Antimicrobial Efficiency of Aloe arborescens and Aloe barbadensis Natural and Commercial Products
Abstract
:1. Introduction
2. Results
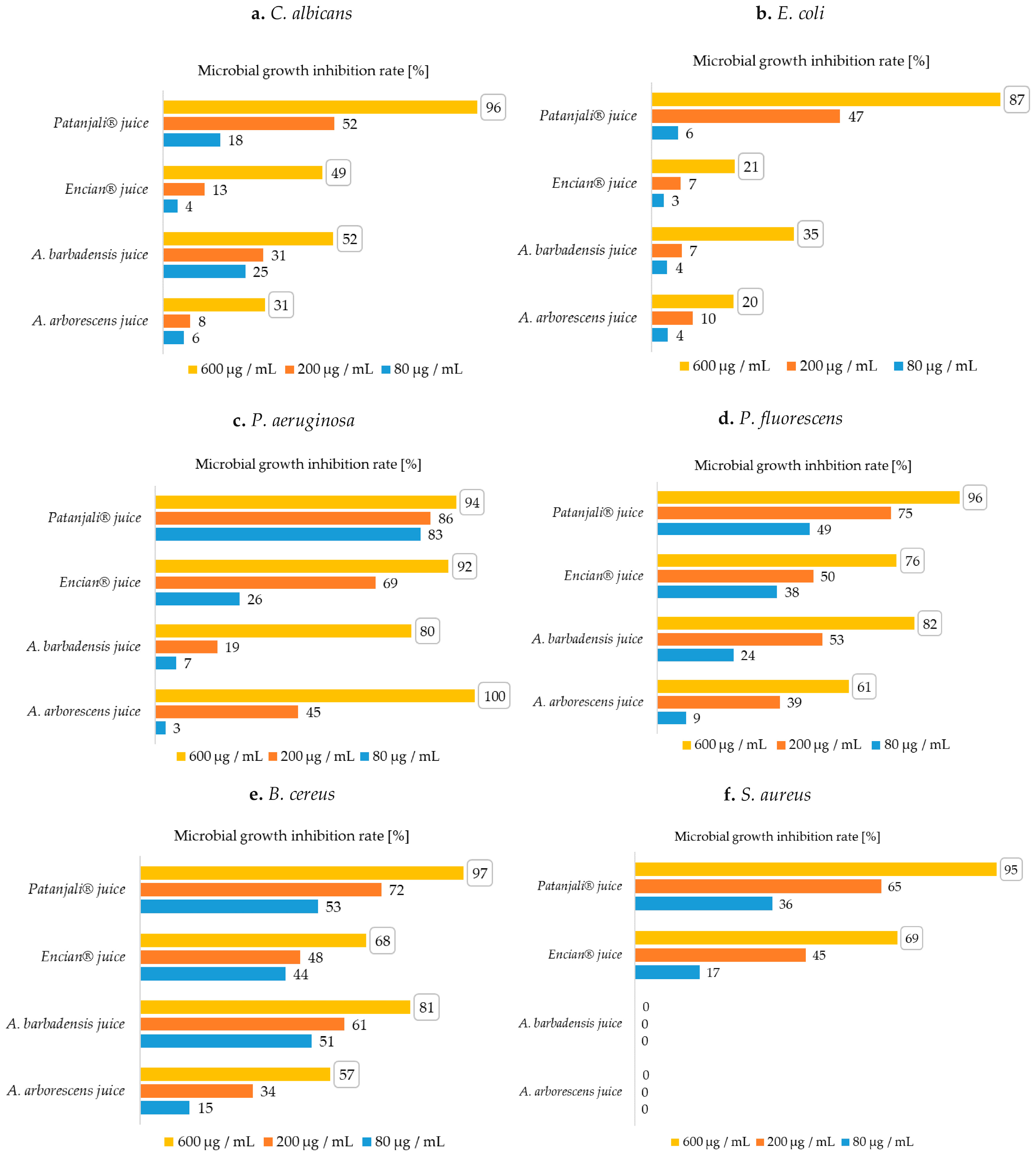
2.1. Antimicrobial Efficacy of Aloe Samples on Fungi
2.2. Antimicrobial Efficacy of Aloe Samples on Gram-Negative Bacteria
2.3. Antimicrobial Efficacy of Aloe Samples on Gram-Positive Bacteria
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Microorganisms
4.3. Plant Material Preparation
4.4. Commercial Products
4.5. General Method for Qualitative Determination of Antimicrobial Activity of Samples
4.6. General Method for Quantitative Determination of the Microbial Growth Inhibition Rate
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Arshad, M.S.; Batool, S.A. Natural Antimicrobials, their Sources and Food Safety. In Food Additives; InTech: London, UK, 2017; pp. 87–102. [Google Scholar] [CrossRef] [Green Version]
- Dolashka, P.; Dolashki, A.; Van Beeumen, J.; Floetenmeyer, M.; Velkova, L.; Stevanovic, S.; Voelter, W. Antimicrobial Activity of Molluscan Hemocyanins from Helix and Rapana Snails. Curr. Pharm. Biotechnol. 2016, 17, 263–270. [Google Scholar] [CrossRef] [PubMed]
- Dolashki, A.; Velkova, L.; Daskalova, E.; Zheleva, N.; Topalova, Y.; Atanasov, V.; Voelter, W.; Dolashka, P. Antimicrobial Activities of Different Fractions from Mucus of the Garden Snail Cornu aspersum. Biomedicines 2020, 8, 315. [Google Scholar] [CrossRef] [PubMed]
- Joseph, B.; Raj, J. Pharmacognostic and Phytochemical Properties of Aloe vera linn—An Overview. Int. J. Pharm. Sci. Rev. Res. 2010, 4, 106–110. [Google Scholar]
- Ravishankar, B.; Shukla, V. Indian Systems of Medicine: A Brief Profile. Afr. J. Tradit. Complement. Altern. Med. 2007, 4, 319–337. [Google Scholar] [CrossRef] [Green Version]
- Reynolds, T. The compounds in Aloë leaf exudates: A review. Bot. J. Linn. Soc. 1985, 90, 157–177. [Google Scholar] [CrossRef]
- Andrea, B.; Dumitrița, R.; Florina, C.; Francisc, D.; Anastasia, V.; Socaci, S.; Adela, P. Comparative analysis of some bioactive compounds in leaves of different Aloe species. BMC Chem. 2020, 14, 67. [Google Scholar] [CrossRef]
- Jia, Y.; Zhao, G.; Jia, J. Preliminary evaluation: The effects of Aloe ferox Miller and Aloe arborescens Miller on wound healing. J. Ethnopharmacol. 2008, 120, 181–189. [Google Scholar] [CrossRef]
- Eshun, K.; He, Q. Aloe Vera: A Valuable Ingredient for the Food, Pharmaceutical and Cosmetic Industries—A Review. Crit. Rev. Food Sci. Nutr. 2004, 44, 91–96. [Google Scholar] [CrossRef]
- Gjerstad, G. Chemical studies of aloe vera juice. Adv. Front. Plant Sci. 1971, 28, 110–112. [Google Scholar]
- Nejatzadeh-Barandozi, F. Antibacterial activities and antioxidant capacity of Aloe vera. Org. Med. Chem. Lett. 2013, 3, 5. [Google Scholar] [CrossRef] [Green Version]
- Kumar, R.; Singh, A.K.; Gupta, A.; Bishayee, A.; Pandey, A.K. Therapeutic potential of Aloe vera-A miracle gift of nature. Phytomedicine 2019, 60, 152996. [Google Scholar] [CrossRef] [PubMed]
- Sahu, P.K.; Giri, D.D.; Singh, R.; Pandey, P.; Gupta, S.; Shrivastava, A.K.; Kumar, A.; Pandey, K.D. Therapeutic and Medicinal Uses of Aloe vera: A Review. Pharmacol. Pharm. 2013, 4, 599–610. [Google Scholar] [CrossRef] [Green Version]
- Grindlay, D.; Reynolds, T. The Aloe vera phenomenon: A review of the properties and modern uses of the leaf parenchyma gel. J. Ethnopharmacol. 1986, 16, 117–151. [Google Scholar] [CrossRef]
- Shelton, R.M. Aloe vera. Its chemical and therapeutic properties. Int. J. Dermatol. 1991, 30, 679–683. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, S.; Sathish Kumar, D.; Arulselvan, P.; Senthilkumar, G.P. In vitro Antibacterial and Antifungal Activities of Ethanolic Extract of Aloe vera Leaf Gel. J. Plant Sci. 2006, 1, 348–355. [Google Scholar] [CrossRef]
- Pandey, R.; Mishra, A. Antibacterial Activities of Crude Extract of Aloe barbadensis to Clinically Isolated Bacterial Pathogens. Appl. Biochem. Biotechnol. 2010, 160, 1356–1361. [Google Scholar] [CrossRef]
- Dagne, E.; Bisrat, D.; Viljoen, A.; Van Wyk, B.E. Chemistry of Aloe Species. Curr. Org. Chem. 2000, 4, 1055–1078. [Google Scholar] [CrossRef]
- Surjushe, A.; Vasani, R.; Saple, D.G. Aloe vera: A short review. Indian J. Dermatol. 2008, 53, 163. [Google Scholar] [CrossRef]
- Hamman, J. Composition and applications of Aloe vera leaf gel. Molecules 2008, 13, 1599–1616. [Google Scholar] [CrossRef] [Green Version]
- Chen, R.; Zhang, J.; Hu, Y.; Wang, S.; Chen, M.; Wang, Y. Potential Antineoplastic Effects of Aloe-emodin: A Comprehensive Review. Am. J. Chin. Med. 2014, 42, 275–288. [Google Scholar] [CrossRef]
- Kumar, S. Evaluating Antimicrobial activity of Aloe vera Plant Extract in Human Life. Biomed. J. Sci. Tech. Res. 2017, 1, 1854–1856. [Google Scholar] [CrossRef] [Green Version]
- Forno-Bell, N.; Bucarey, S.A.; García, D.; Iragüen, D.; Chacón, O.; San Martín, B. Antimicrobial Effects Caused by Aloe barbadensis Miller on Bacteria Associated with Mastitis in Dairy Cattle. Nat. Prod. Commun. 2019, 14. [Google Scholar] [CrossRef] [Green Version]
- Canche-Escamilla, G.; Colli-Acevedo, P.; Borges-Argaez, R.; Quintana-Owen, P.; May-Crespo, J.F.; Cáceres-Farfan, M.; Yam Puc, J.A.; Sansores-Peraza, P.; Vera-Ku, B.M. Extraction of phenolic components from an Aloe vera (Aloe barbadensis Miller) crop and their potential as antimicrobials and textile dyes. Sustain. Chem. Pharm. 2019, 14, 100168. [Google Scholar] [CrossRef]
- Haque, S.D.; Saha, S.K.; Salma, U.; Nishi, M.K.; Rahaman, M.S. Antibacterial Effect of Aloe vera (Aloe barbadensis) leaf gel against Staphylococcus aureus, Pseudomonas aeruginosa, Escherichia coli and Klebsiella pneumoniae. Mymensingh Med. J. 2019, 28, 490–496. [Google Scholar] [CrossRef]
- Ben Moussa, O.; Mahouachi, I.; Dammak, I.; Boulares, M.; Mahmoudi, I.; Hassouna, M. Antimicrobial activities of Aloe barbadensis Miller leaves and their effects on lactic acid bacteria behavior. J. New Sci. 2019, 64, 4008–4016. [Google Scholar]
- Mbajiuka, C.; Obeagu, E.; Eziokwu, O. Antimicrobial effects of Aloe vera on some human pathogens. Int. J. Curr. Microbiol. Appl. Sci. 2014, 3, 1022–1028. [Google Scholar]
- Keikhaie, K.R.; Fazeli-nasab, B.; Jahantigh, H.R.; Hassanshahian, M. Antibacterial Activity of Ethyl Acetate and Methanol Extracts of Securigera securidaca, Withania sominefra, Rosmarinus officinalis and Aloe vera Plants against Important Human Pathogens. J. Med. Bacteriol. 2018, 7, 13–21. [Google Scholar]
- Goudarzi, M.; Fazeli, M.; Azad, M.; Seyedjavadi, S.S.; Mousavi, R. Aloe vera Gel: Effective Therapeutic Agent against Multidrug-Resistant Pseudomonas aeruginosa Isolates Recovered from Burn Wound Infections. Chemother. Res. Pract. 2015, 2015, 639806. [Google Scholar] [CrossRef] [Green Version]
- Alemdar, S.; Agaoglu, S. Investigation of In vitro Antimicrobial Activity of Aloe vera Juice. J. Anim. Vet. Adv. 2009, 8, 99–102. [Google Scholar]
- Cataldi, V.; Di Bartolomeo, S.; Di Campli, E.; Nostro, A.; Cellini, L.; Di Giulio, M. In vitro activity of Aloe vera inner gel against microorganisms grown in planktonic and sessile phases. Int. J. Immunopathol. Pharmacol. 2015, 28, 595–602. [Google Scholar] [CrossRef]
- Cheng, Z.; Su, L.; Moore, J.; Zhou, K.; Luther, M.; Yin, J.-J.; Yu, L. Effects of Postharvest Treatment and Heat Stress on Availability of Wheat Antioxidants. J. Agric. Food Chem. 2006, 54, 5623–5629. [Google Scholar] [CrossRef] [PubMed]
- Kaithwas, G.; Kumar, A.; Pandey, H.; Acharya, A.K.; Singh, M.; Bhatia, D.; Mukerjee, A. Investigation of Comparative Antimicrobial Activity of Aloe vera gel and juice. Pharmacologyonline 2008, 1, 239–243. [Google Scholar]
- Carson, C.F.; Hammer, K.A.; Riley, T.V. Melaleuca alternifolia (Tea Tree) Oil: A Review of Antimicrobial and Other Medicinal Properties. Clin. Microbiol. Rev. 2006, 19, 50–62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petrović, Z.; Čomić, L.; Stefanovic, O.; Simijonović, D.; Petrović, V. Antimicrobial activity of the ionic liquids triethanolamine acetate and diethanolamine chloride, and their corresponding Pd(II) complexes. J. Mol. Liq. 2012, 170, 61–65. [Google Scholar] [CrossRef]
- Andermann, G. Therapeutic Use of DMDM Hydantoin. U.S. Patent 43621689-A, 29 July 1991. [Google Scholar]
- Llabres, C.M.; Ahearn, D.G. Antimicrobial activities of N-chloramines and diazolidinyl urea. Appl. Environ. Microbiol. 1985, 49, 370–373. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Finnegan, S.; Percival, S.L. EDTA: An Antimicrobial and Antibiofilm Agent for Use in Wound Care. Adv. Wound Care 2015, 4, 415–421. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bidossi, A.; Bortolin, M.; Toscano, M.; De Vecchi, E.; Romanò, C.L.; Mattina, R.; Drago, L. In vitro comparison between α-tocopheryl acetate and α-tocopheryl phosphate against bacteria responsible of prosthetic and joint infections. PLoS ONE 2017, 12, e0182323. [Google Scholar] [CrossRef] [Green Version]
- Puschmann, J.; Herbig, M.E.; Müller-Goymann, C.C. Correlation of antimicrobial effects of phenoxyethanol with its free concentration in the water phase of o/w-emulsion gels. Eur. J. Pharm. Biopharm. 2018, 131, 152–161. [Google Scholar] [CrossRef]
- Shen, X.L.; Wu, J.M.; Chen, Y.; Zhao, G. Antimicrobial and physical properties of sweet potato starch films incorporated with potassium sorbate or chitosan. Food Hydrocoll. 2010, 24, 285–290. [Google Scholar] [CrossRef]
- Herman, A.; Tambor, K.; Herman, A. Linalool Affects the Antimicrobial Efficacy of Essential Oils. Curr. Microbiol. 2016, 72, 165–172. [Google Scholar] [CrossRef]
- Bevilacqua, A.; Corbo, M.R.; Sinigaglia, M. In Vitro Evaluation of the Antimicrobial Activity of Eugenol, Limonene, and Citrus Extract against Bacteria and Yeasts, Representative of the Spoiling Microflora of Fruit Juices. J. Food Prot. 2010, 73, 888–894. [Google Scholar] [CrossRef] [PubMed]
- Georgopoulou, M.; Kontakiotis, E.; Nakou, M. Evaluation of the antimicrobial effectiveness of citric acid and sodium hypochlorite on the anaerobic flora of the infected root canal. Int. Endod. J. 1994, 27, 139–143. [Google Scholar] [CrossRef] [PubMed]
- Mousavi, S.; Bereswill, S.; Heimesaat, M.M. Immunomodulatory and Antimicrobial Effects of Vitamin C. Eur. J. Microbiol. Immunol. (Bp) 2019, 9, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Ostergaard, E. Evaluation of the antimicrobial effects of sodium benzoate and dichlorobenzyl alcohol against dental plaque microorganisms. An in vitro study. Acta Odontol. Scand. 1994, 52, 335–345. [Google Scholar] [CrossRef] [PubMed]
- Williamson, E.M. Synergy and other interactions in phytomedicines. Phytomedicine 2001, 8, 401–409. [Google Scholar] [CrossRef] [PubMed]
- Bauer, A.W.; Kirby, W.M.; Sherris, J.C.; Turck, M. Antibiotic susceptibility testing by a standardized single disk method. Am. J. Clin. Pathol. 1966, 45, 493–496. [Google Scholar] [CrossRef] [PubMed]
- Wiegand, I.; Hilpert, K.; Hancock, R.E.W. Agar and broth dilution methods to determine the minimal inhibitory concentration (MIC) of antimicrobial substances. Nat. Protoc. 2008, 3, 163–175. [Google Scholar] [CrossRef] [PubMed]
- Granum, P.E.; Lindbäck, T. Bacillus cereus. In Food Microbiology; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2014; pp. 491–502. ISBN 978-1-68367-058-2. [Google Scholar]
- Mayer, F.L.; Wilson, D.; Hube, B. Candida albicans pathogenicity mechanisms. Virulence 2013, 4, 119–128. [Google Scholar] [CrossRef] [Green Version]
- Bassetti, M.; Vena, A.; Croxatto, A.; Righi, E.; Guery, B. How to manage Pseudomonas aeruginosa infections. Drugs Context 2018, 7, 212527. [Google Scholar] [CrossRef]

| Fungi | Diameter of the Inhibition Zone [mm] | |
|---|---|---|
| ESI® Gel | Fruit of the Earth® Gel | |
| C. albicans | 17 ± 2 15 ± 1 | 18 ± 2 10 ± 1 |
| Gram-Negative Bacteria | Diameter of the Inhibition Zone (mm) | |
|---|---|---|
| ESI® Gel | Fruit of the Earth® Gel | |
| E. coli | 14 ± 2 | 32 ± 2 |
| - | 30 ± 2 | |
| P. aeruginosa | 35 ± 3 | 31 ± 2 |
| 30 ± 2 | 24 ± 2 | |
| P. fluorescens | 20 ± 2 | 45 ± 3 |
| 14 ± 1 | 32 ± 2 | |
| Gram-Positive Bacteria | Diameter of the Inhibition Zone [mm] | |
|---|---|---|
| ESI® Gel | Fruit of the Earth® Gel | |
| B. cereus | 20 ± 2 | 33 ± 3 |
| 17 ± 1 | 23 ± 3 | |
| S. aureus | - | 25 ± 2 |
| - | 21 ± 2 | |
| Microorganism | Optimal Temperature | Initial Concentrations |
|---|---|---|
| C. albicans | 25 °C | 105 CFU/mL 107 CFU/mL |
| E. coli | 37 °C | 106 CFU/mL 107 CFU/mL |
| P. aeruginosa | 37 °C | 106 CFU/mL 108 CFU/ml |
| P. fluorescens | 30 °C | 106 CFU/mL 107 CFU/mL |
| B. cereus | 30 °C | 106 CFU/mL 107 CFU/mL |
| S. aureus | 37 °C | 105 CFU/mL 108 CFU/mL |
| Microorganism | Optimal Temperature | Initial Concentrations |
|---|---|---|
| C. albicans | 25 °C | 106 CFU/mL |
| E. coli | 37 °C | 107 CFU/mL |
| P. aeruginosa | 37 °C | 107 CFU/mL |
| P. fluorescens | 30 °C | 107 CFU/mL |
| B. cereus | 30 °C | 107 CFU/mL |
| S. aureus | 37 °C | 105 CFU/mL |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kupnik, K.; Primožič, M.; Knez, Ž.; Leitgeb, M. Antimicrobial Efficiency of Aloe arborescens and Aloe barbadensis Natural and Commercial Products. Plants 2021, 10, 92. https://doi.org/10.3390/plants10010092
Kupnik K, Primožič M, Knez Ž, Leitgeb M. Antimicrobial Efficiency of Aloe arborescens and Aloe barbadensis Natural and Commercial Products. Plants. 2021; 10(1):92. https://doi.org/10.3390/plants10010092
Chicago/Turabian StyleKupnik, Kaja, Mateja Primožič, Željko Knez, and Maja Leitgeb. 2021. "Antimicrobial Efficiency of Aloe arborescens and Aloe barbadensis Natural and Commercial Products" Plants 10, no. 1: 92. https://doi.org/10.3390/plants10010092
APA StyleKupnik, K., Primožič, M., Knez, Ž., & Leitgeb, M. (2021). Antimicrobial Efficiency of Aloe arborescens and Aloe barbadensis Natural and Commercial Products. Plants, 10(1), 92. https://doi.org/10.3390/plants10010092






