Exploring the Mechanism of Gyejibokryeong-hwan against Atherosclerosis Using Network Pharmacology and Molecular Docking
Abstract
1. Introduction
2. Results
2.1. Components of GBH
2.2. Absorption, Distribution, Metabolism, and Excretion (ADME) Screening of Selected GBH Components
2.3. Relationships between Target Compounds and Genes
2.4. Network Analysis of Potential Compounds and Target Genes
2.5. Pathway Analysis of Potential Targets of GBH
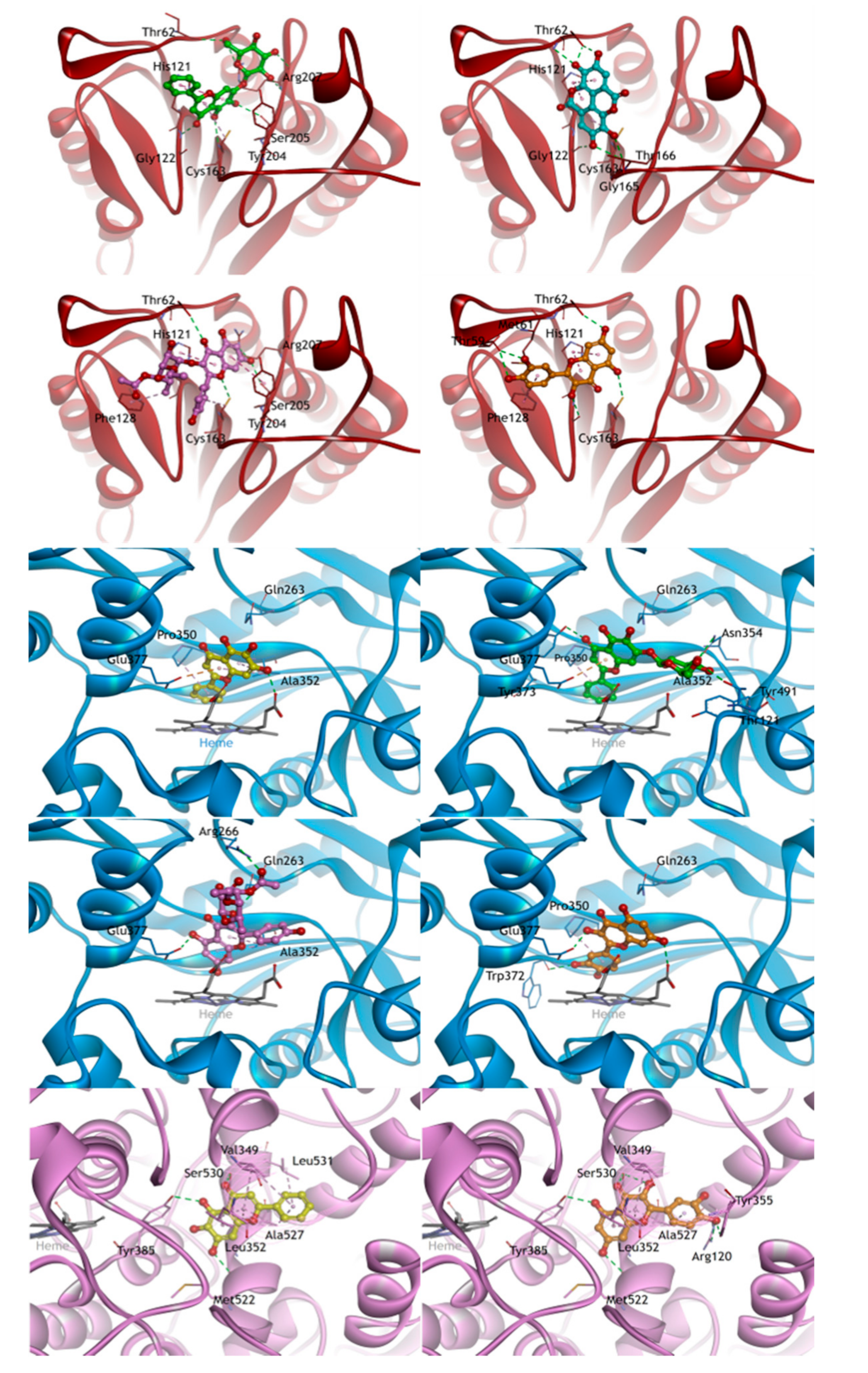
2.6. Molecular Docking Studies
3. Discussion
4. Materials and Methods
4.1. Selection of Compounds in GBH
4.2. Pharmacokinetic ADME Screening
4.3. Target Genes Related to the Selected Ingredients
4.4. Potential Target Genes and Construction of Networks
4.5. Pathway Analyses
4.6. Molecular Docking Studies
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ADME | Absorption, distribution, metabolism, and exertion |
| COX-2 | Cyclooxygenase-2 |
| DAVID | Database for Annotation, Visualization and Integrated Discovery |
| DL | Drug likeness |
| GBH | Gyejibokryeong-hwan |
| iNOS | inducible nitric oxide synthase |
| KEGG | Kyoto Encyclopedia of Genes and Genomes |
| OB | Oral bioavailability |
| TCMSP | Traditional Chinese Medicine System Pharmacology Database and Analysis Platform |
| TNF | Tumor necrosis factor |
References
- WHO. WHO International Standard Terminologies on Traditional Medicine in the Western Pacific Region; WHO Western Pacific Region: Manila, Philippines, 2007. [Google Scholar]
- Park, B.; You, S.; Jung, J.; Lee, J.A.; Yun, K.J.; Lee, M.S. Korean studies on blood stasis: An overview. Evid. Based Complement. Altern. Med. 2015, 2015, 316872. [Google Scholar] [CrossRef]
- Cho, K.H.; Kim, K.P.; Woo, B.C.; Kim, Y.J.; Park, J.Y.; Cho, S.Y.; Park, S.U.; Jung, W.S.; Park, J.M.; Moon, S.K. Relationship between Blood Stasis Syndrome Score and Cardioankle Vascular Index in Stroke Patients. Evid. Based Complement. Altern. Med. 2012, 2012, 696983. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.Y.; Li, C.J.; Hou, M.F.; Chu, P.Y. New Insights into the Role of Inflammation in the Pathogenesis of Atherosclerosis. Int. J. Mol. Sci. 2017, 18, 2034. [Google Scholar] [CrossRef] [PubMed]
- Jaipersad, A.S.; Lip, G.Y.; Silverman, S.; Shantsila, E. The role of monocytes in angiogenesis and atherosclerosis. J. Am. Coll. Cardiol. 2014, 63, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Pan, G.F.; Zhang, X.D.; Wang, J.L.; Wang, W.D.; Zhang, J.Y.; Wang, H.; Liang, R.X.; Sun, X.B. Yindanxinnaotong, a Chinese compound medicine, synergistically attenuates atherosclerosis progress. Sci. Rep. 2015, 5, 12333. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.Y.; Park, W.; Kang, T.W.; Cha, M.H.; Chun, J.M. Network pharmacology-based prediction of active compounds and molecular targets in Yijin-Tang acting on hyperlipidaemia and atherosclerosis. J. Ethnopharmacol. 2018, 221, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Li, J.; Hartstone-Rose, A.; Wang, J.; Li, J.; Janicki, J.S.; Fan, D. Chinese Herbal Compounds for the Prevention and Treatment of Atherosclerosis: Experimental Evidence and Mechanisms. Evid. Based Complement. Altern. Med. 2015, 2015, 752610. [Google Scholar] [CrossRef]
- Shin, H.S.; Han, J.M.; Kim, H.G.; Choi, M.K.; Son, C.G.; Yoo, H.R.; Jo, H.K.; Seol, I.C. Anti-atherosclerosis and hyperlipidemia effects of herbal mixture, Artemisia iwayomogi Kitamura and Curcuma longa Linne, in apolipoprotein E-deficient mice. J. Ethnopharmacol. 2014, 153, 142–150. [Google Scholar] [CrossRef]
- Kim, J.H.; Seo, C.S.; Shin, H.K. Development of validated determination of the eleven marker compounds in Gyejibokryeong-hwan for the quality assessment using HPLC analysis. Arch. Pharm. Res. 2015, 38, 52–62. [Google Scholar] [CrossRef]
- Lei, X.; Chen, J.; Liu, C.X.; Lin, J.; Lou, J.; Shang, H.C. Status and thoughts of Chinese patent medicines seeking approval in the US market. Chin. J. Integr. Med. 2014, 20, 403–408. [Google Scholar] [CrossRef]
- Kim, J.E.; Cho, J.; Kwon, O.; Kim, A.R.; Park, H.J.; Jung, S.Y.; Kim, J.H.; Kim, M.; Lee, H.Y.; Lee, J.H. Effect of Guizhifulingwan (Keishibukuryogan) on climacteric syndrome: Study protocol for a randomized controlled pilot trial. Trials 2017, 18, 135. [Google Scholar] [CrossRef] [PubMed]
- Hikiami, H.; Goto, H.; Sekiya, N.; Hattori, N.; Sakakibara, I.; Shimada, Y.; Terasawa, K. Comparative efficacy of Keishi-bukuryo-gan and pentoxifylline on RBC deformability in patients with “oketsu” syndrome. Phytomedicine 2003, 10, 459–466. [Google Scholar] [CrossRef] [PubMed]
- Terauchi, M.; Akiyoshi, M.; Owa, Y.; Kato, K.; Obayashi, S.; Kubota, T. Effects of the Kampo medication keishibukuryogan on blood pressure in perimenopausal and postmenopausal women. Int. J. Gynaecol. Obstet. 2011, 114, 149–152. [Google Scholar] [CrossRef] [PubMed]
- Sekiya, N.; Goto, H.; Tazawa, K.; Oida, S.; Shimada, Y.; Terasawa, K. Keishi-bukuryo-gan preserves the endothelium dependent relaxation of thoracic aorta in cholesterol-fed rabbit by limiting superoxide generation. Phytother. Res. 2002, 16, 524–528. [Google Scholar] [CrossRef] [PubMed]
- Terawaki, K.; Noguchi, M.; Yuzurihara, M.; Omiya, Y.; Ikarashi, Y.; Kase, Y. Keishibukuryogan, a Traditional Japanese Medicine, Inhibits Platelet Aggregation in Guinea Pig Whole Blood. Evid. Based Complement. Altern. Med. 2015, 2015, 295706. [Google Scholar] [CrossRef]
- Qian, W.; Hasegawa, J.; Tsuno, S.; Endo, Y.; Matsuda, A.; Miura, N. Effects of kampo formulas on the progression of hypercholesterolemia and Fatty liver induced by high-cholesterol diet in rats. Yonago Acta Medica 2014, 57, 147–158. [Google Scholar]
- Tomita, T.; Hirayama, A.; Matsui, H.; Aoyagi, K. Effect of Keishibukuryogan, a Japanese Traditional Kampo Prescription, on Improvement of Microcirculation and Oketsu and Induction of Endothelial Nitric Oxide: A Live Imaging Study. Evid. Based Complement. Altern. Med. 2017, 2017, 3620130. [Google Scholar] [CrossRef]
- Wang, D.; Tian, L.; Shi, C.; Wei, Y.X.; Wang, H.; Liu, T.T.; Gong, M.; Zhang, Y.W.; Yu, R.G.; Wu, X.H. Network pharmacology-based prediction of the active ingredients and mechanism of Shen Gui capsule for application to coronary heart disease. Comput. Biol. Med. 2020, 122, 12. [Google Scholar] [CrossRef]
- Hong, M.; Li, S.; Tan, H.Y.; Cheung, F.; Wang, N.; Huang, J.; Feng, Y. A Network-Based Pharmacology Study of the Herb-Induced Liver Injury Potential of Traditional Hepatoprotective Chinese Herbal Medicines. Molecules 2017, 22, 632. [Google Scholar] [CrossRef]
- Sidders, B.; Karlsson, A.; Kitching, L.; Torella, R.; Karila, P.; Phelan, A. Network-Based Drug Discovery: Coupling Network Pharmacology with Phenotypic Screening for Neuronal Excitability. J. Mol. Biol. 2018, 430, 3005–3015. [Google Scholar] [CrossRef]
- Liu, C.; Liu, L.; Li, J.; Zhang, Y.; Meng, D.-L. Virtual screening of active compounds from jasminum lanceolarium and potential targets against primary dysmenorrhea based on network pharmacology. Nat. Prod. Res. 2020, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Gohlke, H.; Klebe, G. Approaches to the description and prediction of the binding affinity of small-molecule ligands to macromolecular receptors. Angew. Chem. Int. Ed. Engl. 2002, 41, 2644–2676. [Google Scholar] [CrossRef]
- Kuntz, I.D.; Blaney, J.M.; Oatley, S.J.; Langridge, R.; Ferrin, T.E. A geometric approach to macromolecule-ligand interactions. J. Mol. Biol. 1982, 161, 269–288. [Google Scholar] [CrossRef]
- Chen, L.; Wang, D.; Wu, J.; Yu, B.; Zhu, D. Identification of multiple constituents in the traditional Chinese medicine formula GuiZhiFuLing-Wan by HPLC-DAD-MS/MS. J. Pharm. Biomed. Anal. 2009, 49, 267–275. [Google Scholar] [CrossRef] [PubMed]
- Jiagang, D.; Li, C.; Wang, H.; Hao, E.; Du, Z.; Bao, C.; Lv, J.; Wang, Y. Amygdalin mediates relieved atherosclerosis in apolipoprotein E deficient mice through the induction of regulatory T cells. Biochem. Biophys. Res. Commun. 2011, 411, 523–529. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Dai, M.; Jia, W. Paeonol attenuates high-fat-diet-induced atherosclerosis in rabbits by anti-inflammatory activity. Planta Medica 2009, 75, 7–11. [Google Scholar] [CrossRef]
- Li, H.; Jiao, Y.; Xie, M. Paeoniflorin Ameliorates Atherosclerosis by Suppressing TLR4-Mediated NF-kB Activation. Inflammation 2017, 40, 2042–2051. [Google Scholar] [CrossRef]
- Li, W.; Zhi, W.; Zhao, J.; Yao, Q.; Liu, F.; Niu, X. Cinnamaldehyde protects VSMCs against ox-LDL-induced proliferation and migration through S arrest and inhibition of p38, JNK/MAPKs and NF-kB. Vascul. Pharmacol. 2018, 108, 57–66. [Google Scholar] [CrossRef]
- Wu, H.; Song, A.; Hu, W.; Dai, M. The Anti-atherosclerotic Effect of Paeonol against Vascular Smooth Muscle Cell Proliferation by Up-regulation of Autophagy via the AMPK/mTOR Signaling Pathway. Front. Pharmacol. 2017, 8, 948. [Google Scholar] [CrossRef]
- Chandran, U.; Patwardhan, B. Network ethnopharmacological evaluation of the immunomodulatory activity of Withania somnifera. J. Ethnopharmacol. 2017, 197, 250–256. [Google Scholar] [CrossRef]
- Zhang, R.Z.; Yu, S.J.; Bai, H.; Ning, K. TCM-Mesh: The database and analytical system for network pharmacology analysis for TCM preparations. Sci. Rep. 2017, 7, 2821. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Zhang, B. Traditional Chinese medicine network pharmacology: Theory, methodology and application. Chin. J. Nat. Med. 2013, 11, 110–120. [Google Scholar] [CrossRef] [PubMed]
- Boezio, B.; Audouze, K.; Ducrot, P.; Taboureau, O. Network-based Approaches in Pharmacology. Mol. Inform. 2017, 36. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Shi, X.; Huang, Z.; Mao, J.; Mei, W.; Ding, L.; Zhang, L.; Xing, R.; Wang, P. Network Pharmacology Approach to Uncover the Mechanism Governing the Effect of Radix Achyranthis Bidentatae on Osteoarthritis. BMC Complement. Med. Ther. 2020, 20, 121. [Google Scholar] [CrossRef]
- Huang, J.; Cheung, F.; Tan, H.Y.; Hong, M.; Wang, N.; Yang, J.; Feng, Y.; Zheng, Q. Identification of the active compounds and significant pathways of yinchenhao decoction based on network pharmacology. Mol. Med. Rep. 2017, 16, 4583–4592. [Google Scholar] [CrossRef]
- Tang, H.; He, S.; Zhang, X.; Luo, S.; Zhang, B.; Duan, X.; Zhang, Z.; Wang, W.; Wang, Y.; Sun, Y. A Network Pharmacology Approach to Uncover the Pharmacological Mechanism of XuanHuSuo Powder on Osteoarthritis. Evid. Based Complement. Altern. Med. 2016, 2016, 3246946. [Google Scholar] [CrossRef]
- Xiao, L.; Liu, L.; Guo, X.; Zhang, S.; Wang, J.; Zhou, F.; Liu, L.; Tang, Y.; Yao, P. Quercetin attenuates high fat diet-induced atherosclerosis in apolipoprotein E knockout mice: A critical role of NADPH oxidase. Food Chem. Toxicol. 2017, 105, 22–33. [Google Scholar] [CrossRef]
- Feng, H.; Cao, J.; Zhang, G.; Wang, Y. Kaempferol Attenuates Cardiac Hypertrophy via Regulation of ASK1/MAPK Signaling Pathway and Oxidative Stress. Planta Medica 2017, 83, 837–845. [Google Scholar] [CrossRef]
- Che, J.; Liang, B.; Zhang, Y.; Wang, Y.; Tang, J.; Shi, G. Kaempferol alleviates ox-LDL-induced apoptosis by up-regulation of autophagy via inhibiting PI3K/Akt/mTOR pathway in human endothelial cells. Cardiovasc. Pathol. 2017, 31, 57–62. [Google Scholar] [CrossRef]
- Chan, S.H.; Hung, C.H.; Shih, J.Y.; Chu, P.M.; Cheng, Y.H.; Tsai, Y.J.; Lin, H.C.; Tsai, K.L. Baicalein is an available anti-atherosclerotic compound through modulation of nitric oxide-related mechanism under oxLDL exposure. Oncotarget 2016, 7, 42881–42891. [Google Scholar] [CrossRef]
- Tsai, K.L.; Hung, C.H.; Chan, S.H.; Shih, J.Y.; Cheng, Y.H.; Tsai, Y.J.; Lin, H.C.; Chu, P.M. Baicalein protects against oxLDL-caused oxidative stress and inflammation by modulation of AMPK-α. Oncotarget 2016, 7, 72458–72468. [Google Scholar] [CrossRef] [PubMed]
- Mele, L.; Mena, P.; Piemontese, A.; Marino, V.; López-Gutiérrez, N.; Bernini, F.; Brighenti, F.; Zanotti, I.; Del Rio, D. Antiatherogenic effects of ellagic acid and urolithins in vitro. Arch. Biochem. Biophys. 2016, 599, 42–50. [Google Scholar] [CrossRef] [PubMed]
- He, X.W.; Yu, D.; Li, W.L.; Zheng, Z.; Lv, C.L.; Li, C.; Liu, P.; Xu, C.Q.; Hu, X.F.; Jin, X.P. Anti-atherosclerotic potential of baicalin mediated by promoting cholesterol efflux from macrophages via the PPARgamma-LXRα-ABCA1/ABCG1 pathway. Biomed. Pharmacother. 2016, 83, 257–264. [Google Scholar] [CrossRef] [PubMed]
- Tucka, J.; Yu, H.; Gray, K.; Figg, N.; Maguire, J.; Lam, B.; Bennett, M.; Littlewood, T. Akt1 regulates vascular smooth muscle cell apoptosis through FoxO3a and Apaf1 and protects against arterial remodeling and atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 2421–2428. [Google Scholar] [CrossRef] [PubMed]
- Grootaert, M.O.; Schrijvers, D.M.; Hermans, M.; Van Hoof, V.O.; De Meyer, G.R.; Martinet, W. Caspase-3 Deletion Promotes Necrosis in Atherosclerotic Plaques of ApoE Knockout Mice. Oxid. Med. Cell Longev. 2016, 2016, 3087469. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; McLaren, J.E.; Michael, D.R.; Clement, M.; Fielding, C.A.; Ramji, D.P. ERK is integral to the IFN-γ-mediated activation of STAT1, the expression of key genes implicated in atherosclerosis, and the uptake of modified lipoproteins by human macrophages. J. Immunol. 2010, 185, 3041–3048. [Google Scholar] [CrossRef]
- Detmers, P.A.; Hernandez, M.; Mudgett, J.; Hassing, H.; Burton, C.; Mundt, S.; Chun, S.; Fletcher, D.; Card, D.J.; Lisnock, J.; et al. Deficiency in inducible nitric oxide synthase results in reduced atherosclerosis in apolipoprotein E-deficient mice. J. Immunol. 2000, 165, 3430–3435. [Google Scholar] [CrossRef]
- Linton, M.F.; Fazio, S. Cyclooxygenase products and atherosclerosis. Drug Discov. Today. Ther. Strateg. 2008, 5, 25–36. [Google Scholar] [CrossRef]
- Tedgui, A.; Mallat, Z. Cytokines in atherosclerosis: Pathogenic and regulatory pathways. Physiol. Rev. 2006, 86, 515–581. [Google Scholar] [CrossRef]
- Jain, T.; Nikolopoulou, E.A.; Xu, Q.; Qu, A. Hypoxia inducible factor as a therapeutic target for atherosclerosis. Pharmacol. Ther. 2018, 183, 22–33. [Google Scholar] [CrossRef]
- Tsuchiya, K.; Tanaka, J.; Shuiqing, Y.; Welch, C.L.; DePinho, R.A.; Tabas, I.; Tall, A.R.; Goldberg, I.J.; Accili, D. FoxOs integrate pleiotropic actions of insulin in vascular endothelium to protect mice from atherosclerosis. Cell Metab. 2012, 15, 372–381. [Google Scholar] [CrossRef]
- Morello, F.; Perino, A.; Hirsch, E. Phosphoinositide 3-kinase signalling in the vascular system. Cardiovasc. Res. 2009, 82, 261–271. [Google Scholar] [CrossRef]
- Li, T.; Li, D.; Xu, H.; Zhang, H.; Tang, D.; Cao, H. Wen-Xin Decoction ameliorates vascular endothelium dysfunction via the PI3K/AKT/eNOS pathway in experimental atherosclerosis in rats. BMC Complement. Altern. Med. 2016, 16, 27. [Google Scholar] [CrossRef]
- Xing, S.S.; Yang, X.Y.; Zheng, T.; Li, W.J.; Wu, D.; Chi, J.Y.; Bian, F.; Bai, X.L.; Wu, G.J.; Zhang, Y.Z.; et al. Salidroside improves endothelial function and alleviates atherosclerosis by activating a mitochondria-related AMPK/PI3K/Akt/eNOS pathway. Vasc. Pharmacol. 2015, 72, 141–152. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Mu, J.; Zheng, C.; Chen, X.; Guo, Z.; Huang, C.; Fu, Y.; Tian, G.; Shang, H.; Wang, Y. Systems-Pharmacology Dissection of Traditional Chinese Medicine Compound Saffron Formula Reveals Multi-Scale Treatment Strategy for Cardiovascular Diseases. Sci. Rep. 2016, 6, 19809. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Zhu, X.; Bai, H.; Ning, K. Network Pharmacology Databases for Traditional Chinese Medicine: Review and Assessment. Front. Pharmacol. 2019, 10, 123. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wu, L.; Liu, W.; Jin, Y.; Chen, Q.; Wang, L.; Fan, X.; Li, Z.; Cheng, Y. A network pharmacology study of Chinese medicine QiShenYiQi to reveal its underlying multi-compound, multi-target, multi-pathway mode of action. PLoS ONE 2014, 9, e95004. [Google Scholar] [CrossRef]
- Yue, S.J.; Liu, J.; Feng, W.W.; Zhang, F.L.; Chen, J.X.; Xin, L.T.; Peng, C.; Guan, H.S.; Wang, C.Y.; Yan, D. System Pharmacology-Based Dissection of the Synergistic Mechanism of Huangqi and Huanglian for Diabetes Mellitus. Front. Pharmacol. 2017, 8, 694. [Google Scholar] [CrossRef]
- Kuhn, M.; Szklarczyk, D.; Pletscher-Frankild, S.; Blicher, T.H.; von Mering, C.; Jensen, L.J.; Bork, P. STITCH 4: Integration of protein-chemical interactions with user data. Nucleic Acids Res. 2014, 42, D401–D407. [Google Scholar] [CrossRef]
- Choudhary, N.; Singh, V. A census of P. longum’s phytochemicals and their network pharmacological evaluation for identifying novel drug-like molecules against various disease, with a special focus on neurological disorders. PLoS ONE 2018, 13, e0191006. [Google Scholar] [CrossRef]
- Zheng, C.; Pei, T.; Huang, C.; Chen, X.; Bai, Y.; Xue, J.; Wu, Z.; Mu, J.; Li, Y.; Wang, Y. A novel systems pharmacology platform to dissect action mechanisms of traditional Chinese medicines for bovine viral diarrhea disease. Eur. J. Pharm. Sci. 2016, 94, 33–45. [Google Scholar] [CrossRef] [PubMed]
- Guo, R.; Zhang, X.; Su, J.; Xu, H.; Zhang, Y.; Zhang, F.; Li, D.; Zhang, Y.; Xiao, X.; Ma, S.; et al. Identifying potential quality markers of Xin-Su-Ning capsules acting on arrhythmia by integrating UHPLC-LTQ-Orbitrap, ADME prediction and network target analysis. Phytomedicine 2018, 44, 117–128. [Google Scholar] [CrossRef] [PubMed]
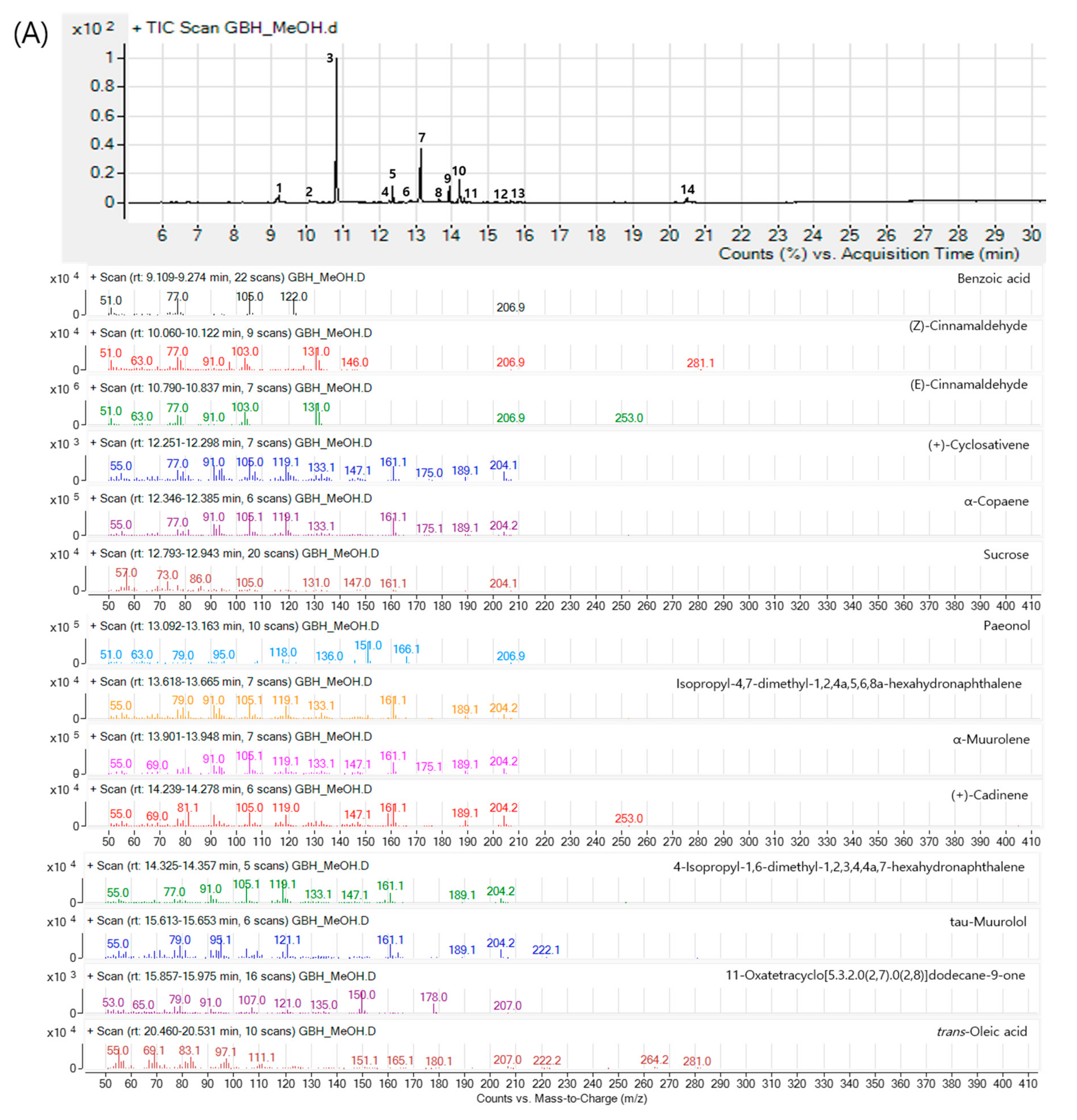
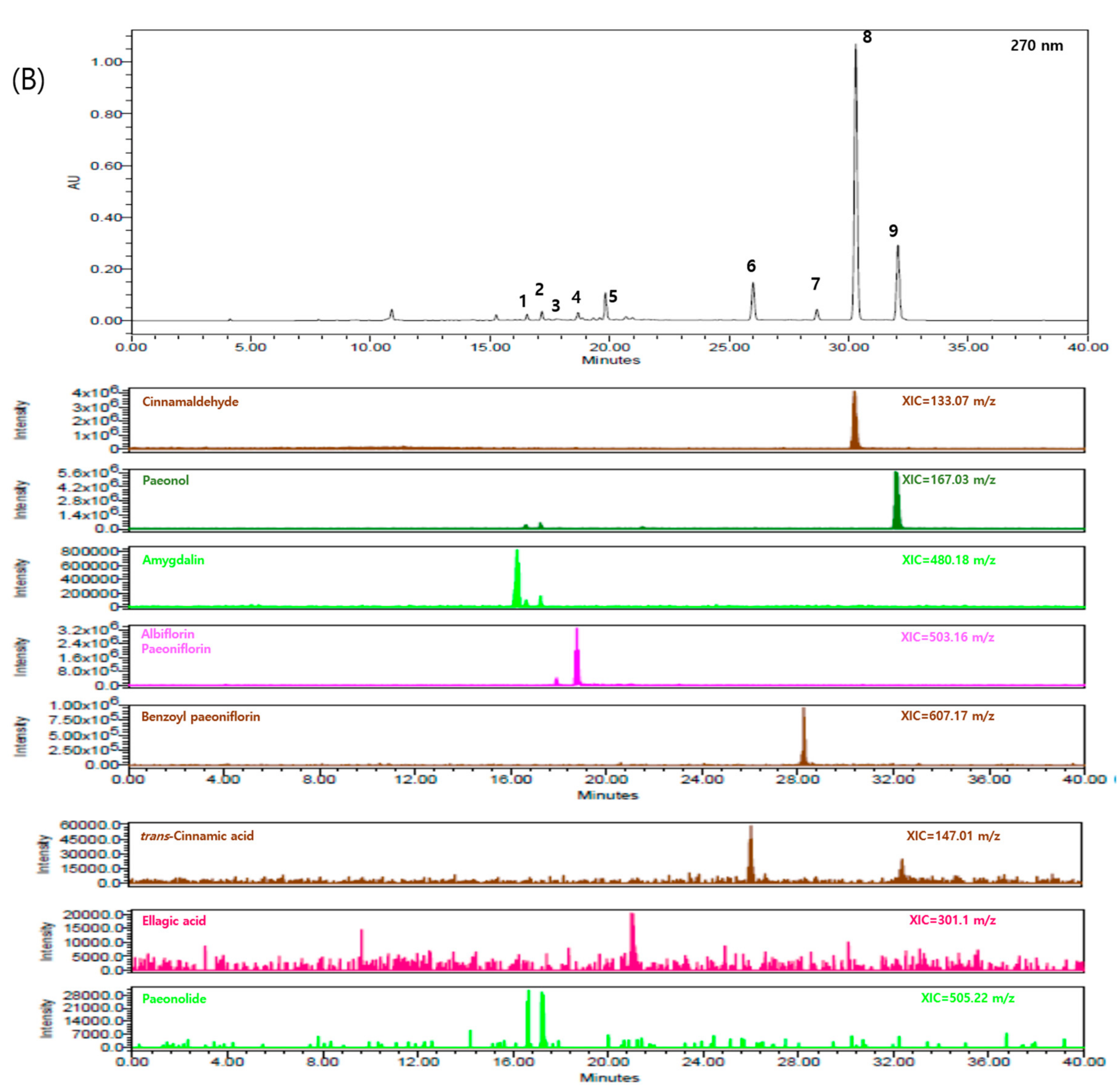
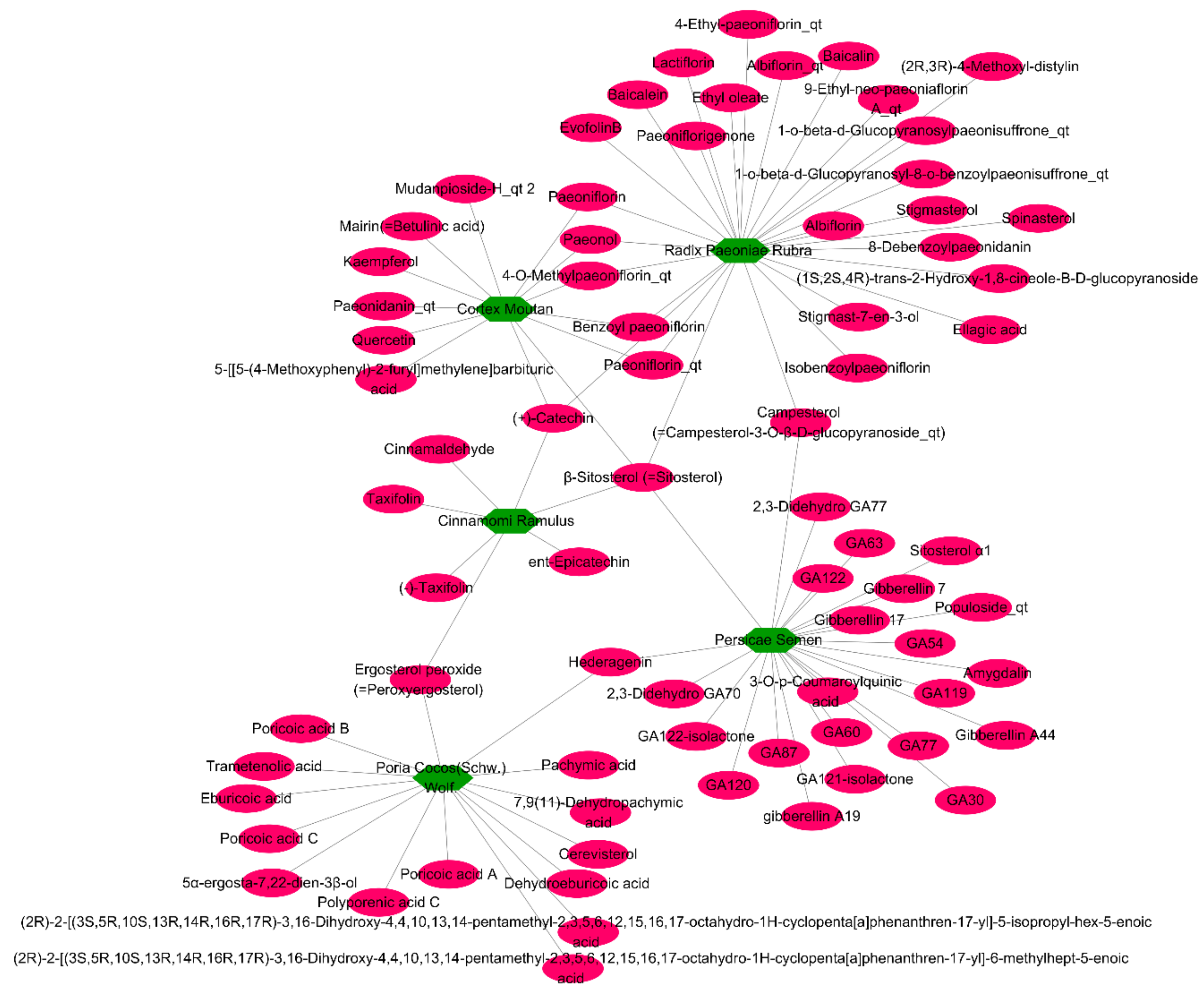
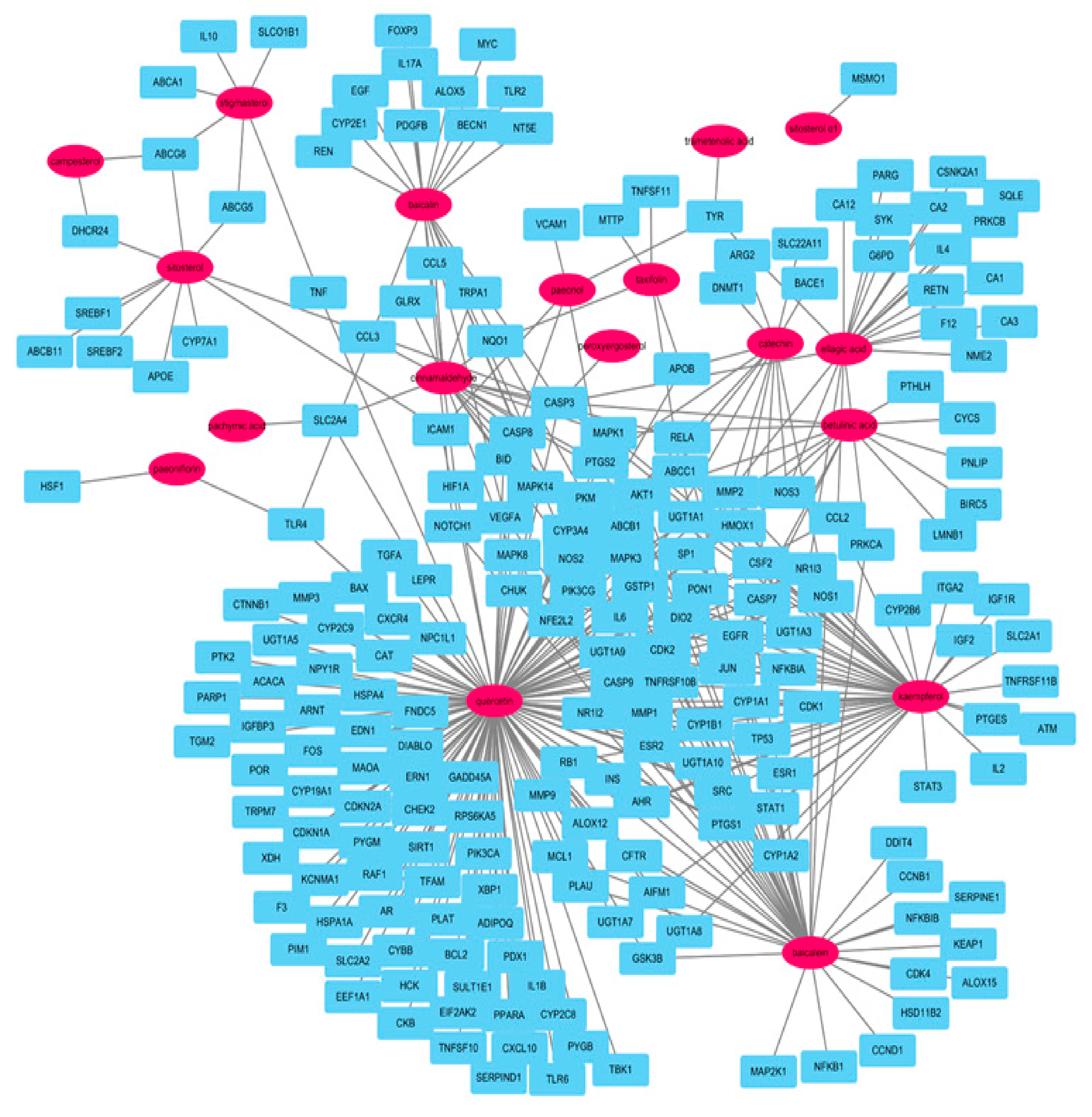
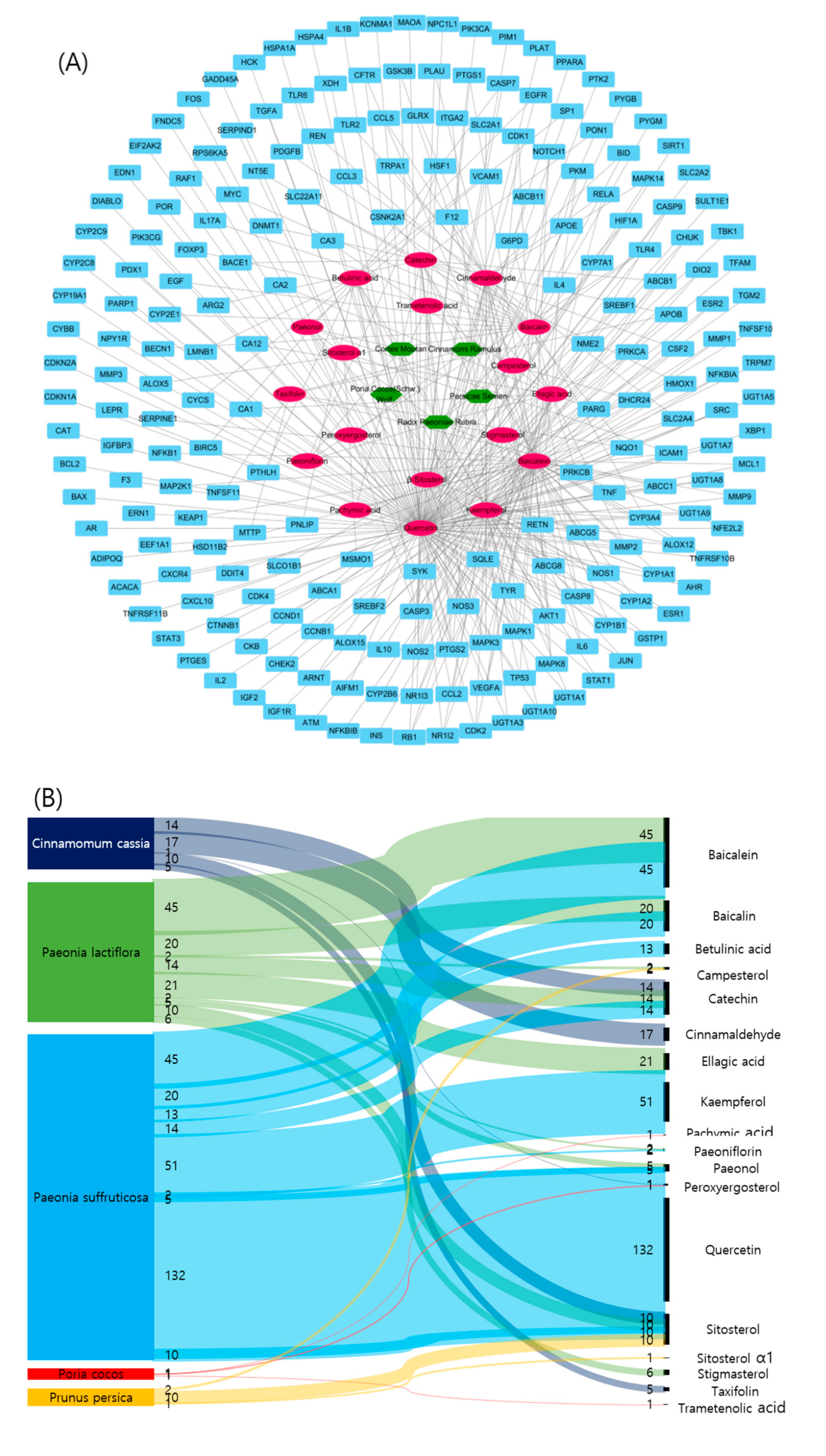
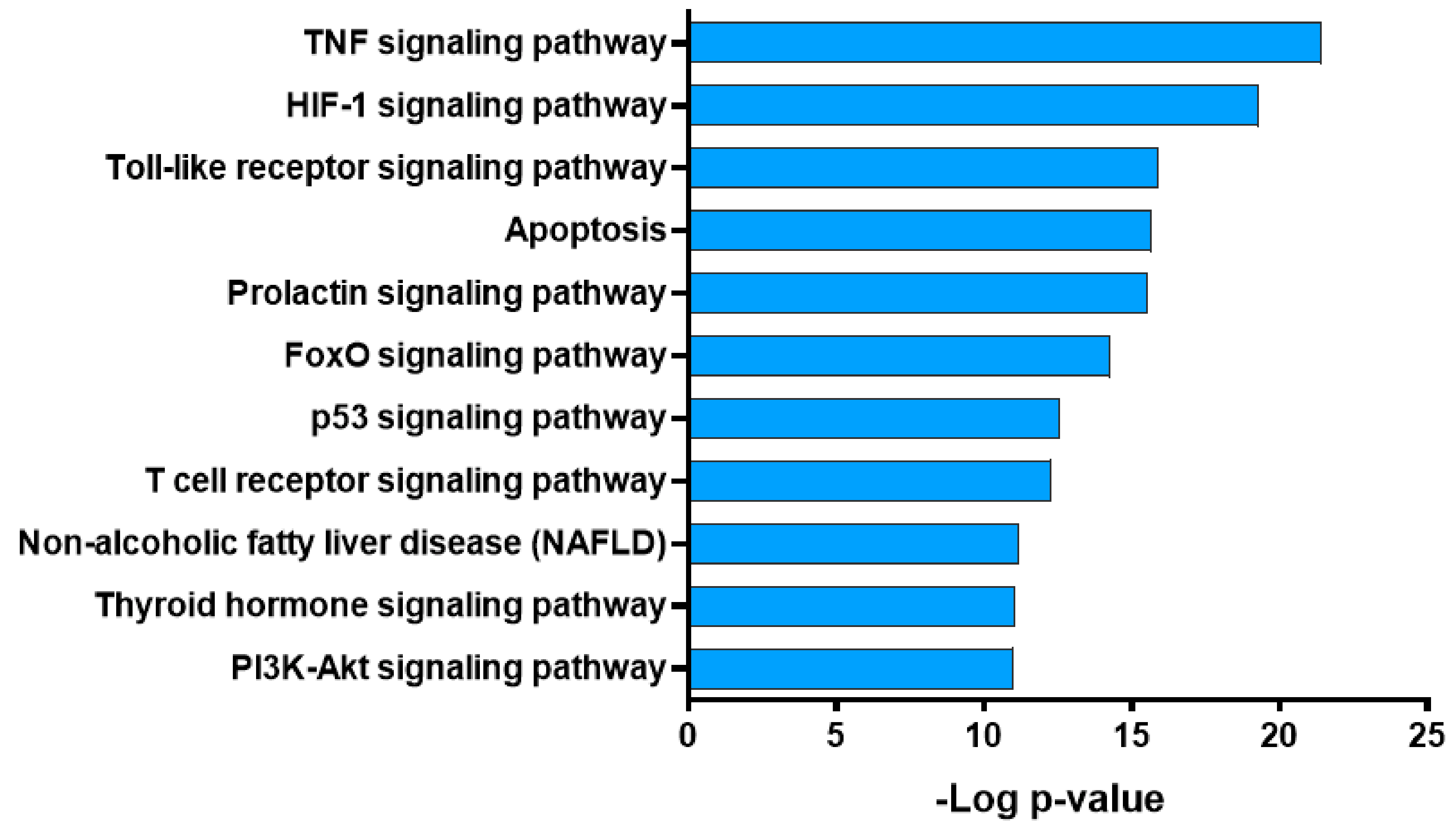
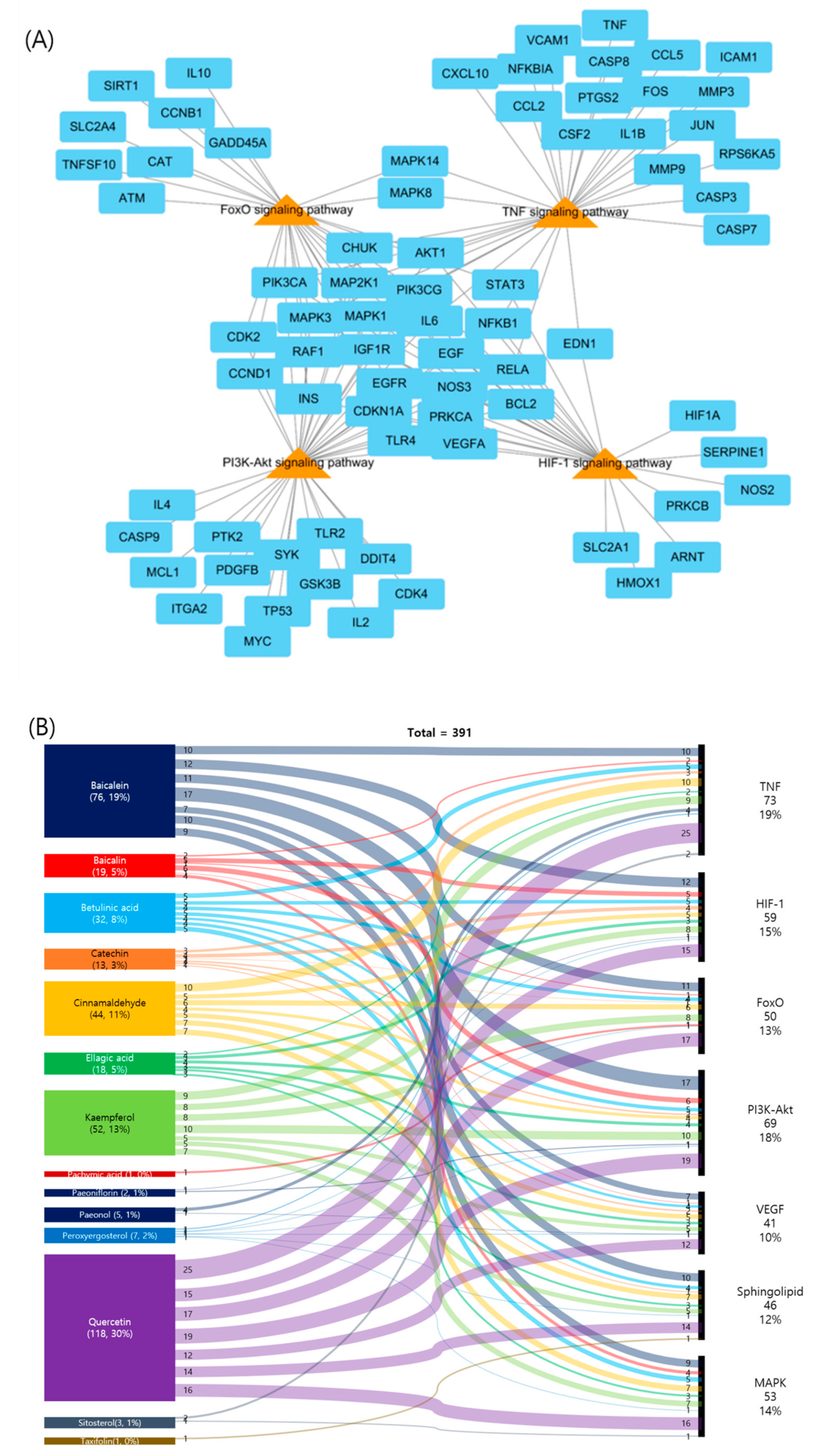
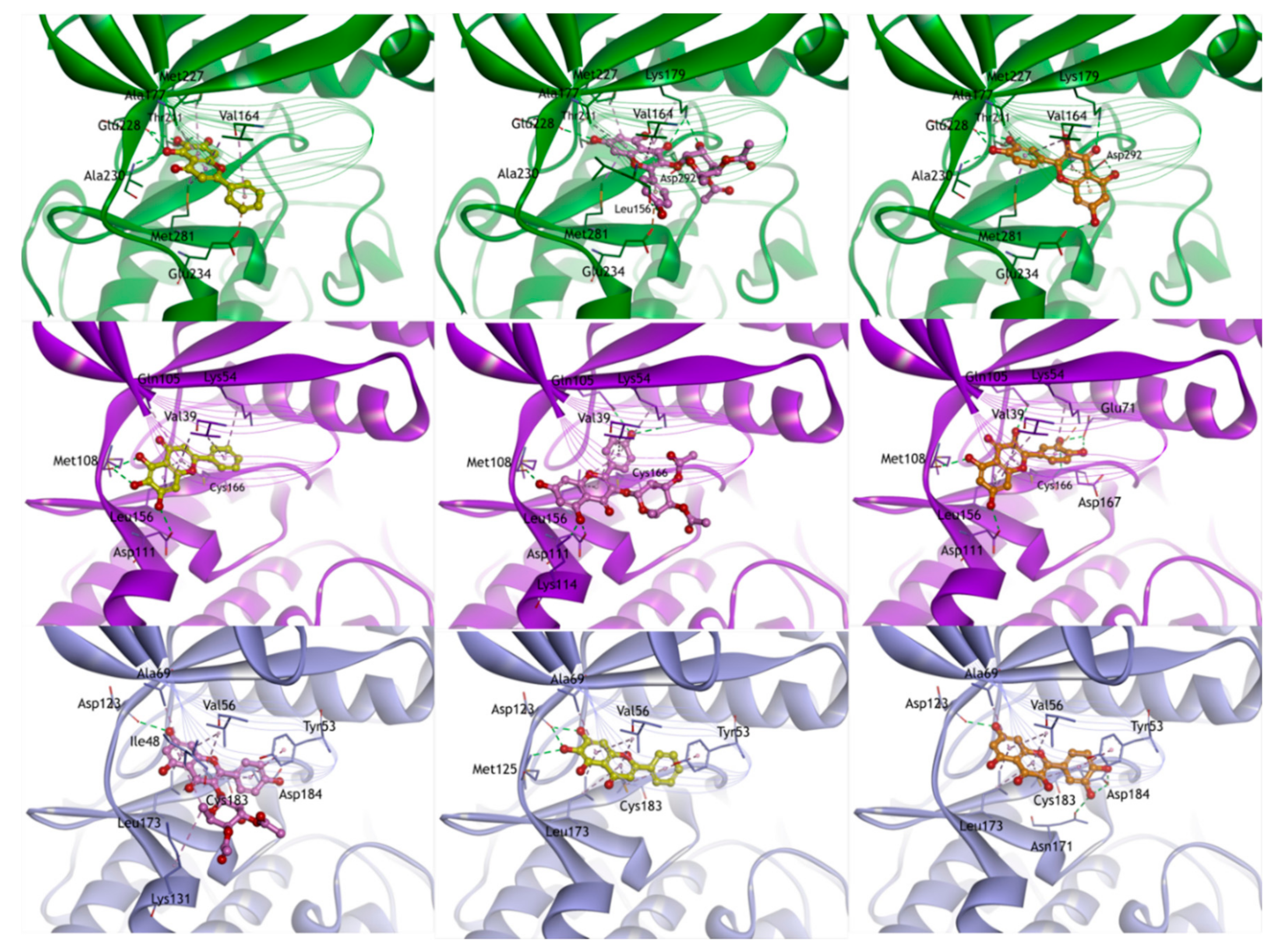

| Components | Genes | Degree | Closeness Centrality | Betweenness Centrality |
|---|---|---|---|---|
| Amygdaline | KLRP | 1 | 1 | 0 |
| Baicalein | ABCC1, AKT1, ALOX12, ALOX15, CCNB1, CCND1, CDK1, CDK2, CDK4, CFTR, CYP1A1, CYP1A2, CYP1B1, CYP3A4, DDIT4, GSK3B, HSD11B2, IL6, INS, KEAP1, MAP2K1, MAPK1, MAPK3, MAPK8, MCL1, MITF, MMP2, MMP9, NFE2L2, NFKB1, NFKBIB, NOS1, NOS2, NOTCH1, NR1I2, PKM, PLAU, PRKCA, PTGS1, PTGS2, RB1, RELA, S100A7, SERPINE1, TNFRSF10B, TP53, UGT2B15, VEGFA | 48 | 0.36213 | 0.13466 |
| Baicalin | ALOX5, BECN1, CASP3, CASP8, CASP10, CDX1, CDX2, CYP2E1, CYP3A4, EGF, FOXP3, HIF1A, IL17A, MYC, NOS2, NOTCH1, NT5E, PDGFB, PKM, REN, TLR2, TLR4, VEGFA | 23 | 0.34969 | 0.09976 |
| Betulinic acid | AKT1, BIRC5, CASP3, CASP7, CYCS, EGFR, LMNB1, MAPK1, MAPK3, NOS3, PNLIP, PTHLH, SP1, TOP1, TOP2A | 15 | 0.33969 | 0.05121 |
| Campesterol | ABCG8, DHCR24, HSD3B2 | 3 | 0.2 | 0.00708 |
| Catechin | ABCB1, APOB, ARG2, BACE1, CSF2, DNMT1, HMOX1, IL6, KIAA1149, NOS1, NOS2, NOS3, PON1, PTGS2, SLC22A11, SLC35A2, SLC47A1, ZFP36 | 18 | 0.33648 | 0.05808 |
| Cinnamaldehyde | AKR1C2, AKT1, BID, CASP3, CASP8, CCL3, CCL5, GRKL, GLRX, IL8, MAPK1, MAPK3, MAPK8, MAPK14, NOS2, NQO1, PTGS2, RELA, SLC2A4, TRPA1 | 20 | 0.35404 | 0.05899 |
| Dehydroeburicoic acid | SPTAN1 | 1 | 1 | 0 |
| Ellagic acid | CA1, CA2, CA3, CA4, CA5A, CA5B, CA6, CA7, CA9, CA12, CA14, CASP3, CCL2, CSNK2A1, F12, G6PD, IL4, MMP2, NME1-NME2, NME2, NOS3, NR1I3, PARG, PGD, PRKACA, PRKCA, PRKCB, RETN, SQLE, SYK, TMPRSS11D, TYR | 32 | 0.35670 | 0.17266 |
| Kaempferol | ABCB1, ABCC1, AHR, AKT1, ALOX12, ATM, CASP3, CASP9, CCL2, CDK1, CDK2, CHUK, CSF2, CYP1A1, CYP1A2, CYP1B1, CYP2B6, CYP3A4, DIO2, ESR1, ESR2, GSTP1, H2AFX, HMOX1, IGF1R, IGF2, IL2, ITGA2, JUN, MAPK1, MAPK3, MMP1, MMP2, NFKBIA, NOS1, NOS2, NR1I2, NR1I3, PTGES, RB1, RPS6KA3, SLC2A1, SRC, STAT1, STAT3, TNFRSF11B, TP53, UGT1A1, UGT1A3, UGT1A7, UGT1A8, UGT1A9, UGT1A10, UGT3A1 | 54 | 0.37748 | 0.12492 |
| Pachymic acid | SLC2A4 | 1 | 0.26611 | 0 |
| Paeoniflorin | HSF1, IL8, TLR4 | 3 | 0.28079 | 0.00720 |
| Paeonol | CASP8, CASP10, ICAM1, PTGS2, TYR, VCAM1 | 6 | 0.31844 | 0.01390 |
| Peroxyergosterol | CASP3 | 1 | 0.31148 | 0 |
| Quercetin | ABCB1, ABCC1, ABCC4, ABCC5, ACACA, ADIPOQ, AHR, AIFM1, AKR1C3, AKT1, ALOX12, AOX1, APAF1, APOB, AR, ARNT, ATP5A1, ATP5B, ATP5C1, BAX, BCL2, BID, CASP3, CASP7, CASP8, CASP9, CAT, CCL2, CD97, CDK2, CDKN1A, CDKN2A, CFTR, CHEK2, CHUK, CKB, CSF2, CTNNB1, CXCL10, CXCR4, CYBB, CYP1A1, CYP1A2, CYP1B1, CYP2C8, CYP2C9, CYP2D6, CYP3A4, CYP19A1, DIABLO, DIO2, EDN1, EEF1A1, EGFR, EIF2AK2, ERN1, ERN2, ESR1, ESR2, F3, FAU, FNDC5, FOS, FOXM1, GADD45A, GLRA1, GSK3B, GSTP1, HCK, HIBCH, HIF1A, HIST3H3, HMOX1, HSPA1A, HSPA4, ICAM1, IGFBP3, IL1B, IL6, IL8, JUN, KCNMA1, KRAS, LEPR, MAOA, MAPK1, MAPK3, MAPK8, MAPK14, MCL1, MMP1, MMP2, MMP3, MMP9, NFE2L2, NFKBIA, NOS1, NOS2, NOS3, NPC1L1, NPY1R, NR1I2, NR1I3, PARP1, PDX1, PIK3CA, PIK3CG, PIM1, PLAT, PLAU, PON1, POR, PPARA, PTGS1, PTGS2, PTK2, PYGB, PYGL, PYGM, RAF1, RB1, RNASEL, RPS6KA5, SERPIND1, SIRT1, SLC2A2, SLC2A4, SP1, SRC, STAT1, STK17B, SULT1A1, SULT1E1, TBK1, TFAM, TGFA, TGM2, TLR4, TLR6, TMPRSS11D, TNF, TNFRSF10B, TNFSF10, TP53, TRPM7, UGT1A1, UGT1A3, UGT1A5, UGT1A7, UGT1A8, UGT1A9, UGT1A10, UGT2A3, UGT2B15, UGT2B4, UGT3A1, VEGFA, XBP1, XDH | 161 | 0.54493 | 0.72132 |
| Stigmasterol | ABCA1, ABCG5, ABCG8, IL8, IL10, SLCO1B1, TNF | 7 | 0.27859 | 0.02904 |
| Sitosterol | ABCB11, ABCG5, ABCG8, APOE, CASP3, CYP7A1, DHCR24, ICAM1, SREBF1, SREBF2 | 10 | 0.32350 | 0.06171 |
| Sitosterol alpha1 | C5orf4, MSMO1 | 2 | 1 | 1 |
| Taxifolin | ABCC1, APOB, MTTP, NQO1, TNFSF11 | 5 | 0.29171 | 0.01483 |
| Trametenolic acid | TYR | 1 | 0.21064 | 0 |
| Targets | PDB Code | Compounds | Docking Score |
|---|---|---|---|
| AKT1 | 6CCY | Baicalein | −6.51 |
| AKT1 | 6CCY | Kaempferol | −5.54 |
| AKT1 | 6CCY | Quercetin | −7.28 |
| CASP3 | 2XYG | Baicalin | −6.07 |
| CASP3 | 2XYG | Ellagic acid | −5.98 |
| CASP3 | 2XYG | Kaempferol | −5.21 |
| CASP3 | 2XYG | Quercetin | −6.21 |
| MAPK1 | 6SLG | Baicalein | −6.55 |
| MAPK1 | 6SLG | Kaempferol | −6.71 |
| MAPK1 | 6SLG | Quercetin | −7.73 |
| MAPK3 | 4QTB | Baicalein | −7.01 |
| MAPK3 | 4QTB | Kaempferol | −6.04 |
| MAPK3 | 4QTB | Quercetin | −7.76 |
| NOS2 | 3E7G | Baicalein | −5.70 |
| NOS2 | 3E7G | Baicalin | −6.10 |
| NOS2 | 3E7G | Kaempferol | −3.44 |
| NOS2 | 3R7G | Quercetin | −7.13 |
| PTGS2 | 5IKR | Baicalein | −8.72 |
| PTGS2 | 5IKR | Quercetin | −9.61 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, A.Y.; Lee, J.-Y.; Chun, J.M. Exploring the Mechanism of Gyejibokryeong-hwan against Atherosclerosis Using Network Pharmacology and Molecular Docking. Plants 2020, 9, 1750. https://doi.org/10.3390/plants9121750
Lee AY, Lee J-Y, Chun JM. Exploring the Mechanism of Gyejibokryeong-hwan against Atherosclerosis Using Network Pharmacology and Molecular Docking. Plants. 2020; 9(12):1750. https://doi.org/10.3390/plants9121750
Chicago/Turabian StyleLee, A Yeong, Joo-Youn Lee, and Jin Mi Chun. 2020. "Exploring the Mechanism of Gyejibokryeong-hwan against Atherosclerosis Using Network Pharmacology and Molecular Docking" Plants 9, no. 12: 1750. https://doi.org/10.3390/plants9121750
APA StyleLee, A. Y., Lee, J.-Y., & Chun, J. M. (2020). Exploring the Mechanism of Gyejibokryeong-hwan against Atherosclerosis Using Network Pharmacology and Molecular Docking. Plants, 9(12), 1750. https://doi.org/10.3390/plants9121750





