Removal of Nitrogen and Phosphorus from Thickening Effluent of an Urban Wastewater Treatment Plant by an Isolated Green Microalga
Abstract
:1. Introduction
2. Results
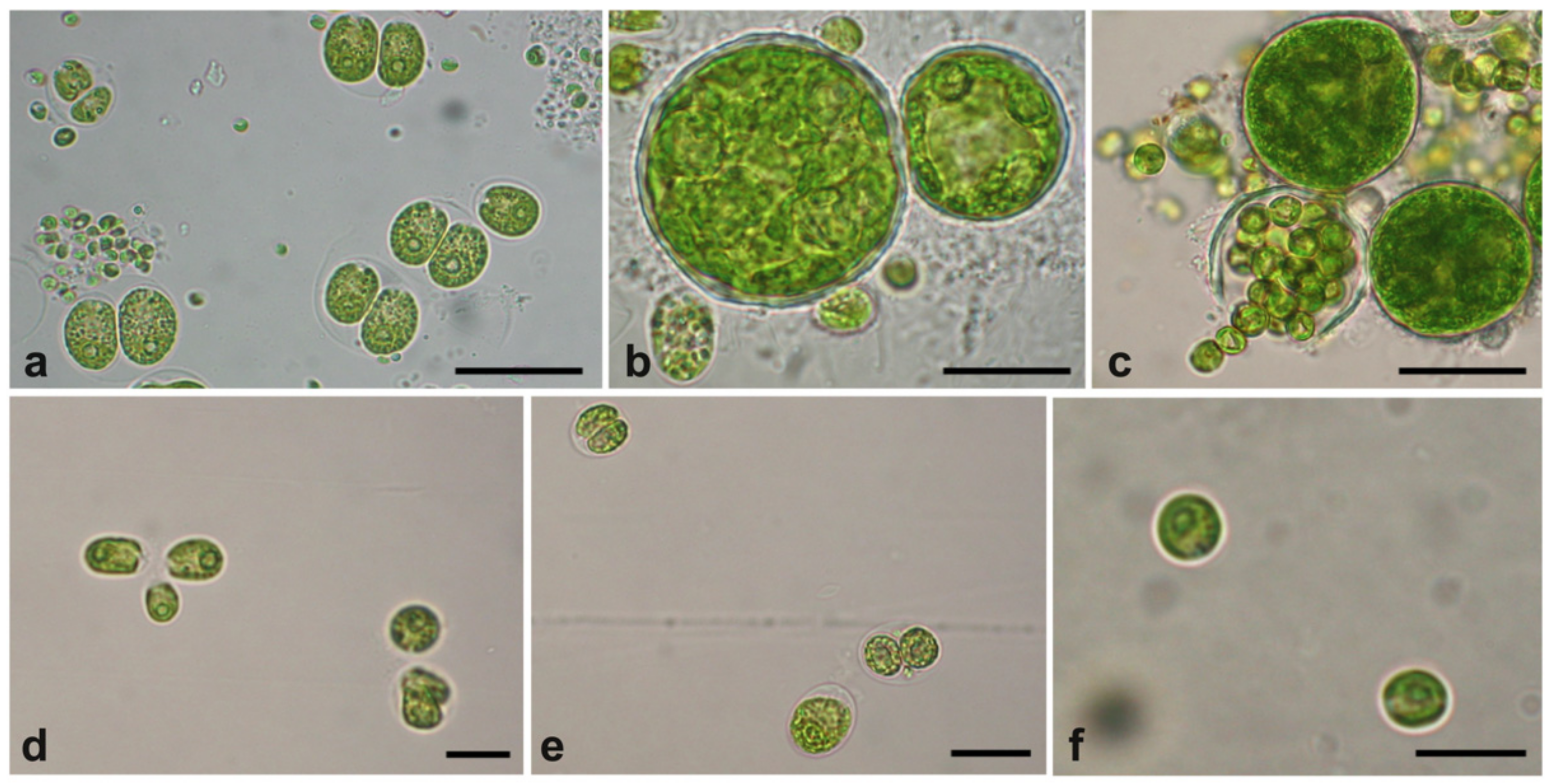
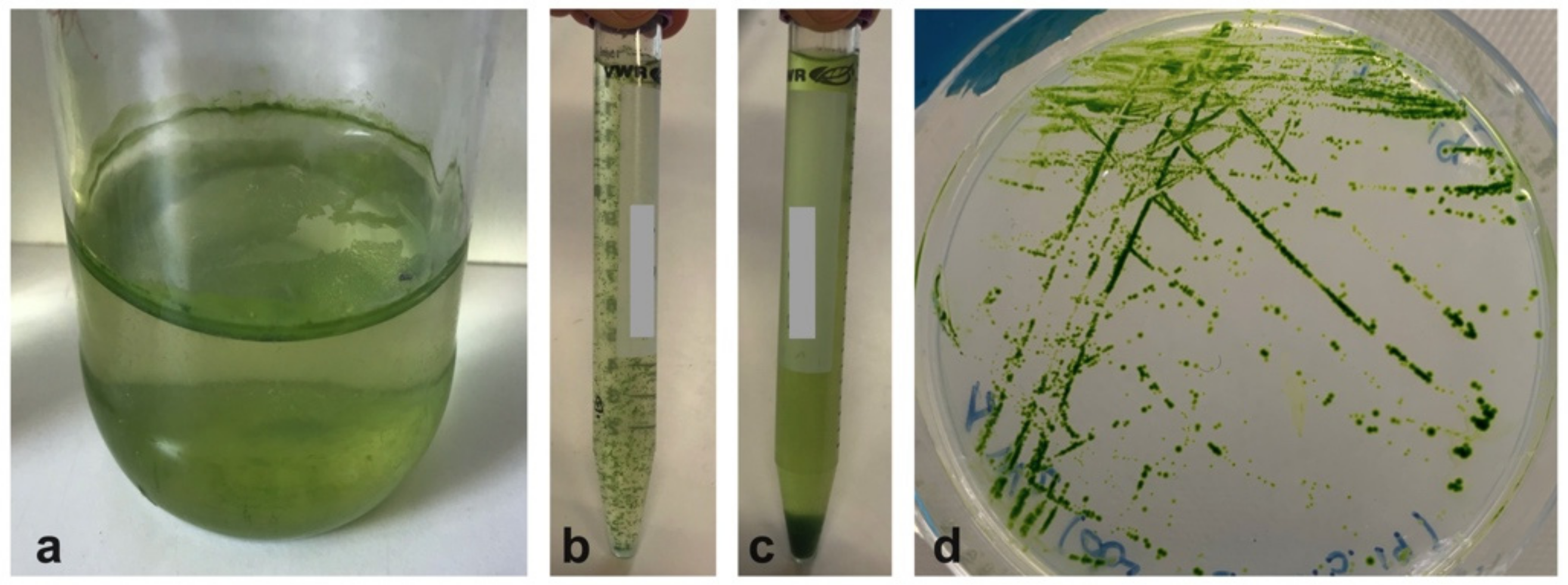
2.1. Microalgae Isolates from the UWW Effluent
2.2. Phytoremediation Experiment
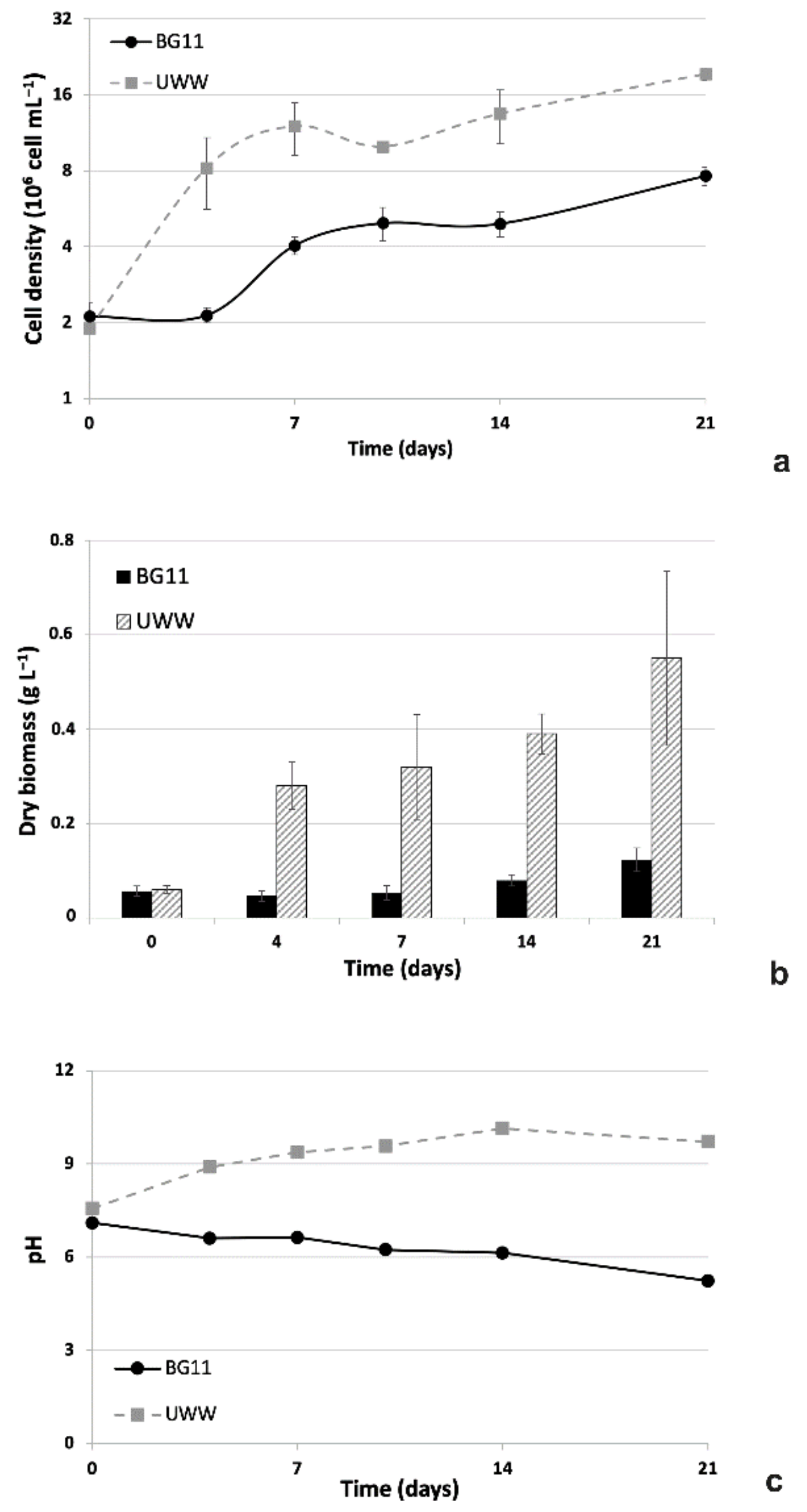
2.2.1. Comparative Growth in UWW and Modified BG11
2.2.2. Comparative Nutrient Removal in UWW and Modified BG11
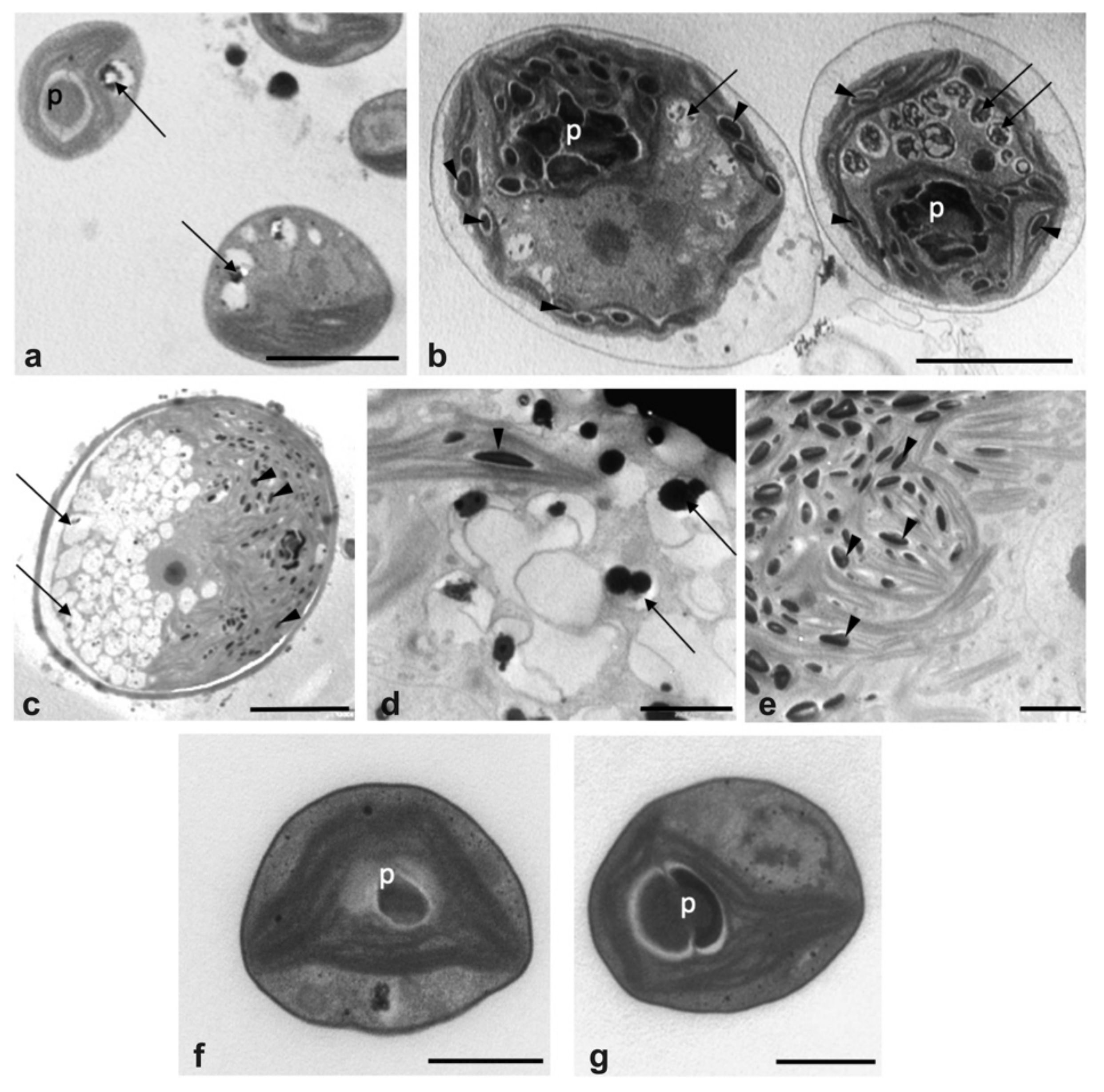
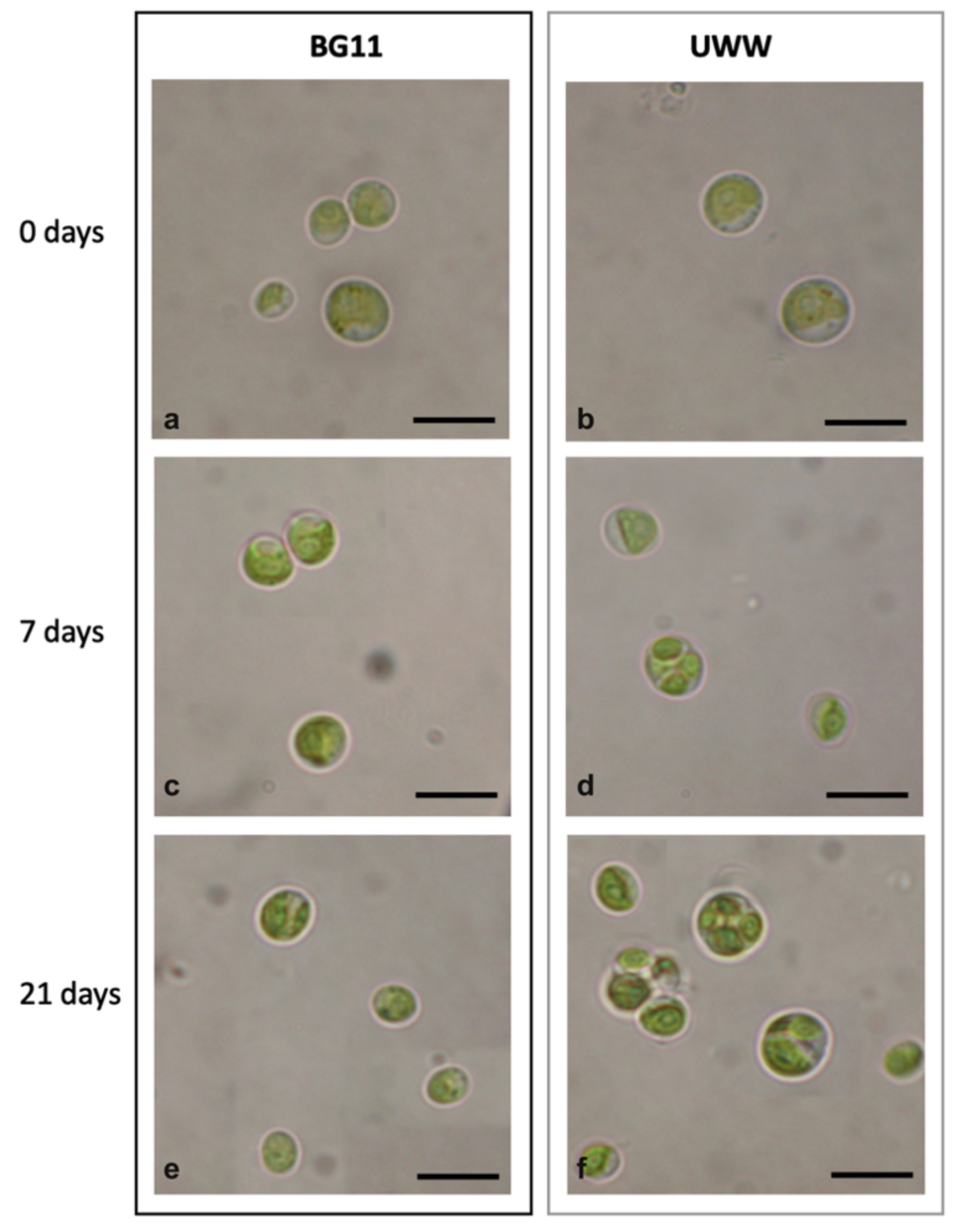
2.2.3. Comparative Morphology of the Microalgal Isolate in UWW and Modified BG11
2.2.4. Comparative Photosynthetic Properties of the Microalgal Isolate in UWW and Modified BG11
3. Discussion
4. Materials and Methods
4.1. Wastewater
4.2. Microalgae Isolation
4.3. Experimental Design
4.4. Light and Transmission Electron Microscopy (TEM)
4.5. Growth and pH Evaluations
4.6. N and P Analysis
4.7. Photosynthetic Pigment Extraction and Quantification
4.8. PSII Maximum Quantum Yield Analysis
4.9. Total protein Extraction and Quantification
4.10. Statistical Analyses of Data
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Rawat, I.; Ranjith Kumar, R.; Mutanda, T.; Bux, F. Dual role of microalgae: Phycoremediation of domestic wastewaters and biomass production for sustainable biofuels production. Appl. Energ. 2011, 88, 3411–3424. [Google Scholar] [CrossRef]
- Nayak, M.; Karemore, A.; Sen, R. Performance evaluation of microalgae for concomitant wastewater bioremediation, CO2 biofixation and lipid biosynthesis for biodiesel application. Algal Res. 2016, 16, 216–223. [Google Scholar] [CrossRef]
- European Commission Directive (1998) 98/15/EC of 27 February. Official Journal of the European Communities. Available online: https://eur-lex.europa.eu/legal-content/en/ALL/?uri=CELEX:31998L0015 (accessed on 22 July 2020).
- United States Environmental Protection Agency; Guidelines for water reuse. 2012. Available online: http://www.epa.gov/ (accessed on 22 July 2020).
- Daverey, A.; Pandey, D.; Verma, P.; Verma, S.; Shah, V.; Dutta, K.; Arunachalam, K. Recent advances in energy efficient biological treatment of municipal wastewater. Bioresour. Technol. Rep. 2019, 7, 100252. [Google Scholar] [CrossRef]
- Arbib, Z.; Ruiz, J.; Álvarez-Díaz, P.; Garrido-Pérez, M.C.; Perales, J.A. Capability of different microalgae species for phytoremediation processes: Wastewater tertiary treatment, CO2 bio-fixation and low cost biofuels production. Water Res. 2014, 49, 474–645. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.-H.; Zhang, T.-Y.; Dao, G.-H.; Xu, X.-Q.; Wang, X.-X.; Hu, H.-Y. Microalgae-based advanced municipal wastewater treatment for reuse in water bodies. Appl. Microbiol. Biotechnol. 2017, 101, 2659–2675. [Google Scholar] [CrossRef]
- Wang, L.; Min, M.; Li, Y.; Chen, P.; Chen, Y.; Liu, Y.; Wang, Y.; Ruan, R. Cultivation of green algae Chlorella sp. in different wastewaters from municipal wastewater treatment plant. Appl. Biochem. Biotechnol. 2010, 162, 1174–1186. [Google Scholar] [CrossRef]
- Wang, B.; Lan, C.Q. Biomass production and nitrogen and phosphorus removal by the green alga Neochloris oleoabundans in simulated wastewater and secondary municipal wastewater effluent. Bioresour. Technol. 2011, 102, 5639–5644. [Google Scholar] [CrossRef]
- Christenson, L.; Sims, R. Production and harvesting of microalgae for wastewater treatment, biofuels, and bioproducts. Biotechnol. Adv. 2011, 29, 686–702. [Google Scholar] [CrossRef]
- Giovanardi, M.; Baldisserotto, C.; Daglia, M.; Ferroni, L.; Sabia, A.; Pancaldi, S. Morpho-physiological aspects of Scenedesmus acutus PVUW12 cultivated with a dairy industry waste and after starvation. Plant Biosyst. 2016, 150, 767–775. [Google Scholar] [CrossRef]
- Avagyan, A.B. Environmental building policy by the use of microalgae and decreasing of risks for Canadian oil sand sector development. Environ. Sci. Pollut. Res. 2017, 24, 20241–20253. [Google Scholar] [CrossRef]
- Álvarez-Díaz, P.D.; Ruiz, J.; Arbib, Z.; Barragán, J.; Garrido Pérez, M.C.; Perales, J.A. Freshwater microalgae selection for simultaneous wastewater nutrient removal and lipid production. Algal Res. 2017, 24, 477–485. [Google Scholar] [CrossRef]
- Singh, R.; Birru, R.; Sibi, G. Nutrient removal efficiencies of Chlorella vulgaris from urban wastewater for reduced eutrophication. J. Environ. Prot. 2017, 8, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Silkina, A.; Ginnever, N.E.; Fernandes, F.F.; Fuentes-Grünewald, F. Large-scale waste bio-remediation using microalgae cultivation as a platform. Energies 2019, 12, 2772. [Google Scholar] [CrossRef] [Green Version]
- Picardo, M.C.; de Medeiros, J.L.; Monteiro, J.G.M.; Moreira Chaloub, R.; Giordano, M.; de Queiroz Fernandes Araújo, O. A methodology for screening of microalgae as a decision making tool for energy and green chemical process applications. Clean Techn. Environ. Policy 2013, 15, 275–291. [Google Scholar] [CrossRef]
- Avagyan, A.B. A contribution to global sustainable development: Inclusion of microalgae and their biomass in production and bio cycles. Clean Techn. Environ. Policy 2008, 10, 313–317. [Google Scholar] [CrossRef] [Green Version]
- Spolaore, P.; Joannis-Cassan, C.; Duran, E.; Isambert, A. Commercial application of microalgae. J. Biosci. Bioeng. 2006, 101, 87–96. [Google Scholar] [CrossRef] [Green Version]
- Brune, D.E.; Lundquist, T.J.; Benemann, J.R. Microalgal biomass for greenhouse gas reductions: Potential for replacement of fossil fuels and animal feeds. J. Environ. Eng. 2009, 135, 1136–1144. [Google Scholar] [CrossRef] [Green Version]
- Baldisserotto, C.; Giovanardi, M.; Ferroni, L.; Pancaldi, S. Growth, morphology and photosynthetic responses of Neochloris oleoabundans during cultivation in a mixotrophic brackish medium and subsequent starvation. Acta Physiol. Plant. 2014, 36, 461–472. [Google Scholar] [CrossRef]
- Baldisserotto, C.; Popovich, C.; Giovanardi, M.; Sabia, A.; Ferroni, L.; Constenla, D.; Leonardi, P.; Pancaldi, S. Photosynthetic aspects and lipid profiles in the mixotrophic alga Neochloris oleoabundans as useful parameters for biodiesel production. Algal Res. 2016, 16, 255–265. [Google Scholar] [CrossRef]
- Caporgno, M.P.; Taleb, A.; Olkiewicz, M.; Font, J.; Pruvost, J.; Legrand, J.; Bengoa, C. Microalgae cultivation in urban wastewater: Nutrient removal and biomass production for biodiesel and methane. Algal Res. 2015, 10, 232–239. [Google Scholar] [CrossRef]
- Acién Fernández, F.G.; Gómez-Serrano, C.; Fernández-Sevilla, J.M. Recovey of nutrients from wastewaters using microalgae. Front. Sustain. Food Syst. 2018, 2, 59. [Google Scholar] [CrossRef] [Green Version]
- Kube, M.; Fefferson, B.; Fan, L.; Roddick, F. The impact of wastewater characteristics, algal species selection and immobilisation on simultaneous nitrogen and phosphorus removal. Algal Res. 2018, 31, 478–488. [Google Scholar] [CrossRef] [Green Version]
- Kasan, N.A.; Shahirah Hashim, F.; Haris, N.; Zakaria, M.Z.; Mohamed, N.N.; Rasdi, N.W.; Wahid, M.E.; Katayama, T.; Takahashi, K.; Jusoh, M. Isolation of freshwater and marine indigenous microalgae species from Terengganu water bodies for potential uses as live feeds in aquaculture industry. Int. Aquat. Res. 2020, 12, 74–83. [Google Scholar] [CrossRef]
- Prajapati, S.K.; Kaushik, P.; Malik, A.; Vijay, V.K. Phycoremediation and biogas potential of native algal isolates from soil and wastewater. Bioresour. Technol. 2013, 135, 232–238. [Google Scholar] [CrossRef] [PubMed]
- McCarty, P.L.; Bae, J.; Kim, J. Domestic wastewater treatment as a net energy producer can this be achieved? Environ. Sci. Technol. 2011, 45, 7100–7106. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Zhang, D.Q.; Le, M.T.; Puah, A.N.; Ng, W.J. Energy utilization in sewage treatment—A review with comparisons. J. Water Clim. Change 2013, 4, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Panepinto, D.; Fiore, S.; Zappone, M.; Genon, G.; Meucci, L. Evaluation of the energy efficiency of a large wastewater treatment plant in Italy. Appl. Energ. 2016, 161, 404–411. [Google Scholar] [CrossRef]
- Li, W.; Li, L.; Qiu, G. Energy consumption and economic cost of typical wastewater treatment systems in Shenzhen, China. J. Clean. Prod. 2017, 163, S374–S378. [Google Scholar] [CrossRef]
- Gabriel Acién, F.; Gómez-Serrano, C.; Morales-Amaral, M.M.; Fernández-Sevilla, J.M.; Molina-Grima, E. Wastewater treatment using microalgae: How realistic a contribution might be to significant urban wastewater treatment? Appl. Microbiol. Biotechnol. 2016, 100, 9013–9022. [Google Scholar] [CrossRef]
- Samorì, G.; Samorì, C.; Guerrini, F.; Pistocchi, R. Growth and nitrogen removal capacity of Desmodesmus communis and of a natural microalgae consortium in a batch culture system in view of urban wastewater treatment: Part I. Water Res. 2013, 47, 791–801. [Google Scholar] [CrossRef]
- Ma, X.; Zhou, W.; Fu, Z.; Cheng, Y.; Min, M.; Liu, Y.; Zhang, Y.; Chen, P.; Ruan, R. Effect of wastewater-borne bacteria on algal growth and nutrients removal in wastewater-based algae cultivation system. Bioresour. Technol. 2014, 167, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Osundeko, O.; Pittmann, J.K. Implications of sludge liquor addition for wastewater-based open pond cultivation of microalgae for biofuel generation and pollutant remediation. Bioresour. Technol. 2014, 152, 355–363. [Google Scholar] [CrossRef] [PubMed]
- Delgadillo-Mirquez, L.; Lopes, F.; Taidi, B.; Pareau, D. Nitrogen and phosphate removal from wastewater with a mixed microalgae and bacteria culture. Biotechnol. Rep. 2016, 11, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Jia, H.; Yuan, Q. Removal of nitrogen from wastewater using microalgae and microalgae–bacteria consortia. Cogent Environ. Sci. 2016, 2, 1275089. [Google Scholar] [CrossRef]
- Fito, J.; Alemu, K. Microalgae–bacteria consortium treatment technology for municipal wastewater management. Nanotechnology Environm. Eng. 2019, 4, 4. [Google Scholar] [CrossRef]
- Tapia, C.; Fermoso, F.G.; Serrano, A.; Torres, A.; Rivas, M.; Ruiz, G.; Vilchez, C.; Cuaresma, M. Potential of a local microalgal strain isolated from anaerobic digester effluents for nutrient removal. J. Appl. Phycol. 2019, 31, 345–353. [Google Scholar] [CrossRef]
- Pancaldi, S.; Ferroni, L.; Baldisserotto, C.; Giovanardi, M.; Mai, L. Impianto e procedimento per lo smaltimento del carico di azoto di reflui organici in allevamenti zootecnici. Brevetto d’invenzione industriale Patent No. 1412445, 15 May 2012. [Google Scholar]
- Bohutskyi, P.; Liu, K.; Nasr, L.K.; Byers, N.; Rosemberg, J.N.; Oyler, G.A.; Betenbaugh, M.J.; Bouwer, E.J. Bioprospecting of microalgae for integrated biomass production and phytoremediation of unsterilized wastewater and anaerobic digestion centrate. Appl. Microbiol. Biotechnol. 2015, 99, 6139–6154. [Google Scholar] [CrossRef]
- George, B.; Pancha, I.; Desai, C.; Chokshi, K.; Paliwal, C.; Ghosh, T.; Mishra, S. Effects of different media composition, light intensity and photoperiod on morphology and physiology of freshwater microalgae Ankistrodesmus falcatus – A potential strain for bio-fuel production. Bioresour. Technol. 2014, 171, 367–374. [Google Scholar] [CrossRef]
- Li, Y.; Chen, Y.F.; Chen, P.; Min, M.; Zhou, W.; Martinez, B.; Zhu, J.; Ruan, R. Characterization of a microalga Chlorella sp. well adapted to highly concentrated municipal wastewater for nutrient removal and biodiesel production. Bioresour. Technol. 2011, 102, 5138–5144. [Google Scholar] [CrossRef]
- Álvarez, X.; Otero, A. Nutrient removal from the centrate of anaerobic digestion of high ammonium industrial wastewater by a semi-continuous culture of Arthrospira sp. and Nostoc sp. PCC 7413. J. Appl. Phycol. 2020, 32, 2785–2794. [Google Scholar] [CrossRef]
- Zhao, X.; Kumar, K.; Gross, M.A.; Kunetz, T.E.; Wen, Z. Evaluation of revolving algae biofilm reactors for nutrients and metals removal from sludge thickening supernatant in a municipal wastewater treatment facility. Water Res. 2018, 143, 467–478. [Google Scholar] [CrossRef] [PubMed]
- Solovchenko, A.E.; Ismagulova, T.T.; Lukyanov, A.A.; Vasilieva, S.G.; Konyukhov, I.V.; Pogosyan, S.I.; Lobakova, E.S.; Gorelova, O. Luxury phosphorus uptake in microalgae. J. Appl. Phycol. 2019, 31, 2755–2770. [Google Scholar] [CrossRef]
- Protist Information Service. Available online: http://protist.i.hosei.ac.jp (accessed on 4 September 2020).
- Ikeda, T.; Takeda, H. Species-specific differences of pyrenoids in Chlorella (Chlorophyta). J. Phycol. 1995, 31, 813–818. [Google Scholar] [CrossRef]
- Barsanti, L.; Gualtieri, P. Algae—Anatomy, Biochemistry, and Biotechnology, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2014; pp. 1–140. [Google Scholar]
- Carpenter, S.R.; Bennett, E.M. Reconsideration of the planetary boundary for phosphorous. Environ. Sci. Lett. 2011, 6, 014009. [Google Scholar] [CrossRef]
- Fedoruk, M.J.; Bronstein, R.; Kerger, B.D. Ammonia exposure and hazard assessment for selected household cleaning product uses. J. Expo. Anal. Environ. Epidemiol. 2005, 15, 534–544. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Conley, D.J.; Paerl, H.W.; Howarth, R.W.; Boesch, D.F.; Seitzinger, S.P.; Havens, K.E.; Lancelot, C.; Likens, G.E. Controlling eutrophication: Nitrogen and phosphorus. Science 2009, 323, 1014. [Google Scholar] [CrossRef] [PubMed]
- Xiong, C.; Guo, Z.; Chen, S.S.; Gao, Q.; Kishe, M.A.; Shen, Q. Understanding the pathway of phosphorus metabolism in urban household consumption system: A case study of Dar es Salaam, Tanzania. J. Clean. Prod. 2020, 274, 122874. [Google Scholar] [CrossRef]
- US-EPA; Wastewater Treatment Fact Sheet: External Carbon Sources for Nitrogen Removal. United States Environmental Protection Agency. 2013. Available online: https://www.epa.gov/sites/production/files/2019-08/documents/external_carbon_surces_for_nitrogen_removal_fact_sheet_p100il8f.pdf (accessed on 4 September 2020).
- Schulze, P.S.C.; Carvalho, C.F.M.; Pereira, H.; Gangadhar, K.N.; Schüler, L.M.; Santos, T.F.; Varela, J.C.S.; Barreira, L. Urban wastewater treatment by Tetraselmis sp. CTP4 (Chlorophyta). Bioresour. Technol. 2017, 223, 175–183. [Google Scholar] [CrossRef]
- Pratt, C.; Parsons, S.A.; Soares, A.; Martin, B.D. Biologically and chemically mediated adsorption and precipitation of phosphorus from wastewater. Curr. Opin. Biotech. 2012, 23, 890–896. [Google Scholar] [CrossRef]
- Kazadi Mbamba, C.; Lindblom, E.; Flores-Alsina, X.; Tait, S.; Anderson, S.; Saagi, R.; Batstone, D.J.; Gernaey, K.V.; Jeppsson, U. Plant-wide model-based analysis of iron dosage strategies for chemical phosphorus removal in wastewater treatment systems. Water Res. 2019, 155, 12–25. [Google Scholar] [CrossRef]
- Dorofeev, A.G.; Nikolaev, Y.A.; Mardanov, A.V.; Pimenov, N.V. Role of phosphate-accumulating bacteria in biological phosphorus removal from wastewater. Appl. Biochem. Microbiol. 2020, 56, 1–14. [Google Scholar] [CrossRef]
- Cabanelas, I.T.D.; Ruiz, J.; Arbib, Z.; Alexandre Chinalia, F.; Garrido-Pére, C.; Rogalla, F.; Andrade Nascimento, I.; Perales, J.A. Comparing the use of different domestic wastewaters for coupling microalgal production and nutrient removal. Bioresour. Technol. 2013, 131, 429–436. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Guo, W.; Yen, H.-W.; Ho, S.-H.; Lo, Y.-C.; Cheng, C.-L.; Ren, N.; Chang, J.-S. Cultivation of Chlorella vulgaris JSC-6 with swine wastewater for simultaneous nutrient/COD removal and carbohydrate production. Bioresour. Technol. 2015, 198, 619–625. [Google Scholar] [CrossRef] [PubMed]
- Mujtaba, G.; Rizwan, M.; Kim, G.; Lee, K. Removal of nutrients and COD through co-culturing activated sludge and immobilized Chlorella vulgaris. Chem. Eng. J. 2018, 343, 155–162. [Google Scholar] [CrossRef]
- Nirmalakhandan, N.; Selvaratnam, T.; Henkanatte-Gedera, S.M.; Tchinda, D.; Abeysiriwardana-Arachchige, I.S.A.; Delanka-Pedige, H.M.K.; Munasinghe-Arachchige, S.P.; Zhang, Y.; Holguin, F.O.; Lammer, P.J. Algal wastewater treatment: Photoautotrophic vs. mixotrophic processes. Algal Res. 2019, 41, 101569. [Google Scholar] [CrossRef]
- Munoz, R.; Guieysse, B. Algal–bacterial processes for the treatment of hazardous contaminants: A review. Water Res. 2006, 40, 2799–2815. [Google Scholar] [CrossRef]
- Zhou, W.; Li, Y.; Min, M.; Hu, B.; Zhang, H.; Ma, X.; Li, L.; Cheng, Y.; Chen, P.; Ruan, R. Growing wastewater-born microalga Auxenochlorella protothecoides UMN280 on concentrated municipal wastewater for simultaneous nutrient removal and energy feedstock production. Appl. Energy 2012, 98, 433–440. [Google Scholar] [CrossRef]
- Yruela, I. Copper in plants. Braz. J. Plant Physiol. 2005, 17, 145–156. [Google Scholar] [CrossRef] [Green Version]
- Gaur, N.; Bhardwaj, V.; Rathi, M. Heavy metals and their effects. JPR BioMedRx An Int. J. 2013, 1, 928–933. [Google Scholar]
- Zhou, G.-J.; Peng, F.-Q.; Zhang, L.-J.; Ying, G.-G. Biosorption of zinc and copper from aqueous solutions by two freshwater green microalgae Chlorella pyrenoidosa and Scenedesmus obliquus. Environ. Sci. Pollut. Res. 2012, 19, 2918–2929. [Google Scholar] [CrossRef]
- Da Silva Ferreira, V.; Sant’Anna, C. Impact of culture conditions on the chlorophyll content of microalgae for biotechnological applications. World J. Miocrobiol. Biotechnol. 2016, 33, 20. [Google Scholar] [CrossRef] [PubMed]
- Roopnarain, A.; Gray, V.M.; Sym, S.D. Phosphorus limitation and starvation effects on cell growth and lipid accumulation in Isochrysis galbana U4 for biodiesel production. Bioresour. Technol. 2014, 156, 408–411. [Google Scholar] [CrossRef]
- Ferroni, L.; Giovanardi, M.; Poggioli, M.; Baldisserotto, C.; Pancaldi, S. Enhanced photosynthetic linear electron flow in mixotrophic green microalga Ettlia oleoabundans UTEX 1185. Plant Physiol. Biochem. 2018, 130, 215–223. [Google Scholar] [CrossRef] [PubMed]
- Villarejo, A.; Orus, M.I.; Martinez, F. Coordination of photosynthetic and respiratory metabolism in Chlorella vulgaris UAM-101 in the light. Physiol. Plantarum 1995, 94, 680–686. [Google Scholar] [CrossRef]
- Hayes, M.; Skomedal, H.; Skjånes, K.; Mazur-Marzec, H.; Torùnska-Sitarz, A.; Catala, M.; Isleten Hosoglu, M.; García-Vaquero, M. Microalgal proteins for feed, food and health. In Microalgae-Based Biofuels and Bioproducts: From Feedstock Cultivation to End-Products; Gonzalez-Fernandez, C., Muñoz, R., Eds.; Woodhead Publishing, an Imprint of Elsevier: Duxford, UK, 2017; pp. 347–368. [Google Scholar]
- Solovchenko, A.; Verschoor, A.M.; Jablonowski, N.D.; Nedbal, L. Phosphorus from wastewater to crops: An alternative path involving microalgae. Biotechnol. Adv. 2016, 34, 550–564. [Google Scholar] [CrossRef] [PubMed]
- Powell, N.; Shilton, A.N.; Pratt, S.; Chisti, Y. Factors influencing luxury uptake of phosphorus by microalgae in waste stabilization ponds. Environ. Sci. Technol. 2008, 42, 287–293. [Google Scholar] [CrossRef] [PubMed]
- Solovchenko, A.; Khozin-Goldberg, I.; Selyakh, I.; Semenova, L.; Ismagulova, T.; Lukyanov, A.; Mamedov, I.; Vinogradova, E.; Karpova, O.; Konyukhov, I.; et al. Phosphorus starvation and luxury uptake in green microalgae revisited. Algal Res. 2019, 43, 101651. [Google Scholar] [CrossRef]
- Zhao, G.; Du, J.; Jia, Y.; Lv, Y.; Han, G.; Tian, X. The importance of bacteria in promoting algal growth in eutrophic lakes with limited available phosphorus. Ecol. Eng. 2012, 42, 107–111. [Google Scholar] [CrossRef]
- Ray, K.; Mukherjee, C.; Ghosh, A.N. A way to curb phosphorus toxicity in the environment: Use of polyphosphate reservoir of cyanobacteria and microalga as a safe alternative phosphorus biofertilizer for Indian agriculture. Environ. Sci. Technol. 2013, 47, 11378–11379. [Google Scholar] [CrossRef]
- Da Silva Cerozi, B.; Fitzsimmons, K. The effect of pH on phosphorus availability and speciation in an aquaponics nutrient solution. Bioresour. Technol. 2016, 219, 778–781. [Google Scholar] [CrossRef] [Green Version]
- Cai, T.; Park, S.Y.; Li, Y. Nutrient recovery from wastewater streams by microalgae: Status and prospects. Renew. Sust. Energ. Rev. 2013, 19, 360–369. [Google Scholar] [CrossRef]
- Shiraiva, Y.; Goyal, A.; Tolbert, N.E. Alkalinization of the medium by unicellular algae during uptake dissolved inorganic carbon. Plant Cell Physiol. 1993, 34, 649–657. [Google Scholar] [CrossRef]
- Grunditz, C.; Delhammar, G. Development of nitrification inhibition assays using pure cultures of Nitrosomonas and Nitrobacter. Water Res. 2001, 35, 433–441. [Google Scholar] [CrossRef]
- Culture Collection of Algae and Protozoa (CCAP, Scotland, UK). Available online: https://www.ccap.ac.uk/media/documents/BG11.pdf (accessed on 30 November 2020).
- Lee, K.; Eisterhold, M.L.; Rindi, F.; Palanisami, S.; Nam, P.K. Isolation and screening of microalgae from natural habitats in the midwestern United States of America for biomass and biodiesel sources. J. Nat. Sci. Biol. Med. 2014, 5, 333–339. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Culture Collection of Algae at the University of Texas at Austin (UTEX, Texas, USA). Available online: Web.biosci.utexas.edu/utex/Media%20PDF/Bristol-medium.pdf (accessed on 30 November 2020).
- Fabregas, J.; Abalde, J. Herrero, C.; Cabezas, B.; Veiga, M. Growth of the marine microalgae Tetraselmis suecica in batch cultures with different salinities. Aquaculture 1984, 42, 207–215. [Google Scholar] [CrossRef] [Green Version]
- Ansari, F.A.; Singh, P.; Guldhe, A.; Bux, F. Microalgal cultivation using aquaculture wastewater: Integrated biomass generation and nutrient remediation. Algal Res. 2017, 21, 169–177. [Google Scholar] [CrossRef]
- Tripathi, R.; Gupta, A.; Thakur, I.S. An integrated approach for phycoremediation of wastewater and sustainable biodiesel production by green microalgae, Scenedesmus sp. ISTGA1. Renew. Energ. 2019, 135, 617–625. [Google Scholar] [CrossRef]
- Baldisserotto, C.; Ferroni, L.; Giovanardi, M.; Pantaleoni, L.; Pancaldi, S. Salinity promotes growth of freshwater Neochloris oleoabundans UTEX 1185 (Sphaeropleales, Chlorophyta): Morphophysiological aspects. Phycologia 2012, 51, 700–710. [Google Scholar] [CrossRef]
- Giovanardi, M.; Ferroni, L.; Baldisserotto, C.; Tedeschi, P.; Maietti, A.; Pantaleoni, L.; Pancaldi, S. Morphophysiological analyses of Neochloris oleoabundans (Chlorophyta) grown mixotrophically in a carbon-rich waste product. Protoplasma 2013, 250, 161–174. [Google Scholar] [CrossRef]
- Popovich, C.A.; Damiani, C.; Constenla, D.; Martínez, A.M.; Freije, H.; Giovanardi, M.; Pancaldi, S.; Leonardi, P.I. Neochloris oleoabundans grown in enriched natural seawater for biodiesel feedstock: Evaluation of its growth and biochemical composition. Bioresour. Technol. 2012, 114, 287–293. [Google Scholar] [CrossRef]
- Bower, C.E.; Holm-Hansen, T. A salycilate-hypochlorite method for determining ammonia in seawater. Can. J. Fish. Aquat. Sci. 1980, 37, 794–798. [Google Scholar] [CrossRef]
- Wellburn, A.R. The spectral determination of chlorophyll-a and chlorophyll-b, as well as total carotenoids, using various solvents with spectrophotometers of different resolution. J. Plant Physiol. 1994, 144, 307–313. [Google Scholar] [CrossRef]
- Ferroni, L.; Baldisserotto, C.; Giovanardi, M.; Pantaleoni, L.; Morosinotto, T.; Pancaldi, S. Revised assignment of room-temperature chlorophyll fluorescence emission bands in single living cells of Chlamydomonas reinhardtii. J. Bioenerg. Biomembr. 2011, 43, 163–173. [Google Scholar] [CrossRef] [PubMed]
- Kalaji, H.M.; Schansker, G.; Ladle, R.J.; Goltsev, V.; Bosa, K.; Allakhverdiev, S.I.; Brestic, M.; Bussotti, F.; Calatayud, A.; Da˛browski, P.; et al. Frequently asked questions about in vivo chlorophyll fluorescence: Practical issues. Photosynth. Res. 2014, 122, 121–158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ivleva, N.B.; Golden, S.S. Protein extraction, fractionation, and purification from cyanobacteria, In: Methods in Molecular Biology™; E. Rosato, E., Ed.; Humana Press: Totowa, NJ, USA, 2007; Volume 362, pp. 365–373. [Google Scholar]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [PubMed]
- Mariotti, F.; Tomé, D.; Mirand, P. Converting nitrogen into protein—Beyond 6.25 and Jones’ factors. Crit. Rev. Food Sci. Nutr. 2008, 48, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Coppens, J.; Grunert, O.; Van Den Hende, S.; Vanhoutte, I.; Boon, N.; Haesaert, G.; De Gelder, L. The use of microalgae as a high-value organic slow-release fertilizer results in tomatoes with increased carotenoid and sugar levels. J. Appl. Phycol. 2016, 28, 2367–2377. [Google Scholar] [CrossRef]
- Henze, M.; Comeu, Y. Wastewater characterization. In Biological Wastewater Treatment—Principle, Modelling and Design; Henze, M., van Loosdrecht, M.C.M., Ekama, G.A., Brdjanovic, D., Eds.; IWA Publishing: London, UK; Cambridge University Press: Cambridge, UK, 2008; pp. 33–52. [Google Scholar]
- Spina, F.; Tigini, V.; Romagnolo, A.; Varese, G.C. Bioremediation of landfill leachate with fungi: Autochthonous vs. allochthonous strains. Life 2018, 8, 27. [Google Scholar] [CrossRef] [Green Version]



| Parameter | Unit | Analytical Method | UWW |
|---|---|---|---|
| Total nitrogen | mg N/L | UNI EN 12260:2004 | 61.8 |
| Ammonium | mg NH4 – N/L | APAT CNR IRSA 4030 A1 Man 29 2003 | 66.5 |
| Nitrate | mg NO3 – N/L | APAT CNR IRSA 4020 Man 29 2003 | <0.5 |
| Nitrite | mg NO2 – N/L | APAT CNR IRSA 4050 Man 29 2003 | <0.04 |
| Total phosphorus | mg P/L | UNI EN ISO 15587-2:2002 + UNI EN ISO 17294-2:2016 | 24.8 |
| BOD-5 | mg O2/L | APHA Standard Methods for the Examination of Water and Wastewater ed 23rd 2017 5210 | 95 |
| COD | mg O2/L | ISO 15705 par 10.2:2002 | 222 |
| Cr | mg/L | UNI EN ISO 15587-2:2002 + UNI EN ISO 17294-2:2016 | <0.02 |
| Cr (VI) | mg/L | APAT CNR IRSA 3150 C Man 29 2003 | <0.02 |
| Cu | mg/L | UNI EN ISO 1187-2_2002 + UNI EN ISO 17294-2:2016 | 0.052 |
| Hg | mg/L | UNI EN ISO 1187-2_2002 + UNI EN ISO 17294-2:2016 | <0.001 |
| Ni | mg/L | UNI EN ISO 1187-2_2002 + UNI EN ISO 17294-2:2016 | <0.01 |
| Pb | mg/L | UNI EN ISO 1187-2_2002 + UNI EN ISO 17294-2:2016 | <0.005 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baldisserotto, C.; Demaria, S.; Accoto, O.; Marchesini, R.; Zanella, M.; Benetti, L.; Avolio, F.; Maglie, M.; Ferroni, L.; Pancaldi, S. Removal of Nitrogen and Phosphorus from Thickening Effluent of an Urban Wastewater Treatment Plant by an Isolated Green Microalga. Plants 2020, 9, 1802. https://doi.org/10.3390/plants9121802
Baldisserotto C, Demaria S, Accoto O, Marchesini R, Zanella M, Benetti L, Avolio F, Maglie M, Ferroni L, Pancaldi S. Removal of Nitrogen and Phosphorus from Thickening Effluent of an Urban Wastewater Treatment Plant by an Isolated Green Microalga. Plants. 2020; 9(12):1802. https://doi.org/10.3390/plants9121802
Chicago/Turabian StyleBaldisserotto, Costanza, Sara Demaria, Ornella Accoto, Roberta Marchesini, Marcello Zanella, Linda Benetti, Francesco Avolio, Michele Maglie, Lorenzo Ferroni, and Simonetta Pancaldi. 2020. "Removal of Nitrogen and Phosphorus from Thickening Effluent of an Urban Wastewater Treatment Plant by an Isolated Green Microalga" Plants 9, no. 12: 1802. https://doi.org/10.3390/plants9121802
APA StyleBaldisserotto, C., Demaria, S., Accoto, O., Marchesini, R., Zanella, M., Benetti, L., Avolio, F., Maglie, M., Ferroni, L., & Pancaldi, S. (2020). Removal of Nitrogen and Phosphorus from Thickening Effluent of an Urban Wastewater Treatment Plant by an Isolated Green Microalga. Plants, 9(12), 1802. https://doi.org/10.3390/plants9121802






