Response of Morphological Characters and Photosynthetic Characteristics of Haloxylon ammodendron to Water and Salt Stress
Abstract
:1. Introduction
2. Materials and Methods
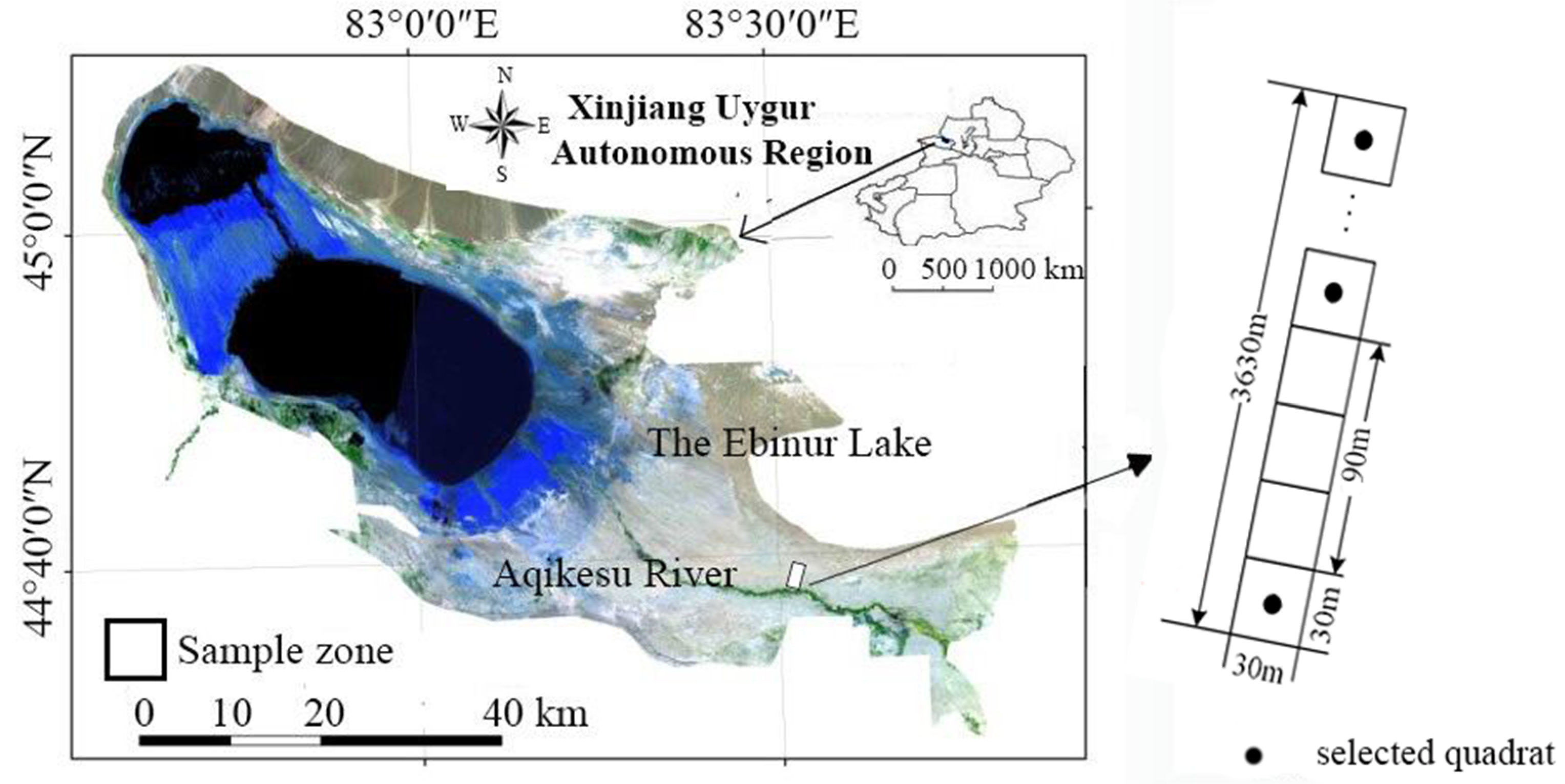
2.1. Study site and Experimental Design
2.2. Collection and Determination of Soil Samples
2.3. Plant Growth Determination
2.4. Photosynthetic Measurement
2.5. Statistical Analysis
3. Results
3.1. Characteristics of Soil Environmental Factors
3.2. Morphological Characters
3.3. Photosynthetic Physiology
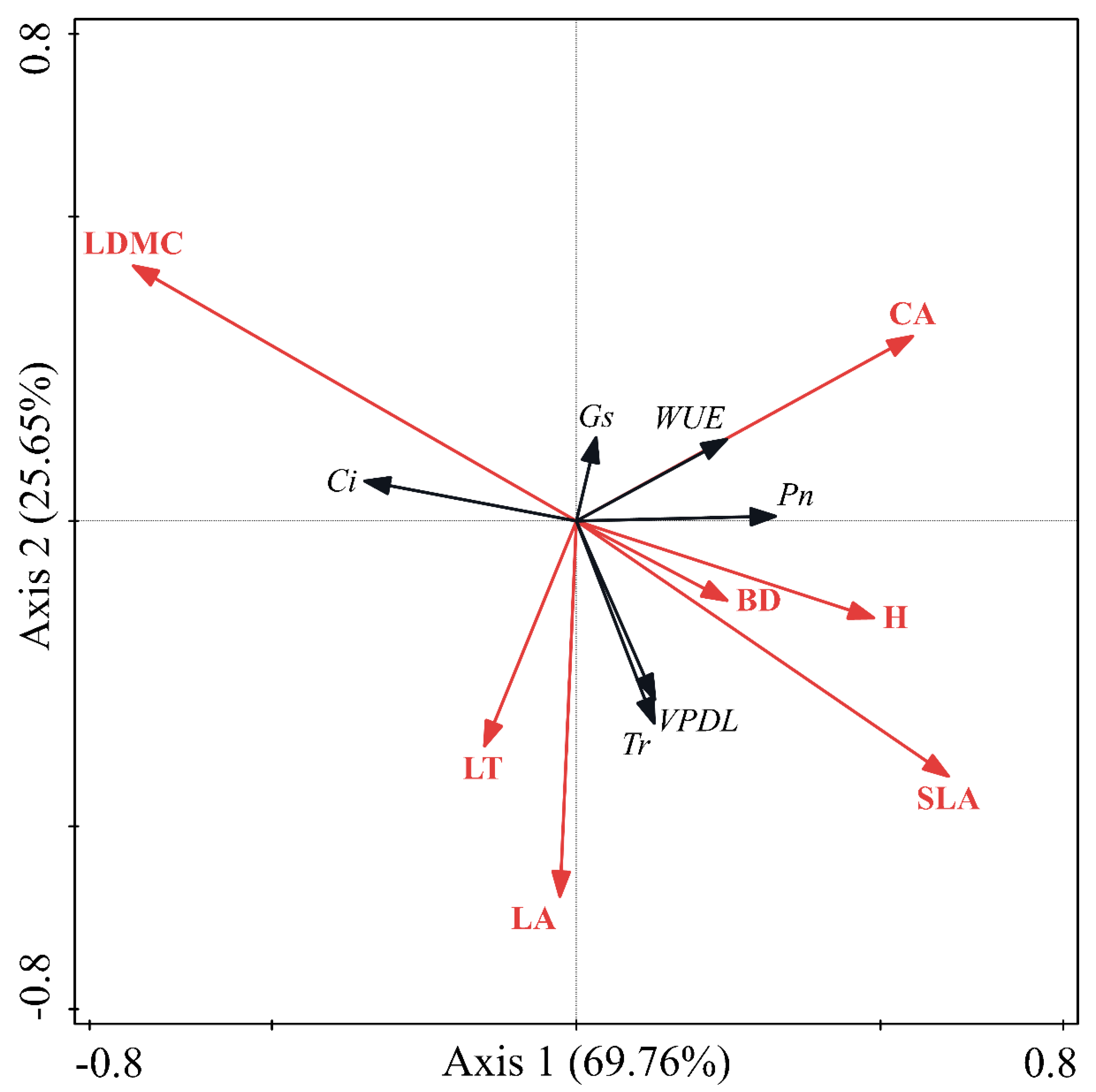
3.4. Relationship between Morphological Characters and Photosynthetic Physiology
4. Discussion
5. Conclusions
- (1)
- The growth of Haloxylon ammodendron is subject to water and salt stress. With the decrease of soil water and salt content, the plant height, base diameter, crown area and specific leaf area of Haloxylon ammodendron all showed downward trends to varying degrees, while the dry matter content of leaves gradually increased.
- (2)
- Soil water and salt content can affect the photosynthesis of Haloxylon ammodendron, and the factors limiting the photosynthetic ability are different under different gradients. The photosynthetic rate of Haloxylon ammodendron in gradient I was much higher than that in other gradients; the photosynthetic rate in gradient I was mainly affected by “stomatal limitation,” while the rates in gradients II, III, and IV were mainly affected by “non-stomatal limitation.”
- (3)
- In arid areas, Haloxylon ammodendron has its own special survival strategy and leaf construction mode. When the soil conditions are good, to cope with the light competition phenomenon caused by the density of plants, Haloxylon ammodendron displays a leaf construction mode with high specific leaf area. This morphology mitigates insufficient light energy absorption of plants in shaded environments and maximizes the photosynthetic income. When the soil conditions worsen, Haloxylon ammodendron chooses the leaf construction mode with low specific leaf area, thereby realizing the optimal distribution of carbon assimilation products and the dissipation of solar energy by the leaves.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lawlor, D.W.; Cornic, G. Photosynthetic carbon assimilation and associated metabolism in relation to water deficits in higher plants. Plant. Cell Environ. 2002, 25, 275–294. [Google Scholar] [CrossRef] [Green Version]
- Bartels, D.; Sunkar, R. Drought and Salt Tolerance in Plants. Crit. Rev. Plant. Sci. 2005, 24, 23–58. [Google Scholar] [CrossRef]
- Chen, T.; Wang, G.M.; Shen, W.W.; Li, X.Z.; Qi, J.M.; Xu, J.T.; Tao, A.F.; Liu, X.Q. Effect of salt stress on the growth and antioxidant enzyme activity of kenaf seedlings. Plant. Sci. J. 2011, 29, 493–501. [Google Scholar] [CrossRef]
- Marcum, K.B.; Anderson, S.J.; Engelke, M.C. Salt Gland Ion Secretion: A Salinity Tolerance Mechanism among Five Zoysiagrass Species. Crop. Sci. 1998, 38, 806–810. [Google Scholar] [CrossRef]
- Muchate, N.S.; Nikalje, G.C.; Rajurkar, N.S.; Suprasanna, P.; Nikam, T.D. Plant Salt Stress: Adaptive Responses, Tolerance Mechanism and Bioengineering for Salt Tolerance. Bot. Rev. 2016, 82, 371–406. [Google Scholar] [CrossRef]
- Li, Q.; Zhao, C.Z.; Zhao, L.C.; Wang, J.W.; Wen, J. The correlation analysis between specific leaf area and photosynthetic efficiency of Phragmites australis in salt marshes of Qinwangchuan. Acta Ecol. Sin. 2019, 39, 7124–7133. [Google Scholar] [CrossRef]
- Zhou, H.; Zhao, W.; Zhang, G. Varying water utilization of Haloxylon ammodendron plantations in a desert-oasis ecotone. Hydrol. Process. 2016, 31, 825–835. [Google Scholar] [CrossRef]
- Hu, R.J.; Fan, Z.L.; Wang, Y.J.; Yang, Q.; Huang, Y.Y. Assessment about the impact of climate change on environment in Xinjiang since recent 50 years. Arid Land Geogr. 2001, 24, 97–103. [Google Scholar] [CrossRef]
- Xin-Gang, D.; Ping, W.; Kai-Jing, Z. A study on precipitation trend and fluctuation mechanism in northwestern China over the past 60 years. Acta Phys. Sin. 2013, 62, 129201. [Google Scholar] [CrossRef]
- Zhao, X.; Tan, K.; Fang, J.Y. NVDI-based interannual and seasonal variations of vegetation activity in Xinjiang during the period of 1982–2006. Arid Zone Res. 2011, 28, 10–16. [Google Scholar] [CrossRef]
- Yan, H. The Response of Two Representative Desert Shrubs to Salt Stress in Northwest arid Region. Ph.D. Thesis, Northwest A &F University, Yangling, China, 2012. [Google Scholar]
- Zhang, J.G.; Lei, J.Q.; Wang, Y.D.; ZhaoiD, Y.; Xu, X.W. Survival and growth of three afforestation species under high saline drip irrigation in the Taklimakan Desert, China. Ecosphere 2016, 7, 01285. [Google Scholar] [CrossRef]
- Yang, G.; Liu, S.; Yan, K.; Tian, L.; Li, P.; Li, X.; He, X. Effect of Drip Irrigation with Brackish Water on the Soil Chemical Properties for a Typical Desert Plant (Haloxylon ammodendron) in the Manas River Basin. Irrig. Drain. 2020, 69, 460–471. [Google Scholar] [CrossRef]
- Qin, L.; Zhang, W.; Yang, L.H.; Zulikeerjiang, A.; Aziguli, A.; Wu, D.Y.; Yi, Y.Y. Effect of dust precipitation on photosyn-thetic characteristics of Haloxylon ammodendron in Zhundong mine area. Environ. Dev. 2020, 32, 203–205, 209. [Google Scholar] [CrossRef]
- Li, Y.J.; Zhao, N.; Cao, Y.E.; Yang, J.J. Effects of coal dust deposition on the physiological properties of plants in an open-pit coal mine. Acta Ecol. Sin. 2018, 38, 8129–8138. [Google Scholar] [CrossRef]
- Wang, H.; Cai, Y.; Yang, Q.; Gong, Y.; Lv, G. Factors that alter the relative importance of abiotic and biotic drivers on the fertile island in a desert-oasis ecotone. Sci. Total. Environ. 2019, 697, 134096. [Google Scholar] [CrossRef]
- Gong, Y.; Ling, H.; Lv, G.; Chen, Y.; Guo, Z.; Cao, J. Disentangling the influence of aridity and salinity on community functional and phylogenetic diversity in local dryland vegetation. Sci. Total. Environ. 2019, 653, 409–422. [Google Scholar] [CrossRef]
- Ye, Z.P.; Yu, Q. A coupled model of stomatal conductance and photosynthesis for winter wheat. Photosynthetica 2008, 46, 637–640. [Google Scholar] [CrossRef]
- Liu, C.J.; Guo, X.; Wang, K.L.; Liu, Q.C.; Sun, Y.K.; Jiang, X.Q.; Liu, Q.H. Ecophysiological responses of Camellia japonica (Naidong) to different light and water conditions. Chin. J. Appl. Ecol. 2018, 29, 1125–1132. [Google Scholar] [CrossRef]
- Wu, J.; Li, J.; Su, Y.; He, Q.; Wang, J.; Qiu, Q.; Ma, J. A morphophysiological analysis of the effects of drought and shade on Catalpa bungei plantlets. Acta Physiol. Plant. 2017, 39, 80. [Google Scholar] [CrossRef]
- Xiao, Y.M. Effects of Phosphorus Nutrition on Growth and Photosynthetic Characteristics of Jerusalem artichoke at Different Growth Stages. Master’s Thesis, Lanzhou University, Lanzhou, China, 2018. [Google Scholar]
- Zhang, C.Y.; Fang, Y.M.; Ji, H.L.; Ma, C.T. Effects of shading on photosynthesis characteristics of Photinia ×frasery and Aucuba japonica var. variegata. Chin. J. Appl. Ecol. 2011, 22, 1743–1749. [Google Scholar] [CrossRef]
- Zhang, X.-R.; Tan, X.-F.; Wang, R.-Q.; Xu, N.-N.; Guo, W.-H. Effects of soil moisture and light intensity on ecophysiological characteristics of Amorpha fruticosa seedlings. J. For. Res. 2013, 24, 293–300. [Google Scholar] [CrossRef]
- Grime, J.P.; Thompson, K.; Hunt, R.; Hodgson, J.; Cornelissen, J.H.C.; Rorison, I.H.; Hendry, G.; Ashenden, T.W.; Askew, A.P.; Band, S.R.; et al. Integrated Screening Validates Primary Axes of Specialisation in Plants. Oikos 1997, 79, 259. [Google Scholar] [CrossRef]
- Wilson, P.J.; Thompson, K.; Hodgson, J.G. Specific leaf area and leaf dry matter content as alternative predictors of plant strategies. New Phytol. 1999, 143, 155–162. [Google Scholar] [CrossRef]
- Li, Y.L.; Cui, J.H.; Su, Y.Z. Specific leaf area and leaf dry matter content of some plants in different dune habitats. Acta Ecol. Sin. 2005, 25, 304–311. [Google Scholar] [CrossRef]
- Du, N.; Wang, R.; Liu, J.; Zhang, X.; Tan, X.; Wang, W.; Chen, H.; Guo, W. Morphological response of Vitex negundo var. heterophylla and Ziziphus jujuba var. spinosa to the combined impact of drought and shade. Agrofor. Syst. 2012, 87, 403–416. [Google Scholar] [CrossRef]
- Wang, F.; Chen, Y.Z.; Wang, X.P.; You, Z.M.; Chen, C.S. Comparison of leaf functional and photosynthetic characteristics in different tea cultivars. J. Tea Sci. 2016, 36, 285–292. [Google Scholar] [CrossRef]
- Anacker, B.L.; Rajakaruna, N.; Ackerly, D.D.; Harrison, S.; E Keeley, J.; Vasey, M.C. Ecological strategies in California chaparral: Interacting effects of soils, climate, and fire on specific leaf area. Plant. Ecol. Divers. 2011, 4, 179–188. [Google Scholar] [CrossRef]
- Zhang, L.; Luo, T.X. Advances in ecological studies on leaf lifespan and associated leaf traits. Acta Phytoecol. Sin. 2004, 28, 844–852. [Google Scholar]
- Chen, G.X.; Xia, X.; Lv, S.C.; Zhang, X.T.; Gong, Z.P. Effects of phosphorus nutrition on photosynthesis and yield of soy-bean by sanding method. Soybean Sci. 2017, 36, 575–582. [Google Scholar]
- León-Sánchez, L.; Nicolás, E.; Nortes, P.A.; Maestre, F.T.; Querejeta, J.I. Photosynthesis and growth reduction with warming are driven by nonstomatal limitations in a Mediterranean semi-arid shrub. Ecol. Evol. 2016, 6, 2725–2738. [Google Scholar] [CrossRef] [Green Version]
- Salmon, Y.; Lintunen, A.; Dayet, A.; Chan, T.; Dewar, R.C.; Vesala, T.; Hölttä, T. Leaf carbon and water status control stomatal and nonstomatal limitations of photosynthesis in trees. New Phytol. 2020, 226, 690–703. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Xia, J.B.; Zhou, D.X.; Zhao, Z.G.; Dong, L.S. Photosynthesis characteristics of Periploca sepium under drought stress in shell-sand habitat in the Yellow River Delta. J. Desert Res. 2019, 39, 139–148. [Google Scholar] [CrossRef]
- Brugnoli, E.; Lauteri, M. Effects of Salinity on Stomatal Conductance, Photosynthetic Capacity, and Carbon Isotope Discrimination of Salt-Tolerant (Gossypium hirsutum L.) and Salt-Sensitive (Phaseolus vulgaris L.) C3 Non-Halophytes. Plant. Physiol. 1991, 95, 628–635. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, R.J.; Chen, X.Z.; Yue, C.L.; Li, H.P.; Wang, J.; Guo, L.; Yang, L. Effects of drought stress on the photosynthetic characteristics of Viburnum japonicum seedlings. Acta Ecol. Sin. 2018, 38, 2041–2047. [Google Scholar] [CrossRef]
- Jiang-Bao, X. Critical responses of photosynthetic efficiency in Campsis radicans (L.) Seem to soil water and light intensities. Afr. J. Biotechnol. 2011, 10, 17748–17754. [Google Scholar] [CrossRef]
- Wan, L.; Xing, Z.; Chang, X.; Liu, J.; Zhang, G. Research on Light Response Curve Fitting Model of Four Chamaenerion Plants on the Serzilla Mountains. Am. J. Plant. Sci. 2018, 9, 1630–1645. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Zeng, R.; Liao, H. Improving crop nutrient efficiency through root architecture modifications. J. Integr. Plant. Biol. 2015, 58, 193–202. [Google Scholar] [CrossRef] [Green Version]
- Li, M.; Xu, J.; Wang, X.; Fu, H.; Zhao, M.; Wang, H.; Shi, L. Photosynthetic characteristics and metabolic analyses of two soybean genotypes revealed adaptive strategies to low-nitrogen stress. J. Plant. Physiol. 2018, 229, 132–141. [Google Scholar] [CrossRef]
- Yuan, J.C.; Zhao, D.Y.; Xu, K.; Yan, S.; Chen, C.G. Effects of different phosphorus levels on photosynthesis and chlorophyll fluorescence characteristics of Fuji apple. South China Fruits 2017, 46, 112–114. [Google Scholar]

| Soil Factors | Method |
|---|---|
| Soil water content | Drying method |
| Soil pH | Electrode potential method |
| Soil salt content | Residue from an oven drying method |
| Soil organic matter | Potassium dichromate volumetric method-external heating method |
| Soil total phosphorus | Molybdenum-antimony spectrophotometry method |
| Soil total nitrogen | Kjeldahl nitrogen determination method |
| Soil Environmental Factors, Mean (SE) | |||||||
|---|---|---|---|---|---|---|---|
| Clustering Result | Sample Number | WC (%) | SC (g/kg) | pH | SOM (g/kg) | TN (g/kg) | TP (g/kg) |
| I | 1–8,10,22 | 18.160a (2.095) | 9.574a (1.996) | 8.519a (0.162) | 8.933a (3.362) | 1.504a (0.431) | 0.636a (0.047) |
| II | 9,11,13–15,17,18,23,25,27,30 | 10.324b (1.490) | 6.615b (1.028) | 8.177b (0.128) | 2.685b (0.807) | 0.547b (0.141) | 0.506b (0.046) |
| III | 12,16,20,21,24,26,28,29,31,32,34,37 | 8.840b (1.800) | 4.580c (0.267) | 8.100b (0.116) | 1.757c (0.389) | 0.390c (0.055) | 0.475c (0.034) |
| IV | 19,33,35,36,38–62 | 3.551c (1.563) | 2.551d (0.756) | 7.784c (0.138) | 1.135d (0.348) | 0.223d (0.053) | 0.382d (0.027) |
| Water and Salt Gradient | BD (cm2) | H (m) | CA (m2) |
|---|---|---|---|
| I | 22.730 ± 2.709a | 4.100 ± 0.278a | 33.860 ± 4.958a |
| II | 18.710 ± 2.701ab | 3.270 ± 0.194b | 20.870 ± 5.201b |
| III | 12.930 ± 0.973b | 2.830 ± 0.160bc | 11.010 ± 1.974c |
| IV | 10.210 ± 0.466b | 2.520 ± 0.094c | 8.730 ± 0.750c |
| Water and Salt Gradient | LT (mm) | LA (cm2) | LDMC (%) | SLA (cm2/g) |
|---|---|---|---|---|
| I | 1.049 ± 0.029a | 3.186 ± 0.132a | 28.107 ± 0.559a | 111.113 ± 2.892a |
| II | 1.015 ± 0.048a | 3.063 ± 0.371a | 28.576 ± 0.547a | 100.940 ± 3.606ab |
| III | 1.078 ± 0.046a | 3.575 ± 0.414a | 29.400 ± 0.770a | 92.566 ± 2.857b |
| IV | 1.057 ± 0.030a | 3.187 ± 0.188a | 36.434 ± 1.377b | 76.396 ± 3.488c |
| Gradient | Pn max (μmol·m−2·s−1) | AQY (mol·mol−1) | LCP (μmol·m−2·s−1) | LSP (μmol·m−2·s−1) | Rd (μmol·m−2·s−1) |
|---|---|---|---|---|---|
| I | 20.919 ± 1.058a | 0.039 ± 0.0020a | 244.943 ± 37.923a | 1981.105 ± 128.396ac | 8.480 ± 1.351a |
| II | 15.824 ± 1.046b | 0.036 ± 0.002a | 473.184 ± 43.066b | 2259.406 ± 134.515ab | 13.523 ± 1.230b |
| III | 16.430 ± 0.886b | 0.038 ± 0.001a | 379.480 ± 42.779b | 1844.776 ± 61.077c | 11.751 ± 1.176b |
| IV | 16.390 ± 0.688b | 0.038 ± 0.001a | 281.991 ± 23.226a | 1920.300 ± 56.869c | 8.753 ± 0.616a |
| Item | Axis1 | Axis2 | |
|---|---|---|---|
| Eigenvalues | 0.074 | 0.027 | |
| Morphology-photosynthetic correlations | 0.328 | 0.354 | |
| Cumulative percentage variance | Morphological data | 7.4 | 10.2 |
| Morphology-photosynthetic relationship | 69.8 | 95.4 | |
| Sum of all eigenvalues | 1 | ||
| Sum of all canonical eigenvalues | 0.11 | ||
| Item | Axis 1 | Axis 2 | Explained (%) | P |
|---|---|---|---|---|
| LDMC | −0.2392 | −0.1482 | 4.4 | 0.07 |
| SLA | 0.2008 | 0.1484 | 3.3 | 0.134 |
| CA | 0.1815 | 0.1074 | 2.5 | 0.212 |
| H | 0.1606 | −0.0564 | 1.9 | 0.288 |
| LA | −0.0088 | −0.2178 | 1.1 | 0.5 |
| LT | −0.0495 | −0.1307 | 0.7 | 0.648 |
| BD | 0.0813 | −0.0461 | 0.5 | 0.752 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, D.; Lv, G.; Qie, Y.; Wang, H.; Yang, F.; Jiang, L. Response of Morphological Characters and Photosynthetic Characteristics of Haloxylon ammodendron to Water and Salt Stress. Sustainability 2021, 13, 388. https://doi.org/10.3390/su13010388
Hu D, Lv G, Qie Y, Wang H, Yang F, Jiang L. Response of Morphological Characters and Photosynthetic Characteristics of Haloxylon ammodendron to Water and Salt Stress. Sustainability. 2021; 13(1):388. https://doi.org/10.3390/su13010388
Chicago/Turabian StyleHu, Dong, Guanghui Lv, Yadong Qie, Hengfang Wang, Fang Yang, and Lamei Jiang. 2021. "Response of Morphological Characters and Photosynthetic Characteristics of Haloxylon ammodendron to Water and Salt Stress" Sustainability 13, no. 1: 388. https://doi.org/10.3390/su13010388
APA StyleHu, D., Lv, G., Qie, Y., Wang, H., Yang, F., & Jiang, L. (2021). Response of Morphological Characters and Photosynthetic Characteristics of Haloxylon ammodendron to Water and Salt Stress. Sustainability, 13(1), 388. https://doi.org/10.3390/su13010388





