Increased Risk of Hospitalization for Pancreatic Cancer in the First 8 Years after a Gestational Diabetes Mellitus regardless of Subsequent Type 2 Diabetes: A Nationwide Population-Based Study
Abstract
Simple Summary
Abstract
1. Background
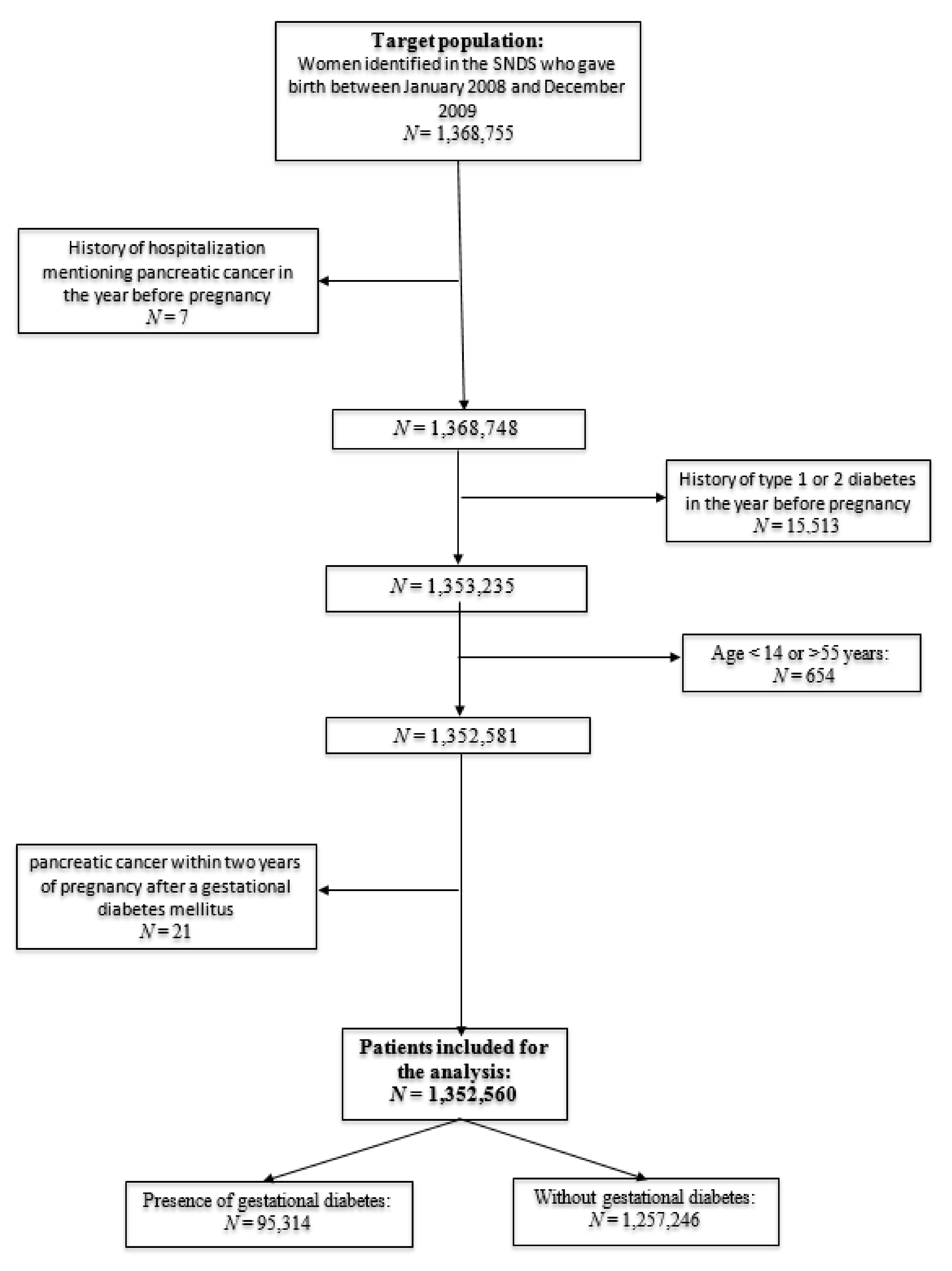
2. Methods
2.1. Data Sources and Study Design
2.2. Exposure
2.3. Outcome
2.4. Variables
2.5. Statistical Analyses
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Drouillard, A.; Manfredi, S.; Lepage, C.; Bouvier, A.-M. Epidemiology of pancreatic cancer. Bull. Cancer JANV 2018, 105, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://seer.cancer.gov/statfacts/html/pancreas.html (accessed on 13 November 2020).
- Rawla, P.; Sunkara, T.; Gaduputi, V. Epidemiology of Pancreatic Cancer: Global Trends, Etiology and Risk Factors. World J. Oncol. 2019, 10, 10–27. [Google Scholar] [CrossRef] [PubMed]
- Huxley, R.; Ansarymoghaddam, A.; De González, A.B.; Barzi, F.; Woodward, M.J. Type-II diabetes and pancreatic cancer: A meta-analysis of 36 studies. Br. J. Cancer 2005, 92, 2076–2083. [Google Scholar] [CrossRef] [PubMed]
- Elena, J.W.; Steplowski, E.; Yu, K.; Hartge, P.; Tobias, G.S.; Brotzman, M.J.; Chanock, S.J.; Stolzenberg-Solomon, R.Z.; Arslan, A.A.; Bueno-De-Mesquita, H.B.; et al. Diabetes and risk of pancreatic cancer: A pooled analysis from the pancreatic cancer cohort consortium. Cancer Causes Control 2013, 24, 13–25. [Google Scholar] [CrossRef] [PubMed]
- Chodick, G.; Heymann, A.D.; Rosenmann, L.; Green, M.S.; Flash, S.; Porath, A.; Kokia, E.; Shalev, V. Diabetes and risk of incident cancer: A large population-based cohort study in Israel. Cancer Causes Control 2010, 21, 879–887. [Google Scholar] [CrossRef]
- Everhart, J.; Wright, D. Diabetes mellitus as a risk factor for pancreatic cancer. A meta-analysis. JAMA 1995, 273, 9. [Google Scholar] [CrossRef]
- Zhou, X.H.; for the DECODE Study Group; Qiao, Q.; Zethelius, B.; Pyörälä, K.; Söderberg, S.; Pajak, A.; Stehouwer, C.D.A.; Heine, R.J.; Jousilahti, P.; et al. Diabetes, prediabetes and cancer mortality. Diabetologia 2010, 53, 1867–1876. [Google Scholar] [CrossRef]
- Biadgo, B.; Abebe, M. Type 2 Diabetes Mellitus and Its Association with the Risk of Pancreatic Carcinogenesis: A Review. Korean J. Gastroenterol. 2016, 67, 168–177. [Google Scholar] [CrossRef]
- Rahn, S.; Zimmermann, V.; Viol, F.; Knaack, H.; Stemmer, K.; Peters, L.; Lenk, L.; Ungefroren, H.; Saur, D.; Schäfer, H.; et al. Diabetes as risk factor for pancreatic cancer: Hyperglycemia promotes epithelial-mesenchymal-transition and stem cell properties in pancreatic ductal epithelial cells. Cancer Lett. 2018, 415, 129–150. [Google Scholar] [CrossRef]
- Eibl, G.; Cruz-Monserrate, Z.; Korc, M.; Petrov, M.S.; Goodarzi, M.O.; Fisher, W.E.; Habtezion, A.; Lugea, A.P.; Pandol, S.J.; Hart, P.A.; et al. Diabetes Mellitus and Obesity as Risk Factors for Pancreatic Cancer. J. Acad. Nutr. Diet. 2018, 118, 555–567. [Google Scholar] [CrossRef]
- Paternoster, S.; Falasca, M. The intricate relationship between diabetes, obesity and pancreatic cancer. Biochim. Biophys. Acta (BBA) Bioenerg. 2020, 1873, 188326. [Google Scholar] [CrossRef] [PubMed]
- Bellamy, L.; Casas, J.-P.; Hingorani, A.D.; Williams, D. Type 2 diabetes mellitus after gestational diabetes: A systematic review and meta-analysis. Lancet 2009, 373, 1773–1779. [Google Scholar] [CrossRef]
- Kaul, P.; Savu, A.; Nerenberg, K.A.; Donovan, L.E.; Chik, C.L.; Ryan, E.A.; Johnson, J.A. Impact of gestational diabetes mellitus and high maternal weight on the development of diabetes, hypertension and cardiovascular disease: A population-level analysis. Diabet. Med. 2015, 32, 164–173. [Google Scholar] [CrossRef] [PubMed]
- Retnakaran, R.; Shah, B.R. Role of Type 2 Diabetes in Determining Retinal, Renal, and Cardiovascular Outcomes in Women With Previous Gestational Diabetes Mellitus. Diabetes Care 2016, 40, 101–108. [Google Scholar] [CrossRef]
- Dawson, S.I. Long-term risk of malignant neoplasm associated with gestational glucose intolerance. Cancer 2003, 100, 149–155. [Google Scholar] [CrossRef]
- Fuchs, O.; Sheiner, E.; Meirovitz, M.; Davidson, E.; Sergienko, R.; Kessous, R. The association between a history of gestational diabetes mellitus and future risk for female malignancies. Arch. Gynecol. Obstet. 2017, 295, 731–736. [Google Scholar] [CrossRef]
- Trabert, B.; Troisi, R.; Grotmol, T.; Ekbom, A.; Engeland, A.; Gissler, M.; Glimelius, I.; Madanat-Harjuoja, L.; Sørensen, H.T.; Tretli, S.; et al. Associations of pregnancy-related factors and birth characteristics with risk of endometrial cancer: A Nordic population-based case–control study. Int. J. Cancer 2020, 146, 1523–1531. [Google Scholar] [CrossRef]
- Tong, G.-X.; Cheng, J.; Chai, J.; Geng, Q.-Q.; Chen, P.-L.; Shen, X.-R.; Liang, H.; Wang, D. Association Between Gestational Diabetes Mellitus and Subsequent Risk of Cancer: A Systematic Review of Epidemiological Studies. Asian Pac. J. Cancer Prev. 2014, 15, 4265–4269. [Google Scholar] [CrossRef]
- Perrin, M.C.; Terry, M.B.; Kleinhaus, K.R.; Deutsch, L.; Yanetz, R.; Tiram, E.; Calderon-Margalit, R.; Friedlander, Y.; Paltiel, O.; Harlap, S. Gestational diabetes as a risk factor for pancreatic cancer: A prospective cohort study. BMC Med. 2007, 5, 25. [Google Scholar] [CrossRef]
- Sella, T.; Chodick, G.; Barchana, M.; Heymann, A.D.; Porath, A.; Kokia, E.; Shalev, V. Gestational diabetes and risk of incident primary cancer: A large historical cohort study in Israel. Cancer Causes Control 2011, 22, 1513–1520. [Google Scholar] [CrossRef]
- Peng, Y.-S.; Lin, J.-R.; Cheng, B.-H.; Ho, C.; Lin, Y.-H.; Shen, C.-H.; Tsai, M.-H. Incidence and relative risk for developing cancers in women with gestational diabetes mellitus: A nationwide cohort study in Taiwan. BMJ Open 2019, 9, e024583. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yan, P.; Fu, T.; Yuan, J.; Yang, G.; Liu, Y.; Zhang, Z.-J. The association between gestational diabetes mellitus and cancer in women: A systematic review and meta-analysis of observational studies. Diabetes Metab. 2020, 46, 461–471. [Google Scholar] [CrossRef] [PubMed]
- Pannala, R.; Leirness, J.B.; Bamlet, W.R.; Basu, A.; Petersen, G.M.; Chari, S.T. Prevalence and Clinical Profile of Pancreatic Cancer–Associated Diabetes Mellitus. Gastroenterology 2008, 134, 981–987. [Google Scholar] [CrossRef] [PubMed]
- Tuppin, P.; Rudant, J.; Constantinou, P.; Gastaldi-Ménager, C.; Rachas, A.; de Roquefeuil, L.; Maura, G.; Caillol, H.; Tajahmady, A.; Coste, J.; et al. Value of a national administrative database to guide public decisions: From the système national d’information interrégimes de l’Assurance Maladie (SNIIRAM) to the système national des données de santé (SNDS) in France. Rev. Epidemiol. Sante Publique 2017, 65 (Suppl. 4), S149–S167. [Google Scholar] [CrossRef]
- Goueslard, K.; Cottenet, J.; Benzenine, E.; Tubert-Bitter, P.; Quantin, C. Validation study: Evaluation of the metrological quality of French hospital data for perinatal algorithms. BMJ Open 2020, 10, e035218. [Google Scholar] [CrossRef]
- Fosse-Edorh, S.; Rigou, A.; Morin, S.; Fezeu, L.; Mandereau-Bruno, L.; Fagot-Campagna, A. Algorithms based on medico-administrative data in the field of endocrine, nutritional and metabolic diseases, especially diabetes. Rev. Epidemiol. Sante Publique 2017, 65 (Suppl. 4), S168–S173. [Google Scholar] [CrossRef]
- VanderWeele, T.J.; Ding, P. Sensitivity Analysis in Observational Research: Introducing the E-Value. Ann. Intern. Med. 2017, 167, 268–274. [Google Scholar] [CrossRef]
- Ma, R.C.; Chan, J.C. Type 2 diabetes in East Asians: Similarities and differences with populations in Europe and the United States. Ann. N. Y. Acad. Sci. 2013, 1281, 64–91. [Google Scholar] [CrossRef]
- Noto, H.; Tsujimoto, T.; Noda, M. Significantly increased risk of cancer in diabetes mellitus patients: A meta-analysis of epidemiological evidence in Asians and non-Asians. J. Diabetes Investig. 2011, 3, 24–33. [Google Scholar] [CrossRef]
- Pergolini, I.; Schorn, S.; Jäger, C.; Goess, R.; Novotny, A.; Friess, H.; Ceyhan, G.O.; Demir, I.E. Diabetes mellitus in intraductal papillary mucinous neoplasms: A systematic review and meta-analysis. Surgery 2020. [Google Scholar] [CrossRef]
- Fuentes, S.; CONSTANCES-Diab Group; Cosson, E.; Mandereau-Bruno, L.; Fagot-Campagna, A.; Bernillon, P.; Goldberg, M.; Fosse-Edorh, S. Identifying diabetes cases in health administrative databases: A validation study based on a large French cohort. Int. J. Public Health 2018, 64, 441–450. [Google Scholar] [CrossRef] [PubMed]
- Bousquet, P.-J.; Caillet, P.; Coeuret-Pellicer, M.; Goulard, H.; Kudjawu, Y.C.; Le Bihan, C.; Lecuyer, A.; Séguret, F. Using cancer case identification algorithms in medico-administrative databases: Literature review and first results from the REDSIAM Tumors group based on breast, colon, and lung cancer. Rev. Epidemiol. Sante Publique 2017, 65 (Suppl. 4), S236–S242. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.W.; Azoulay, L.; Huang, A.; Paterson, M.; Wu, F.; Secrest, M.H.; Filion, K.B. Identification of incident pancreatic cancer in Ontario administrative health data: A validation study. Pharmacoepidemiol. Drug Saf. 2020, 29, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Pierron, A.; Revert, M.; Goueslard, K.; Vuagnat, A.; Cottenet, J.; Benzenine, E.; Fresson, J.; Quantin, C. Evaluation of the metrological quality of the medico-administrative data for perinatal indicators: A pilot study in 3 university hospitals]. Rev. Epidemiol. Sante Publique 2015, 63, 237–246. [Google Scholar] [CrossRef]
- Yadav, D.; Lowenfels, A.B. The Epidemiology of Pancreatitis and Pancreatic Cancer. Gastroenterology 2013, 144, 1252–1261. [Google Scholar] [CrossRef]
- Torloni, M.R.; Betrán, A.P.; Horta, B.L.; Nakamura, M.U.; Atallah, A.N.; Moron, A.F.; Valente, O. Prepregnancy BMI and the risk of gestational diabetes: A systematic review of the literature with meta-analysis. Obes. Rev. 2009, 10, 194–203. [Google Scholar] [CrossRef]
- Johansen, D.; Stocks, T.; Jonsson, H.; Lindkvist, B.; Björge, T.; Concin, H.; Almquist, M.; Häggström, C.; Engeland, A.; Ulmer, H.; et al. Metabolic Factors and the Risk of Pancreatic Cancer: A Prospective Analysis of almost 580,000 Men and Women in the Metabolic Syndrome and Cancer Project. Cancer Epidemiol. Biomark. Prev. 2010, 19, 2307–2317. [Google Scholar] [CrossRef]
- Goueslard, K.; Cottenet, J.; Mariet, A.-S.; Sagot, P.; Petit, J.-M.; Quantin, C. Early screening for type 2 diabetes following gestational diabetes mellitus in France: Hardly any impact of the 2010 guidelines. Acta Diabetol. 2017, 54, 645–651. [Google Scholar] [CrossRef]
- Quaresima, P.; Visconti, F.; Chiefari, E.; Puccio, L.; Foti, D.; Venturella, R.; Vero, R.; Brunetti, A.; Di Carlo, C. Barriers to Postpartum Glucose Intolerance Screening in an Italian Population. Int. J. Environ. Res. Public Health 2018, 15, 2853. [Google Scholar] [CrossRef]
- Boyle, D.I.; Versace, V.L.; Dunbar, J.; Scheil, W.; Janus, E.; Oats, J.J.N.; Skinner, T.C.; Shih, S.; O’Reilly, S.; Sikaris, K.; et al. Results of the first recorded evaluation of a national gestational diabetes mellitus register: Challenges in screening, registration, and follow-up for diabetes risk. PLoS ONE 2018, 13, e0200832. [Google Scholar] [CrossRef]
- Bernstein, J.A.; Quinn, E.; Ameli, O.; Craig, M.; Heeren, T.; Iverson, R.; Jack, B.; Lee-Parritz, A.; McCloskey, L. Onset of T2DM after gestational diabetes: What the prevention paradox tells us about risk. Prev. Med. 2018, 113, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Goueslard, K.; Cottenet, J.; Mariet, A.-S.; Giroud, M.; Cottin, Y.; Petit, J.-M.; Quantin, C. Early cardiovascular events in women with a history of gestational diabetes mellitus. Cardiovasc. Diabetol. 2016, 15, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Collège National des Gynécologues et Obstétriciens Français, Société Francophone du Diabète. Gestational diabetes. J. Gynecol. Obstet. Biol. Reprod 2010, 39 (Suppl. 2), S139, S338–S342. [Google Scholar]
- American Diabetes Association. 14.Management of Diabetes in Pregnancy: Standards of Medical Care in Diabetes—2020. Diabetes Care 2020, 43 (Suppl. 1), S183–S192. [Google Scholar] [CrossRef]
- Capasso, M.; Franceschi, M.; Rodriguez-Castro, K.I.; Crafa, P.; Cambiè, G.; Miraglia, C.; Barchi, A.; Nouvenne, A.; Leandro, G.; Meschi, T.; et al. Epidemiology and risk factors of pancreatic cancer. Acta Biomed. 2018, 89 (Suppl. 9), 141–146. [Google Scholar]
- Midha, S.; Chawla, S.; Garg, P.K. Modifiable and non-modifiable risk factors for pancreatic cancer: A review. Cancer Lett. 2016, 381, 269–277. [Google Scholar] [CrossRef]
| Variables | GDM+ N = 95,314 (7.05%) | GDM− N = 1,257,246 (92.95%) | p | ||
|---|---|---|---|---|---|
| Age * (years) | <0.0001 | ||||
| <26 | 18,095 | 19.0% | 299,873 | 23.9% | |
| 26–29 | 26,013 | 27.3% | 352,910 | 28.1% | |
| 30–33 | 24,279 | 25.5% | 313,954 | 25.0% | |
| ≥34 | 26,927 | 28.2% | 290,509 | 23.1% | |
| Hospitalization with Pancreatic Cancer | 18 | 0.02% | 108 | 0.01% | 0.004 |
| Subsequent type 2 diabetes | 8184 | 8.6% | 6840 | 0.5% | <0.0001 |
| Tobacco consumption ** | 7818 | 8.2% | 86,403 | 6.9% | <0.0001 |
| Variables | Non-Adjusted | Cox Model 1 * | Cox Model 2 ** | |||
|---|---|---|---|---|---|---|
| HR | 95% CI | HR | 95% CI | HR | 95% CI | |
| GDM | 2.59 | 1.57–4.26 | 1.81 | 1.06–3.10 | 1.77 | 1.03–3.03 |
| Age *** | ||||||
| <26 | 0.60 | 0.28–1.27 | 0.61 | 0.28–1.30 | 0.56 | 0.26–1.21 |
| 26–29 | 1 | 1 | 1 | |||
| 30–33 | 1.85 | 1.06–3.22 | 1.83 | 1.05–3.19 | 1.88 | 1.08–3.27 |
| >34 | 3.77 | 2.28–6.23 | 3.60 | 2.18–5.96 | 3.67 | 2.22–6.08 |
| Type 2 diabetes | 8.06 | 3.76–17.32 | 4.85 | 2.13–11.06 | 4.74 | 2.08–10.79 |
| Tobacco consumption | 2.8 | 1.72–4.56 | - | - | 3.22 | 1.97–5.26 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Simon, J.; Goueslard, K.; Arveux, P.; Bechraoui-Quantin, S.; Petit, J.-M.; Quantin, C. Increased Risk of Hospitalization for Pancreatic Cancer in the First 8 Years after a Gestational Diabetes Mellitus regardless of Subsequent Type 2 Diabetes: A Nationwide Population-Based Study. Cancers 2021, 13, 308. https://doi.org/10.3390/cancers13020308
Simon J, Goueslard K, Arveux P, Bechraoui-Quantin S, Petit J-M, Quantin C. Increased Risk of Hospitalization for Pancreatic Cancer in the First 8 Years after a Gestational Diabetes Mellitus regardless of Subsequent Type 2 Diabetes: A Nationwide Population-Based Study. Cancers. 2021; 13(2):308. https://doi.org/10.3390/cancers13020308
Chicago/Turabian StyleSimon, Julien, Karine Goueslard, Patrick Arveux, Sonia Bechraoui-Quantin, Jean-Michel Petit, and Catherine Quantin. 2021. "Increased Risk of Hospitalization for Pancreatic Cancer in the First 8 Years after a Gestational Diabetes Mellitus regardless of Subsequent Type 2 Diabetes: A Nationwide Population-Based Study" Cancers 13, no. 2: 308. https://doi.org/10.3390/cancers13020308
APA StyleSimon, J., Goueslard, K., Arveux, P., Bechraoui-Quantin, S., Petit, J.-M., & Quantin, C. (2021). Increased Risk of Hospitalization for Pancreatic Cancer in the First 8 Years after a Gestational Diabetes Mellitus regardless of Subsequent Type 2 Diabetes: A Nationwide Population-Based Study. Cancers, 13(2), 308. https://doi.org/10.3390/cancers13020308






