Visceral Adipose Tissue of Prediabetic and Diabetic Females Shares a Set of Similarly Upregulated microRNAs Functionally Annotated to Inflammation, Oxidative Stress and Insulin Signaling
Abstract
1. Introduction
2. Materials and Methods
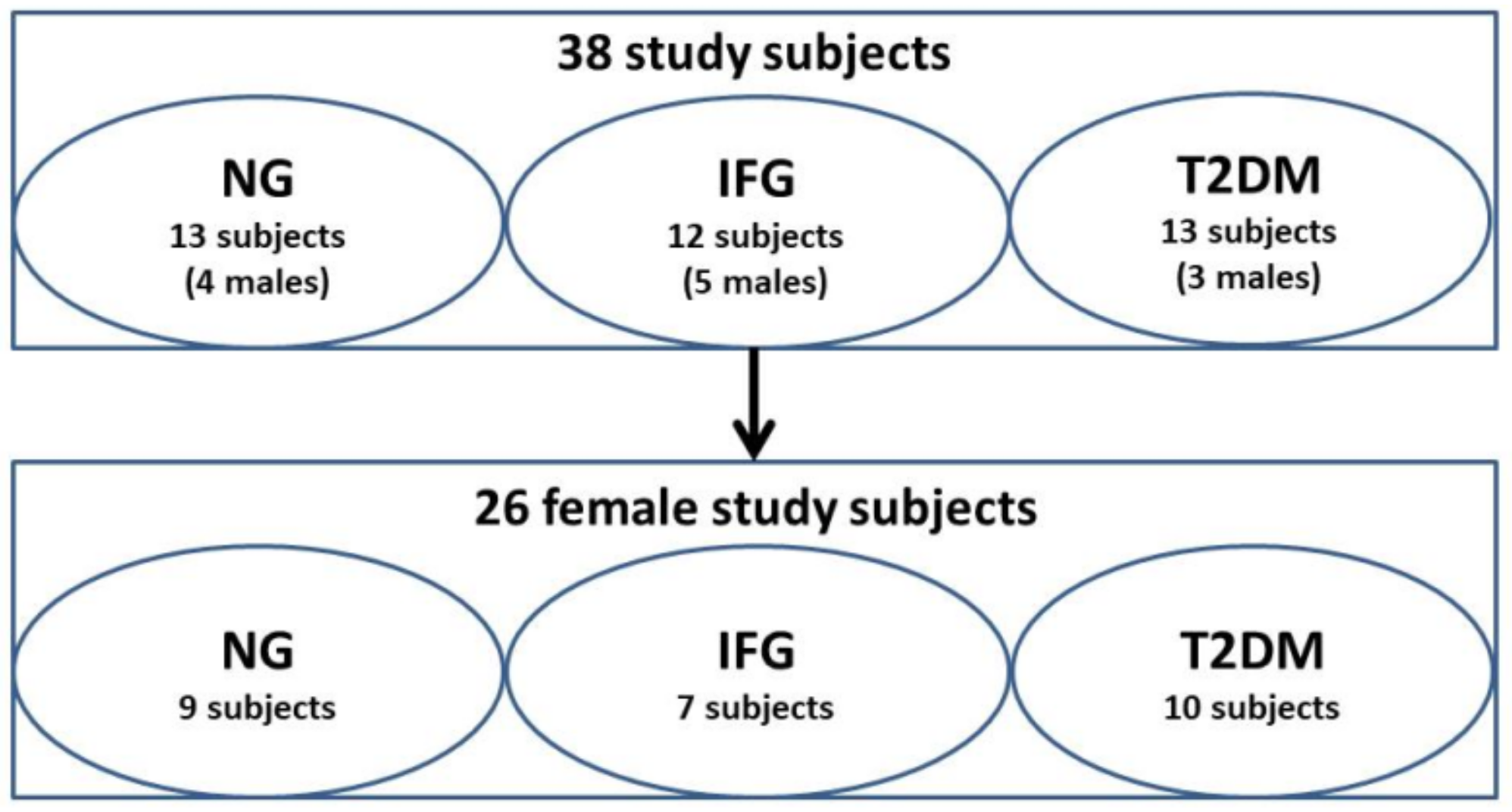
2.1. Study Subjects and Ethics
2.2. Biochemical and Anthropometric Measurements
2.3. VAT Sample Processing and Total RNA Isolation
2.4. Molecules Selection
2.5. Reverse Transcription and Expression Profiling
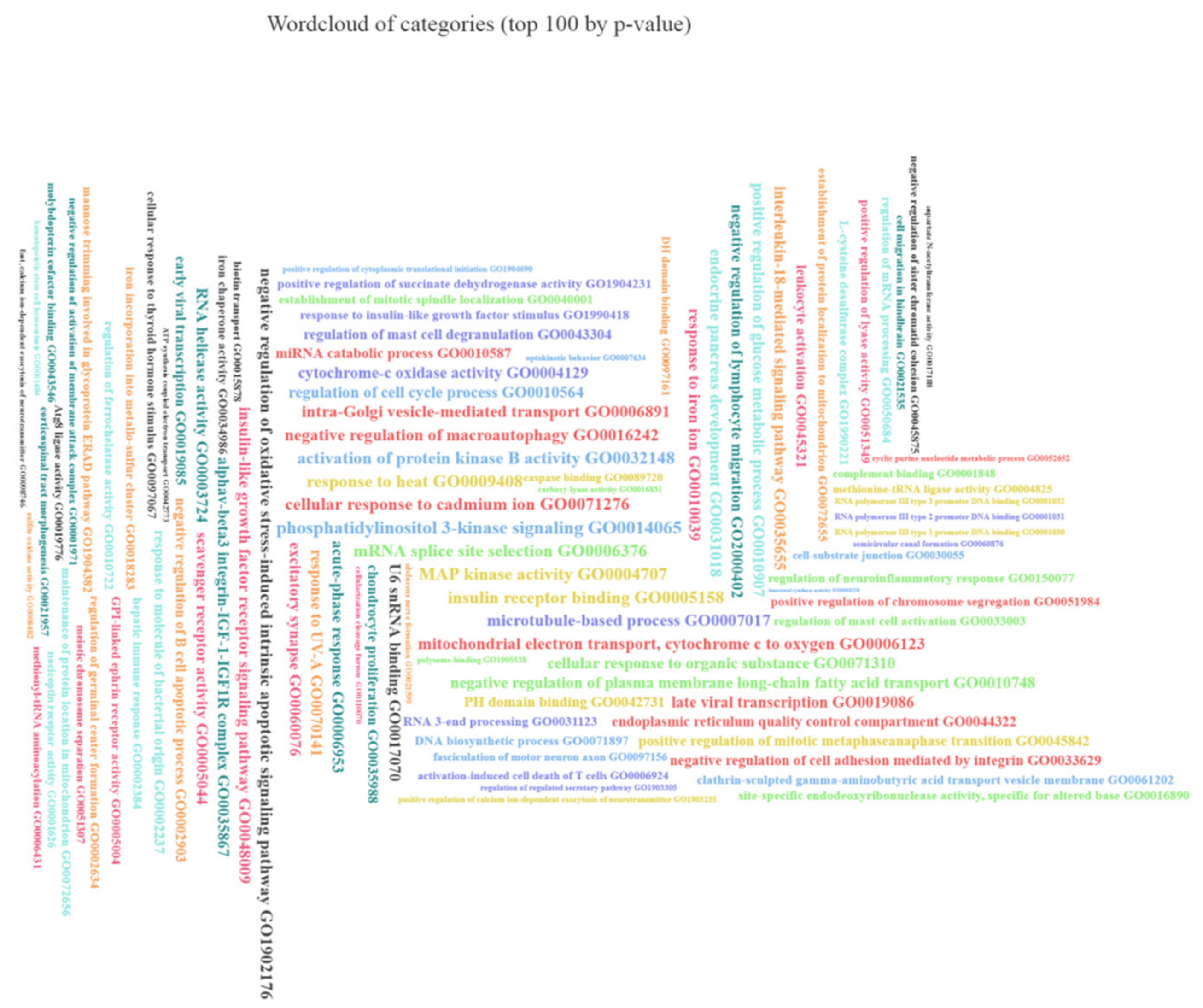
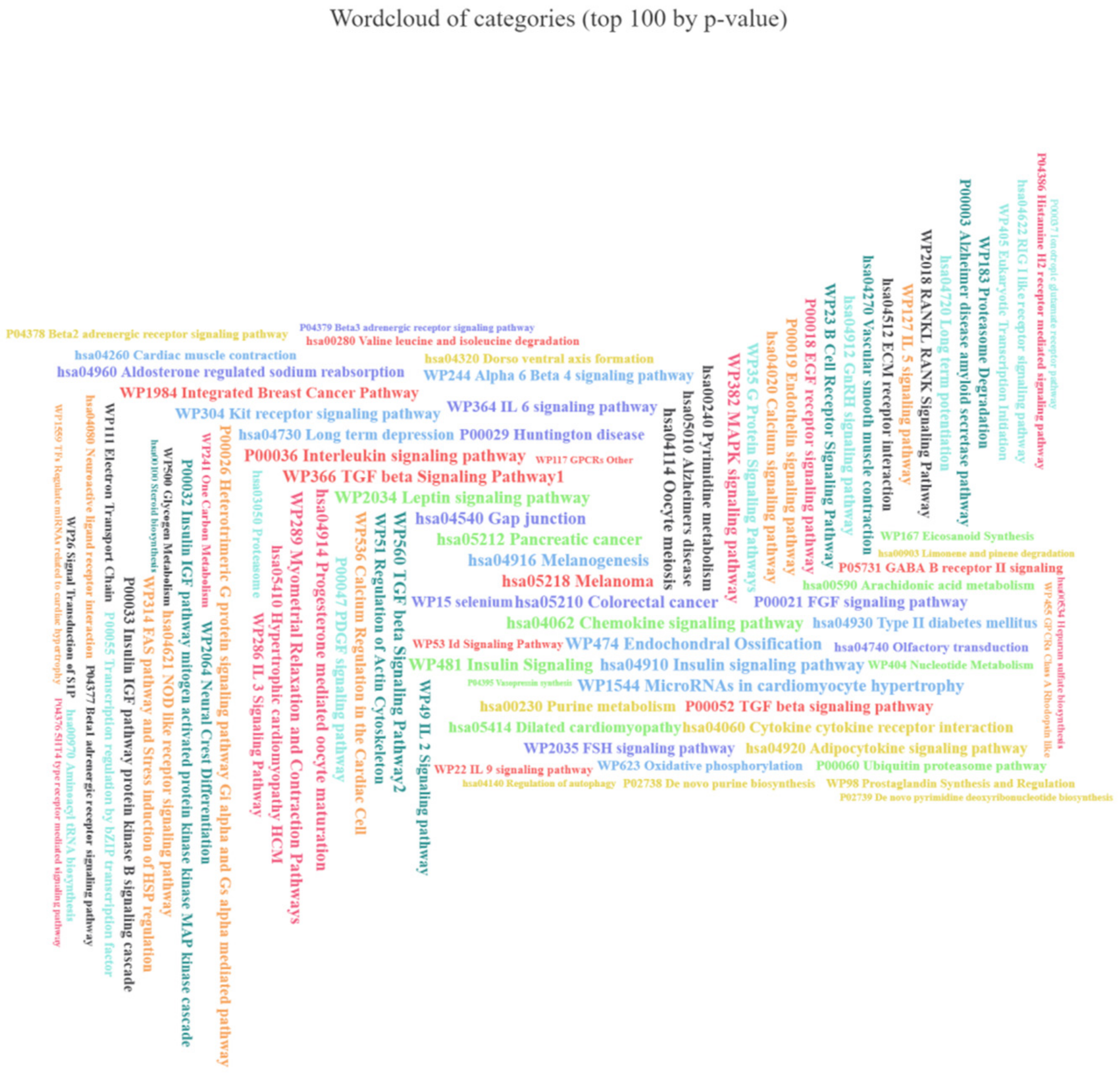
2.6. Functional Enrichment Analysis
2.7. Statistical Analysis
3. Results
3.1. Description of Study Participants
3.2. The Expression of the Set of miRNAs Is Changed in VAT of T2DM and IFG Patients in Comparison to NG Subjects, Yet Slightly or Uncorrelated with Parameters of Glucose Metabolism (FPG, HbA1c, FI, HOMA-IR and TG/HDL)
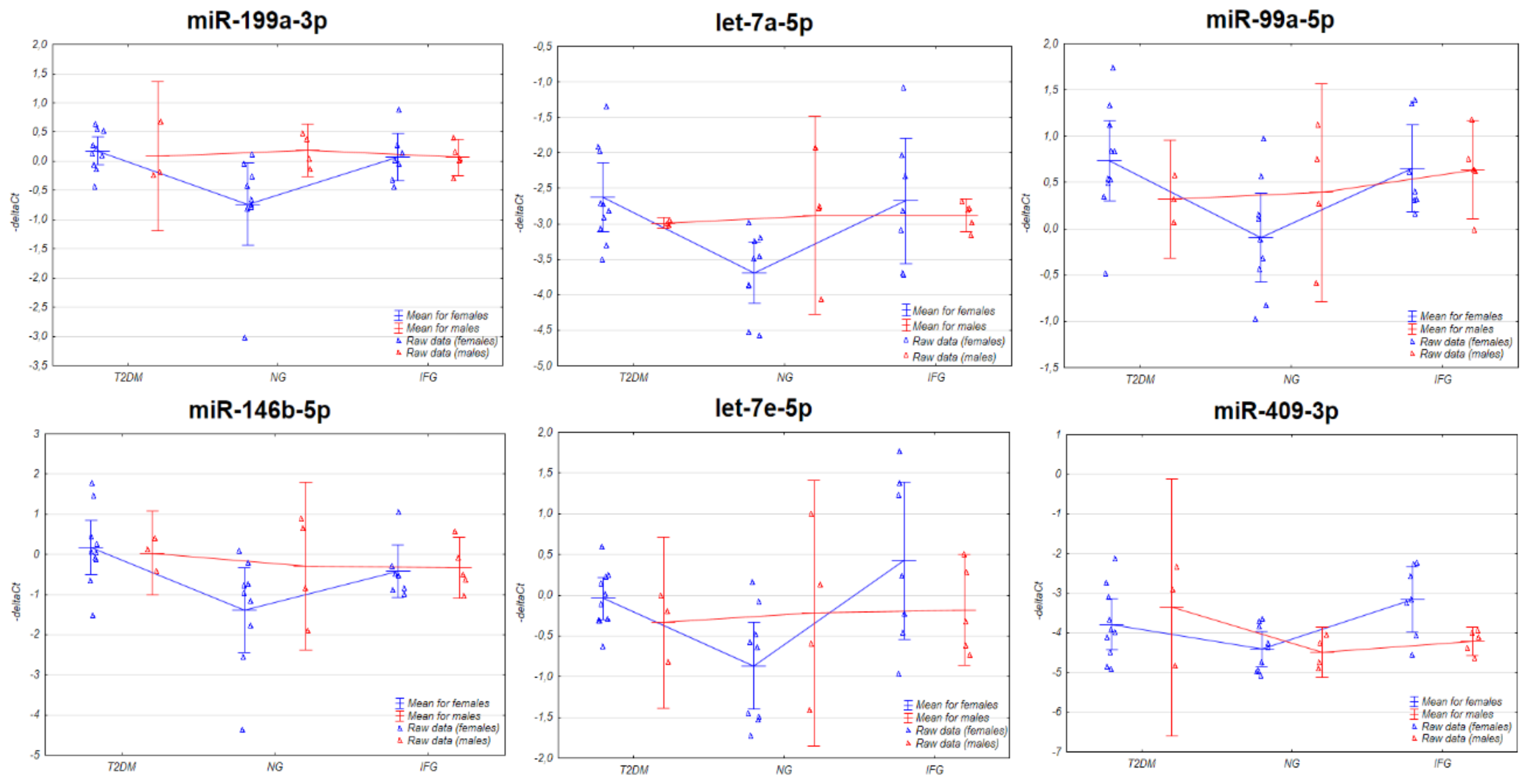
3.3. The Expression of the miRNAs in VAT Appears to Be Connected with Sex-Specific Differences
3.3.1. Two-Way ANOVA
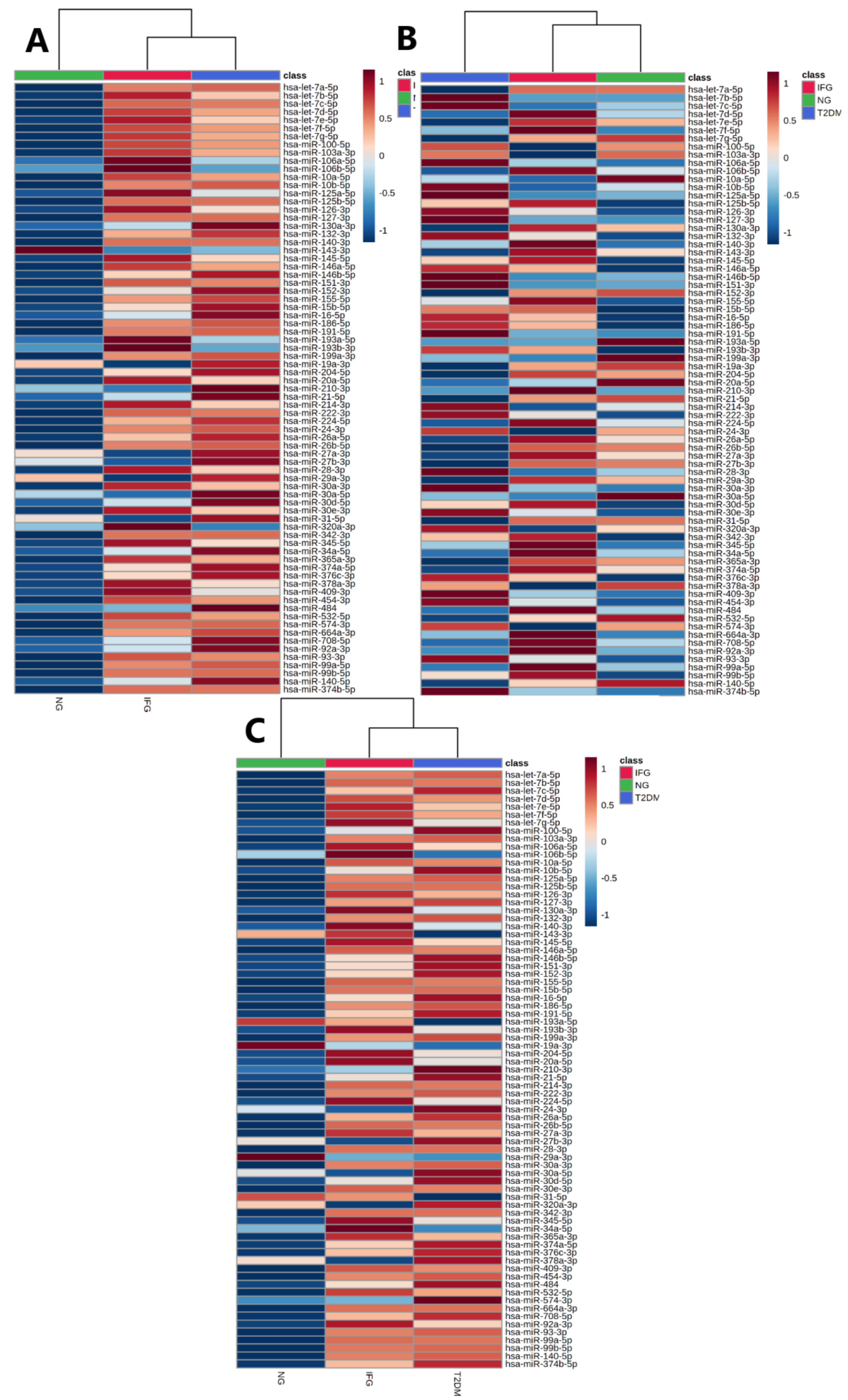
3.3.2. Euclidean Hierarchical Clustering
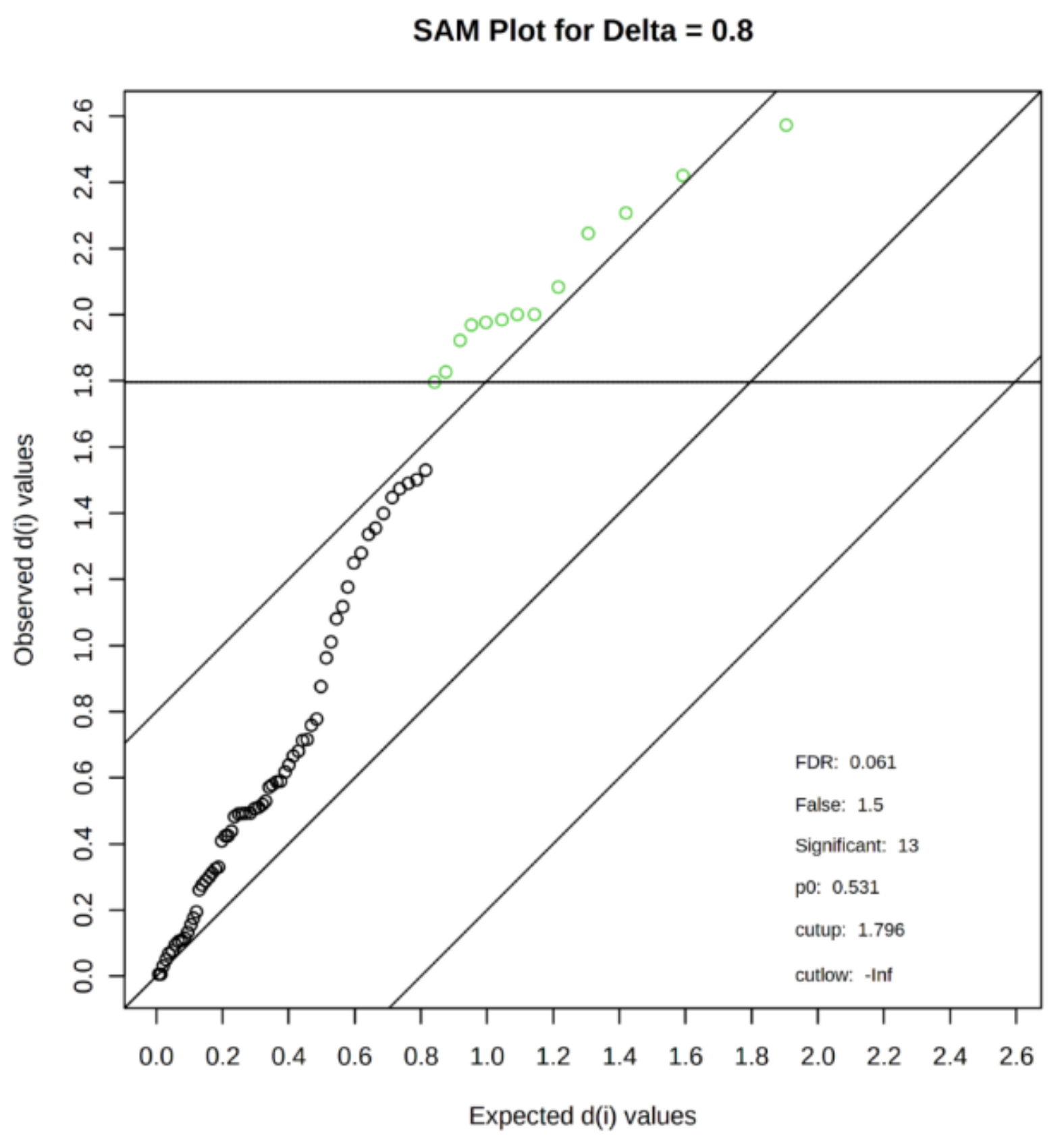
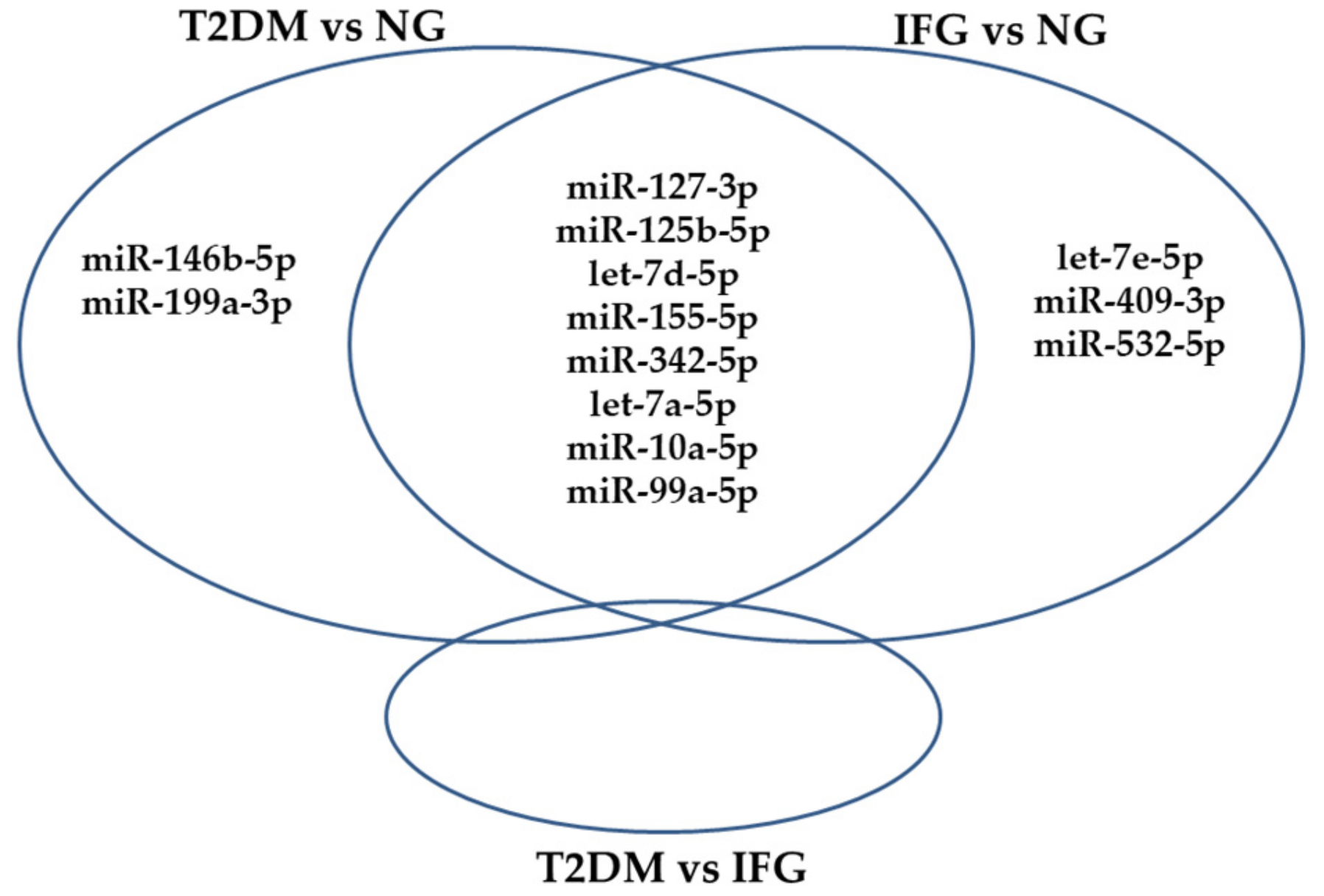
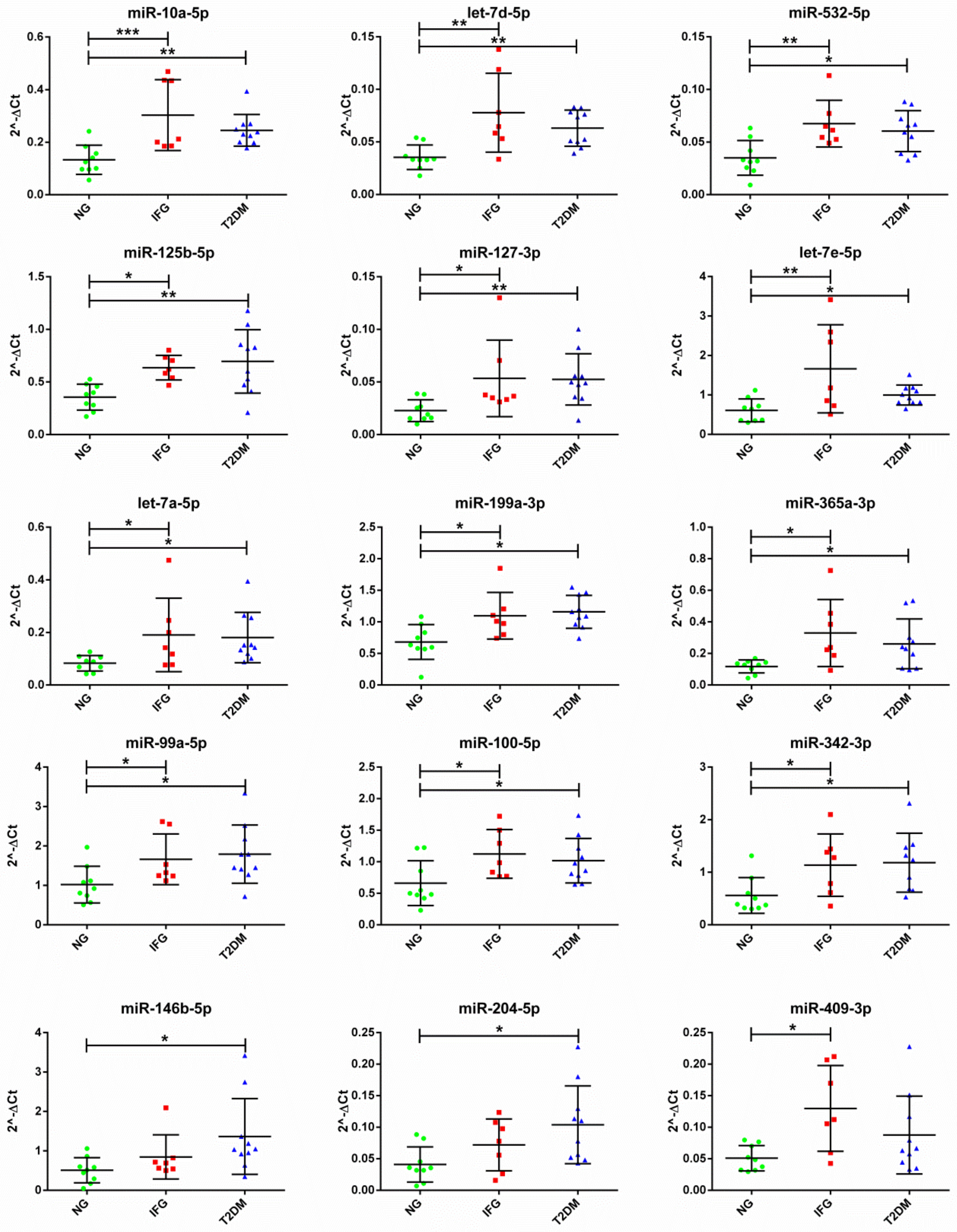
3.4. The Set of Similarly Upregulated miRNAs Is Shared by T2DM and IFG Female Patients in VAT
3.5. The Expression of Majority of Differentially Expressed miRNAs Is Significantly Correlated with Important Biochemical and Anthropometric Parameters in VAT of T2DM, IFG and NG Females
Spearman’s Rank Correlations among Differentially Expressed miRNAs and Biochemical and Anthropometric Parameters
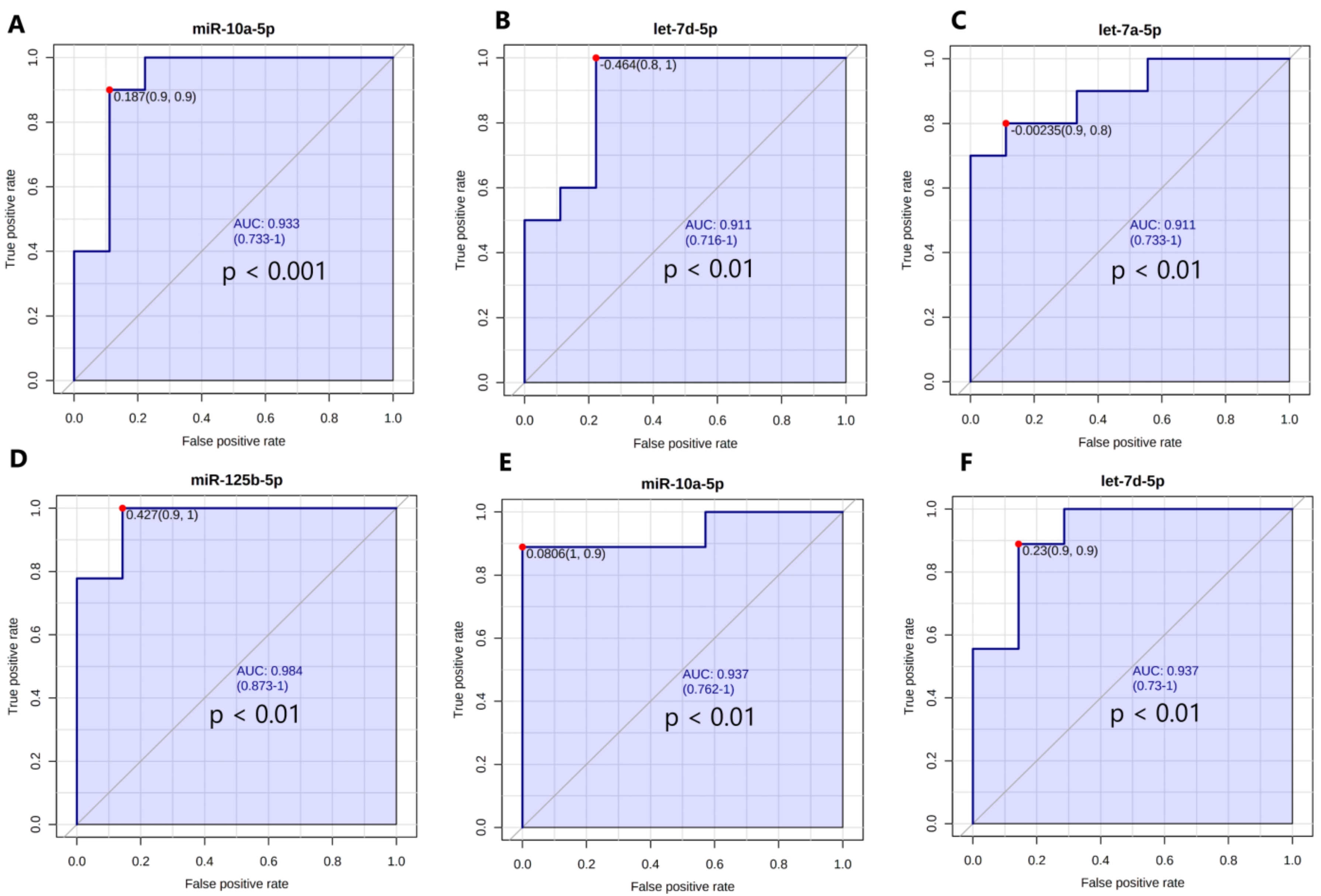
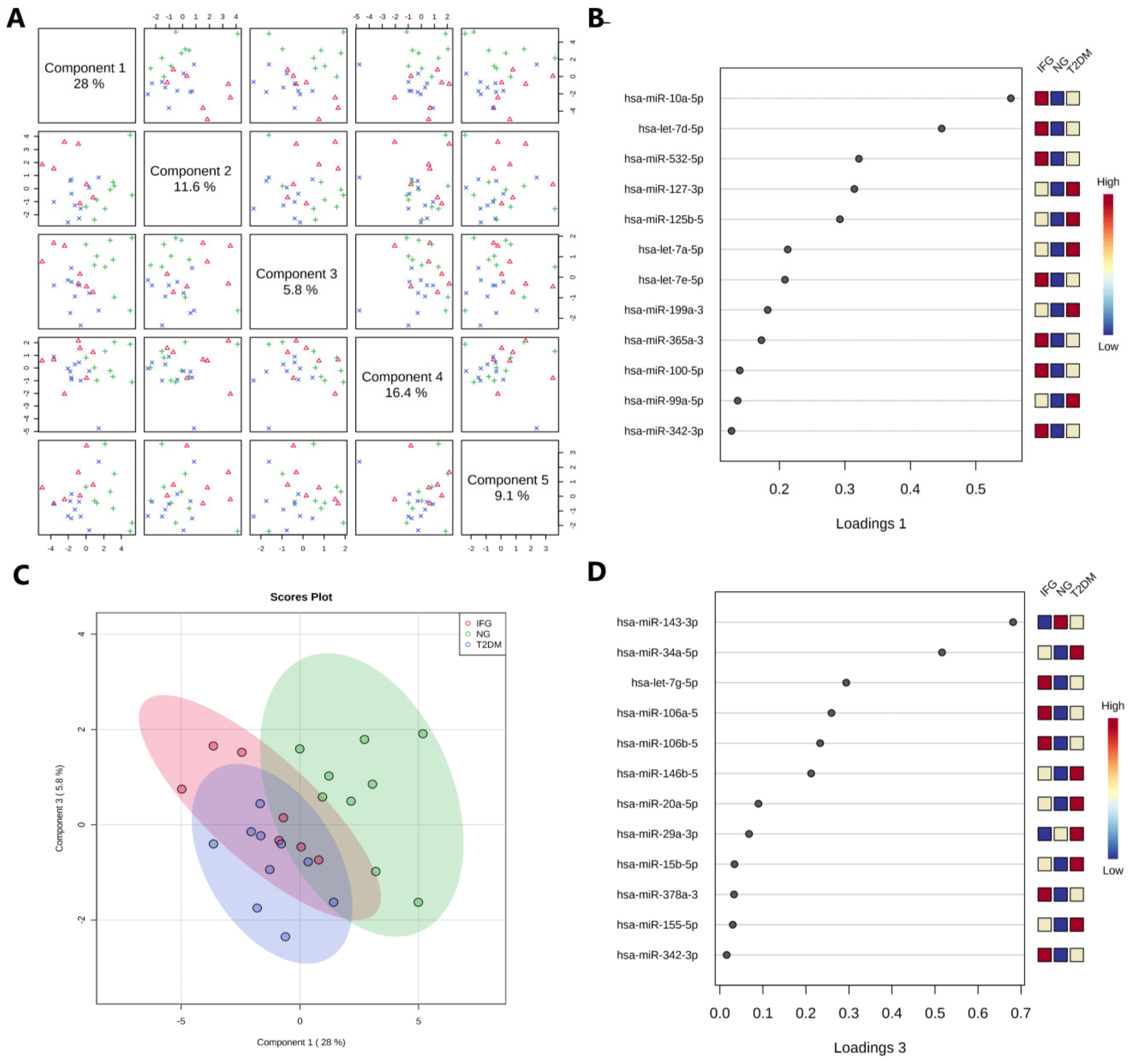
3.6. The Expression Levels of the Set of miRNAs Distinguish NG Female Subjects from T2DM and IFG Female Patients
3.6.1. Univariate Receiving Operating Characteristic (ROC) Curve Analysis
3.6.2. Sparse Partial Least Squares-Discriminant Analysis (sPLS-DA)
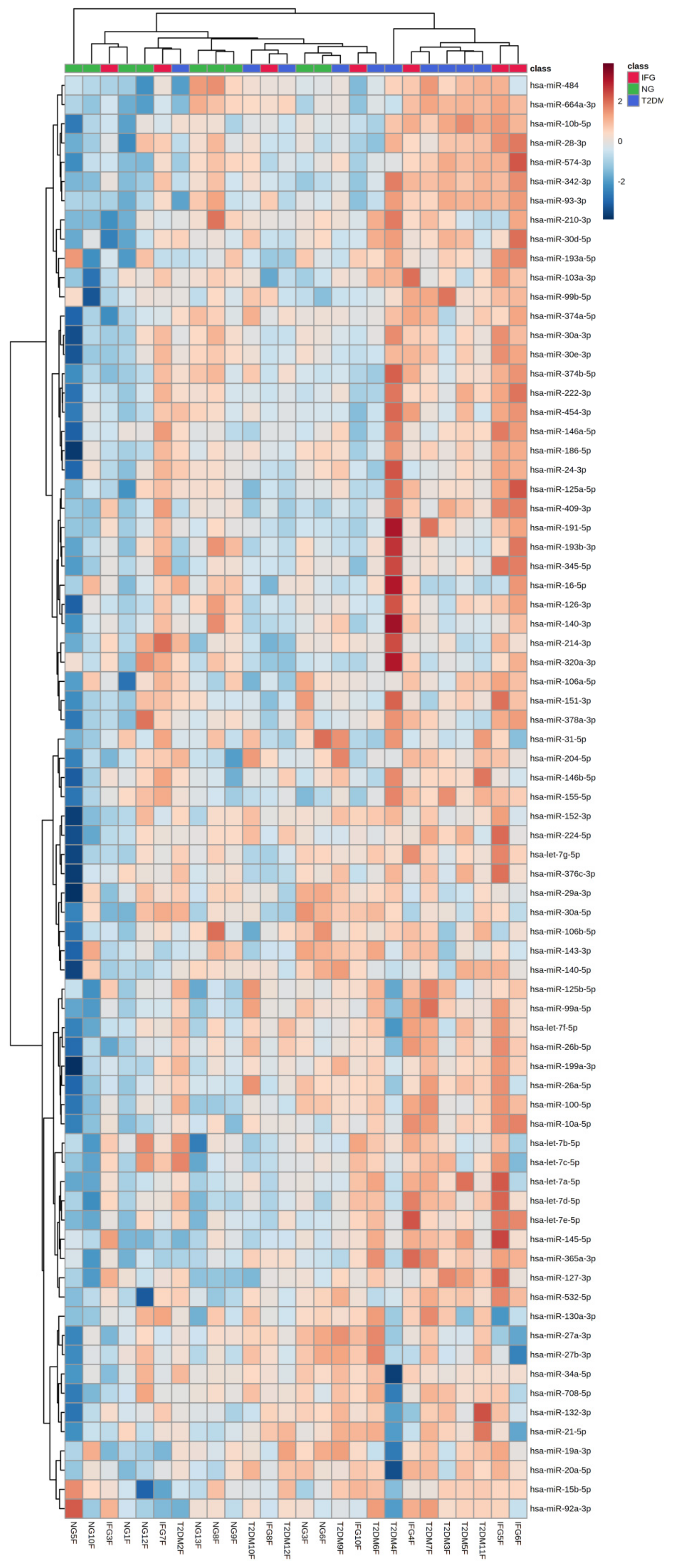
3.6.3. Euclidean Hierarchical Clustering
3.7. The Set of 11 Top Differentially Expressed miRNAs in VAT of Both T2DM and IFG Female Patients While Compared to NG Subjects is Functionally Annotated to Signaling Pathways Associated with Inflammation, Oxidative Stress and Insulin Signaling
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A


References
- Grundy, S.M. Pre-diabetes, metabolic syndrome, and cardiovascular risk. J. Am. Coll. Cardiol. 2012, 59, 635–643. [Google Scholar] [CrossRef] [PubMed]
- Bansal, N. Prediabetes diagnosis and treatment: A review. World J. Diabetes 2015, 6, 296. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.J.; Ma, F.; Lou, H.P.; Chen, Y. Visceral adiposity index is associated with pre-diabetes and type 2 diabetes mellitus in Chinese adults aged 20–50. Ann. Nutr. Metab. 2016, 68, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Kintscher, U.; Hartge, M.; Hess, K.; Foryst-Ludwig, A.; Clemenz, M.; Wabitsch, M.; Fischer-Posovszky, P.; Barth, T.F.; Dragun, D.; Skurk, T. T-lymphocyte infiltration in visceral adipose tissue: A primary event in adipose tissue inflammation and the development of obesity-mediated insulin resistance. Arterioscler. Thromb. Vasc. Biol. 2008, 28, 1304–1310. [Google Scholar] [CrossRef]
- Brahimaj, A.; Ligthart, S.; Ghanbari, M.; Ikram, M.A.; Hofman, A.; Franco, O.H.; Kavousi, M.; Dehghan, A. Novel inflammatory markers for incident pre-diabetes and type 2 diabetes: The Rotterdam Study. Eur. J. Epidemiol. 2017, 32, 217–226. [Google Scholar] [CrossRef]
- Samaras, K.; Botelho, N.K.; Chisholm, D.J.; Lord, R.V. Subcutaneous and visceral adipose tissue gene expression of serum adipokines that predict type 2 diabetes. Obesity 2010, 18, 884–889. [Google Scholar] [CrossRef]
- Burgos-Morón, E.; Abad-Jiménez, Z.; de Marañón, A.M.; Iannantuoni, F.; Escribano-López, I.; López-Domènech, S.; Salom, C.; Jover, A.; Mora, V.; Roldan, I. Relationship between oxidative stress, ER stress, and inflammation in type 2 diabetes: The battle continues. J. Clin. Med. 2019, 8, 1385. [Google Scholar] [CrossRef]
- Ighodaro, O.M. Molecular pathways associated with oxidative stress in diabetes mellitus. Biomed. Pharmacother. 2018, 108, 656–662. [Google Scholar] [CrossRef]
- Włodarski, A.; Strycharz, J.; Wróblewski, A.; Kasznicki, J.; Drzewoski, J.; Śliwińska, A. The Role of microRNAs in Metabolic Syndrome-Related Oxidative Stress. Int. J. Mol. Sci. 2020, 21, 6902. [Google Scholar] [CrossRef]
- Ghasemi, A.; Hashemy, S.I.; Azimi-Nezhad, M.; Dehghani, A.; Saeidi, J.; Mohtashami, M. The cross-talk between adipokines and miRNAs in health and obesity-mediated diseases. Clin. Chim. Acta 2019, 499, 41–53. [Google Scholar] [CrossRef]
- Lorente-Cebrián, S.; González-Muniesa, P.; Milagro, F.I.; Martínez, J.A. MicroRNAs and other non-coding RNAs in adipose tissue and obesity: Emerging roles as biomarkers and therapeutic targets. Clin. Sci. 2019, 133, 23–40. [Google Scholar] [CrossRef] [PubMed]
- Ying, W.; Riopel, M.; Bandyopadhyay, G.; Dong, Y.; Birmingham, A.; Seo, J.B.; Ofrecio, J.M.; Wollam, J.; Hernandez-Carretero, A.; Fu, W. Adipose tissue macrophage-derived exosomal miRNAs can modulate in vivo and in vitro insulin sensitivity. Cell 2017, 171, 372–384.e12. [Google Scholar] [CrossRef] [PubMed]
- Lopez, Y.O.N.; Garufi, G.; Seyhan, A.A. Altered levels of circulating cytokines and microRNAs in lean and obese individuals with prediabetes and type 2 diabetes. Mol. Biosyst. 2017, 13, 106–121. [Google Scholar] [CrossRef] [PubMed]
- Yan, S.; Wang, T.; Huang, S.; Di, Y.; Huang, Y.; Liu, X.; Luo, Z.; Han, W.; An, B. Differential expression of microRNAs in plasma of patients with prediabetes and newly diagnosed type 2 diabetes. Acta Diabetol. 2016, 53, 693–702. [Google Scholar] [CrossRef] [PubMed]
- Prabu, P.; Rome, S.; Sathishkumar, C.; Aravind, S.; Mahalingam, B.; Shanthirani, C.S.; Gastebois, C.; Villard, A.; Mohan, V.; Balasubramanyam, M. Circulating miRNAs of ‘Asian Indian phenotype’identified in subjects with impaired glucose tolerance and patients with type 2 diabetes. PLoS ONE 2015, 10, e0128372. [Google Scholar] [CrossRef]
- American Diabetes Association. 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes-2020. Diabetes Care 2020, 43, S14–S31. [Google Scholar] [CrossRef]
- Wenclewska, S.; Szymczak-Pajor, I.; Drzewoski, J.; Bunk, M.; Śliwińska, A. Vitamin D supplementation reduces both oxidative DNA damage and insulin resistance in the elderly with metabolic disorders. Int. J. Mol. Sci. 2019, 20, 2891. [Google Scholar] [CrossRef]
- Strycharz, J.; Świderska, E.; Wróblewski, A.; Podolska, M.; Czarny, P.; Szemraj, J.; Balcerczyk, A.; Drzewoski, J.; Kasznicki, J.; Śliwińska, A. Hyperglycemia Affects miRNAs Expression Pattern during Adipogenesis of Human Visceral Adipocytes—Is Memorization Involved? Nutrients 2018, 10, 1774. [Google Scholar] [CrossRef]
- Świderska, E.; Podolska, M.; Strycharz, J.; Szwed, M.; Abramczyk, H.; Brożek-Płuska, B.; Wróblewski, A.; Szemraj, J.; Majsterek, I.; Drzewoski, J. Hyperglycemia changes expression of key adipogenesis markers (C/EBPα and PPARγ) and morphology of differentiating Human visceral adipocytes. Nutrients 2019, 11, 1835. [Google Scholar] [CrossRef]
- Pfaffl, M.W.; Tichopad, A.; Prgomet, C.; Neuvians, T.P. Determination of stable housekeeping genes, differentially regulated target genes and sample integrity: BestKeeper–Excel-based tool using pair-wise correlations. Biotechnol. Lett. 2004, 26, 509–515. [Google Scholar] [CrossRef]
- Kern, F.; Fehlmann, T.; Solomon, J.; Schwed, L.; Backes, C.; Meese, E.; Keller, A. miEAA 2.0: Integrating multi-species microRNA enrichment analysis and workflow management systems. bioRxiv 2020. [Google Scholar] [CrossRef] [PubMed]
- Lu, T.-P.; Lee, C.-Y.; Tsai, M.-H.; Chiu, Y.-C.; Hsiao, C.K.; Lai, L.-C.; Chuang, E.Y. miRSystem: An integrated system for characterizing enriched functions and pathways of microRNA targets. PLoS ONE 2012, 7, e42390. [Google Scholar] [CrossRef] [PubMed]
- Howe, E.; Holton, K.; Nair, S.; Schlauch, D.; Sinha, R.; Quackenbush, J. Mev: Multiexperiment viewer. In Biomedical Informatics for Cancer Research; Springer: Berlin/Heidelberg, Germany, 2010; pp. 267–277. [Google Scholar]
- Pang, Z.; Chong, J.; Li, S.; Xia, J. MetaboAnalystR 3.0: Toward an Optimized Workflow for Global Metabolomics. Metabolites 2020, 10, 186. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Zhang, Q.; Ma, X.; Wang, J.; Liang, T. miRNA and mRNA expression analysis reveals potential sex-biased miRNA expression. Sci. Rep. 2017, 7, 39812. [Google Scholar] [CrossRef]
- Mentzel, C.M.J.; Anthon, C.; Jacobsen, M.J.; Karlskov-Mortensen, P.; Bruun, C.S.; Jørgensen, C.B.; Gorodkin, J.; Cirera, S.; Fredholm, M. Gender and obesity specific microRNA expression in adipose tissue from lean and obese pigs. PLoS ONE 2015, 10, e0131650. [Google Scholar] [CrossRef]
- Link, J.C.; Hasin-Brumshtein, Y.; Cantor, R.M.; Chen, X.; Arnold, A.P.; Lusis, A.J.; Reue, K. Diet, gonadal sex, and sex chromosome complement influence white adipose tissue miRNA expression. BMC Genom. 2017, 18, 1–11. [Google Scholar] [CrossRef]
- Brovkina, O.; Nikitin, A.; Khodyrev, D.; Shestakova, E.; Sklyanik, I.; Panevina, A.; Stafeev, Y.S.; Menshikov, M.; Kobelyatskaya, A.; Yurasov, A. Role of microRNAs in the regulation of subcutaneous white adipose tissue in obese patients without type 2 diabetes. Front. Endocrinol. 2019, 10, 840. [Google Scholar] [CrossRef]
- Li, Y.B.; Wu, Q.; Liu, J.; Fan, Y.Z.; Yu, K.F.; Cai, Y. miR-199a-3p is involved in the pathogenesis and progression of diabetic neuropathy through downregulation of SerpinE2. Mol. Med. Rep. 2017, 16, 2417–2424. [Google Scholar] [CrossRef]
- Jiang, L.Q.; Franck, N.; Egan, B.; Sjögren, R.J.; Katayama, M.; Duque-Guimaraes, D.; Arner, P.; Zierath, J.R.; Krook, A. Autocrine role of interleukin-13 on skeletal muscle glucose metabolism in type 2 diabetic patients involves microRNA let-7. Am. J. Physiol. Endocrinol. Metab. 2013, 305, E1359–E1366. [Google Scholar] [CrossRef]
- Krause, C.; Geißler, C.; Tackenberg, H.; El Gammal, A.T.; Wolter, S.; Spranger, J.; Mann, O.; Lehnert, H.; Kirchner, H. Multi-layered epigenetic regulation of IRS2 expression in the liver of obese individuals with type 2 diabetes. Diabetologia 2020, 63, 2182–2193. [Google Scholar] [CrossRef]
- Satake, E.; Pezzolesi, M.G.; Dom, Z.I.M.; Smiles, A.M.; Niewczas, M.A.; Krolewski, A.S. Circulating miRNA profiles associated with hyperglycemia in patients with type 1 diabetes. Diabetes 2018, 67, 1013–1023. [Google Scholar] [CrossRef] [PubMed]
- Oger, F.; Gheeraert, C.; Mogilenko, D.; Benomar, Y.; Molendi-Coste, O.; Bouchaert, E.; Caron, S.; Dombrowicz, D.; Pattou, F.; Duez, H. Cell-specific dysregulation of microRNA expression in obese white adipose tissue. J. Clin. Endocrinol. Metab. 2014, 99, 2821–2833. [Google Scholar] [CrossRef] [PubMed]
- Chartoumpekis, D.V.; Zaravinos, A.; Ziros, P.G.; Iskrenova, R.P.; Psyrogiannis, A.I.; Kyriazopoulou, V.E.; Habeos, I.G. Differential expression of microRNAs in adipose tissue after long-term high-fat diet-induced obesity in mice. PLoS ONE 2012, 7, e34872. [Google Scholar] [CrossRef] [PubMed]
- Ortega, F.J.; Moreno-Navarrete, J.M.; Pardo, G.; Sabater, M.; Hummel, M.; Ferrer, A.; Rodriguez-Hermosa, J.I.; Ruiz, B.; Ricart, W.; Peral, B. MiRNA expression profile of human subcutaneous adipose and during adipocyte differentiation. PLoS ONE 2010, 5, e9022. [Google Scholar] [CrossRef]
- Jaiswal, A.; Reddy, S.S.; Maurya, M.; Maurya, P.; Barthwal, M.K. MicroRNA-99a mimics inhibit M1 macrophage phenotype and adipose tissue inflammation by targeting TNFα. Cell. Mol. Immunol. 2019, 16, 495–507. [Google Scholar]
- Pek, S.L.T.; Sum, C.F.; Lin, M.X.; Cheng, A.K.S.; Wong, M.T.K.; Lim, S.C.; Tavintharan, S. Circulating and visceral adipose miR-100 is down-regulated in patients with obesity and Type 2 diabetes. Mol. Cell. Endocrinol. 2016, 427, 112–123. [Google Scholar] [CrossRef]
- Cho, Y.K.; Son, Y.; Kim, S.-N.; Song, H.-D.; Kim, M.; Park, J.-H.; Jung, Y.-S.; Ahn, S.-Y.; Saha, A.; Granneman, J.G. MicroRNA-10a-5p regulates macrophage polarization and promotes therapeutic adipose tissue remodeling. Mol. Metab. 2019, 29, 86–98. [Google Scholar] [CrossRef]
- Essandoh, K.; Li, Y.; Huo, J.; Fan, G.-C. MiRNA-mediated macrophage polarization and its potential role in the regulation of inflammatory response. Shock Augusta Ga. 2016, 46, 122. [Google Scholar] [CrossRef]
- Ying, H.; Kang, Y.; Zhang, H.; Zhao, D.; Xia, J.; Lu, Z.; Wang, H.; Xu, F.; Shi, L. MiR-127 modulates macrophage polarization and promotes lung inflammation and injury by activating the JNK pathway. J. Immunol. 2015, 194, 1239–1251. [Google Scholar] [CrossRef]
- Weisberg, S.P.; McCann, D.; Desai, M.; Rosenbaum, M.; Leibel, R.L.; Ferrante, A.W. Obesity is associated with macrophage accumulation in adipose tissue. J. Clin. Investig. 2003, 112, 1796–1808. [Google Scholar] [CrossRef]
- Ortega, F.J.; Moreno, M.; Mercader, J.M.; Moreno-Navarrete, J.M.; Fuentes-Batllevell, N.; Sabater, M.; Ricart, W.; Fernández-Real, J.M. Inflammation triggers specific microRNA profiles in human adipocytes and macrophages and in their supernatants. Clin. Epigenetics 2015, 7, 49. [Google Scholar] [CrossRef] [PubMed]
- Gu, N.; You, L.; Shi, C.; Yang, L.; Pang, L.; Cui, X.; Ji, C.; Zheng, W.; Guo, X. Expression of miR-199a-3p in human adipocytes is regulated by free fatty acids and adipokines. Mol. Med. Rep. 2016, 14, 1180–1186. [Google Scholar] [CrossRef] [PubMed]
- Jones, A.; Danielson, K.M.; Benton, M.C.; Ziegler, O.; Shah, R.; Stubbs, R.S.; Das, S.; Macartney-Coxson, D. miRNA signatures of insulin resistance in obesity. Obesity 2017, 25, 1734–1744. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Wei, S.; Xu, F.; Cai, X.; Wang, H.; Ding, R. MicroRNA-532-5p is implicated in the regulation of osteoporosis by forkhead box O1 and osteoblast differentiation. BMC Musculoskelet. Disord. 2020, 21, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Frost, R.J.; Olson, E.N. Control of glucose homeostasis and insulin sensitivity by the Let-7 family of microRNAs. Proc. Natl. Acad. Sci. USA 2011, 108, 21075–21080. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.-W.; Guo, R.-W.; Lv, J.-L.; Wang, X.-M.; Ye, J.-S.; Lu, N.-H.; Liang, X.; Yang, L.-X. MicroRNA-99a inhibits insulin-induced proliferation, migration, dedifferentiation, and rapamycin resistance of vascular smooth muscle cells by inhibiting insulin-like growth factor-1 receptor and mammalian target of rapamycin. Biochem. Biophys. Res. Commun. 2017, 486, 414–422. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.H.; Zhang, C.L.; Dong, F.S.; Zhang, Y.M. miR-99a suppresses the metastasis of human non-small cell lung cancer cells by targeting AKT1 signaling pathway. J. Cell. Biochem. 2015, 116, 268–276. [Google Scholar] [CrossRef]
- Jin, Y.; Tymen, S.D.; Chen, D.; Fang, Z.J.; Zhao, Y.; Dragas, D.; Dai, Y.; Marucha, P.T.; Zhou, X. MicroRNA-99 family targets AKT/mTOR signaling pathway in dermal wound healing. PLoS ONE 2013, 8, e64434. [Google Scholar] [CrossRef]
- Li, P.; Fan, C.; Cai, Y.; Fang, S.; Zeng, Y.; Zhang, Y.; Lin, X.; Zhang, H.; Xue, Y.; Guan, M. Transplantation of brown adipose tissue up-regulates miR-99a to ameliorate liver metabolic disorders in diabetic mice by targeting NOX4. Adipocyte 2020, 9, 57–67. [Google Scholar]
- Kurylowicz, A.; Owczarz, M.; Polosak, J.; Jonas, M.; Lisik, W.; Jonas, M.; Chmura, A.; Puzianowska-Kuznicka, M. SIRT1 and SIRT7 expression in adipose tissues of obese and normal-weight individuals is regulated by microRNAs but not by methylation status. Int. J. Obes. 2016, 40, 1635–1642. [Google Scholar] [CrossRef]
- Chen, L.; Dai, Y.-M.; Ji, C.-B.; Yang, L.; Shi, C.-M.; Xu, G.-F.; Pang, L.-X.; Huang, F.-Y.; Zhang, C.-M.; Guo, X.-R. MiR-146b is a regulator of human visceral preadipocyte proliferation and differentiation and its expression is altered in human obesity. Mol. Cell. Endocrinol. 2014, 393, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Gu, M.; Ma, Y.; Peng, Y. LncRNA TUG1 reduces inflammation and enhances insulin sensitivity in white adipose tissue by regulating miR-204/SIRT1 axis in obesity mice. Mol. Cell. Biochem. 2020, 475, 171–183. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.-L.; Chen, T.; Xiong, J.-L.; Wu, D.; Xi, Q.-Y.; Luo, J.-Y.; Sun, J.-J.; Zhang, Y.-L. miR-146b inhibits glucose consumption by targeting IRS1 gene in porcine primary adipocytes. Int. J. Mol. Sci. 2018, 19, 783. [Google Scholar] [CrossRef] [PubMed]
- Strycharz, J.; Rygielska, Z.; Swiderska, E.; Drzewoski, J.; Szemraj, J.; Szmigiero, L.; Sliwinska, A. SIRT1 as a therapeutic target in diabetic complications. Curr. Med. Chem. 2018, 25, 1002–1035. [Google Scholar] [CrossRef]
- Zhang, Y.; Ma, Y.; Gu, M.; Peng, Y. lncRNA TUG1 promotes the brown remodeling of white adipose tissue by regulating miR-204-targeted SIRT1 in diabetic mice. Int. J. Mol. Med. 2020, 46, 2225–2234. [Google Scholar] [CrossRef]
- Shi, C.; Zhu, L.; Chen, X.; Gu, N.; Chen, L.; Zhu, L.; Yang, L.; Pang, L.; Guo, X.; Ji, C. IL-6 and TNF-α induced obesity-related inflammatory response through transcriptional regulation of miR-146b. J. Interferon Cytokine Res. 2014, 34, 342–348. [Google Scholar] [CrossRef]
- Pfeiffer, D.; Roßmanith, E.; Lang, I.; Falkenhagen, D. miR-146a, miR-146b, and miR-155 increase expression of IL-6 and IL-8 and support HSP10 in an in vitro sepsis model. PLoS ONE 2017, 12, e0179850. [Google Scholar] [CrossRef]
- Wang, Y.; He, Y.; Bai, H.; Dang, Y.; Gao, J.; Lv, P. Phosphoinositide-dependent kinase 1–associated glycolysis is regulated by miR-409-3p in clear cell renal cell carcinoma. J. Cell. Biochem. 2019, 120, 126–134. [Google Scholar] [CrossRef]
- Zhang, G.; Liu, Z.; Xu, H.; Yang, Q. miR-409-3p suppresses breast cancer cell growth and invasion by targeting Akt1. Biochem. Biophys. Res. Commun. 2016, 469, 189–195. [Google Scholar] [CrossRef]
- Liu, S.; Li, B.; Xu, J.; Hu, S.; Zhan, N.; Wang, H.; Gao, C.; Li, J.; Xu, X. SOD1 Promotes Cell Proliferation and Metastasis in Non-small Cell Lung Cancer via an miR-409-3p/SOD1/SETDB1 Epigenetic Regulatory Feedforward Loop. Front. Cell Dev. Biol. 2020, 8, 213. [Google Scholar] [CrossRef]
- Liu, X.; Zhou, F.; Yang, Y.; Wang, W.; Niu, L.; Zuo, D.; Li, X.; Hua, H.; Zhang, B.; Kou, Y. MiR-409-3p and MiR-1896 co-operatively participate in IL-17-induced inflammatory cytokine production in astrocytes and pathogenesis of EAE mice via targeting SOCS3/STAT3 signaling. Glia 2019, 67, 101–112. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.M.; Chen, L.H.; Hong, M.; Chen, Y.Y.; Yang, X.R.; Tang, S.M.; Yuan, Q.F.; Chen, W.W. Serum microRNA profiling and bioinformatics analysis of patients with type 2 diabetes mellitus in a Chinese population. Mol. Med. Rep. 2017, 15, 2143–2153. [Google Scholar] [CrossRef] [PubMed]


| Sub. | NG (9F/4M) | IFG (7F/5M) | T2DM (10F/3M) | |
|---|---|---|---|---|
| Age [years] | A | 66 (61, 71) | 65 (61.5, 77) | 68 (64, 72) |
| F | 66 (61, 69) | 66 (63, 77) | 67 (64, 70) | |
| M | 65.5 (60, 71) | 64 (60, 80) | 72 (62, 77) | |
| FPG [mmol/L] | A | 5.06 (4.82, 5.14) | 5.99 (5.95, 6.42) (**) | 7.46 (7.07, 8.12) (***) |
| F | 5.09 (4.85, 5.15) | 5.98 (5.93, 6.64) | 7.4 (7.07, 8.12) (***) | |
| M | 4.46 (3.65, 5.10) | 6 (5.98, 6.13) | 10.15 (5.58, 19.11) (*) | |
| FI [pmol/L] & | A | 32.4 (25.30, 58.70) | 48.75 (38.2, 76.95) | 80.4 (40.3, 136.1) |
| F | 37.3 (29.00, 72.00) | 43.7 (36.10, 67.60) | 103.1 (74.7, 136.1) | |
| M | 24.75 (11.50, 32.30) | 50.9 (46.60, 172.90) | - | |
| HOMA-IR & | A | 1.05 (0.79, 2.09) | 1.89 (1.59, 3.12) | 3.71 (1.72, 8.34) |
| F | 1.22 (0.95, 2.37) | 1.63 (1.59, 2.57) | 5.35 (3.37, 8.34) (*) | |
| M | 0.74 (0.28, 1.05) | 1.95 (1.83, 6.20) | - | |
| HbA1c [%] | A | 5.5 (5.4, 5.5) | 5.8 (5.7, 5.9) (**) | 6.7 (6, 7) (***), (#) |
| F | 5.5 (5.4, 5.6) | 5.9 (5.7, 5.9) | 6.45 (6, 7) (***) | |
| M | 5.35 (5.1, 5.5) | 5.8 (5.7, 5.8) | 6.7 (5.9, 9.1) (**) | |
| TC [mmol/L] | A | 4.98 (4.27, 5.39) | 3.49 (2.94, 3.93) (*) | 4.72 (2.89, 5.38) |
| F | 4.98 (4.31, 5.39) | 3.62 (3.27, 4.10) | 3.98 (2.89, 5.38) | |
| M | 4.06 (2.87, 5.66) | 2.9 (2.20, 3.80) | 4.72 (2.87, 5.39) | |
| LDL-C [mmol/L] | A | 2.95 (2.64, 3.75) | 1.77 (1.24, 2.28) (*) | 2.66 (1.2, 2.93) |
| F | 3.37 (2.68, 3.75) | 2.02 (1.36, 2.50) | 2.05 (0.85, 2.78) | |
| M | 2.17 (1.27, 3.55) | 1.31 (0.89, 2.20) | 2.93 (1.22, 3.40) | |
| HDL-C [mmol/L] | A | 1.22 (1.11, 1.35) | 1.11 (0.92, 1.36) | 1.19 (1.07, 1.25) |
| F | 1.22 (1.11, 1.29) | 1.15 (0.87, 1.60) | 1.19 (1.07, 1.25) | |
| M | 1.26 (1.07, 1.40) | 1.06 (0.96, 1.16) | 1.29 (0.67, 1.61) | |
| TG [mmol/L] | A | 1.08 (1.01, 1.49) | 1.06 (0.71, 1.27) | 1.53 (1.25, 2.2) (#) |
| F | 1.06 (1.01, 1.18) | 1.11 (0.85, 1.63) | 1.7 (1.25, 2.31) (*) | |
| M | 1.56 (1.07, 1.66) | 0.72 (0.69, 1.16) | 1.43 (0.79, 1.86) | |
| TG/HDL | A | 2.14 (1.79, 2.57) | 1.92 (1.33, 2.89) | 2.93 (2.65, 4.27) |
| F | 2.01 (1.79, 2.17) | 1.99 (1.34, 4.29) | 3.21 (2.78, 4.27) (*) | |
| M | 2.56 (1.91, 3.27) | 1.84 (1.32, 2.51) | 2.65 (1.40, 4.89) | |
| BMI [kg/m2] | A | 27.44 (25.95, 27.99) | 27.83 (25.48, 31.02) | 30.84 (27.34, 33.20) |
| F | 27.51 (27.01, 27.99) | 28.84 (25.00, 32.72) | 30.31 (27.34, 33.91) | |
| M | 26.7 (25.84, 28.08) | 26.12 (25.95, 30.86) | 31.14 (24.88, 31.14) | |
| Body mass [kg] | A | 74 (70, 80) | 77.5 (71, 91) | 77 (72, 89) |
| F | 72 (69, 74) | 73 (64, 88) | 75.5 (70, 85) | |
| M | 80 (76, 84) | 80 (75, 96) | 90 (77, 90) | |
| Height [m] | A | 1.64 (1.61, 1.7) | 1.68 (1.59, 1.75) | 1.6 (1.58, 1.63) |
| F | 1.64 (1.61, 1.64) | 1.6 (1.58, 1.65) | 1.6 (1.57, 1.62) | |
| M | 1.72 (1.7, 1.75) | 1.75 (1.7, 1.76) | 1.7 (1.7, 1.76) | |
| WHR | A | 0.94 (0.86, 0.96) | 0.93 (0.90, 0.96) | 1.02 (0.92, 1.06) |
| F | 0.9 (0.85, 0.94) | 0.92 (0.84, 0.95) | 0.97 (0.95, 1.02) | |
| M | 1.00 (0.95, 1.05) | 0.96 (0.92, 0.97) | 1.06 (1.04, 1.09) | |
| Waist circ. [cm] | A | 100 (89.0, 103.0) | 97.5 (85.0, 105.0) | 104 (99.0, 112.0) |
| F | 90 (88.0, 100.0) | 95 (80.0, 105.0) | 102.5 (98.0, 109.0) | |
| M | 103.5 (102.5, 104.5) | 100 (90.0, 105.0) | 115 (102.0, 117.0) | |
| Hips circ. [cm] | A | 105 (98.0, 110.0) | 102.5 (97.0, 112.0) | 107 (104.0, 110.0) |
| F | 105 (104.0, 111.0) | 102 (96.0, 114.0) | 108.5 (104.0, 110.0) | |
| M | 104 (97.5, 110) | 103 (98.0, 110.0) | 107 (98.0, 109.0) |
| miRNA | One-Way ANOVA | SAM | ||
|---|---|---|---|---|
| Raw p | FDR | Raw p | FDR | |
| miR-127-3p | 0.0056 | 0.1439 | 0.0033 | 0.0560 |
| miR-125b-5p | 0.0077 | 0.1439 | 0.0048 | 0.0560 |
| let-7d-5p | 0.0097 | 0.1439 | 0.0068 | 0.0560 |
| miR-155-5p | 0.0110 | 0.1439 | 0.0080 | 0.0560 |
| miR-342-3p | 0.0152 | 0.1439 | 0.0112 | 0.0560 |
| miR-532-5p | 0.0180 | 0.1439 | 0.0132 | 0.0560 |
| miR-10a-5p | 0.0180 | 0.1439 | 0.0132 | 0.0560 |
| let-7a-5p | 0.0186 | 0.1439 | 0.0139 | 0.0560 |
| miR-99a-5p | 0.0189 | 0.1439 | 0.0140 | 0.0560 |
| miR-146b-5p | 0.0192 | 0.1439 | 0.0144 | 0.0560 |
| let-7e-5p | 0.0211 | 0.1439 | 0.0155 | 0.0560 |
| miR-409-3p | 0.0256 | 0.1571 | 0.0185 | 0.0613 |
| miR-199a-3p | 0.0272 | 0.1571 | 0.02 | 0.0613 |
| miRNA | One-Way ANOVA | SAM | ||
|---|---|---|---|---|
| Raw p Value | FDR | Raw p Value | FDR | |
| miR-10a-5p | 0.0003 | 0.0204 | 1.333E-4 | 0.0054 |
| let-7d-5p | 0.0009 | 0.0328 | 6.666E-4 | 0.0136 |
| miR-532-5p | 0.0031 | 0.0552 | 0.0021 | 0.0254 |
| miR-127-3p | 0.0034 | 0.0552 | 0.0025 | 0.0254 |
| miR-125b-5p | 0.0042 | 0.0552 | 0.0036 | 0.0254 |
| let-7a-5p | 0.0044 | 0.0552 | 0.0037 | 0.0254 |
| let-7e-5p | 0.0075 | 0.0753 | 0.0060 | 0.0333 |
| miR-199a-3p | 0.0087 | 0.0753 | 0.0073 | 0.0333 |
| miR-365a-3p | 0.0090 | 0.0753 | 0.0073 | 0.0333 |
| miR-99a-5p | 0.0124 | 0.0831 | 0.0104 | 0.0377 |
| miR-100-5p | 0.0128 | 0.0831 | 0.0105 | 0.0377 |
| miR-342-3p | 0.0143 | 0.0831 | 0.0119 | 0.0377 |
| miR-146b-5p | 0.0144 | 0.0831 | 0.0120 | 0.0377 |
| miR-204-5p | 0.0183 | 0.0950 | 0.0149 | 0.0418 |
| miR-409-3p | 0.0190 | 0.0950 | 0.0153 | 0.0418 |
| Category | Term | Raw p Value | Empirical p Value |
|---|---|---|---|
| KEGG | CYTOKINE-CYTOKINE_RECEPTOR_INTERACTION | 1.01 × 10−3 | 3.03 × 10−4 |
| REACTOME | CHEMOKINE_RECEPTORS_BIND_CHEMOKINES | 3.72 × 10−3 | 8.25 × 10−3 |
| REACTOME | CLASS_A_1_(RHODOPSIN-LIKE_RECEPTORS) | 7.34 × 10−3 | 1.24 × 10−2 |
| BIOCARTA | BIOCARTA_NKT_PATHWAY | 1.12 × 10−2 | 1.24 × 10−2 |
| KEGG | JAK-STAT_SIGNALING_PATHWAY | 1.13 × 10−2 | 1.57 × 10−2 |
| BIOCARTA | BIOCARTA_INFLAM_PATHWAY | 1.20 × 10−2 | 1.73 × 10−2 |
| REACTOME | GPCR_LIGAND_BINDING | 7.99 × 10−3 | 2.19 × 10−2 |
| PAD | ATR_SIGNALING_PATHWAY | 2.07 × 10−2 | 2.75 × 10−2 |
| PAD | REGULATION_OF_RHOA_ACTIVITY | 2.77 × 10−2 | 2.89 × 10−2 |
| REACTOME | PEPTIDE_LIGAND-BINDING_RECEPTORS | 1.96 × 10−2 | 4.72 × 10−2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Strycharz, J.; Wróblewski, A.; Zieleniak, A.; Świderska, E.; Matyjas, T.; Rucińska, M.; Pomorski, L.; Czarny, P.; Szemraj, J.; Drzewoski, J.; et al. Visceral Adipose Tissue of Prediabetic and Diabetic Females Shares a Set of Similarly Upregulated microRNAs Functionally Annotated to Inflammation, Oxidative Stress and Insulin Signaling. Antioxidants 2021, 10, 101. https://doi.org/10.3390/antiox10010101
Strycharz J, Wróblewski A, Zieleniak A, Świderska E, Matyjas T, Rucińska M, Pomorski L, Czarny P, Szemraj J, Drzewoski J, et al. Visceral Adipose Tissue of Prediabetic and Diabetic Females Shares a Set of Similarly Upregulated microRNAs Functionally Annotated to Inflammation, Oxidative Stress and Insulin Signaling. Antioxidants. 2021; 10(1):101. https://doi.org/10.3390/antiox10010101
Chicago/Turabian StyleStrycharz, Justyna, Adam Wróblewski, Andrzej Zieleniak, Ewa Świderska, Tomasz Matyjas, Monika Rucińska, Lech Pomorski, Piotr Czarny, Janusz Szemraj, Józef Drzewoski, and et al. 2021. "Visceral Adipose Tissue of Prediabetic and Diabetic Females Shares a Set of Similarly Upregulated microRNAs Functionally Annotated to Inflammation, Oxidative Stress and Insulin Signaling" Antioxidants 10, no. 1: 101. https://doi.org/10.3390/antiox10010101
APA StyleStrycharz, J., Wróblewski, A., Zieleniak, A., Świderska, E., Matyjas, T., Rucińska, M., Pomorski, L., Czarny, P., Szemraj, J., Drzewoski, J., & Śliwińska, A. (2021). Visceral Adipose Tissue of Prediabetic and Diabetic Females Shares a Set of Similarly Upregulated microRNAs Functionally Annotated to Inflammation, Oxidative Stress and Insulin Signaling. Antioxidants, 10(1), 101. https://doi.org/10.3390/antiox10010101





