Regenerative Medicine for Equine Musculoskeletal Diseases
Abstract
Simple Summary
Abstract
1. Introduction
1.1. Equine Musculoskeletal Disease: Clinical Need and Burden of Disease
1.2. Regenerative Medicine Overview: Development of the Field, First Successes, Challenges Preventing Wide-Spread Implementation
1.2.1. Mesenchymal Stem Cells
1.2.2. Autologous Blood Products
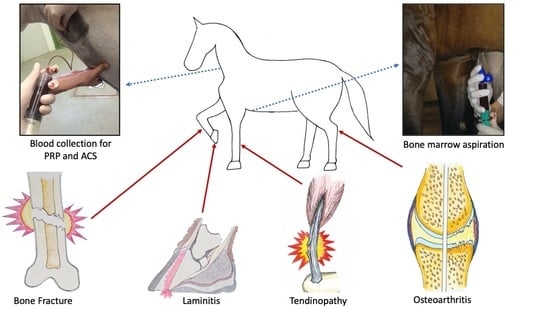
2. Regenerative Therapies by Disease Area
2.1. Tendon/Ligament
2.1.1. Clinical Need and Burden of Disease
2.1.2. Regenerative Therapies
2.2. Osteoarthritis
2.2.1. Clinical Need and Burden of Disease
2.2.2. Regenerative Therapies
2.3. Meniscus
2.3.1. Clinical Need and Burden of Disease
2.3.2. Regenerative Therapies
2.4. Bone
2.4.1. Clinical Need and Burden of Disease
2.4.2. Regenerative Therapies
2.5. Laminitis
2.5.1. Clinical Need and Burden of Disease
2.5.2. Regenerative Therapies
3. Future Perspectives
3.1. Regulatory
3.2. Novel Regenerative Therapies
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Egenvall, A.; Bonnett, B.; Wattle, O.; Emanuelson, U. Veterinary-care events and costs over a 5-year follow-up period for warmblooded riding horses with or without previously recorded locomotor problems in Sweden. Prev. Vet. Med. 2008, 83, 130–143. [Google Scholar] [CrossRef] [PubMed]
- Murray, R.C.; Walters, J.M.; Snart, H.; Dyson, S.J.; Parkin, T.D.H. Identification of risk factors for lameness in dressage horses. Vet. J. 2010, 184, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Bertuglia, A.; Bullone, M.; Rossotto, F.; Gasparini, M. Epidemiology of musculoskeletal injuries in a population of harness Standardbred racehorses in training. BMC Vet. Res. 2014, 10, 11. [Google Scholar] [CrossRef] [PubMed]
- Van Weeren, P.R.; Back, W. Musculoskeletal Disease in Aged Horses and Its Management. Vet. Clin. N. Am. Equine Pract. 2016, 32, 229–247. [Google Scholar] [CrossRef]
- Egenvall, A.; Tranquille, C.A.; Lönnell, A.C.; Bitschnau, C.; Oomen, A.; Hernlund, E.; Montavon, S.; Franko, M.A.; Murray, R.C.; Weishaupt, M.A.; et al. Days-lost to training and competition in relation to workload in 263 elite show-jumping horses in four European countries. Prev. Vet. Med. 2013, 112, 387–400. [Google Scholar] [CrossRef]
- Jeffcott, L.B.; Rossdale, P.D.; Freestone, J.; Frank, C.J.; Towers-Clark, P.F. An assessment of wastage in thoroughbred racing from conception to 4 years of age. Equine Vet. J. 1982, 14, 185–198. [Google Scholar] [CrossRef]
- Rossdale, P.; Hopes, R.; Digby, N.; Offord, K. Epidemiological study of wastage among racehorses 1982 and 1983. Vet. Rec. 1985, 116, 66. [Google Scholar] [CrossRef]
- Olivier, A.; Nurton, J.P.; Guthrie, A.J. An epizoological study of wastage in Thoroughbred racehorses in Gauteng, South Africa. J. S. Afr. Vet. Assoc. 1997, 68, 125–127. [Google Scholar] [CrossRef]
- Ramzan, P.H.L.; Palmer, L. Musculoskeletal injuries in Thoroughbred racehorses: A study of three large training yards in Newmarket, UK (2005–2007). Vet. J. 2011, 187, 325–329. [Google Scholar] [CrossRef]
- Ireland, J.L.; McGowan, C.M.; Clegg, P.D.; Chandler, K.J.; Pinchbeck, G.L. A survey of health care and disease in geriatric horses aged 30years or older. Vet. J. 2012, 192, 57–64. [Google Scholar] [CrossRef]
- Ireland, J.L.; Clegg, P.D.; McGowan, C.M.; McKane, S.A.; Chandler, K.J.; Pinchbeck, G.L. Disease prevalence in geriatric horses in the United Kingdom: Veterinary clinical assessment of 200 cases. Equine Vet. J. 2012, 44, 101–106. [Google Scholar] [CrossRef] [PubMed]
- Jönsson, L.; Roepstorff, L.; Egenvall, A.; Näsholm, A.; Dalin, G.; Philipsson, J. Prevalence of clinical findings at examinations of young Swedish warmblood ridinghorses. Acta Vet. Scand. 2013, 55, 34. [Google Scholar] [CrossRef] [PubMed]
- Dyson, S. Lameness and poor performance in the sport horse: Dressage, show jumping and horse trials. J. Equine Vet. Sci. 2002, 22, 145–150. [Google Scholar] [CrossRef]
- Murray, R.C.; Dyson, S.J.; Tranquille, C.; Adams, V. Association of type of sport and performance level with anatomical site of orthopaedic injury diagnosis. Equine Vet. J. 2006, 38, 411–416. [Google Scholar] [CrossRef] [PubMed]
- Dabareiner, R.M.; Cohen, N.D.; Carter, G.K.; Nunn, S.; Moyer, W. Musculoskeletal problems associated with lameness and poor performance among horses used for barrel racing: 118 cases (2000–2003). J. Am. Vet. Med. Assoc. 2005, 227, 1646–1650. [Google Scholar] [CrossRef] [PubMed]
- Ely, E.R.; Avella, C.S.; Price, J.S.; Smith, R.K.W.; Wood, J.L.N.; Verheyen, K.L.P. Descriptive epidemiology of fracture, tendon and suspensory ligament injuries in National Hunt racehorses in training. Equine Vet. J. 2009, 41, 372–378. [Google Scholar] [CrossRef]
- Reed, S.R.; Jackson, B.F.; McIlwraith, C.W.M.; Wright, I.M.; Pilsworth, R.; Knapp, S.; Wood, J.L.N.; Price, J.S.; Verheyen, K.L.P. Descriptive epidemiology of joint injuries in Thoroughbred racehorses in training. Equine Vet. J. 2012, 44, 13–19. [Google Scholar] [CrossRef]
- Ireland, J.L.; Clegg, P.D.; McGowan, C.M.; Platt, L.; Pinchbeck, G.L. Factors associated with mortality of geriatric horses in the United Kingdom. Prev. Vet. Med. 2011, 101, 204–218. [Google Scholar] [CrossRef]
- Mason, C.; Dunnill, P. A brief definition of regenerative medicine. Regen. Med. 2008, 3, 1–5. [Google Scholar] [CrossRef]
- Katari, R.; Peloso, A.; Orlando, G. Tissue Engineering and Regenerative Medicine: Semantic Considerations for an Evolving Paradigm. Front. Bioeng. Biotechnol. 2015, 2, 57. [Google Scholar] [CrossRef]
- Geburek, F.; Gaus, M.; Van Schie, H.T.M.; Rohn, K.; Stadler, P.M. Effect of intralesional platelet-rich plasma (PRP) treatment on clinical and ultrasonographic parameters in equine naturally occurring superficial digital flexor tendinopathies—A randomized prospective controlled clinical trial. BMC Vet. Res. 2016, 12, 191. [Google Scholar] [CrossRef] [PubMed]
- Geburek, F.; Roggel, F.; van Schie, H.T.M.; Beineke, A.; Estrada, R.; Weber, K.; Hellige, M.; Rohn, K.; Jagodzinski, M.; Welke, B.; et al. Effect of single intralesional treatment of surgically induced equine superficial digital flexor tendon core lesions with adipose-derived mesenchymal stromal cells: A controlled experimental trial. Stem Cell Res. Ther. 2017, 8, 129. [Google Scholar] [CrossRef] [PubMed]
- Giunta, K.; Donnell, J.R.; Donnell, A.D.; Frisbie, D.D. Prospective randomized comparison of platelet rich plasma to extracorporeal shockwave therapy for treatment of proximal suspensory pain in western performance horses. Res. Vet. Sci. 2019, 126, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, M.W.J.; O’Kane, S. Scar-free healing: From embryonic mechanisms to adult therapeutic intervention. Philos. Trans. R. Soc. B Biol. Sci. 2004, 359, 839–850. [Google Scholar] [CrossRef] [PubMed]
- Degen, K.E.; Gourdie, R.G. Embryonic wound healing: A primer for engineering novel therapies for tissue repair. Birth Defects Res. Part C Embryo Today Rev. 2012, 96, 258–270. [Google Scholar] [CrossRef]
- Cowin, A.J.; Brosnan, M.P.; Holmes, T.M.; Ferguson, M.W.J. Endogenous inflammatory response to dermal wound healing in the fetal and adult mouse. Dev. Dyn. 1998, 212, 385–393. [Google Scholar] [CrossRef]
- Kornicka, K.; Geburek, F.; Röcken, M.; Marycz, K. Stem Cells in Equine Veterinary Practice—Current Trends, Risks, and Perspectives. J. Clin. Med. 2019, 8, 675. [Google Scholar] [CrossRef]
- Dominici, M.; Blanc, K.L.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.; Krause, D.; Deans, R.; Keating, A.; Prockop, D.; Horwitz, E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef]
- Smith, R.K.W.; Korda, M.; Blunn, G.W.; Goodship, A.E. Isolation and implantation of autologous equine mesenchymal stem cells from bone marrow into the superficial digital flexor tendon as a potential novel treatment. Equine Vet. J. 2003, 35, 99–102. [Google Scholar] [CrossRef]
- Lombana, K.G.; Goodrich, L.R.; Phillips, J.N.; Kisiday, J.D.; Ruple-Czerniak, A.; McIlwraith, C.W. An Investigation of Equine Mesenchymal Stem Cell Characteristics from Different Harvest Sites: More Similar Than Not. Front. Vet. Sci. 2015, 2, 66. [Google Scholar] [CrossRef]
- Barrachina, L.; Remacha, A.R.; Romero, A.; Vitoria, A.; Albareda, J.; Prades, M.; Roca, M.; Zaragoza, P.; Vázquez, F.J.; Rodellar, C. Assessment of effectiveness and safety of repeat administration of proinflammatory primed allogeneic mesenchymal stem cells in an equine model of chemically induced osteoarthritis. BMC Vet. Res. 2018, 14, 241. [Google Scholar] [CrossRef] [PubMed]
- Lange-Consiglio, A.; Corradetti, B.; Meucci, A.; Perego, R.; Bizzaro, D.; Cremonesi, F. Characteristics of equine mesenchymal stem cells derived from amnion and bone marrow: In vitro proliferative and multilineage potential assessment. Equine Vet. J. 2013, 45, 737–744. [Google Scholar] [CrossRef] [PubMed]
- Hoynowski, S.M.; Fry, M.M.; Gardner, B.M.; Leming, M.T.; Tucker, J.R.; Black, L.; Sand, T.; Mitchell, K.E. Characterization and differentiation of equine umbilical cord-derived matrix cells. Biochem. Biophys. Res. Commun. 2007, 362, 347–353. [Google Scholar] [CrossRef] [PubMed]
- Desancé, M.; Contentin, R.; Bertoni, L.; Gomez-Leduc, T.; Branly, T.; Jacquet, S.; Betsch, J.-M.; Batho, A.; Legendre, F.; Audigié, F.; et al. Chondrogenic Differentiation of Defined Equine Mesenchymal Stem Cells Derived from Umbilical Cord Blood for Use in Cartilage Repair Therapy. Int. J. Mol. Sci. 2018, 19, 537. [Google Scholar] [CrossRef]
- Loon, V.J.F.V.; Scheffer, C.J.W.; Genn, H.J.; Hoogendoorn, A.C.; Greve, J.W. Clinical follow-up of horses treated with allogeneic equine mesenchymal stem cells derived from umbilical cord blood for different tendon and ligament disorders. Vet. Q. 2014, 34, 92–97. [Google Scholar] [CrossRef]
- Carrade, D.D.; Owens, S.D.; Galuppo, L.D.; Vidal, M.A.; Ferraro, G.L.; Librach, F.; Buerchler, S.; Friedman, M.S.; Walker, N.J.; Borjesson, D.L. Clinicopathologic findings following intra-articular injection of autologous and allogeneic placentally derived equine mesenchymal stem cells in horses. Cytotherapy 2011, 13, 419–430. [Google Scholar] [CrossRef]
- Carrade, D.D.; Lame, M.W.; Kent, M.S.; Clark, K.C.; Walker, N.J.; Borjesson, D.L. Comparative Analysis of the Immunomodulatory Properties of Equine Adult-Derived Mesenchymal Stem Cells. Cell Med. 2012, 4, 1–12. [Google Scholar] [CrossRef]
- Romero, A.; Barrachina, L.; Ranera, B.; Remacha, A.R.; Moreno, B.; de Blas, I.; Sanz, A.; Vázquez, F.J.; Vitoria, A.; Junquera, C.; et al. Comparison of autologous bone marrow and adipose tissue derived mesenchymal stem cells, and platelet rich plasma, for treating surgically induced lesions of the equine superficial digital flexor tendon. Vet. J. 2017, 224, 76–84. [Google Scholar] [CrossRef]
- Lovati, A.B.; Corradetti, B.; Consiglio, A.L.; Recordati, C.; Bonacina, E.; Bizzaro, D.; Cremonesi, F. Comparison of equine bone marrow-, umbilical cord matrix and amniotic fluid-derived progenitor cells. Vet. Res. Commun. 2011, 35, 103–121. [Google Scholar] [CrossRef]
- Gale, A.L.; Linardi, R.L.; McClung, G.; Mammone, R.M.; Ortved, K.F. Comparison of the Chondrogenic Differentiation Potential of Equine Synovial Membrane-Derived and Bone Marrow-Derived Mesenchymal Stem Cells. Front. Vet. Sci. 2019, 6, 178. [Google Scholar] [CrossRef]
- Toupadakis, C.A.; Wong, A.; Genetos, D.C.; Cheung, W.K.; Borjesson, D.L.; Ferraro, G.L.; Galuppo, L.D.; Leach, J.K.; Owens, S.D.; Yellowley, C.E. Comparison of the osteogenic potential of equine mesenchymal stem cells from bone marrow, adipose tissue, umbilical cord blood, and umbilical cord tissue. Am. J. Vet. Res. 2010, 71, 1237–1245. [Google Scholar] [CrossRef] [PubMed]
- Guest, D.J.; Smith, M.R.W.; Allen, W.R. Equine embryonic stem-like cells and mesenchymal stromal cells have different survival rates and migration patterns following their injection into damaged superficial digital flexor tendon. Equine Vet. J. 2010, 42, 636–642. [Google Scholar] [CrossRef] [PubMed]
- McClellan, A.; Paterson, Y.Z.; Paillot, R.; Guest, D.J. Equine Fetal, Adult, and Embryonic Stem Cell-Derived Tenocytes are All Immune Privileged but Exhibit Different Immune Suppressive Properties In Vitro. Stem Cells Dev. 2019, 28, 1413–1423. [Google Scholar] [CrossRef] [PubMed]
- Barberini, D.J.; Freitas, N.P.P.; Magnoni, M.S.; Maia, L.; Listoni, A.J.N.; Heckler, M.C.; Sudano, M.J.; Golim, M.A.; da Landim-Alvarenga, F.C.; Amorim, R.M. Equine mesenchymal stem cells from bone marrow, adipose tissue and umbilical cord: Immunophenotypic characterization and differentiation potential. Stem Cell Res. Ther. 2014, 5, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Koerner, J.; Nesic, D.; Romero, J.D.; Brehm, W.; Mainil-Varlet, P.; Grogan, S.P. Equine peripheral blood-derived progenitors in comparison to bone marrow-derived mesenchymal stem cells. Stem Cells 2006, 24, 1613–1619. [Google Scholar] [CrossRef] [PubMed]
- Reed, S.A.; Johnson, S.E. Equine umbilical cord blood contains a population of stem cells that express Oct4 and differentiate into mesodermal and endodermal cell types. J. Cell. Physiol. 2008, 215, 329–336. [Google Scholar] [CrossRef] [PubMed]
- Kisiday, J.D.; Kopesky, P.W.; Evans, C.H.; Grodzinsky, A.J.; McIlwraith, C.W.; Frisbie, D.D. Evaluation of adult equine bone marrow- and adipose-derived progenitor cell chondrogenesis in hydrogel cultures. J. Orthop. Res. 2008, 26, 322–331. [Google Scholar] [CrossRef]
- Vidal, M.A.; Walker, N.J.; Napoli, E.; Borjesson, D.L. Evaluation of Senescence in Mesenchymal Stem Cells Isolated from Equine Bone Marrow, Adipose Tissue, and Umbilical Cord Tissue. Stem Cells Dev. 2012, 21, 273–283. [Google Scholar] [CrossRef]
- Carter-Arnold, J.L.; Neilsen, N.L.; Amelse, L.L.; Odoi, A.; Dhar, M.S. In vitro analysis of equine, bone marrow-derived mesenchymal stem cells demonstrates differences within age- and gender-matched horses. Equine Vet. J. 2013, 46, 589–595. [Google Scholar] [CrossRef]
- Arnhold, S.J.; Goletz, I.; Klein, H.; Stumpf, G.; Beluche, L.A.; Rohde, C.; Addicks, K.; Litzke, L.F. Isolation and characterization of bone marrow-derived equine mesenchymal stem cells. Am. J. Vet. Res. 2007, 68, 1095–1105. [Google Scholar] [CrossRef]
- Hass, R.; Kasper, C.; Böhm, S.; Jacobs, R. Different populations and sources of human mesenchymal stem cells (MSC): A comparison of adult and neonatal tissue-derived MSC. Cell Commun. Signal. 2011, 9, 12. [Google Scholar] [CrossRef] [PubMed]
- Rogers, I.; Casper, R.F. Umbilical cord blood stem cells. Best Pract. Res Clin. Obstet. 2004, 18, 893–908. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Khan, D.; Delling, J.; Tobiasch, E. Mechanisms Underlying the Osteo- and Adipo-Differentiation of Human Mesenchymal Stem Cells. Sci. World J. 2012, 2012, 793823. [Google Scholar] [CrossRef] [PubMed]
- Barlow, S.; Brooke, G.; Chatterjee, K.; Price, G.; Pelekanos, R.; Rossetti, T.; Doody, M.; Venter, D.; Pain, S.; Gilshenan, K.; et al. Comparison of Human Placenta- and Bone MarrowDerived Multipotent Mesenchymal Stem Cells. Stem Cells Dev. 2008, 17, 1095–1108. [Google Scholar] [CrossRef]
- Caplan, A.I. Mesenchymal stem cells. J. Orthop. Res. 1991, 9, 641–650. [Google Scholar] [CrossRef]
- Gnecchi, M.; He, H.; Liang, O.D.; Melo, L.G.; Morello, F.; Mu, H.; Noiseux, N.; Zhang, L.; Pratt, R.E.; Ingwall, J.S.; et al. Paracrine action accounts for marked protection of ischemic heart by Akt-modified mesenchymal stem cells. Nat. Med. 2005, 11, 367–368. [Google Scholar] [CrossRef]
- Caplan, A.I.; Correa, D. The MSC: An injury drugstore. Cell Stem Cell 2011, 9, 11–15. [Google Scholar] [CrossRef]
- Espinosa, G.; Plaza, A.; Schenffeldt, A.; Alarcón, P.; Gajardo, G.; Uberti, B.; Morán, G.; Henríquez, C. Equine bone marrow-derived mesenchymal stromal cells inhibit reactive oxygen species production by neutrophils. Vet. Immunol. Immunopathol. 2019, 221, 109975. [Google Scholar] [CrossRef]
- Bastos, F.Z.; Barussi, F.C.M.; Leite, L.M.B.; Jamur, V.R.; de Soares, A.A.; Senegaglia, A.C.; Junior, P.V.M. Quality control and immunomodulatory potential for clinical-grade equine bone marrow-derived mesenchymal stromal cells and conditioned medium. Res. Vet. Sci. 2020, 132, 407–415. [Google Scholar] [CrossRef]
- Barrachina, L.; Remacha, A.R.; Romero, A.; Vázquez, F.J.; Albareda, J.; Prades, M.; Ranera, B.; Zaragoza, P.; Martín-Burriel, I.; Rodellar, C. Effect of inflammatory environment on equine bone marrow derived mesenchymal stem cells immunogenicity and immunomodulatory properties. Vet. Immunol. Immunopathol. 2016, 171, 57–65. [Google Scholar] [CrossRef]
- Khatab, S.; van Osch, G.J.; Kops, N.; Bastiaansen-Jenniskens, Y.M.; Bos, P.K.; Verhaar, J.A.; Bernsen, M.R.; van Buul, G.M. Mesenchymal stem cell secretome reduces pain and prevents cartilage damage in a murine osteoarthritis model. Eur. Cells Mater. 2018, 36, 218–230. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Leijten, J.C.H.; Georgi, N.; Post, J.N.; van Blitterswijk, C.A.; Karperien, M. Trophic Effects of Mesenchymal Stem Cells Increase Chondrocyte Proliferation and Matrix Formation. Tissue Eng. Part A 2011, 17, 1425–1436. [Google Scholar] [CrossRef]
- Baglio, S.R.; Pegtel, D.M.; Baldini, N. Mesenchymal stem cell secreted vesicles provide novel opportunities in (stem) cell-free therapy. Front. Physiol. 2012, 3, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Schnabel, L.; Boone, L.; Peroni, J. Regenerative Medicine. In Equine Surgery, 5th ed.; Auer, J., Kümmerle, J., Prange, T., Eds.; Saunders Elsevier: St. Louis, MO, USA, 2019. [Google Scholar]
- Ionita, C.R.; Troillet, A.R.; Vahlenkamp, T.W.; Winter, K.; Brehm, W.; Ionita, J.-C. Comparison of humoral insulin-like growth factor-1, platelet-derived growth factor-BB, transforming growth factor-β 1, and interleukin-1 receptor antagonist concentrations among equine autologous blood-derived preparations. Am. J. Vet. Res. 2016, 77, 898–905. [Google Scholar] [CrossRef] [PubMed]
- Hessel, L.N.; Bosch, G.; van Weeren, P.R.; Ionita, J.C. Equine autologous platelet concentrates: A comparative study between different available systems. Equine Vet. J. 2014, 47, 319–325. [Google Scholar] [CrossRef] [PubMed]
- Mageed, M.; Ionita, C.; Kissich, C.; Brehm, W.; Winter, K.; Ionita, J.-C. Influence of cryopreservation and mechanical stimulation on equine Autologous Conditioned Plasma (ACP). Tier Rztliche Praxis G Gro Tiere Nutztiere 2015, 43, 97–104. [Google Scholar] [CrossRef]
- McLellan, J.; Plevin, S. Does it matter which platelet-rich plasma we use? Equine Vet. Educ. 2011, 23, 101–104. [Google Scholar] [CrossRef]
- Sundman, E.A.; Cole, B.J.; Fortier, L.A. Growth Factor and Catabolic Cytokine Concentrations Are Influenced by the Cellular Composition of Platelet-Rich Plasma. Am. J. Sports Med. 2011, 39, 2135–2140. [Google Scholar] [CrossRef]
- Schnabel, L.V.; Mohammed, H.O.; Miller, B.J.; McDermott, W.G.; Jacobson, M.S.; Santangelo, K.S.; Fortier, L.A. Platelet rich plasma (PRP) enhances anabolic gene expression patterns in flexor digitorum superficialis tendons. J. Orthop. Res. 2007, 25, 230–240. [Google Scholar] [CrossRef]
- Bramono, D.S.; Richmond, J.C.; Weitzel, P.P.; Kaplan, D.L.; Altman, G.H. Matrix Metalloproteinases and Their Clinical Applications in Orthopaedics. Clin. Orthop. Relat. Res. 2004, 428, 272–285. [Google Scholar] [CrossRef]
- Zimmermann, R.; Jakubietz, R.; Jakubietz, M.; Strasser, E.; Schlegel, A.; Wiltfang, J.; Eckstein, R. Different preparation methods to obtain platelet components as a source of growth factors for local application. Transfusion 2001, 41, 1217–1224. [Google Scholar] [CrossRef] [PubMed]
- Hraha, T.H.; Doremus, K.M.; McIlwraith, C.W.; Frisbie, D.D. Autologous conditioned serum: The comparative cytokine profiles of two commercial methods (IRAP and IRAP II) using equine blood. Equine Vet. J. 2011, 43, 516–521. [Google Scholar] [CrossRef]
- Marques-Smith, P.; Kallerud, A.S.; Johansen, G.M.; Boysen, P.; Jacobsen, A.M.; Reitan, K.M.; Henriksen, M.M.; Löfgren, M.; Fjordbakk, C.T. Is clinical effect of autologous conditioned serum in spontaneously occurring equine articular lameness related to ACS cytokine profile? BMC Vet. Res. 2020, 16, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Rutgers, M.; Saris, D.B.; Dhert, W.J.; Creemers, L.B. Cytokine profile of autologous conditioned serum for treatment of osteoarthritis, in vitro effects on cartilage metabolism and intra-articular levels after injection. Arthritis Res. 2015, 12, R114. [Google Scholar] [CrossRef] [PubMed]
- De Ascurra, J.L.; Ehrle, A.; Einspanier, R.; Lischer, C. Influence of Incubation Time and Incubation Tube on the Cytokine and Growth Factor Concentrations of Autologous Conditioned Serum in Horses. J. Equine Vet. Sci. 2019, 75, 30–34. [Google Scholar] [CrossRef] [PubMed]
- Lasarzik, J.; Bondzio, A.; Rettig, M.; Estrada, R.; Klaus, C.; Ehrle, A.; Einspanier, R.; Lischer, C.J. Evaluation of Two Protocols Using Autologous Conditioned Serum for Intra-articular Therapy of Equine Osteoarthritis-A Pilot Study Monitoring Cytokines and Cartilage-Specific Biomarkers. J. Equine Vet. Sci. 2018, 60, 35–42.e2. [Google Scholar] [CrossRef]
- McClain, A.K.; McCarrel, T.M. The effect of four different freezing conditions and time in frozen storage on the concentration of commonly measured growth factors and enzymes in equine platelet-rich plasma over six months. BMC Vet. Res. 2019, 15, 1–9. [Google Scholar] [CrossRef]
- Hauschild, G.; Geburek, F.; Gosheger, G.; Eveslage, M.; Serrano, D.; Streitbürger, A.; Johannlükens, S.; Menzel, D.; Mischke, R. Short term storage stability at room temperature of two different platelet-rich plasma preparations from equine donors and potential impact on growth factor concentrations. BMC Vet. Res. 2017, 13, 1–9. [Google Scholar] [CrossRef]
- Spaas, J.H.; Guest, D.J.; de Walle, G.R.V. Tendon regeneration in human and equine athletes: Ubi Sumus-Quo Vadimus (where are we and where are we going to)? Sports Med. 2012, 42, 871–890. [Google Scholar] [CrossRef]
- Dakin, S.G. A review of the healing processes in equine superficial digital flexor tendinopathy. Equine Vet. Educ. 2016, 29, 516–520. [Google Scholar] [CrossRef]
- Clegg, P.D. Musculoskeletal disease and injury, now and in the future. Part 2: Tendon and ligament injuries. Equine Vet. J. 2012, 44, 371–375. [Google Scholar] [CrossRef] [PubMed]
- Dowling, B.A.; Dart, A.J.; Hodgson, D.R.; Smith, R.K. Superficial digital flexor tendonitis in the horse. Equine Vet. J. 2000, 32, 369–378. [Google Scholar] [CrossRef] [PubMed]
- Thorpe, C.T.; Clegg, P.D.; Birch, H.L. A review of tendon injury: Why is the equine superficial digital flexor tendon most at risk? Equine Vet. J. 2010, 42, 174–180. [Google Scholar] [CrossRef] [PubMed]
- Kasashima, Y.; Takahashi, T.; Smith, R.K.W.; Goodship, A.E.; Kuwano, A.; Ueno, T.; Hirano, S. Prevalence of superficial digital flexor tendonitis and suspensory desmitis in Japanese Thoroughbred flat racehorses in 1999. Equine Vet. J. 2004, 36, 346–350. [Google Scholar] [CrossRef] [PubMed]
- Pinchbeck, G.L.; Clegg, P.D.; Proudman, C.J.; Stirk, A.; Morgan, K.L.; French, N.P. Horse injuries and racing practices in National Hunt racehorses in the UK: The results of a prospective cohort study. Vet. J. 2004, 167, 45–52. [Google Scholar] [CrossRef]
- Richardson, L.E.; Dudhia, J.; Clegg, P.D.; Smith, R. Stem cells in veterinary medicine--attempts at regenerating equine tendon after injury. Trends Biotechnol. 2007, 25, 409–416. [Google Scholar] [CrossRef]
- Dakin, S.G.; Dudhia, J.; Smith, R.K.W. Resolving an inflammatory concept: The importance of inflammation and resolution in tendinopathy. Vet. Immunol. Immunopathol. 2014, 158, 121–127. [Google Scholar] [CrossRef]
- Walden, G.; Liao, X.; Donell, S.; Raxworthy, M.J.; Riley, G.P.; Saeed, A. A Clinical, Biological, and Biomaterials Perspective into Tendon Injuries and Regeneration. Tissue Eng. Part B Rev. 2017, 23, 44–58. [Google Scholar] [CrossRef]
- Dakin, S.G.; Dudhia, J.; Werling, N.J.; Werling, D.; Abayasekara, D.R.E.; Smith, R.K.W. Inflamm-Aging and Arachadonic Acid Metabolite Differences with Stage of Tendon Disease. PLoS ONE 2012, 7, e48978. [Google Scholar] [CrossRef]
- Dakin, S.G.; Werling, D.; Hibbert, A.; Abayasekara, D.R.E.; Young, N.J.; Smith, R.K.W.; Dudhia, J. Macrophage sub-populations and the lipoxin A4 receptor implicate active inflammation during equine tendon repair. PLoS ONE 2012, 7, e32333. [Google Scholar] [CrossRef]
- Dakin, S.G.; Dudhia, J.; Smith, R.K.W. Science in brief: Resolving tendon inflammation. A new perspective. Equine Vet. J. 2013, 45, 398–400. [Google Scholar] [CrossRef] [PubMed]
- Smith, R.K.W.; Werling, N.J.; Dakin, S.G.; Alam, R.; Goodship, A.E.; Dudhia, J. Beneficial Effects of Autologous Bone Marrow-Derived Mesenchymal Stem Cells in Naturally Occurring Tendinopathy. PLoS ONE 2013, 8, e75697. [Google Scholar] [CrossRef]
- Smith, R.K.W. Mesenchymal stem cell therapy for equine tendinopathy. Disabil. Rehabil. 2008, 30, 1752–1758. [Google Scholar] [CrossRef] [PubMed]
- Lam, K.H.; Parkin, T.D.H.; Riggs, C.M.; Morgan, K.L. Descriptive analysis of retirement of Thoroughbred racehorses due to tendon injuries at the Hong Kong Jockey Club (1992–2004). Equine Vet. J. 2007, 39, 143–148. [Google Scholar] [CrossRef]
- Crovace, A.; Lacitignola, L.; Rossi, G.; Francioso, E. Histological and Immunohistochemical Evaluation of Autologous Cultured Bone Marrow Mesenchymal Stem Cells and Bone Marrow Mononucleated Cells in Collagenase-Induced Tendinitis of Equine Superficial Digital Flexor Tendon. Vet. Med. Int. 2010, 2010, 250978. [Google Scholar] [CrossRef]
- Usunier, B.; Benderitter, M.; Tamarat, R.; Chapel, A. Management of Fibrosis: The Mesenchymal Stromal Cells Breakthrough. Stem Cells Int. 2014, 2014, 340257. [Google Scholar] [CrossRef]
- Linard, C.; Busson, E.; Holler, V.; Strup-Perrot, C.; Lacave-Lapalun, J.-V.; Lhomme, B.; Prat, M.; Devauchelle, P.; Sabourin, J.-C.; Simon, J.-M.; et al. Repeated autologous bone marrow-derived mesenchymal stem cell injections improve radiation-induced proctitis in pigs. Stem Cells Transl. Med. 2013, 2, 916–927. [Google Scholar] [CrossRef]
- Conze, P.; van Schie, H.T.; van Weeren, R.; Staszyk, C.; Conrad, S.; Skutella, T.; Hopster, K.; Rohn, K.; Stadler, P.; Geburek, F. Effect of autologous adipose tissue-derived mesenchymal stem cells on neovascularization of artificial equine tendon lesions. Regen. Med. 2014, 9, 743–757. [Google Scholar] [CrossRef]
- Durgam, S.; Stewart, M. Evidence Supporting Intralesional Stem Cell Therapy to Improve Equine Flexor Tendon Healing. Vet. Évid. 2016, 2. [Google Scholar] [CrossRef]
- Taylor, S.E.; Smith, R.K.W.; Clegg, P.D. Mesenchymal stem cell therapy in equine musculoskeletal disease: Scientific fact or clinical fiction? Equine Vet. J. 2007, 39, 172–180. [Google Scholar] [CrossRef]
- Torrent, A.; Spriet, M.; Espinosa-Mur, P.; Clark, K.C.; Whitcomb, M.B.; Borjesson, D.L.; Galuppo, L.D. Ultrasound-guided injection of the cranial tibial artery for stem cell administration in horses. Equine Vet. J. 2019, 51, 681–687. [Google Scholar] [CrossRef] [PubMed]
- Ahrberg, A.B.; Horstmeier, C.; Berner, D.; Brehm, W.; Gittel, C.; Hillmann, A.; Josten, C.; Rossi, G.; Schubert, S.; Winter, K.; et al. Effects of mesenchymal stromal cells versus serum on tendon healing in a controlled experimental trial in an equine model. BMC Musculoskelet 2018, 19, 230. [Google Scholar] [CrossRef] [PubMed]
- Bue, M.D.; Ricco, S.; Ramoni, R.; Conti, V.; Gnudi, G.; Grolli, S. Equine adipose-tissue derived mesenchymal stem cells and platelet concentrates: Their association in vitro and in vivo. Vet. Res. Commun. 2008, 32 (Suppl. S1), S51–S55. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, A.M.; Badial, P.R.; Álvarez, L.E.C.; Yamada, A.L.M.; Borges, A.S.; Deffune, E.; Hussni, C.A.; Alves, A.L.G. Equine tendonitis therapy using mesenchymal stem cells and platelet concentrates: A randomized controlled trial. Stem Cell Res. Ther. 2013, 4, 85. [Google Scholar] [CrossRef] [PubMed]
- Shojaee, A.; Parham, A. Strategies of tenogenic differentiation of equine stem cells for tendon repair: Current status and challenges. Stem Cell Res. Ther. 2019, 10, 181. [Google Scholar] [CrossRef]
- Consiglio, A.L.; Rossi, D.; Tassan, S.; Perego, R.; Cremonesi, F.; Parolini, O. Conditioned medium from horse amniotic membrane-derived multipotent progenitor cells: Immunomodulatory activity in vitro and first clinical application in tendon and ligament injuries in vivo. Stem Cells Dev. 2013, 22, 3015–3024. [Google Scholar] [CrossRef]
- Brandão, J.S.; Alvarenga, M.L.; Pfeifer, J.P.H.; dos Santos, V.H.; Fonseca-Alves, C.E.; Rodrigues, M.; Laufer-Amorim, R.; Castillo, J.A.L.; Alves, A.L.G. Allogeneic mesenchymal stem cell transplantation in healthy equine superficial digital flexor tendon: A study of the local inflammatory response. Res. Vet. Sci. 2018, 118, 423–430. [Google Scholar] [CrossRef]
- Chamberlain, G.; Fox, J.; Ashton, B.; Middleton, J. Concise review: Mesenchymal stem cells: Their phenotype, differentiation capacity, immunological features, and potential for homing. Stem Cells 2007, 25, 2739–2749. [Google Scholar] [CrossRef]
- Lange-Consiglio, A.; Tassan, S.; Corradetti, B.; Meucci, A.; Perego, R.; Bizzaro, D.; Cremonesi, F. Investigating the efficacy of amnion-derived compared with bone marrow–derived mesenchymal stromal cells in equine tendon and ligament injuries. Cytotherapy 2013, 15, 1011–1020. [Google Scholar] [CrossRef]
- Bonilla-Gutiérrez, A.F.; Castillo-Franz, C.; López, C.; Álvarez, M.E.; Giraldo, C.E.; Carmona, J.U. Equine suspensory ligament and tendon explants cultured with platelet-rich gel supernatants release different anti-inflammatory and anabolic mediators. Biomed. Pharm. 2018, 108, 476–485. [Google Scholar] [CrossRef]
- Smith, J.J.; Ross, M.W.; Smith, R.K.W. Anabolic effects of acellular bone marrow, platelet rich plasma, and serum on equine suspensory ligament fibroblasts in vitro. Vet. Comp. Orthop. Traumatol. 2006, 19, 43–47. [Google Scholar] [PubMed]
- Schnabel, L.V.; Mohammed, H.O.; Jacobson, M.S.; Fortier, L.A. Effects of platelet rich plasma and acellular bone marrow on gene expression patterns and DNA content of equine suspensory ligament explant cultures. Equine Vet. J. 2008, 40, 260–265. [Google Scholar] [CrossRef] [PubMed]
- Bosch, G.; Moleman, M.; Barneveld, A.; van Weeren, P.R.; van Schie, H.T.M. The effect of platelet-rich plasma on the neovascularization of surgically created equine superficial digital flexor tendon lesions. Scand. J. Med. Sci. Sports 2011, 21, 554–561. [Google Scholar] [CrossRef] [PubMed]
- Castelijns, G.; Crawford, A.; Schaffer, J.; Ortolano, G.A.; Beauregard, T.; Smith, R.K.W. Evaluation of a filter-prepared platelet concentrate for the treatment of suspensory branch injuries in horses. Vet. Comp. Orthop. 2011, 24, 363–369. [Google Scholar] [CrossRef]
- Zuffova, K.; Krisova, S.; Zert, Z. Platelet rich plasma treatment of superficial digital flexor tendon lesions in racing Thoroughbreds. Vet. Med. 2013, 58, 230–239. [Google Scholar] [CrossRef]
- Waselau, M.; Sutter, W.W.; Genovese, R.L.; Bertone, A.L. Intralesional injection of platelet-rich plasma followed by controlled exercise for treatment of midbody suspensory ligament desmitis in Standardbred racehorses. J. Am. Vet. Med. Assoc. 2008, 232, 1515–1520. [Google Scholar] [CrossRef]
- Geburek, F.; Lietzau, M.; Beineke, A.; Rohn, K.; Stadler, P.M. Effect of a single injection of autologous conditioned serum (ACS) on tendon healing in equine naturally occurring tendinopathies. Stem Cell Res. Ther. 2015, 6, 79. [Google Scholar] [CrossRef]
- McIlwraith, C.W.; Frisbie, D.D. The horse as a model of naturally occurring osteoarthritis. Bone Jt. 2012, 1, 297–309. [Google Scholar] [CrossRef]
- Oke, S.; McIlwraith, C.W.; Moyer, W. Review of the Economic Impact of Osteoarthritis and Oral Joint-Health Supplements in Horses. Jt. AAEP 2010, 56, 15. [Google Scholar]
- Ireland, J.L.; Clegg, P.D.; McGowan, C.M.; McKane, S.A.; Pinchbeck, G.L. A cross-sectional study of geriatric horses in the United Kingdom. Part 1: Demographics and management practices. Equine Vet. J. 2010, 43, 30–36. [Google Scholar] [CrossRef]
- Loeser, R.F.; Goldring, S.R.; Scanzello, C.R.; Goldring, M.B. Osteoarthritis: A disease of the joint as an organ. Arthritis Rheum. 2012, 64, 1697–1707. [Google Scholar] [CrossRef] [PubMed]
- Goldring, M.B.; Otero, M.; Plumb, D.A.; Dragomir, C.; Favero, M.; Hachem, K.E.; Hashimoto, K.; Roach, H.I.; Olivotto, E.; Borzí, R.M.; et al. Roles of inflammatory and anabolic cytokines in cartilage metabolism: Signals and multiple effectors converge upon MMP-13 regulation in osteoarthritis. Eur. Cells Mater. 2011, 21, 202–220. [Google Scholar] [CrossRef] [PubMed]
- Berenbaum, F. Osteoarthritis as an inflammatory disease (osteoarthritis is not osteoarthrosis!). Osteoarthr. Cartil. 2013, 21, 16–21. [Google Scholar] [CrossRef] [PubMed]
- Mobasheri, A.; Kalamegam, G.; Musumeci, G.; Batt, M.E. Chondrocyte and mesenchymal stem cell-based therapies for cartilage repair in osteoarthritis and related orthopaedic conditions. Maturitas 2014, 78, 188–198. [Google Scholar] [CrossRef]
- Raman, S.; FitzGerald, U.; Murphy, J.M. Interplay of Inflammatory Mediators with Epigenetics and Cartilage Modifications in Osteoarthritis. Front. Bioeng. Biotechnol. 2018, 6, 1361. [Google Scholar] [CrossRef]
- Holt, D.W.; Henderson, M.L.; Stockdale, C.E.; Farrell, J.T.; Kooyman, D.L.; Bridgewater, L.C.; Seegmiller, R.E. Osteoarthritis-like changes in the heterozygous sedc mouse associated with the HtrA1–Ddr2–Mmp-13 degradative pathway: A new model of osteoarthritis. Osteoarthr. Cartil. 2012, 20, 430–439. [Google Scholar] [CrossRef]
- Sandell, L.J.; Aigner, T. Articular cartilage and changes in Arthritis: Cell biology of osteoarthritis. Arthritis Res. Ther. 2001, 3, 107. [Google Scholar] [CrossRef]
- Goldring, M.B.; Goldring, S.R. Osteoarthritis. J. Cell. Physiol. 2007, 213, 626–634. [Google Scholar] [CrossRef]
- Sellam, J.; Berenbaum, F. The role of synovitis in pathophysiology and clinical symptoms of osteoarthritis. Nat. Rev. Rheumatol. 2010, 6, 625–635. [Google Scholar] [CrossRef]
- Mobasheri, A.; Rayman, M.P.; Gualillo, O.; Sellam, J.; van der Kraan, P.; Fearon, U. The role of metabolism in the pathogenesis of osteoarthritis. Nat. Rev. Rheumatol. 2017, 13, 302–311. [Google Scholar] [CrossRef]
- Sokolove, C.M.L.J. Role of inflammation in the pathogenesis of osteoarthritis: Latest findings and interpretations. Ther. Adv. Musculoskelet. Dis. 2013, 5, 77–94. [Google Scholar] [CrossRef] [PubMed]
- Rahmati, M.; Mobasheri, A.; Mozafari, M. Inflammatory mediators in osteoarthritis: A critical review of the state-of-the-art, current prospects, and future challenges. Bone 2016, 85, 81–90. [Google Scholar] [CrossRef] [PubMed]
- De Lange-Brokaar, B.J.E.; Ioan-Facsinay, A.; van Osch, G.J.V.M.; Zuurmond, A.M.; Schoones, J.; Toes, R.E.M.; Huizinga, T.W.J.; Kloppenburg, M. Synovial inflammation, immune cells and their cytokines in osteoart. Osteoarthr. Cartil. 2012, 20, 1484–1499. [Google Scholar] [CrossRef]
- Aigner, T.; Söder, S.; Gebhard, P.M.; McAlinden, A.; Haag, J. Mechanisms of Disease: Role of chondrocytes in the pathogenesis of osteoarthritis—structure, chaos and senescence. Nat. Clin. Pract. Rheumatol. 2007, 3, 391–399. [Google Scholar] [CrossRef] [PubMed]
- Minguzzi, M.; Cetrullo, S.; D’Adamo, S.; Silvestri, Y.; Flamigni, F.; Borzí, R.M. Emerging Players at the Intersection of Chondrocyte Loss of Maturational Arrest, Oxidative Stress, Senescence and Low-Grade Inflammation in Osteoarthritis. Oxidative Med. Cell. Longev. 2018, 2018, 1–17. [Google Scholar] [CrossRef]
- Houard, X.; Goldring, M.B.; Berenbaum, F. Homeostatic Mechanisms in Articular Cartilage and Role of Inflammation in Osteoarthritis. Curr. Rheumatol. Rep. 2013, 15, 375. [Google Scholar] [CrossRef]
- Sherwood, J. Osteoarthritis year in review 2018: Biology. Osteoarthr. Cartil. 2018, 27, 365–370. [Google Scholar] [CrossRef]
- Kraus, V.B.; McDaniel, G.; Huebner, J.L.; Stabler, T.V.; Pieper, C.F.; Shipes, S.W.; Petry, N.A.; Low, P.S.; Shen, J.; McNearney, T.A.; et al. Direct in vivo evidence of activated macrophages in human osteoarthritis. Osteoarthr. Cartil. 2016, 24, 1613–1621. [Google Scholar] [CrossRef]
- Manferdini, C.; Paolella, F.; Gabusi, E.; Gambari, L.; Piacentini, A.; Filardo, G.; Fleury-Cappellesso, S.; Barbero, A.; Murphy, M.; Lisignoli, G. Adipose stromal cells mediated switching of the pro-inflammatory profile of M1-like macrophages is facilitated by PGE2: In vitro evaluation. Osteoarthr. Cartil. 2017, 25, 1161–1171. [Google Scholar] [CrossRef]
- Manferdini, C.; Paolella, F.; Gabusi, E.; Silvestri, Y.; Gambari, L.; Cattini, L.; Filardo, G.; Fleury-Cappellesso, S.; Lisignoli, G. From osteoarthritic synovium to synovial-derived cells characterization: Synovial macrophages are key effector cells. Arthritis Res. Ther. 2016, 18, 1697. [Google Scholar] [CrossRef]
- Utomo, L.; van Osch, G.J.V.M.; Bayon, Y.; Verhaar, J.A.N.; Bastiaansen-Jenniskens, Y.M. Guiding synovial inflammation by macrophage phenotype modulation: An in vitro study towards a therapy for osteoarthritis. Osteoarthr. Cartil. 2016, 24, 1629–1638. [Google Scholar] [CrossRef] [PubMed]
- Utomo, L.; Bastiaansen-Jenniskens, Y.M.; Verhaar, J.A.N.; van Osch, G.J.V.M. Cartilage inflammation and degeneration is enhanced by pro-inflammatory (M1) macrophages in vitro, but not inhibited directly by anti-inflammatory (M2) macrophages. Osteoarthr. Cartil. 2016, 24, 2162–2170. [Google Scholar] [CrossRef] [PubMed]
- Fahy, N.; de Melle, M.L.V.; Lehmann, J.; Wei, W.; Grotenhuis, N.; Farrell, E.; van der Kraan, P.M.; Murphy, J.M.; Bastiaansen-Jenniskens, Y.M.; van Osch, G.J.V.M. Human osteoarthritic synovium impacts chondrogenic differentiation of MSCs via macrophage polarisation state. Osteoarthr. Cartil. 2014, 22, 1167–1175. [Google Scholar] [CrossRef] [PubMed]
- Mathiessen, P.G.C.A. Synovitis in osteoarthritis: Current understanding with therapeutic implications. Arthritis Res. Ther. 2017, 19, 338. [Google Scholar] [CrossRef]
- Vinatier, C.; Domínguez, E.; Guicheux, J.; Caramés, B. Role of the Inflammation-Autophagy-Senescence Integrative Network in Osteoarthritis. Front. Physiol. 2018, 9, 1. [Google Scholar] [CrossRef] [PubMed]
- Goldring, M.B.; Goldring, S.R. Articular cartilage and subchondral bone in the pathogenesis of osteoarthritis. Ann. N. Y. Acad. Sci. 2010, 1192, 230–237. [Google Scholar] [CrossRef]
- Chan, P.M.B.; Zhu, L.; Wen, C.Y.; Chiu, K.Y. Subchondral bone proteomics in osteoarthritis: Current status and perspectives. J. Orthop. Transl. 2015, 3, 71–77. [Google Scholar] [CrossRef]
- Li, G.; Yin, J.; Gao, J.; Cheng, T.S.; Pavlos, N.J.; Zhang, C.; Zheng, M.H. Subchondral bone in osteoarthritis: Insight into risk factors and microstructural changes. Arthritis Res. Ther. 2013, 15, 223. [Google Scholar] [CrossRef]
- Fahy, N.; Farrell, E.; Ritter, T.; Ryan, A.E.; Murphy, J.M. Immune Modulation to Improve Tissue Engineering Outcomes for Cartilage Repair in the Osteoarthritic Joint. Tissue Eng. Part B Rev. 2015, 21, 55–66. [Google Scholar] [CrossRef]
- Jeon, O.H.; Kim, C.; Laberge, R.-M.; Demaria, M.; Rathod, S.; Vasserot, A.P.; Chung, J.W.; Kim, D.H.; Poon, Y.; David, N.; et al. Local clearance of senescent cells attenuates the development of post-traumatic osteoarthritis and creates a pro-regenerative environment. Nat. Med. 2017, 23, 775–781. [Google Scholar] [CrossRef]
- Goodrich, L.R.; Nixon, A.J. Medical treatment of osteoarthritis in the horse—A review. Vet. J. 2006, 171, 51–69. [Google Scholar] [CrossRef] [PubMed]
- Grauw, J.C.; Visser-Meijer, M.C.; Lashley, F.; Meeus, P.; Weeren, P.R. Intra-articular treatment with triamcinolone compared with triamcinolone with hyaluronate: A randomised open-label multicentre clinical trial in 80 lame horses. Equine Vet. J. 2016, 48, 152–158. [Google Scholar] [CrossRef] [PubMed]
- Broeckx, S.; Suls, M.; Beerts, C.; Vandenberghe, A.; Seys, B.; Wuertz-Kozak, K.; Duchateau, L.; Spaas, J.H. Allogenic mesenchymal stem cells as a treatment for equine degenerative joint disease: A pilot study. Curr. Stem Cell Res. Ther. 2014, 9, 497–503. [Google Scholar] [CrossRef] [PubMed]
- Hill, J.A.; Cassano, J.M.; Goodale, M.B.; Fortier, L.A. Antigenicity of mesenchymal stem cells in an inflamed joint environment. Am. J. Vet. Res. 2017, 78, 867–875. [Google Scholar] [CrossRef] [PubMed]
- Colbath, A.C.; Dow, S.W.; Hopkins, L.S.; Phillips, J.N.; McIlwraith, C.W.; Goodrich, L.R. Single and repeated intra-articular injections in the tarsocrural joint with allogeneic and autologous equine bone marrow-derived mesenchymal stem cells are safe, but did not reduce acute inflammation in an experimental interleukin-1β model of synovitis. Equine Vet. J. 2020, 52, 601–612. [Google Scholar] [CrossRef]
- Cokelaere, S.; Malda, J.; van Weeren, P.R. Cartilage defect repair in horses: Current strategies and recent developments in regenerative medicine of the equine joint with emphasis on the surgical approach. Vet. J. 2016, 214, 61–71. [Google Scholar] [CrossRef]
- Nixon, A.J.; Rickey, E.; Butler, T.J.; Scimeca, M.S.; Moran, N.; Matthews, G.L. A chondrocyte infiltrated collagen type I/III membrane (MACI® implant) improves cartilage healing in the equine patellofemoral joint model. Osteoarthr. Cartil. 2015, 23, 648–660. [Google Scholar] [CrossRef]
- Nixon, A.J.; Sparks, H.D.; Begum, L.; McDonough, S.; Scimeca, M.S.; Moran, N.; Matthews, G.L. Matrix-Induced Autologous Chondrocyte Implantation (MACI) Using a Cell-Seeded Collagen Membrane Improves Cartilage Healing in the Equine Model. J. Bone Jt. Surg. 2017, 99, 1987–1998. [Google Scholar] [CrossRef]
- Frisbie, D.D.; Bowman, S.M.; Colhoun, H.A.; DiCarlo, E.F.; Kawcak, C.E.; McIlwraith, C.W. Evaluation of autologous chondrocyte transplantation via a collagen membrane in equine articular defects—Results at 12 and 18 months. Osteoarthr. Cartil. 2008, 16, 667–679. [Google Scholar] [CrossRef]
- Bekkers, J.E.J.; Tsuchida, A.I.; van Rijen, M.H.P.; Vonk, L.A.; Dhert, W.J.A.; Creemers, L.B.; Saris, D.B.F. Single-stage cell-based cartilage regeneration using a combination of chondrons and mesenchymal stromal cells: Comparison with microfracture. Am. J. Sports Med. 2013, 41, 2158–2166. [Google Scholar] [CrossRef]
- Dahlin, R.L.; Kinard, L.A.; Lam, J.; Needham, C.J.; Lu, S.; Kasper, F.K.; Mikos, A.G. Articular chondrocytes and mesenchymal stem cells seeded on biodegradable scaffolds for the repair of cartilage in a rat osteochondral defect model. Biomaterials 2014, 35, 7460–7469. [Google Scholar] [CrossRef] [PubMed]
- Jo, C.H.; Lee, Y.G.; Shin, W.H.; Kim, H.; Chai, J.W.; Jeong, E.C.; Kim, J.E.; Shim, H.; Shin, J.S.; Shin, I.S.; et al. Intra-Articular Injection of Mesenchymal Stem Cells for the Treatment of Osteoarthritis of the Knee: A Proof-of-Concept Clinical Trial. Stem Cells 2014, 32, 1254–1266. [Google Scholar] [CrossRef] [PubMed]
- Menarim, B.C.; Gillis, K.H.; Oliver, A.; Mason, C.; Ngo, Y.; Werre, S.R.; Barrett, S.H.; Luo, X.; Byron, C.R.; Dahlgren, L.A. Autologous bone marrow mononuclear cells modulate joint homeostasis in an equine in vivo model of synovitis. FASEB J. 2019, 33, 14337–14353. [Google Scholar] [CrossRef] [PubMed]
- Frisbie, D.D.; Kisiday, J.D.; Kawcak, C.E.; Werpy, N.M.; McIlwraith, C.W. Evaluation of adipose-derived stromal vascular fraction or bone marrow-derived mesenchymal stem cells for treatment of osteoarthritis. J. Orthop. Res. 2009, 27, 1675–1680. [Google Scholar] [CrossRef] [PubMed]
- Delco, M.L.; Goodale, M.; Talts, J.F.; Pownder, S.L.; Koff, M.F.; Miller, A.D.; Nixon, B.; Bonassar, L.J.; Lundgren-Åkerlund, E.; Fortier, L.A. Integrin α10β1-Selected Mesenchymal Stem Cells Mitigate the Progression of Osteoarthritis in an Equine Talar Impact Model. Am. J. Sports Med. 2019, 48, 612–623. [Google Scholar] [CrossRef]
- Yamada, A.L.M.; de Carvalho, A.M.; Moroz, A.; Deffune, E.; Watanabe, M.J.; Hussni, C.A.; Rodrigues, C.A.; Alves, A.L.G. Mesenchymal stem cell enhances chondral defects healing in horses. Stem Cell Discov 2013, 2013, 218–225. [Google Scholar] [CrossRef][Green Version]
- Broeckx, S.Y.; Martens, A.M.; Bertone, A.L.; Brantegem, L.V.; Duchateau, L.; Hecke, L.V.; Dumoulin, M.; Oosterlinck, M.; Chiers, K.; Hussein, H.; et al. The use of equine chondrogenic-induced mesenchymal stem cells as a treatment for osteoarthritis: A randomised, double-blinded, placebo-controlled proof-of-concept study. Equine Vet. J. 2019, 51, 787–794. [Google Scholar] [CrossRef]
- Barrachina, L.; Remacha, A.R.; Romero, A.; Zaragoza, P.; Vázquez, F.J.; Rodellar, C. Differentiation of equine bone marrow derived mesenchymal stem cells increases the expression of immunogenic genes. Vet. Immunol. Immunopathol. 2018, 200, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Mariñas-Pardo, L.; García-Castro, J.; Rodríguez-Hurtado, I.; Rodríguez-García, M.I.; Núñez-Naveira, L.; Hermida-Prieto, M. Allogeneic Adipose-Derived Mesenchymal Stem Cells (Horse Allo 20) for the Treatment of Osteoarthritis-Associated Lameness in Horses: Characterization, Safety, and Efficacy of Intra-Articular Treatment. Stem Cells Dev. 2018, 27, 1147–1160. [Google Scholar] [CrossRef]
- Zayed, M.; Newby, S.; Misk, N.; Donnell, R.; Dhar, M. Xenogenic Implantation of Equine Synovial Fluid-Derived Mesenchymal Stem Cells Leads to Articular Cartilage Regeneration. Stem Cells Int. 2018, 2018, 1–9. [Google Scholar] [CrossRef]
- Joswig, A.-J.; Mitchell, A.; Cummings, K.J.; Levine, G.J.; Gregory, C.A.; Smith, R.; Watts, A.E. Repeated intra-articular injection of allogeneic mesenchymal stem cells causes an adverse response compared to autologous cells in the equine model. Stem Cell Res. Ther. 2017, 8, 42. [Google Scholar] [CrossRef] [PubMed]
- Pezzanite, L.M.; Fortier, L.A.; Antczak, D.F.; Cassano, J.M.; Brosnahan, M.M.; Miller, D.; Schnabel, L.V. Equine allogeneic bone marrow-derived mesenchymal stromal cells elicit antibody responses in vivo. Stem Cell Res. Ther. 2015, 6, 54. [Google Scholar] [CrossRef] [PubMed]
- Berglund, A.K.; Schnabel, L.V. Allogeneic major histocompatibility complex-mismatched equine bone marrow-derived mesenchymal stem cells are targeted for death by cytotoxic anti-major histocompatibility complex antibodies. Equine Vet. J. 2017, 49, 539–544. [Google Scholar] [CrossRef] [PubMed]
- Pigott, J.H.; Ishihara, A.; Wellman, M.L.; Russell, D.S.; Bertone, A.L. Inflammatory effects of autologous, genetically modified autologous, allogeneic, and xenogeneic mesenchymal stem cells after intra-articular injection in horses. Vet. Comp. Orthop. 2013, 26, 453–460. [Google Scholar] [CrossRef][Green Version]
- Barrachina, L.; Cequier, A.; Romero, A.; Vitoria, A.; Zaragoza, P.; Vázquez, F.J.; Rodellar, C. Allo-antibody production after intraarticular administration of mesenchymal stem cells (MSCs) in an equine osteoarthritis model: Effect of repeated administration, MSC inflammatory stimulation, and equine leukocyte antigen (ELA) compatibility. Stem Cell Res. Ther. 2020, 11, 52. [Google Scholar] [CrossRef] [PubMed]
- Carmona, J.U.; Ríos, D.L.; López, C.; Álvarez, M.E.; Pérez, J.E.; Bohórquez, M.E. In vitro effects of platelet-rich gel supernatants on histology and chondrocyte apoptosis scores, hyaluronan release and gene expression of equine cartilage explants challenged with lipopolysaccharide. BMC Vet. Res. 2016, 12, 135. [Google Scholar] [CrossRef]
- Kisiday, J.D.; McIlwraith, C.W.; Rodkey, W.G.; Frisbie, D.D.; Steadman, J.R. Effects of Platelet-Rich Plasma Composition on Anabolic and Catabolic Activities in Equine Cartilage and Meniscal Explants. Cartilage 2012, 3, 245–254. [Google Scholar] [CrossRef]
- Pichereau, F.; Décory, M.; Ramos, G.C. Autologous Platelet Concentrate as a Treatment for Horses with Refractory Fetlock Osteoarthritis. J. Equine Vet. Sci. 2014, 34, 489–493. [Google Scholar] [CrossRef]
- Carmona, J.U.; Argüelles, D.; Climent, F.; Prades, M. Autologous Platelet Concentrates as a Treatment of Horses with Osteoarthritis: A Preliminary Pilot Clinical Study. J. Equine Vet. Sci. 2007, 27, 167–170. [Google Scholar] [CrossRef]
- Garbin, L.C.; Olver, C.S. Platelet-Rich Products and Their Application to Osteoarthritis. J. Equine Vet. Sci. 2020, 86, 102820. [Google Scholar] [CrossRef]
- Smit, Y.; Marais, H.J.; Thompson, P.N.; Mahne, A.T.; Goddard, A. Clinical findings, synovial fluid cytology and growth factor concentrations after intra-articular use of a platelet-rich product in horses with osteoarthritis. J. S. Afr. Vet. Assoc. 2019, 90, 26–29. [Google Scholar] [CrossRef] [PubMed]
- Smiley, S.T.; King, J.A.; Hancock, W.W. Fibrinogen Stimulates Macrophage Chemokine Secretion through Toll-Like Receptor 4. J. Immunol. 2001, 167, 2887–2894. [Google Scholar] [CrossRef] [PubMed]
- Flick, M.J.; LaJeunesse, C.M.; Talmage, K.E.; Witte, D.P.; Palumbo, J.S.; Pinkerton, M.D.; Thornton, S.; Degen, J.L. Fibrin(ogen) exacerbates inflammatory joint disease through a mechanism linked to the integrin αMβ2 binding motif. J. Clin. Investig. 2007, 117, 3224–3235. [Google Scholar] [CrossRef] [PubMed]
- Lindemann, S.; Tolley, N.D.; Dixon, D.A.; McIntyre, T.M.; Prescott, S.M.; Zimmerman, G.A.; Weyrich, A.S. Activated platelets mediate inflammatory signaling by regulated interleukin 1β synthesis. J. Cell Biol. 2001, 154, 485–490. [Google Scholar] [CrossRef] [PubMed]
- Boilard, E.; Nigrovic, P.A.; Larabee, K.; Watts, G.F.M.; Coblyn, J.S.; Weinblatt, M.E.; Massarotti, E.M.; Remold-O’Donnell, E.; Farndale, R.W.; Ware, J.; et al. Platelets Amplify Inflammation in Arthritis via Collagen-Dependent Microparticle Production. Science 2010, 327, 580–583. [Google Scholar] [CrossRef]
- Hottz, E.D.; Monteiro, A.P.T.; Bozza, F.A.; Bozza, P.T. Inflammasome in Platelets: Allying Coagulation and Inflammation in Infectious and Sterile Diseases? Med. Inflamm. 2015, 2015, 1–7. [Google Scholar] [CrossRef]
- Del Conde, I.; Crúz, M.A.; Zhang, H.; López, J.A.; Afshar-Kharghan, V. Platelet activation leads to activation and propagation of the complement system. J. Exp. Med. 2005, 201, 871–879. [Google Scholar] [CrossRef]
- Wang, Q.; Rozelle, A.L.; Lepus, C.M.; Scanzello, C.R.; Song, J.J.; Larsen, D.M.; Crish, J.F.; Bebek, G.; Ritter, S.Y.; Lindstrom, T.M.; et al. Identification of a central role for complement in osteoarthritis. Nat. Med. 2011, 17, 1674–1679. [Google Scholar] [CrossRef]
- Hamad, O.A.; Ekdahl, K.N.; Nilsson, P.H.; Andersson, J.; Magotti, P.; Lambris, J.D.; Nilsson, B. Complement activation triggered by chondroitin sulfate released by thrombin receptor-activated platelets. J. Thromb. Haemost. 2008, 6, 1413–1421. [Google Scholar] [CrossRef]
- Browning, S.R.; Weiser, A.M.; Woolf, N.; Golish, S.R.; SanGiovanni, T.P.; Scuderi, G.J.; Carballo, C.; Hanna, L.S. Platelet-Rich Plasma Increases Matrix Metalloproteinases in Cultures of Human Synovial Fibroblasts. J. Bone Jt. Surg. 2012, 94, e172. [Google Scholar] [CrossRef]
- Jansen, N.W.D.; Roosendaal, G.; Bijlsma, J.W.J.; DeGroot, J.; Theobald, M.; Lafeber, F.P.J.G. Degenerated and healthy cartilage are equally vulnerable to blood-induced damage. Ann. Rheum. Dis. 2008, 67, 1468. [Google Scholar] [CrossRef] [PubMed]
- Van Meegeren, M.E.R.; Roosendaal, G.; Rijbroek, A.D.B.; Schutgens, R.E.G.; Lafeber, F.P.J.G.; Mastbergen, S.C. Coagulation aggravates blood-induced joint damage in dogs. Arthritis Rheum. 2012, 64, 3231–3239. [Google Scholar] [CrossRef] [PubMed]
- Akassoglou, K. Coagulation takes center stage in inflammation. Blood 2015, 125, 419–420. [Google Scholar] [CrossRef] [PubMed]
- Moraes, A.P.L.; Moreira, J.J.; Brossi, P.M.; Machado, T.S.L.; Michelacci, Y.M.; Baccarin, R.Y.A. Short- and long-term effects of platelet-rich plasma upon healthy equine joints: Clinical and laboratory aspects. Can. Vet. J. Rev. Vét. Can. 2015, 56, 831–838. [Google Scholar]
- Textor, J.A.; Tablin, F. Intra-Articular Use of a Platelet-Rich Product in Normal Horses: Clinical Signs and Cytologic Responses. Vet. Surg. 2013, 42, 499–510. [Google Scholar] [CrossRef]
- Moreira, J.J.; Moraes, A.P.L.; Brossi, P.M.; Machado, T.S.L.; Michelacci, Y.M.; Massoco, C.O.; Baccarin, R.Y.A. Autologous processed plasma: Cytokine profile and effects upon injection into healthy equine joints. J. Vet. Sci. 2015, 16, 47–49. [Google Scholar] [CrossRef]
- Bertone, A.L.; Ishihara, A.; Zekas, L.J.; Wellman, M.L.; Lewis, K.B.; Schwarze, R.A.; Barnaba, A.R.; Schmall, M.L.; Kanter, P.M.; Genovese, R.L. Evaluation of a single intra-articular injection of autologous protein solution for treatment of osteoarthritis in horses. Am. J. Vet. Res. 2014, 75, 141–151. [Google Scholar] [CrossRef]
- Tyrnenopoulou, P.; Diakakis, N.; Karayannopoulou, M.; Savvas, I.; Koliakos, G. Evaluation of intra-articular injection of autologous platelet lysate (PL) in horses with osteoarthritis of the distal interphalangeal joint. Vet. Q. 2016, 36, 56–62. [Google Scholar] [CrossRef]
- Mirza, M.H.; Bommala, P.; Richbourg, H.A.; Rademacher, N.; Kearney, M.T.; Lopez, M.J. Gait Changes Vary among Horses with Naturally Occurring Osteoarthritis Following Intra-articular Administration of Autologous Platelet-Rich Plasma. Front. Vet. Sci. 2016, 3, 29. [Google Scholar] [CrossRef]
- Meijer, H.; Reinecke, J.; Becker, C.; Tholen, G.; Wehling, P. The production of anti-inflammatory cytokines in whole blood by physico-chemical induction. Inflamm. Res. 2003, 52, 404–407. [Google Scholar] [CrossRef]
- Frisbie, D.D.; Kawcak, C.E.; Werpy, N.M.; Park, R.D.; McIlwraith, C.W. Clinical, biochemical, and histologic effects of intra-articular administration of autologous conditioned serum in horses with experimentally induced osteoarthritis. Am. J. Vet. Res. 2007, 68, 290–296. [Google Scholar] [CrossRef] [PubMed]
- Frisbie, D.D.; Ghivizzani, S.C.; Robbins, P.D.; Evans, C.H.; McIlwraith, C.W. Treatment of experimental equine osteoarthritis by in vivo delivery of the equine interleukin-1 receptor antagonist gene. Gene Ther. 2002, 9, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Fjordbakk, C.T.; Johansen, G.M.; Løvås, A.C.; Oppegård, K.L.; Storset, A.K. Surgical stress influences cytokine content in autologous conditioned serum. Equine Vet. J. 2014, 47, 212–217. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, A.V.; Boone, L.H.; Pondugula, S.R.; Caldwell, F.; Wooldridge, A.A. Effects of Autologous Conditioned Serum, Autologous Protein Solution, and Triamcinolone on Inflammatory and Catabolic Gene Expression in Equine Cartilage and Synovial Explants Treated With IL-1β in Co-culture. Front. Vet. Sci. 2020, 7, 323. [Google Scholar] [CrossRef] [PubMed]
- Renström, P.; Johnson, R.J. Anatomy and Biomechanics of the Menisci. Clin. Sport Med. 1990, 9, 523–538. [Google Scholar] [CrossRef]
- Makris, E.A.; Hadidi, P.; Athanasiou, K.A. The knee meniscus: Structure-function, pathophysiology, current repair techniques, and prospects for regeneration. Biomaterials 2011, 32, 7411–7431. [Google Scholar] [CrossRef] [PubMed]
- Dubuc, J.; Girard, C.; Richard, H.; Lasalle, J.D.; Laverty, S. Equine meniscal degeneration is associated with medial femorotibial osteoarthritis. Equine Vet. J. 2018, 50, 133–140. [Google Scholar] [CrossRef]
- McCoy, A.M.; Smith, R.L.; Herrera, S.; Kawcak, C.E.; McIlwraith, C.W.; Goodrich, L.R. Long-term outcome after stifle arthroscopy in 82 Western performance horses (2003–2010). Vet. Surg. 2019, 48, 956–965. [Google Scholar] [CrossRef]
- Cohen, J.M.; Richardson, D.W.; McKnight, A.L.; Ross, M.W.; Boston, R.C. Long-Term Outcome in 44 Horses with Stifle Lameness After Arthroscopic Exploration and Debridement. Vet. Surg. 2009, 38, 543–551. [Google Scholar] [CrossRef]
- Hendrix, S.M.; Baxter, G.M.; McIlwraith, C.W.M.; Hendrickson, D.A.; Goodrich, L.R.; Frisbie, D.D.; Trotter, G.W. Concurrent or sequential development of medial meniscal and subchondral cystic lesions within the medial femorotibial joint in horses (1996–2006). Equine Vet. J. 2010, 42, 5–9. [Google Scholar] [CrossRef]
- Walmsley, J.P. Diagnosis and Treatment of Ligamentous and Meniscal Injuries in the Equine Stifle. Vet. Clin. N. Am. Equine Pract. 2005, 21, 651–672. [Google Scholar] [CrossRef] [PubMed]
- Walmsley, J.P.; Phillips, T.J.; Townsend, H.G. Meniscal tears in horses: An evaluation of clinical signs and arthroscopic treatment of 80 cases. Equine Vet. J. 2003, 35, 402–406. [Google Scholar] [CrossRef] [PubMed]
- Walmsley, J.P. Vertical tears of the cranial horn of the meniscus and its cranial ligament in the equine femorotibial joint: 7 cases and their treatment by arthroscopic surgery. Equine Vet. J. 1995, 27, 20–25. [Google Scholar] [CrossRef] [PubMed]
- Fowlie, J.G.; Arnoczky, S.P.; Lavagnino, M.; Stick, J.A. Stifle extension results in differential tensile forces developing between abaxial and axial components of the cranial meniscotibial ligament of the equine medial meniscus: A mechanistic explanation for meniscal tear patterns. Equine Vet. J. 2012, 44, 554–558. [Google Scholar] [CrossRef]
- Fowlie, J.G.; Arnoczky, S.P.; Stick, J.A.; Pease, A.P. Meniscal translocation and deformation throughout the range of motion of the equine stifle joint: An in vitro cadaveric study. Equine Vet. J. 2011, 43, 259–264. [Google Scholar] [CrossRef] [PubMed]
- Adrian, A.M.; Barrett, M.F.; Werpy, N.M.; Kawcak, C.E.; Chapman, P.L.; Goodrich, L.R. A comparison of arthroscopy to ultrasonography for identification of pathology of the equine stifle. Equine Vet. J. 2017, 49, 314–321. [Google Scholar] [CrossRef]
- Daglish, J.; Frisbie, D.D.; Selberg, K.T.; Barrett, M.F. High field magnetic resonance imaging is comparable with gross anatomy for description of the normal appearance of soft tissues in the equine stifle. Vet. Radiol. Ultrasound 2018, 59, 721–736. [Google Scholar] [CrossRef]
- Murphy, J.M.; Fink, D.J.; Hunziker, E.B.; Barry, F.P. Stem cell therapy in a caprine model of osteoarthritis. Arthritis Rheum. 2003, 48, 3464–3474. [Google Scholar] [CrossRef]
- Ribitsch, I.; Reboredo, J.; Kremer, A.; Ade, N.; Schramel, J.P.; Peham, C.; Egerbacher, M.; Jenner, F.; Walles, H. Adaption of a vascularized meniscus model as a potential model for equine meniscus regeneration. J. Tissue Eng. Regen. Med. 2014, 8, 84. [Google Scholar] [CrossRef][Green Version]
- Kremer, A.; Ribitsch, I.; Reboredo, J.; Dürr, J.; Egerbacher, M.; Jenner, F.; Walles, H. Three-Dimensional Coculture of Meniscal Cells and Mesenchymal Stem Cells in Collagen Type I Hydrogel on a Small Intestinal Matrix-A Pilot Study Toward Equine Meniscus Tissue Engineering. Tissue Eng. Part A 2017, 23, 390–402. [Google Scholar] [CrossRef]
- Fox, D.B.; Warnock, J.J.; Stoker, A.M.; Luther, J.K.; Cockrell, M. Effects of growth factors on equine synovial fibroblasts seeded on synthetic scaffolds for avascular meniscal tissue engineering. Res. Vet. Sci. 2010, 88, 326–332. [Google Scholar] [CrossRef] [PubMed]
- Coninck, T.D.; Elsner, J.J.; Linder-Ganz, E.; Cromheecke, M.; Shemesh, M.; Huysse, W.; Verdonk, R.; Verstraete, K.; Verdonk, P. In-vivo evaluation of the kinematic behavior of an artificial medial meniscus implant: A pilot study using open-MRI. Clin. Biomech. 2014, 29, 898–905. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Vijayavenkataraman, S.; Liu, H. An Overview of Scaffold Design and Fabrication Technology for Engineered Knee Meniscus. Materials 2017, 10, 29. [Google Scholar] [CrossRef] [PubMed]
- Moran, C.J.; Withers, D.P.; Kurzweil, P.R.; Verdonk, P.C. Clinical Application of Scaffolds for Partial Meniscus Replacement. Sports Med. Arthrosc. 2015, 23, 156–161. [Google Scholar] [CrossRef]
- Myers, K.; Sgaglione, N.; Goodwillie, A. Meniscal Scaffolds. J. Knee Surg. 2014, 27, 435–442. [Google Scholar] [CrossRef]
- Scotti, C.; Hirschmann, M.T.; Antinolfi, P.; Martin, I.; Peretti, G.M. Meniscus repair and regeneration: Review on current methods and research potential. Eur. Cells Mater. 2013, 26, 150–170. [Google Scholar] [CrossRef]
- Tucker, B.; Khan, W.; Al-Rashid, M.; Al-Khateeb, H. Tissue Engineering for the Meniscus: A Review of the Literature. Open Orthop. J. 2012, 6, 348–351. [Google Scholar] [CrossRef] [PubMed]
- Sandmann, G.H.; Adamczyk, C.; Garcia, E.G.; Doebele, S.; Buettner, A.; Milz, S.; Imhoff, A.B.; Vogt, S.; Burgkart, R.; Tischer, T. Biomechanical comparison of menisci from different species and artificial constructs. BMC Musculoskelet. Disord. 2013, 14, 324. [Google Scholar] [CrossRef] [PubMed]
- Ferris, D.; Frisbie, D.; Kisiday, J.; McIlwraith, C.W. In vivo healing of Meniscal Lacerations Using Bone Marrow-Derived Mesenchymal Stem Cells and Fibrin Glue. Stem Cells Int. 2012, 2012, 1–9. [Google Scholar] [CrossRef] [PubMed]
- González-Fernández, M.L.; Pérez-Castrillo, S.; Sánchez-Lázaro, J.A.; Prieto-Fernández, J.G.; López-González, M.E.; Lobato-Pérez, S.; Colaço, B.J.; Olivera, E.R.; Villar-Suárez, V. Assessment of regeneration in meniscal lesions by use of mesenchymal stem cells derived from equine bone marrow and adipose tissue. Am. J. Vet. Res. 2016, 77, 779–788. [Google Scholar] [CrossRef]
- Fortier, L.A.; Nixon, A.J. New Surgical Treatments for Osteochondritis Dissecans and Subchondral Bone Cysts. Vet. Clin. N. Am. Equine Pract. 2005, 21, 673–690. [Google Scholar] [CrossRef] [PubMed]
- Jackson, W.A.; Stick, J.A.; Nickels, F.A. The Effect of Compacted Cancellous Bone Grafting on the Healing of Subchondral Bone Defects of the Medial Femoral Condyle in Horses. Vet. Surg. 2000, 29, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Richardson, D.W. Complications of Orthopaedic Surgery in Horses. Vet. Clin. N. Am. Equine Pract. 2008, 24, 591–610. [Google Scholar] [CrossRef]
- Milner, P.I.; Clegg, P.D.; Stewart, M.C. Stem Cell–based Therapies for Bone Repair. Vet. Clin. N. Am. Equine Pract. 2011, 27, 299–314. [Google Scholar] [CrossRef] [PubMed]
- Pfeiffenberger, M.; Bartsch, J.; Hoff, P.; Ponomarev, I.; Barnewitz, D.; Thöne-Reineke, C.; Buttgereit, F.; Gaber, T.; Lang, A. Hypoxia and mesenchymal stromal cells as key drivers of initial fracture healing in an equine in vitro fracture hematoma model. PLoS ONE 2019, 14, e0214276. [Google Scholar] [CrossRef] [PubMed]
- Govoni, K.E. Horse Species Symposium: Use of mesenchymal stem cells in fracture repair in horses. J. Anim. Sci. 2015, 93, 871. [Google Scholar] [CrossRef]
- Diloksumpan, P.; Bolaños, R.V.; Cokelaere, S.; Pouran, B.; Grauw, J.; Rijen, M.; Weeren, R.; Levato, R.; Malda, J. Orthotopic Bone Regeneration within 3D Printed Bioceramic Scaffolds with Region-Dependent Porosity Gradients in an Equine Model. Adv. Healthc. Mater. 2020, 9, 1901807. [Google Scholar] [CrossRef] [PubMed]
- Ball, A.N.; Phillips, J.N.; McIlwraith, C.W.; Kawcak, C.E.; Samulski, R.J.; Goodrich, L.R. Genetic modification of scAAV-equine-BMP-2 transduced bone-marrow-derived mesenchymal stem cells before and after cryopreservation: An “off-the-shelf” option for fracture repair. J. Orthop. Res. 2019, 37, 1310–1317. [Google Scholar] [CrossRef]
- Golafshan, N.; Vorndran, E.; Zaharievski, S.; Brommer, H.; Kadumudi, F.B.; Dolatshahi-Pirouz, A.; Gbureck, U.; van Weeren, R.; Castilho, M.; Malda, J. Tough magnesium phosphate-based 3D-printed implants induce bone regeneration in an equine defect model. Biomaterials 2020, 261, 120302. [Google Scholar] [CrossRef]
- Perrier, M.; Lu, Y.; Nemke, B.; Kobayashi, H.; Peterson, A.; Markel, M. Acceleration of Second and Fourth Metatarsal Fracture Healing with Recombinant Human Bone Morphogenetic Protein-2/Calcium Phosphate Cement in Horses. Vet. Surg. 2008, 37, 648–655. [Google Scholar] [CrossRef]
- Payne, R.J.; Compston, P.C. Short- and long-term results following standing fracture repair in 34 horses. Equine Vet. J. 2012, 44, 721–725. [Google Scholar] [CrossRef]
- Verheyen, K.; Price, J.; Lanyon, L.; Wood, J. Exercise distance and speed affect the risk of fracture in racehorses. Bone 2006, 39, 1322–1330. [Google Scholar] [CrossRef]
- Ahern, B.J.; Richardson, D.W.; Boston, R.C.; Schaer, T.P. Orthopedic Infections in Equine Long Bone Fractures and Arthrodeses Treated by Internal Fixation: 192 Cases (1990–2006). Vet. Surg. 2010, 39, 588–593. [Google Scholar] [CrossRef]
- Bischofsberger, A.S.; Fürst, A.; Auer, J.; Lischer, C. Surgical management of complete diaphyseal third metacarpal and metatarsal bone fractures: Clinical outcome in 10 mature horses and 11 foals. Equine Vet. J. 2009, 41, 465–473. [Google Scholar] [CrossRef]
- Maeda, Y.; Hanada, M.; Oikawa, M. Epidemiology of racing injuries in Thoroughbred racehorses with special reference to bone fractures: Japanese experience from the 1980s to 2000s. J. Equine Sci. 2016, 27, 81–97. [Google Scholar] [CrossRef]
- Verheyen, K.L.P.; Wood, J.L.N. Descriptive epidemiology of fractures occurring in British Thoroughbred racehorses in training. Equine Vet. J. 2004, 36, 167–173. [Google Scholar] [CrossRef]
- Wylie, C.E.; McManus, P.; McDonald, C.; Jorgensen, S.; McGreevy, P. Thoroughbred fatality and associated jockey falls and injuries in races in New South Wales and the Australian Capital Territory, Australia: 2009–2014. Vet. J. 2017, 227, 1–7. [Google Scholar] [CrossRef]
- Rosanowski, S.M.; Chang, Y.-M.; Stirk, A.J.; Verheyen, K.L.P. Risk factors for race-day fatality in flat racing Thoroughbreds in Great Britain (2000 to 2013). PLoS ONE 2018, 13, e0194299. [Google Scholar] [CrossRef]
- Johnson, B.J.; Stover, S.M.; Daft, B.M.; Kinde, H.; Read, D.H.; Barr, B.C.; Anderson, M.; Moore, J.; Woods, L.; Stolz, J.; et al. Causes of death in racehorses over a 2 year period. Equine Vet. J. 1994, 26, 327–330. [Google Scholar] [CrossRef]
- Georgopoulos, S.P.; Parkin, T.D.H. Risk factors associated with fatal injuries in Thoroughbred racehorses competing in flat racing in the United States and Canada. J. Am. Vet. Med. Assoc. 2016, 249, 931–939. [Google Scholar] [CrossRef]
- Zambruno, T.; Georgopoulos, S.P.; Boden, L.A.; Parkin, T.D.H. Association between the administration of phenylbutazone prior to racing and musculoskeletal and fatal injuries in Thoroughbred racehorses in Argentina. J. Am. Vet. Med. Assoc. 2020, 257, 642–647. [Google Scholar] [CrossRef]
- Nixon, A. General Considerations for Fracture Repair. In Equine Fracture Repair; Nixon, A., Ed.; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2019; ISBN 978-1-119-10875-7. [Google Scholar]
- Levine, D.G.; Richardson, D.W. Clinical use of the locking compression plate (LCP) in horses: A retrospective study of 31 cases (2004–2006). Equine Vet. J. 2007, 39, 401–406. [Google Scholar] [CrossRef]
- Lescun, T.B.; McClure, S.R.; Ward, M.P.; Downs, C.; Wilson, D.A.; Adams, S.B.; Hawkins, J.F.; Reinertson, E.L. Evaluation of transfixation casting for treatment of third metacarpal, third metatarsal, and phalangeal fractures in horses: 37 cases (1994–2004). J. Am. Vet. Med. Assoc. 2007, 230, 1340–1349. [Google Scholar] [CrossRef]
- James, F.M.; Richardson, D.W. Minimally invasive plate fixation of lower limb injury in horses: 32 cases (1999–2003). Equine Vet. J. 2006, 38, 246–251. [Google Scholar] [CrossRef]
- Donati, B.; Fürst, A.; Chicca, F.D.; Jackson, M. Plate Removal after Internal Fixation of Limb Fractures: A Retrospective Study of Indications and Complications in 48 Horses. Vet. Comp. Orthop. 2020, 34, 59–67. [Google Scholar] [CrossRef]
- Janicek, J.C.; McClure, S.R.; Lescun, T.B.; Witte, S.; Schultz, L.; Whittal, C.R.; Whitfield-Cargile, C. Risk factors associated with cast complications in horses: 398 cases (1997–2006). J. Am. Vet. Med. Assoc. 2013, 242, 93–98. [Google Scholar] [CrossRef]
- Stewart, S.; Richardson, D.; Boston, R.; Schaer, T.P. Risk Factors Associated With Survival to Hospital Discharge of 54 Horses With Fractures of the Radius. Vet. Surg. 2015, 44, 1036–1041. [Google Scholar] [CrossRef]
- Németh, F.; Back, W. The use of the walking cast to repair fractures in horses and ponies. Equine Vet. J. 1991, 23, 32–36. [Google Scholar] [CrossRef]
- Jackson, M.; Kummer, M.; Auer, J.; Hagen, R.; Fuerst, A. Treatment of type 2 and 4 olecranon fractures with locking compression plate osteosynthesis in horses: A prospective study (2002?2008). Vet. Comp. Orthop. 2011, 24, 57–61. [Google Scholar] [CrossRef][Green Version]
- Jacobs, C.C.; Levine, D.G.; Richardson, D.W. Use of locking compression plates in ulnar fractures of 18 horses*. Vet. Surg. 2017, 46, 242–248. [Google Scholar] [CrossRef]
- Kawcak, C.E.; Trotter, G.W.; Powers, B.E.; Park, R.D.; Turner, A.S. Comparison of Bone Healing by Demineralized Bone Matrix and Autogenous Cancellous Bone in Horses. Vet. Surg. 2000, 29, 218–226. [Google Scholar] [CrossRef]
- Florin, M.; Arzdorf, M.; Linke, B.; Auer, J.A. Assessment of Stiffness and Strength of 4 Different Implants Available for Equine Fracture Treatment: A Study on a 20° Oblique Long-Bone Fracture Model Using a Bone Substitute. Vet. Surg. 2005, 34, 231–238. [Google Scholar] [CrossRef]
- Fürst, A.E.; Keller, R.; Kummer, M.; Manera, C.; Salis, B.V.; Auer, J.; Bettschart-Wolfensberger, R. Evaluation of a new full-body animal rescue and transportation sling in horses: 181 horses (1998–2006). J. Vet. Emerg. Crit. Car. 2008, 18, 619–625. [Google Scholar] [CrossRef]
- Brianza, S.; Brighenti, V.; Lansdowne, J.L.; Schwieger, K.; Bouré, L. Finite element analysis of a novel pin-sleeve system for external fixation of distal limb fractures in horses. Vet. J. 2011, 190, 260–267. [Google Scholar] [CrossRef]
- Cruz, A.M.; Rubio-Martinez, L.; Dowling, T. New Antimicrobials, Systemic Distribution, and Local Methods of Antimicrobial Delivery in Horses. Vet. Clin. N. Am. Equine Pract. 2006, 22, 297–322. [Google Scholar] [CrossRef]
- Auer, J. Surgical Equipment and Implants for Fracture Repair. In Equine Fracture Repair; Nixon, A., Ed.; John Wiley & Sons: Hoboken, NJ, USA, 2019; pp. 107–126. ISBN 978-1-119-10875-7. [Google Scholar]
- Sullivan, E.K.; Klein, L.V.; Richardson, D.W.; Ross, M.W.; Orsini, J.A.; Nunamaker, D.M. Use of a pool-raft system for recovery of horses from general anesthesia: 393 horses (1984–2000). J. Am. Vet. Med. Assoc. 2002, 221, 1014–1018. [Google Scholar] [CrossRef]
- Liebig, B.E.; Kisiday, J.D.; Bahney, C.S.; Ehrhart, N.P.; Goodrich, L.R. The platelet-rich plasma and mesenchymal stem cell milieu: A review of therapeutic effects on bone healing. J. Orthop. Res. 2020, 1–36. [Google Scholar] [CrossRef]
- Deschaseaux, F.; Sensébé, L.; Heymann, D. Mechanisms of bone repair and regeneration. Trends Mol. Med. 2009, 15, 417–429. [Google Scholar] [CrossRef]
- Schindeler, A.; McDonald, M.M.; Bokko, P.; Little, D.G. Bone remodeling during fracture repair: The cellular picture. Semin. Cell Dev. Biol. 2008, 19, 459–466. [Google Scholar] [CrossRef]
- Ishihara, A.; Zekas, L.J.; Weisbrode, S.E.; Bertone, A.L. Comparative efficacy of dermal fibroblast-mediated and direct adenoviral bone morphogenetic protein-2 gene therapy for bone regeneration in an equine rib model. Gene Ther. 2010, 17, 733–744. [Google Scholar] [CrossRef]
- Seo, J.; Kambayashi, Y.; Itho, M.; Haneda, S.; Yamada, K.; Furuoka, H.; Tabata, Y.; Sasaki, N. Effects of a synovial flap and gelatin/β-tricalcium phosphate sponges loaded with mesenchymal stem cells, bone morphogenetic protein-2, and platelet rich plasma on equine osteochondral defects. Res. Vet. Sci. 2015, 101, 140–143. [Google Scholar] [CrossRef]
- McDuffee, L.A.; Pack, L.; Lores, M.; Wright, G.M.; Esparza-Gonzalez, B.; Masaoud, E. Osteoprogenitor Cell Therapy in an Equine Fracture Model. Vet. Surg. 2012, 41, 773–783. [Google Scholar] [CrossRef]
- Leonardi, F.; Angelone, M.; Biacca, C.; Battaglia, B.; Pecorari, L.; Conti, V.; Costa, G.L.; Ramoni, R.; Grolli, S. Platelet-Rich Plasma combined with a sterile 3D polylactic acid scaffold for postoperative management of complete hoof wall resection for keratoma in four horses. J. Equine Vet. Sci. 2020, 103178. [Google Scholar] [CrossRef]
- Baird, A.; Lindsay, T.; Everett, A.; Iyemere, V.; Paterson, Y.Z.; McClellan, A.; Henson, F.M.D.; Guest, D.J. Osteoblast differentiation of equine induced pluripotent stem cells. Biol. Open 2018, 7, bio033514. [Google Scholar] [CrossRef]
- McDuffee, L.A.; Gonzalez, B.P.E.; Nino-Fong, R.; Aburto, E. Evaluation of an in vivo heterotopic model of osteogenic differentiation of equine bone marrow and muscle mesenchymal stem cells in fibrin glue scaffold. Cell Tissue Res. 2014, 355, 327–335. [Google Scholar] [CrossRef]
- Markel, M. Bone Grafts and Bone Substitutes. In Equine Fracture Repair; Nixon, A., Ed.; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2019; pp. 163–172. ISBN 978-1-119-10875-7. [Google Scholar]
- Seo, J.; Tsuzuki, N.; Haneda, S.; Yamada, K.; Furuoka, H.; Tabata, Y.; Sasaki, N. Osteoinductivity of gelatin/β-tricalcium phosphate sponges loaded with different concentrations of mesenchymal stem cells and bone morphogenetic protein-2 in an equine bone defect model. Vet. Res. Commun. 2014, 38, 73–80. [Google Scholar] [CrossRef]
- Gianakos, A.; Zambrana, L.; Savage-Elliott, I.; Lane, J.M.; Kennedy, J.G. Platelet-Rich Plasma in the Animal Long-Bone Model: An Analysis of Basic Science Evidence. Orthopedics 2015, 38, e1079–e1090. [Google Scholar] [CrossRef]
- Faillace, V.; Tambella, A.M.; Fratini, M.; Paggi, E.; Dini, F.; Laus, F. Use of autologous platelet-rich plasma for a delayed consolidation of a tibial fracture in a young donkey. J. Vet. Med. Sci. 2017, 79. [Google Scholar] [CrossRef]
- Katz, L.M.; Bailey, S.R. A review of recent advances and current hypotheses on the pathogenesis of acute laminitis. Equine Vet. J. 2012, 44, 752–761. [Google Scholar] [CrossRef]
- Bailey, S.R.; Marr, C.M.; Elliott, J. Current research and theories on the pathogenesis of acute laminitis in the horse. Vet. J. 2004, 167, 129–142. [Google Scholar] [CrossRef]
- Wylie, C.E.; Collins, S.N.; Verheyen, K.L.P.; Newton, J.R. Risk factors for equine laminitis: A case-control study conducted in veterinary-registered horses and ponies in Great Britain between 2009 and 2011. Vet. J. 2013, 198, 57–69. [Google Scholar] [CrossRef]
- Wylie, C.E.; Collins, S.N.; Verheyen, K.L.P.; Newton, J.R. Frequency of equine laminitis: A systematic review with quality appraisal of published evidence. Vet. J. 2011, 189, 248–256. [Google Scholar] [CrossRef]
- Menzies-Gow, N. Laminitis in horses. Practice 2018, 40, 411. [Google Scholar] [CrossRef]
- Yang, Q.; Lopez, M.J. The Equine Hoof: Laminitis, Progenitor (Stem) Cells, and Therapy Development. Toxicol. Pathol. 2019, 41. [Google Scholar] [CrossRef]
- Van Eps, A.W. Acute Laminitis: Medical and Supportive Therapy. Vet. Clin. N. Am. Equine Pract. 2010, 26, 103–114. [Google Scholar] [CrossRef]
- Eustace, R.A. Clinical Presentation, Diagnosis, and Prognosis of Chronic Laminitis in Europe. Vet. Clin. N. Am. Equine Pract. 2010, 26, 391–405. [Google Scholar] [CrossRef]
- Hunt, R.J.; Wharton, R.E. Clinical Presentation, Diagnosis, and Prognosis of Chronic Laminitis in North America. Vet. Clin. N. Am. Equine Pract. 2010, 26, 141–153. [Google Scholar] [CrossRef]
- Belknap, J.K.; Black, S.J. Sepsis-related laminitis. Equine Vet. J. 2012, 44, 738–740. [Google Scholar] [CrossRef]
- Belknap, J.K.; Giguère, S.; Pettigrew, A.; Cochran, A.M.; Eps, A.W.; Pollitt, C.C. Lamellar pro-inflammatory cytokine expression patterns in laminitis at the developmental stage and at the onset of lameness: Innate vs. adaptive immune response. Equine Vet. J. 2007, 39, 42–47. [Google Scholar] [CrossRef]
- Donaldson, M.T.; Jorgensen, A.J.R.; Beech, J. Evaluation of suspected pituitary pars intermedia dysfunction in horses with laminitis. J. Am. Vet. Med. Assoc. 2004, 224, 1123–1127. [Google Scholar] [CrossRef]
- Wylie, C.E.; Collins, S.N.; Verheyen, K.L.P.; Newton, J.R. Risk factors for equine laminitis: A systematic review with quality appraisal of published evidence. Vet. J. 2012, 193, 58–66. [Google Scholar] [CrossRef]
- Menzies-Gow, N.J.; Katz, L.M.; Barker, K.J.; Elliott, J.; Brauwere, M.N.D.; Jarvis, N.; Marr, C.M.; Pfeiffer, D.U. Epidemiological study of pasture-associated laminitis and concurrent risk factors in the South of England. Vet. Rec. 2010, 167, 690. [Google Scholar] [CrossRef]
- Laat, M.A.; Reiche, D.B.; Sillence, M.N.; McGree, J.M. Incidence and risk factors for recurrence of endocrinopathic laminitis in horses. J. Vet. Intern. Med. 2019, 33, 1473–1482. [Google Scholar] [CrossRef]
- Virgin, J.E.; Goodrich, L.R.; Baxter, G.M.; Rao, S. Incidence of support limb laminitis in horses treated with half limb, full limb or transfixation pin casts: A retrospective study of 113 horses (2000–2009). Equine Vet. J. Suppl 2011, 43, 7–11. [Google Scholar] [CrossRef]
- Parsons, C.S.; Orsini, J.A.; Krafty, R.; Capewell, L.; Boston, R. Risk factors for development of acute laminitis in horses during hospitalization: 73 cases (1997–2004). J. Am. Vet. Med. Assoc. 2007, 230, 885–889. [Google Scholar] [CrossRef]
- Eustace, R.A.; Emery, S.L.; Cripps, P.J. A Retrospective Study of 23 Cases of Coronary Band Separation Longer than 8 cm as a Sequel to Severe Laminitis. J. Equine Vet. Sci. 2012, 32, 235–244. [Google Scholar] [CrossRef]
- Kullmann, A.; Holcombe, S.J.; Hurcombe, S.D.; Roessner, H.A.; Hauptman, J.G.; Geor, R.J.; Belknap, J. Prophylactic digital cryotherapy is associated with decreased incidence of laminitis in horses diagnosed with colitis. Equine Vet. J. 2014, 46, 554–559. [Google Scholar] [CrossRef]
- Eps, A.W.; Pollitt, C.C. Equine laminitis model: Cryotherapy reduces the severity of lesions evaluated seven days after induction with oligofructose. Equine Vet. J. 2009, 41, 741–746. [Google Scholar] [CrossRef]
- Steelman, S.M.; Johnson, D.; Wagner, B.; Stokes, A.M.; Chowdhary, B.P. Cellular and humoral immunity in chronic equine laminitis. Vet. Immunol. Immunopathol. 2013, 153, 217–226. [Google Scholar] [CrossRef]
- Matthay, M.A.; Pati, S.; Lee, J. Concise Review: Mesenchymal Stem (Stromal) Cells: Biology and Preclinical Evidence for Therapeutic Potential for Organ Dysfunction Following Trauma or Sepsis. Stem Cells 2017, 35, 316–324. [Google Scholar] [CrossRef]
- Monsel, A.; Zhu, Y.; Gennai, S.; Hao, Q.; Liu, J.; Lee, J.W. Cell-based Therapy for Acute Organ Injury. Anesthesiology 2014, 121, 1099–1121. [Google Scholar] [CrossRef]
- Angelone, M.; Conti, V.; Biacca, C.; Battaglia, B.; Pecorari, L.; Piana, F.; Gnudi, G.; Leonardi, F.; Ramoni, R.; Basini, G.; et al. The Contribution of Adipose Tissue-Derived Mesenchymal Stem Cells and Platelet-Rich Plasma to the Treatment of Chronic Equine Laminitis: A Proof of Concept. Int. J. Mol. Sci. 2017, 18, 2122. [Google Scholar] [CrossRef]
- Sole, A.; Spriet, M.; Galuppo, L.D.; Padgett, K.A.; Borjesson, D.L.; Wisner, E.R.; Brosnan, R.J.; Vidal, M.A. Scintigraphic evaluation of intra-arterial and intravenous regional limb perfusion of allogeneic bone marrow-derived mesenchymal stem cells in the normal equine distal limb using 99mTc-HMPAO. Equine Vet. J. 2012, 44, 594–599. [Google Scholar] [CrossRef]
- Carmona, J.U.; López, C.; Samudio, I.J. Autologous Platelet Concentrates as an Adjunctive Treatment for Chronic Laminitis in a Mare with Pituitary Pars Intermedia Dysfunction. J. Equine Vet. Sci. 2013, 33, 191–195. [Google Scholar] [CrossRef]
- Carmona, J.U.; Gómez, W.A.; López, C. Could Platelet-Rich Plasma Be a Clinical Treatment for Horses With Laminitis? J. Equine Vet. Sci. 2018, 61, 46–57. [Google Scholar] [CrossRef]
- Faltus, T.; Emmerich, I.; Brehm, W. Zellbasierte Tiertherapien—Arzneimittelrechtliche Einordnung, Straf- und berufsrechtliche Fallstricke. Dtsch. Tierärzteblatt 2015, 63, 1414–1419. [Google Scholar]
- European Medicines Agency. First Stem Cell-Based Veterinary Medicine Recommended for Marketing Authorisation; EMA/CVMP/399992/2018; European Medicines Agency: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Hellingman, C.A.; Verwiel, E.T.P.; Slagt, I.; Koevoet, W.; Poublon, R.M.L.; Nolst-Trenité, G.J.; de Jong, R.J.B.; Jahr, H.; van Osch, G.J.V.M. Differences in Cartilage-Forming Capacity of Expanded Human Chondrocytes From Ear and Nose and Their Gene Expression Profiles. Cell Transplant. 2011, 20, 925–940. [Google Scholar] [CrossRef]
- Murphy, J.M.; Dixon, K.; Beck, S.; Fabian, D.; Feldman, A.; Barry, F. Reduced chondrogenic and adipogenic activity of mesenchymal stem cells from patients with advanced osteoarthritis. Arthritis Rheumatol. 2002, 46, 704–713. [Google Scholar] [CrossRef]
- Toh, W.S.; Brittberg, M.; Farr, J.; Foldager, C.B.; Gomoll, A.H.; Hui, J.H.P.; Richardson, J.B.; Roberts, S.; Spector, M. Cellular senescence in aging and osteoarthritis. Acta Orthop. 2016, 87, 6–14. [Google Scholar] [CrossRef]
- Severino, V.; Alessio, N.; Farina, A.; Sandomenico, A.; Cipollaro, M.; Peluso, G.; Galderisi, U.; Chambery, A. Insulin-like growth factor binding proteins 4 and 7 released by senescent cells promote premature senescence in mesenchymal stem cells. Cell Death Dis. 2013, 4, e911. [Google Scholar] [CrossRef]
- Barry, F.; Murphy, M. Mesenchymal stem cells in joint disease and repair. Nat. Rev. Rheumatol. 2013, 9, 584–594. [Google Scholar] [CrossRef]
- Harrell, C.R.; Markovic, B.S.; Fellabaum, C.; Arsenijevic, A.; Volarevic, V. Mesenchymal stem cell-based therapy of osteoarthritis: Current knowledge and future perspectives. Biomed. Pharmacother. 2019, 109, 2318–2326. [Google Scholar] [CrossRef] [PubMed]
- Berglund, A.K.; Fortier, L.A.; Antczak, D.F.; Schnabel, L.V. Immunoprivileged no more: Measuring the immunogenicity of allogeneic adult mesenchymal stem cells. Stem Cell Res. Ther. 2017, 8, 288. [Google Scholar] [CrossRef] [PubMed]
- Schnabel, L.V.; Pezzanite, L.M.; Antczak, D.F.; Felippe, M.J.B.; Fortier, L.A. Equine bone marrow-derived mesenchymal stromal cells are heterogeneous in MHC class II expression and capable of inciting an immune response in vitro. Stem Cell Res. Ther. 2014, 5, 13. [Google Scholar] [CrossRef] [PubMed]
- Caplan, A.I.; Dennis, J.E. Mesenchymal stem cells as trophic mediators. J. Cell. Biochem. 2006, 98, 1076–1084. [Google Scholar] [CrossRef]
- Gnecchi, M.; Danieli, P.; Malpasso, G.; Ciuffreda, M.C. Paracrine Mechanisms of Mesenchymal Stem Cells in Tissue Repair. Methods Mol. Biol. 2016, 1416, 123–146. [Google Scholar]
- Baldari, S.; Rocco, G.D.; Piccoli, M.; Pozzobon, M.; Muraca, M.; Toietta, G. Challenges and Strategies for Improving the Regenerative Effects of Mesenchymal Stromal Cell-Based Therapies. Int. J. Mol. Sci. 2017, 18, 2087. [Google Scholar] [CrossRef]
- Fakurazi, S.; Ghofar, H.A.A.; Nordin, N.; Muhammad, S.A. Harnessing stem cell secretome towards cell-free therapeutic strategies. Stem Cells 2020, 237–258. [Google Scholar]
- Muhammad, S.A.; Nordin, N.; Mehat, M.Z.; Fakurazi, S. Comparative efficacy of stem cells and secretome in articular cartilage regeneration: A systematic review and meta-analysis. Cell Tissue Res. 2018, 5, e37976. [Google Scholar] [CrossRef]
- Lener, T.; Gimona, M.; Aigner, L.; Börger, V.; Buzas, E.; Camussi, G.; Chaput, N.; Chatterjee, D.; Court, F.A.; Portillo, H.A.D.; et al. Applying extracellular vesicles based therapeutics in clinical trials-an ISEV position paper. J. Extracell. Vesicles 2015, 4, 30087. [Google Scholar] [CrossRef]
- Doeppner, T.R.; Herz, J.; Görgens, A.; Schlechter, J.; Ludwig, A.-K.; Radtke, S.; de Miroschedji, K.; Horn, P.A.; Giebel, B.; Hermann, D.M. Extracellular Vesicles Improve Post-Stroke Neuroregeneration and Prevent Postischemic Immunosuppression. Stem Cells Transl. Med. 2015, 4, 1131–1143. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Chuah, S.J.; Lai, R.C.; Hui, J.H.P.; Lim, S.-K.; Toh, W.S. MSC exosomes mediate cartilage repair by enhancing proliferation, attenuating apoptosis and modulating immune reactivity. Biomaterials 2018, 156, 16–27. [Google Scholar] [CrossRef] [PubMed]
- Vonk, L.A.; van Dooremalen, S.F.J.; Liv, N.; Klumperman, J.; Coffer, P.J.; Saris, D.B.F.; Lorenowicz, M.J. Mesenchymal Stromal/stem Cell-derived Extracellular Vesicles Promote Human Cartilage Regeneration In Vitro. Theranostics 2018, 8, 906–920. [Google Scholar] [CrossRef] [PubMed]
- Mocchi, M.; Dotti, S.; Bue, M.D.; Villa, R.; Bari, E.; Perteghella, S.; Torre, M.L.; Grolli, S. Veterinary Regenerative Medicine for Musculoskeletal Disorders: Can Mesenchymal Stem/Stromal Cells and Their Secretome Be the New Frontier? Cells 2020, 9, 1453. [Google Scholar] [CrossRef] [PubMed]
- Bundgaard, L.; Stensballe, A.; Elbæk, K.J.; Berg, L.C. Mass spectrometric analysis of the in vitro secretome from equine bone marrow-derived mesenchymal stromal cells to assess the effect of chondrogenic differentiation on response to interleukin-1β treatment. Stem Cell Res. Ther. 2020, 11, 187. [Google Scholar] [CrossRef]
- Kornicka-Garbowska, K.; Pędziwiatr, R.; Woźniak, P.; Kucharczyk, K.; Marycz, K. Microvesicles isolated from 5-azacytidine-and-resveratrol-treated mesenchymal stem cells for the treatment of suspensory ligament injury in horse—A case report. Stem Cell Res. Ther. 2019, 10, 394. [Google Scholar] [CrossRef]
- Zhang, W.; Ouyang, H.; Dass, C.R.; Xu, J. Current research on pharmacologic and regenerative therapies for osteoarthritis. Bone Res. 2016, 4, 15040. [Google Scholar] [CrossRef]
- Malda, J.; Boere, J.; van de Lest, C.H.A.; van Weeren, P.R.; Wauben, M.H.M. Extracellular vesicles—New tool for joint repair and regeneration. Nat. Rev. Rheumatol. 2016, 12, 243–249. [Google Scholar] [CrossRef]
- Rohani, L.; Johnson, A.A.; Arnold, A.; Stolzing, A. The aging signature: A hallmark of induced pluripotent stem cells? Aging Cell 2014, 13, 2–7. [Google Scholar] [CrossRef]
- Iismaa, S.E.; Kaidonis, X.; Nicks, A.M.; Bogush, N.; Kikuchi, K.; Naqvi, N.; Harvey, R.P.; Husain, A.; Graham, R.M. Comparative regenerative mechanisms across different mammalian tissues. NPJ Regen. Med. 2018, 3, 6. [Google Scholar] [CrossRef]
- Godwin, J.W.; Pinto, A.R.; Rosenthal, N.A. Chasing the recipe for a pro-regenerative immune system. Semin. Cell Dev. Biol. 2017, 61, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.J.; Zheng, L. Rejuvenation: An integrated approach to regenerative medicine. Regen. Med. Res. 2013, 1, 7. [Google Scholar] [CrossRef] [PubMed]
- Badylak, S. Perspective: Work with, not against, biology. Nature 2016, 540, S55. [Google Scholar] [CrossRef] [PubMed]
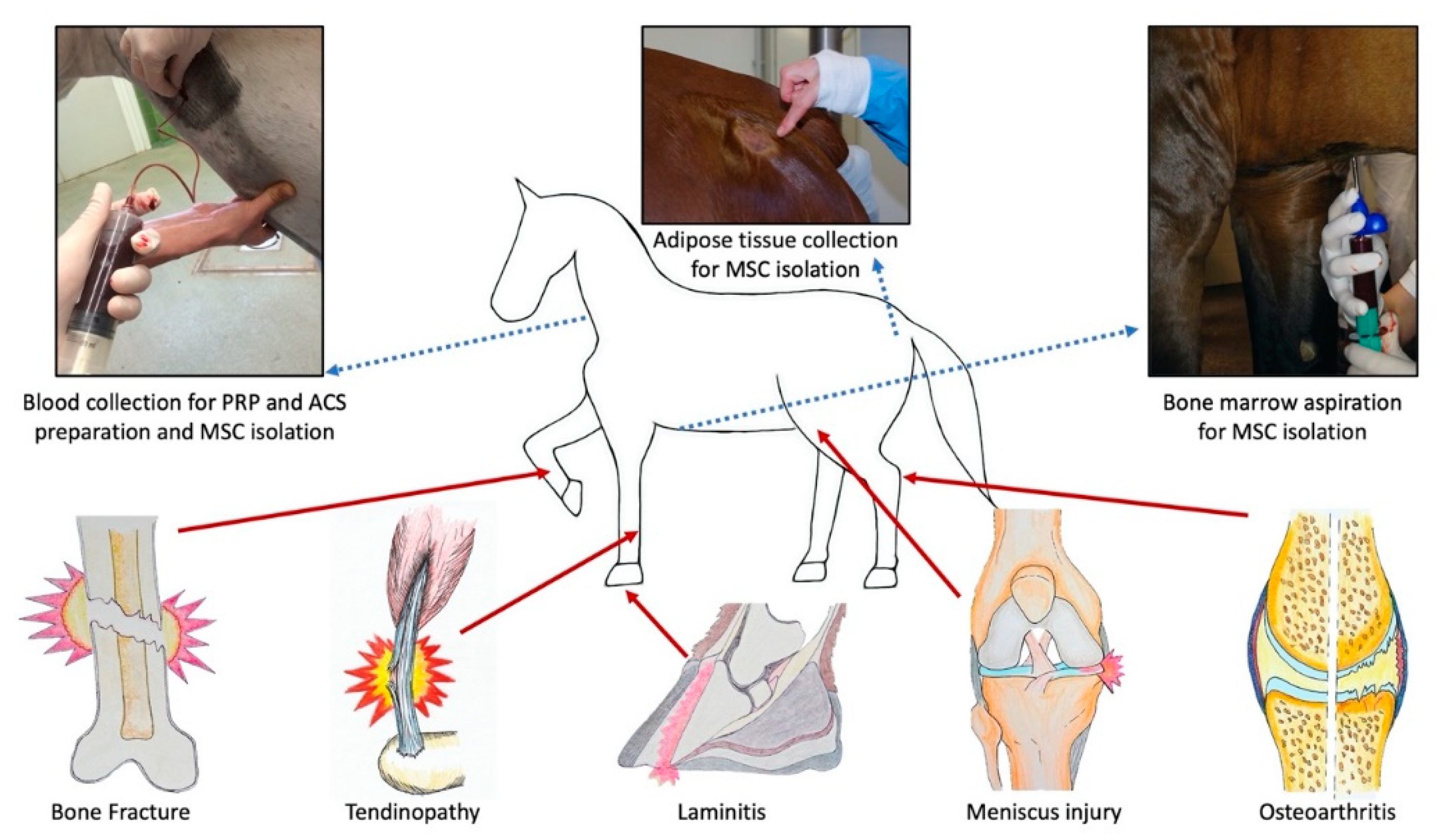
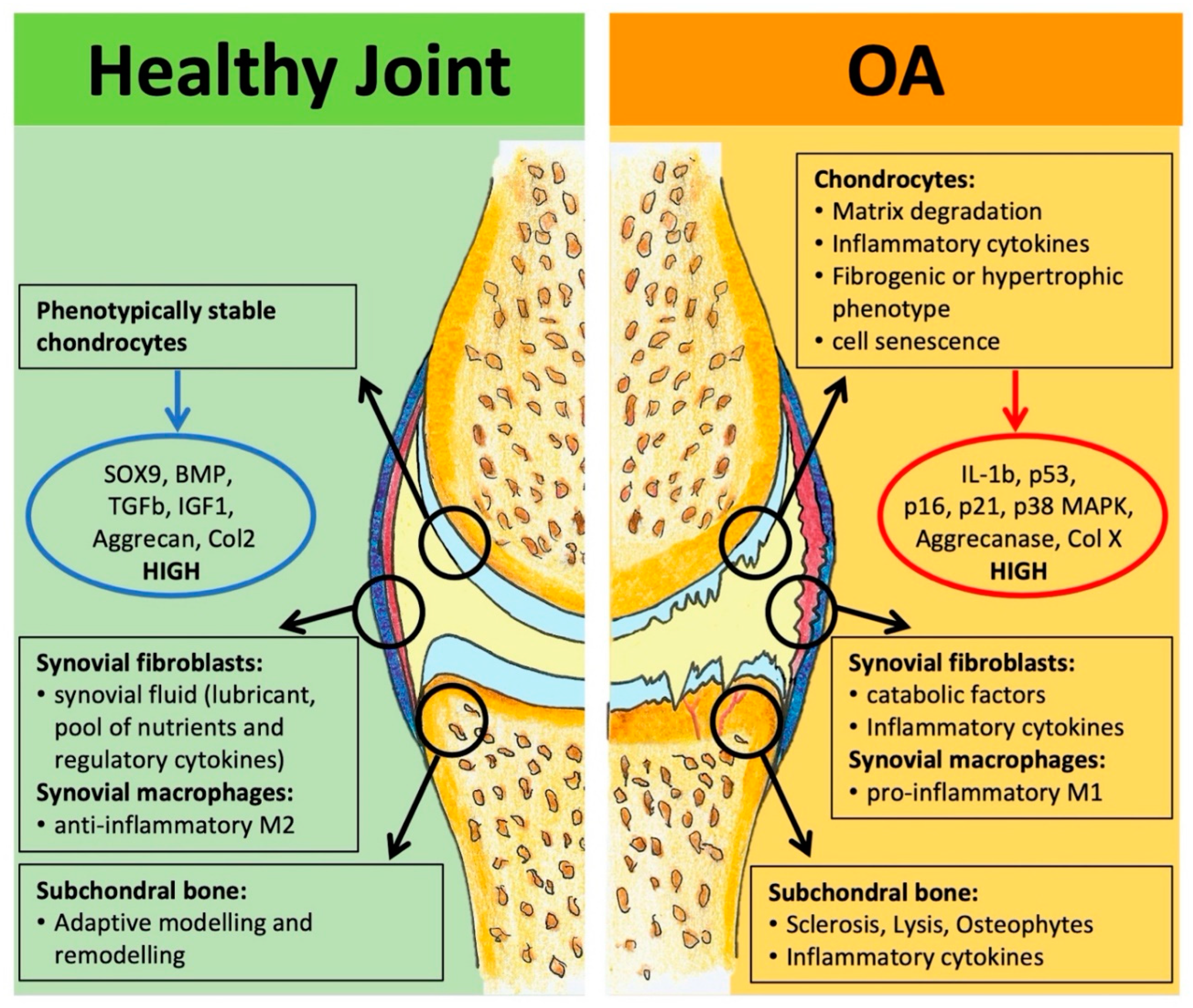

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ribitsch, I.; Oreff, G.L.; Jenner, F. Regenerative Medicine for Equine Musculoskeletal Diseases. Animals 2021, 11, 234. https://doi.org/10.3390/ani11010234
Ribitsch I, Oreff GL, Jenner F. Regenerative Medicine for Equine Musculoskeletal Diseases. Animals. 2021; 11(1):234. https://doi.org/10.3390/ani11010234
Chicago/Turabian StyleRibitsch, Iris, Gil Lola Oreff, and Florien Jenner. 2021. "Regenerative Medicine for Equine Musculoskeletal Diseases" Animals 11, no. 1: 234. https://doi.org/10.3390/ani11010234
APA StyleRibitsch, I., Oreff, G. L., & Jenner, F. (2021). Regenerative Medicine for Equine Musculoskeletal Diseases. Animals, 11(1), 234. https://doi.org/10.3390/ani11010234