Antioxidants and Exercise Performance: With a Focus on Vitamin E and C Supplementation
Abstract
1. Introduction
2. Antioxidants and Sports Performance
2.1. Reactive Oxygen and Nitrogen Species
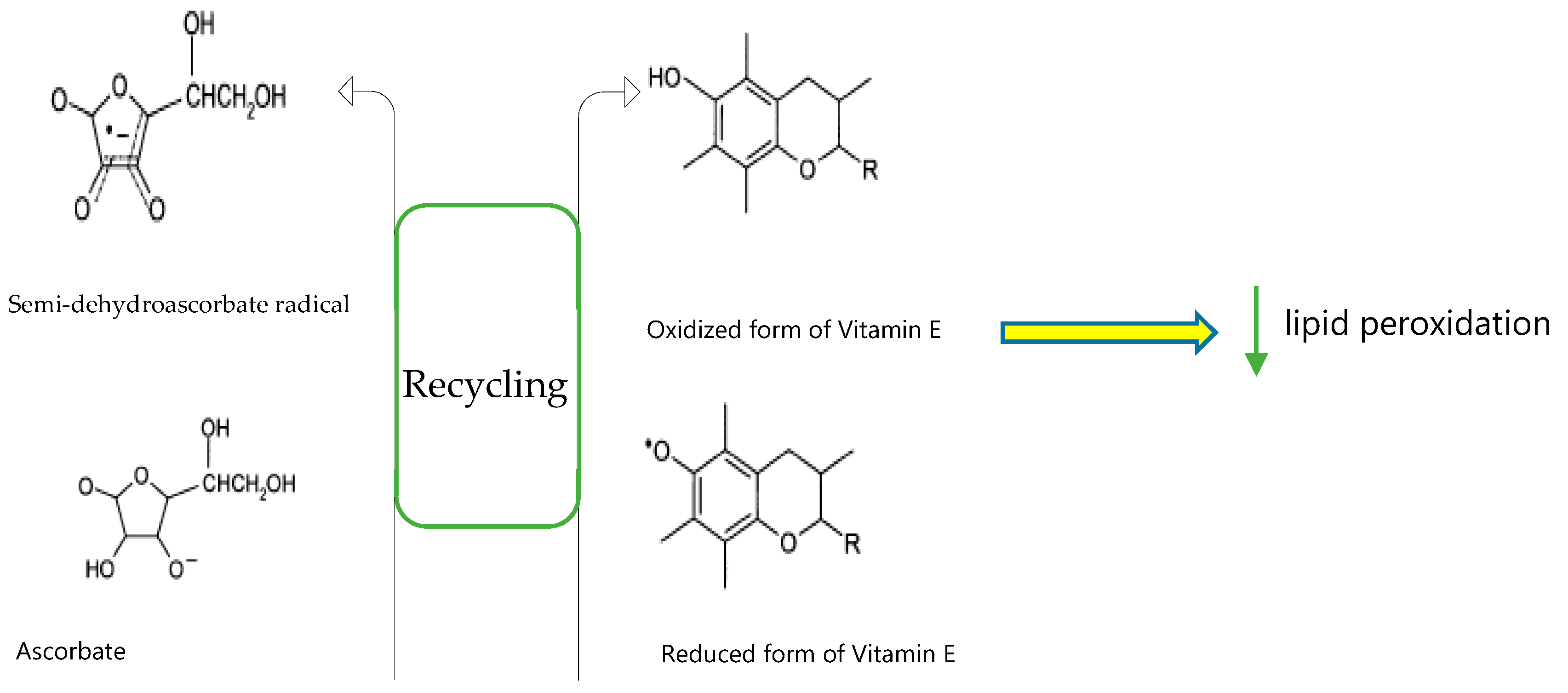
2.2. Exogenous and Endogenous Antioxidants
2.3. Antioxidants and Impaired Adaptations to Training
3. Search Strategy
4. Vitamin E as An Antioxidant Supplement
4.1. Food Sources Versus Supplements
4.2. Supplementation with Vitamin E Alone and Combined with Vitamin C and Exercise Performance
4.3. Acute Versus Chronic Supplementation with Vitamin E
5. Supplementation with Vitamin C and Exercise Performance
6. Effects Vitamin E and C Supplementation: Environment and Physiological Factors
6.1. Altitude Training
6.2. Mitochondrial Biogenesis and Antioxidant Induction
6.3. Skeletal Muscle Contraction Force
6.4. Skeletal Muscle Hypertrophy
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Beil, L. Tough to swallow: Athletes who use antioxidant supplements may not be getting the boost they expect. Sci. News 2015, 187, 24–27. [Google Scholar] [CrossRef]
- Knapik, J.J.; Steelman, R.A.; Hoedebecke, S.S.; Austin, K.G.; Farina, E.K.; Lieberman, H.R. Prevalence of dietary supplement use by athletes: Systematic review and meta-analysis. Sports Med. 2016, 46, 103–123. [Google Scholar] [CrossRef]
- Martinez-Ferran, M.; Sanchis-Gomar, F.; Lavie, C.J.; Lippi, G.; Pareja-Galeano, H. Do antioxidant vitamins prevent exercise-induced muscle damage? A systematic review. Antioxidants 2020, 9, 372. [Google Scholar] [CrossRef] [PubMed]
- Mertens, E.; Kuijsten, A.; Dofková, M.; Mistura, L.; D’Addezio, L.; Turrini, A.; Dubuisson, C.; Favret, S.; Havard, S.; Trolle, E. Geographic and socioeconomic diversity of food and nutrient intakes: A comparison of four European countries. Eur. J. Nutr. 2019, 58, 1475–1493. [Google Scholar] [CrossRef] [PubMed]
- de Sousa, E.F.; Da Costa, T.H.; Nogueira, J.A.; Vivaldi, L.J. Assessment of nutrient and water intake among adolescents from sports federations in the Federal District, Brazil. Br. J. Nutr. 2008, 99, 1275–1283. [Google Scholar] [CrossRef] [PubMed]
- Wardenaar, F.; Brinkmans, N.; Ceelen, I.; Van Rooij, B.; Mensink, M.; Witkamp, R.; De Vries, J. Micronutrient intakes in 553 Dutch elite and sub-elite athletes: Prevalence of low and high intakes in users and non-users of nutritional supplements. Nutrients 2017, 9, 142. [Google Scholar] [CrossRef] [PubMed]
- Merry, T.L.; Ristow, M. Do antioxidant supplements interfere with skeletal muscle adaptation to exercise training? J. Physiol. 2016, 594, 5135–5147. [Google Scholar] [CrossRef]
- Bentley, D.J.; Ackerman, J.; Clifford, T.; Slattery, K.S. Acute and chronic effects of antioxidant supplementation on exercise performance. In Antioxidants in Sport Nutrition, 1st ed.; CRC Press: Boca Raton FL, USA, 2015; p. 141. [Google Scholar]
- Morrison, D.; Hughes, J.; Della Gatta, P.A.; Mason, S.; Lamon, S.; Russell, A.P.; Wadley, G.D. Vitamin C and E supplementation prevents some of the cellular adaptations to endurance-training in humans. Free Radic. Biol. Med. 2015, 89, 852–862. [Google Scholar] [CrossRef]
- Paulsen, G.; Hamarsland, H.; Cumming, K.; Johansen, R.; Hulmi, J.; Børsheim, E.; Wiig, H.; Garthe, I.; Raastad, T. Vitamin C and E supplementation alters protein signalling after a strength training session, but not muscle growth during 10 weeks of training. J. Physiol. 2014, 592, 5391–5408. [Google Scholar] [CrossRef]
- Gomez-Cabrera, M.-C.; Domenech, E.; Romagnoli, M.; Arduini, A.; Borras, C.; Pallardo, F.V.; Sastre, J.; Vina, J. Oral administration of vitamin C decreases muscle mitochondrial biogenesis and hampers training-induced adaptations in endurance performance. Am. J. Clin. Nutr. 2008, 87, 142–149. [Google Scholar] [CrossRef]
- Ristow, M.; Zarse, K.; Oberbach, A.; Klöting, N.; Birringer, M.; Kiehntopf, M.; Stumvoll, M.; Kahn, C.R.; Blüher, M. Antioxidants prevent health-promoting effects of physical exercise in humans. Proc. Natl. Acad. Sci. USA 2009, 106, 8665–8670. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Marcos, P.J.; Auwerx, J. Regulation of PGC-1α, a nodal regulator of mitochondrial biogenesis. Am. J. Clin. Nutr. 2011, 93, 884S–890S. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, V.H.; Valente, H.F.; Casal, S.I.; Marques, A.F.; Moreira, P.A. Antioxidants do not prevent postexercise peroxidation and may delay muscle recovery. Med. Sci. Sports Exerc. 2009, 41, 1752–1760. [Google Scholar] [CrossRef]
- Peternelj, T.-T.; Coombes, J.S. Antioxidant supplementation during exercise training. Sports Med. 2011, 41, 1043–1069. [Google Scholar] [CrossRef] [PubMed]
- Pham-Huy, L.A.; He, H.; Pham-Huy, C. Free radicals, antioxidants in disease and health. Int. J. Biomed. Sci. 2008, 4, 89. [Google Scholar] [PubMed]
- Powers, S.K.; Sollanek, K.J.; Wiggs, M.P. Endurance Exercise and Antioxidant Supplementation: Sense or Nonsense?—Part 2. Sports Sci. 2014, 27, 1–4. [Google Scholar]
- Simon-Schnass, I. Oxidative stress at high altitude and effect of vitamin E. In Nutritional Needs in Cold and High Altitude Environments; National Academy Press: Washington, DC, USA, 1996; p. 393Á/418. [Google Scholar]
- Braakhuis, A.J.; Hopkins, W.G. Impact of dietary antioxidants on sport performance: A review. Sports Med. 2015, 45, 939–955. [Google Scholar] [CrossRef]
- Avery, N.G.; Kaiser, J.L.; Sharman, M.J.; Scheett, T.E.; Barnes, D.M.; Gomez, A.L.; Kraemer, W.J.; Volek, J.S. Effects of vitamin E supplementation on recovery from repeated bouts of resistance exercise. J. Strength Cond. Res. 2003, 17, 801–809. [Google Scholar]
- de Oliveira, D.C.; Rosa, F.T.; Simões-Ambrósio, L.; Jordao, A.A.; Deminice, R. Antioxidant vitamin supplementation prevents oxidative stress but does not enhance performance in young football athletes. Nutrition 2019, 63, 29–35. [Google Scholar] [CrossRef]
- Neubauer, O.; Yfanti, C. Antioxidants in athlete’s basic nutrition: Considerations towards a guideline for the intake of vitamin C and vitamin E. In Antioxidants in Sport Nutrition; CRC Press/Taylor & Francis: Boca Raton, FL, USA, 2015. [Google Scholar]
- Powers, S.K.; Jackson, M.J. Exercise-induced oxidative stress: Cellular mechanisms and impact on muscle force production. Physiol. Rev. 2008, 88, 1243–1276. [Google Scholar] [CrossRef]
- Rosa, T.S.; Neves, R.V.P.; Deus, L.A.; Sousa, C.V.; da Silva Aguiar, S.; de Souza, M.K.; Moraes, M.R.; Rosa, É.C.C.C.; Andrade, R.V.; Korhonen, M.T. Sprint and endurance training in relation to redox balance, inflammatory status and biomarkers of aging in master athletes. Nitr. Oxid. 2020, 102, 42–51. [Google Scholar] [CrossRef] [PubMed]
- Margaritelis, N.; Paschalis, V.; Theodorou, A.; Kyparos, A.; Nikolaidis, M. Redox basis of exercise physiology. Redox. Biol. 2020, 35, 101499. [Google Scholar] [CrossRef] [PubMed]
- Nikolaidis, M.G.; Kerksick, C.M.; Lamprecht, M.; McAnulty, S.R. Does vitamin C and E supplementation impair the favorable adaptations of regular exercise? Oxid. Med. Cell. Longev. 2012, 2012, 707941. [Google Scholar] [CrossRef] [PubMed]
- Michalczyk, M.; Czuba, M.; Zydek, G.; Zając, A.; Langfort, J. Dietary recommendations for cyclists during altitude training. Nutrition 2016, 8, 377. [Google Scholar] [CrossRef]
- Koivisto, A.; Paulsen, G.; Paur, I.; Garthe, I.; Tønnessen, E.; Raastad, T.; Bastani, N.; Hallén, J.; Blomhoff, R.; Bøhn, S. Antioxidant-rich foods and response to altitude training: A randomized controlled trial in elite endurance athletes. Scand. J. Med. Sci. Sports 2018, 28, 1982–1995. [Google Scholar] [CrossRef]
- Takanami, Y.; Iwane, H.; Kawai, Y.; Shimomitsu, T. Vitamin E supplementation and endurance exercise. Sports Med. 2000, 29, 73–83. [Google Scholar] [CrossRef]
- Sacheck, J.M.; Decker, E.A.; Clarkson, P.M. The effect of diet on vitamin E intake and oxidative stress in response to acute exercise in female athletes. Eur. J. Appl. Physiol. 2000, 83, 40–46. [Google Scholar] [CrossRef]
- Koivisto, A.E.; Olsen, T.; Paur, I.; Paulsen, G.; Bastani, N.E.; Garthe, I.; Raastad, T.; Matthews, J.; Blomhoff, R.; Bøhn, S.K. Effects of antioxidant-rich foods on altitude-induced oxidative stress and inflammation in elite endurance athletes: A randomized controlled trial. PLoS ONE 2019, 14, e0217895. [Google Scholar] [CrossRef]
- Capó, X.; Martorell, M.; Busquets-Cortés, C.; Sureda, A.; Riera, J.; Drobnic, F.; Tur, J.; Pons, A. Effects of dietary almond-and olive oil-based docosahexaenoic acid-and vitamin E-enriched beverage supplementation on athletic performance and oxidative stress markers. Food Funct. 2016, 7, 4920–4934. [Google Scholar] [CrossRef]
- Capó, X.; Martorell, M.; Sureda, A.; Riera, J.; Drobnic, F.; Tur, J.A.; Pons, A. Effects of almond-and olive oil-based Docosahexaenoic-and vitamin E-enriched beverage dietary supplementation on inflammation associated to exercise and age. Nutrition 2016, 8, 619. [Google Scholar] [CrossRef]
- Hoene, M.; Irmler, M.; Beckers, J.; Hrabě de Angelis, M.; Häring, H.-U.; Weigert, C. A vitamin E-enriched antioxidant diet interferes with the acute adaptation of the liver to physical exercise in mice. Nutrients 2018, 10, 547. [Google Scholar] [CrossRef] [PubMed]
- Górnicka, M.; Ciecierska, A.; Hamulka, J.; Drywień, M.E.; Frackiewicz, J.; Górnicki, K.; Wawrzyniak, A. α-Tocopherol protects the heart, muscles, and testes from lipid peroxidation in growing male rats subjected to physical efforts. Oxid. Med. Cell. Longev. 2019, 2019, 8431057. [Google Scholar] [CrossRef]
- Yi, M.; Fu, J.; Zhou, L.; Gao, H.; Fan, C.; Shao, J.; Xu, B.; Wang, Q.; Li, J.; Huang, G. The effect of almond consumption on elements of endurance exercise performance in trained athletes. J. Int. Soc. Sports Nutr. 2014, 11, 18. [Google Scholar] [CrossRef] [PubMed]
- Esquius, L.; Segura, R.; Oviedo, G.R.; Massip-Salcedo, M.; Javierre, C. Effect of Almond Supplementation on Non-Esterified Fatty Acid Values and Exercise Performance. Nutrition 2020, 12, 635. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-P.; Mar, G.-Y.; Ng, L.-T. Effects of tocotrienol-rich fraction on exercise endurance capacity and oxidative stress in forced swimming rats. Eur. J. Appl. Physiol. 2009, 107, 587–595. [Google Scholar] [CrossRef]
- Powers, S.K.; De Ruisseau, K.C.; Quindry, J.; Hamilton, K.L. Dietary antioxidants and exercise. J. Sports Sci. 2004, 22, 81–94. [Google Scholar] [CrossRef]
- Paulsen, G.; Cumming, K.T.; Holden, G.; Hallén, J.; Rønnestad, B.R.; Sveen, O.; Skaug, A.; Paur, I.; Bastani, N.E.; Østgaard, H.N. Vitamin C and E supplementation hampers cellular adaptation to endurance training in humans: A double-blind, randomised, controlled trial. J. Physiol. 2014, 592, 1887–1901. [Google Scholar] [CrossRef]
- Akova, B.; SuÈrmen-GuÈr, E.; GuÈr, H.; Dirican, M.; SarandoÈl, E.; Küçükoglu, S. Exercise-induced oxidative stress and muscle performance in healthy women: Role of vitamin E supplementation and endogenous oestradiol. Eur. J. Appl. Physiol. 2001, 84, 141–147. [Google Scholar] [CrossRef]
- Zoppi, C.C.; Hohl, R.; Silva, F.C.; Lazarim, F.L.; Neto, J.A.; Stancanneli, M.; Macedo, D.V. Vitamin C and e supplementation effects in professional soccer players under regular training. J. Int. Soc. Sports Nutr. 2006, 3, 37. [Google Scholar] [CrossRef]
- Silva, L.A.; Pinho, C.A.; Silveira, P.C.; Tuon, T.; De Souza, C.T.; Dal-Pizzol, F.; Pinho, R.A. Vitamin E supplementation decreases muscular and oxidative damage but not inflammatory response induced by eccentric contraction. J. Physiol. Sci. 2010, 60, 51. [Google Scholar] [CrossRef]
- Strobel, N.A.; Peake, J.M.; Matsumoto, A.; Marsh, S.A.; Coombes, J.S.; Wadley, G.D. Antioxidant supplementation reduces skeletal muscle mitochondrial biogenesis. Med. Sci. Sports Exerc. 2011, 43, 1017–1024. [Google Scholar] [CrossRef] [PubMed]
- Venditti, P.; Napolitano, G.; Barone, D.; Di Meo, S. Vitamin E supplementation modifies adaptive responses to training in rat skeletal muscle. Free Radic. Res. 2014, 48, 1179–1189. [Google Scholar] [CrossRef] [PubMed]
- Braakhuis, A.J.; Hopkins, W.G.; Lowe, T.E. Effects of dietary antioxidants on training and performance in female runners. Eur. J. Sport Sci. 2014, 14, 160–168. [Google Scholar] [CrossRef] [PubMed]
- Roberts, L.A.; Beattie, K.; Close, G.L.; Morton, J.P. Vitamin C consumption does not impair training-induced improvements in exercise performance. Int. J. Sports Physiol. Perform. 2011, 6, 58–69. [Google Scholar] [CrossRef]
- Yfanti, C.; Åkerström, T.; Nielsen, S.; Nielsen, A.R.; Mounier, R.; Mortensen, O.H.; Lykkesfeldt, J.; Rose, A.J.; Fischer, C.P.; Pedersen, B.K. Antioxidant supplementation does not alter endurance training adaptation. Med. Sci. Sports. Exerc. 2010, 42, 1388–1395. [Google Scholar] [CrossRef]
- Clifford, T.; Jeffries, O.; Stevenson, E.J.; Davies, K.A.B. The effects of vitamin C and E on exercise-induced physiological adaptations: A systematic review and Meta-analysis of randomized controlled trials. Crit. Rev. Food Sci. Nutr. 2019. [Google Scholar] [CrossRef]
- Bjørnsen, T.; Salvesen, S.; Berntsen, S.; Hetlelid, K.; Stea, T.; Lohne-Seiler, H.; Rohde, G.; Haraldstad, K.; Raastad, T.; Køpp, U. Vitamin C and E supplementation blunts increases in total lean body mass in elderly men after strength training. Scand. J. Med. Sci. Sports 2016, 26, 755–763. [Google Scholar] [CrossRef]
- Bobeuf, F.; Labonté, M.; Khalil, A.; Dionne, I.J. Effects of resistance training combined with antioxidant supplementation on fat-free mass and insulin sensitivity in healthy elderly subjects. Diabetes Res. Clin. Pract. 2010, 87, e1–e3. [Google Scholar] [CrossRef]
- Bobeuf, F.; Labonte, M.; Dionne, I.; Khalil, A. Combined effect of antioxidant supplementation and resistance training on oxidative stress markers, muscle and body composition in an elderly population. J. Nutr. Health Aging 2011, 15, 883–889. [Google Scholar] [CrossRef]
- Chou, C.-C.; Sung, Y.-C.; Davison, G.; Chen, C.-Y.; Liao, Y.-H. Short-term high-dose vitamin C and E supplementation attenuates muscle damage and inflammatory responses to repeated taekwondo competitions: A randomized placebo-controlled trial. Int. J. Med. Sci. 2018, 15, 1217. [Google Scholar] [CrossRef]
- Cumming, K.T.; Raastad, T.; Sørstrøm, A.; Paronetto, M.P.; Mercatelli, N.; Ugelstad, I.; Caporossi, D.; Paulsen, G. Vitamin C and E supplementation does not affect heat shock proteins or endogenous antioxidants in trained skeletal muscles during 12 weeks of strength training. BMC Nutr. 2017, 3, 70. [Google Scholar] [CrossRef] [PubMed]
- Dutra, M.T.; Alex, S.; Mota, M.R.; Sales, N.B.; Brown, L.E.; Bottaro, M. Effect of strength training combined with antioxidant supplementation on muscular performance. Appl. Physiol. Nutr. Metab. 2018, 43, 775–781. [Google Scholar] [CrossRef] [PubMed]
- Paoli, A.; Bianco, A.; Grimaldi, K.A. The ketogenic diet and sport: A possible marriage? Exerc. Sport Sci. Rev. 2015, 43, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Hamułka, J.; Górnicka, M.; Sulich, A.; Frąckiewicz, J. Weight loss program is associated with decrease α-tocopherol status in obese adults. Clin. Nutr. 2019, 38, 1861–1870. [Google Scholar] [CrossRef] [PubMed]
- Mulligan, A.; Lentjes, M.; Hayhoe, R.; Khaw, K.; Welch, A. Ratio of plasma α: γ-tocopherol and associations with indices of skeletal muscle mass. Proc. Nutr. Soc. 2018, 77. [Google Scholar] [CrossRef]
- Mulligan, A.; Lentjes, M.; Luben, R.; Khaw, K.; Welch, A. Dietary vitamin E intake is associated with greater fat-free mass and percentage fat-free mass in the EPIC-Norfolk cohort. Proc. Nutr. Soc. 2016, 75. [Google Scholar] [CrossRef]
- Yfanti, C.; Tsiokanos, A.; Fatouros, I.G.; Theodorou, A.A.; Deli, C.K.; Koutedakis, Y.; Jamurtas, A.Z. Chronic eccentric exercise and antioxidant supplementation: Effects on lipid profile and insulin sensitivity. J. Sports Sci. Med. 2017, 16, 375. [Google Scholar]
- Theodorou, A.A.; Nikolaidis, M.G.; Paschalis, V.; Koutsias, S.; Panayiotou, G.; Fatouros, I.G.; Koutedakis, Y.; Jamurtas, A.Z. No effect of antioxidant supplementation on muscle performance and blood redox status adaptations to eccentric training. Am. J. Clin. Nutr. 2011, 93, 1373–1383. [Google Scholar] [CrossRef]
- Gillam, I.; Telford, R.; Skinner, S. Is there a critical tissue vitamin E level to maintain cell membrane integrity and assist recovery in elite endurance trained athletes? J. Sci. Med. Sport 2018, 21, S29. [Google Scholar] [CrossRef]
- Traber, M.G.; Stevens, J.F. Vitamins C and E: Beneficial effects from a mechanistic perspective. Free Radic. Biol. Med. 2011, 51, 1000–1013. [Google Scholar] [CrossRef]
- Jungert, A.; Neuhäuser-Berthold, M. Interrelation between Plasma Concentrations of Vitamins C and E along the Trajectory of Ageing in Consideration of Lifestyle and Body Composition: A Longitudinal Study over Two Decades. Nutrients 2020, 12, 2944. [Google Scholar] [CrossRef] [PubMed]
- Yeum, K.-J.; Beretta, G.; Krinsky, N.I.; Russell, R.M.; Aldini, G. Synergistic interactions of antioxidant nutrients in a biological model system. Nutrition 2009, 25, 839–846. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, I.M.; Gilmore, W.S.; Benzie, I.F.; Mulholland, C.W.; Strain, J. Interactions between vitamins C and E in human subjects. Br. J. Nutr. 2000, 84, 261–267. [Google Scholar] [CrossRef] [PubMed]
- Shafat, A.; Butler, P.; Jensen, R.; Donnelly, A. Effects of dietary supplementation with vitamins C and E on muscle function during and after eccentric contractions in humans. Eur. J. Appl. Physiol. 2004, 93, 196–202. [Google Scholar] [CrossRef]
- Nalbant, Ö.; Toraman, N.F.; Öğü, C.; Aydın, H.; Kaçar, C.; Özkaya, Y.G. Vitamin E and aerobic exercise: Effects on physical performance in older adults. Aging Clin. Exp. Res. 2009, 21, 111–121. [Google Scholar] [CrossRef]
- Yfanti, C.; Nielsen, A.R.; Åkerström, T.; Nielsen, S.; Rose, A.J.; Richter, E.A.; Lykkesfeldt, J.; Fischer, C.P.; Pedersen, B.K. Effect of antioxidant supplementation on insulin sensitivity in response to endurance exercise training. Appl. Physiol. Nutr. Metab. 2011, 300, E761–E770. [Google Scholar] [CrossRef]
- Yfanti, C.; Fischer, C.P.; Nielsen, S.; Åkerström, T.; Nielsen, A.R.; Veskoukis, A.S.; Kouretas, D.; Lykkesfeldt, J.; Pilegaard, H.; Pedersen, B.K. Role of vitamin C and E supplementation on IL-6 in response to training. J. Appl. Physiol. 2012, 112, 990–1000. [Google Scholar] [CrossRef]
- He, F.; Hockemeyer, J.; Sedlock, D. Does combined antioxidant vitamin supplementation blunt repeated bout effect. Int. J. Sports Med. 2015, 36, 407–413. [Google Scholar] [CrossRef]
- Santos, S.; Silva, E.; Caris, A.; Lira, F.; Tufik, S.; Dos Santos, R. Vitamin E supplementation inhibits muscle damage and inflammation after moderate exercise in hypoxia. J. Hum. Nutr. Diet. 2016, 29, 516–522. [Google Scholar] [CrossRef]
- Wyckelsma, V.L.; Venckunas, T.; Brazaitis, M.; Gastaldello, S.; Snieckus, A.; Eimantas, N.; Baranauskiene, N.; Subocius, A.; Skurvydas, A.; Pääsuke, M. Vitamin C and E Treatment Blunts Sprint Interval Training–Induced Changes in Inflammatory Mediator-, Calcium-, and Mitochondria-Related Signaling in Recreationally Active Elderly Humans. Antioxidants 2020, 9, 879. [Google Scholar] [CrossRef]
- Coombes, J.S.; Powers, S.K.; Rowell, B.; Hamilton, K.L.; Dodd, S.L.; Shanely, R.A.; Sen, C.K.; Packer, L. Effects of vitamin E and α-lipoic acid on skeletal muscle contractile properties. J. Appl. Physiol. 2001, 90, 1424–1430. [Google Scholar] [CrossRef] [PubMed]
- Ryan, M.J.; Dudash, H.J.; Docherty, M.; Geronilla, K.B.; Baker, B.A.; Haff, G.G.; Cutlip, R.G.; Alway, S.E. Vitamin E and C supplementation reduces oxidative stress, improves antioxidant enzymes and positive muscle work in chronically loaded muscles of aged rats. Exp. Gerontol. 2010, 45, 882–895. [Google Scholar] [CrossRef] [PubMed]
- Kyparos, A.; Sotiriadou, S.; Mougios, V.; Cheva, A.; Barbanis, S.; Karkavelas, G.; Arsos, G.; Albani, M.; Matziari, C. Effect of 5-day vitamin E supplementation on muscle injury after downhill running in rats. Eur. J. Appl. Physiol. 2011, 111, 2557–2569. [Google Scholar] [CrossRef] [PubMed]
- Al-Hashem, F.H. Potential roles for vitamins E and C in combination in modulating exhaustive swimming and high altitude-associated lung injury in rats. Saudi Med. J. 2012, 33, 367–374. [Google Scholar] [PubMed]
- Picklo, M.J.; Thyfault, J.P. Vitamin E and vitamin C do not reduce insulin sensitivity but inhibit mitochondrial protein expression in exercising obese rats. Appl. Physiol. Nutr. Metab. 2015, 40, 343–352. [Google Scholar] [CrossRef] [PubMed]
- Goswami, A.R.; Ghosh, T. Vitamin E Reduces Hypobaric Hypoxia-Induced Immune Responses in Male Rats. High Alt. Med. Biol. 2019, 20, 12–21. [Google Scholar] [CrossRef]
- Fagan, M.M.; Harris, P.; Adams, A.; Pazdro, R.; Krotky, A.; Call, J.; Duberstein, K.J. Form of vitamin E supplementation effects oxidative and inflammatory response in exercising horses. J. Equine Vet. Sci. 2020, 91, 103103. [Google Scholar] [CrossRef]
- Hoffman, R.M.; Olesiak, M.; Harris, M.B. Effects of vitamin E supplementation and exercise on endothelial function in rat aortas. FASEB 2013. [Google Scholar] [CrossRef]
- Roberts II, L.J.; Oates, J.A.; Linton, M.F.; Fazio, S.; Meador, B.P.; Gross, M.D.; Shyr, Y.; Morrow, J.D. The relationship between dose of vitamin E and suppression of oxidative stress in humans. Free Radic. Biol. Med. 2007, 43, 1388–1393. [Google Scholar] [CrossRef]
- Merry, T.L.; McConell, G.K. Do reactive oxygen species regulate skeletal muscle glucose uptake during contraction? Exerc. Sport Sci. Rev. 2012, 40, 102–105. [Google Scholar] [CrossRef]
- Jackson, M.J. Redox regulation of adaptive responses in skeletal muscle to contractile activity. Free Radic. Biol. Med. 2009, 47, 1267–1275. [Google Scholar] [CrossRef] [PubMed]
- Mason, S.A.; Morrison, D.; McConell, G.K.; Wadley, G.D. Muscle redox signalling pathways in exercise. Role of antioxidants. Free Radic. Biol. Med. 2016, 98, 29–45. [Google Scholar] [CrossRef] [PubMed]
- Lamb, G.D.; Westerblad, H. Acute effects of reactive oxygen and nitrogen species on the contractile function of skeletal muscle. J. Physiol. 2011, 589, 2119–2127. [Google Scholar] [CrossRef] [PubMed]
- Evans, L.W.; Zhang, F.; Omaye, S.T. Vitamin C Supplementation Reduces Exercise-Induced Oxidative Stress and Increases Peak Muscular Force. Food. Nutr. Sci. 2017, 8, 812–822. [Google Scholar] [CrossRef]
- Margaritelis, N.V.; Paschalis, V.; Theodorou, A.A.; Kyparos, A.; Nikolaidis, M.G. Antioxidants in personalized nutrition and exercise. Adv. Nutr. 2018, 9, 813–823. [Google Scholar] [CrossRef] [PubMed]
- Paschalis, V.; Theodorou, A.A.; Kyparos, A.; Dipla, K.; Zafeiridis, A.; Panayiotou, G.; Vrabas, I.S.; Nikolaidis, M.G. Low vitamin C values are linked with decreased physical performance and increased oxidative stress: Reversal by vitamin C supplementation. Eur. J. Nutr. 2016, 55, 45–53. [Google Scholar] [CrossRef]
- Thompson, D.; Williams, C.; McGregor, S.J.; Nicholas, C.W.; McArdle, F.; Jackson, M.J.; Powell, J.R. Prolonged vitamin C supplementation and recovery from demanding exercise. Int. J. Sport Nutr. Exerc. Metab. 2001, 11, 466–481. [Google Scholar] [CrossRef]
- Bryer, S.; Goldfarb, A.H. Effect of high dose vitamin C supplementation on muscle soreness, damage, function, and oxidative stress to eccentric exercise. Int. J. Sport Nutr. Exerc. Metab. 2006, 16, 270–280. [Google Scholar] [CrossRef]
- Close, G.L.; Ashton, T.; Cable, T.; Doran, D.; Holloway, C.; McArdle, F.; MacLaren, D.P. Ascorbic acid supplementation does not attenuate post-exercise muscle soreness following muscle-damaging exercise but may delay the recovery process. Br. J. Nutr. 2006, 95, 976–981. [Google Scholar] [CrossRef]
- Connolly, D.; Lauzon, C.; Agnew, J.; Dunn, M.; Reed, B. The effects of vitamin C supplementation on symptoms of delayed onset muscle soreness. J. Sports. Med. Fit. 2006, 46, 462. [Google Scholar]
- Scalzo, R.L.; Bauer, T.A.; Harrall, K.; Moreau, K.; Ozemek, C.; Herlache, L.; McMillin, S.; Huebschmann, A.G.; Dorosz, J.; Reusch, J.E. Acute vitamin C improves cardiac function, not exercise capacity, in adults with type 2 diabetes. Diabetol. Metab. Syndr. 2018, 10, 7. [Google Scholar] [CrossRef] [PubMed]
- Kobayaski, Y. Effect of Vitamin E on Aerobic Work Performance in Man during Acute Exposure to Hypoxic Hypoxia. Ph.D. Thesis, University of New Mexico, Albuquerque, NM, USA, 1974. [Google Scholar]
- Simon-Schnass, I.; Pabst, H. Influence of vitamin E on physical performance. Int. J. Vitam. Nutr. Res. 1988, 58, 49–54. [Google Scholar] [PubMed]
- Subudhi, A.W.; Jacobs, K.A.; Hagobian, T.A.; Fattor, J.A.; Fulco, C.S.; Muza, S.R.; Rock, P.B.; Hoffman, A.R.; Cymerman, A.; Friedlander, A.L. Antioxidant supplementation does not attenuate oxidative stress at high altitude. Aviat Space Environ. Med. 2004, 75, 881–888. [Google Scholar] [PubMed]
- Lee, G.Y.; Han, S.N. The role of vitamin E in immunity. Nutrients 2018, 10, 1614. [Google Scholar] [CrossRef]
- Siques, P.; Brito, J.; Pena, E. Reactive oxygen species and pulmonary vasculature during hypobaric hypoxia. Front. Physiol. 2018, 9, 865. [Google Scholar] [CrossRef]
- Smita, K.; Qadar Pasha, M.; Jain, S. Oxidative stress and histopathological evaluation of rat lung tissue during hypobaric hypoxia. J. Proteom. Bioinform. 2015, 8, 108–115. [Google Scholar]
- Larsen, S.; Nielsen, J.; Hansen, C.N.; Nielsen, L.B.; Wibrand, F.; Stride, N.; Schroder, H.D.; Boushel, R.; Helge, J.W.; Dela, F. Biomarkers of mitochondrial content in skeletal muscle of healthy young human subjects. J. Physiol. 2012, 590, 3349–3360. [Google Scholar] [CrossRef]
- Reid, M.B. Free radicals and muscle fatigue: Of ROS, canaries, and the IOC. Free Radic. Biol. Med. 2008, 44, 169–179. [Google Scholar] [CrossRef]
- Nocella, C.; Cammisotto, V.; Pigozzi, F.; Borrione, P.; Fossati, C.; D’Amico, A.; Cangemi, R.; Peruzzi, M.; Gobbi, G.; Ettorre, E. Impairment between oxidant and antioxidant systems: Short-and long-term implications for athletes’ health. Nutrients 2019, 11, 1353. [Google Scholar] [CrossRef]
- Gomez-Cabrera, M.C.; Ristow, M.; Viña, J. Antioxidant supplements in exercise: Worse than useless? Am. J. Physiol. Endocrinol. Metab. 2012, 302, E476–E477. [Google Scholar] [CrossRef]
- Valenzuela, P.L.; Morales, J.S.; Emanuele, E.; Pareja-Galeano, H.; Lucia, A. Supplements with purported effects on muscle mass and strength. Eur. J. Nutr. 2019, 58, 2983–3008. [Google Scholar] [CrossRef] [PubMed]
| Study | Participants | Exercise | Timing | Supplementation | Result |
|---|---|---|---|---|---|
| Morrison et al. 2015 [9] | Healthy, young men (n = 11) | Ten, 4 min intervals at 90% VO2 peak interspersed by 2 min active rest at 50 W | Once per day for 4 weeks | Vitamin C (2 × 500 mg/day) and vitamin E (400 IU/day) (placebo added) |
VO2 peak and W max were significantly increased with no effect of supplementation. Rate of perceived exertion was reduced independent of supplementation |
| Paulsen et al. 2014 [10] | Recreationally strength trained men (n = 21) and women (n = 11) | 4 × 10 RM leg press and knee extension with 1 min rest between sets and 3 min rest between exercises | 1–3 h before training and 1 h following training | Vitamin C (1000 mg/day) and vitamin E (235 mg/day) (placebo added) | Supplementation did not blunt muscle hypertrophy, but measurements of muscle strength were lower following supplementation |
| Ristow et al. 2009 [12] | Previously untrained (n = 19) and pretrained (n = 20) healthy, young males | Four-week physical exercise intervention | Once per day for 4 weeks | Vitamin C (1000 mg/day) and vitamin E (400 IU/day) (placebo added) | Exercise increased parameters of insulin sensitivity in both groups only in absence of antioxidants |
| de Oliveira et al. 2019 [21] | Football athletes (n = 21) | A protocol consisting of plyometric jumping and strength resistance sets to exhaustion | After 7 days of supplementation, athletes were submitted to training protocol | vitamin C (500 mg/d) and E (400 UI/d) for 15 days | Although antioxidant supplementation reduced oxidative stress, it did not attenuate elevated markers of muscle damage or muscle soreness do not exert any ergogenic effect on football performance |
| Paulsen et al. 2014 [40] | Recreationally endurance trained (n = 45) and untrained (n = 14) men and women | VO2 max treadmill test at 5.3% elevation, and 20 shuttle run beep test | 1–3 h before every training session and 1 h after on training days, and in the morning and evening on non-training days | Ascorbic acid (1000 mg/day) and DL α-tocopherol acetate (235 mg/day) (placebo added) | VO2 max and shuttle test performance was increased in both groups |
| Akova et al. 2001 [41] | Sedentary, healthy women (n = 18) | Submaximal cycling (50%) followed by maximal concentric-eccentric combined contractions to measure maximal work force of the dominant knee | Once per day across the duration of two menstrual cycles | Alpha-tocopherol (300 mg/day) (placebo added) | No effect of vitamin E on muscle performance |
| Zoppi et al. 2006 [42] | Young male soccer players (n = 5) | Lactate maximum speed protocol, one rep max two-legged knee extension, and a 30 m maximal sprint test | Four equal doses every day for 90 days | Ascorbic acid (1000 mg/day) and α-tocopherol (800 mg/day) (placebo added) | No effect on aerobic capacity, strength, or speed |
| Yfanti et al. 2010 [48] | Healthy, physically active males (n = 21) | Incremental exercise test on a cadence-independent cycle ergometer for VO2 max and Pmax | Once per day at breakfast for 4 weeks prior to training then for 12 weeks during cycling training | Vitamin C (500 mg/day) and vitamin E (400 IU/day) (placebo added) | No effect on Pmax or VO2 max |
| Bjørnsen et al. 2016 [50] | Elderly men (n = 34) | One repetition max (1 RM) leg extension | 500 mg vitamin C and 117.5 mg vitamin E before and after training 3 times per week for 3 weeks | Vitamin C (1000 mg/day) and vitamin E (235 mg/day) (placebo added) | Supplementation blunted muscular some adaptations (lean mass gains) |
| Bobeuf et al. 2011 [52] | Sedentary older men (n = 27) and women (n = 30) | Resistance training 3 days per week for 6 months | Daily for 6 months | Vitamin C (1000 mg/day) and vitamin E (400 IU/day) (placebo added) | No effect on strength gains |
| Chou et al. 2018 [53] | Elite male taekwondo (TKD) athletes (n = 18) | Four TKD matches against weight matched competitors | Twice daily 3 days before and the day of competition | Vitamin C (2000 mg/day) and vitamin E (1400 IU/day) (placebo added) | Supplementation attenuated circulating creatine kinase and myoglobin. Antioxidant supplementation suppressed exercise provided RBC hemolysis and systemic inflammation |
| Cumming et al. 2017 [54] | Physically active male (n = 18) and female (n = 10) | A 4x10 RM leg press and knee extension with 1 min rest between sets and 3 min rest between exercises | Two pills 1–3 h before training and 2 pills in the first hour after training | Vitamin C (1000 mg/day) and vitamin E (235 mg/day) (placebo added) | No effect on acute stress responses |
| Yfanti et al. 2017 [60] | Healthy men (n = 16) | Isometric knee extensor peak at 90° knee flexion | Once per day at breakfast for 5 weeks prior to training and for 4 weeks during eccentric exercise training | Vitamin C (1 g/day) and vitamin E (400 IU/day) (placebo added) | Peak torque increased in both groups with no significant difference between groups |
| Theodorou et al. 2011 [61] | Recreationally trained healthy men (n = 28) | Five sets of 15 eccentric maximal contractions with each leg on an isokinetic dynamometer | Once daily for 11 weeks at breakfast | Vitamin C (1 g/day) and vitamin E (400 IU/day) (placebo added) | No significant supplement and time effect on muscle function, redox status, or hemolysis observed |
| Shafat et al. 2004 [67] | Moderately active males (n = 12) | Thirty sets of 10 eccentric knee extensions | Once per day for 37 days | Vitamin C (500 mg/day) and vitamin E (1200 IU/day) (placebo added) | Supplementation reduced the deficit in muscle function experienced during and after bouts of eccentric muscle contractions |
| Nalbant et al. 2009 [68] | Older adults (n = 57) | three sessions of walking exercise per week | Six months | Vitamin E (900 IU/day) | Exercise alone or combined with vitamin E supplementation improved 6-min walk, chair stand, arm curl tests. |
| Yfanti et al. 2010 [69] | Moderately trained young men (n = 21) | VO2 max cadence dependent cycle test | Daily supplementation for 16 weeks | Vitamin C (500 mg/day) and vitamin E (400 IU/day) (placebo added) | No effect on VO2 max or maximum power output |
| Yfanti et al. 2012 [70] | Healthy, physically active males (n = 21) | One hour at 65% max power on a cycle ergometer | Once per day at breakfast for 16 weeks | Vitamin C (500 mg/day) and vitamin E (400 IU/day) (placebo added) | Supplementation does not further decrease interlukin-6 levels following endurance training |
| He et al. 2015 [71] | Moderately trained males (n = 22) | 40 min downhill run at 65–70% VO2 max | Daily for 2 weeks | Vitamin C (1000 mg/day) and vitamin E (400 IU/day) | Supplementation enhanced repeated bout effect as evidenced by attenuation of biomarkers of muscle damage and greater antioxidant capacity |
| Santos et al. 2018 [72] | Healthy, physically active males (n = 9) | VO2 max treadmill test to exhaustion under normal conditions and hypoxia conditions | One hour before exercise | Vitamin E (250 mg) (placebo added) | A reduction was seen in creatine kinase (CK) and lactate dehydrogenase levels. |
| Wyckelsma et al. 2020 [73] | Recreationally active elderly (mean age 65, n = 18) | nine sessions (three sessions/week for 3 weeks) | On the training days, tablets were taken at least 1 h before the training session | vitamin C (1 g daily) and vitamin E (235 mg daily), treatments were initiated 7 days before the first sprint interval training (SIT) session | Supplementation with antioxidants vitamin C and E blunts SIT-induced cellular signaling in skeletal muscle of elderly individuals |
| Study | Participants | Exercise | Supplementation | Result |
|---|---|---|---|---|
| Hoene et al. 2018 [34] | Male mice | One hour of treadmill running | Diet supplemented with 100 mg/kg vitamin C and 2000 IU/kg vitamin E (as _-tocopheryl acetate) for 4 weeks | The increase in circulatory free fatty acids 1-h post exercise was blunted vitamin E. Upregulation of several exercise-responsive transcripts was attenuated by vitamin E. |
| Górnicka et al. 2019 [35] | Male Wistar rats | Fifteen minutes of treadmill running/day | Two mg/day of vitamin E as α-tocopherol acetate for 14 days | Vitamin E supplementation significantly reduced thiobarbituric acid reactive substance (TBARS) in the muscles and heart. |
| Lee et al. 2009 [38] | Male Wistar rats | Forced swimming 10 h after the last treatment | 25 or 50 mg/kg of tocotrienol-rich fraction (TRF), or 25 mg/kg D-α-tocopherol (T-25) for 28 days | TRF improved endurance capacity indicated by longer duration of swimming and reduce the exercise-induced oxidative stress. |
| Strobel et al. 2011 [44] | Male Wistar rats | Treadmill running 4 days/week for 14 weeks | Vitamin E (1000 mg/kg) and α-lipoic acid (1.6 g/kg) fortified feed | No effect on the changes of exercise induced markers of mitochondrial biogenesis and mitochondrial proteins |
| Venditti et al. 2014 [45] | Male Wistar rats | Swimming 5 days/week for 10 weeks | Vitamin E (700 mg/kg) fortified food | Vitamin E supplementation attenuated training induced declines in mitochondrial respiration. |
| Coombes et al. 2001 [74] | Antiox rats | A fatigue protocol (30 min) | 10,000 IU vitamin E/kg diet and 1.65 g/kg and α-lipoic acid for 8 weeks | A decline in skeletal muscle force production at low stimulation frequencies |
| Ryan et al. 2010 [75] | Young and aged Male rats | Three times weekly for 4.5 weeks using 80 maximal stretch–shortening contractions per session | Diet supplemented with Vitamin E (DL-alpha tocopheryl acetate; 30,000 mg/kg) and Vitamin C (Lascorbic acid; 2% by weight) | Supplementing with vitamin E and C reduced oxidative damage markers (e.g., malondialdehyde) associated with aging. |
| Kyparos et al. 2011 [76] | Male Wistar rats | 90 min of intermittent downhill running on a motor-driven treadmill | Vitamin E was administered by daily intraperitoneal injections of 100 mg/kg body mass of DL-α-tocopheryl acetate for 5 consecutive days prior to exercise | Vitamin E supplementation resulted in a higher soleus muscle single-twitch tension immediately post-exercise compared to the placebo condition. No effect of vitamin E supplementation on eccentric exercise-induced muscle damage. |
| Al-Hashem 2012 [77] | Male rats | Acute forced exhaustive swimming stress for a duration of 2.5 h in glass tanks (in both low and high altitude) | A single dose of 25 mg/kg of vitamin E and 20 mg/kg of vitamin C 1 h before the experimental procedure | Co-ingestion of vitamin E and C resulted in lower activities of SOD and catalase in the lungs under high and low altitude conditions. measured at both altitudes |
| Picklo et al. 2015 [78] | Male obese rats | Running 5days/week for 12 weeks on a motorized wheel | Dietary supplementation with vitamin E (0.4 g α-tocopherol acetate/kg) and vitamin C (0.5 g/kg) during a high fat diet | No difference in insulin area under curve was found between the conditions. Exercise combined with vitamin C and vitamin E resulted in a higher mitochondrial DNA content versus exercise alone or high fat diet alone conditions. |
| Goswami and Ghosh. 2019 [79] | Male Albino rats | The rats were exposed to simulated conditions of hypobaric hypoxia (HH) for 8 h daily for 6 consecutive days | Two equal daily doses of vitamin E (first half of the total dose, i.e., 10, 20, and 30 mg/kg of vitamin E) was given orally 5–6 min before the exposure to HH and the second half was given immediately after | Vitamin E in a dose-dependent manner blocked some of the immune changes such as, phagocytic activity of white blood cell, cytotoxic activity of splenic mononuclear cell. Also, corticosterone levels was reduced by vitamin E. |
| Fagan et al. 2020 [80] | horses | Six-week conditioning program | Horses divided to three groups and fed the control diet plus (1) 1000 IU/day synthetic α [44] tocopherol, (2) 4000 IU/day synthetic α-tocopherol, or (3) 4000 IU/day RRR-α-tocopherol (natural source) | Vitamin E improved some oxidative and inflammatory responses. |
| Study | Participants | Exercise | Timing | Supplementation | Result |
|---|---|---|---|---|---|
| Gomez-Cabrera et al. 2008 [11] | Human (15 men) and male Wistar rats | For humans: Static bicycle on 3 days/week for 8 weeks; for animals 5 days/week on an animal treadmill | For 8 weeks | For humans: 1 g/day vitamin C and for rats 0.24 mg/cm2 body surface area | Vitamin C reduced factors are PGC-1, nuclear respiratory factor 1, and mitochondrial transcription factor A. The exercise-induced expression of cytochrome C, SD, and glutathione peroxidase were blocked by vitamin C. |
| Roberts et al. 2011 [47] | Recreationally active males (n = 15) | high-intensity interval running protocol, 4 times per week | For 4 weeks | 1 g/day | Training-induced improvements of VO2max, 10 km time trial, and running economy were not affected by vitamin C. |
| Evans et al. 2017 [87] | Nine persons naive to resistance exercise (RE) | One RE bout was performed pre-supplementation and one was performed post-supplementation; RE bouts consisted of a warmup set of bodyweight pushups, three working sets (WS) of 10 isokinetic contracting push-pull repetitions, and one maximal effort set (ME) of five isokinetic contracting push-pull repetitions | For 28 days | 250 mg every 12 h | Vitamin C supplementation increased peak muscular pushing force (PMF) and reduced exercise-induced oxidative stress. |
| Paschalis et al. 2016 [89] | recreationally trained healthy males (n = 20) | aerobic exercise to exhaustion | For 30 days | three vitamin C tablets/day (each tablet contained 333 mg of vitamin C) | The low vitamin C group had lower VO2 max values than the high vitamin C group. Vitamin C supplementation in this group marginally increased VO2 max. |
| Thompson et al. 2001 [90] | Active men (n = 16) | Aerobic: A prolonged intermittent shuttle-running test for 90 min | For 14 days | 400 mg/day | Vitamin C supplementation had modest beneficial effects on muscle soreness and muscle function |
| Bryer and Goldfarb 2006 [91] | healthy men (n = 18) | Seventy eccentric elbow extensions with their non-dominant arm | for 2 weeks prior and 4 days after eccentric exercise | 3 g/day | Vitamin C reduced muscle soreness and delayed CK increase. Muscle force and range of motion were not affected. |
| close et al. 2006 [92] | Physically active male subjects (n = 20) | Aerobic: Downhill running for 30 min at 60% VO2 max | For 2 h pre-, and for 14 days after exercise | 1 g/day | Vitamin C attenuated RONS production following downhill running. No effect on DOMS was seen. |
| Connolly et al. 2006 [93] | 24 subjects (male and female) | Anaerobic: 40 (2 × 20) maximal eccentric contractions of the elbow flexors | For 8 days (3 days prior to an exercise bouts and 5 days after) | 3 g/day | Vitamin C had no effect on muscle soreness and muscle strength |
| Scalzo et al. 2018 [94] | Adults with T2D (n = 31) and healthy adults (n = 21) | Peak oxygen uptake was determined via graded exercise to exhaustion | Single dose | IV infusion of vitamin C (7.5 g) | Acute vitamin C infusion did not change VO2 peak |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Higgins, M.R.; Izadi, A.; Kaviani, M. Antioxidants and Exercise Performance: With a Focus on Vitamin E and C Supplementation. Int. J. Environ. Res. Public Health 2020, 17, 8452. https://doi.org/10.3390/ijerph17228452
Higgins MR, Izadi A, Kaviani M. Antioxidants and Exercise Performance: With a Focus on Vitamin E and C Supplementation. International Journal of Environmental Research and Public Health. 2020; 17(22):8452. https://doi.org/10.3390/ijerph17228452
Chicago/Turabian StyleHiggins, Madalyn Riley, Azimeh Izadi, and Mojtaba Kaviani. 2020. "Antioxidants and Exercise Performance: With a Focus on Vitamin E and C Supplementation" International Journal of Environmental Research and Public Health 17, no. 22: 8452. https://doi.org/10.3390/ijerph17228452
APA StyleHiggins, M. R., Izadi, A., & Kaviani, M. (2020). Antioxidants and Exercise Performance: With a Focus on Vitamin E and C Supplementation. International Journal of Environmental Research and Public Health, 17(22), 8452. https://doi.org/10.3390/ijerph17228452







