, J.; Joosten, H.J.; Dederen, P.; Zethof, T.J.; Groenink, L.; Van Der Gugten, J.; Olivier, B. Stress-induced hyperthermia in the mouse: C-fos expression, corticosterone and temperature changes. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2004, 28, 699–707. [Google Scholar] [CrossRef]
- Pattij, T.; Hijzen, T.H.; Groenink, L.; Oosting, R.S.; Van Der Gugten, J.; Maes, R.A.; Hen, R.; Olivier, B. Stress-induced hyperthermia in the 5-HT1A receptor knockout mouse is normal. Biol. Psychiatry 2001, 49, 569–574. [Google Scholar] [CrossRef]
- Gomez, F.; García-García, L. Anxiogenic-like effects of fluoxetine render adult male rats vulnerable to the effects of a novel stress. Pharmacol. Biochem. Behav. 2017, 153, 32–44. [Google Scholar] [CrossRef] [PubMed]
- Eliwa, H.; Brizard, B.; Le Guisquet, A.-M.; Hen, R.; Belzung, C.; Surget, A. Adult neurogenesis augmentation attenuates anhedonia and HPA axis dysregulation in a mouse model of chronic stress and depression. Psychoneuroendocrinology 2020, 124, 105097. [Google Scholar] [CrossRef] [PubMed]
- Coutens, B.; Rekik, K.; Harster, A.; Etienne, P.; Noirot, V.; Frances, B.; Moulédous, L.; Guiard, B.P. A Citrus Based Sensory Functional Food Ingredient Induces Antidepressant-like Effects: Possible Involvement of an Interplay between the Olfactory and the Ser-otonergic Systems. Neuroscience 2020, 451, 149–163. [Google Scholar] [CrossRef]
- De Montigny, C.; Blier, P. Effects of antidepressant treatments on 5-HT neurotransmission: Electrophysiological and clinical studies. Adv. Biochem. Psychopharmacol. 1984, 39, 223–239. [Google Scholar]
- Richardson-Jones, J.W.; Craige, C.P.; Guiard, B.P.; Stephen, A.; Metzger, K.L.; Kung, H.F.; Gardier, A.M.; Dranovsky, A.; David, D.J.; Beck, S.G.; et al. 5-HT1A Autoreceptor Levels Determine Vulnerability to Stress and Response to Antidepressants. Neuron 2010, 65, 40–52. [Google Scholar] [CrossRef]
- Uhr, M.; Steckler, T.; Yassouridis, A.; Holsboer, F. Penetration of Amitriptyline, but Not of Fluoxetine, into Brain is Enhanced in Mice with Blood-Brain Barrier Deficiency Due to Mdr1a P-Glycoprotein Gene Disruption. Neuropsychopharmacology 2000, 22, 380–387. [Google Scholar] [CrossRef]
- Porsolt, R.D.; Le Pichon, M.; Jalfre, M. Depression: A new animal model sensitive to antidepressant treatments. Nat. Cell Biol. 1977, 266, 730–732. [Google Scholar] [CrossRef]
- Zemdegs, J.; Martin, H.; Pintana, H.; Bullich, S.; Manta, S.; Marqués, M.A.; Moro, C.; Layé, S.; Ducrocq, F.; Chattipakorn, N.; et al. Metformin Promotes Anxiolytic and Antidepressant-Like Responses in Insulin-Resistant Mice by Decreasing Circulating Branched-Chain Amino Acids. J. Neurosci. 2019, 39, 5935–5948. [Google Scholar] [CrossRef]
- Paxinos, G.; Franklin, K. The Mouse Brain in Stereotaxic Coordinates, 5th ed.; Elsevier: Amsterdam, The Netherlands, 2019. [Google Scholar]

| Control Non-stressed Animals (Sham) | 5d-RFSS/Veh | 5d-RFSS/AMI | ||
|---|---|---|---|---|
| Induction Phase Swim stress | D1 | ● | ● | |
| D2 | ● | ● | ||
| D3 | ● | ● | ||
| D4 | ● | ● | ||
| D5 | ● | ● | ||
| Treatments for three weeks | D5-D26 | Veh | Veh | AMI |
| Test Phase | OF (D26) | ● | ● | ● |
| NSF (D28) | ● | ● | ● | |
| TST (D30) | ● | ● | ● | |
| FST (D32) | ● | ● | ● | |
| HPA axis | SIH | ● | ● | ● |
| CORT | ● | ● | ● | |
| Serotonergic system | Electrophysiology | ● | ● | ● |
| Microdialysis | ● | ● | ● |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bullich, S.; Delcourte, S.; Haddjeri, N.; Guiard, B.P. Learned Immobility Produces Enduring Impairment of the HPA Axis Reactivity in Mice without Replicating the Broad Spectrum of Depressive-Like Phenotype. Int. J. Mol. Sci. 2021, 22, 937. https://doi.org/10.3390/ijms22020937
Bullich S, Delcourte S, Haddjeri N, Guiard BP. Learned Immobility Produces Enduring Impairment of the HPA Axis Reactivity in Mice without Replicating the Broad Spectrum of Depressive-Like Phenotype. International Journal of Molecular Sciences. 2021; 22(2):937. https://doi.org/10.3390/ijms22020937
Chicago/Turabian StyleBullich, Sébastien, Sarah Delcourte, Nasser Haddjeri, and Bruno P. Guiard. 2021. "Learned Immobility Produces Enduring Impairment of the HPA Axis Reactivity in Mice without Replicating the Broad Spectrum of Depressive-Like Phenotype" International Journal of Molecular Sciences 22, no. 2: 937. https://doi.org/10.3390/ijms22020937
APA StyleBullich, S., Delcourte, S., Haddjeri, N., & Guiard, B. P. (2021). Learned Immobility Produces Enduring Impairment of the HPA Axis Reactivity in Mice without Replicating the Broad Spectrum of Depressive-Like Phenotype. International Journal of Molecular Sciences, 22(2), 937. https://doi.org/10.3390/ijms22020937






