Assessing the Effects of Land Use on Surface Water Quality in the Lower uMfolozi Floodplain System, South Africa
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area Background
2.2. Data Collection
2.3. Water and Soil Lab Analyses
2.4. Statistical Analysis
3. Results
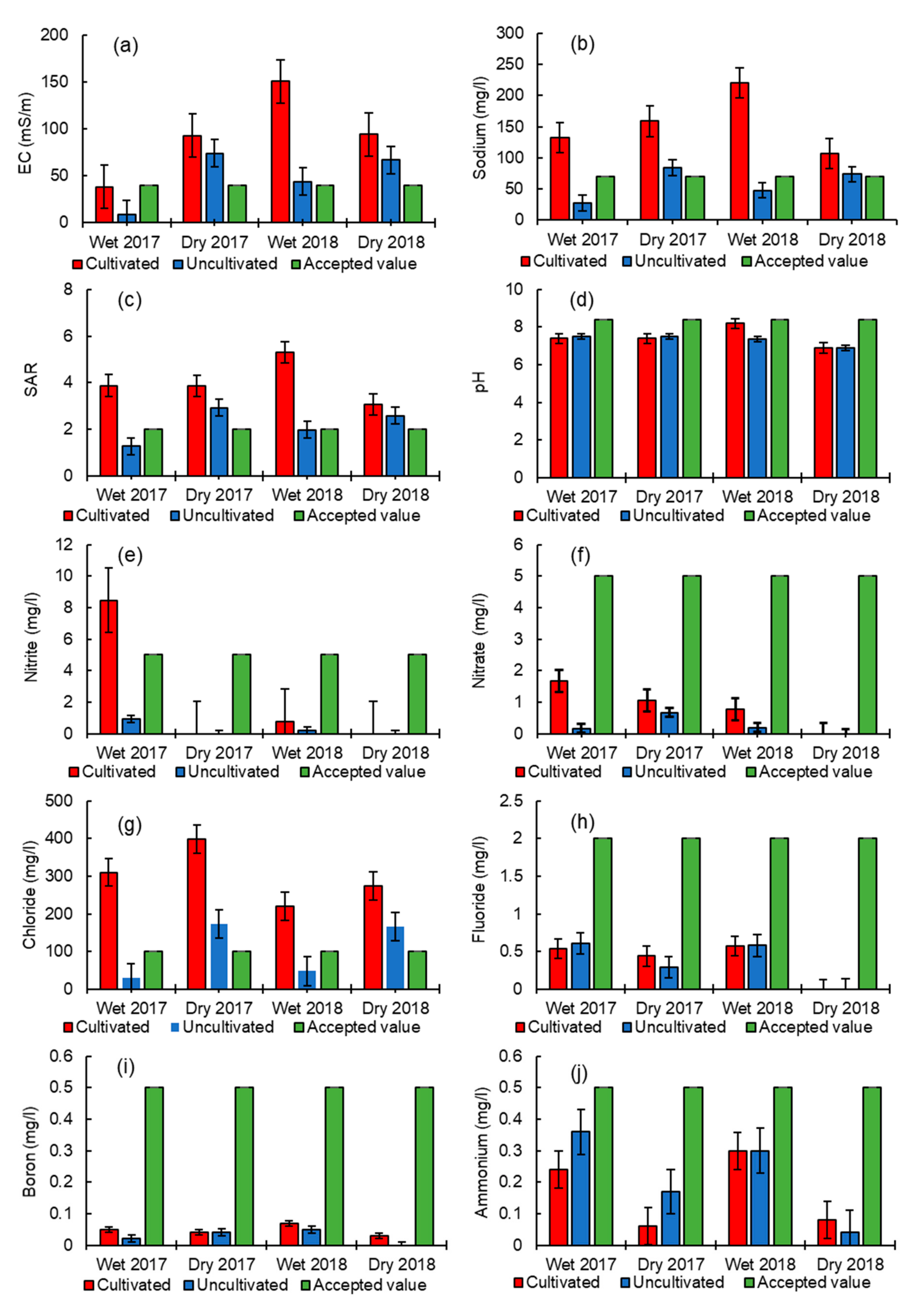
3.1. Comparison of Water Quality Analyses between Cultivated and Uncultivated Sites and Seasonal Dynamics
3.2. Comparison of Water Quality with South African Water Quality (SAWQ) Guidelines for Irrigation
3.3. Cultivation and Soil Quality
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Verma, M.; Negandhi, D. Valuing ecosystem services of wetlands—A tool for effective policy formulation and poverty alleviation. Hydrol. Sci. J. 2011, 56, 1622–1639. [Google Scholar] [CrossRef]
- Bhatta, L.; Chaudhary, S.; Pandit, A.; Baral, H.; Das, P.; Stork, N. Ecosystem Service Changes and Livelihood Impacts in the Maguri-Motapung Wetlands of Assam, India. Land 2016, 5, 15. [Google Scholar] [CrossRef]
- Maltby, E.; Acreman, M.C. Ecosystem services of wetlands: Pathfinder for a new paradigm. Hydrol. Sci. J. 2011, 56, 1341–1359. [Google Scholar] [CrossRef]
- Barbier, E. Valuing Ecosystem Services for Coastal Wetland Protection and Restoration: Progress and Challenges. Resources 2013, 2, 213–230. [Google Scholar] [CrossRef]
- Moomaw, W.R.; Chmura, G.; Davies, G.T.; Finlayson, C.; Middleton, B.A.; Natali, S.M.; Perry, J.; Roulet, N.; Sutton-Grier, A.E. Wetlands in a changing climate: Science, policy and management. Wetlands 2018, 38, 183–205. [Google Scholar] [CrossRef]
- Smardon, R. Wetland Ecology Principles and Conservation, Second Edition. Water 2014, 6, 813–817. [Google Scholar] [CrossRef]
- Semlitsch, R.D. Principles for management of aquatic breeding amphibians. Widlife Manag. 2000, 64, 615–631. [Google Scholar] [CrossRef]
- Russi, D.; Brink, P.; Farmer, A.; Badura, T.; Coates, D.; Förster, J.; Kumar, R.; Davidson, N. The Economics of Ecosystems and Biodiversity for Water and Wetlands; IEEP: London, UK; Brussels, Belgium; Ramsar Secretariat: Gland, Switzerland, 2013; p. 77. [Google Scholar]
- Gardner, R.C.; Barchiesi, S.; Beltrame, C.; Finlayson, C.M.; Galewski, T.; Harrison, I.; Paganini, M.; Perennou, C.; Pritchard, D.E.; Rosenqvist, A.; et al. State of the World’s Wetlands and their Services to People: A Compilation of Recent Analyses; Ramsar Convention Secretariat: Gland, Switzerland, 2015; p. 19. [Google Scholar]
- Zedler, J.B.; Kercher, S. Wetland Resources: Status, Trends, Ecosystem Services, and Restorability. Annu. Rev. Environ. Resour. 2005, 30, 39–74. [Google Scholar] [CrossRef]
- Bassi, N.; Kumar, M.D.; Sharma, A.; Pardha-Saradhi, P. Status of wetlands in India: A review of extent, ecosystem benefits, threats and management strategies. J. Hydrol. Reg. Stud. 2014, 2, 1–19. [Google Scholar] [CrossRef]
- An, S.; Li, H.; Guan, B.; Zhou, C.; Wang, Z.; Deng, Z.; Zhi, Y.; Liu, Y.; Xu, C.; Fang, S. China’s natural wetlands: Past problems, current status, and future challenges. Ambio A J. Hum. Environ. 2007, 36, 335–342. [Google Scholar] [CrossRef]
- Capon, S.J.; Lynch, A.J.J.; Bond, N.; Chessman, B.C.; Davis, J.; Davidson, N.; Finlayson, M.; Gell, P.A.; Hohnberg, D.; Humphrey, C. Regime shifts, thresholds and multiple stable states in freshwater ecosystems; A critical appraisal of the evidence. Sci. Total Environ. 2015, 534, 122–130. [Google Scholar] [CrossRef] [PubMed]
- Finlayson, C.; Gitay, H.; Bellio, M.; Van Dam, R.; Taylor, I. Climate variability and change and other pressures on wetlands and waterbirds: Impacts and adaptation. In Water Birds Around World; Boerec, G.C., Gailbraithand, C.A., Stroud, D.A., Eds.; The Stationery Office: Edinburgh, UK, 2006; pp. 88–97. [Google Scholar]
- Junk, W.J.; An, S.; Finlayson, C.; Gopal, B.; Květ, J.; Mitchell, S.A.; Mitsch, W.J.; Robarts, R.D. Current state of knowledge regarding the world’s wetlands and their future under global climate change: A synthesis. Aquat. Sci. 2013, 75, 151–167. [Google Scholar] [CrossRef]
- Bennett, E.M.; Peterson, G.D.; Gordon, L.J. Understanding relationships among multiple ecosystem services. Ecol. Lett. 2009, 12, 1394–1404. [Google Scholar] [CrossRef] [PubMed]
- Bwapwa, J.K. Review on main issues causing deterioration of water quality and water scarcity: Case study of South Africa. Environ. Manag. Sustain. Dev. 2018, 7, 14. [Google Scholar] [CrossRef]
- McKergow, L.A.; Weaver, D.M.; Prosser, I.P.; Grayson, R.B.; Reed, A.E. Before and after riparian management: Sediment and nutrient exports from a small agricultural catchment, Western Australia. J. Hydrol. 2003, 270, 253–272. [Google Scholar] [CrossRef]
- Selemani, J.; Zhang, J.; Muzuka, A.; Njau, K.N.; Zhang, G.; Mzuza, M.; Maggid, A. Nutrients’ distribution and their impact on Pangani River Basin’s ecosystem–Tanzania. Environ. Technol. 2018, 39, 702–716. [Google Scholar] [CrossRef]
- Munyika, S.; Kongo, V.; Kimwaga, R. River health assessment using macroinvertebrates and water quality parameters: A case of the Orange River in Namibia. Phys. Chem. Earthparts A/B/C 2014, 76, 140–148. [Google Scholar] [CrossRef]
- Matson, P.A.; Parton, W.J.; Power, A.; Swift, M. Agricultural intensification and ecosystem properties. Science 1997, 277, 504–509. [Google Scholar] [CrossRef]
- Heathwaite, A.L.; Johnes, P. Contribution of nitrogen species and phosphorus fractions to stream water quality in agricultural catchments. Hydrol. Process. 1996, 10, 971–983. [Google Scholar] [CrossRef]
- Tockner, K.; Bunn, S.; Gordon, C.; Naiman, R.; Quinn, G.; Stanford, J. Aquatic Ecosystems: Trends and Global Prospects; Flood plains: Critically threatened ecosystems; Polunin, N., Ed.; Cambridge University Press: Cambridge, UK, 2008; pp. 45–62. [Google Scholar] [CrossRef]
- SANBI. Life: The State of South Africa’s Biodiversity 2012; South African National Biodiversity Institute: Pretoria, South African, 2013; p. 54.
- Taiwo, O.J. Farmers’ choice of wetland agriculture: Checking wetland loss and degradation in Lagos State, Nigeria. GeoJournal 2013, 78, 103–115. [Google Scholar] [CrossRef]
- Ojanuga, A.; Okusami, T.A.; Lekwa, G. Wetland Soils of Nigeria: Status of Knowledge and Potentials; Soil Science Society of Nigeria: Enugu, Nigeria, 2003. [Google Scholar]
- Akpan-Idiok, A.; Ogbaji, P.; Abtahi, A.; Khormali, F.; Anand, R.; Seghal, J.; Sharma, P.; Al-Farraj, A.; Allison, L.; Akpan-Idiok, A. Watershed ecological engineering for sustainable increase for food production and restoration of degraded environment in West Africa. Int. J. Agric. Res. 2001, 8, 1643–1658. [Google Scholar]
- Nagabhatla, N.; Brakel, M.L.V. Landscape Level Characterization of Seasonal Floodplains under Community Based Aquaculture: Illustrating a Case of the Ganges and the Mekong Delta; The WorldFish Center: Penang, Malaysia, 2010. [Google Scholar]
- DWAF. South African Water Quality Guidelines; Agricultural Use; Department of Water Affairs and Forestry: Pretoria, South Africa, 1996; Volume 4. Available online: https://www.iwa-network.org/filemanager-uploads/WQ_Compendium/Database/Selected_guidelines/041.pdf (accessed on 1 June 2020).
- EPA. SA Water Quality Guidelines. Available online: http://www.waternet.co.za/policy/g_wq.html (accessed on 1 June 2020).
- Hansen, M.; Faran, T.; O’Byrne, D. The best laid plans: Using the capability approach to assess neoliberal conservation in South Africa—The case of the iSimangaliso Wetland Park. J. Environ. Dev. 2015, 24, 395–417. [Google Scholar] [CrossRef]
- Ellery, W.; Grenfell, M.; Grenfell, S.; Kotze, D.; McCarthy, T.; Tooth, S.; Grundling, P.-S.; Beckedahl, H.; Maitre, D.l.; Ramsay, L. The Origin and Evolution of Wetlands; Water Research Commission Research Programme Report; Wetland Rehabilitation: Pretoria, South Africa, 2008; Available online: http://www.wrc.org.za/wp-content/uploads/mdocs/TT%20334%20web1.pdf (accessed on 24 April 2020).
- Garden, S. Wetland Geomorphology and Floodplain Dynamics on the Hydrologically Variable Mfolozi River. Ph.D. Thesis, KwaZulu-Natal, University of KwaZulu-Natal, Durban, South Africa, 2008. [Google Scholar]
- Morgenthal, T.; Kellner, K.; Van Rensburg, L.; Newby, T.; Van der Merwe, J. Vegetation and habitat types of the Umkhanyakude Node. S. Afr. J. Bot. 2006, 72, 1–10. [Google Scholar] [CrossRef]
- Schulze, R.E. South African Atlas of Agrohydrology and Climatology; Water Research Commission: Pretoria, South Africa, 1997. [Google Scholar]
- Grenfell, S.; Ellery, W. Hydrology, sediment transport dynamics and geomorphology of a variable flow river: The Mfolozi River, South Africa. Water SA 2009, 35, 271–282. [Google Scholar] [CrossRef][Green Version]
- Keddy, P.A. Wetland Ecology: Principles and Conservation; Cambridge University Press: Cambridge, UK, 2010. [Google Scholar]
- Vivier, L.; Cyrus, D.; Owen, R.; Jerling, H. Fish assemblages in the Mfolozi–Msunduzi estuarine system, KwaZulu-Natal, South Africa, when not linked to the St Lucia mouth. Afr. J. Aquat. Sci. 2010, 35, 141–154. [Google Scholar] [CrossRef]
- Searle, A. Sugarcane at Umfolozi, South Africa: Contributing to the sustainability of an environmentally and socially sensitive area. Int. Farm Manag. Assoc. Ifma19 2013, 2, 1–11. [Google Scholar]
- Leslie, G.; Moodley, S. Progress in the use of insecticides for the control of the sugarcane thrips Fulmekiola serrata (Kobus)(Thysanoptera: Thripidae) in South Africa. In Proceedings of the South African Sugarcane Technologists’ Association, Durban, South Africa; Cary, NC, USA, 26–28 August 2009; pp. 437–440. [Google Scholar]
- Chigor, V.N.; Sibanda, T.; Okoh, A.I. Studies on the bacteriological qualities of the Buffalo River and three source water dams along its course in the Eastern Cape Province of South Africa. Environ. Sci. Pollut. Res. 2013, 20, 4125–4136. [Google Scholar] [CrossRef]
- APHA. Standard Methods for the Examination of Water and Wastewater; American Public Health Association: Washington, DC, USA, 2005. [Google Scholar]
- Polese, L.; Dores, E.F.G.d.C.; Jardim, E.F.G.; Navickiene, S.; Ribeiro, M.L. Determination of herbicides residues in soil by small scale extraction. Eclética Química 2002, 27. [Google Scholar] [CrossRef]
- Pfaff, J.D. Method 300.0 Determination of Inorganic Anions by Ion Chromatography; US Environmental Protection Agency, Office of Research and Development, Environmental Monitoring Systems Laboratory: Las Vegas, NV, USA, 1993; Volume 28.
- Silva, S.; Baffi, C.; Spalla, S.; Cassinari, C.; Lodigiani, P. Method for the determination of CEC and exchangeable bases in calcareous soils. Agrochimica 2010, 54, 103–114. [Google Scholar]
- Wright, A.F.; Bailey, J.S. Organic carbon, total carbon, and total nitrogen determinations in soils of variable calcium carbonate contents using a Leco CN-2000 dry combustion analyzer. Commun. Soil Sci. Plant Anal. 2001, 32, 3243–3258. [Google Scholar] [CrossRef]
- SABS. South African National Standards and Relared Publications; SABS Standards Division: Pretoria, South Africa, 2019; ISBN 978-0-626-37224-8. Available online: https://www.sabs.co.za/Standard-Sales/docs/SABS_Catalogue_of_February_2019_abridged.pdf (accessed on 24 April 2020).
- Verhoeven, J.T.; Setter, T.L. Agricultural use of wetlands: Opportunities and limitations. Ann. Bot. 2009, 105, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Orhan, U.; Kılınç, E. Estimating soil texture with laser-guided Bouyoucos. Automatika 2020, 61, 1–10. [Google Scholar] [CrossRef]
- Rao, E.P.; Puttanna, K. Nitrates, agriculture and environment. Curr. Sci. 2000, 79, 1163–1168. [Google Scholar]
- Koegelenberg, F. Irrigation User’s Manual-Chapter 5: Water; Agricultural Research Council: Silverton, South Africa, 2004. [Google Scholar]
- Morway, E.D.; Gates, T.K. Regional assessment of soil water salinity across an intensively irrigated river valley. J. Irrig. Drain. Eng. 2012, 138, 393–405. [Google Scholar] [CrossRef]
- La Mora-Orozco, D.; González-Acuña, I.J.; Saucedo-Terán, R.A.; Flores-López, H.E.; Rubio-Arias, H.O.; Ochoa-Rivero, J.M. Removing organic matter and nutrients from pig farm wastewater with a constructed wetland system. Int. J. Environ. Res. Public Health 2018, 15, 1031. [Google Scholar] [CrossRef]
- Hussain, B.; Ratan, C.; Dutta, A. Study of the Limnology and Ichthyology of Dhir Beel at Dhubri. Assamindia Int. Res. J. Biol. Sci. 2015, 4, 40–48. [Google Scholar]
- Shabalala, A.N.; Combrinck, L.; McCrindle, R. Effect of farming activities on seasonal variation of water quality of Bonsma Dam, KwaZulu-Natal. S. Afr. J. Sci. 2013, 109, 1–7. [Google Scholar] [CrossRef]
- Mmualefe, L.C.; Torto, N. Water quality in the Okavango Delta. Water SA 2011, 37. [Google Scholar] [CrossRef][Green Version]
- Ngabirano, H.; Byamugisha, D.; Ntambi, E. Effects of seasonal variations in physical parameters on quality of gravity flow water in Kyanamira Sub-County, Kabale District, Uganda. J. Water Resour. Prot. 2016, 8, 1297–1309. [Google Scholar] [CrossRef]
- Lorenz, K.; Iwanyshyn, M.; Olson, B.; Kalischuk, A.; Pentland, J.; Hofer, B.; Sommerville, A. Livestock Manure Impacts on Groundwater Quality in Alberta 2008 to 2011; Alberta Agriculture and Rural Development: Lethbridgeab, AB, Canada, 2014; p. 316. [Google Scholar]
- Acosta, J.A.; Faz, A.; Jansen, B.; Kalbitz, K.; Martínez-Martínez, S. Assessment of salinity status in intensively cultivated soils under semiarid climate, Murcia, SE Spain. J. Arid Environ. 2011, 75, 1056–1066. [Google Scholar] [CrossRef]
- Sainato, C.M.; Losinno, B.N.; Malleville, H.J. Assessment of contamination by intensive cattle activity through electrical resistivity tomography. J. Appl. Geophys. 2012, 76, 82–91. [Google Scholar] [CrossRef]
- EPA. Monitoring and Assessment. In Conductivity. Available online: https://archive.epa.gov/water/archive/web/html/vms59.html (accessed on 24 April 2020).
- Thilagavathi, R.; Chidambaram, S.; Prasanna, M.; Thivya, C.; Singaraja, C. A study on groundwater geochemistry and water quality in layered aquifers system of Pondicherry region, southeast India. Appl. Water Sci. 2012, 2, 253–269. [Google Scholar] [CrossRef]
- Gamvroula, D.; Alexakis, D.; Stamatis, G. Diagnosis of groundwater quality and assessment of contamination sources in the Megara basin (Attica, Greece). Arab. J. Geosci. 2013, 6, 2367–2381. [Google Scholar] [CrossRef]
- Davies, O. Tidal influence on the physico-chemistry quality of Okpoka Creek, Nigeria. Int. J. Biol. Sci. Appl. 2014, 1, 113–123. [Google Scholar]
- Chen, J.; Wang, F.; Meybeck, M.; He, D.; Xia, X.; Zhang, L. Spatial and temporal analysis of water chemistry records (1958–2000) in the Huanghe (Yellow River) basin. Glob. Biogeochem. Cycles 2005, 19. [Google Scholar] [CrossRef]
- Zhang, S.R.; Lu, X.X.; Higgitt, D.L.; Chen, C.T.A.; Sun, H.G.; Han, J.T. Water chemistry of the Zhujiang (Pearl River): Natural processes and anthropogenic influences. J. Geophys. Res. Earth Surf. 2007, 112. [Google Scholar] [CrossRef]
- Sampaio, F.G.; de Lima Boijink, C.; dos Santos, L.R.B.; Oba, E.T.; Kalinin, A.L.; Rantin, F.T. The combined effect of copper and low pH on antioxidant defenses and biochemical parameters in neotropical fish pacu, Piaractus mesopotamicus (Holmberg, 1887). Ecotoxicology 2010, 19, 963–976. [Google Scholar] [CrossRef]
- CSIR. A CSIR Perspective on Water in South Africa—2010; CSIR: Pretoria, South Africa, 2010. Available online: https://researchspace.csir.co.za/dspace/bitstream/handle/10204/5760/Wall11_2010.pdf?s (accessed on 24 April 2020).
- Brainwood, M.A.; Burgin, S.; Maheshwari, B. Temporal variations in water quality of farm dams: Impacts of land use and water sources. Agric. Water Manag. 2004, 70, 151–175. [Google Scholar] [CrossRef]
- Mosher, J.J.; Klein, G.C.; Marshall, A.G.; Findlay, R.H. Influence of bedrock geology on dissolved organic matter quality in stream water. Org. Geochem. 2010, 41, 1177–1188. [Google Scholar] [CrossRef]
- Zaman, M.; Shahid, S.A.; Heng, L. Guideline for Salinity Assessment, Mitigation and Adaptation Using Nuclear and Related Techniques; Springer: Berlin, Germany, 2018. [Google Scholar]
- Gholami, S.; Srikantaswamy, S. Analysis of agricultural impact on the Cauvery river water around KRS dam. World Appl. Sci. J. 2009, 6, 1157–1169. [Google Scholar]
- Van de Graaff, R.; Patterson, R.A. Explaining the mysteries of salinity, sodicity, SAR and ESP in on-site practice. In Proceedings of the On-Site ‘01 Conference: Advancing on-Site Wastewater Systems, University of New England, Armidale, NSW, Australia, 25–27 September 2001; Patterson, R.A., Jones, M.J., Eds.; Lanfax Laboratories: Armidale, Australia, 2001; pp. 25–27. [Google Scholar]
- Mandal, S.; Dutta, S.K.; Pramanik, S.; Kole, R. Assessment of river water quality for agricultural irrigation. Int. J. Environ. Sci. Technol. 2019, 16, 451–462. [Google Scholar] [CrossRef]
- Rahman, M.M.; Rahman, M.T.; Rahaman, M.S.; Rahman, F.; Ahmad, J.U.; Shakera, B.; Halim, M.A. Water quality of the world’s largest mangrove forest. Can Chem. Trans. 2013, 1, 141–156. [Google Scholar]
- Cloern, J.E.; Abreu, P.C.; Carstensen, J.; Chauvaud, L.; Elmgren, R.; Grall, J.; Greening, H.; Johansson, J.O.R.; Kahru, M.; Sherwood, E.T. Human activities and climate variability drive fast-paced change across the world’s estuarine–coastal ecosystems. Glob. Chang. Biol. 2016, 22, 513–529. [Google Scholar] [CrossRef] [PubMed]
- Bewket, W.; Stroosnijder, L. Effects of agroecological land use succession on soil properties in Chemoga watershed, Blue Nile basin, Ethiopia. Geoderma 2003, 111, 85–98. [Google Scholar] [CrossRef]
- Marzaioli, R.; D’Ascoli, R.; De Pascale, R.; Rutigliano, F.A. Soil quality in a Mediterranean area of Southern Italy as related to different land use types. Appl. Soil Ecol. 2010, 44, 205–212. [Google Scholar] [CrossRef]
- Tilahun, G. Soil fertility status as influenced by different land uses in Maybar areas of South Wello Zone, North Ethiopia. Master’s Thesis, Haramaya University, Awaday, Ethiopia, 2007; 40p. [Google Scholar]
- Minjibir, S.A. The Effect of Late and Early Burning on Soil Physicochemical Properties, Plant Species Composition and Abundance at Falgore Game Reserve, Kano. Master’s Thesis, Ahmadu Bello University, Zaria, Nigeria, 2011; p. 68. Available online: http://kubanni.abu.edu.ng/jspui/bitstream/123456789/2688/1/THE%20EFFECT%20OF%20LATE%20AND%20EARLY%20BURNING%20ON%20SOIL%20PHYSICO-CHEMICAL%20PROPERTIES%2C%20PLANT%20SPECIES%20COMPOSITION%20AND%20ABUNDANCE%20AT%20FALGORE%20GAME%20RESERVE%2C%20KANO.pdf (accessed on 24 April 2020).
- Mutyavaviri, F. Impact of Cultivation on Soil and Species Composition of Monavale Vlei; Department of Biological Sciences: Harare, Zimbabwe, 2006. [Google Scholar]
- Mekonnen, T.; Aticho, A. The driving forces of Boye wetland degradation and its bird species composition, Jimma, Southwestern Ethiopia. J. Ecol. Nat. Environ. 2011, 3, 365–369. [Google Scholar]
- Dube, T.; Chitiga, M. Human impacts on macrophyte diversity, water quality and some soil properties in the Madikane and Dufuya wetlands of Lower Gweru, Zimbabwe. Appl. Ecol. Environ. Res. 2011, 9, 85–99. [Google Scholar] [CrossRef]
- Brady, N.C.; Weil, R.R.; Weil, R.R. The Nature and Properties of Soils; Prentice Hall: Upper Saddle River, NJ, USA, 2008; Volume 13. [Google Scholar]
- Liu, X.-R.; Dong, Y.-S.; Ren, J.-Q.; Li, S.-G. Drivers of soil net nitrogen mineralization in the temperate grasslands in Inner Mongolia, China. Nutr. Cycl. Agr. 2010, 87, 59–69. [Google Scholar] [CrossRef]

| Variables | Statistics | WET SEASON CULTIVATED | WET SEASON UNCULTIVATED | DRY SEASON CULTIVATED | DRY SEASON UNCULTIVATED | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| * Mar 17–** Mar 18 Cult | * Mar 17–**Mar 18 Uncult | * Jul 17–** Jul 18 Cult | * Jul 17–** Jul 18 Uncult | ||||||||||
| * N = 17 | ** N = 17 | Avg | * N = 10 | ** N = 10 | Avg | * N = 14 | ** N = 13 | Avg | * N = 10 | ** N = 10 | Avg | ||
| NH4+ (mg−/L) | Mean | 0.24 | 0.31 | 0.28 | 0.36 | 0.34 | 0.33 | 0.06 | 0.08 | 0.07 | 0.17 | 0.04 | 0.105 |
| Maximum | 0.93 | 0.79 | 0.86 | 0.70 | 1.66 | 1.2 | 0.30 | 0.27 | 0.29 | 0.96 | 0.12 | 0.56 | |
| Minimum | 0.08 | 0.08 | 0.08 | 0.15 | 0.05 | 0.1 | 0.01 | 0.01 | 0.01 | 0.01 | 0.02 | 0.015 | |
| Median | 0.15 | 0.27 | 0.225 | 0.34 | 0.07 | 0.21 | 0.05 | 0.04 | 0.05 | 0.08 | 0.02 | 0.05 | |
| SD | 0.21 | 0.21 | 0.21 | 0.17 | 0.57 | 0.38 | 0.07 | 0.09 | 0.08 | 0.30 | 0.04 | 0.17 | |
| p-value * | 0.163 | 0.186 | |||||||||||
| p-value ** | 0.006 | 0.006 | |||||||||||
| B(mg/L) | Mean | 0.05 | 0.07 | 0.06 | 0.02 | 0.06 | 0.04 | 0.04 | < 0.01 | 0.025 | 0.04 | < 0.01 | 0.025 |
| Maximum | 0.15 | 0.10 | 0.125 | 0.04 | 0.07 | 0.06 | 0.05 | < 0.01 | 0.03 | 0.06 | < 0.01 | 0.035 | |
| Minimum | 0.03 | 0.05 | 0.04 | 0.00 | 0.03 | 0.02 | 0.02 | < 0.01 | 0.015 | 0.02 | < 0.01 | 0.015 | |
| Median | 0.04 | 0.06 | 0.05 | 0.02 | 0.06 | 0.04 | 0.04 | < 0.01 | 0.025 | 0.04 | < 0.01 | 0.201 | |
| SD | 0.03 | 0.01 | 0.02 | 0.02 | 0.01 | 0.02 | 0.01 | < 0.01 | 0.01 | 0.01 | < 0.01 | 0.01 | |
| p-value * | 0.108 | 0.359 | - | ||||||||||
| p-value ** | 0.807 | 0.000 | - | ||||||||||
| Cl− (mg/L) | Mean | 310.36 | 435.36 | 265.28 | 30.07 | 67.30 | 38.99 | 397.71 | 273.88 | 335.8 | 172.87 | 166.61 | 169.74 |
| Maximum | 417.18 | 857.59 | 373.1 | 50.98 | 84.50 | 52.90 | 622.49 | 303.30 | 462.9 | 221.82 | 183.78 | 202.8 | |
| Minimum | 26.4 | 337.43 | 109.2 | 20.27 | 40.14 | 27.75 | 188.91 | 159.12 | 174 | 123.70 | 123.20 | 123.45 | |
| Median | 349.5 | 377.26 | 275.75 | 23.9 | 71.13 | 36.85 | 379.85 | 284.05 | 332 | 179.68 | 170.66 | 175.2 | |
| SD | 107.70 | 134.55 | 121,13 | 12.63 | 16.66 | 14.65 | 153.83 | 41.24 | 97.5 | 32.73 | 18.26 | 25.495 | |
| p-value * | 0.020 | 0.020 | |||||||||||
| p-value ** | 0.004 | 0.012 | |||||||||||
| NO3− (mg/L) | Mean | 1.57 | 0.83 | 1.19 | 0.18 | 0.20 | 0.19 | 1.05 | < 0.060 | 0.555 | 0.71 | < 0.060 | 0.385 |
| Maximum | 5.96 | 3.27 | 4.49 | 0.36 | 0.50 | 0.43 | 3.44 | < 0.060 | 1.75 | 2.88 | < 0.060 | 1.471 | |
| Minimum | 0.05 | 0 | 0.03 | 0.06 | 0.06 | 0.06 | 0.10 | < 0.060 | 0.08 | 0.14 | < 0.060 | 0.1 | |
| Median | 0.67 | 0.65 | 0.60 | 0.16 | 0.09 | 0.15 | 0.57 | < 0.060 | 0.315 | 0.37 | < 0.060 | 0.215 | |
| SD | 1.88 | 0.78 | 1.33 | 0.10 | 0.18 | 0.14 | 1.12 | < 0.060 | 0.59 | 0.88 | < 0.060 | 0.47 | |
| p-value * | 0.135 | 0.056 | - | ||||||||||
| p-value ** | 0.798 | 0.001 | - | ||||||||||
| NO2- (mg/L) | Mean | 8.69 | < 0.022 | 4.46 | 1.05 | 0.23 | 2.76 | < 0.022 | < 0.022 | 0.022 | < 0.022 | < 0.022 | 0.022 |
| Maximum | 24.10 | < 0.022 | 12.16 | 2.77 | 0.93 | 1.85 | < 0.022 | < 0.022 | 0.022 | < 0.022 | < 0.022 | 0.022 | |
| Minimum | 0 | < 0.022 | 0.111 | 0 | 0.00 | 0.00 | < 0.022 | < 0.022 | 0.022 | < 0.022 | < 0.022 | 0.022 | |
| Median | 9.90 | < 0.022 | 5.061 | 0.85 | 0.00 | 0.43 | < 0.022 | < 0.022 | 0.022 | < 0.022 | < 0.022 | 0.022 | |
| SD | 7.60 | < 0.022 | 3.91 | 1.16 | 0.35 | 0.76 | < 0.022 | < 0.022 | 0.022 | < 0.022 | < 0.022 | 0.022 | |
| p-value * | 0.085 | 0.000 | |||||||||||
| p-value ** | 0.001 | 0.187 | |||||||||||
| PO43−(mg/L) | Mean | 0.93 | 1.46 | 1.17 | 0.15 | 0.49 | 0.33 | 2.05 | < 0.109 | 1.08 | 1.90 | < 0.109 | 1.00 |
| Maximum | 8.01 | 4.25 | 6.15 | 0.24 | 0.87 | 0.57 | 4.00 | < 0.109 | 2.06 | 3.65 | < 0.109 | 1.88 | |
| Minimum | 0.00 | 0.00 | 0.00 | 0.05 | 0.18 | 0.13 | 0.86 | < 0.109 | 0.49 | 0.43 | < 0.109 | 0.27 | |
| Median | 0.12 | 1.26 | 0.58 | 0.17 | 0.46 | 0.34 | 1.71 | < 0.109 | 0.91 | 2.12 | < 0.109 | 1.11 | |
| SD | 2.05 | 1.44 | 1.75 | 0.07 | 0.24 | 0.16 | 0.85 | < 0.109 | 0.48 | 0.99 | < 0.109 | 0.55 | |
| p-value * | 0.189 | 0.621 | - | ||||||||||
| p-value ** | 0.002 | 0.000 | |||||||||||
| F (mg/L) | Mean | 0.53 | 0.56 | 0.55 | 0.64 | 0.58 | 0.61 | 0.44 | < 0.030 | 0.24 | 0.28 | < 0.030 | 0.16 |
| Maximum | 1.24 | 1.22 | 1.21 | 0.80 | 0.76 | 0.78 | 1.26 | < 0.030 | 0.65 | 0.41 | < 0.030 | 0.22 | |
| Minimum | 0.21 | 0.27 | 0.24 | 0.43 | 0.41 | 0.41 | 0.15 | < 0.030 | 0.09 | 0.13 | < 0.030 | 0.08 | |
| Median | 0.50 | 0.51 | 0.51 | 0.63 | 0.58 | 0.59 | 0.35 | < 0.030 | 0.19 | 0.31 | < 0.030 | 0.17 | |
| SD | 0.29 | 0.26 | 0.28 | 0.12 | 0.13 | 0.13 | 0.28 | < 0.030 | 0.16 | 0.10 | < 0.030 | 0.07 | |
| p-value * | 0.629 | 0.180 | - | ||||||||||
| p-value ** | 0.065 | 0.000 | |||||||||||
| pH | Mean | 6.69 | 8.19 | 7.44 | 6.64 | 7.36 | 7 | 8.11 | 7.93 | 7.15 | 7.74 | 7.74 | 7.20 |
| Maximum | 6.89 | 9.20 | 8.01 | 6.86 | 7.85 | 7.33 | 8.58 | 8.39 | 7.75 | 8.60 | 7.92 | 7.80 | |
| Minimum | 6.46 | 7.33 | 6.90 | 6.43 | 7.13 | 6.77 | 7.59 | 7.59 | 6.55 | 7.25 | 7.58 | 6.80 | |
| Median | 6.44 | 7.94 | 7.19 | 6.63 | 7.28 | 6.96 | 7.94 | 7.94 | 7.25 | 7.64 | 7.73 | 7.20 | |
| SD | 0.15 | 0.53 | 0.34 | 0.19 | 0.22 | 0.21 | 0.27 | 0.22 | 0.25 | 0.46 | 0.13 | 0.30 | |
| p-value * | 0.038 | 0.305 | |||||||||||
| p-value ** | 0.100 | 0.048 | |||||||||||
| SAR | Mean | 3.71 | 5.24 | 4.51 | 1.26 | 1.97 | 1.62 | 3.86 | 3.07 | 3.47 | 2.88 | 2.58 | 2.73 |
| Maximum | 4.77 | 6.96 | 5.85 | 1.60 | 2.24 | 1.90 | 5.05 | 4.50 | 4.80 | 3.27 | 2.97 | 3.12 | |
| Minimum | 1.45 | 4.62 | 3.05 | 0.97 | 1.51 | 1.235 | 2.18 | 1.80 | 2.00 | 2.25 | 1.41 | 1.83 | |
| Median | 3.91 | 4.83 | 4.45 | 1.27 | 2.06 | 1.7 | 4.08 | 2.90 | 3.50 | 2.97 | 3.73 | 3.35 | |
| SD | 0.82 | 0.74 | 0.78 | 0.21 | 0.26 | 0.24 | 0.91 | 0.80 | 0.86 | 0.35 | 0.50 | 0.43 | |
| p-value * | 0.014 | 0.009 | |||||||||||
| p-value ** | 0.014 | 0.020 | |||||||||||
| Na (mg/L) | Mean | 132.63 | 216.73 | 176.42 | 27.84 | 47.90 | 37.87 | 158.67 | 107.1 | 132.90 | 83.57 | 73.65 | 78.65 |
| Maximum | 192.27 | 329 | 260.65 | 35.85 | 54.90 | 45.4 | 252 | 159 | 205.50 | 102 | 86.80 | 94.4 | |
| Minimum | 27.74 | 192 | 109.85 | 24.66 | 35.20 | 29.95 | 78.40 | 52.80 | 65.60 | 67.50 | 38.50 | 53 | |
| Median | 142.87 | 201 | 172.45 | 25.59 | 49.80 | 37.4 | 162 | 101.50 | 131.75 | 83.20 | 76.25 | 79.85 | |
| SD | 41.17 | 36.16 | 38.67 | 3.88 | 7.37 | 5.63 | 61.32 | 29.60 | 45.46 | 10.46 | 14.82 | 12.6i | |
| p-value * | 0.017 | 0.033 | |||||||||||
| p-value ** | 0.031 | 0.014 | |||||||||||
| EC (mS/m) | Mean | 44.20 | 150.87 | 97.54 | 9.13 | 43.88 | 26.51 | 92.92 | 94.44 | 93.65 | 74.07 | 66.84 | 70.45 |
| Maximum | 54.10 | 229 | 141.55 | 14.20 | 49 | 31.6 | 143.10 | 119.70 | 131.40 | 84.80 | 72 | 78.4 | |
| Minimum | 28.60 | 135 | 81.8 | 5 | 38 | 21.5 | 52 | 60.70 | 56.35 | 55 | 54.10 | 54.55 | |
| Median | 47.10 | 143 | 95.05 | 9.50 | 44.50 | 27 | 90.50 | 94.40 | 92.45 | 81.60 | 68.25 | 74.95 | |
| SD | 7.55 | 23.67 | 15.61 | 3.02 | 4.02 | 3.52 | 32.22 | 13.83 | 22.92 | 12.75 | 5.81 | 9.28 | |
| p-value * | 0.015 | 0.063 | |||||||||||
| p-value ** | 0.270 | 0.011 | |||||||||||
| Temperature (°C) | Mean | 26.2 | - | 26.45 | - | 17.55 | 16.32 | 16.50 | 19.84 | 16.46 | 18.18 | ||
| Maximum | 30 | - | 29.20 | - | 24.60 | 17.60 | 18.05 | 24.20 | 17.40 | 20.8 | |||
| Minimum | 24.4 | - | 24.70 | - | 13.50 | 15.60 | 14.55 | 16.50 | 15.60 | 16.05 | |||
| Median | 26.45 | - | 26.30 | - | 17.20 | 16.05 | 16.13 | 19.20 | 16.60 | 18 | |||
| SD | 1.67 | - | 1.32 | - | 2.84 | 0.63 | 1.74 | 2.93 | 0.64 | 1.79 | |||
| p-value * | - | - | 0.008 | ||||||||||
| p-value ** | |||||||||||||
| Texture | Mean (%) ± Std. Deviation | p-Value | |
|---|---|---|---|
| Cultivated | Uncultivated | ||
| Sand | 13.43 ± 15.32 | 73.63 ± 15.32 | 0.03 |
| Silt | 41.97 ± 10.42 | 17.02 ± 15.31 | 0.51 |
| Clay | 44.60 ± 14.58 | 9.35 ± 7.88 | 0.04 |
| Parameters | Statistics | March 2017 | July 2017 | March 2018 | July 2018 | ||||
|---|---|---|---|---|---|---|---|---|---|
| Cult | Uncult | Cult | Uncult | Cult | Uncult | Cult | Uncult | ||
| EC (mS/m) | N | 17 | 10 | 14 | 10 | 17 | 10 | 13 | 10 |
| Mean | 57.4 | 31.7 | 14.2 | 11.2 | 25 | 19.8 | 27.4 | 16.5 | |
| Maximum | 84 | 85 | 19 | 22 | 44 | 19.8 | 84 | 20 | |
| Minimum | 11 | 12 | 9 | 2 | 16 | 3 | 2 | 14 | |
| Median | 62 | 23 | 14 | 11.5 | 22.5 | 19.8 | 17.3 | 17.5 | |
| SD | 21.1 | 23.9 | 2.6 | 6.8 | 8.2 | 11.1 | 29.8 | 2.3 | |
| p-value | 0.30 | 0.54 | 0.33 | <0.01 | |||||
| CEC (cmol(+)/kg) | N | 17 | 10 | 14 | 10 | 17 | 10 | 13 | 10 |
| Mean | 25.9 | 31.2 | 29.7 | 25.9 | 19.8 | 32.8 | 22.1 | 37.8 | |
| Maximum | 43.2 | 44.2 | 37.8 | 36.8 | 19.8 | 39.9 | 32.4 | 49.6 | |
| Minimum | 3.1 | 9.2 | 11.7 | 12.9 | 3 | 15.5 | 5.6 | 31.4 | |
| Median | 27.5 | 32.9 | 30.7 | 28.6 | 19.8 | 33.6 | 30.8 | 36.2 | |
| SD | 13.9 | 11.4 | 7.1 | 8.3 | 11.1 | 6.9 | 7.1 | 5.5 | |
| p-value | 0.98 | 0.65 | 0.83 | 0.69 | |||||
| pH | N | - | - | - | - | 17 | 10 | 13 | 10 |
| Mean | - | - | - | - | 0.36 | 0.37 | 0.34 | 0.34 | |
| Maximum | - | - | - | - | 0.36 | 0.40 | 0.38 | 0.37 | |
| Minimum | - | - | - | - | 0.32 | 0.36 | 0.32 | 0.31 | |
| Median | - | - | - | - | 0.37 | 0.37 | 0.33 | 0.34 | |
| SD | 0.07 | 0.01 | 0.02 | 0.02 | |||||
| p-value | 0.13 | 0.59 | |||||||
| Organic matter (%) | N | - | - | - | - | 17 | 10 | 13 | 10 |
| Mean | - | - | - | - | 6.2 | 5.7 | 6.6 | 5.8 | |
| Maximum | - | - | - | - | 6.2 | 6 | 6.9 | 6.1 | |
| Minimum | - | - | - | - | 4.8 | 4.8 | 6.3 | 5.5 | |
| Median | - | - | - | - | 5.9 | 5.7 | 6.2 | 5.8 | |
| SD | - | - | - | - | 1.1 | 0.36 | 0.23 | 0.22 | |
| p-value | - | - | 0.08 | 0.52 | |||||
| N-NO3- (mg/kg) | N | 17 | 10 | 14 | 10 | 17 | 10 | 13 | 10 |
| Mean | 9.5 | 7.4 | 6.4 | 5.5 | 8.9 | 11.5 | 8.5 | 11.7 | |
| Maximum | 23.1 | 19.2 | 8 | 7.2 | 8.9 | 12.5 | 10.8 | 12.9 | |
| Minimum | 11.2 | 1.2 | 4.1 | 3.4 | 4.9 | 9.9 | 6.1 | 10.4 | |
| Median | 5.6 | 5.9 | 6.2 | 5.2 | 10.1 | 11.6 | 10.2 | 11.8 | |
| SD | 8.1 | 5.9 | 1.2 | 0.9 | 1.86 | 0.89 | 1.16 | 0.86 | |
| p-value | 0.05 | 0.08 | 0.01 | 0.02 | |||||
| Total carbon (%) | N | 17 | 10 | 14 | 10 | 17 | 10 | 13 | 10 |
| Mean | 4.6 | 5.1 | 5.5 | 6.4 | 14.3 | 8.3 | 12.6 | 9 | |
| Maximum | 7.5 | 8.8 | 7.2 | 8 | 36.7 | 34.5 | 33.5 | 26.8 | |
| Minimum | 1 | 1.2 | 3.4 | 4.1 | 0.3 | 2.6 | 0.23 | 4.1 | |
| Median | 5.3 | 4.5 | 5.2 | 6.2 | 13.5 | 7.5 | 12.4 | 8.1 | |
| SD | 2.2 | 2.3 | 0.9 | 1.2 | 10.8 | 5.7 | 9.4 | 4.6 | |
| p-value | 0.94 | 0.22 | 0.03 | 0.92 | |||||
| Total nitrogen (%) | N | 17 | 10 | 14 | 10 | 17 | 10 | 13 | 10 |
| Mean | 0.07 | 0.10 | 0.09 | 0.15 | 1.5 | 2.8 | 1.5 | 2.6 | |
| Maximum | 0.12 | 0.13 | 0.13 | 0.73 | 2.4 | 3.9 | 2.9 | 3.8 | |
| Minimum | 0.04 | 0.05 | 0.01 | 0.07 | 0.6 | 1.6 | 0.03 | 1.2 | |
| Median | 0.07 | 0.10 | 0.09 | 0.11 | 1.5 | 2.6 | 0.1 | 2.6 | |
| SD | 0.02 | 0.03 | 0.02 | 0.16 | 0.5 | 0.6 | 0.07 | 0.7 | |
| p-value | 0.76 | 0.03 | 0.17 | 0.06 | |||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dlamini, M.; Chirima, G.; Jovanovic, N.; Adam, E. Assessing the Effects of Land Use on Surface Water Quality in the Lower uMfolozi Floodplain System, South Africa. Int. J. Environ. Res. Public Health 2021, 18, 561. https://doi.org/10.3390/ijerph18020561
Dlamini M, Chirima G, Jovanovic N, Adam E. Assessing the Effects of Land Use on Surface Water Quality in the Lower uMfolozi Floodplain System, South Africa. International Journal of Environmental Research and Public Health. 2021; 18(2):561. https://doi.org/10.3390/ijerph18020561
Chicago/Turabian StyleDlamini, Mandla, George Chirima, Nebo Jovanovic, and Elhadi Adam. 2021. "Assessing the Effects of Land Use on Surface Water Quality in the Lower uMfolozi Floodplain System, South Africa" International Journal of Environmental Research and Public Health 18, no. 2: 561. https://doi.org/10.3390/ijerph18020561
APA StyleDlamini, M., Chirima, G., Jovanovic, N., & Adam, E. (2021). Assessing the Effects of Land Use on Surface Water Quality in the Lower uMfolozi Floodplain System, South Africa. International Journal of Environmental Research and Public Health, 18(2), 561. https://doi.org/10.3390/ijerph18020561







