Correlations on Phenolic Screening Related to In Vitro and In Ovo Assessment of Ocimum basilicum L. Hydro-Alcoholic Extracts Used as Skin Active Ingredient
Abstract
1. Introduction
2. Results
2.1. Extraction of Ocimum basilicum L.
2.2. Phenolic Composition of Basil Hydro-Alcoholic Extracts
2.2.1. Total Phenolic, Flavonoid/Flavonols Contents and Condensed Tannins
2.2.2. LC-MS Assessment of Hydro-Alcoholic Basil Extracts
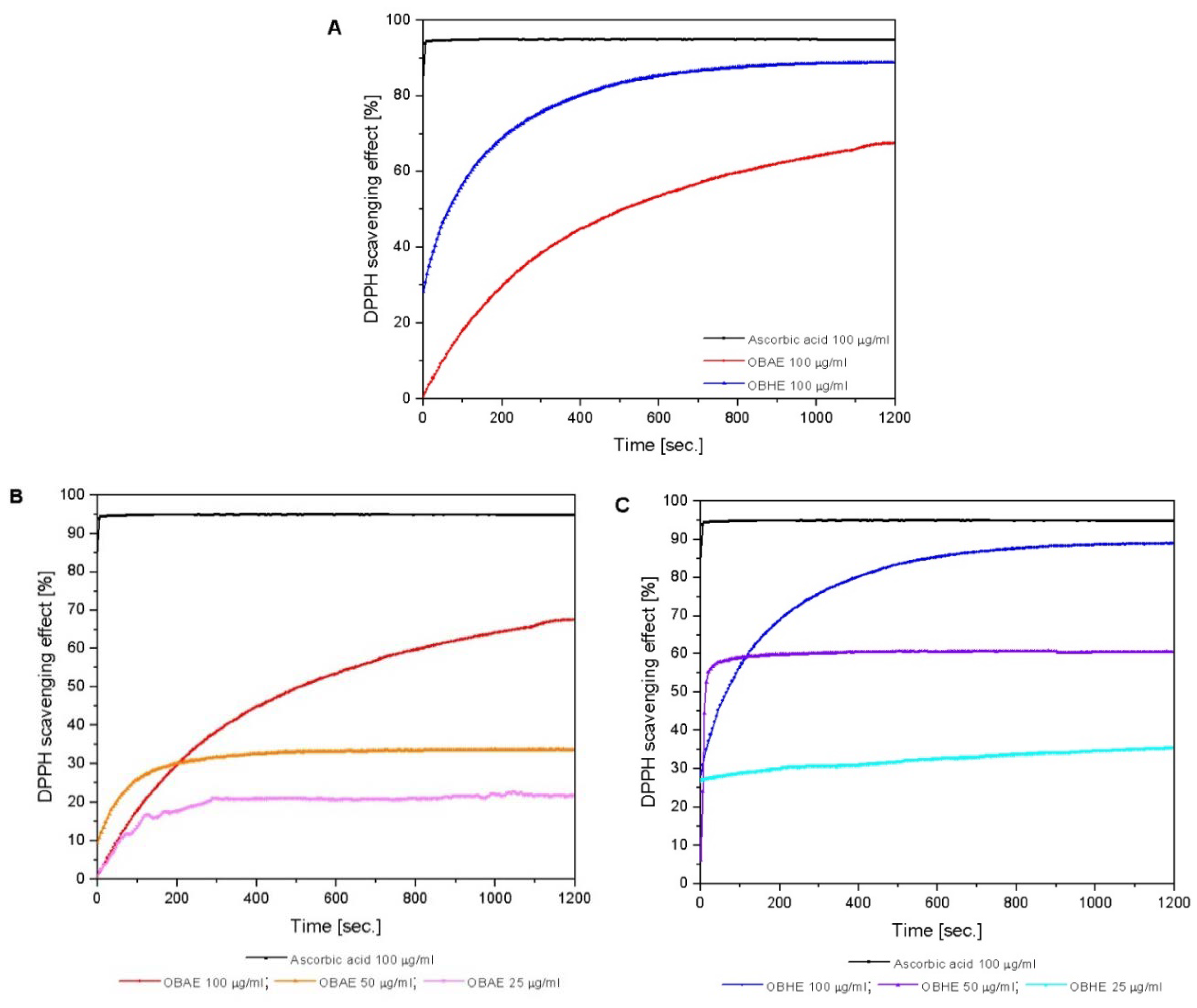
2.2.3. Total Antioxidant Activity
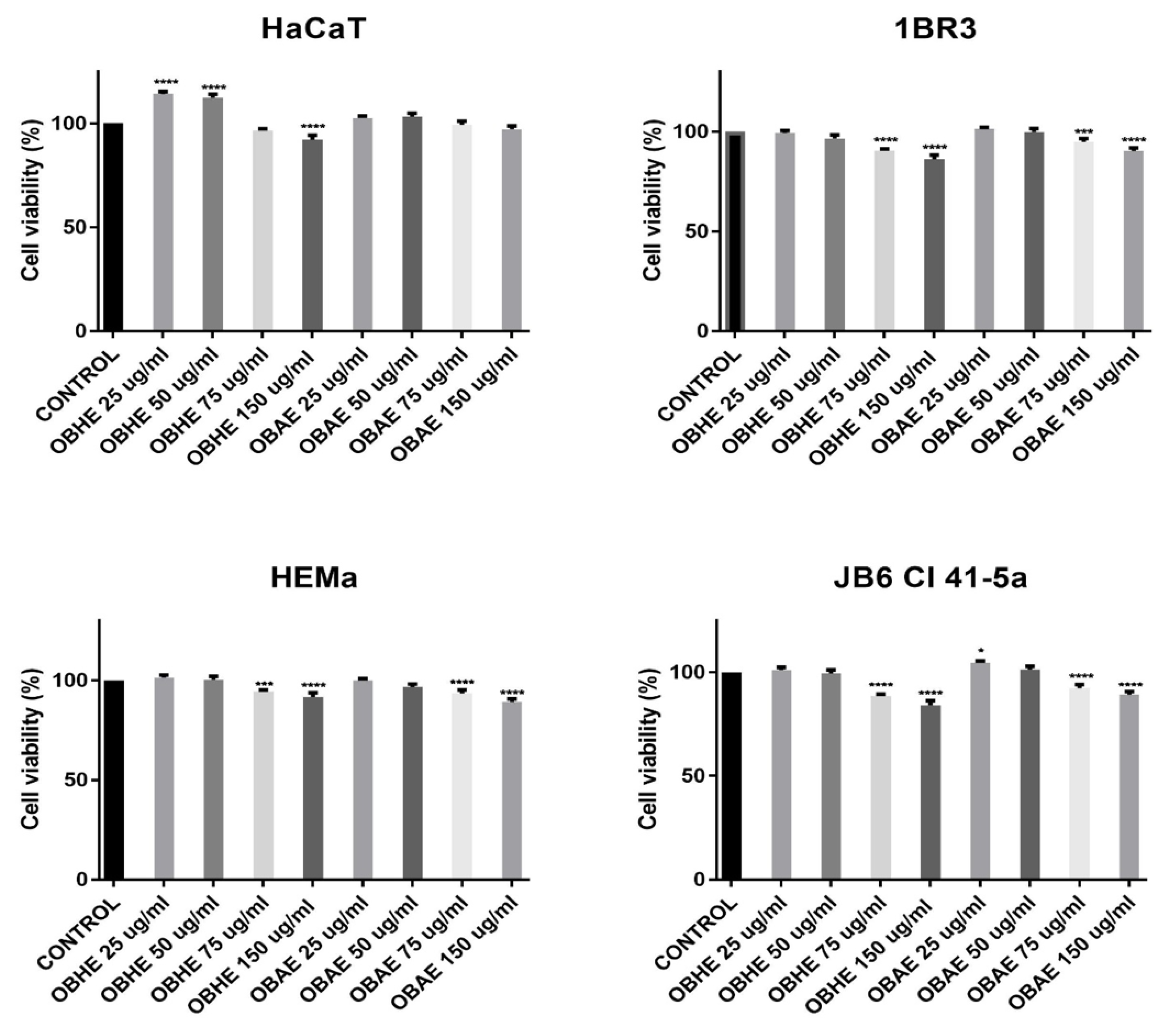
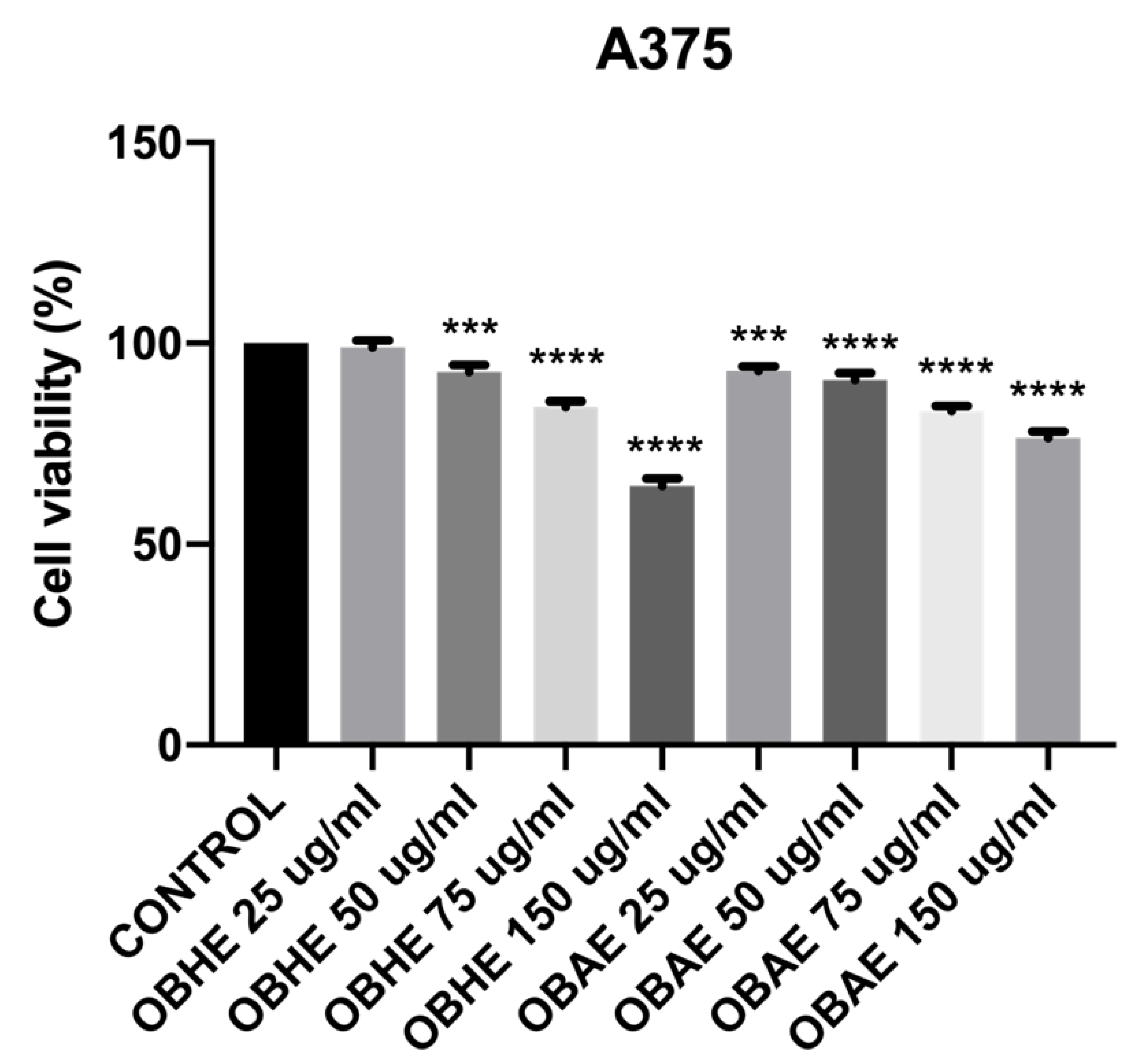
2.3. Bioactivity of Basil Hydro-Alcoholic Extracts
Cytotoxicity of OBHE and OBAE
2.4. In Ovo Assessment of Hydro-Alcoholic Basil Extracts
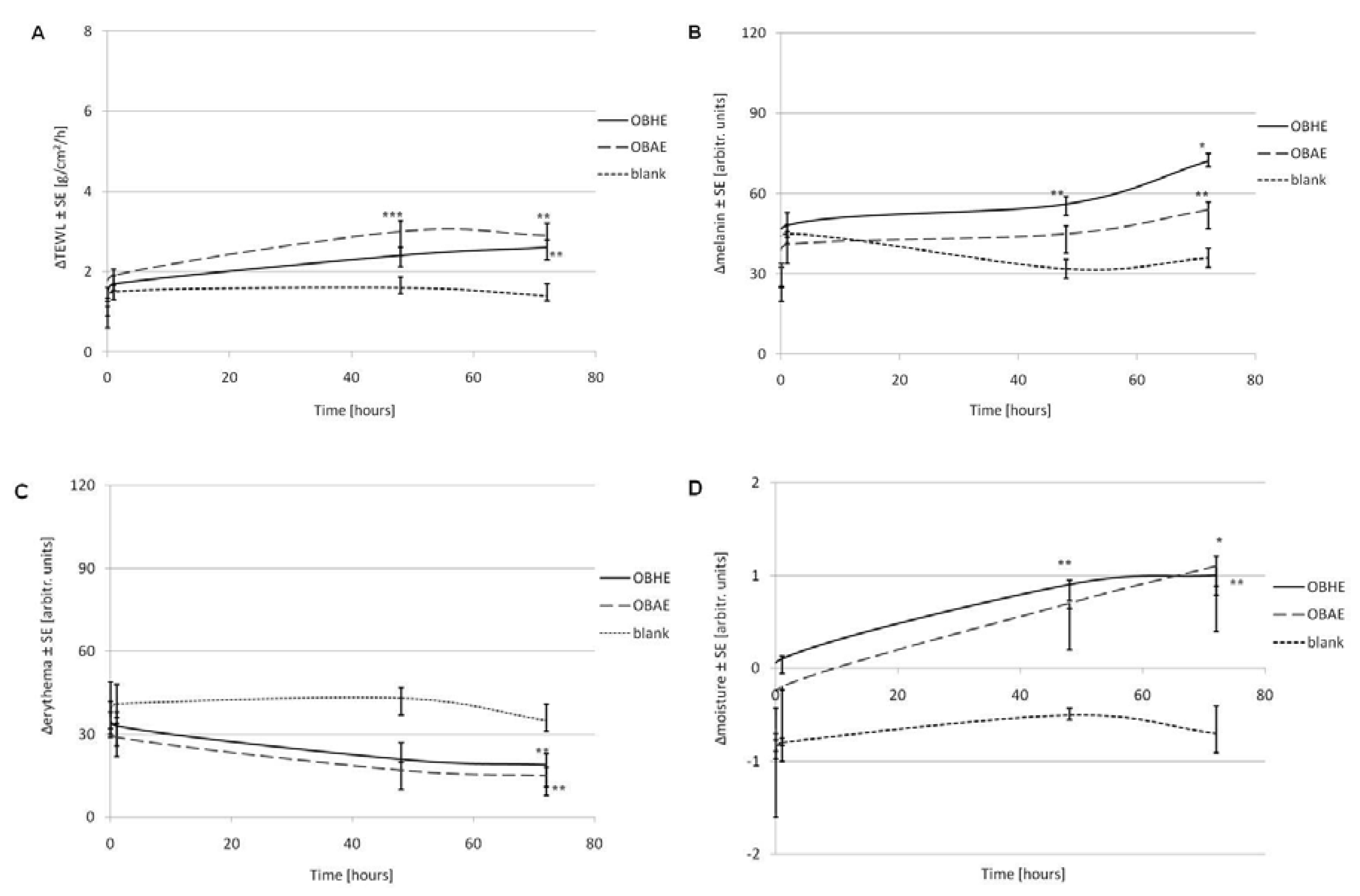
2.5. Assessment of Skin Biophysical Parameters after Treatment with Hydro-Alcoholic Basil Extracts
3. Discussion
4. Materials and Methods
4.1. Materials
4.1.1. Reagents and Culture Media
4.1.2. Cell Lines
4.2. Methods
4.2.1. Plant Extracts Preparation
4.2.2. Physicochemical Characterization of Plant Extracts
Total Phenolic Content Determination (TP)
The Total Flavonoid and Flavonols (TF and TFv) Content
The Total Condensed Tannins Content (TT)
Identification of Phenolic Compounds by Liquid Chromatography—Mass Spectrometry (LC-MS)
4.2.3. Antioxidant Activity (AOA) Evaluation
4.2.4. Cell Culture Conditions
4.2.5. Cell Viability Assessment by the Means of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium Bromide (MTT) Assay
4.2.6. HET-CAM Assay
4.2.7. Skin Biophysical Parameters Assessment
4.2.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sakr, S.A.; Nooh, H.Z. Effect of Ocimum basilicum extract on cadmium-induced testicular histomorphometric and immunohistochemical alterations in albino rats. Anat. Cell Biol. 2013, 46, 122–130. [Google Scholar] [CrossRef] [PubMed]
- Lawal, O.A.; Ogunwande, I.A. 5—Essential Oils from the Medicinal Plants of Africa. Med. Plant Res. Afr. Pharmacol. Chem. 2013, 2013, 203–224. [Google Scholar]
- Ahmed, A.F.; Attia, F.A.; Liu, Z.; Li, C.; Wei, J.; Kang, W. Antioxidant activity and total phenolic content of essential oils and extracts of sweet basil (Ocimum basilicum L.) plants. Food Sci. Hum. Wellness 2019, 8, 299–305. [Google Scholar] [CrossRef]
- Castaño, A.M.V.; Cifuentes, M.C.B.; Rinćon, D.J.C. Antioxidant activity of two varieties of Ocimum basilicum L. for potential use in phytocosmetics. Rev. Fac. Nac. Agron. Medellín 2016, 69, 7965–7973. [Google Scholar] [CrossRef]
- Joshi, R.K. Chemical composition and antimicrobial activity of the essential oil of Ocimum basilicum L. (sweet basil) from Western Ghats of North West Karnataka. Indian Anc. Sci. Life 2014, 33, 151–156. [Google Scholar] [CrossRef]
- Zarlaha, A.; Kourkoumelis, N.; Stanojkovic, T.; Kovala-Demertzi, D. Cytotoxic activity of essential oil and extracts of Ocimum basilicum against human carcinoma cells. Molecular docking study of isoeugenol as a potent cox and lox inhibitor. Dig. J. Nanomater. Biostructures 2014, 9, 907–917. [Google Scholar]
- Sanchez-Suarez, J.; Riveros, I.; Delgado, G. Evaluation of the leishmanicidal and cytotoxic potential of essential oils derived from ten Colombian plants. Iran. J. Parasitol. 2013, 8, 129–136. [Google Scholar]
- Martinez-Velazquez, M.; Castillo-Herrera, G.A.; Rosario-Cruz, R.; Flores-Fernandez, J.M.; Lopez-Ramirez, J. Acaricidal effect and chemical composition of essential oils extracted from Cuminum cyminum, Pimenta dioica and Ocimum basilicum against the cattle tick Rhipicephalus (Boophilus) microplus (Acari: Ixodidae). Parasitol Res. 2011, 108, 481–487. [Google Scholar] [CrossRef]
- Hanif, M.A.; Al-Maskari, M.Y.; Al-Maskari, A.; Al-Shukaili, A.; Al-Maskari, A.Y.; Al-Sabahi, J.N. Essential oil composition, antimicrobial and antioxidant activities of unexplored Omani basil. J. Med. Plants Res. 2011, 5, 751–757. [Google Scholar]
- Jakowienko, P.; Wójcik-Stopczyńska, B.; Jadczak, D. Antifungal activity of essential oils from two varieties of sweet basil (Ocimum basilicum L.). Veg. Crop. Res. Bull. 2011, 74, 97–106. [Google Scholar] [CrossRef]
- Mohammed, A.B.A.; Yagi, S.; Tzanova, T.; Schohn, H.; Abdelgadir, H.; Stefanucci, A.; Mollica, A.; Mahomoodally, M.F.; Adlan, T.A.; Zengin, G. Chemical profile, antiproliferative, antioxidant and enzyme inhibition activities of Ocimum basilicum L. and Pulicaria undulata (L.) C.A. Mey. grown in Sudan. S. Afr. J. Bot. 2020, 132, 403–409. [Google Scholar] [CrossRef]
- Farouk, A.; Fikry, R.; Mohsen, M. Chemical composition and antioxidant activity of Ocimum basilicum L. essential oil cultivated in Madinah Monawara, Saudi Arabia and its comparison to the Egyptian chemotype. J. Essent. Oil Bear Plants 2016, 19, 1119–1128. [Google Scholar] [CrossRef]
- Savithramma, N.; Rao, M.L.; Suhrulatha, D. Screening of medicinal plants for secondary metabolites. Middle East J. Sci. Res. 2011, 8, 579–584. [Google Scholar]
- Tomar, U.S.; Daniel, V.; Shrivastava, K.; Panwar, M.S.; Pant, P. Comparative evaluation and antimicrobial activity of Ocimum basilicum Linn. (Labiatae). J. Glob. Pharmacol. Technol. 2010, 2, 49–53. [Google Scholar]
- Yang, L.; Lyu, L.; Wu, W.; Lei, D.; Tu, Y.; Xu, D.; Feng, J.; He, L. Genome-wide identification of long non-coding RNA and mRNA profiling using RNA sequencing in subjects with sensitive skin. Oncotarget 2017, 8, 114894–114910. [Google Scholar] [CrossRef] [PubMed]
- Misery, L.; Stander, S.; Szepietowski, J.C.; Reich, A.; Wallengren, J.; Evers, A.W.; Takamori, K.; Brenaut, E.; Le Gall-Ianotto, C.; Fluhr, J.; et al. Definition of Sensitive Skin: An Expert Position Paper from the Special Interest Group on Sensitive Skin of the International Forum for the Study of Itch. Acta Derm. Venereol. 2017, 97, 4–6. [Google Scholar] [CrossRef]
- Guerra-Tapia, A.; Serra-Baldrich, E.; Cabezas, L.P.; González-Guerra, E.; López-Estebaranz, J. Diagnosis and treatment of sensitive skin syndrome: An algorithm for clinical practice. Actas Dermo Sifiliogr. 2019, 110, 800–808. [Google Scholar] [CrossRef]
- Wang, X.; Su, Y.; Zheng, B.; Wen, S.; Liu, D.; Ye, L.; Yan, Y.; Elias, P.M.; Yang, B.; Man, M.Q. Gender-related characterization of sensitive skin in normal young Chinese. J. Cosmet. Dermatol. 2020, 19, 1137–1142. [Google Scholar] [CrossRef]
- Wang, Y.; Viennet, C.; Jeudy, A.; Fanian, F.; He, L.; Humbert, P. Assessment of the efficacy of a new complex antisensitive skin cream. J. Cosmet. Dermatol. 2018, 17, 1101–1107. [Google Scholar] [CrossRef]
- Zhang, Y.; Jin, Y.; Humbert, P.; Fan, X.; Cha, Y.; Guo, Y.; He, L. An herbal cream reduces erythema of sensitive skin. J. Cosmet. Dermatol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Lan, C.E.; Hung, Y.T.; Fang, A.H.; Ching-Shuang, W. Effects of irradiance on UVA-induced skin aging. J. Dermatol. Sci. 2019, 94, 220–228. [Google Scholar] [CrossRef] [PubMed]
- Marwat, S.K.; Khan, M.S.; Ghulam, S.; Anwar, N.; Mustafa, G.; Usman, K. Phytochemical constituents and pharmacological activities of sweet Basil-Ocimum basilicum L. (Lamiaceae). Asian J. Chem. 2011, 23, 3773–3782. [Google Scholar]
- Yoshikawa, M.; Okano, Y.; Masaki, H. An Ocimum basilicum Extract Containing Rosmarinic Acid Restores the Disruption of Collagen Fibers Caused by Repetitive UVA Irradiation of Dermal Fibroblasts. J. Oleo Sci. 2020, 69, 1487–1495. [Google Scholar] [CrossRef] [PubMed]
- Rasul, A.; Akhtar, N. Formulation and in vivo evaluation for anti-aging effects of an emulsion containing basil extract using non- invasive biophysical techniques. DARU J. Pharm. Sci. 2011, 19, 344–350. [Google Scholar]
- World Health Organization. The World Medicines Situation 2011—Traditional Medicines: Global Situation, Issues and Challenges; World Health Organization: Geneva, Switzerland, 2011. [Google Scholar]
- Koparde, A.A.; Doijad, R.C.; Magdum, C.S. Natural Products in Drug Discovery. Pharmacognosy-Medicinal Plants; Intech Open: London, UK, 2019; pp. 1–20. [Google Scholar]
- Acheampong, A.; Okyem, S.; Osei Akoto, C.; Baah, A.K. Antioxidant, antimicrobial and FTIR analysis of methanol root extract of Cnestis ferruginea and ethanol root extract of Citrus limon. J. Pharmacogn. Phytochem. 2018, 7, 2938–2946. [Google Scholar]
- Bilal, A.; Jahan, N.; Ahmed, A.; Bilal, S.N.; Habib, S.; Hajra, S. Phytochemical and pharmacological studies on Ocimum basilicum Linn—A review. Int. J. Curr. Res. Rev. 2012, 4, 74–83. [Google Scholar]
- Vlase, L.; Benedec, D.; Hanganu, D. Evaluation of Antioxidant and Antimicrobial Activities and Phenolic Profile for Hyssopus officinalis, Ocimum basilicum and Teucrium chamaedrys. Molecules 2014, 19, 5490–5507. [Google Scholar] [CrossRef]
- Benedec, D.; Vlase, L.; Hanganu, D.; Oniga, I. Antioxidant potential and polyphenolic content of Romanian Ocimum basilicum. Dig. J. Nanomater. Bios. 2012, 7, 1263–1270. [Google Scholar]
- Yesiloglu, Y.; Sit, L. Antioxidant properties of various solvent extracts from purple basil. Spectrochim. Acta Part Mol. Biomol. Spectrosc. 2012, 95, 100–106. [Google Scholar] [CrossRef]
- Rezzoug, M.; Bakchiche, B.; Gherib, A. Chemical composition and bioactivity of essential oils and ethanolic extracts of Ocimum basilicum L. and Thymus algeriensis Boiss. & Reut. from the Algerian Saharan Atlas. BMC Complement Altern. Med. 2019, 19, 146. [Google Scholar]
- Elansary, H.O.; Mahmoud, E.A. In vitro antioxidant and antiproliferative activities of six international basil cultivars. Nat. Prod. Res. 2015, 29, 2149–2154. [Google Scholar] [CrossRef] [PubMed]
- Boniface, P.K.; Ferreira, S.B.; Kaiser, C.R. Recent trends in phytochemistry, ethnobotany and pharmacological significance of Alchornea cordifolia (Schumach. & Thonn.) Muell. Arg. J. Ethnopharmacol. 2016, 191, 216–244. [Google Scholar]
- Bihari, C.G.; Behera, M.; Kumar, J.P.; Kumar, T.S. Pharmacognostical and phytochemical investigation of various tulsi plants available in south eastern Odisha. Int. J. Res. Pharm. Biomed. Sci. 2011, 2, 605–610. [Google Scholar]
- Kwee, E.M.; Niemeyer, E.D. Variations in phenolic composition and antioxidant properties among 15 basil (Ocimum basilicum L.) cultivars. Food Chem. 2011, 128, 1044–1050. [Google Scholar] [CrossRef]
- Mihaylova, D.; Popova, A.; Alexieva, I. The effect of extraction time on the antioxidant activity of fresh Bulgarian Melissa officinalis L. J. BioSci. Biotechnol. 2015, 2015, 115–118. [Google Scholar]
- Lobo, V.; Patil, A.; Phatak, A.; Chandra, N. Free radicals, antioxidants and functional foods: Impact on human health. Pharmacogn. Rev. 2010, 4, 118–126. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Ou, B.; Prior, R.L. The chemistry behind antioxidant capacity assays. J. Agric. Food Chem. 2005, 53, 1841–1856. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.S.; Yang, M.R.; Lee, O.H.; Kang, S.N. Antioxidant Activities of Hot Water Extracts from Various Spices. Int. J. Mol. Sci. 2011, 12, 4120–4131. [Google Scholar] [CrossRef]
- Weidner, C.; Rousseau, M.; Plauth, A.; Wowro, S.J.; Fischer, C.; Abdel-Aziz, H.; Sauer, S. Melissa officinalis extract induces apoptosis and inhibits proliferation in colon cancer cells through formation of reactive oxygen species. Phytomedicine 2015, 22, 262–270. [Google Scholar] [CrossRef]
- Yin, X.; Singer, S.D.; Qiao, H.; Liu, Y.; Jiao, C.; Wang, H.; Li, Z.; Fei, Z.; Wang, Y.; Fan, C. Insights into the mechanisms underlying ultraviolet-C induced resveratrol metabolism in grapevine (V. amurensis Rupr.) Cv. “Tonghua-3”. Front. Plant Sci. 2016, 7, 503. [Google Scholar]
- Gangopadhyay, M.; Gantait, S.; Palchoudhury, S.; Ali, M.N.; Mondal, C.; Pal, A.K. UVC-priming mediated modulation of forskolin biosynthesis key genes against Macrophomina root rot of Coleus forskohlii A tissue culture based sustainable approach. Phytochem. Lett. 2016, 17, 36–44. [Google Scholar] [CrossRef]
- Nazir, M.; Tungmunnithum, D.; Bose, S.; Drouet, S.; Garros, L.; Giglioli-Guivarc’H, N.; Abbasi, B.H.; Hano, C. Differential Production of Phenylpropanoid Metabolites in Callus Cultures of Ocimum basilicum L. with Distinct In Vitro Antioxidant Activities and In Vivo Protective Effects against UV stress. J. Agric. Food Chem. 2019, 67, 1847–1859. [Google Scholar] [CrossRef] [PubMed]
- Nazir, M.; Asad-Ullah, M.; Mumtaz, S.; Siddiquah, A.; Shah, M.; Drouet, S.; Hano, C.; Abbasi, B.H. Interactive Effect of Melatonin and UV-C on Phenylpropanoid Metabolite Production and Antioxidant Potential in Callus Cultures of Purple Basil (Ocimum basilicum L. var purpurascens). Molecules 2020, 25, 1072. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Alarcon, C.; Denicola, A. Evaluating the antioxidant capacity of natural products: A review on chemical and cellular based assays. Anal Chim. Acta. 2013, 763, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Maulik, N.; McFadden, D.; Otani, H.; Thirunavukkarasu, M.; Parinandi, N.L. Antioxidants in longevity and medicine. Oxid Med. Cell. Longev. 2013, 2013, 820679. [Google Scholar] [CrossRef]
- Toda, S. Polyphenol content and antioxidant effects in herb teas. Chin Med. 2011, 2, 29–31. [Google Scholar] [CrossRef]
- Nazir, S.; Jan, H.; Tungmunnithum, D.; Drouet, S.; Zia, M.; Hano, C.; Abbasi, B.H. Callus Culture of Thai Basil Is an Effective Biological System for the Production of Antioxidants. Molecules 2020, 25, 4859. [Google Scholar] [CrossRef]
- Jackson, A.L.; Loeb, L.A. The contribution of endogenous sources of DNA damage to the multiple mutations in cancer. Mutat Res. 2001, 477, 7–21. [Google Scholar] [CrossRef]
- Storz, P. Reactive oxygen species in tumor progression. Front Biosci. J. Virtual Libr. 2005, 10, 1881–1896. [Google Scholar] [CrossRef]
- Al-Ali, K.H.; El-Beshbishy, H.A.; El-Badry, A.A.; Alkhalaf, M. Cytotoxic activity of methanolic extract of Mentha longifolia and Ocimum basilicum against human breast cancer. Pak. J. Biol. Sci. 2013, 16, 1744–1750. [Google Scholar]
- Arshad Qamar, K.; Dar, A.; Siddiqui, S.B.; Kabir, N.; Aslam, H.; Ahmed, S.; Erum, S.; Habib, S.; Begum, S. Anticancer Activity of Ocimum basilicum and the Effect of Ursolic Acid on the Cytoskeleton of MCF-7 Human Breast Cancer Cells. Lett. Drug Des. Discov. 2010, 7, 726–736. [Google Scholar] [CrossRef]
- Kathirvel, P.; Ravi, S. Chemical composition of the essential oil from basil (Ocimum basilicum Linn.) and its in vitro cytotoxicity against HeLa and HEp-2 human cancer cell lines and NIH 3T3 mouse embryonic fibroblasts. Nat. Prod. Res. 2012, 26, 1112–1118. [Google Scholar] [CrossRef] [PubMed]
- Canadian Council on Animal Care. Available online: www.iivs.org/pages/methods/CAMVA.summary.sheet.pdf (accessed on 3 October 2020).
- Silva, O.; Rougier, A.; Dossou, K.G. The HET-CAM test: A study of irritation potential of chemicals and formulations (L’OREAL). Altern. Labor. Anim. 1992, 20, 432–437. [Google Scholar] [CrossRef]
- Niazi, F.; Maryam, T.P.; Shahrokh-Abadi, K. To investigate the effect of basil (Ocimum basilicum) on angiogenesis chorioalantoic membrane chick. Res Med. 2017, 41, 71–76. [Google Scholar]
- Joshua, P.; Minoza, G.; Joshua, P.M.G.; Geneva, E.; Daryl May, C.C.; Blanes, D. Evaluation of antiangiogenic property of Ocimum basilica ethanolic leaf extract by using duck embryo chorioallantoic membrane (cam) assay and its morphometric analysis. Evaluation of antiangiogenic property of Ocimum morphometric analysis. Int. J. Herb. Med. 2016, 4, 22–26. [Google Scholar]
- Mazzarello, V.; Gavini, E.; Rassu, G.; Donadu, M.G.; Usai, D.; Piu, G.; Pomponi, V.; Sucato, F.; Zanetti, S.; Montesu, M.A. Clinical Assessment of New Topical Cream Containing Two Essential Oils Combined with Tretinoin in the Treatment of Acne. Clin. Cosmet. Investig. Dermatol. 2020, 13, 233–239. [Google Scholar] [CrossRef]
- Jadoon, S.; Karim, S.; Asad, M.H.H.B.; Akram, M.R.; Khan, A.K.; Malik, A.; Chen, C.; Murtaza, G. Anti-Aging Potential of Phytoextract Loaded-Pharmaceutical Creams for Human Skin Cell Longetivity. Oxid. Med. Cell. Longev. 2015. [Google Scholar] [CrossRef]
- Borcan, F.; Chirita-Emandi, A.; Andreescu, N.I.; Borcan, L.C.; Albulescu, R.C.; Puiu, M.; Tomescu, M.C. Synthesis and preliminary characterization of polyurethane nanoparticles with ginger extract as a possible cardiovascular protector. Int. J. Nanomed. 2019, 14, 3691–3703. [Google Scholar] [CrossRef]
- Salmah, I.; Mahmood, A.A.; Sidik, K. Synergistic Effects of Alcoholic Extract of Sweet Basil (Ocimum basilicum L.) Leaves and Honey on Cutaneous Wound Healing in Rats. Int. J. Mol. Med. Adv. Sci. 2005, 1, 220–224. [Google Scholar]
- Sengupta, R. Combined wound healing activity of Calendula officinalis and Basil leaves. J. Pharmacogn. Phytochem. 2017, 6, 173–176. [Google Scholar]
- Pandey, A.K.; Singh, P.; Tripathi, N.N. Chemistry and bioactivities of essential oils of some Ocimum species: An overview. Asian Pac. J. Trop. Biomed. 2014, 4, 682–694. [Google Scholar] [CrossRef]
- Selvakkumar, C.; Gayathri, B.; Sanathkumar Vinaykumar, K.; Lakshmi, B.S.; Balakrishnan, A. Potential Anti-inflammatory Properties of Crude Alcoholic Extract of Ocimum basilicum L. in Human Peripheral Blood Mononuclear Cells. J. Health Sci. 2007, 53, 500–505. [Google Scholar] [CrossRef]
- Pansanga, S.; Maphanta, S.; Tuntijarukorn, P.; Viyoch, J. Skin irritation test of a microemulsion containing essential oil isolated from Ocimum basilicum. Sci. Asia 2010, 36, 355–358. [Google Scholar] [CrossRef]
- Modaresi, M.; Pouriyanzadeh, A.; Asadi-Samani, M. Antiepileptic activity of hydroalcoholic extract of basil in mice. J. HerbMed Pharmacol. 2014, 3, 57–60. [Google Scholar]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventos, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar]
- Rouphael, Y.; Bernardi, J.; Cardarelli, M.; Bernardo, L.; Kane, D.; Colla, G.; Lucini, L. Phenolic Compounds and Sesquiterpene Lactones Profile in Leaves of Nineteen Artichoke Cultivars. J. Agric. Food Chem. 2016, 64, 8540–8548. [Google Scholar] [CrossRef]
- Cosarca, S.L.; Moaca, E.A.; Tanase, C.; Muntean, D.L.; Pavel, I.Z.; Dehelean, C.A. Spruce and beech bark aqueous extracts: Source of polyphenols, tannins and antioxidants correlated to in vitro antitumor potential on two different cell lines. Wood Sci. Technol. 2019, 53, 313–333. [Google Scholar] [CrossRef]
- Miliauskas, G.; Venskutonis, P.R.; Van Beek, T.A. Screening of radical scavenging activity of some medicinal and aromatic plant extracts. Food Chem. 2004, 85, 231–237. [Google Scholar] [CrossRef]
- Avram, S.; Coricovac, D.E.; Pavel, I.Z.; Pinzaru, I.; Ghiulai, R.; Baderca, F.; Soica, C.; Muntean, D.; Branisteanu, D.E.; Spandidos, D.A.; et al. Standardization of A375 human melanoma models on chicken embryo chorioallantoic membrane and Balb/c nude mice. Oncol Rep. 2017, 38, 89–99. [Google Scholar] [CrossRef]
- Interagency Coordinating Committee on the Validation of Alternative Methods (ICCVAM). ICCVAM-Recommended Test Method Protocol: Hen’s Egg Test—Chorioallantoic Membrane (HET-CAM) Test Method. ICCVAM Test Method Eval. Rep. 2010, 13, B30–B38. [Google Scholar]
- Kishore, A.S.; Surekha, P.A.; Sekhar, P.V.; Srinivas, A.; Murthy, P.B. Hen egg chorioallantoic membrane bioassay: An in vitro alternative to draize eye irritation test for pesticide screening. Int. J. Toxicol. 2008, 27, 449–453. [Google Scholar] [CrossRef] [PubMed]
- Luepke, N.P. Hen’s egg chorioallantoic membrane test for irritation potential. Food Chem. Toxicol. 1985, 23, 287–291. [Google Scholar] [CrossRef]
- Misery, L.; Jourdan, E.; Huet, F.; Brenaut, E.; Cadars, B.; Virassamynaik, S.; Sayag, M.; Taieb, C. Sensitive skin in France: A study on prevalence, relationship with age and skin type and impact on quality of life. J. Eur. Acad. Dermatol. Venerol. 2018, 32, 791–795. [Google Scholar] [CrossRef] [PubMed]


| Basil Extract | Extract Yield (%) | TP (mg GAE/g dm) | TF (mg RE/g dm) | TFv (mg RE/g dm) | TT (%) |
|---|---|---|---|---|---|
| OBAE | 17.2 | 177.36 ± 2.65 | 26.58 ± 1.33 | 2.39 ± 0.46 | 8.27 ± 2.86 |
| OBHE | 20.3 | 231.62 ± 0.42 | 48.22 ± 2.05 | 9.43 ± 1.51 | 11.18 ± 1.69 |
| Compound | Retention Time (min) | Conc. (µg/g) OBAE | Conc. (µg/g) OBHE |
|---|---|---|---|
| Gallic acid | 5.123 | 2.134 | 4.206 |
| Protocatechuic acid | 11.506 | 0.638 | 0.020 |
| Caffeic acid | 18.150 | 0.046 | 0.148 |
| Epicathechin | 23.322 | 30.468 | 11.043 |
| Coumaric acid | 24.573 | 0.316 | 2.108 |
| Rutin | 25.615 | 4.684 | 24.869 |
| Resveratrol | 29.632 | 4.335 | 16.207 |
| Quercetin | 31.322 | 11.332 | 37.228 |
| Kaempferol | 34.391 | 40.085 | 1040.025 |
| Ascorbic Acid | Ocimum Basilicum L. Aerial Part (OBAE) | Ocimum Basilicum L. Leaves (OBHE) | |||
|---|---|---|---|---|---|
| Conc. [μg/mL] | % Inhibition | Conc. [μg/mL] | % Inhibition | Conc. [μg/mL] | % Inhibition |
| 100 | 94.82 ± 0.052 | 100 | 67.51 ± 0.052 | 100 | 88.87 ± 0.045 |
| 50 | 50.17 ± 0.052 | 50 | 33.60 ± 0.014 | 50 | 60.52 ± 0.056 |
| 25 | 31.37 ± 0.045 | 25 | 21.77 ± 0.045 | 25 | 35.53 ± 0.052 |
| Samples | Irritation Score (IS) | Irritation Category |
|---|---|---|
| H2O | 0 | No irritation |
| SLS 0.5% | 18.7 | Strong irritation |
| DMSO 0.5% | 0.81 | No irritation |
| OBAE 150 µg/mL | 4.48 | Weak irritation |
| OBHE 150 µg/mL | 2.38 | Weak irritation |
Sample Availability: Samples of the compounds are not available from the authors. | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Faur, A.; Watz, C.; Moacă, E.-A.; Avram, Ş.; Borcan, F.; Pinzaru, I.; Iftode, A.; Nicolov, M.; Popovici, R.A.; Raica, M.; et al. Correlations on Phenolic Screening Related to In Vitro and In Ovo Assessment of Ocimum basilicum L. Hydro-Alcoholic Extracts Used as Skin Active Ingredient. Molecules 2020, 25, 5442. https://doi.org/10.3390/molecules25225442
Faur A, Watz C, Moacă E-A, Avram Ş, Borcan F, Pinzaru I, Iftode A, Nicolov M, Popovici RA, Raica M, et al. Correlations on Phenolic Screening Related to In Vitro and In Ovo Assessment of Ocimum basilicum L. Hydro-Alcoholic Extracts Used as Skin Active Ingredient. Molecules. 2020; 25(22):5442. https://doi.org/10.3390/molecules25225442
Chicago/Turabian StyleFaur, Alin, Claudia Watz, Elena-Alina Moacă, Ştefana Avram, Florin Borcan, Iulia Pinzaru, Andrada Iftode, Mirela Nicolov, Ramona Amina Popovici, Marius Raica, and et al. 2020. "Correlations on Phenolic Screening Related to In Vitro and In Ovo Assessment of Ocimum basilicum L. Hydro-Alcoholic Extracts Used as Skin Active Ingredient" Molecules 25, no. 22: 5442. https://doi.org/10.3390/molecules25225442
APA StyleFaur, A., Watz, C., Moacă, E.-A., Avram, Ş., Borcan, F., Pinzaru, I., Iftode, A., Nicolov, M., Popovici, R. A., Raica, M., Szuhanek, C. A., & Dehelean, C. (2020). Correlations on Phenolic Screening Related to In Vitro and In Ovo Assessment of Ocimum basilicum L. Hydro-Alcoholic Extracts Used as Skin Active Ingredient. Molecules, 25(22), 5442. https://doi.org/10.3390/molecules25225442











