The S. pombe CDK5 Orthologue Pef1 Cooperates with Three Cyclins, Clg1, Pas1 and Psl1, to Promote Pre-Meiotic DNA Replication
Abstract
1. Introduction
2. Materials and Methods
2.1. Yeast Strains, Growth Conditions and Induction of Meiosis
2.2. Construction of Modified Strains and Gene Expression Plasmids
2.3. Flow Cytometry Analysis
2.4. Protein Preparation and Immunoblotting
2.5. RNA Preparation and Reverse-Transcription Quantitative Real-Time PCR (RT-qPCR)
2.6. Fluorescence Microscopy
2.7. Statistical Analysis
3. Results
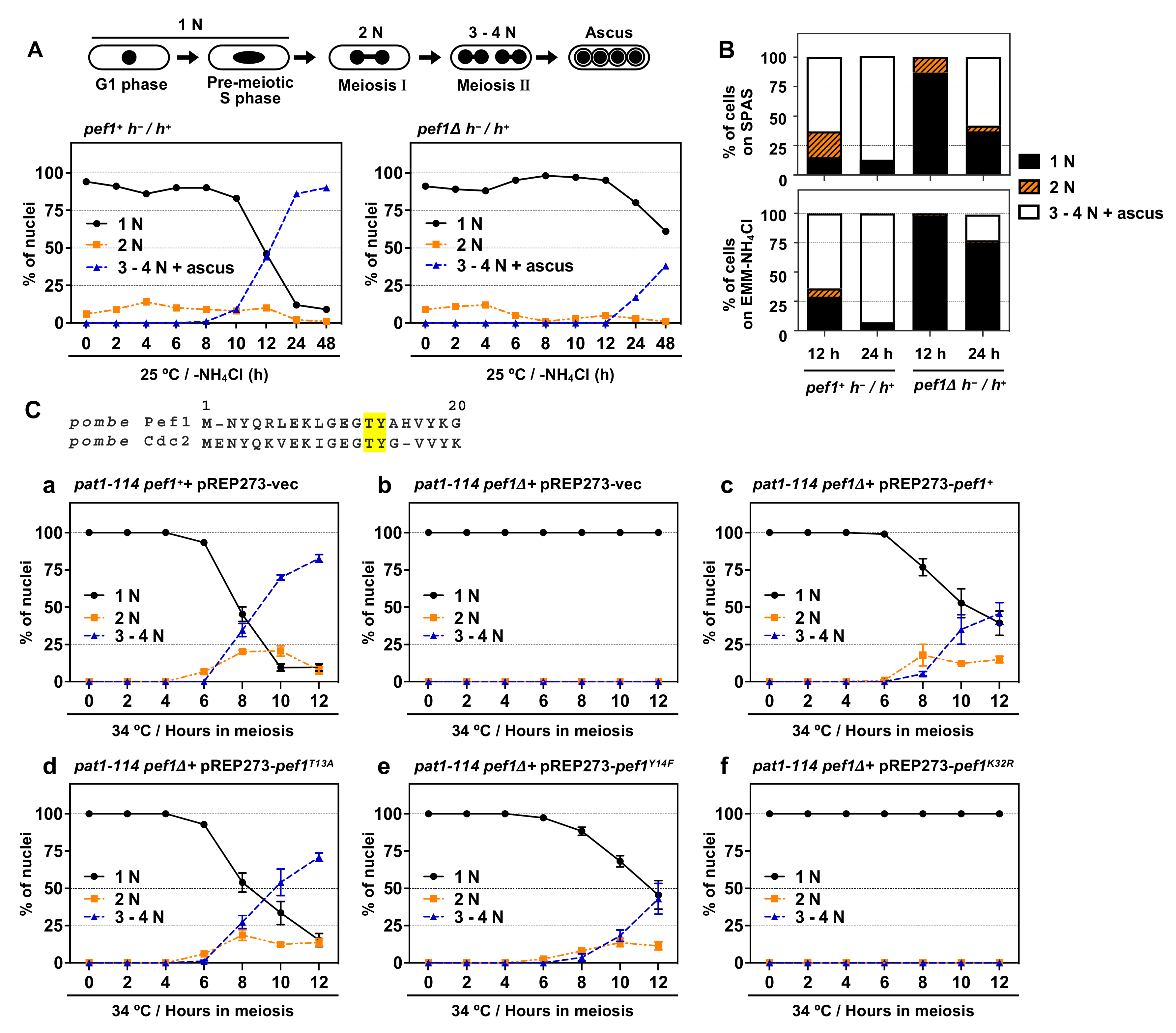
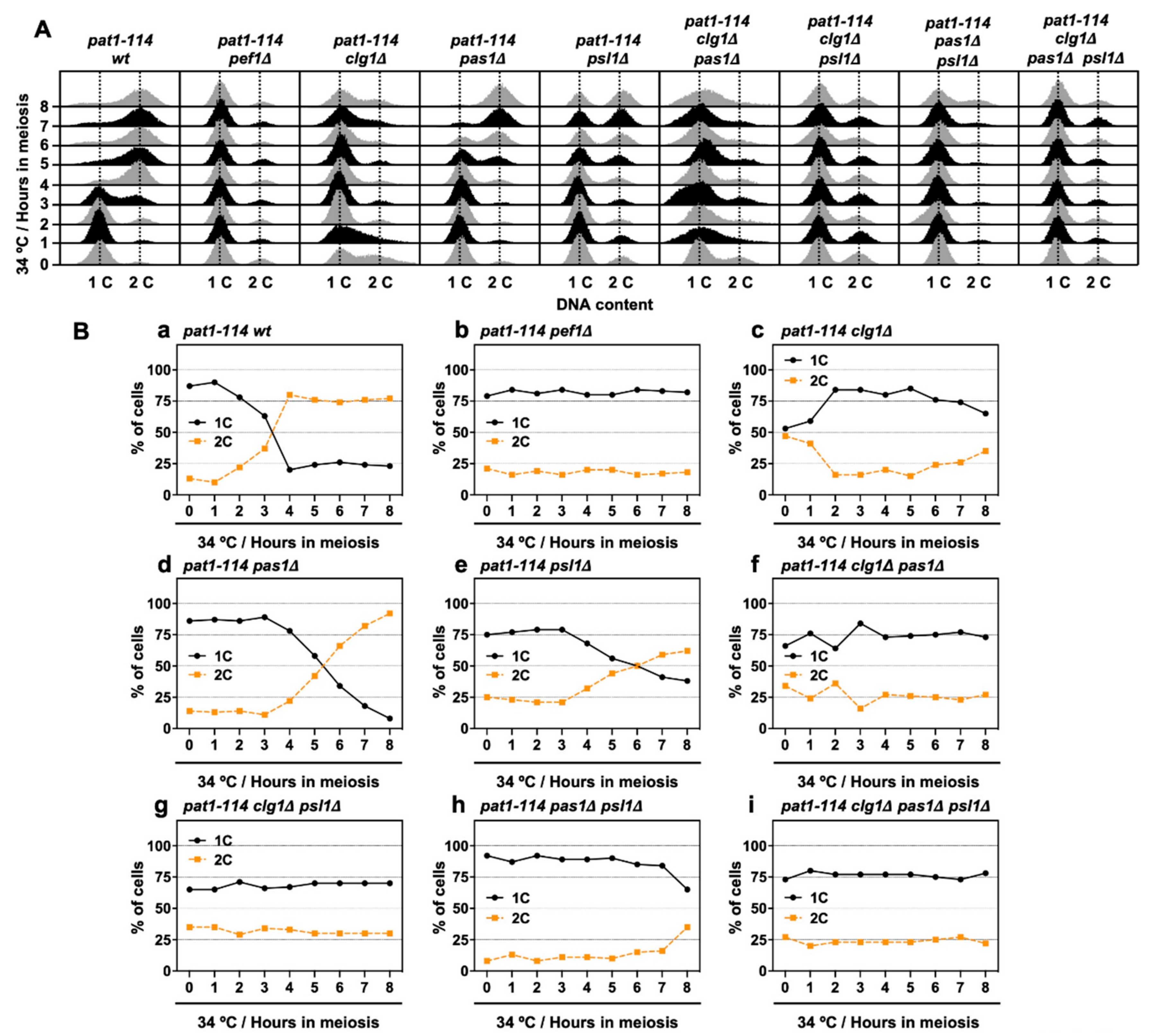
3.1. Pef1 is Required for Meiotic Progression
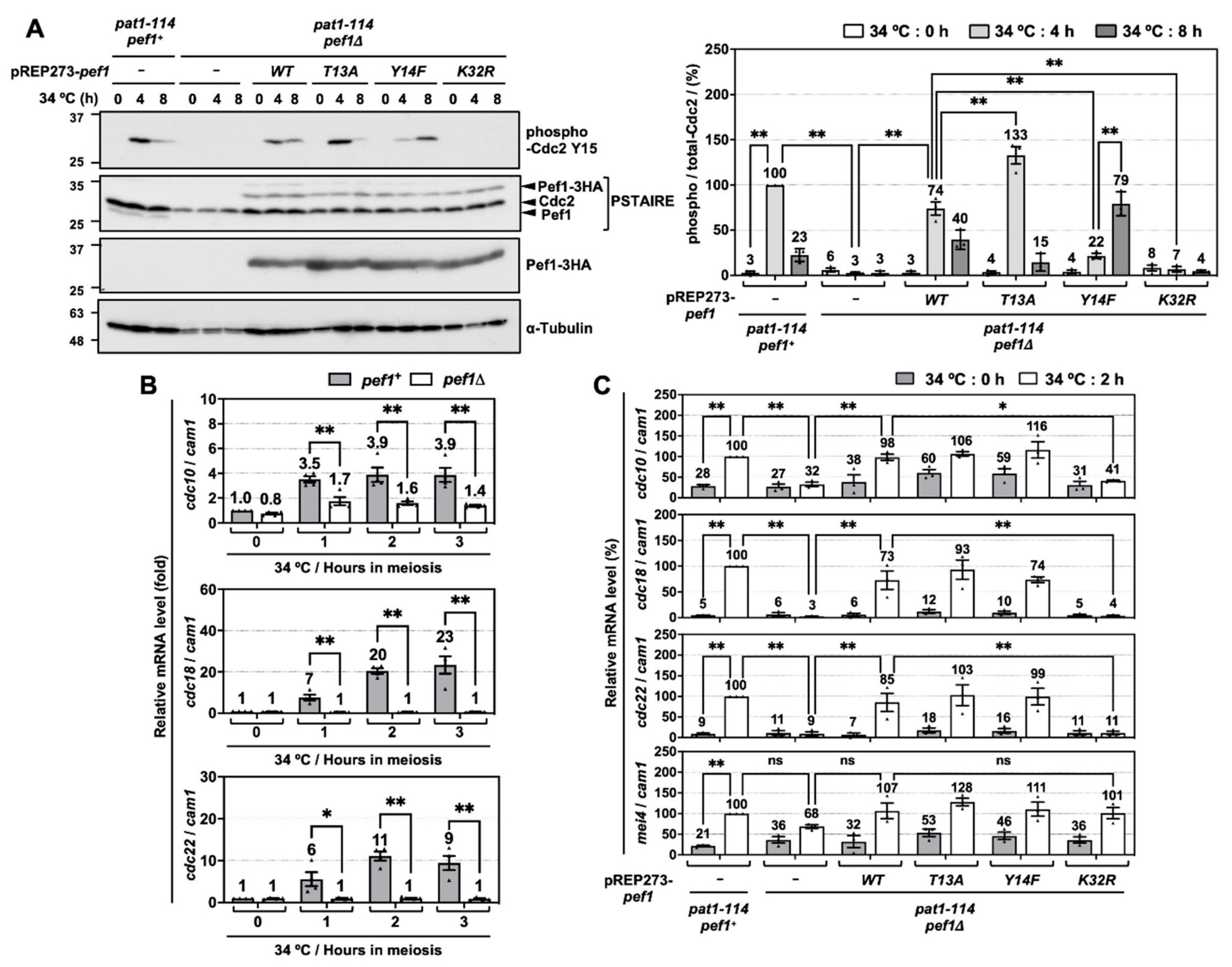
3.2. Pef1 Regulates the Phosphorylation of Tyr-15 in Cdc2 and Promotes the Expression of DNA Replication Factors
3.3. Pef1 Deletion Interrupts Pre-Meiotic Chromosomal DNA Replication
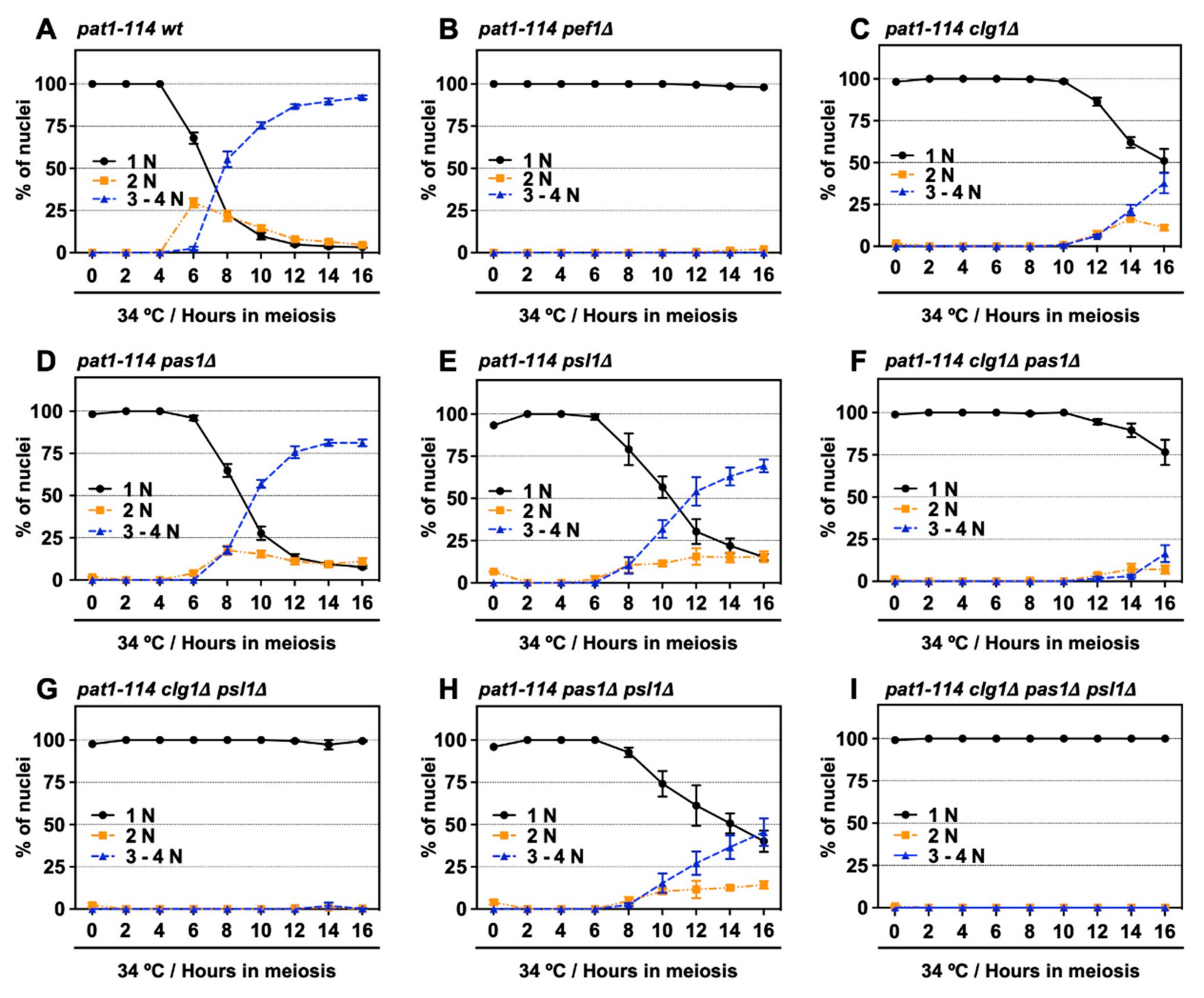
3.4. Double Deletion of clg1 and psl1 Disturbs Meiotic Nuclear Division
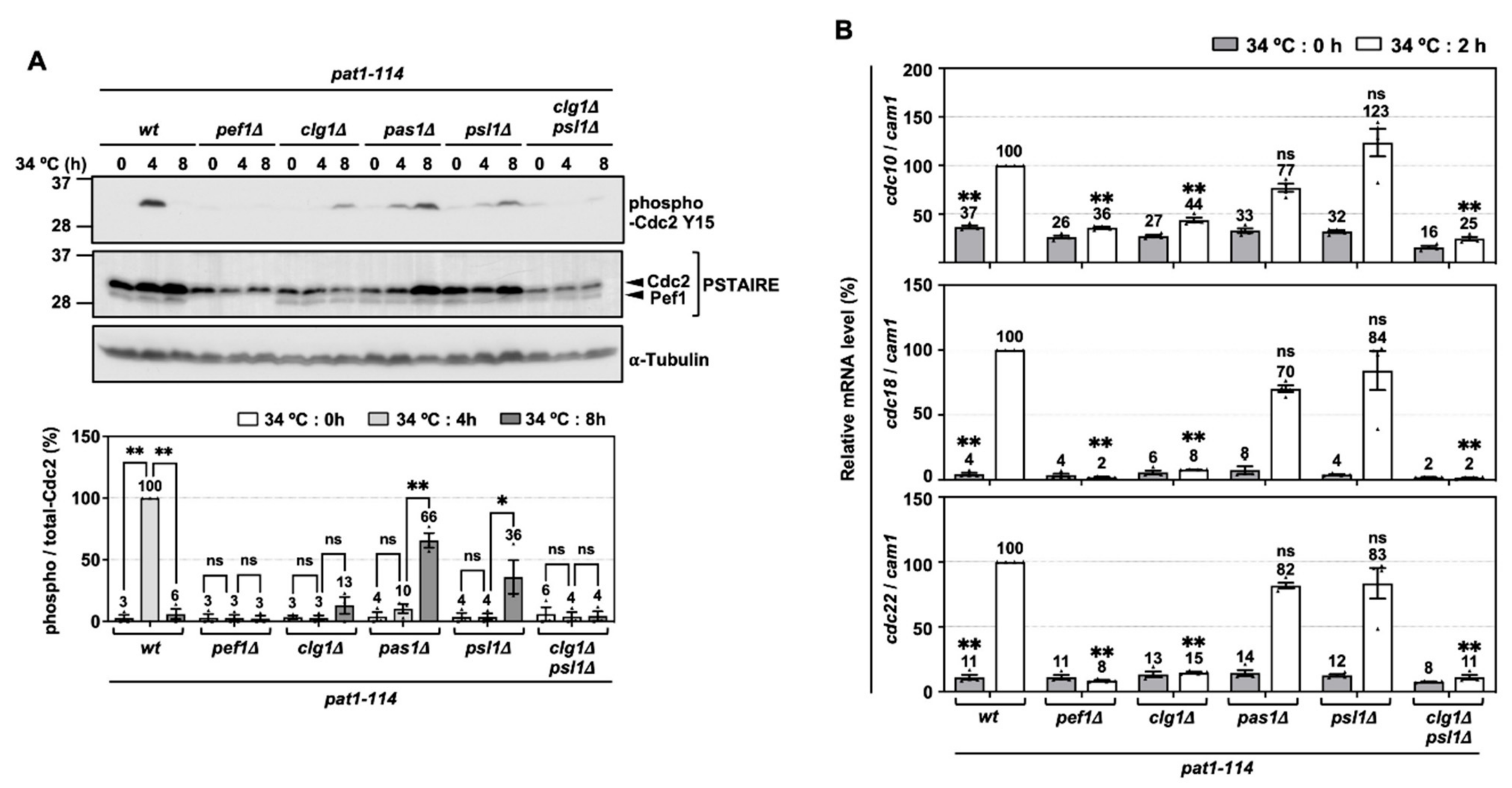
3.5. Cdc2 Phosphorylation and DNA Replication Factors Are Silenced by the Double Deletion of clg1 and psl1
3.6. Deletion of clg1 Stalls Initiation of Pre-Meiotic DNA Replication
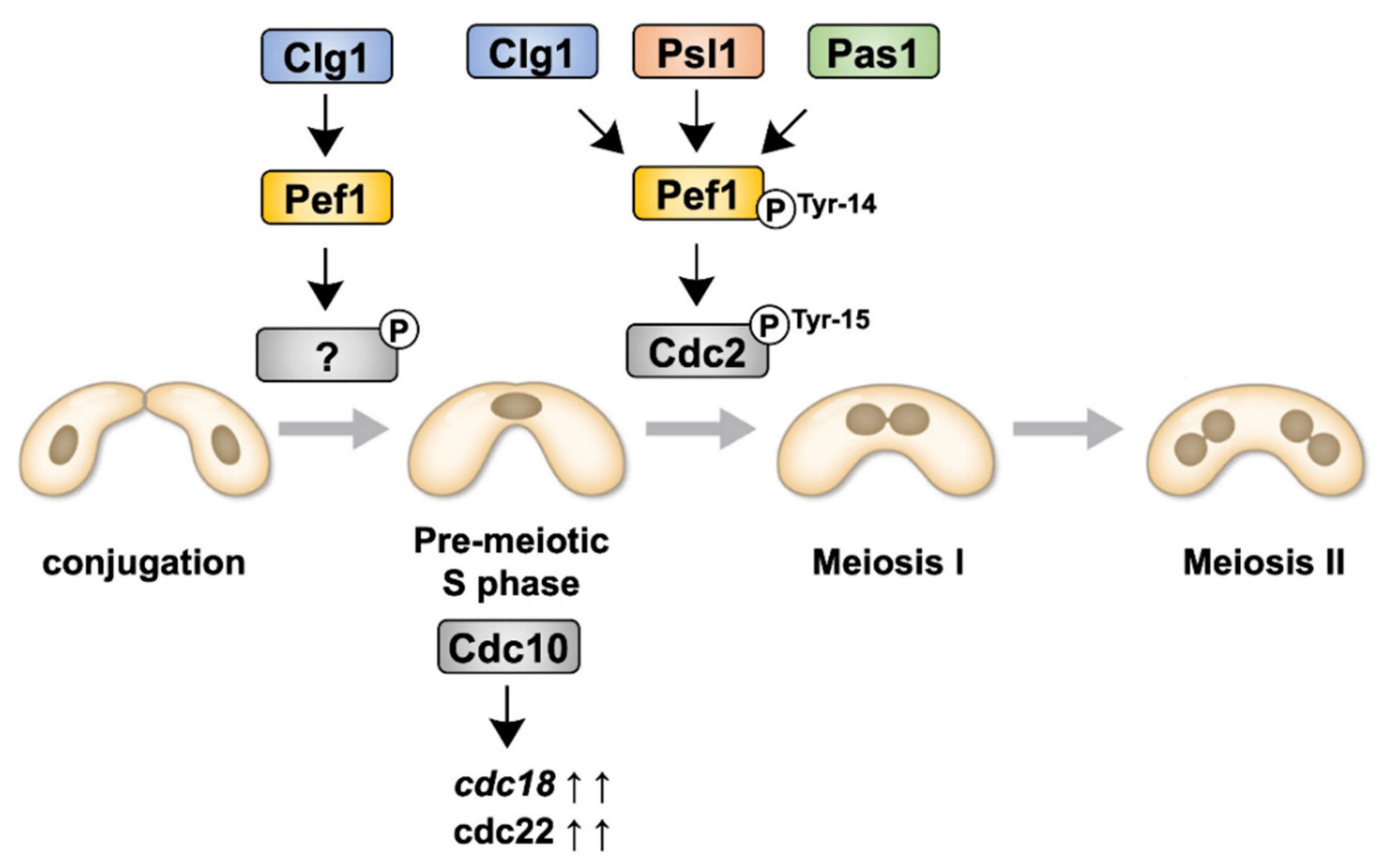
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gray, S.; Cohen, P.E. Control of meiotic crossovers: From double-strand break formation to designation. Annu. Rev. Genet. 2016, 50, 175–210. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, V.V.; Hochwagen, A. The meiotic checkpoint network: Step-by-step through meiotic prophase. Cold Spring Harb. Perspect. Biol. 2014, 6, a016675. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, Y.; Yamamoto, M.S. pombe mei2+ encodes an RNA- binding protein essential for premeiotic DNA synthesis and meiosis I, which cooperates with a novel RNA species meiRNA. Cell 1994, 78, 487–498. [Google Scholar] [CrossRef]
- Watanabe, Y.; Shinozaki-Yabana, S.; Chikashige, Y.; Hiraoka, Y.; Yamamoto, M. Phosphorylation of RNA-binding protein controls cell cycle switch from mitotic to meiotic in fission yeast. Nature 1997, 386, 187–190. [Google Scholar] [CrossRef]
- Yamashita, A.; Watanabe, Y.; Nukina, N.; Yamamoto, M. RNA-assisted nuclear transport of the meiotic regulator Mei2p in fission yeast. Cell 1998, 95, 115–123. [Google Scholar] [CrossRef]
- Harigaya, Y.; Yamamoto, M. Molecular mechanisms underlying the mitosis-meiosis decision. Chromosome Res. 2007, 15, 523–537. [Google Scholar] [CrossRef]
- Malumbres, M. Cyclin-dependent kinases. Genome Biol. 2014, 15, 122. [Google Scholar] [CrossRef]
- Murakami, H.; Nurse, P. DNA replication and damage checkpoints and meiotic cell cycle controls in the fission and budding yeasts. Biochem. J. 2000, 349, 1–12. [Google Scholar] [CrossRef]
- Bustamante-Jaramillo, L.F.; Ramos, C.; Alonso, L.; Sesmero, A.; Segurado, M.; Martín-Castellanos, C. CDK contribution to DSB formation and recombination in fission yeast meiosis. PLoS Genet. 2019, 15, e1007876. [Google Scholar] [CrossRef]
- Gutiérrez-Escribano, P.; Nurse, P. A single cyclin-CDK complex is sufficient for both mitotic and meiotic progression in fission yeast. Nat. Commun. 2015, 6, 6871. [Google Scholar] [CrossRef]
- Lincoln, A.J.; Wickramasinghe, D.; Stein, P.; Schultz, R.M.; Palko, M.E.; De Miguel, M.P.; Tessarollo, L.; Donovan, P.J. Cdc25b phosphatase is required for resumption of meiosis during oocyte maturation. Nat. Genet. 2002, 30, 446–449. [Google Scholar] [CrossRef] [PubMed]
- Han, S.J.; Chen, R.; Paronetto, M.P.; Conti, M. Wee1B is an oocyte-specific kinase involved in the control of meiotic arrest in the mouse. Curr. Biol. 2005, 15, 1670–1676. [Google Scholar] [CrossRef] [PubMed]
- Nakajo, N.; Yoshitome, S.; Iwashita, J.; Iida, M.; Uto, K.; Ueno, S.; Okamoto, K.; Sagata, N. Absence of Wee1 ensures the meiotic cell cycle in Xenopus oocytes. Genes Dev. 2000, 14, 328–338. [Google Scholar] [PubMed]
- Cao, L.; Chen, F.; Yang, X.; Xu, W.; Xie, J.; Yu, L. Phylogenetic analysis of CDK and cyclin proteins in premetazoan lineages. BMC Evol. Biol. 2014, 14, 10. [Google Scholar] [CrossRef] [PubMed]
- Dhavan, R.; Tsai, L.H. A decade of CDK5. Nat. Rev. Mol. Cell Biol. 2001, 2, 749–759. [Google Scholar] [CrossRef]
- Tanaka, K.; Okayama, H. A pcl-like cyclin activates the Res2p-Cdc10p cell cycle “start” transcriptional factor complex in fission yeast. Mol. Biol. Cell 2000, 11, 2845–2862. [Google Scholar] [CrossRef]
- Chen, B.R.; Li, Y.; Eisenstatt, J.R.; Runge, K.W. Identification of a lifespan extending mutation in the Schizosaccharomyces pombe cyclin gene clg1+ by direct selection of long-lived mutants. PLoS ONE 2013, 8, e69084. [Google Scholar] [CrossRef]
- Matsuda, S.; Kikkawa, U.; Uda, H.; Nakashima, A. The S. pombe CDK5 ortholog Pef1 regulates sexual differentiation through control of the TORC1 pathway and autophagy. J. Cell Sci. 2020, 133, jcs247817:1–jcs247817:17. [Google Scholar] [CrossRef]
- Moreno, S.; Klar, A.; Nurse, P. Molecular genetic analysis of fission yeast Schizosaccharomyces pombe. Methods Enzymol. 1991, 194, 795–823. [Google Scholar]
- Sabatinos, S.A.; Forsburg, S.L. Molecular Genetics of Schizosaccharomyces pombe. Methods Enzymol. 2010, 470, 759–795. [Google Scholar]
- Okazaki, K.; Okazaki, N.; Kume, K.; Jinno, S.; Tanaka, K.; Okayama, H. High-frequency transformation method and library transducing vectors for cloning mammalian cDNAs by trans-complementation of Schizosaccharomyces pombe. Nucleic Acids Res. 1990, 18, 6485–6489. [Google Scholar] [CrossRef] [PubMed]
- Bähler, J.; Wu, J.Q.; Longtine, M.S.; Shah, N.G.; McKenzie, A.; Steever, A.B.; Wach, A.; Philippsen, P.; Pringle, J.R. Heterologous modules for efficient and versatile PCR-based gene targeting in Schizosaccharomyces pombe. Yeast 1998, 14, 943–951. [Google Scholar] [CrossRef]
- Sato, M.; Dhut, S.; Toda, T. New drug-resistant cassettes for gene disruption and epitope tagging in Schizosaccharomyces pombe. Yeast 2005, 22, 583–591. [Google Scholar] [CrossRef] [PubMed]
- Boddy, M.N.; Furnari, B.; Mondesert, O.; Russell, P. Replication checkpoint enforced by kinases Cds1 and Chk1. Science 1998, 280, 909–912. [Google Scholar] [CrossRef]
- Zeng, Y.; Forbes, K.C.; Wu, Z.; Moreno, S.; Piwnica-Worms, H.; Enoch, T. Replication checkpoint requires phosphorylation of the phosphatase Cdc25 by Cds1 or Chk1. Nature 1998, 395, 507–510. [Google Scholar] [CrossRef]
- Furnari, B.; Blasina, A.; Boddy, M.N.; McGowan, C.H.; Russell, P. Cdc25 inhibited in vivo and in vitro by checkpoint kinases Cds1 and Chk1. Mol. Biol. Cell 1999, 10, 833–845. [Google Scholar] [CrossRef]
- Bähler, J. Cell-cycle control of gene expression in budding and fission yeast. Annu. Rev. Genet. 2005, 39, 69–94. [Google Scholar] [CrossRef]
- Dutta, C.; Patel, P.K.; Rosebrock, A.; Oliva, A.; Leatherwood, J.; Rhind, N. The DNA replication checkpoint directly regulates MBF-dependent G1/S transcription. Mol. Cell. Biol. 2008, 28, 5977–5985. [Google Scholar] [CrossRef]
- Cunliffe, L.; White, S.; McInerny, C.J. DSC1-MCB regulation of meiotic transcription in Schizosaccharomyces pombe. Mol. Genet. Genomics 2004, 271, 60–71. [Google Scholar] [CrossRef]
- Kelly, T.J.; Martin, G.S.; Forsburg, S.L.; Stephen, R.J.; Russo, A.; Nurse, P. The fission yeast cdc18+ gene product couples S phase to START and mitosis. Cell 1993, 74, 371–382. [Google Scholar] [CrossRef]
- Lowndes, N.F.; McInerny, C.J.; Johnson, A.L.; Fantes, P.A.; Johnston, L.H. Control of DNA synthesis genes in fission yeast by the cell-cycle gene cdc10+. Nature 1992, 355, 449–453. [Google Scholar] [CrossRef] [PubMed]
- Ayté, J.; Schweitzer, C.; Zarzov, P.; Nurse, P.; DeCaprio, J.A. Feedback regulation of the MBF transcription factor by cyclin Cig2. Nat. Cell Biol. 2001, 3, 1043–1050. [Google Scholar] [CrossRef] [PubMed]
- De Bruin, R.A.; Kalashnikova, T.I.; Aslanian, A.; Wohlschlegel, J.; Chahwan, C.; Yates, J.R.; Russell, P.; Wittenberg, C. DNA replication checkpoint promotes G1-S transcription by inactivating the MBF repressor Nrm1. Proc. Natl. Acad. Sci. USA 2008, 105, 11230–11235. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Escoda, B.; Ivanova, T.; Calvo, I.A.; Alves-Rodrigues, I.; Hidalgo, E.; Ayté, J. Yox1 links MBF-dependent transcription to completion of DNA synthesis. EMBO Rep. 2011, 12, 84–89. [Google Scholar]
- Caetano, C.; Klier, S.; de Bruin, R.A. Phosphorylation of the MBF repressor Yox1p by the DNA replication checkpoint keeps the G1/s cell-cycle transcriptional program active. PLoS ONE 2011, 6, e17211. [Google Scholar] [CrossRef] [PubMed]
- Horie, S.; Watanabe, Y.; Tanaka, K.; Nishiwaki, S.; Fujioka, H.; Abe, H.; Yamamoto, M.; Shimoda, C. The Schizosaccharomyces pombe mei4+ gene encodes a meiosis-specific transcription factor containing a forkhead DNA-binding domain. Mol. Cell. Biol. 1998, 18, 2118–2129. [Google Scholar] [CrossRef]
- Murakami-Tonami, Y.; Ohtsuka, H.; Aiba, H.; Murakami, H. Regulation of wee1+ expression during meiosis in fission yeast. Cell Cycle 2014, 13, 2853–2858. [Google Scholar] [CrossRef]
- Lee, K.Y.; Rosales, J.L.; Lee, B.C.; Chung, S.H.; Fukui, Y.; Lee, N.S.; Lee, K.Y.; Jeong, Y.G. Cdk5/p35 expression in the mouse ovary. Mol. Cells 2004, 17, 17–22. [Google Scholar]
- Ino, H.; Ishizuka, T.; Chiba, T.; Tatibana, M. Expression of CDK5 (PSSALRE kinase), a neural cdc2-related protein kinase, in the mature and developing mouse central and peripheral nervous systems. Brain Res. 1994, 661, 196–206. [Google Scholar] [CrossRef]
- Mikolcevic, P.; Sigl, R.; Rauch, V.; Hess, M.W.; Pfaller, K.; Barisic, M.; Pelliniemi, L.J.; Boesl, M.; Geley, S. Cyclin-dependent kinase 16/PCTAIRE kinase 1 is activated by cyclin Y and is essential for spermatogenesis. Mol. Cell. Biol. 2012, 32, 868–879. [Google Scholar] [CrossRef]
- Iino, Y.; Hiramine, Y.; Yamamoto, M. The role of cdc2 and other genes in meiosis in Schizosaccharomyces pombe. Genetics 1995, 140, 1235–1245. [Google Scholar] [PubMed]
- Malapeira, J.; Moldón, A.; Hidalgo, E.; Smith, G.R.; Nurse, P.; Ayté, J. A meiosis-specific cyclin regulated by splicing is required for proper progression through meiosis. Mol. Cell. Biol. 2005, 25, 6330–6337. [Google Scholar] [CrossRef] [PubMed]
- Murakami-Tonami, Y.; Yamada-Namikawa, C.; Tochigi, A.; Hasegawa, N.; Kojima, H.; Kunimatsu, M.; Nakanishi, M.; Murakami, H.M. Mei4p coordinates the onset of meiosis I by regulating cdc25+ in fission yeast. Proc. Natl. Acad. Sci. USA 2007, 104, 14688–14693. [Google Scholar] [CrossRef] [PubMed]
- Chang, K.H.; Vincent, F.; Shah, K. Deregulated Cdk5 triggers aberrant activation of cell cycle kinases and phosphatases inducing neuronal death. J. Cell Sci. 2012, 125, 5124–5137. [Google Scholar] [CrossRef] [PubMed]
- Chica, N.; Rozalén, A.E.; Pérez-Hidalgo, L.; Rubio, A.; Novak, B.; Moreno, S. Nutritional control of cell size by the Greatwall-Endosulfine-PP2A·B55 pathway. Curr. Biol. 2016, 26, 319–330. [Google Scholar] [CrossRef]
- Yang, Z.; Geng, J.; Yen, W.L.; Wang, K.; Klionsky, D.J. Positive or negative roles of different cyclin-dependent kinase Pho85-cyclin complexes orchestrate induction of autophagy in Saccharomyces cerevisiae. Mol. Cell 2010, 38, 250–264. [Google Scholar] [CrossRef]
- Zukerberg, L.R.; Patrick, G.N.; Nikolic, M.; Humbert, S.; Wu, C.L.; Lanier, L.M.; Gertler, F.B.; Vidal, M.; Van Etten, R.A.; Tsai, L.H. Cables links Cdk5 and c-Abl and facilitates Cdk5 tyrosine phosphorylation, kinase upregulation, and neurite outgrowth. Neuron 2000, 26, 633–646. [Google Scholar] [CrossRef]
- Sasaki, Y.; Cheng, C.; Uchida, Y.; Nakajima, O.; Ohshima, T.; Yagi, T.; Taniguchi, M.; Nakayama, T.; Kishida, R.; Kudo, Y.; et al. Fyn and Cdk5 mediate semaphorin-3A signaling, which is involved in regulation of dendrite orientation in cerebral cortex. Neuron 2002, 35, 907–920. [Google Scholar] [CrossRef]
- Fu, W.Y.; Chen, Y.; Sahin, M.; Zhao, X.S.; Shi, L.; Bikoff, J.B.; Lai, K.O.; Yung, W.H.; Fu, A.K.; Greenberg, M.E.; et al. Cdk5 regulates EphA4-mediated dendritic spine retraction through an ephexin1-dependent mechanism. Nat. Neurosci. 2007, 10, 67–76. [Google Scholar] [CrossRef]
- Cheung, Z.H.; Chin, W.H.; Chen, Y.; Ng, Y.P.; Ip, N.Y. Cdk5 is involved in BDNF-stimulated dendritic growth in hippocampal neurons. PLoS Biol. 2007, 5, e63. [Google Scholar] [CrossRef]
- Lin, H.; Lin, T.Y.; Juang, J.L. Abl deregulates Cdk5 kinase activity and subcellular localization in Drosophila neurodegeneration. Cell Death Differ. 2007, 14, 607–615. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, H.; Saito, T.; Sato, K.; Furusawa, K.; Hosokawa, T.; Tsutsumi, K.; Asada, A.; Kamada, S.; Ohshima, T.; Hisanaga, S. Phosphorylation of cyclin-dependent kinase 5 (Cdk5) at Tyr-15 is inhibited by Cdk5 activators and does not contribute to the activation of Cdk5. J. Biol. Chem. 2014, 289, 19627–19636. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, K.; Futcher, B.; Leatherwood, J. mmi1 and rep2 mRNAs are novel RNA targets of the Mei2 RNA-binding protein during early meiosis in Schizosaccharomyces pombe. Open Biol. 2018, 8, 180110:1–180110:10. [Google Scholar] [CrossRef] [PubMed]
- Sugiyama, A.; Tanaka, K.; Okazaki, K.; Nojima, H.; Okayama, H. A zinc finger protein controls the onset of premeiotic DNA synthesis of fission yeast in a Mei2-independent cascade. EMBO J. 1994, 13, 1881–1887. [Google Scholar] [CrossRef]
- Valbuena, N.; Moreno, S. TOR and PKA pathways synergize at the level of the Ste11 transcription factor to prevent mating and meiosis in fission yeast. PLoS ONE 2010, 5, e11514. [Google Scholar] [CrossRef]
- Kamei, H.; Saito, T.; Ozawa, M.; Fujita, Y.; Asada, A.; Bibb, J.A.; Saido, T.C.; Sorimachi, H.; Hisanaga, S. Suppression of calpain-dependent cleavage of the CDK5 activator p35 to p25 by site-specific phosphorylation. J. Biol. Chem. 2007, 282, 1687–1694. [Google Scholar] [CrossRef]
- Zachariae, W. Destruction with a box: Substrate recognition by the anaphase-promoting complex. Mol. Cell 2004, 13, 2–3. [Google Scholar] [CrossRef]
- Yamano, H. APC/C: Current understanding and future perspectives. F1000Research 2019, 8, 725:1–725:15. [Google Scholar] [CrossRef]

| Strain | Genotype | Source/Reference |
|---|---|---|
| L972 | h− | National-Bio-Resource-Project |
| FY7052 | h− pat1-114 | National-Bio-Resource-Project |
| FY7122 | h− pat1-114 leu1- | National-Bio-Resource-Project |
| AN0353 | h−/h+ ade6-M210/ade6-M216 | This study |
| AN0579 | h− pat1-114 mei2::hphMX | This study |
| MS009 | h- pef1::hphMX | [18] |
| MS030 | h− pat1-114 pef1::hphMX | This study |
| MS053 | h− pat1-114 pef1-3HA:hphMX | This study |
| MS065 | h− psl1::hphMX | [18] |
| MS067 | h− clg1::hphMX | [18] |
| MS069 | h− pas1::hphMX | [18] |
| MS073 | h+ pef1::natR ade6M210 | This study |
| MS100 | h−/h+ pef1::hphMX/pef1::natR ade6M210 diploid | This study |
| MS113 | h- clg1::hphMX psl1::hphMX | [18] |
| MS115 | h− clg1::hphMX pas1::kanMX psl1::hphMX | [18] |
| MS157 | h− pas1::KanMX psl1::hphMX | [18] |
| MS159 | h− pas1::KanMX clg1::hphMX | [18] |
| MS105 | h− pat1-114 clg1::hphMX | This study |
| MS106 | h− pat1-114 pas1::kanMX | This study |
| MS107 | h− pat1-114 psl1::hphMX | This study |
| MS119-1 | h− pat1-114 clg1::hphMX psl1::hphMX | This study |
| MS126-2 | h− pat1-114 clg1::hphMX pas1::kanMX psl1::hphMX | This study |
| MS155 | h− pat1-114 clg1::hphMX pas1::kanMX | This study |
| MS156 | h− pat1-114 psl1::kanMX pas1::kanMX | This study |
| MS254 | h− pat1-114 leu1- pef1::hphMX | This study |
| Gene | Forward Primer (5′ to 3′) | Reverse Primer (5′ to 3′) |
|---|---|---|
| cdc10 | AACAAACTTTGGCAACGAAA | AACACGTAAGAATCCAGCAA |
| cdc18 | TTTGTGTCTTCGAGATCGTT | TGTCTCCAACAGCTGTAATG |
| cdc22 | TTTTATGGCTGGAAGAAGGG | CTCTTCTTTAGTGGGCTCTG |
| mei4 | ATGTCCCTGATTTAACACCC | TGATTAAGTTCAGGGCTGTC |
| cam1 | CCCGAAAAATGAAGGATACC | CTTGGAAGAAATGACACGAG |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matsuda, S.; Kikkawa, U.; Nakashima, A. The S. pombe CDK5 Orthologue Pef1 Cooperates with Three Cyclins, Clg1, Pas1 and Psl1, to Promote Pre-Meiotic DNA Replication. Biomolecules 2021, 11, 89. https://doi.org/10.3390/biom11010089
Matsuda S, Kikkawa U, Nakashima A. The S. pombe CDK5 Orthologue Pef1 Cooperates with Three Cyclins, Clg1, Pas1 and Psl1, to Promote Pre-Meiotic DNA Replication. Biomolecules. 2021; 11(1):89. https://doi.org/10.3390/biom11010089
Chicago/Turabian StyleMatsuda, Shinya, Ushio Kikkawa, and Akio Nakashima. 2021. "The S. pombe CDK5 Orthologue Pef1 Cooperates with Three Cyclins, Clg1, Pas1 and Psl1, to Promote Pre-Meiotic DNA Replication" Biomolecules 11, no. 1: 89. https://doi.org/10.3390/biom11010089
APA StyleMatsuda, S., Kikkawa, U., & Nakashima, A. (2021). The S. pombe CDK5 Orthologue Pef1 Cooperates with Three Cyclins, Clg1, Pas1 and Psl1, to Promote Pre-Meiotic DNA Replication. Biomolecules, 11(1), 89. https://doi.org/10.3390/biom11010089





