Anti-Proliferative and Apoptotic Induction Effect of Elateriospermum Extract on Human Lung Cancer Cell Line A549 †
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Hot and Cold Water Extraction
2.3. Cells, Reagent, and Antibodies
2.4. Cell Viability Assay
2.5. Analysis of Apoptosis by Hoechst 33358
2.6. Detection of Intracellular Reactive Oxygen Species (ROS)
3. Results
3.1. Inhibitory Effects of E. tapos Extracts on A549 Cell Line
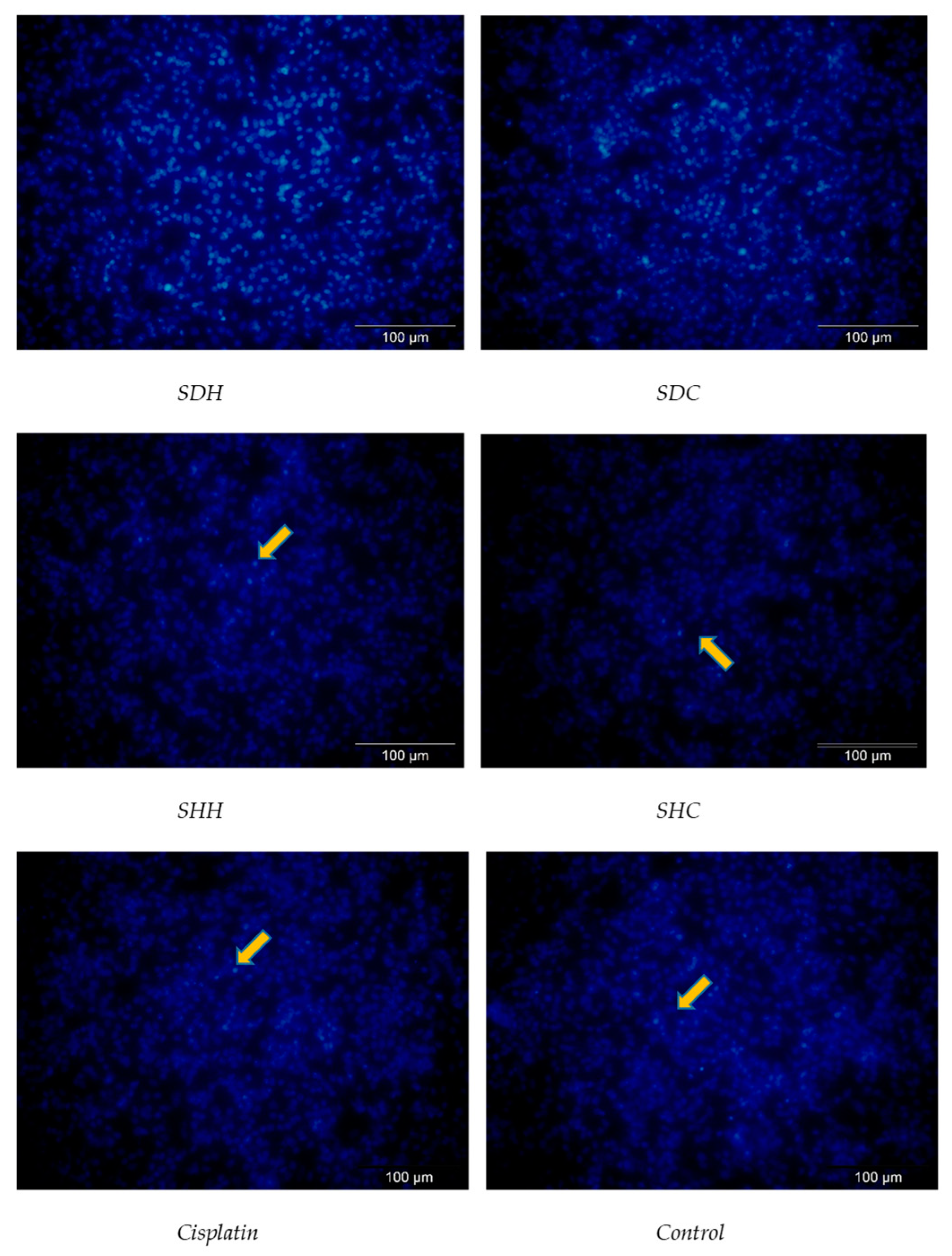
3.2. Effects of E. tapos Extracts on Hoechst 33358 Staining
3.3. The Effects E. tapos Extracts on ROS on Treated A549 Cell Line
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Yong, O.Y.; Salimon, J. Characteristics of Elateriospermum tapos seed oil as a new source of oilseed. Ind. Crop. Prod. 2006, 24, 146–151. [Google Scholar] [CrossRef]
- Husin, N.; Tan, N.A.H.; Muhamad, I.I.; Nawi, N.M. Physicochemical and Biochemical Characteristics of the Underutilized Elateriospermum Tapos. J. Teknol. 2013, 64, 57–61. [Google Scholar] [CrossRef][Green Version]
- Lim, T.K. Elateriospermum tapos. In Edible Medicinal and Non-Medicinal Plants; Springer: Berlin/Heidelberg, Germany, 2012; pp. 472–475. [Google Scholar]
- Dandona, P.; Kumar, V.; Aljada, A.; Ghanim, H.; Syed, T.; Hofmayer, D.; Mohanty, P.; Tripathy, D.; Garg, R. Angiotensin II Receptor Blocker Valsartan Suppresses Reactive Oxygen Species Generation in Leukocytes, Nuclear Factor-κB, in Mononuclear Cells of Normal Subjects: Evidence of an Antiinflammatory Action. J. Clin. Endocrinol. Metab. 2003, 88, 4496–4501. [Google Scholar] [CrossRef] [PubMed]
- Perumal, K.V.; Ja’Afar, N.L.; Balan, S.S.; Abidin, A.Z.; Arapoc, D.J.; Shafie, N.H.; Bahari, H. Preventive effect of Elateriospermum tapos seed extract against obese Sprague Dawley rats. Adv. Tradit. Med. 2019, 20, 107–113. [Google Scholar] [CrossRef]
- Sita, T.; Thanaset, S.; Prasan, S.; Auamporn, R. Antioxidant and antiproliferative activities of ethanolic extracts of Elateriospermum tapos Blume (Euphorbiaceae). J. Med. Plants Res. 2018, 12, 474–482. [Google Scholar] [CrossRef]
- Pattamadilok, D.; Suttisri, R. Seco-Terpenoids and Other Constituents fromElateriospermum tapos. J. Nat. Prod. 2008, 71, 292–294. [Google Scholar] [CrossRef] [PubMed]
- Attafi, I.M.; Bakheet, S.A.; Korashy, H.M.; AlBakheet, S.A. The role of NF-κB and AhR transcription factors in lead-induced lung toxicity in human lung cancer A549 cells. Toxicol. Mech. Methods 2019, 30, 197–207. [Google Scholar] [CrossRef] [PubMed]
- Torre, L.A.; Siegel, R.L.; Jemal, A. Lung Cancer Statistics. Lung Cancer Pers. Med. 2015, 893, 1–19. [Google Scholar] [CrossRef]
- Homet, B.; Ribas, A. New drug targets in metastatic melanoma. J. Pathol. 2013, 232, 134–141. [Google Scholar] [CrossRef] [PubMed]
- Goldstraw, P.; Chansky, K.; Crowley, J.J.; Rami-Porta, R.; Asamura, H.; Eberhardt, W.E.E.; Nicholson, A.G.; Groome, P.; Mitchell, A.; Bolejack, V.; et al. The IASLC Lung Cancer Staging Project: Proposals for Revision of the TNM Stage Groupings in the Forthcoming (Eighth) Edition of the TNM Classification for Lung Cancer. J. Thorac. Oncol. 2016, 11, 39–51. [Google Scholar] [CrossRef] [PubMed]
- Lim, T.K. Edible Medicinal and Non-Medicinal Plants; Springer: Dordrecht, The Netherlands, 2012; Volume 1, pp. 285–292. [Google Scholar]
- Thiagarajan, S.K.; Arapoc, D.J.; Shafie, N.H.; Yong, Y.K.; Bahari, H.; Adam, Z.; Ei, T. Momordica charantia (Indian and Chinese Bitter Melon) Extracts Inducing Apoptosis in Human Lung Cancer Cell Line A549 via ROS-Mediated Mitochodria Injury. Evid. Based Complement. Altern. Med. 2019, 2019, 2821597–2821599. [Google Scholar] [CrossRef] [PubMed]
- Redza-Dutordoir, M.; Averill-Bates, D.A. Activation of apoptosis signalling pathways by reactive oxygen species. Biochim. Biophys. Acta (BBA) Bioenerg. 2016, 1863, 2977–2992. [Google Scholar] [CrossRef] [PubMed]
- Nor-Liyana, J.; Siroshini, K.T.; Nurul-Syahirah, M.B.; Chang, W.L.; Nurul-Husna, S.; Daryl, J.A.; Hasnah, B. Phytochemical analysis of Elateriospermum tapos and its inhibitory effects on alpha-amylase, alpha-glucosidase and pancreatic lipase. J. Trop. For. Sci. 2019, 31, 240–248. [Google Scholar]
- Zhang, H.-W.; Hu, J.-J.; Fu, R.-Q.; Liu, X.; Zhang, Y.-H.; Li, J.; Liu, L.; Li, Y.-N.; Deng, Q.; Luo, Q.-S.; et al. Flavonoids inhibit cell proliferation and induce apoptosis and autophagy through downregulation of PI3Kγ mediated PI3K/AKT/mTOR/p70S6K/ULK signaling pathway in human breast cancer cells. Sci. Rep. 2018, 8, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Badroon, N.A.; Majid, N.A.; Alshawsh, M.A. Antiproliferative and Apoptotic Effects of Cardamonin against Hepatocellular Carcinoma HepG2 Cells. Nutrients 2020, 12, 1757. [Google Scholar] [CrossRef] [PubMed]
- Abotaleb, M.; Samuel, S.M.; Varghese, E.; Varghese, S.; Kubatka, P.; Liskova, A.; Büsselberg, D. Flavonoids in Cancer and Apoptosis. Cancers 2018, 11, 28. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Jiang, C.; Xiong, C.; Ruan, J. DEDC, a new flavonoid induces apoptosis via a ROS-dependent mechanism in human neuroblastoma SH-SY5Y cells. Toxicol. Vitr. 2012, 26, 16–23. [Google Scholar] [CrossRef]

| E. tapos | Solvents | Cancer cell line A549 (µg/mL) | Normal cell line MRC5 (µg/mL) | |
|---|---|---|---|---|
| Plant extracts | Seed (SD) | Hot Aqueous (H) | 45.8 ± 0.15 | >500 |
| Cold Aqueous (C) | 28.6 ± 0.18 | >500 | ||
| Shell (SH) | Hot Aqueous (H) | 49.8 ± 0.06 | >500 | |
| Cold Aqueous (C) | 75.9 ± 0.19 | >500 | ||
| Standard drug | Cisplatin | 17.3 ± 0.01 | 13.2 ± 0.05 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thiagarajan, S.K.; Perumal, K.V.; Shafie, N.H.; Kadir, K.K.A.; Bahari, H. Anti-Proliferative and Apoptotic Induction Effect of Elateriospermum Extract on Human Lung Cancer Cell Line A549. Proceedings 2020, 61, 4. https://doi.org/10.3390/IECN2020-06985
Thiagarajan SK, Perumal KV, Shafie NH, Kadir KKA, Bahari H. Anti-Proliferative and Apoptotic Induction Effect of Elateriospermum Extract on Human Lung Cancer Cell Line A549. Proceedings. 2020; 61(1):4. https://doi.org/10.3390/IECN2020-06985
Chicago/Turabian StyleThiagarajan, Siroshini K., Kokila Vani Perumal, Nurul Husna Shafie, Khairul Kamilah Abdul Kadir, and Hasnah Bahari. 2020. "Anti-Proliferative and Apoptotic Induction Effect of Elateriospermum Extract on Human Lung Cancer Cell Line A549" Proceedings 61, no. 1: 4. https://doi.org/10.3390/IECN2020-06985
APA StyleThiagarajan, S. K., Perumal, K. V., Shafie, N. H., Kadir, K. K. A., & Bahari, H. (2020). Anti-Proliferative and Apoptotic Induction Effect of Elateriospermum Extract on Human Lung Cancer Cell Line A549. Proceedings, 61(1), 4. https://doi.org/10.3390/IECN2020-06985




