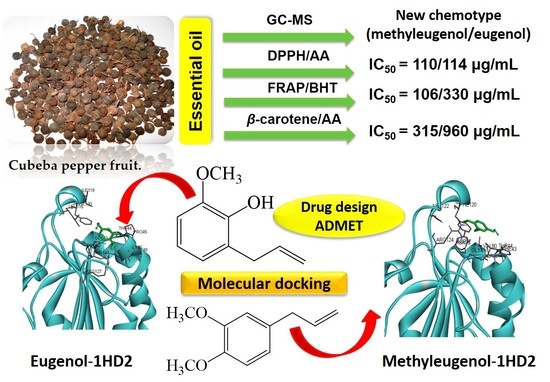
Antioxidant Activities of a New Chemotype of Piper cubeba L. Fruit Essential Oil (Methyleugenol/Eugenol): In Silico Molecular Docking and ADMET Studies
Abstract
:1. Introduction
2. Results
2.1. Essential Oil Characterization
2.2. Antioxidant Activity
2.2.1. DPPH Test
2.2.2. FRAP Test
2.2.3. β-Carotene Bleaching Test
2.3. Molecular Docking Study
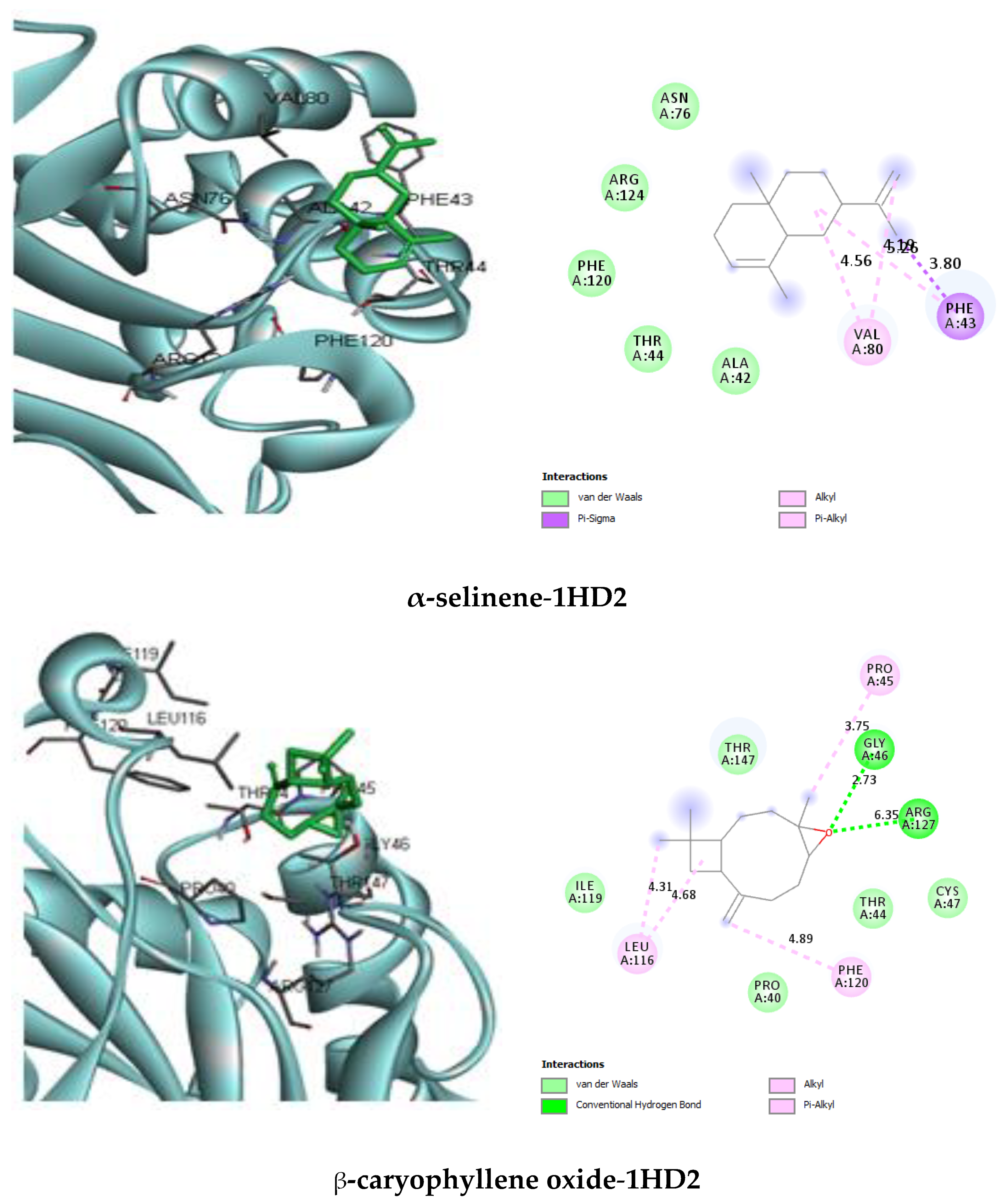
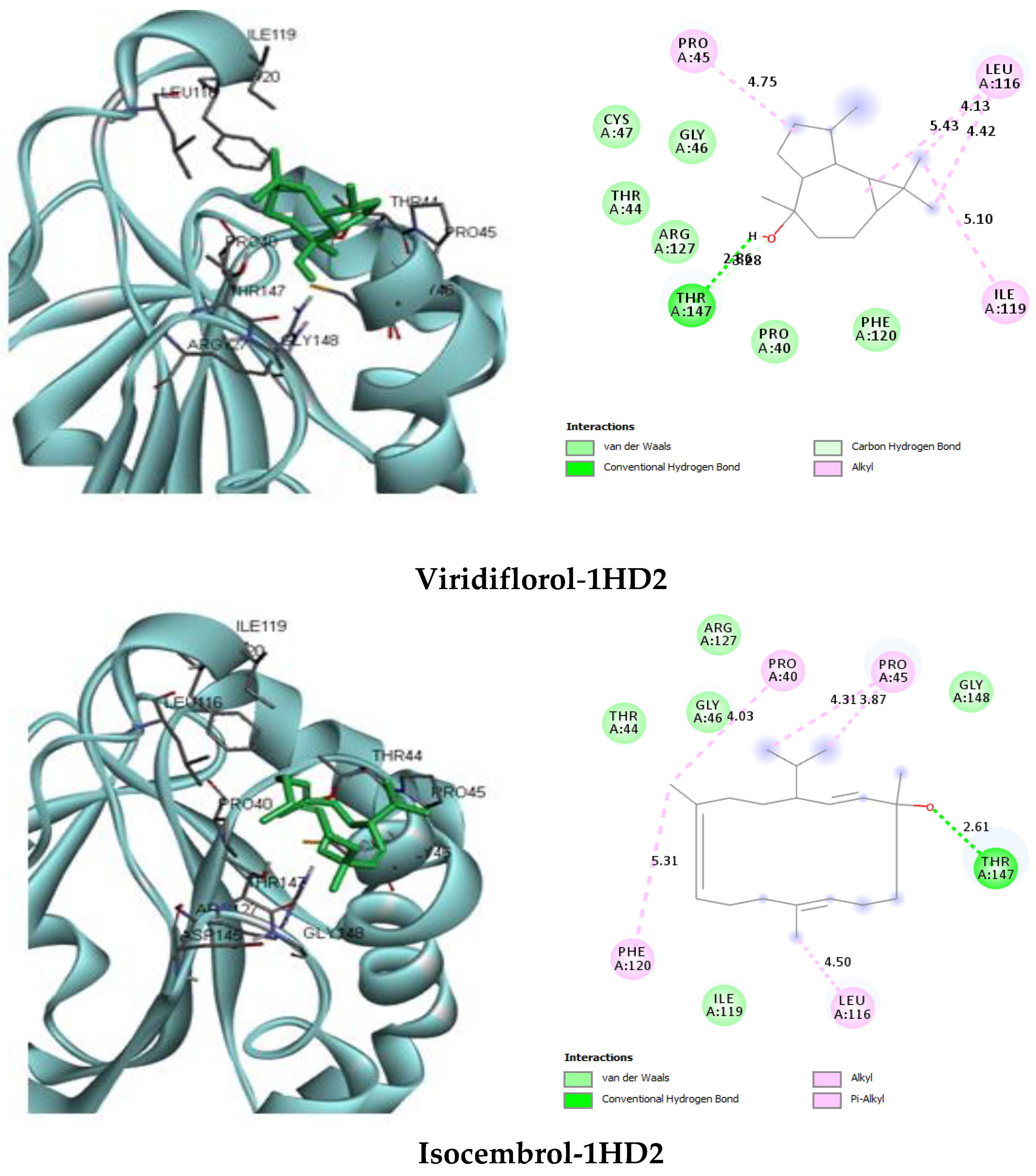
2.3.1. Protein–Ligand Complex Interactions
2.3.2. ADME Analysis
3. Discussion
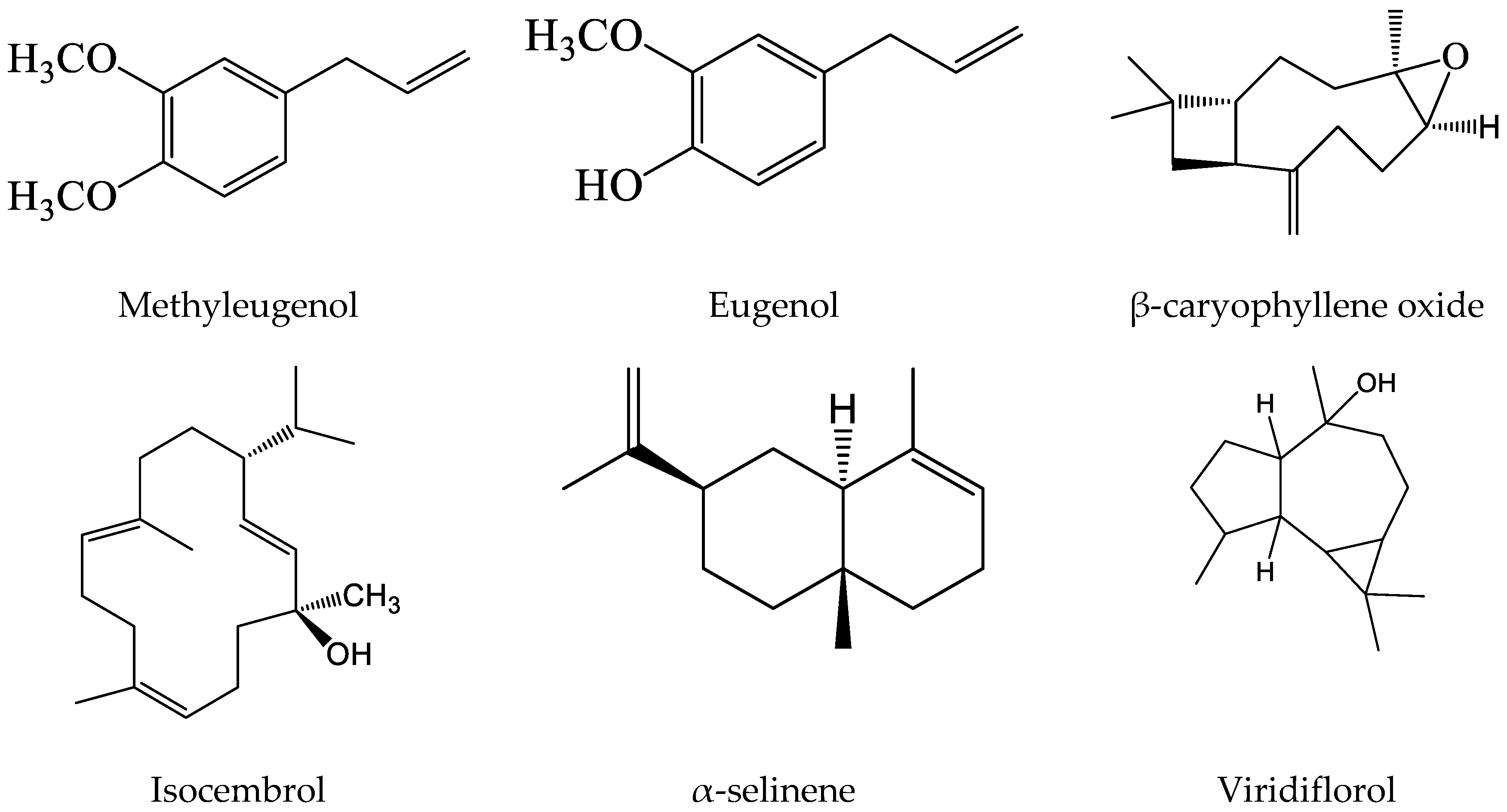
3.1. Chemical Composition Analysis vs. Beneficial Role
3.2. Antioxidant Potential
3.3. In Silico Study
4. Materials and Methods
4.1. Reagents, Plant Material, and Essential Oil Extraction
4.2. Characterization of Essential Oils by GC–MS
4.3. In Vitro Antioxidant Activity
4.3.1. DPPH (1,1-Diphenyl-2-picrylhydrazyl) Radical Scavenging activity
4.3.2. Ferric Reducing/Antioxidant Power (FRAP) Assay
4.3.3. β-Carotene Bleaching Test
4.4. Computational Approach
4.5. ADMET Analysis
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Bayir, H. Reactive oxygen species. Crit. Care Med. 2005, 33, S498–S501. [Google Scholar] [CrossRef]
- Alfadda, A.A.; Sallam, R.M. Reactive ox ygen species in health and disease. J. Biomed. Biotechnol. 2012, 2012, 936486. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Zhong, S.; Liao, X.; Chen, J.; He, T.; Lai, S.; Jia, Y. A Meta-Analysis of Oxidative Stress Markers in Depression. PLoS ONE 2015, 10, e0138904. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, C.A.; Ni, D.; Rosenkrans, Z.T.; Cai, W. Scavenging of reactive oxygen and nitrogen species with nanomaterials. Nano Res. 2018, 11, 4955–4984. [Google Scholar] [CrossRef] [PubMed]
- Al-Salahi, R.; Taie, H.A.A.; Bakheit, A.H.; Marzouk, M.; Almehizia, A.A.; Herqash, R.; Abuelizz, H.A. Antioxidant activities and molecular docking of 2-thioxobenzo[g]quinazoline derivatives. Pharmacol. Rep. 2019, 71, 695–700. [Google Scholar] [CrossRef] [PubMed]
- Rhee, S.G. Cell signaling. H2O2, a necessary evil for cell signaling. Science 2006, 312, 1882–1883. [Google Scholar] [CrossRef]
- Bae, Y.S.; Oh, H.; Rhee, S.G.; Yoo, Y.D. Regulation of reactive oxygen species generation in cell signaling. Mol. Cells 2011, 32, 491–509. [Google Scholar] [CrossRef] [Green Version]
- Kadri, A.; Zarai, Z.; Ben Chobba, I.; Gharsallah, N.; Damak, M.; Bekir, A. Chemical composition and in vitro antioxidant activities of Thymelaea hirsuta L. essential oil from Tunisia. Afri. J. Biotechnol. 2011, 15, 2930–2935. [Google Scholar]
- Bakari, S.; Daoud, A.; Felhi, S.; Smaoui, S.; Gharsallah, N.; Kadri, A. Proximate analysis, mineral composition, phytochemical contents, antioxidant and antimicrobial activities and GC-MS investigation of various solvent extracts of cactus cladode. Food Sci. Technol. (Campinas) 2017, 27, 286–293. [Google Scholar] [CrossRef] [Green Version]
- Ben Mefteh, F.; Daoud, A.; Bouket, A.C.; Thissera, B.; Kadri, Y.; Cherif-Silini, H.; Eshelli, M.; Alenezi, F.N.; Vallat, A.; Oszako, T.; et al. Date Palm Trees Root-Derived Endophytes as Fungal Cell Factories for Diverse Bioactive Metabolites. Int. J. Mol. Sci. 2018, 19, 1986. [Google Scholar] [CrossRef] [Green Version]
- Gad-Elkareem, M.A.M.; Abdelgadir, E.H.; Badawy, O.M.; Kadri, A. Potential antidiabetic effect of ethanolic and aqueous-ethanolic extracts of Ricinus communis leaves on streptozotocin-induced diabetes in rats. PeerJ. 2019, 7, e6441. [Google Scholar] [CrossRef] [Green Version]
- Snoussi, M.; Dehmani, A.; Noumi, E.; Flamini, G.; Papetti, A. Chemical composition and antibiofilm activity of Petroselinum crispum and Ocimum basilicum essential oils against Vibrio spp. Strains. Micro. Pathog. 2016, 90, 13–21. [Google Scholar] [CrossRef]
- Noumi, E.; Snoussi, M.; Merghni, A.; Nazzaro, F.; Quindós, G.; Akdamar, G.; Mastouri, M.; Al-Sieni, A.; Ceylan, O. Phytochemical composition, anti-biofilm and anti-quorum sensing potential of fruit, stem and leaves of Salvadora persica L. methanolic extracts. Micro. Pathog. 2017, 109, 169–176. [Google Scholar] [CrossRef]
- Noumi, A.; Merghni, A.; Alreshidi, M.M.; Haddad, O.; Akmadar, G.; De Martino, L.; Mastouri, M.; Ceylan, O.; Snoussi, M.; Al-sieni, A.; et al. Chromobacterium violaceum and Pseudomonas aeruginosa PAO1: Models for Evaluating Anti-Quorum Sensing Activity of Melaleuca alternifolia Essential Oil and Its Main Component Terpinen-4-ol. Molecules 2018, 23, 2672. [Google Scholar] [CrossRef] [Green Version]
- Snoussi, M.; Noumi, E.; Punchappady-Devasya, R.; Trabelsi, N.; Kanekar, S.; Nazarro, F.; Fratianni, F.; Flamini, G.; De Feo, V.; Al-sieni, A. Antioxidant properties and anti-quorum sensing potential of Carum copticum essential oil and phenolics against Chromobacterium violaceum. J. Food Sci. Technol. 2018, 55, 2824–2832. [Google Scholar] [CrossRef]
- Mseddi, K.; Alimi, F.; Noumi, E.; Veettil, V.N.; Deshpande, S.; Adnan, M.; Hamdi, A.; Elkahoui, S.; Alghamdi, A.; Kadri, A.; et al. Thymus musilii Velen. as a promising source of potent bioactive compounds with its pharmacological properties: In vitro and in silico analysis. Arab. J. Chem. 2020, 13, 6782–6801. [Google Scholar] [CrossRef]
- Bos, J.L.; Rehmann, H.; Wittinghofer, A. GEFs and GAPs: Critical Elements in the Control of Small G Proteins. Cell 2007, 129, 865–877. [Google Scholar] [CrossRef] [Green Version]
- Rendon, A.; Schäkel, K. Psoriasis Pathogenesis and Treatment. Int. J. Mol. Sci. 2019, 20, 1475. [Google Scholar] [CrossRef] [Green Version]
- Choi, E.-M.; Hwang, J.K. Investigations of anti-inflammatory and antinociceptive activities of Piper cubeba, Physalis angulata and Rosa hybrid. J. Ethnopharmacol. 2003, 89, 171–175. [Google Scholar] [CrossRef]
- Khan, M.; Siddiqui, M. Antimicrobial activity of Piper fruits. Nat. Prod. Rad. 2007, 6, 111–113. [Google Scholar]
- Zaidi, S.F.H.; Yamada, K.; Kadowaki, M.; Usmanghani, K.; Sugiyama, T. Bactericidal activity of medicinal plants, employed for the treatment of gastrointestinal ailments, against Helicobacter pylori. J. Ethnopharmacol. 2009, 121, 286–291. [Google Scholar] [CrossRef]
- Ahmad, Q.Z.; Jahan, N.; Ahmad, G.; Tajuddin. Nephroprotective effect of Kabab chini (Piper cubeba) in gentamycin-induced nephrotoxicity. Saudi J. Kidney Dis. Transplant. 2012, 23, 773–781. [Google Scholar] [CrossRef]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry; Allured Publishing: Carol Stream, IL, USA, 2007. [Google Scholar]
- Neelman Khatkar, A.; Sharma, K.K. Phenylpropanoids and its derivatives: Biological activities and its role in food, pharmaceutical and cosmetic industries. Crit. Rev. Food Sci. Nutr. 2020, 60, 2655–2675. [Google Scholar]
- Macchia, M.; Pagano, A.; Ceccarini, L.; Benvenuti, S.; Cioni, P.L.; Flamini, G. Agronomic and phytochimic characteristics in some genotypes of Ocimum basilicum L. Acta Hort. 2006, 723, 143–149. [Google Scholar] [CrossRef]
- Murningsih, T.; Chairul, C.; Kuncari, E.S. Methyl Eugenol, Chemotype of essential Oils of Melaleuca spp. (Myrtaceae) Growing in Cibodas Botanical Garden. Berita Biologi. 2009, 9, 809–816. (In Bahasa) [Google Scholar]
- Binu, P.; Abhilash, S.; Vineetha, R.C.; Arathi, P.; Harikumaran Nair, R. Protective Role of Eugenol on Acute Promyelocyte Leukemia Drug Arsenic Trioxide Induced Renal Injury in Wistar Rats. Indian J. Physiol. Pharmacol. 2018, 62, 120–131. [Google Scholar]
- Kim, S.S.; Oh, O.J.; Min, H.Y.; Park, E.J.; Kim, Y.; Park, H.J.; Han, Y.N.; Lee, S.K. Eugenol suppresses cyclooxygenase-2 expression in lipopolysaccharide-stimulated mouse macrophage RAW264.7 cells. Life Sci. 2003, 73, 337–348. [Google Scholar] [CrossRef]
- Dervis, E.; Yurt Kilcar, A.; Medine, E.I.; Tekin, V.; Cetkin, B.; Uygur, E.; Muftuler, F.Z.B. In vitro incorporation of radioiodinated eugenol on adenocarcinoma cell lines (Caco2, MCF7, and PC3). Cancer Biother Radiopharm. 2017, 32, 75–81. [Google Scholar] [CrossRef]
- Hamed, S.F.; Sadek, Z.; Edris, A. Antioxidant and antimicrobial activities of clove bud essential oil and eugenol nanoparticles in alcohol-free microemulsion. J. Oleo Sci. 2012, 61, 641–648. [Google Scholar] [CrossRef]
- Darvishi, E.; Omidi, M.; Bushehri, A.A.S.; Golshani, A.; Smith, M.L. The antifungal eugenol perturbs dual aromatic and branched-chain amino acid permeases in the cytoplasmic membrane of yeast. PLoS ONE 2013, 8, e76028. [Google Scholar] [CrossRef] [Green Version]
- Taher, Y.A.; Samud, A.M.; El-Taher, F.E.; Ben-Husin, G.; Elmezogi, J.S.; Al-Mehdawi, B.F.; Salem, H.A. Experimental evaluation of anti-inflammatory, antinociceptive and antipyretic activities of clove oil in mice. Libyan J. Med. 2015, 10, 28685. [Google Scholar] [CrossRef] [PubMed]
- Tsuchiya, H. Anesthetic agents of plant origin: A review of phytochemicals with anesthetic activity. Molecules 2017, 22, 1369. [Google Scholar] [CrossRef] [PubMed]
- Baldisserotto, B.; Parodi, T.V.; Stevens, E.D. Lack of postexposure analgesic efficacy of low concentrations of eugenol in Zebrafish. Vet. Anaesth. Analg. 2018, 45, 48–56. [Google Scholar] [CrossRef]
- Bos, R.; Woerdenbag, H.J.; Kayser, O.; Quax, W.J.; Ruslan, K.; Elfami. Essential oil constituents of Piper cubeba L. fils. from Indonesia. J. Essent. Oil Res. 2007, 19, 14–17. [Google Scholar] [CrossRef]
- Burfield, T. Natural Aromatic Materials: Odours & Origins, 2nd ed.; The Atlantic Institute of Aromatherapy: Tampa, FL, USA, 2017. [Google Scholar]
- Lawrence, B.M. Progress in essential oils: Cubeb oil. Perfum. Flavor. 2016, 41, 54–57. [Google Scholar]
- Andriana, Y.; Xuan, T.D.; Quy, T.N.; Tran, H.D.; Le, Q.T. Biological Activities and Chemical Constituents of Essential Oils from Piper cubeba Bojer and Piper nigrum L. Molecules 2019, 24, 1876. [Google Scholar] [CrossRef] [Green Version]
- Padmakumari, K.P.; Sasidharan, I.; Sreekumar, M.M. Composition and antioxidant activity of essential oil of pimento (Pimenta dioica (L) Merr.) from Jamaica. Nat. Prod. Res. 2011, 25, 152–160. [Google Scholar] [CrossRef]
- Gogoi, R.; Loying, R.; Sarma, N.; Begum, T.; Pandey, S.K.; Lal, M. Comparative Analysis of In Vitro Biological Activities of Methyl Eugenol Rich Cymbopogon khasianus Hack., Leaf Essential Oil with Pure Methyl Eugenol Compound. Curr. Pharm. Biotechnol. 2020, 21, 927–938. [Google Scholar] [CrossRef]
- Gülçin, I. Antioxidant Activity of Eugenol: A Structure–Activity Relationship Study. J. Med. Food 2011, 14, 975–985. [Google Scholar] [CrossRef]
- Assaeed, A.; Elshamy, A.; El Gendy, A.N.; Dar, B.; Al-Rowaily, S.; Abd-ElGawad, A. Sesquiterpenes-Rich Essential Oil from Above Ground Parts of Pulicaria somalensis Exhibited Antioxidant Activity and Allelopathic Effect on Weeds. Agronomy 2020, 10, 399. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.L.; Zhang, L.F.; Xu, J.G.; Hu, Q.P. Comparison study on antioxidant, DNA damage protective and antibacterial activities of eugenol and isoeugenol against several foodborne pathogens. Food Nutr. Res. 2017, 61, 1353356. [Google Scholar] [CrossRef] [Green Version]
- Radonic, A.; Milos, M. Chemical Composition and In Vitro Evaluation of Antioxidant Effect of Free Volatile Compounds from Satureja montana L. Free Radic. Res. 2003, 37, 673–679. [Google Scholar] [CrossRef] [PubMed]
- Tepe, B.; Sihoglu-Tepe, A.; Daferera, D.; Polissiou, M.; Sokmen, A. Chemical composition and antioxidant activity of the essential oil of Clinopodium vulgare L. Food Chem. 2007, 103, 766–770. [Google Scholar] [CrossRef]
- Hindalgo, M.E.; Rosa, C. Antioxidant capacity of eugenol derivatives. Quim. Nova 2009, 32, 1467–1470. [Google Scholar] [CrossRef]
- Findik, E.; Ceylan, M.; Elmastas, M. Isoeugenol-based novel potent antioxidants: Chemistry Synthesis and reactivity. Eur. J. Med. 2011, 46, 4618–4624. [Google Scholar] [CrossRef]
- Hammami, S.; Jmii, H.; Mokni, R.E.; Khmiri, A.; Faidi, K.; Dhaouadi, H.; El Aouni, M.H.; Aouni, M.; Joshi, R.K. Essential Oil Composition, Antioxidant, Cytotoxic and Antiviral Activities of Teucrium pseudochamaepitys Growing Spontaneously in Tunisia. Molecules 2015, 20, 20426–20433. [Google Scholar] [CrossRef]
- Balboa, E.M.; Conde, E.; Moure, A.; Falqué, E.; Domínguez, H. In vitro antioxidant properties of crude extracts and compounds from brown algae. Food Chem. 2013, 138, 1764–1785. [Google Scholar] [CrossRef]
- Marican, H.; Edros, R.; Mohammad, M.; Salleh, S. Antimicrobial activity of tropical soft corals found in the Northern Straits of Malacca. Int. J. Eng. Technol. Sci. 2016, 6, 1–10. [Google Scholar]
- Mayachiew, P.; Devahastin, S. Antimicrobial and antioxidant activities of Indian gooseberry and galangal extracts. LWT-Food Sci. Technol. 2008, 41, 1153–1159. [Google Scholar] [CrossRef]
- Trevizana, L.N.F.; Nascimento, K.F.; Santos, J.A.; Aparecida, C.; Kassuya, L.; Cardoso, C.A.L.; Vieira, M.C.; Moreira, F.M.F.; Croda, J.; Formagio, A.S.N. Anti-inflammatory, antioxidant and anti-Mycobacterium tuberculosis activity of viridiflorol: The major constituent of Allophylus edulis (A. St.-Hil., A. Juss. & Cambess.) Radlk. J. Ethnopharmacol. 2016, 192, 510–515. [Google Scholar]
- De Clercq, E.; Naesens, L.; De Bolle, L.; Schols, D.; Zhang, Y.; Neyts, J. Antiviral agents active against human herpesviruses HHV-6, HHV-7 and HHV-8. Rev. Med. Virol. 2001, 11, 381–395. [Google Scholar] [CrossRef] [PubMed]
- Eze, F.U.; Okoro, U.C.; Ugwu, D.I.; Okafor, S.N. Biological Activity Evaluation of Some New Benzenesulphonamide Derivatives. Front. Chem. 2019, 7, 634. [Google Scholar] [CrossRef] [PubMed]
- Bakari, S.; Ncir, M.; Felhi, S.; Hajlaoui, H.; Gharsallah, N.; Saoudi, M.; Kadri, A. Chemical composition and in vitro evaluation of total phenolic, flavonoid, and antioxidant properties of essential oil and solvent extract from the aerial parts of Teucrium polium grown in Tunisia. Food Sci. Biotechnol. 2015, 24, 1943–1949. [Google Scholar] [CrossRef]
- Felhi, S.; Baccouch, N.; Ben Salah, H.; Smaoui, S.; Allouche, N.; Gharsallah, N.; Kadri, A. Nutritional constituents, phytochemical profiles, in vitro antioxidant and antimicrobial properties and gas chromatography-mass spectrometry (GC-MS) analysis of various solvent extracts from grape seeds (Vitis vinifera L.). Food Sci. Biotechnol. 2016, 25, 1537–1544. [Google Scholar] [CrossRef] [PubMed]
- Morris, G.M.; Huey, R.; Olson, A.J. Using AutoDock for ligand-receptor docking. Curr. Protoc. Bioinform. 2008, 24, 8–14. [Google Scholar] [CrossRef]
- Pedretti, A.; Villa, L.; Vistoli, G. VEGA—an open platform to develop chemo-bio-informatics applications, using plug-in architecture and script programming. J. Comput. Aided Mol. Des. 2004, 18, 167–173. [Google Scholar] [CrossRef]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef] [Green Version]
- Dassault Systemes BIOVIA, BIOVIA Discovery Studio Visualizer; v16.1.0.15350; Dassault Systemes: San Diego, CA, USA, 2015.
- Kadri, A.; Aouadi, K. In vitro antimicrobial and α-glucosidase inhibitory potential of enantiopure cycloalkylglycine derivatives: Insights into their in silico pharmacokinetic, druglikeness, and medicinal chemistry properties. J. App. Pharm. Sci. 2020, 10, 107–115. [Google Scholar]
- Othman, I.M.M.; Gad-Elkareem, M.A.M.; Anouar, E.H.; Aouadi, K.; Kadri, A.; Snoussi, M. Design, synthesis ADMET and molecular docking of new imidazo [4,5-b]pyridine-5-thione derivatives as potential tyrosyl-tRNA synthetase inhibitors. Bioorg. Chem. 2020, 102, 104105. [Google Scholar] [CrossRef]
- Ghannay, S.; Kadri, A.; Aouadi, K. Synthesis, in vitro antimicrobial assessment, and computational investigation of pharmacokinetic and bioactivity properties of novel trifluoromethylated compounds using in silico ADME and toxicity prediction tools. Monatsh. Chem. 2020, 151, 267–280. [Google Scholar] [CrossRef]



| N° | Compounds | RI a | RI b | Rt (mn) | % Area |
|---|---|---|---|---|---|
| 1 | β-myrcene | 990 | 985 | 8.57 | 1.23 |
| 2 | Limonene | 1032 | 1031 | 9.52 | 0.12 |
| 3 | 1,8-cineole | 1035 | 1039 | 9.68 | 2.94 |
| 4 | β-ocimene | 1049 | 1043 | 9.95 | 0.30 |
| 5 | α-terpinolene | 1089 | 1088 | 11.25 | 1.41 |
| 6 | Linalool | 1102 | 1098 | 12.88 | 0.22 |
| 7 | Terpinen-4-ol | 1177 | 1177 | 13.14 | 1.80 |
| 8 | p-cymene-8-ol | 1183 | 1183 | 13.47 | 3.50 |
| 9 | α-terpineol | 1186 | 1189 | 13.55 | 0.96 |
| 10 | Estragole | 1196 | 1195 | 14.14 | 0.15 |
| 11 | Citronellol | 1224 | 1228 | 14.19 | 0.10 |
| 12 | (E)-geraniol | 1248 | 1276 | 14.74 | 0.19 |
| 13 | Eugenol (main compound 2) | 1359 | 1356 | 17.30 | 33.95 |
| 14 | β-elemene | 1393 | 1391 | 17.73 | 0.66 |
| 15 | Methyleugenol (main compound 1) | 1404 | 1404 | 18.29 | 41.31 |
| 16 | (E)-caryophyllene | 1419 | 1418 | 18.45 | 5.65 |
| 17 | α-humulene | 1454 | 1444 | 19.06 | 1.14 |
| 18 | Germacrene D | 1486 | 1484 | 19.55 | 0.15 |
| 19 | α-selinene | 1490 | 1515 | 19.81 | 0.47 |
| 20 | δ-cadinene | 1526 | 1534 | 20.24 | 0.19 |
| 21 | Spathulenol | 1578 | 1573 | 21.37 | 0.18 |
| 22 | β-caryophyllene oxide | 1583 | 1580 | 21.52 | 0.96 |
| 23 | Viridiflorol | 1593 | 1591 | 22.72 | 0.39 |
| 24 | Isocembrol | 2044 | 2046 | 22.95 | 0.16 |
| Total identified % | 98.13 | ||||
| Phenylpropanoids | 75.41 | ||||
| Oxygenated monoterpenes | 9.71 | ||||
| Hydrocarbonated sesquiterpenes | 8.26 | ||||
| Hydrocarbonated monoterpenes | 3.06 | ||||
| Oxygenated sesquiterpenes | 1.69 | ||||
| Samples | IC50 (μg/mL) | ||
|---|---|---|---|
| DPPH | FRAP | β-Carotene | |
| Cubeba pepper EO | 110.00 ± 0.08 a | 106.00 ± 0.11 a | 315.00 ± 2.08 a |
| Butylated hydroxytoluene (BHT) | - | - | 930.00 ± 0.02 b |
| Ascorbic acid (AA) | 114.00 ± 0.70 a | 330.00 ± 0.60 b | - |
| N° | Compounds | Receptor/Binding Energy (kcal/mole) (kcal/mol) |
|---|---|---|
| 1 | β-myrcene | −3.7 |
| 2 | Limonene | −3.9 |
| 3 | 1,8-cineole | −4.3 |
| 4 | β-ocimene | −4.0 |
| 5 | α-terpinolene | −4.0 |
| 6 | Linalool | −4.2 |
| 7 | Terpinen-4-ol | −4.4 |
| 8 | p-cymene-8-ol | −4.1 |
| 9 | α-terpineol | −4.3 |
| 10 | Estragole | −4.1 |
| 11 | Citronellol | −4.0 |
| 12 | (E)-geraniol | −3.9 |
| 13 | Eugenol (main compound 2) | −4.7 |
| 14 | β-elemene | −4.6 |
| 15 | Methyleugenol (main compound 1) | −4.3 |
| 16 | (E)-caryophyllene | −4.9 |
| 17 | α-humulene | −4.9 |
| 18 | Germacrene D | −5.0 |
| 19 | α-selinene | −5.1 |
| 20 | δ-cadinene | −3.9 |
| 21 | Spathulenol | −5.0 |
| 22 | β-caryophyllene oxide | −5.8 |
| 23 | Viridiflorol | −5.1 |
| 24 | Isocembrol | −5.4 |
| Compounds Receptor vs. Targets | Interacting Residues 1HD2 | Binding Energy (kcal/mol) |
|---|---|---|
| Viridiflorol | van der Waals: Gly46, Cys47, Phe120, Arg127. H bond: Thr147 (2.86). C–H bond: Thr147 (3.28). Alkyl: Pro45 (4.75), Leu116 (4.13) (4.42) (5.43), Ile119 (5.10) | −5.1 |
| Methyleugenol (main compound 1) | van der Waals: Ala42, Thr44, Asn76, Phe120. H bond: Gly46 (2.21) (2.41), Cys47 (2.27) (2.88). C–H bond: Pro45 (3.45). Alkyl/Pi–Alkyl: Phe43 (4.24), Val80 (4.00) (4.43) | −4.3 |
| Isocembrol | van der Waals: Thr44, Gly46, Ile119, Arg127, Gly148. H bond: Thr147 (2.61). Alkyl/Pi–Alkyl: Pro40 (4.03), Pro45 (3.87) (4.31), Leu116 (4.50), Phe120 (5.31) | −5.4 |
| Eugenol (main compound 2) | van der Waals: Thr44, Ile119, Phe120, Thr147. H bond: Gly46 (2.73), Arg127 (6.35). Alkyl/Pi–Alkyl: Pro45 (3.75), Leu116 (4.31) (4.68), Phe120 (4.89) | −4.7 |
| α-selinene | van der Waals: Ala42, Thr44, Asn76, Phe120, Arg124. Pi-Sigma: Phe43 (3.80). Alkyl/Pi–Alkyl: Phe43 (5.26), Val80 (4.19) (4.56) | −5.1 |
| β-caryophyllene oxide | van der Waals: Pro40, Thr44, Cys47, Ile119, Thr147. H bond: Gly46 (2.73), Arg127 (6.35). Alkyl/Pi–Alkyl: Pro45 (3.75), Leu116 (4.31) (4.68), Phe120 (4.89) | −5.8 |
| Entry | 1 | 2 | 3 | 4 | 5 | 6 |
|---|---|---|---|---|---|---|
| Pharmacokinetics/Druglikeness | ||||||
| Lipinski | Yes | Yes | Yes | Yes | Yes | Yes |
| TPSA (Å2) | 18.46 | 29.46 | 12.53 | 20.23 | 0.00 | 20.23 |
| Consensus Log Po/w | 2.58 | 2.25 | 3.68 | 4.75 | 4.40 | 3.42 |
| Bioavailability Score | 0.55 | 0.55 | 0.55 | 0.55 | 0.55 | 0.55 |
| GI absorption | High | High | High | High | Low | High |
| BBB permeant | Yes | Yes | Yes | No | No | Yes |
| P–gp substrate | No | No | No | No | No | No |
| CYP1A2 inhibitor | Yes | Yes | No | No | No | No |
| CYP2C19 inhibitor | No | No | Yes | Yes | Yes | Yes |
| CYP2C9 inhibitor | No | No | Yes | Yes | Yes | No |
| CYP2D6 inhibitor | No | No | No | No | No | No |
| CYP3A4 inhibitor | No | No | No | Yes | No | No |
| Log Kp (cm/s) a | −5.60 | −5.69 | −5.12 | −4.47 | −3.85 | −5.00 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alminderej, F.; Bakari, S.; Almundarij, T.I.; Snoussi, M.; Aouadi, K.; Kadri, A. Antioxidant Activities of a New Chemotype of Piper cubeba L. Fruit Essential Oil (Methyleugenol/Eugenol): In Silico Molecular Docking and ADMET Studies. Plants 2020, 9, 1534. https://doi.org/10.3390/plants9111534
Alminderej F, Bakari S, Almundarij TI, Snoussi M, Aouadi K, Kadri A. Antioxidant Activities of a New Chemotype of Piper cubeba L. Fruit Essential Oil (Methyleugenol/Eugenol): In Silico Molecular Docking and ADMET Studies. Plants. 2020; 9(11):1534. https://doi.org/10.3390/plants9111534
Chicago/Turabian StyleAlminderej, Fahad, Sana Bakari, Tariq I. Almundarij, Mejdi Snoussi, Kaïss Aouadi, and Adel Kadri. 2020. "Antioxidant Activities of a New Chemotype of Piper cubeba L. Fruit Essential Oil (Methyleugenol/Eugenol): In Silico Molecular Docking and ADMET Studies" Plants 9, no. 11: 1534. https://doi.org/10.3390/plants9111534