Acetone Vapor-Sensing Properties of Chitosan-Polyethylene Glycol Using Surface Plasmon Resonance Technique
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Fabrication of Au/Chitosan-PEG SPR Sensor Film
2.3. Characterization
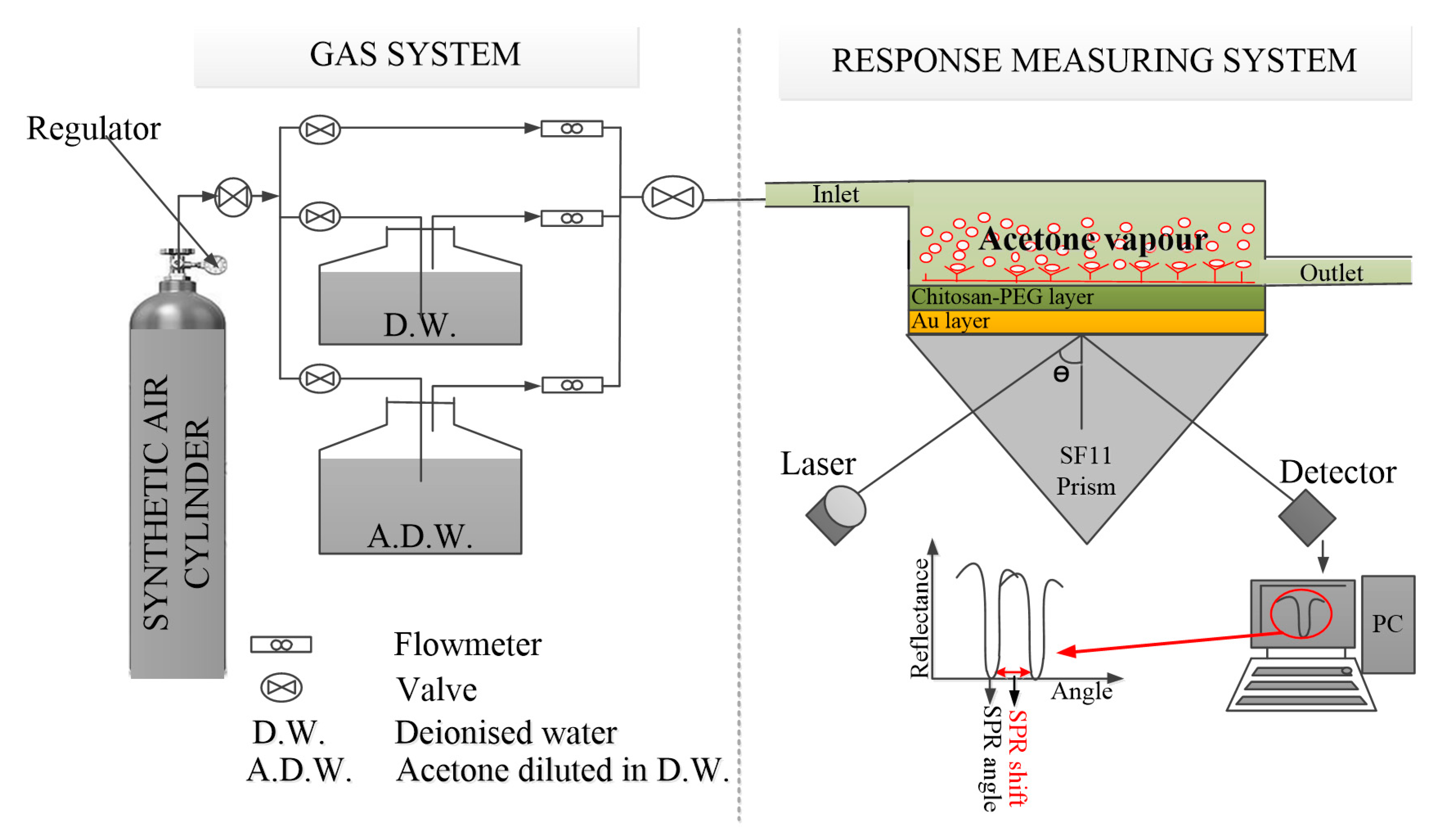
2.4. SPR Measurement
3. Results
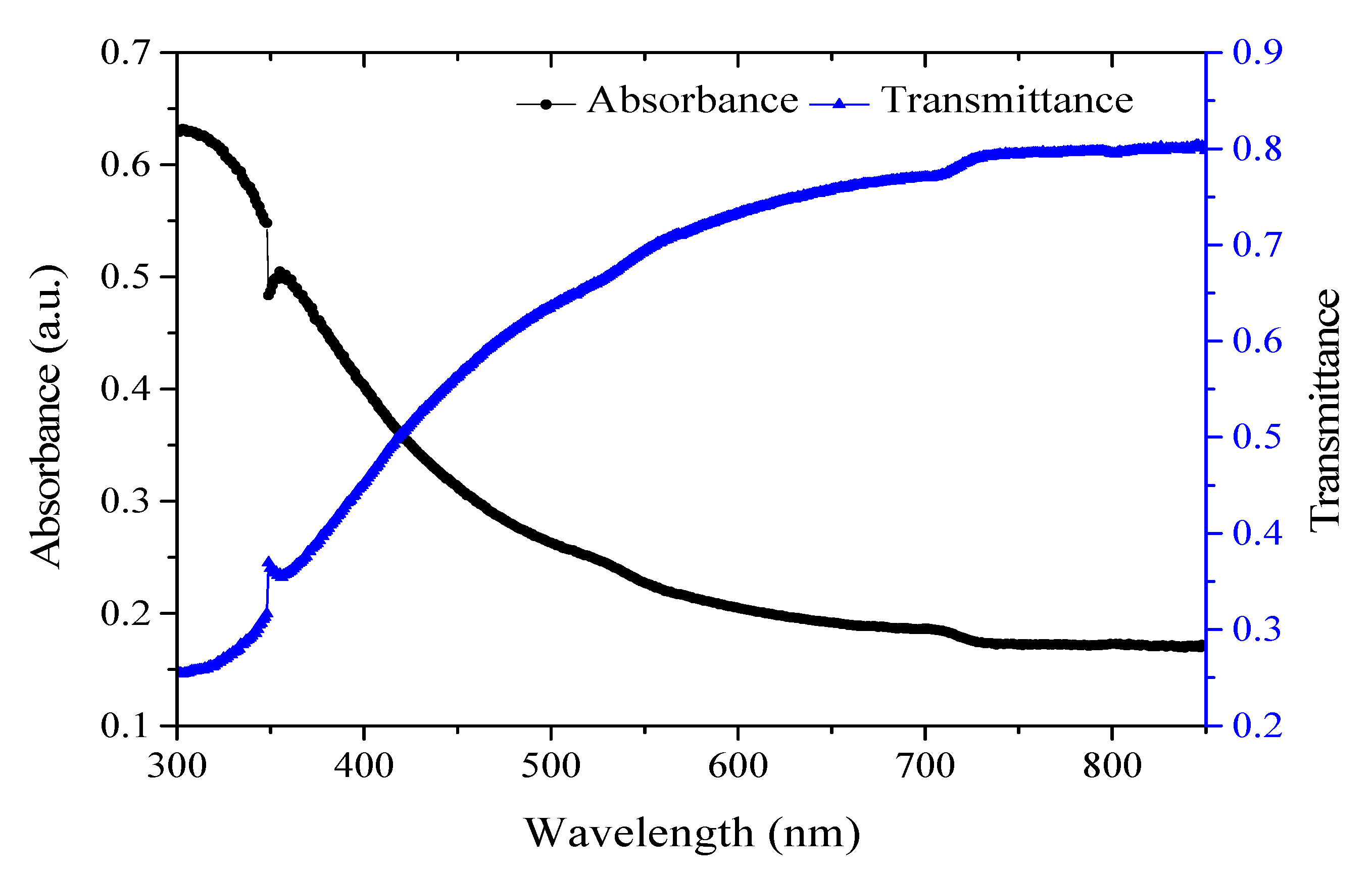
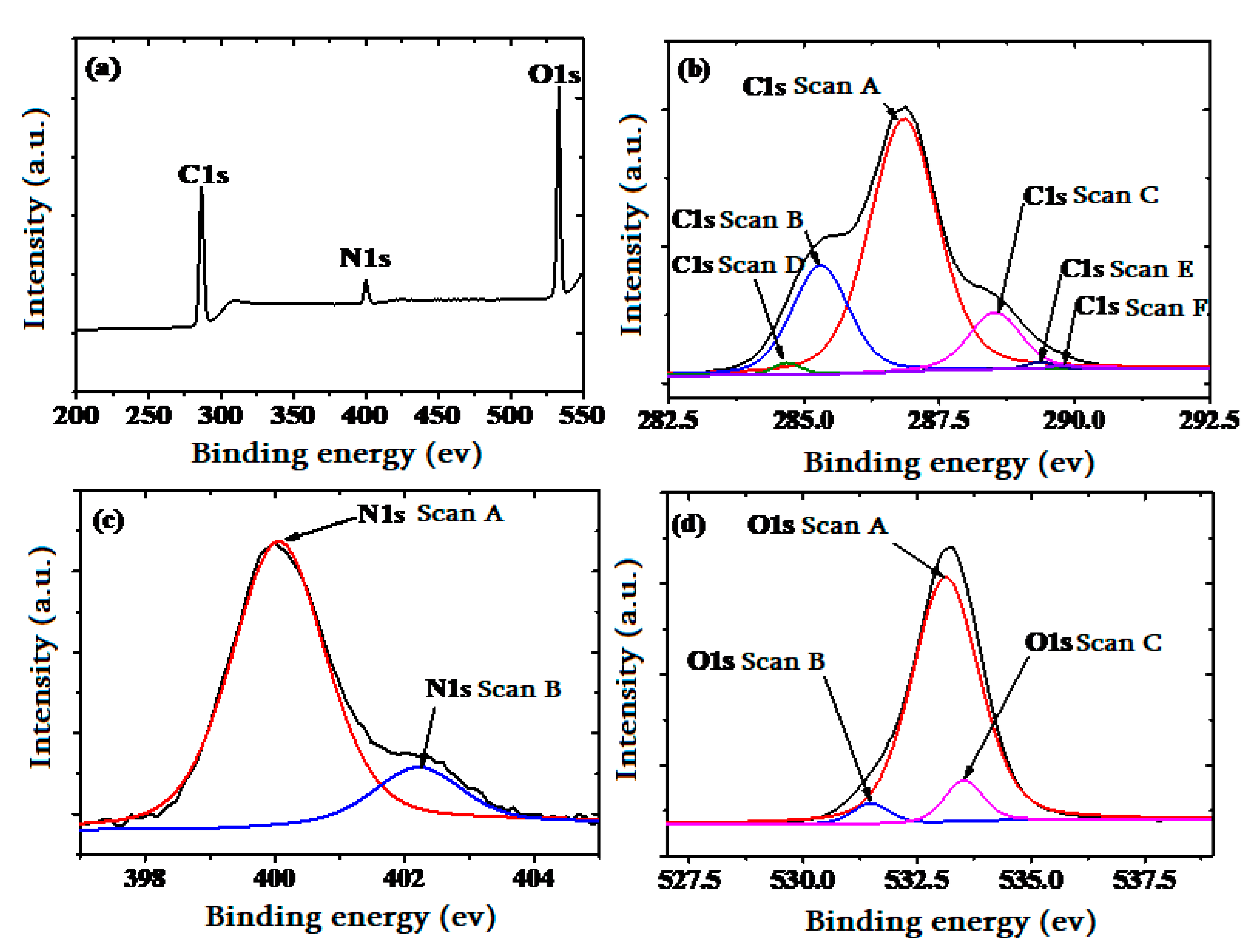
3.1. Structural, Morphological, and Chemical Compositional Characterization of the Sensing Layer
3.2. Acetone Detection Measurement
3.2.1. Optimization of Experimental Conditions
3.2.2. SPR Response on the Chitosan-PEG-Based Sensor to Different Acetone Vapor Concentrations in Air
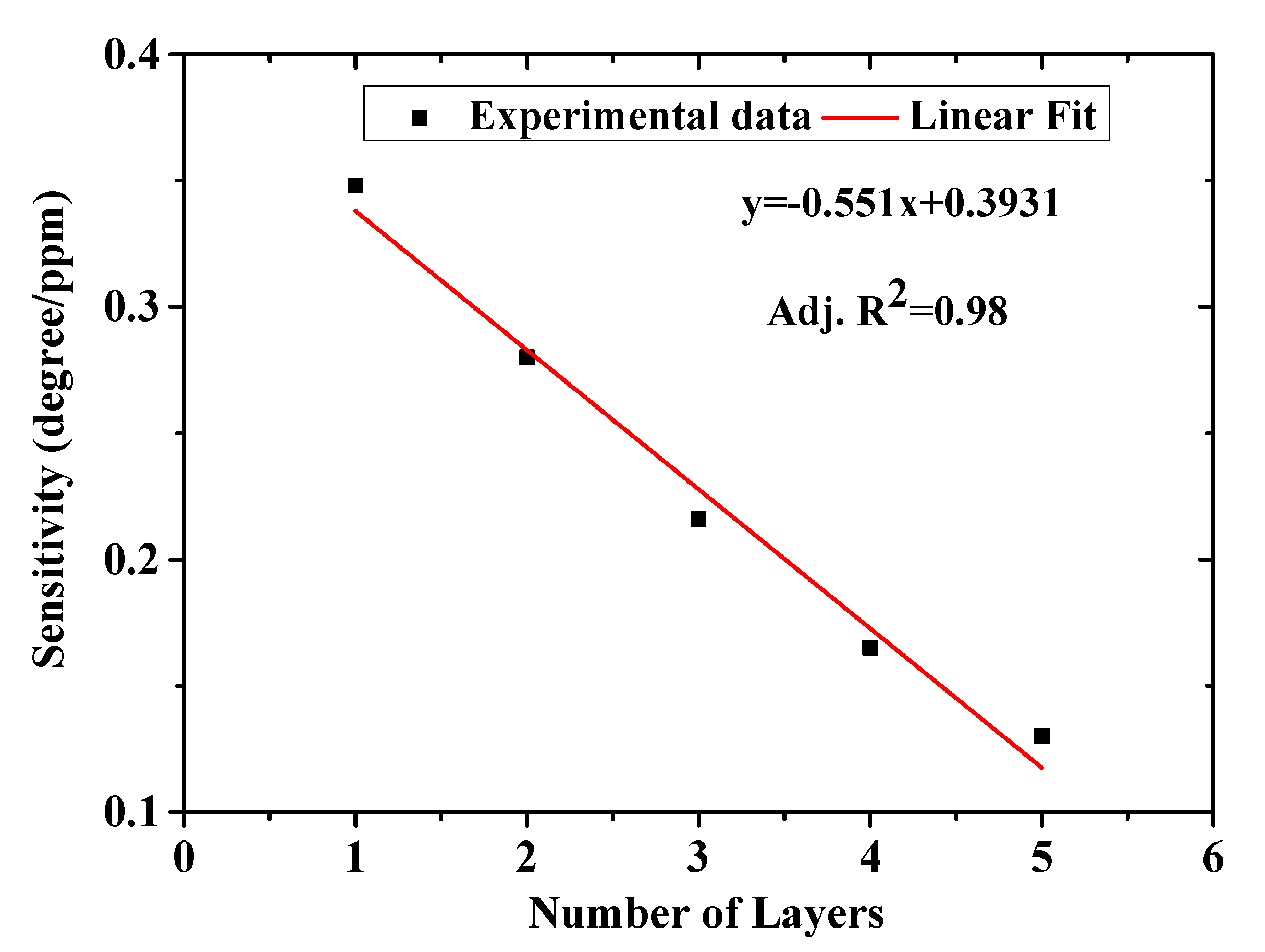
3.2.3. Thickness Variation of Layers and Lowest Detection Limit (LOD) of the Chitosan-PEG Films
3.2.4. SPR Angle Versus Time Graph of Single-Layer Chitosan-PEG-Based SPR Sensor for the Detection of Acetone Vapor
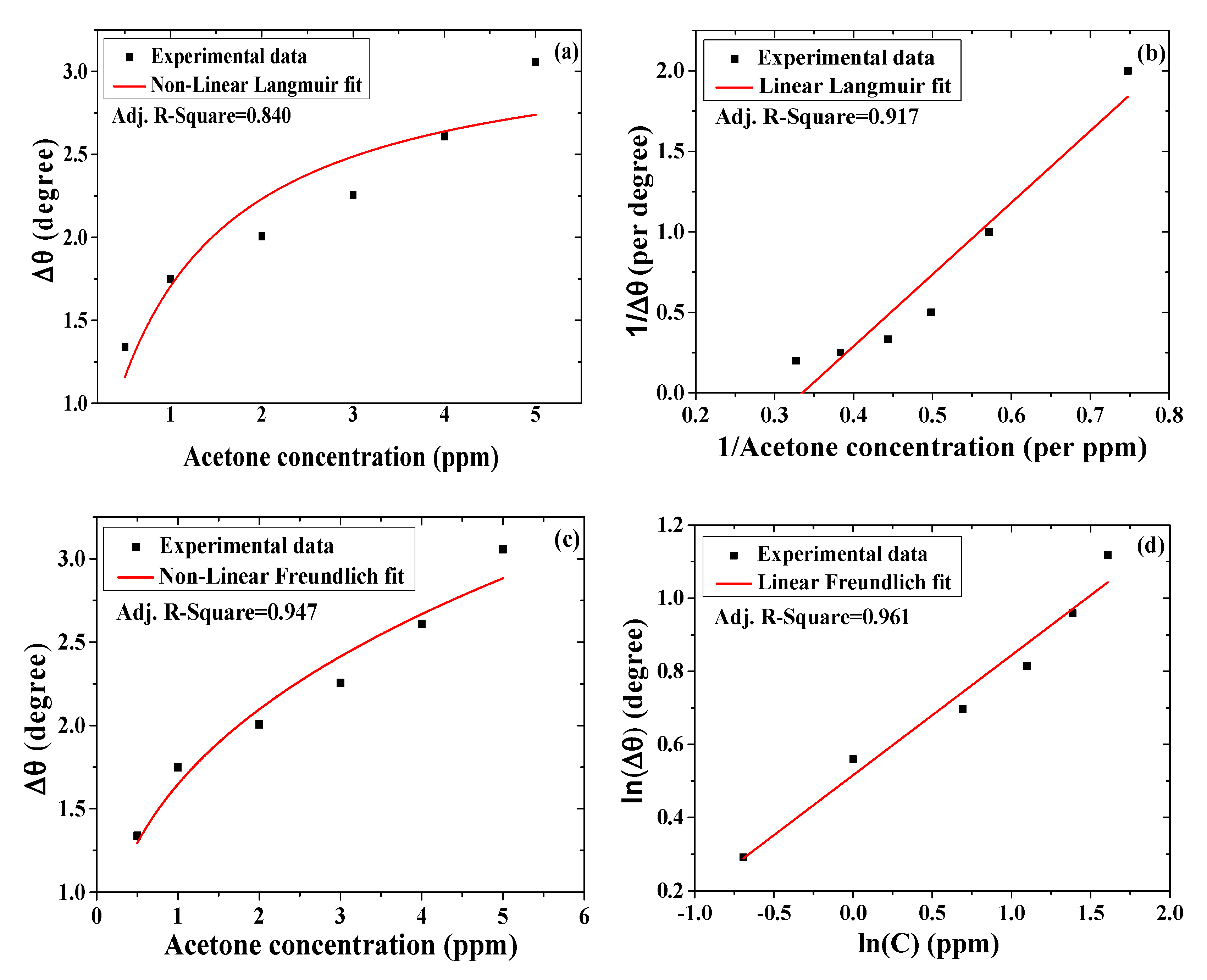
3.2.5. Binding Affinity of Acetone toward SPR Sensor with Single Layer of Chitosan-PEG
3.2.6. Detection Mechanism and Selectivity Test of the Single-Layer Chitosan-PEG-Based SPR Acetone Vapor Sensor
3.2.7. Selectivity of Chitosan-PEG-Based SPR Sensor to Acetone Vapor
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rahman, K.U.; Mehmood, S. Diabetes mellitus—A challenging metabolic disorder of the modern world. Pure Appl. Biol. 2019, 8, 1684–1689. [Google Scholar]
- American Diabetes Association. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes—2020. Diabetes Care 2019, 43, S14–S31. [Google Scholar] [CrossRef] [Green Version]
- World Health Organization. Diabetes. Available online: https://www.who.int/news-room/fact-sheets/detail/diabetes (accessed on 15 August 2020).
- Saeedi, P.; Petersohn, I.; Salpea, P.; Malanda, B.; Karuranga, S.; Unwin, N.; Colagiuri, S.; Guariguata, L.; Motala, A.A.; Ogurtsova, K.; et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res. Clin. Pract. 2019, 157, 107843. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Q.; Wang, D. Room temperature acetone sensor based on nanostructured K2W7O22. In Proceedings of the 2016 IEEE SENSORS, Orlando, FL, USA, 30 October–3 November 2016. [Google Scholar] [CrossRef]
- Saraoğlu, H.M.; Kocan, M. Determination of Blood Glucose Level-Based Breath Analysis by a Quartz Crystal Microbalance Sensor Array. IEEE Sens. J. 2009, 10, 104–109. [Google Scholar] [CrossRef]
- Rydosz, A.; Wincza, K.; Gruszczynski, S. Microsystem in LTCC technology for the detection of acetone in healthy and diabetes breath. In Proceedings of the 2016 IEEE ANDESCON, Arequipa, Peru, 19–21 October 2016; pp. 1–4. [Google Scholar] [CrossRef]
- Usman, F.; Dennis, J.O.; Ahmed, A.Y.; Meriaudeau, F.; Ayodele, O.B.; Rabih, A.A.S. A Review of Biosensors for Non-Invasive Diabetes Monitoring and Screening in Human Exhaled Breath. IEEE Access 2018, 7, 5963–5974. [Google Scholar] [CrossRef]
- Cantalini, C.; Pelino, M.; Sun, H.; Faccio, M.; Santucci, S.; Lozzi, L.; Passacantando, M. Cross sensitivity and stability of NO2 sensors from WO3 thin film. Sens. Actuators B Chem. 1996, 35, 112–118. [Google Scholar] [CrossRef]
- Usman, F.; Dennis, J.O.; Meriaudeau, F. Development of a Surface Plasmon Resonance Acetone Sensor for Noninvasive Screening and Monitoring of Diabetes. IOP Conf. Ser. Mater. Sci. Eng. 2018, 383, 012024. [Google Scholar] [CrossRef]
- Masson, J.-F. Surface Plasmon Resonance Clinical Biosensors for Medical Diagnostics. ACS Sens. 2017, 2, 16–30. [Google Scholar] [CrossRef]
- Santos, J.L.; Farahi, F. Handbook of Optical Sensors; CRC Press: Boca Raton, FL, USA, 2014. [Google Scholar]
- Lesňák, M.; Staněk, F.; Hlavatý, I.; Pištor, J.; Procházka, J. SPR Method and Its Utilisation for Low Alcohols Concentrations Determination. J. Mod. Phys. 2015, 6, 363–368. [Google Scholar] [CrossRef] [Green Version]
- Usman, F.; Dennis, J.O.; Seong, K.C.; Ahmed, A.Y.; Ferrell, T.L.; Fen, Y.W.; Sadrolhosseini, A.R.; Ayodele, O.B.; Meriaudeau, F.; Saidu, A. Enhanced Sensitivity of Surface Plasmon Resonance Biosensor Functionalized with Doped Polyaniline Composites for the Detection of Low-Concentration Acetone Vapour. J. Sens. 2019, 2019, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Omar, N.A.S.; Fen, Y.W.; Abdullah, J.; Kamil, Y.M.; Daniyal, W.M.E.M.M.; Sadrolhosseini, A.R.; Mahdi, M.A. Sensitive Detection of Dengue Virus Type 2 E-Proteins Signals Using Self-Assembled Monolayers/Reduced Graphene Oxide-PAMAM Dendrimer Thin Film-SPR Optical Sensor. Sci. Rep. 2020, 10, 2374. [Google Scholar] [CrossRef]
- Rabih, A.A.S.; Dennis, J.O.; Ahmed, A.Y.; Khir, M.H.M.; Ahmed, M.G.A.; Idris, A.; Mian, M.U. MEMS-Based Acetone Vapor Sensor for Non-Invasive Screening of Diabetes. IEEE Sens. J. 2018, 18, 9486–9500. [Google Scholar] [CrossRef]
- Fan, H.; Wang, L.; Zhao, K.; Li, N.; Shi, Z.; Ge, Z.; Jin, Z. Fabrication, Mechanical Properties, and Biocompatibility of Graphene-Reinforced Chitosan Composites. Biomacromolecules 2010, 11, 2345–2351. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Koh, J. Physiochemical and optical properties of chitosan based graphene oxide bionanocomposite. Int. J. Biol. Macromol. 2014, 70, 559–564. [Google Scholar] [CrossRef] [PubMed]
- Kyzas, G.Z.; Bikiaris, D.N. Recent Modifications of Chitosan for Adsorption Applications: A Critical and Systematic Review. Mar. Drugs 2015, 13, 312–337. [Google Scholar] [CrossRef]
- Zhang, M.; Li, X.; Gong, Y.; Zhao, N.; Zhang, X. Properties and biocompatibility of chitosan films modified by blending with PEG. Biomaterials 2002, 23, 2641–2648. [Google Scholar] [CrossRef]
- Kolhe, P.; Kannan, R.M. Improvement in Ductility of Chitosan through Blending and Copolymerization with PEG: FTIR Investigation of Molecular Interactions. Biomacromolecules 2003, 4, 173–180. [Google Scholar] [CrossRef] [Green Version]
- Mohamed, R.R.; Seoudi, R.S.; Sabaa, M.W. Synthesis and Characterization of Cross-linked Polyethylene Glycol/Carboxymethyl Chitosan Hydrogels. Adv. Polym. Technol. 2014, 34. [Google Scholar] [CrossRef]
- Sudhakar, Y.; Selvakumar, M. Miscibility of chitosan and poly(ethyleneglycol) blends in buffer solution. e-Polymers 2012, 12, 1037–1050. [Google Scholar] [CrossRef]
- Akmaz, S.; Adıgüzel, E.D.; Yasar, M.; Erguven, O. The Effect of Ag Content of the Chitosan-Silver Nanoparticle Composite Material on the Structure and Antibacterial Activity. Adv. Mater. Sci. Eng. 2013, 2013, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Slepička, P.; Elashnikov, R.; Ulbrich, P.; Staszek, M.; Kolska, Z.; Svorcik, V. Stabilization of sputtered gold and silver nanoparticles in PEG colloid solutions. J. Nanopart. Res. 2015, 17, 11. [Google Scholar] [CrossRef]
- Bin Ahmad, M.; Tay, M.Y.; Shameli, K.; Hussein, M.Z.; Lim, J.J. Green Synthesis and Characterization of Silver/Chitosan/Polyethylene Glycol Nanocomposites without any Reducing Agent. Int. J. Mol. Sci. 2011, 12, 4872–4884. [Google Scholar] [CrossRef] [PubMed]
- Mohandoss, R.; Renganathan, B.; Annalakshmi, O.; Ganesan, A. Gamma radiation impact on the fiber optic acetone gas sensing behaviour of magnesium tetraborate. Opt. Fiber Technol. 2019, 52, 101935. [Google Scholar] [CrossRef]
- Usman, F.; Dennis, J.O.; Seong, K.C.; Ahmed, A.Y.; Meriaudeau, F.; Ayodele, O.B.; Tobi, A.R.; Rabih, A.A.S.; Yar, A. Synthesis and characterisation of a ternary composite of polyaniline, reduced graphene-oxide and chitosan with reduced optical band gap and stable aqueous dispersibility. Results Phys. 2019, 15, 102690. [Google Scholar] [CrossRef]
- Nasution, T.I.; Nainggolan, I.; Hutagalung, S.D.; Ahmad, K.R.; Ahmad, Z. The sensing mechanism and detection of low concentration acetone using chitosan-based sensors. Sens. Actuators B Chem. 2013, 177, 522–528. [Google Scholar] [CrossRef]
- Li, R.; Liu, L.; Yang, F. Preparation of polyaniline/reduced graphene oxide nanocomposite and its application in adsorption of aqueous Hg(II). Chem. Eng. J. 2013, 229, 460–468. [Google Scholar] [CrossRef]
- Anderson Materials Evaluation. Elemental Composition Analysis. Available online: www.andersonmaterials.com/elemental-composition-analysis.html (accessed on 1 August 2020).
- Agarwal, S.; Giri, P.; Prajapati, Y.K.; Chakrabarti, P. Effect of Surface Roughness on the Performance of Optical SPR Sensor for Sucrose Detection: Fabrication, Characterization, and Simulation Study. IEEE Sens. J. 2016, 16, 8865–8873. [Google Scholar] [CrossRef]
- Lokman, N.F.; Azeman, N.H.; Suja, F.B.; Zan, M.S.D.; Bakar, A.A.A. Sensitivity Enhancement of Pb(II) Ion Detection in Rivers Using SPR-Based Ag Metallic Layer Coated with Chitosan–Graphene Oxide Nanocomposite. Sensors 2019, 19, 5159. [Google Scholar] [CrossRef] [Green Version]
- Kamaruddin, N.H.; Bakar, A.A.A.; Mobarak, N.N.; Zan, M.S.D.; Arsad, N. Binding Affinity of a Highly Sensitive Au/Ag/Au/Chitosan-Graphene Oxide Sensor Based on Direct Detection of Pb2+ and Hg2+ Ions. Sensors 2017, 17, 2277. [Google Scholar] [CrossRef] [Green Version]
- Oldenbroek, K.; van der Waaij, L. Textbook Animal Breeding and Genetics for BSc Students; Centre for Genetic Resources The Netherlands and Animal Breeding and Genomics Centre: Wageningen, The Netherlands, 2015. [Google Scholar]
- Statistics How To. Available online: https://www.statisticshowto.com/relative-standard-deviation/ (accessed on 12 April 2020).
- Manera, M.G.; Valli, L.; Conoci, S.; Rella, R. Thin layer porphyrinogen for alcohol-vapor optical sensors. J. Porphyr. Phthalocyanines 2009, 13, 1140–1147. [Google Scholar] [CrossRef]
- Thomsen, V.; Schatzlein, D.; Mercuro, D. Limits of detection in spectroscopy. Spectroscopy 2003, 18, 112–114. [Google Scholar]
- Kumar, R.; Jaiswal, M.; Singh, O.; Gupta, A.; Ansari, M.S.; Mittal, J.; Ansari, M.S. Selective and Reversible Sensing of Low Concentration of Carbon Monoxide Gas Using Nb-Doped OMS-2 Nanofibers at Room Temperature. IEEE Sens. J. 2019, 19, 7201–7206. [Google Scholar] [CrossRef]
- Kong, L.; Enders, A.; Rahman, T.S.; Dowben, P.A. Molecular adsorption on graphene. J. Phys. Condens. Matter 2014, 26, 443001. [Google Scholar] [CrossRef]
- Liu, Z.; Yang, T.; Dong, Y.; Wang, X. A Room Temperature VOCs Gas Sensor Based on a Layer by Layer Multi-Walled Carbon Nanotubes/Poly-ethylene Glycol Composite. Sensors 2018, 18, 3113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alwahib, A.A.; Sadrolhosseini, A.R.; An’Amt, M.N.; Lim, H.N.; Yaacob, M.H.; Abu Bakar, M.H.; Ming, H.N.; Mahdi, M.A. Reduced Graphene Oxide/Maghemite Nanocomposite for Detection of Hydrocarbons Vapor Using Surface Plasmon Resonance. IEEE Photonics J. 2016, 8, 1. [Google Scholar] [CrossRef]
- Ayawei, N.; Ebelegi, A.N.; Wankasi, D. Modelling and Interpretation of Adsorption Isotherms. J. Chem. 2017, 2017, 3039817. [Google Scholar] [CrossRef]
- Chen, X. Modeling of Experimental Adsorption Isotherm Data. Information 2015, 6, 14–22. [Google Scholar] [CrossRef] [Green Version]
- Salyk, O.; Castello, P.; Harskamp, F. A facility for characterization and testing of hydrogen sensors. Meas. Sci. Technol. 2006, 17, 3033–3041. [Google Scholar] [CrossRef]
- De Sá, A.; Abreu, A.S.; Moura, I.; Machado, A.V. Polymeric Materials for Metal Sorption from Hydric Resources. In Water Purification; Elsevier: Amsterdam, The Netherlands, 2017; pp. 289–322. [Google Scholar]
- Desta, M.B. Batch Sorption Experiments: Langmuir and Freundlich Isotherm Studies for the Adsorption of Textile Metal Ions onto Teff Straw (Eragrostis tef) Agricultural Waste. J. Thermodyn. 2013, 2013, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Belhachemi, M.; Addoun, F. Comparative adsorption isotherms and modeling of methylene blue onto activated carbons. Appl. Water Sci. 2011, 1, 111–117. [Google Scholar] [CrossRef] [Green Version]
- Sunberg, R.J. A simple procedure to convert parts per million (ppm) to molarity (m). J. Chem. Educ. 1986, 63, 714. [Google Scholar] [CrossRef]
- Daniyal, W.M.E.M.M.; Fen, Y.W.; Anas, N.A.A.; Omar, N.A.S.; Ramdzan, N.S.M.; Nakajima, H.; Mahdi, M.A. Enhancing the sensitivity of a surface plasmon resonance-based optical sensor for zinc ion detection by the modification of a gold thin film. RSC Adv. 2019, 9, 41729–41736. [Google Scholar] [CrossRef] [Green Version]
- Raja, M.; Sadhasivam, B.; Naik, J.R.; Dhamodharan, R.; Ramanujam, K.; Murugan, R.; Ramavath, J.N.; Raghavachari, D. A chitosan/poly(ethylene glycol)-ran-poly(propylene glycol) blend as an eco-benign separator and binder for quasi-solid-state supercapacitor applications. Sustain. Energy Fuels 2019, 3, 760–773. [Google Scholar] [CrossRef]
- Nguyen, V.H.; Lamiel, C.; Kharismadewi, D.; Tran, V.C.; Shim, J.-J. Covalently bonded reduced graphene oxide/polyaniline composite for electrochemical sensors and capacitors. J. Electroanal. Chem. 2015, 758, 148–155. [Google Scholar] [CrossRef]
- Dambies, L.; Guimon, C.; Yiacoumi, S.; Guibal, E. Characterization of metal ion interactions with chitosan by X-ray photoelectron spectroscopy. Colloids Surf. A Physicochem. Eng. Asp. 2001, 177, 203–214. [Google Scholar] [CrossRef]
- Alamdari, S.; Sasani Ghamsari, M.; Jafar Tafreshi, M. Synthesis, characterization, and gas sensing properties of In-doped ZnO nanopowders. Nanochem. Res. 2017, 2, 198–204. [Google Scholar]
- Wang, J.-C.; Shi, W.; Sun, X.-Q.; Wu, F.-Y.; Li, Y.; Hou, Y. Enhanced Photo-Assisted Acetone Gas Sensor and Efficient Photocatalytic Degradation Using Fe-Doped Hexagonal and Monoclinic WO3 Phase−Junction. Nanomaterials 2020, 10, 398. [Google Scholar] [CrossRef] [Green Version]
- Usman, F.; Dennis, J.O.; Meriaudeau, F.; Ahmed, A.Y.; Seong, K.C.; Fen, Y.W.; Sadrolhosseini, A.R.; Abdulkadir, B.A.; Ayinla, R.T.; Daniyal, W.M.E.M.M.; et al. Dependence of the Optical Constant Parameters of p-Toluene Sulfonic Acid-Doped Polyaniline and Its Composites on Dispersion Solvents. Molecules 2020, 25, 4414. [Google Scholar] [CrossRef]
- Nalwa, H.S. Handbook of Low and High Dielectric Constant Materials and Their Applications; Elsevier: Amsterdam, The Netherlands, 1999. [Google Scholar]
- Fox, M. Optical Properties of Solids; American Association of Physics Teachers: College Park, MD, USA, 2002. [Google Scholar]
- Fukui, K. Detection and measurements of odor by sintered tin oxide gas sensor. Sens. Actuators B Chem. 1991, 5, 27–32. [Google Scholar]






| Material | Ra (nm) | RMS (nm) |
|---|---|---|
| Glass | 0.16 | 0.22 |
| Gold | 2.13 | 4.05 |
| Chitosan-PEG | 5.87 | 9.29 |
| Number of Layers | FWHM (Degree) | Sensitivity (Degree/ppm) | FOM (per ppm) |
|---|---|---|---|
| 1 | 4.86 | 0.348 | 0.07 |
| 2 | 5.12 | 0.280 | 0.05 |
| 2 | 7.81 | 0.216 | 0.03 |
| 4 | 8.85 | 0.165 | 0.02 |
| 5 | indefinite | 0.130 | 0 |
| Model | Format | Parameter | Value | Standard Error |
|---|---|---|---|---|
| Langmuir | Non-linear | Δθmax | 3.227 | 0.328 |
| 1/KA | 0.893 | 0.320 | ||
| Adj. R2 | 0.840 | - | ||
| Reduced chi-square | 0.060 | - | ||
| KA | 1.120 | - | ||
| KD | 0.893 | - | ||
| Langmuir | Linear | Residual sum of squares | 0.160 | - |
| Adj. R2 | 0.917 | - | ||
| Intercept | 0.334 | 0.306 | ||
| Slope | 4.458 | 0.595 | ||
| Δθmax | 2.994 | - | ||
| KA | 0.075 | - | ||
| KD | 13.333 | - | ||
| Freundlich | Non-linear | KF | 1.647 | 0.082 |
| n | 2.874 | 0.337 | ||
| Adj. R2 | 0.947 | - | ||
| Reduced chi-square | 0.020 | - | ||
| Freundlich | Linear | Residual sum of squares | 0.014 | - |
| Adj. R2 | 0.961 | - | ||
| Intercept | 0.516 | 0.031 | ||
| Slope | 0.328 | 0.030 | ||
| KF | 1.430 | - | ||
| n | 3.049 | - |
| Hydrocarbon | Chemical Formula | Carbon Number | Evaporation Rate n-butyl acetate = 1.0 |
|---|---|---|---|
| Acetone | C3H6O | 3 | 14.4 |
| Ethanol | C2H6O | 2 | 1.7 |
| Propanol | C3H8O | 3 | 1.3 |
| Methanol | CH4O | 1 | 4.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Usman, F.; Dennis, J.O.; Mkawi, E.M.; Al-Hadeethi, Y.; Meriaudeau, F.; Fen, Y.W.; Sadrolhosseini, A.R.; Ferrell, T.L.; Alsadig, A.; Sulieman, A. Acetone Vapor-Sensing Properties of Chitosan-Polyethylene Glycol Using Surface Plasmon Resonance Technique. Polymers 2020, 12, 2586. https://doi.org/10.3390/polym12112586
Usman F, Dennis JO, Mkawi EM, Al-Hadeethi Y, Meriaudeau F, Fen YW, Sadrolhosseini AR, Ferrell TL, Alsadig A, Sulieman A. Acetone Vapor-Sensing Properties of Chitosan-Polyethylene Glycol Using Surface Plasmon Resonance Technique. Polymers. 2020; 12(11):2586. https://doi.org/10.3390/polym12112586
Chicago/Turabian StyleUsman, Fahad, John Ojur Dennis, E. M. Mkawi, Yas Al-Hadeethi, Fabrice Meriaudeau, Yap Wing Fen, Amir Reza Sadrolhosseini, Thomas L. Ferrell, Ahmed Alsadig, and Abdelmoneim Sulieman. 2020. "Acetone Vapor-Sensing Properties of Chitosan-Polyethylene Glycol Using Surface Plasmon Resonance Technique" Polymers 12, no. 11: 2586. https://doi.org/10.3390/polym12112586
APA StyleUsman, F., Dennis, J. O., Mkawi, E. M., Al-Hadeethi, Y., Meriaudeau, F., Fen, Y. W., Sadrolhosseini, A. R., Ferrell, T. L., Alsadig, A., & Sulieman, A. (2020). Acetone Vapor-Sensing Properties of Chitosan-Polyethylene Glycol Using Surface Plasmon Resonance Technique. Polymers, 12(11), 2586. https://doi.org/10.3390/polym12112586








