Bayesian Spatial Survival Analysis of Duration to Cure among New Smear-Positive Pulmonary Tuberculosis (PTB) Patients in Iran, during 2011–2018
Abstract
1. Introduction
2. Material and Methods
2.1. Design
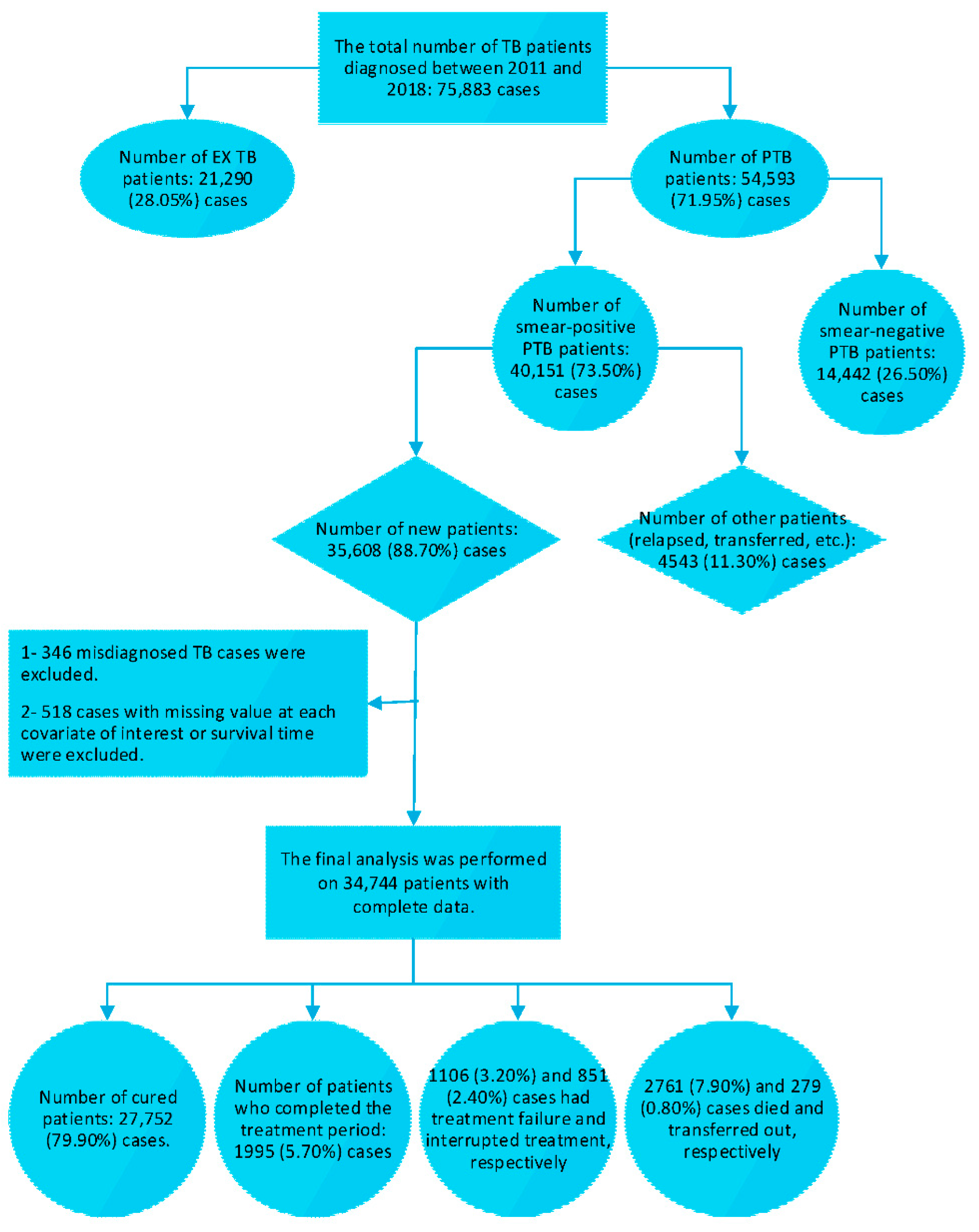
2.2. Study Participant and Procedure
2.3. Survival Time Description
2.4. Statistical analysis
- (a)
- is added to the survival model as non-spatial frailty (spatially uncorrelated random effects), and it follows an independent normal distribution.
- (b)
- We consider a conditional autoregressive (CAR) model for spatial random effects, which was proposed by Besag et al. [44] as follows:
- (c)
- We also considered the convolution prior model for that was first introduced by Besag et al. [44]. This model incorporates both spatially correlated and uncorrelated random effects in order to consider the spatial correlation between clusters and the correlation of observations within clusters. A purely spatial structure of random effects can be considered as follows:
2.5. Bayesian Inference
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Ethics Statement
References
- Pablos-Méndez, A.; Knirsch, C.A.; Barr, R.G.; Lerner, B.H.; Frieden, T.R. Nonadherence in tuberculosis treatment: Predictors and consequences in New York City. Am. J. Med. 1997, 102, 164–170. [Google Scholar] [CrossRef]
- Bradford, W.Z.; Martin, J.N.; Reingold, A.L.; Schecter, G.F.; Hopewell, P.C.; Small, P.M. The changing epidemiology of acquired drug-resistant tuberculosis in San Francisco, USA. Lancet 1996, 348, 928–931. [Google Scholar] [CrossRef]
- Cegielski, J.P.; Dalton, T.; Yagui, M.; Wattanaamornkiet, W.; Volchenkov, G.V.; Via, L.E.; Van Der Walt, M.; Tupasi, T.; Smith, S.E.; Odendaal, R.; et al. Extensive Drug Resistance Acquired During Treatment of Multidrug-Resistant Tuberculosis. Clin. Infect. Dis. 2014, 59, 1049–1063. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Global Tuberculosis Report 2017; Report No.: 9241565055; World Health Organization: Geneva, Switzerland, 2018. [Google Scholar]
- Jensen, P.A.; Lambert, L.A.; Iademarco, M.F.; Ridzon, R. Guidelines for preventing the transmission of Mycobacterium tuberculosis in health-care settings, 2005. MMWR Recomm Rep. 2005, 54, 1–141. [Google Scholar] [PubMed]
- Agarwal, S.; Chauhan, L. Tuberculosis Control in India: Directorate General of Health Services; Ministry of Health and Family Welfare, Government of India: New Delhi, India, 2005.
- Telzak, E.E.; Fazal, B.A.; Pollard, C.L.; Turett, G.S.; Justman, J.E.; Blum, S. Factors Influencing Time to Sputum Conversion Among Patients with Smear-Positive Pulmonary Tuberculosis. Clin. Infect. Dis. 1997, 25, 666–670. [Google Scholar] [CrossRef] [PubMed]
- Nasehi, M.; Mirhaghani, L. National Guidelines for TB Control, 1st ed.; Iran Ministry of Health and Medical Education: Tehran, Iran, 2009; pp. 59–64.
- WHO. Treatment of Tuberculosis: Guidelines; World Health Organization: Geneva, Switzerland, 2010. [Google Scholar]
- WHO. Guidelines for the Programmatic Management of Drug-Resistant Tuberculosis: Emergency Update 2008; World Health Organization: Geneva, Switzerland, 2008. [Google Scholar]
- WHO. World Health Statistics; World Health Organization: Geneva, Switzerland, 2015. [Google Scholar]
- Uplekar, M.; Weil, D.; Lonnroth, K.; Jaramillo, E.; Lienhardt, C.; Dias, H.M.; Falzon, D.; Floyd, K.; Gargioni, G.; Getahun, H.; et al. WHO′s new end TB strategy. Lancet 2015, 385, 1799–1801. [Google Scholar] [CrossRef]
- Castro, K.G.; Nicol, M.P. Tuberculosis eradication: Renewed commitment and global investment required. Lancet Infect. Dis. 2018, 18, 228–229. [Google Scholar]
- Annabel, B.; Anna, D.; Hannah, M. Global Tuberculosis Report 2019; World Health Organization: Geneva, Switzerland, 2019. [Google Scholar]
- Tavakoli, A. Incidence and Prevalence of Tuberculosis in Iran and Neighboring Countries. Zahedan J. Res. Med. Sci. 2017, 19, 19. [Google Scholar] [CrossRef]
- Yassin, M.A.; Datiko, D.G.; Shargie, E.B. Ten-year experiences of the tuberculosis control programme in the southern region of Ethiopia. Int. J. Tuberc. Lung Dis. 2006, 10, 1166–1171. [Google Scholar]
- WHO. What is DOTS?: A Guide to Understanding the WHO-Recommended TB Control Strategy Known as DOTS; World Health Organization: Geneva, Switzerland, 1999. [Google Scholar]
- Falzon, D.; Schünemann, H.J.; Harausz, E.; González-Angulo, L.; Lienhardt, C.; Jaramillo, E.; Weyer, K. World Health Organization treatment guidelines for drug-resistant tuberculosis, 2016 update. Eur. Respir. J. 2017, 49, 1602308. [Google Scholar] [CrossRef]
- WHO. WHO Consolidated Guidelines on Tuberculosis: Tuberculosis Preventive Treatment: Module 1: Prevention: Tuberculosis Preventive Treatment; World Health Organization: Geneva, Switzerland, 2020. [Google Scholar]
- Atif, M.; Sulaiman, S.A.S.; Shafie, A.A.; Babar, Z.U. Duration of treatment in pulmonary tuberculosis: Are international guidelines on the management of tuberculosis missing something? Public Health 2015, 129, 777–782. [Google Scholar] [CrossRef] [PubMed]
- Lienhardt, C.; Raviglione, M.C.; Spigelman, M.; Hafner, R.; Jaramillo, E.; Hoelscher, M.; Zumla, A.; Gheuens, J. New Drugs for the Treatment of Tuberculosis: Needs, Challenges, Promise, and Prospects for the Future. J. Infect. Dis. 2012, 205, S241–S249. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Lienhardt, C.; McIlleron, H.; Nunn, A.J.; Wang, X. Global tuberculosis drug development pipeline: The need and the reality. Lancet 2010, 375, 2100–2109. [Google Scholar] [CrossRef]
- Nahid, P.; Jarlsberg, L.G.; Rudoy, I.; De Jong, B.C.; Unger, A.; Kawamura, L.M.; Osmond, D.H.; Hopewell, P.C.; Daley, C.L. Factors associated with mortality in patients with drug-susceptible pulmonary tuberculosis. BMC Infect. Dis. 2011, 11, 1. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, S.; Carlin, B.P.; Gelfand, A.E. Hierarchical Modeling and Analysis for Spatial Data; CRC Press: Boca Raton, FL, USA, 2014. [Google Scholar]
- Ziegel, E.R. Survival Analysis: A Self-Learning Text. Technometrics 2006, 48, 317. [Google Scholar]
- Zhou, H.; Hanson, T. Bayesian Spatial Survival Models. Nonparametric Bayesian Inference in Biostatistics; Springer: Berlin/Heidelberg, Germany, 2015; pp. 215–246. [Google Scholar]
- Zhang, J.; Lawson, A.B. Bayesian parametric accelerated failure time spatial model and its application to prostate cancer. J. Appl. Stat. 2010, 38, 591–603. [Google Scholar] [CrossRef]
- Banerjee, S.; Wall, M.M.; Carlin, B.P. Frailty modeling for spatially correlated survival data, with application to infant mortality in Minnesota. Biostatistics 2003, 4, 123–142. [Google Scholar] [CrossRef]
- Aswi, A.; Cramb, S.M.; Duncan, E.W.; Hu, W.; White, G.; Mengersen, K. Bayesian Spatial Survival Models for Hospitalisation of Dengue: A Case Study of Wahidin Hospital in Makassar, Indonesia. Int. J. Environ. Res. Public Health 2020, 17, 878. [Google Scholar] [CrossRef]
- Asad, M.; Mahmood, A.; Usman, M. A machine learning-based framework for Predicting Treatment Failure in tuberculosis: A case study of six countries. Tuberculosis 2020, 123, 101944. [Google Scholar] [CrossRef]
- Kalhori, S.R.N.; Zeng, X.-J. Evaluation and Comparison of Different Machine Learning Methods to Predict Outcome of Tuberculosis Treatment Course. J. Intell. Learn. Syst. Appl. 2013, 5, 184–193. [Google Scholar] [CrossRef][Green Version]
- Sauer, C.M.; Sasson, D.; Paik, K.E.; McCague, N.; Celi, L.A.; Sanchez Fernandez, I.; Illigens, B.M. Feature selection and prediction of treatment failure in tuberculosis. PLoS ONE 2018, 13, e0207491. [Google Scholar] [CrossRef] [PubMed]
- Killian, J.A.; Wilder, B.; Sharma, A.; Choudhary, V.; Dilkina, B.; Tambe, M. Learning to prescribe interventions for tuberculosis patients using digital adherence data. In Proceedings of the 25th ACM SIGKDD International Conference on Knowledge Discovery & Data Mining, Anchorage, AK, USA, 4–8 August 2019. [Google Scholar]
- Ahmad, R.; Xie, L.; Pyle, M.; Fernandez-Suarez, M.; Broger, T.; Steinberg, D.; Ame, S.M.; Lucero, M.G.; Szucs, M.J.; MacMullan, M.; et al. A rapid triage test for active pulmonary tuberculosis in adult patients with persistent cough. Sci. Transl. Med. 2019, 11, eaaw8287. [Google Scholar] [CrossRef] [PubMed]
- Seyedagha, S.H.; Kavousi, A.; Baghestani, A.R.; Nasehi, M. Evaluation of Effect of Geographical Pattern on Improvement of Patients with Pulmonary Tuberculosis Based on A Parametric Accelerated Failure Time Model. Iran. J. Epidemiol. 2017, 13, 199–209. [Google Scholar]
- Dangisso, M.H.; Datiko, D.G.; Lindtjorn, B. Accessibility to tuberculosis control services and tuberculosis programme performance in southern Ethiopia. Glob. Health Action 2015, 8, 29443. [Google Scholar] [CrossRef] [PubMed]
- Tanrikulu, A.C.; Acemoglu, H.; Palanci, Y.; Dagli, C.E. Tuberculosis in Turkey: High altitude and other socio-economic risk factors. Public Health 2008, 122, 613–619. [Google Scholar] [CrossRef]
- Gardiner, C.F.; Webb, G.B.; Ryder, C.T. Tuberculosis Mortality in Relation to Altitude. Trans. Am. Clim. Clin. Assoc. 1923, 39, 197–208. [Google Scholar]
- Vree, M.; Nguyen, H.B.; Sy, D.N.; Co, N.; Cobelens, F.G.J.; Borgdorff, M.W. Low tuberculosis notification in mountainous Vietnam is not due to low case detection: A cross-sectional survey. BMC Infect. Dis. 2007, 7, 109. [Google Scholar] [CrossRef]
- Vargas, M.H.; Furuya, M.E.Y.; Pérez-Guzmán, C. Effect of altitude on the frequency of pulmonary tuberculosis. Int. J. Tuberc. Lung Dis. 2004, 8, 1321–1324. [Google Scholar]
- Rogers, F.B. The rise and decline of the altitude therapy of tuberculosis. Bull. Hist. Med. 1969, 43, 1–16. [Google Scholar]
- WHO. Definitions and Reporting Framework for Tuberculosis–2013 Revision; Report No.: 9241505346; World Health Organization: Geneva, Switzerland, 2013. [Google Scholar]
- Christensen, R.; Johnson, W.; Branscum, A.; Hanson, T.E. Bayesian Ideas and Data Analysis: An Introduction for Scientists and Statisticians; CRC Press: Boca Raton, FL, USA, 2011. [Google Scholar]
- Besag, J.; York, J. Bayesian image restoration, with two applications in spatial statistics. Ann. Inst. Stat. Math. 1991, 43, 1–20. [Google Scholar] [CrossRef]
- Finkelstein, D.M. A Proportional Hazards Model for Interval-Censored Failure Time Data. Biometrics 1986, 42, 845–854. [Google Scholar] [CrossRef] [PubMed]
- Spiegelhalter, D.J.; Best, N.G.; Carlin, B.P.; Van Der Linde, A. Bayesian measures of model complexity and fit. J. R. Stat. Soc. Ser. B 2002, 64, 583–639. [Google Scholar] [CrossRef]
- Rodrigues, A.S.; Calsavara, V.F.; Tomazella, V.L.D. Modeling cure fraction with frailty term in latent risk: A Bayesian approach. arXiv 2018, arXiv:180308128. [Google Scholar]
- Jawetz, M. Medical Microbiology Twenty, 26th ed.; Lange: Irvine, CA, USA, 2013; p. 313. [Google Scholar]
- Khazaei, S.; Hassanzadeh, J.; Rezaeian, S.; Ghaderi, E.; Khazaei, S.; Hafshejani, A.M.; Salehiniya, H.; Zahiri, A. Treatment outcome of new smear positive pulmonary tuberculosis patients in Hamadan, Iran: A registry-based cross-sectional study. Egypt. J. Chest Dis. Tuberc. 2016, 65, 825–830. [Google Scholar] [CrossRef]
- McClelland, E.E.; Smith, J.M. Gender Specific Differences in the Immune Response to Infection. Arch. Immunol. Ther. Exp. 2011, 59, 203–213. [Google Scholar] [CrossRef]
- Hertz, D.; Schneider, B. Sex Differences in Tuberculosis. Seminars in Immunopathology; Springer: Berlin/Heidelberg, Germany, 2019. [Google Scholar]
- Lesourd, B. Immune response during disease and recovery in the elderly. Proc. Nutr. Soc. 1999, 58, 85–98. [Google Scholar] [CrossRef] [PubMed]
- Molaeipoor, L.; Poorolajal, J.; Mohraz, M.; Esmailnasab, N. Predictors of tuberculosis and human immunodeficiency virus co-infection: A case-control study. Epidemiol. Health 2014, 36, e2014024. [Google Scholar] [CrossRef]
- Chiang, C.Y.; Slama, K.; Enarson, D.A. Associations between tobacco and tuberculosis [Educational Series: Tobacco and tuberculosis. Serialised guide. Tobacco cessation interventions for tuberculosis patients. Number 1 in the series]. Int. J. Tuberc. Lung Dis. 2007, 11, 258–262. [Google Scholar]
- Atif, M.; Toghrayee, Z.; Sulaiman, S.A.S.; Shafie, A.A.; Low, H.C. Missing data analysis in longitudinal studies: Findings from a quality of life study in Malaysian tuberculosis patients. Appl. Res. Qual. Life 2015, 10, 95–105. [Google Scholar] [CrossRef]
- Mansoer, J.R.; Kibuga, D.K.; Borgdorff, M.W. Altitude: A determinant for tuberculosis in Kenya? Int. J. Tuberc. Lung Dis. 1999, 3, 156–161. [Google Scholar]
- Saito, M.; Bamrah, S.; Argüello, D.F.; Pan, W.K.; Bautista, C.T.; Tsiouris, S.J.; Martinez-Carrasco, G.; Martín, C.A.; Gilman, R.H. Comparison of altitude effect on Mycobacterium tuberculosis infection between rural and urban communities in Peru. Am. J. Trop. Med. Hyg. 2006, 75, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Olender, S.; Gilman, R.H.; Moro, P.; Bautista, C.T.; Saito, M.; Caviedes, L.; Apgar, J.; Gillenwater, K.; Hsieh, E.; Lescano, A.G. Low prevalence and increased household clustering of Mycobacterium tuberculosis infection in high altitude villages in Peru. Am. J. Trop. Med. Hyg. 2003, 68, 721–727. [Google Scholar] [CrossRef] [PubMed]
- Zenk, S.F.; Vollmer, M.; Schercher, E.; Kallert, S.; Kubis, J.; Stenger, S. Hypoxia promotes Mycobacterium tuberculosis-specific up-regulation of granulysin in human T cells. Med. Microbiol. Immunol. 2016, 205, 219–229. [Google Scholar] [CrossRef] [PubMed]
- Grosset, J.H. Bacteriology of tuberculosis. Lung Biol. Health Dis. 1993, 66, 49–74. [Google Scholar]
- Sever, J.L.; Youmans, G.P. The Relation of Oxygen Tension to Virulence of Tubercle Bacilli and to Acquired Resistance in Tuberculosis. J. Infect. Dis. 1957, 101, 193–202. [Google Scholar] [CrossRef]
- Corona, F.; Martinez, J.L. Phenotypic resistance to antibiotics. Antibiotics 2013, 2, 237–255. [Google Scholar] [CrossRef]
- Hui, L.; Rong, W.; Zheng-ping, J.; Juan, X.; Hua, X. Effects of high altitude exposure on physiology and pharmacokinetics. Curr. Drug Metab. 2016, 17, 559–565. [Google Scholar] [CrossRef]
- Dobner, J.; Kaser, S. Body mass index and the risk of infection—From underweight to obesity. Clin. Microbiol. Infect. 2018, 24, 24–28. [Google Scholar] [CrossRef]
- Campillo, B.; Paillaud, E.; Uzan, I.; Merlier, I.; Abdellaoui, M.; Perennec, J.; Louarn, F.; Bories, P. Value of body mass index in the detection of severe malnutrition: Influence of the pathology and changes in anthropometric parameters. Clin. Nutr. 2004, 23, 551–559. [Google Scholar] [CrossRef]
- Azarkar, Z.; Sharifzadeh, G.; Ebrahimzadeh, A.; Olumi, S. Time to sputum smear conversion in smear-positive pulmonary tuberculosis patients and factors for delayed conversion. Iran. J. Med. Sci. 2016, 41, 44. [Google Scholar]
- Kayigamba, F.R.; Bakker, M.I.; Mugisha, V.; De Naeyer, L.; Gasana, M.; Cobelens, F.; van der Loeff, M.S. Adherence to tuberculosis treatment, sputum smear conversion and mortality: A retrospective cohort study in 48 Rwandan clinics. PLoS ONE 2013, 8, e73501. [Google Scholar] [CrossRef]
- Gopi, P.G.; Chandrasekaran, V.; Subramani, R.; Santha, T.; Thomas, A.; Selvakumar, N.; Narayanan, P.R. Association of conversion & cure with initial smear grading among new smear positive pulmonary tuberculosis patients treated with Category I regimen. Indian J. Med. Res. 2006, 123, 807–814. [Google Scholar] [PubMed]
- Parikh, R.; Nataraj, G.; Kanade, S.; Khatri, V.; Mehta, P. Time to sputum conversion in smear positive pulmonary TB patients on category I DOTS and factors delaying it. J. Assoc. Physicians India 2012, 60, 22–26. [Google Scholar] [PubMed]
- Yimer, S.A.; Bjune, G.; Alene, G. Diagnostic and treatment delay among pulmonary tuberculosis patients in Ethiopia: A cross sectional study. BMC Infect. Dis. 2005, 5, 112. [Google Scholar] [CrossRef] [PubMed]
- Virenfeldt, J.; Rudolf, F.; Camara, C.; Furtado, A.; Gomes, V.; Aaby, P.; Petersen, E.; Wejse, C. Treatment delay affects clinical severity of tuberculosis: A longitudinal cohort study. BMJ Open 2014, 4, e004818. [Google Scholar] [CrossRef]
- Lusignani, L.S.; Quaglio, G.; Atzori, A.; Nsuka, J.; Grainger, R.; Palma, M.D.C.; Putoto, G.; Manenti, F. Factors associated with patient and health care system delay in diagnosis for tuberculosis in the province of Luanda, Angola. BMC Infect. Dis. 2013, 13, 168. [Google Scholar] [CrossRef]
- Rafiee, S.; Besharat, S.; Jabbari, A.; Golalipour, F.; Nasermoaadeli, A. Epidemiology of tuberculosis in northeast of Iran: A population-based study. Iran. J. Med. Sci. 2009, 34, 193–197. [Google Scholar]
- World Health Organization. World Malaria Report 2015; World Health Organization: Geneva, Switzerland, 2016. [Google Scholar]
- Mitnick, C.D.; Appleton, S.C.; Shin, S.S. Epidemiology and Treatment of Multidrug Resistant Tuberculosis. Semin. Respir. Crit. Care Med. 2008, 29, 499–524. [Google Scholar] [CrossRef][Green Version]
- Pourhossein, B.; Irani, A.D.; Mostafavi, E. Major infectious diseases affecting the Afghan immigrant population of Iran: A systematic review and meta-analysis. Epidemiol. Health 2015, 37, 37. [Google Scholar] [CrossRef]
- Levy, O.; Wynn, J.L. A prime time for trained immunity: Innate immune memory in newborns and infants. Neonatology 2014, 105, 136–141. [Google Scholar] [CrossRef]
- Schröder, P.C.; Li, J.; Wong, G.W.K.; Schaub, B. The rural-urban enigma of allergy: What can we learn from studies around the world? Pediatr. Allergy Immunol. 2015, 26, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Yemaneberhan, H.; Bekele, Z.; Venn, A.; Lewis, S.; Parry, E.; Britton, J. Prevalence of wheeze and asthma and relation to atopy in urban and rural Ethiopia. Lancet 1997, 350, 85–90. [Google Scholar] [CrossRef]
- Apostol, A.C.; Jensen, K.D.; Beaudin, A.E. Training the Fetal Immune System through Maternal Inflammation—A Layered Hygiene Hypothesis. Front. Immunol. 2020, 11, 123. [Google Scholar] [CrossRef] [PubMed]
- Horne, D.J.; Johnson, C.O.; Oren, E.; Spitters, C.; Narita, M. How soon can smear positive TB patients be released from inpatient isolation? Infection control and hospital epidemiology. Off. J. Soc. Hosp. Epidemiol. Am. 2010, 31, 78. [Google Scholar] [CrossRef] [PubMed]
- Leroux, B.G.; Lei, X.; Breslow, N. Estimation of Disease Rates in Small Areas: A New Mixed Model for Spatial Dependence. Statistical Models in Epidemiology, the Environment, and Clinical Trials; Springer: Berlin/Heidelberg, Germany, 2000; pp. 179–191. [Google Scholar]

| Characteristics | Overall | Cured | P | |
|---|---|---|---|---|
| No | Yes | |||
| Age | <0.001 * <0.001 * | |||
| Mean ± SD | 51.26 ± 21.45 | 54.03 ± 21.75 | 50.56 ± 21.34 | |
| <15 y | 503 (1.40) | 79 (15.70) | 424 (84.30) | |
| 15–35 y | 10,317 (29.70) | 1803 (17.50) | 8514 (82.50) | |
| 35–63 y | 11,789 (33.90) | 2373 (20.10) | 94.16 (79.90) | |
| >63 y | 12,135 (34.90) | 2730 (22.60) | 9398 (77.40) | |
| Weight | 54.60 ± 13.09 | 53.49 ± 13.33 | 54.88 ± 13.01 | <0.001 * |
| Gender | <0.001 * | |||
| Female | 16,06 4(46.20) | 2548 (15.90) | 13,516 (84.10) | |
| Male | 18,68 0(53.80) | 4444 (23.80) | 14,23 6(76.20) | |
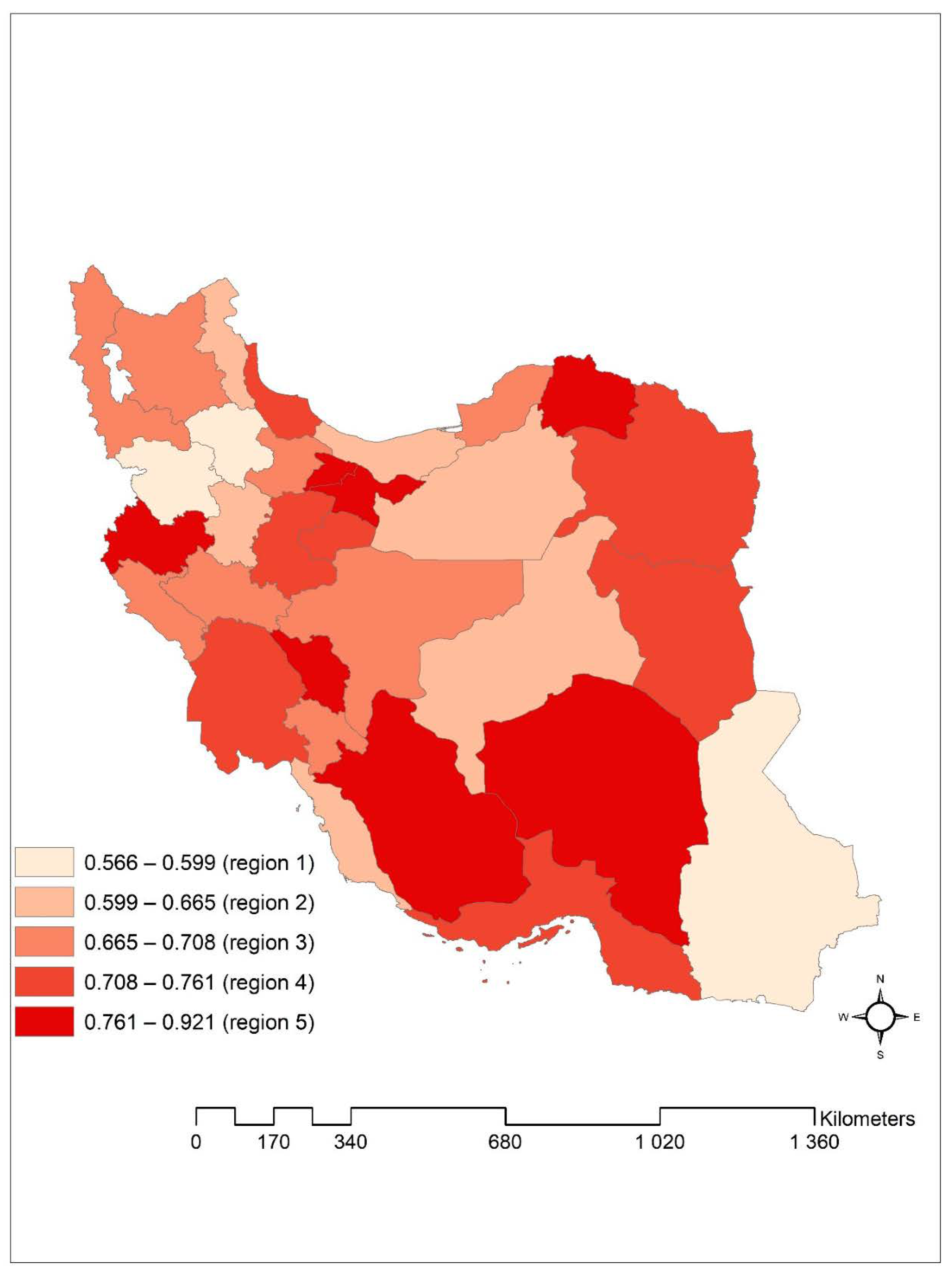
| Altitude (meter) | ||||
| Median (Q1–Q3) | 1027 (133–1302) | 1062 (214–1312) | 1027 (128–1264) | <0.001 * |
| 750 | 13,692 (39.40) | 2275 (16.60) | 11,417 (83.40) | <0.001 * |
| >750 | 21,052 (60.60) | 4717 (22.40) | 16,335 (77.60) | |
| Bacilli density in initial smear | <0.001 * | |||
| 1–9 Basil | 2226 (6.40) | 439 (19.70) | 1787 (80.30) | |
| 1+ | 11,759 (33.80) | 2217 (18.90) | 954 2(81.10) | |
| 2+ | 7786 (22.40) | 1453 (18.70) | 633 3(81.30) | |
| 3+ | 12,97 3(37.30) | 2883 (22.20) | 10,09 0(77.80) | |
| Delayed diagnosis (month) | <0.001 * | |||
| <1 | 10,935 (31.50) | 2074 (19.00) | 8861 (81.00) | |
| 1–3 | 15,174 (43.70) | 2991 (19.70) | 12,183 (80.30) | |
| >3 | 8635 (24.90) | 1927 (22.30) | 6708 (77.70) | |
| Nationality | 0.007 * | |||
| Other | 4935 (14.20) | 1063 (21.50) | 3872 (78.50) | |
| Iranian | 29,809 (85.80) | 5929 (19.90) | 23,88 0(80.10) | |
| Prison condition | 0.42 | |||
| No | 32,93 1(94.80) | 6614 (20.10) | 26,317 (79.90) | |
| Yes | 1813 (5.20) | 378 (20.80) | 1435 (79.20) | |
| Location | <0.001 * | |||
| Rural | 11,578 (33.30) | 2027 (17.50) | 955 1(82.50) | |
| Urban | 23,166 (66.70) | 4965 (21.40) | 18,20 1(78.60) | |
| Model | Independent | CAR Prior | Convolution Prior |
|---|---|---|---|
| DIC | |||
| Log logistic | 25,110 | 25,080 | 24,810 |
| Weibull | 30,000 | 29,970 | 29,890 |
| Log normal | 26,350 | 26,330 | 26,290 |
| Characteristics (Ref) | Mean (SD) | (95% CI) | Time Ratio |
|---|---|---|---|
| Age | 0.00009 (0.0001) | (−0.0002, 0.0004) | 1.00009 |
| Weight | −0.002 (0.0003) | (−0.003, −0.001) | 0.998 * |
| Gender (female) | |||
| male | 0.09 (0.007) | (0.07, 0.11) | 1.09 * |
| Altitude (750) | |||
| >750 | 0.05 (0.015) | (0.02, 0.08) | 1.05 * |
| bacilli density in initial smear (1–9 Basil & 1+) | |||
| 2+ | 0.02 (0.009) | (0.008, 0.04) | 1.02 * |
| 3+ | 0.09 (0.007) | (0.07, 0.10) | 1.09 * |
| Delayed diagnosis (<1 month) | |||
| 1–3 | 0.01 (0.008) | (−0.005, 0.02) | 1.01 |
| >3 | 0.02 (0.009) | (0.01, 0.04) | 1.02 * |
| Nationality (Other) | |||
| Iranian | 0.02 (0.011) | (0.001, 0.04) | 1.02 * |
| Prison condition (No) | |||
| Yes | −0.008 (0.015) | (−0.03, 0.02) | 0.992 |
| Location (Rural) | |||
| Urban | 0.02 (0.008) | (0.006, 0.03) | 1.02 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nazar, E.; Baghishani, H.; Doosti, H.; Ghavami, V.; Aryan, E.; Nasehi, M.; Sharafi, S.; Esmaily, H.; Yazdani Charati, J. Bayesian Spatial Survival Analysis of Duration to Cure among New Smear-Positive Pulmonary Tuberculosis (PTB) Patients in Iran, during 2011–2018. Int. J. Environ. Res. Public Health 2021, 18, 54. https://doi.org/10.3390/ijerph18010054
Nazar E, Baghishani H, Doosti H, Ghavami V, Aryan E, Nasehi M, Sharafi S, Esmaily H, Yazdani Charati J. Bayesian Spatial Survival Analysis of Duration to Cure among New Smear-Positive Pulmonary Tuberculosis (PTB) Patients in Iran, during 2011–2018. International Journal of Environmental Research and Public Health. 2021; 18(1):54. https://doi.org/10.3390/ijerph18010054
Chicago/Turabian StyleNazar, Eisa, Hossein Baghishani, Hassan Doosti, Vahid Ghavami, Ehsan Aryan, Mahshid Nasehi, Saeid Sharafi, Habibollah Esmaily, and Jamshid Yazdani Charati. 2021. "Bayesian Spatial Survival Analysis of Duration to Cure among New Smear-Positive Pulmonary Tuberculosis (PTB) Patients in Iran, during 2011–2018" International Journal of Environmental Research and Public Health 18, no. 1: 54. https://doi.org/10.3390/ijerph18010054
APA StyleNazar, E., Baghishani, H., Doosti, H., Ghavami, V., Aryan, E., Nasehi, M., Sharafi, S., Esmaily, H., & Yazdani Charati, J. (2021). Bayesian Spatial Survival Analysis of Duration to Cure among New Smear-Positive Pulmonary Tuberculosis (PTB) Patients in Iran, during 2011–2018. International Journal of Environmental Research and Public Health, 18(1), 54. https://doi.org/10.3390/ijerph18010054





