1. Introduction
Obesity is an increasingly growing pandemic with significant public health implications. This pandemic has been initiated by a sedentary lifestyle and continuous availability of high caloric food options, with higher prevalence in women than in men, and is associated with such disorders as cardiovascular disease, certain types of cancer and type 2 diabetes [
1]. Evidence has been increasing in recent years that stress, particularly an increase in the glucocorticoid stress hormone cortisol, plays a role in the development of obesity [
2]. A glucocorticoid secreted by the cortex of the adrenal gland is corticosterone. Corticosterone is formed by the adrenocorticotropic hormone (ACTH) in response to adrenal cortex stimulation and is the precursor of aldosterone. Cortisol is known to induce redistribution to the abdominal area of white adipose tissue and often increases appetite with a desire for energy-dense food (“comfort food”) [
3]. Abdominal obesity, metabolic syndrome (MetS) and ultimately cardiovascular diseases (CVDs) arise in patients that are chronically exposed to elevated levels of glucocorticoids, such as in Cushing’s syndrome. Interestingly, the obesity pandemic in our modern society coincides with an increase in factors that stimulate the production of cortisol, such as chronic stress, high glycemic index food intake and decreased sleep levels [
2]. This demonstrates a vicious cycle in which increased glucocorticoid production, obesity and stress combine and exacerbate each other [
4].
Increased use of traditional herbal medicine has contributed to the prevalent side effects of conventional obesity treatment, such as headache, constipation, heart arrhythmia and more diseases. As a result, procedures using natural ingredients with comparatively healthy and fewer side effects are gaining interest in curing obesity [
5].
Elateriospermum tapos is a tropical canopy found mostly in Southeast Asia, including Peninsular Thailand, Peninsular Malaysia, Sumatra, Java and Borneo’s tropical rainforests. White and sticky latex, traditionally used in the healing of wounds and broken soles in the treatment of feet [
6,
7], is present in
E. tapos bark, leaves and fruit stalks. The predominant polyunsaturated fatty acid,
E. tapos linolenic acid seed oil, has been of great benefit in the treatment of chronic diseases [
6]. In 2019, Nor Liyana et al. [
8] showed that
E. tapos shell hot water extraction contains high levels of phenols and flavonoids, highlighting the potential of
E. tapos as an anti-obesity agent [
9]. The study has been extended, and the anti-obesity effect of
E. tapos shell extraction has been shown to decrease body weight and calorie intake in rats fed a high-fat diet [
10]. Further research by Santhra et al. [
11] on the effects of
E. tapos seed and shell extraction on offspring at post-natal day 21 of obese dams showed that weight loss was promoted by the extraction of
E. tapos in the dams and their offspring. This research aimed to investigate the impact of
E. tapos in ameliorating obesity development and stress hormone levels among adult male offspring.
2. Materials and Methods
2.1. Plant Material
E. tapos fruit was collected from Pahang, Malaysia, and the Biodiversity Unit, Institute of Bioscience, Universiti Putra Malaysia (voucher number SK3154/17) carried out the identification of the species. The fruit was dried overnight for 24 h in an oven with the temperature of 60 °C, followed by seed segregation from the shell, grounded and sieved. Fifty grams of mashed
E. tapos (seed or shell) were added to 500 mL of distilled water in a 1 L Scott bottle wrapped in aluminum foil and held for 24 h in a water bath at 70 °C followed by a filtration process using Whatman paper No 1. The filtered solution was then concentrated using the freeze dryer (Scanvac). Until further use, the prepared sample was held at −20 °C [
10,
11,
12].
2.2. Experimental Animal and Diet
All animal-related procedures were conducted under the approval of the Management & Science University’s Animal Care and Use Committee (AE-MSU-073). In this study, thirty female Sprague Dawley rats weighing between 150 and 200 g were used. The rats were housed in plastic boxes (22 cm height × 65 cm length × 40 cm width) with two rats in each box. All rats were acclimatized for 1 week in a temperature-controlled room (22 ± 3 °C) on a 12/12 h light/dark cycle. The rats were fed a standard chow diet, and water was available ad libitum.
Six rats that were fed with regular chow were allocated to the normal diet group (Group 1, DND) and the rest of the rats were assigned to the high-fat diet group (HFD), which were fed with both high-fat diets and selected cafeteria foods like 440 kcal/100 g cake, 260 kcal/100 g sausage and 566 kcal/100 g extruded savory snacks. In the cafeteria diet, nutritional products were chosen to reflect the variety, palatability and energy density of the modern western diet.
Obesity was verified after 5 weeks by comparing the significant 15 percent bodyweight difference between the DND and HFD groups [
13]. The HFD groups were further divided into 4 groups according to their treatment: Group 2, DNC (dams negative control, normal saline); Group 3, DPC (dams positive control, 200 mg/kg BW of orlistat); Group 4, DTX1 (dams treatment 1, 200 mg/kg BW of
E. tapos seed supplementation); and Group 5, DTX2 (dams treatment 2, 200 mg/kg BW of
E. tapos shell supplementation). The treatment period was continued for 6 weeks. Calorie intake and bodyweight were measured weekly.
2.3. High-Fat Diet
The high-fat diet was composed of 414 kcal/100 g, with 17% protein, 40% fat and 43% carbohydrate. All ingredients were blended and contained 6% corn oil (Vecorn,Yee Lee Corporation Berhad, Kuala Lumpur, Malaysia), 6% ghee (Crispo, Crispo-Tato (M) Sdn Bhd, Kuala Lumpur, Malaysia), 20% milk powder (Dutch lady, Dutch Lady Milk Industries Berhad, Selangor, Malaysia) and 68% standard chow pellet (Gold Coin Feedmills (M) Sdn Bhd, Selangor, Malaysia). Standard chow pellet contained 306.2 kcal/100 g with 21% protein, 3% fat and 48.8% carbohydrate [
10,
11].
2.4. Mating, Gestation and Offspring
Female rats were mating after 6 weeks of treatment, and vaginal smears were conducted at 8 a.m. the next morning to search for sperm as evidence of successful mating, and this was designated as day 0 of gestation. Litter sizes were equalized to 8 to 12 pups per dam within 2 days of birth. The offspring were designated according to their dam’s group: Group 1a (OND, offspring normal diet) and Group 1b (OCD, offspring cafeteria diet) both from Group 1 dams; Group 2a (ONC, offspring negative control); Group 3a (OPC, offspring positive control); Group 4a (OTX1, offspring treatment 1, E. tapos seed); and Group 5a (OTX2, offspring treatment 2, E. tapos shell). After the weaning phase, offspring were fed standard chow and cafeteria diet except for Group 1a offspring which were fed with standard chow only.
2.5. Blood and Tissue Collection
Fasting blood samples were obtained from each animal at week 12 of age in the control and test group via cardiac puncture after each rat had been anesthetized. Each blood sample was collected into heparin tubes and centrifuged at 3500 rpm for 15 min to obtain the plasma. Plasma was collected into a plain tube and stored at −20 °C until further analysis.
2.6. Determination of Corticosterone and ACTH in Plasma
Corticosterone and ACTH concentration of 12-weeks-old female offspring were determined with ELISA kit (Elabscience Biotechnology Co.,Ltd, USA) utilized the principle of competitive ELISA. The micro ELISA plate provided in this kit was pre-coated with human corticosterone or ACTH. During the reaction, rat corticosterone or ACTH in the sample or standard competes with a fixed amount of human corticosterone or ACTH on the solid phase supporter for sites on the biotinylated detection Ab specific to human ACTH. Excess conjugate and unbound sample or standard were washed from the plate, and avidin conjugated to horseradish peroxidase (HRP) were added to each microplate well and incubated. Then a TMB substrate solution was added to each well. The enzyme-substrate reaction was terminated by the addition of stop solution, and the color change was measured spectrophotometrically at a wavelength of 450 nm ± 2 nm. The concentration of rat corticosterone and ACTH in the samples was then determined by comparing the optical density of the samples to the standard curve.
2.7. Statistical Analysis
Data were analyzed with IBM Statistics 25.0 Windows. Results were expressed as mean ± standard error of mean (SEM). Data normality was evaluated using a normality test. Dams’ bodyweight, offspring bodyweight and level of corticosterone and ACTH in plasma were analyzed by one-way ANOVA followed by post-hoc LSD. Probability of p < 0.05 was considered as statistically significant.
4. Discussion
For proper development during childhood and adulthood, nutrition given during the prenatal process is important. In adulthood, offspring fed a high-fat diet are at high risk of obesity and metabolic disorders. In our study, we clarified the potential of
E. tapos to improve the effect of maternal obesity on dams and the bodyweight of their male offspring and the plasma level of stress hormones which are ACTH and corticosterone. A study conducted by Baranowska et al. [
14] found that maternal HFD had contributed to obesity and metabolic disorders in both male and female offspring during mating, pregnancy and lactation. The finding that male adult offspring from HFD dams were more obese compared to offspring from normal diet and treatment groups was confirmed in our research. As early as week 4 of age, male offspring on HFD had higher bodyweight.
However, at six weeks of age onward, adult male offspring from dams treated with
E. tapos shell (OTX2) had the lowest bodyweight among all groups. Recent research by Nor Liyana et al. [
8] indicated that
E. tapos shell is a potent source of natural anti-oxidant and has a high compound of flavonoids and phenols. Similarly, in this research, compared to seed extraction,
E. tapos shell extract had better anti-obesity activity. Before pregnancy, treatment with
E. tapos shell extraction can reduce the bodyweight of obese female rats. In the second generation of female rats, the anti-obesity effect of the
E. tapos shell may be observed. The
E. tapos seed and shell have also been reported to act as an inhibitor of pancreatic lipase [
8]. Pancreatic lipase inhibition may decrease fat ingestion and thus energy absorption, which is one of the main targets thought to mediate obesity [
15]. This study showed that obese dams will produce high-bodyweight offspring which leads to more complications in serious illnesses. The current research showed that the DNC group fed a five-week HFD and cafeteria diet had an increase in the bodyweight and adiposity of their male offspring. A comparable study by Santhra et al. [
11] also showed that pre-pregnancy supplementation of
E. tapos shell extraction among obese dams showed an anti-obesity effect on their offspring as early as post-natal day 21. This finding highlights the importance of human beings changing their way of eating and having a healthy lifestyle to generate a physically and mentally healthy new generation. In their reproductive age, supplementation of
E. tapos among females will help cut off the obesity chain in the cycle of human life.
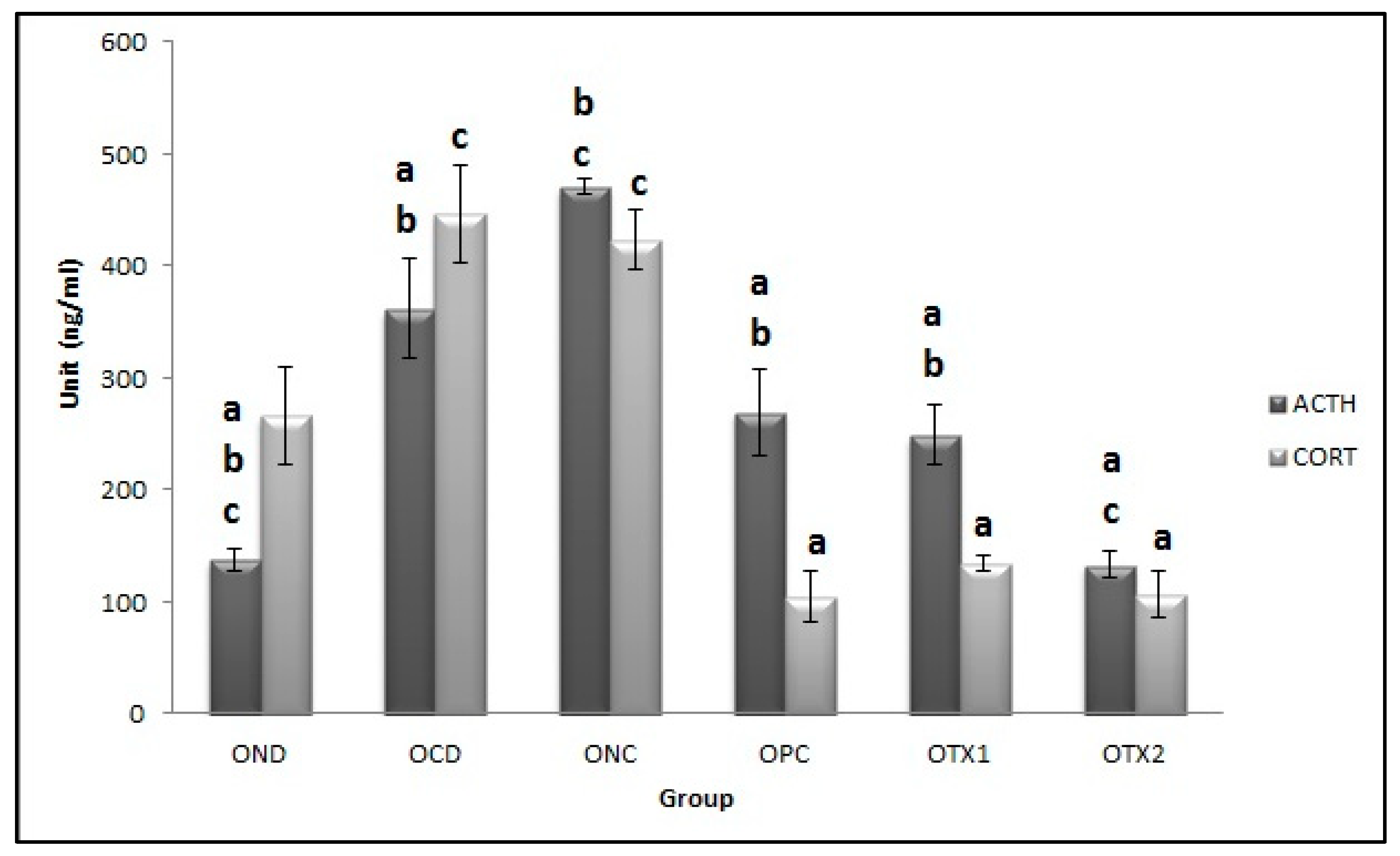
Higher cortisol concentrate has been causally related physiologically to fat build-up and weight increment, as glucocorticoids facilitate pre-adipocyte conversion to mature adipocytes. Research by Rebuffé-Scrive and colleagues proved that male Sprague Dawley rats that had been stressed for 28 days had significantly more enlarged adipocytes than controls and had a propensity to show a heavier abdominal wall fat pad [
16]. This finding was consistent with our findings that the male offspring from HFD dams had higher total bodyweight, ACTH and corticosterone plasma level as compared to male offspring from the normal diet dam group. A similar study by Buchenauer et al. [
17] showed that obese rats had a significantly higher corticosterone level as compared to lean rats. Cortisol also drives insulin resistance via the increase of adipocytokines and the secretion of pro-inflammatory cytokines. Stress-related cortisol concentrations play a significant role in adipocyte biology and weight gain, potentially implicating it as a key component in the development of obesity [
18]. In our research, the increased level of plasma ACTH and corticosterone in offspring from HFD dams had been ameliorated by the supplementation of
E. tapos seed and shell. This was shown by the lower level of ACTH and corticosterone in male adult offspring from dams supplemented with
E. tapos seed and shell compared to offspring from the HFD group. Previous research that used obese Zucker rats found that 11β-HSD1 was elevated in adipose cells, probably enhancing the initiation of local glucocorticoids and thus stimulating obesity. Other research, in rodent models of obesity of various etiologies, has mostly confirmed increased 11β-HSD1 activity and mRNA levels selectively in fat tissue but not in the hepar. Genetically modified mice selectively over-expressing 11β-HSD1 in fat tissue had features of visceral obesity and chronic diseases such as diabetes mellitus, hyperlipidemia and high blood pressure [
18].
E. tapos seed and shell possibly act in the deactivation of corticosterone by suppressing the 11β-HSD1 expression.
Therefore, this research proved that E. tapos seed and shell supplementation in obese dams prior to pregnancy has the potential to alleviate the stress hormone in their offspring. Data from this study suggested that E. tapos seed and shell extraction may attenuate the corticosterone and ACTH level, therefore reducing stress among obese rats which leads to reduced fat accumulation and weight gain. In order to augment the success of E. tapos weight-loss treatments and ameliorate the stress hormone, suggested further research is to analyze the effect of E. tapos on the hormone of the hypothalamic-pituitary-adrenal (HPA) axis to investigate the effect of E. tapos in controlling stress hormone which commonly causes obesity and memory and cognitive deficit in the mother and her offspring.