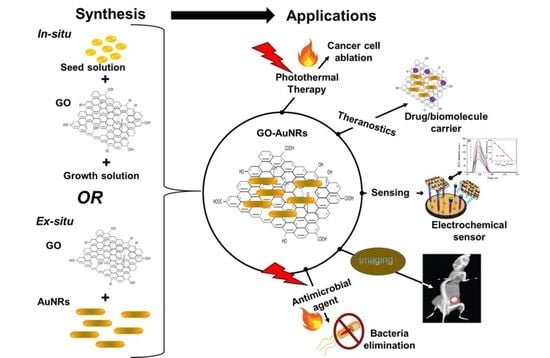
Graphene Oxide-Coated Gold Nanorods: Synthesis and Applications
Abstract
:1. Introduction
2. Gold Nanorods Synthesis
2.1. Hard Template Method
2.2. Electrochemical Method
2.3. Photochemical Method
2.4. Seed-Mediated Growth Method
2.4.1. Seed-Mediated Method without AgNO3
2.4.2. Seed-Mediated Method with AgNO3
2.5. Seedless Growth Method
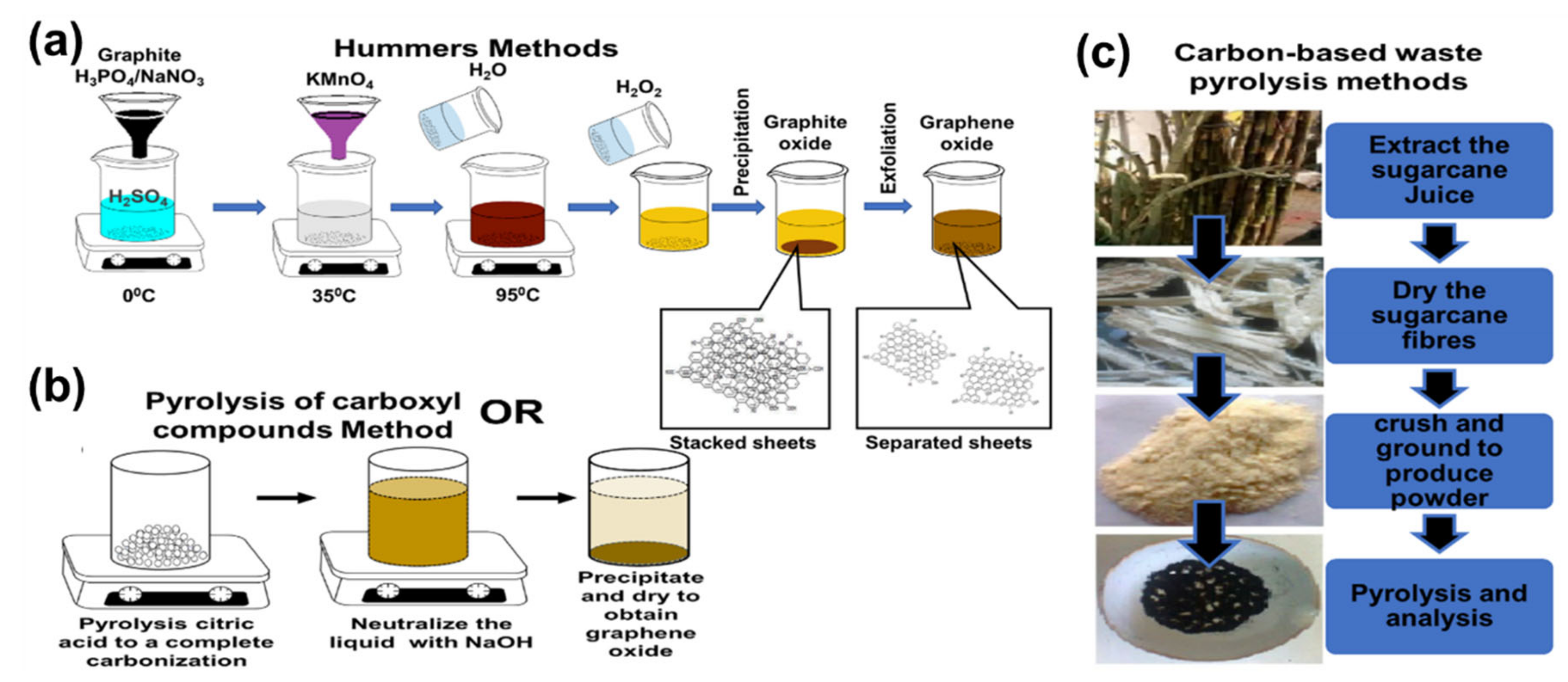
3. Graphene Oxide Synthesis
4. Graphene Oxide–Gold Nanorod: Coating and Properties
4.1. Ex Situ Coating Approach
4.2. In Situ Coating Approach
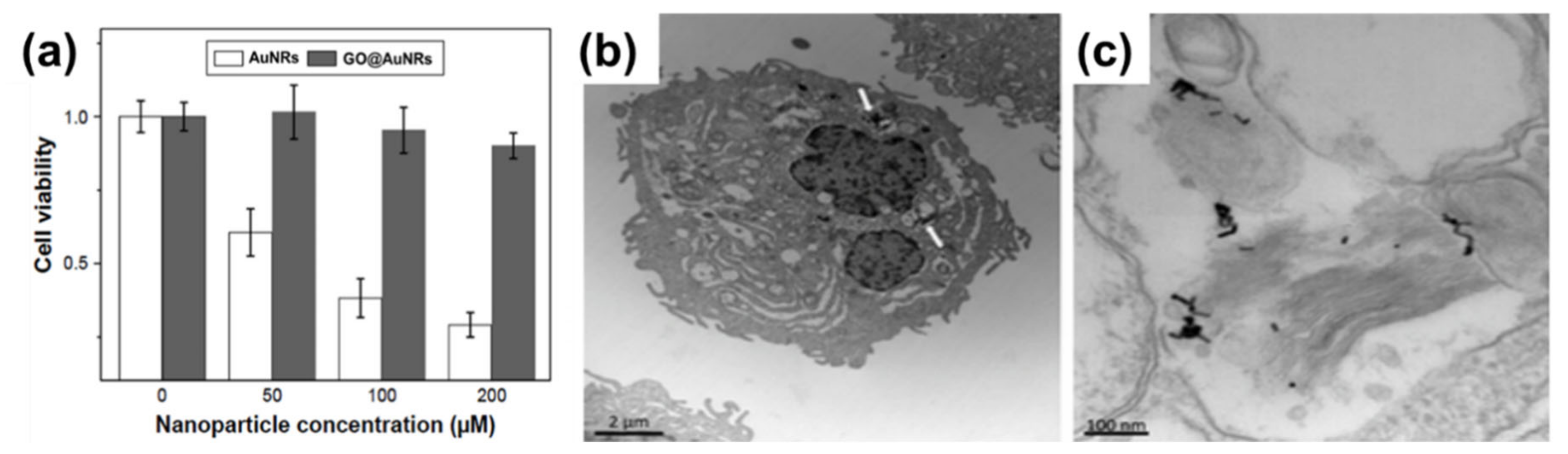
5. Biocompatibility
6. Photothermal Properties
7. Applications
7.1. Biomedical Application: Theranostic Agent
7.2. Sensors
7.3. Other Applications
8. Conclusions, Remarks, and Future Prospects
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Riley, R.S.; Day, E.S. Gold nanoparticle-mediated photothermal therapy: Applications and opportunities for multimodal cancer treatment. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2017, 9, e1449. [Google Scholar] [CrossRef] [PubMed]
- Moussawi, R.N.; Patra, D. Synthesis of Au nanorods through prereduction with curcumin: Preferential enhancement of Au nanorod formation prepared from CTAB-capped over citrate-capped Au seeds. J. Phys. Chem. C 2015, 199, 19458–19468. [Google Scholar] [CrossRef]
- De Melo-Diogo, D.; Pais-Silva, C.; Dias, D.R.; Moreira, A.F.; Correia, I.J. Strategies to improve cancer photothermal therapy mediated by nanomaterials. Adv. Healthc. Mater. 2017, 6, 1700073. [Google Scholar] [CrossRef] [PubMed]
- Burrows, N.D.; Harvey, S.; Idesis, F.A.; Murphy, C.J. Understanding the seed-mediated growth of gold nanorods through a fractional factorial design of experiments. Langmuir 2017, 33, 1891–1907. [Google Scholar] [CrossRef]
- Ahmad, M.Z.; Akhter, S.; Rahman, Z.; Akhter, S.; Anwar, M.; Mallik, N.; Ahmad, F.J. Nanometric gold in cancer nanotechnology: Current status and future prospect. J. Pharm. Pharmacol. 2013, 65, 634–651. [Google Scholar] [CrossRef]
- Pattani, V.P.; Tunnell, J.W. Nanoparticle-mediated photothermal therapy: A comparative study of heating for different particle types. Lasers Surg. Med. 2012, 44, 675–684. [Google Scholar] [CrossRef] [Green Version]
- Kennedy, L.C.; Bickford, L.R.; Lewinski, N.A.; Coughlin, A.J.; Hu, Y.; Day, E.S.; West, J.L.; Drezek, R.A. A New Era for Cancer Treatment: Gold-Nanoparticle-Mediated Thermal Therapies. Small 2011, 7, 169–183. [Google Scholar] [CrossRef]
- Vines, J.B.; Yoon, J.-H.; Ryu, N.-E.; Lim, D.-J.; Park, H. Gold Nanoparticles for Photothermal Cancer Therapy. Front. Chem. 2019, 7, 167. [Google Scholar] [CrossRef] [Green Version]
- Hlapisi, N.; Motaung, T.E.; Linganiso, L.Z.; Oluwafemi, O.S.; Songca, S.P. Encapsulation of gold nanorods with porphyrins for the potential treatment of cancer and bacterial diseases: A critical review. Bioinorg. Chem. Appl. 2019, 2019, 7147128. [Google Scholar] [CrossRef]
- Huang, X.; Neretina, S.; El-Sayed, M.A. Gold nanorods: From synthesis and properties to biological and biomedical applications. Adv. Mater. 2009, 21, 4880–4910. [Google Scholar] [CrossRef]
- Elahi, N.; Kamali, M.; Baghersad, M.H. Recent biomedical applications of gold nanoparticles: A review. Talanta 2018, 184, 537–556. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.R.; Rahman, M.A.; Wu, Y.; Han, T.; Peng, X.; Mackey, M.A.; Wang, D.; Shin, H.J.; Chen, Z.G.; Xiao, H. Efficacy, long-term toxicity, and mechanistic studies of gold nanorods photothermal therapy of cancer in xenograft mice. Proc. Natl. Acad. Sci. USA 2017, 114, E3110–E3118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, J.; Bender, C.M.; Murphy, C.J. Dependence of the gold nanorod aspect ratio on the nature of the directing surfactant in aqueous solution. Langmuir 2003, 19, 9065–9070. [Google Scholar] [CrossRef]
- Kou, X.; Zhang, S.; Tsung, C.K.; Yang, Z.; Yeung, M.H.; Stucky, G.D.; Sun, L.; Wang, J.; Yan, C. One-Step Synthesis of Large-Aspect-Ratio Single-Crystalline Gold Nanorods by Using CTPAB and CTBAB Surfactants. Chem. Eur. J. 2007, 13, 2929–2936. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Yao, J.; Luo, J.; Duan, X.; Yao, Y.; Liu, T. Effect of Growth Temperature on Tailoring the Size and Aspect Ratio of Gold Nanorods. Langmuir 2017, 33, 7479–7485. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Juste, J.; Pastoriza-Santos, I.; Liz-Marzán, L.M.; Mulvaney, P. Gold nanorods: Synthesis, characterization and applications. Coord. Chem. Rev. 2005, 249, 1870–1901. [Google Scholar] [CrossRef]
- Allen, J.M.; Xu, J.; Blahove, M.; Canonico-May, S.A.; Santaloci, T.J.; Braselton, M.E.; Stone, J.W. Synthesis of less toxic gold nanorods by using dodecylethyldimethylammonium bromide as an alternative growth-directing surfactant. J. Colloid Interface Sci. 2017, 505, 1172–1176. [Google Scholar] [CrossRef]
- Wang, Z.L.; Mohamed, M.B.; Link, S.; El-Sayed, M.A. Crystallographic facets and shapes of gold nanorods of different aspect ratios. Surf. Sci. 1999, 440, L809–L814. [Google Scholar] [CrossRef]
- Chang, H.-H.; Murphy, C.J. Mini gold nanorods with tunable plasmonic peaks beyond 1000 nm. Chem. Mater. 2018, 30, 1427–1435. [Google Scholar] [CrossRef]
- Nasrollahzadeh, M.; Babaei, F.; Fakhri, P.; Jaleh, B. Synthesis, characterization, structural, optical properties and catalytic activity of reduced graphene oxide/copper nanocomposites. RSC Adv. 2015, 5, 10782–10789. [Google Scholar] [CrossRef]
- Pinto, A.M.; Gonçalves, I.C.; Magalhães, F.D. Graphene-based materials biocompatibility: A review. Colloids Surf. B 2013, 111, 188–202. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, O.N.; Fernando, K.S.; Wang, B.; Brown, N.A.; Luo, P.G.; McNamara, N.D.; Vangsness, M.; Sun, Y.-P.; Bunker, C.E. Graphene oxide: A nonspecific enhancer of cellular growth. ACS Nano 2011, 5, 8100–8107. [Google Scholar] [CrossRef] [PubMed]
- Dideikin, A.T.; Vul’, A.Y. Graphene Oxide and Derivatives: The Place in Graphene Family. Front. Phys. 2019, 6, 149. [Google Scholar] [CrossRef]
- Singh, D.P.; Herrera, C.E.; Singh, B.; Singh, S.; Singh, R.K.; Kumar, R. Graphene oxide: An efficient material and recent approach for biotechnological and biomedical applications. Mater. Sci. Eng. C 2018, 86, 173–197. [Google Scholar] [CrossRef] [PubMed]
- Muazim, K.; Hussain, Z. Graphene oxide—A platform towards theranostics. Mater. Sci. Eng. C 2017, 76, 1274–1288. [Google Scholar] [CrossRef]
- Lebepe, T.C.; Parani, S.; Vuyelwa, N.; Kodama, T.; Oluwafemi, O.S. Cytotoxicity evaluation of Graphene Oxide against Adherent and Suspension cancer cells. Mater. Lett. 2020, 279, 128470. [Google Scholar] [CrossRef]
- Liu, J.; Cui, L.; Losic, D. Graphene and graphene oxide as new nanocarriers for drug delivery applications. Acta Biomater. 2013, 9, 9243–9257. [Google Scholar] [CrossRef]
- McCallion, C.; Burthem, J.; Rees-Unwin, K.; Golovanov, A.; Pluen, A. Graphene in therapeutics delivery: Problems, solutions and future opportunities. Eur. J. Pharm. Biopharm. 2016, 104, 235–250. [Google Scholar] [CrossRef]
- Dembereldorj, U.; Choi, S.Y.; Ganbold, E.O.; Song, N.W.; Kim, D.; Choo, J.; Lee, S.Y.; Kim, S.; Joo, S.W. Gold Nanorod-Assembled PEGylated Graphene-Oxide Nanocomposites for Photothermal Cancer Therapy. Photochem. Photobiol. 2014, 90, 659–666. [Google Scholar] [CrossRef]
- Khan, M.S.; Pandey, S.; Bhaisare, M.L.; Gedda, G.; Talib, A.; Wu, H.-F. Graphene oxide@gold nanorods for chemo-photothermal treatment and controlled release of doxorubicin in mice Tumor. Colloids Surf. B Biointerfaces 2017, 160, 543–552. [Google Scholar] [CrossRef]
- Lim, D.-K.; Barhoumi, A.; Wylie, R.G.; Reznor, G.; Langer, R.S.; Kohane, D.S. Enhanced Photothermal Effect of Plasmonic Nanoparticles Coated with Reduced Graphene Oxide. Nano Lett. 2013, 13, 4075–4079. [Google Scholar] [CrossRef]
- Moon, H.; Kumar, D.; Kim, H.; Sim, C.; Chang, J.-H.; Kim, J.-M.; Kim, H.; Lim, D.-K. Amplified Photoacoustic Performance and Enhanced Photothermal Stability of Reduced Graphene Oxide Coated Gold Nanorods for Sensitive Photoacoustic Imaging. ACS Nano 2015, 9, 2711–2719. [Google Scholar] [CrossRef]
- Robinson, J.T.; Tabakman, S.M.; Liang, Y.; Wang, H.; Sanchez Casalongue, H.; Vinh, D.; Dai, H. Ultrasmall Reduced Graphene Oxide with High Near-Infrared Absorbance for Photothermal Therapy. J. Am. Chem. Soc. 2011, 133, 6825–6831. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Yang, X.; Jacobson, O.; Lin, L.; Huang, P.; Niu, G.; Ma, Q.; Chen, X. Sequential drug release and enhanced photothermal and photoacoustic effect of hybrid reduced graphene oxide-loaded ultrasmall gold nanorod vesicles for cancer therapy. ACS Nano 2015, 9, 9199–9209. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Turcheniuk, K.; Dumych, T.; Bilyy, R.; Turcheniuk, V.; Bouckaert, J.; Vovk, V.; Chopyak, V.; Zaitsev, V.; Mariot, P.; Prevarskaya, N.; et al. Plasmonic photothermal cancer therapy with gold nanorods/reduced graphene oxide core/shell nanocomposites. RSC Adv. 2016, 6, 1600–1610. [Google Scholar] [CrossRef] [Green Version]
- Xu, C.; Yang, D.; Mei, L.; Li, Q.; Zhu, H.; Wang, T. Targeting chemophotothermal therapy of hepatoma by gold nanorods/graphene oxide core/shell nanocomposites. ACS Appl. Mater. Interfaces 2013, 5, 12911–12920. [Google Scholar] [CrossRef]
- Qiu, X.; You, X.; Chen, X.; Chen, H.; Dhinakar, A.; Liu, S.; Guo, Z.; Wu, J.; Liu, Z. Development of graphene oxide-wrapped gold nanorods as robust nanoplatform for ultrafast near-infrared SERS bioimaging. Int. J. Nanomed. 2017, 12, 4349–4360. [Google Scholar] [CrossRef] [Green Version]
- Sun, B.; Wu, J.; Cui, S.; Zhu, H.; An, W.; Fu, Q.; Shao, C.; Yao, A.; Chen, B.; Shi, D. In situ synthesis of graphene oxide/gold nanorods theranostic hybrids for efficient tumor computed tomography imaging and photothermal therapy. Nano Res. 2017, 10, 37–48. [Google Scholar] [CrossRef]
- Zhang, Z.; Shi, J.; Song, Z.; Zhu, X.; Zhu, Y.; Cao, S. A synergistically enhanced photothermal transition effect from mesoporous silica nanoparticles with gold nanorods wrapped in reduced graphene oxide. J. Mater. Sci. 2018, 53, 1810–1823. [Google Scholar] [CrossRef]
- Arvand, M.; Gholizadeh, T.M. Gold nanorods–graphene oxide nanocomposite incorporated carbon nanotube paste modified glassy carbon electrode for voltammetric determination of indomethacin. Sens. Actuators B 2013, 186, 622–632. [Google Scholar] [CrossRef]
- Azimzadeh, M.; Rahaie, M.; Nasirizadeh, N.; Ashtari, K.; Naderi-Manesh, H. An electrochemical nanobiosensor for plasma miRNA-155, based on graphene oxide and gold nanorod, for early detection of breast cancer. Biosens. Bioelectron. 2016, 77, 99–106. [Google Scholar] [CrossRef]
- Cao, J.-T.; Yang, J.-J.; Zhao, L.-Z.; Wang, Y.-L.; Wang, H.; Liu, Y.-M.; Ma, S.-H. Graphene oxide@gold nanorods-based multiple-assisted electrochemiluminescence signal amplification strategy for sensitive detection of prostate specific antigen. Biosens. Bioelectron. 2018, 99, 92–98. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Chen, L.; Li, J.; Lin, M.; You, H.; Wang, W. Label-free colorimetric sensor for ultrasensitive detection of heparin based on color quenching of gold nanorods by graphene oxide. Biosens. Bioelectron. 2012, 34, 227–231. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Fang, X.; Shi, A.; Wang, J.; Zhang, Y. An electrochemical DNA biosensor based on gold nanorods decorated graphene oxide sheets for sensing platform. Anal. Biochem. 2013, 443, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Jayabal, S.; Viswanathan, P.; Ramaraj, R. Reduced graphene oxide–gold nanorod composite material stabilized in silicate sol–gel matrix for nitric oxide sensor. RSC Adv. 2014, 4, 33541–33548. [Google Scholar] [CrossRef]
- Cepak, V.M.; Martin, C.R. Preparation and stability of template-synthesized metal nanorod sols in organic solvents. J. Phys. Chem. B 1998, 102, 9985–9990. [Google Scholar] [CrossRef]
- Foss, C.A., Jr.; Hornyak, G.L.; Stockert, J.A.; Martin, C.R. Template-synthesized nanoscopic gold particles: Optical spectra and the effects of particle size and shape. J. Phys. Chem. 1994, 98, 2963–2971. [Google Scholar] [CrossRef]
- Martin, C.R. Nanomaterials: A membrane-based synthetic approach. Science 1994, 266, 1961–1966. [Google Scholar] [CrossRef]
- Chen, H.; Shao, L.; Li, Q.; Wang, J. Gold nanorods and their plasmonic properties. Chem. Soc. Rev. 2013, 42, 2679–2724. [Google Scholar] [CrossRef]
- Yu, Y.-Y.; Chang, S.-S.; Lee, C.-L.; Wang, C.C. Gold nanorods: Electrochemical synthesis and optical properties. J. Phys. Chem. B 1997, 101, 6661–6664. [Google Scholar] [CrossRef]
- Gole, A.; Murphy, C.J. Seed-mediated synthesis of gold nanorods: Role of the size and nature of the seed. Chem. Mater. 2004, 16, 3633–3640. [Google Scholar] [CrossRef]
- Ye, X.; Gao, Y.; Chen, J.; Reifsnyder, D.C.; Zheng, C.; Murray, C.B. Seeded growth of monodisperse gold nanorods using bromide-free surfactant mixtures. Nano Lett. 2013, 13, 2163–2171. [Google Scholar] [CrossRef] [PubMed]
- Ye, X.; Zheng, C.; Chen, J.; Gao, Y.; Murray, C.B. Using binary surfactant mixtures to simultaneously improve the dimensional tunability and monodispersity in the seeded growth of gold nanorods. Nano Lett. 2013, 13, 765–771. [Google Scholar] [CrossRef]
- Zijlstra, P.; Bullen, C.; Chon, J.W.; Gu, M. High-temperature seedless synthesis of gold nanorods. J. Phys. Chem. B 2006, 110, 19315–19318. [Google Scholar] [CrossRef]
- Kim, F.; Sohn, K.; Wu, J.; Huang, J. Chemical synthesis of gold nanowires in acidic solutions. J. Am. Chem. Soc. 2008, 130, 14442–14443. [Google Scholar] [CrossRef]
- Wu, H.-Y.; Chu, H.-C.; Kuo, T.-J.; Kuo, C.-L.; Huang, M.H. Seed-mediated synthesis of high aspect ratio gold nanorods with nitric acid. Chem. Mater. 2005, 17, 6447–6451. [Google Scholar] [CrossRef]
- Khlebtsov, B.N.; Khanadeev, V.A.; Ye, J.; Sukhorukov, G.B.; Khlebtsov, N.G. Overgrowth of gold nanorods by using a binary surfactant mixture. Langmuir 2014, 30, 1696–1703. [Google Scholar] [CrossRef] [PubMed]
- Jana, N.R.; Gearheart, L.; Murphy, C.J. Seed-mediated growth approach for shape-controlled synthesis of spheroidal and rod-like gold nanoparticles using a surfactant template. Adv. Mater. 2001, 13, 1389–1393. [Google Scholar] [CrossRef]
- Lai, J.; Zhang, L.; Niu, W.; Qi, W.; Zhao, J.; Liu, Z.; Zhang, W.; Xu, G. One-pot synthesis of gold nanorods using binary surfactant systems with improved monodispersity, dimensional tunability and plasmon resonance scattering properties. Nanotechnology 2014, 25, 125601. [Google Scholar] [CrossRef]
- Nikoobakht, B.; El-Sayed, M.A. Evidence for Bilayer Assembly of Cationic Surfactants on the Surface of Gold Nanorods. Langmuir 2001, 17, 6368–6374. [Google Scholar] [CrossRef]
- Roach, L.; Ye, S.; Moorcroft, S.C.; Critchley, K.; Coletta, P.L.; Evans, S.D. Morphological control of seedlessly-synthesized gold nanorods using binary surfactants. Nanotechnology 2018, 29, 135601. [Google Scholar] [CrossRef]
- Ali, M.R.; Snyder, B.; El-Sayed, M.A. Synthesis and optical properties of small Au nanorods using a seedless growth technique. Langmuir 2012, 28, 9807–9815. [Google Scholar] [CrossRef]
- Jana, N.R.; Gearheart, L.; Murphy, C.J. Wet chemical synthesis of high aspect ratio cylindrical gold nanorods. J. Phys. Chem. B 2001, 105, 4065–4067. [Google Scholar] [CrossRef]
- Park, K.; Hsiao, M.-s.; Yi, Y.-J.; Izor, S.; Koerner, H.; Jawaid, A.; Vaia, R.A. Highly Concentrated Seed-Mediated Synthesis of Monodispersed Gold Nanorods. ACS Appl. Mater. Interfaces 2017, 9, 26363–26371. [Google Scholar] [CrossRef] [PubMed]
- Scarabelli, L.; Sánchez-Iglesias, A.; Pérez-Juste, J.; Liz-Marzán, L.M. A “Tips and Tricks” Practical Guide to the Synthesis of Gold Nanorods. ACS J. Phys. Chem. Lett. 2015, 6, 4270–4279. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, X.; Zhao, Y.; Xue, X.; Huo, S.; Chen, F.; Zou, G.; Liang, X.-J. Seedless synthesis of high aspect ratio gold nanorods with high yield. J. Mater. Chem. A 2014, 2, 3528–3535. [Google Scholar] [CrossRef]
- Requejo, K.I.; Liopo, A.V.; Zubarev, E.R. Synthesis of Gold Nanorods Using Poly (vinylpyrrolidone) of Different Molecular Weights as an Additive. ChemistrySelect 2018, 3, 12192–12197. [Google Scholar] [CrossRef]
- Ye, X.; Jin, L.; Caglayan, H.; Chen, J.; Xing, G.; Zheng, C.; Doan-Nguyen, V.; Kang, Y.; Engheta, N.; Kagan, C.R. Improved size-tunable synthesis of monodisperse gold nanorods through the use of aromatic additives. ACS Nano 2012, 6, 2804–2817. [Google Scholar] [CrossRef]
- Zhu, J.; Yong, K.-T.; Roy, I.; Hu, R.; Ding, H.; Zhao, L.; Swihart, M.T.; He, G.S.; Cui, Y.; Prasad, P.N. Additive controlled synthesis of gold nanorods (GNRs) for two-photon luminescence imaging of cancer cells. Nanotechnology 2010, 21, 285106. [Google Scholar] [CrossRef]
- Nikoobakht, B.; El-Sayed, M.A. Preparation and growth mechanism of gold nanorods (NRs) using seed-mediated growth method. Chem. Mater. 2003, 15, 1957–1962. [Google Scholar] [CrossRef]
- Varón, M.; Arbiol, J.; Puntes, V.F. High aspect ratio gold nanorods grown with platinum seeds. J. Phys. Chem. C 2015, 119, 11818–11825. [Google Scholar] [CrossRef] [Green Version]
- Foss, C.A.; Hornyak, G.L.; Stockert, J.A.; Martin, C.R. Optically transparent nanometal composite membranes. Adv. Mater. 1993, 5, 135–136. [Google Scholar] [CrossRef]
- Bao, Z.; Sun, Z.; Li, Z.; Tian, L.; Ngai, T.; Wang, J. Plasmonic Gold− Superparamagnetic Hematite Heterostructures. Langmuir 2011, 27, 5071–5075. [Google Scholar] [CrossRef]
- Govindaraju, S.; Yun, K. Synthesis of gold nanomaterials and their cancer-related biomedical applications: An update. 3 Biotech 2018, 8, 113. [Google Scholar] [CrossRef] [PubMed]
- Grabinski, C.; Schaeublin, N.; Wijaya, A.; D’Couto, H.; Baxamusa, S.H.; Hamad-Schifferli, K.; Hussain, S.M. Effect of gold nanorod surface chemistry on cellular response. ACS Nano 2011, 5, 2870–2879. [Google Scholar] [CrossRef] [Green Version]
- Rao, H.; Xue, X.; Wang, H.; Xue, Z. Gold nanorod etching-based multicolorimetric sensors: Strategies and applications. J. Mater. Chem. C 2019, 7, 4610–4621. [Google Scholar] [CrossRef]
- Liopo, A.; Wang, S.; Derry, P.J.; Oraevsky, A.A.; Zubarev, E.R. Seedless synthesis of gold nanorods using dopamine as a reducing agent. RSC Adv. 2015, 5, 91587–91593. [Google Scholar] [CrossRef]
- Cao, J.; Sun, T.; Grattan, K.T. Gold nanorod-based localized surface plasmon resonance biosensors: A review. Sens. Actuators B 2014, 195, 332–351. [Google Scholar] [CrossRef]
- Tong, W.; Walsh, M.J.; Mulvaney, P.; Etheridge, J.; Funston, A.M. Control of Symmetry Breaking Size and Aspect Ratio in Gold Nanorods: Underlying Role of Silver Nitrate. J. Phys. Chem. C 2017, 121, 3549–3559. [Google Scholar] [CrossRef]
- Xu, Y.; Chen, L.; Ye, X.; Wang, X.; Yu, J.; Zhao, Y.; Cao, M.; Xia, Z.; Sun, B.; Zhang, Q. Cooperative interactions among CTA+, Br–and Ag+ during seeded growth of gold nanorods. Nano Res. 2017, 10, 2146–2155. [Google Scholar] [CrossRef]
- Vigderman, L.; Zubarev, E.R. High-yield synthesis of gold nanorods with longitudinal SPR peak greater than 1200 nm using hydroquinone as a reducing agent. Chem. Mater. 2013, 25, 1450–1457. [Google Scholar] [CrossRef]
- Morasso, C.; Picciolini, S.; Schiumarini, D.; Mehn, D.; Ojea-Jiménez, I.; Zanchetta, G.; Vanna, R.; Bedoni, M.; Prosperi, D.; Gramatica, F. Control of size and aspect ratio in hydroquinone-based synthesis of gold nanorods. J. Nanopart. Res. 2015, 17, 330. [Google Scholar] [CrossRef]
- Zhang, L.; Xia, K.; Lu, Z.; Li, G.; Chen, J.; Deng, Y.; Li, S.; Zhou, F.; He, N. Efficient and facile synthesis of gold nanorods with finely tunable plasmonic peaks from visible to near-IR range. Chem. Mater. 2014, 26, 1794–1798. [Google Scholar] [CrossRef]
- Su, G.; Yang, C.; Zhu, J.-J. Fabrication of gold nanorods with tunable longitudinal surface plasmon resonance peaks by reductive dopamine. Langmuir 2015, 31, 817–823. [Google Scholar] [CrossRef] [PubMed]
- Zweifel, D.A.; Wei, A. Sulfide-arrested growth of gold nanorods. Chem. Mater. 2005, 17, 4256–4261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, D.K.; Miller, N.R.; Korgel, B.A. Iodide in CTAB prevents gold nanorod formation. Langmuir 2009, 25, 9518–9524. [Google Scholar] [CrossRef]
- Rayavarapu, R.G.; Ungureanu, C.; Krystek, P.; van Leeuwen, T.G.; Manohar, S. Iodide impurities in hexadecyltrimethylammonium bromide (CTAB) products: Lot− lot variations and influence on gold nanorod synthesis. Langmuir 2010, 26, 5050–5055. [Google Scholar] [CrossRef] [PubMed]
- Sau, T.; Murphy, C.J. Role of ions in the colloidal synthesis of gold nanowires. Philos. Mag. 2007, 87, 2143–2158. [Google Scholar] [CrossRef]
- Si, S.; Leduc, C.; Delville, M.H.; Lounis, B. Short gold nanorod growth revisited: The critical role of the bromide counterion. ChemPhysChem 2012, 13, 193–202. [Google Scholar] [CrossRef]
- Wen, T.; Hu, Z.; Liu, W.; Zhang, H.; Hou, S.; Hu, X.; Wu, X. Copper-ion-assisted growth of gold nanorods in seed-mediated growth: Significant narrowing of size distribution via tailoring reactivity of seeds. Langmuir 2012, 28, 17517–17523. [Google Scholar] [CrossRef]
- Keul, H.A.; Moeller, M.; Bockstaller, M.R. Effect of solvent isotopic replacement on the structure evolution of gold nanorods. J. Phys. Chem. C 2008, 112, 13483–13487. [Google Scholar] [CrossRef]
- Wang, W.; Li, J.; Lan, S.; Rong, L.; Liu, Y.; Sheng, Y.; Zhang, H.; Yang, B. Seedless synthesis of gold nanorods using resveratrol as a reductant. Nanotechnology 2016, 27, 165601. [Google Scholar] [CrossRef] [PubMed]
- Requejo, K.I.; Liopo, A.V.; Derry, P.J.; Zubarev, E.R. Accelerating gold nanorod synthesis with nanomolar concentrations of poly (vinylpyrrolidone). Langmuir 2017, 33, 12681–12688. [Google Scholar] [CrossRef] [PubMed]
- Requejo, K.I.; Liopo, A.; Zubarev, E.R. Gold Nanorods Synthesis with Small Thiolated Molecules. Langmuir 2020, 36, 3758–3769. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.T.; LaChance, A.M.; Zeng, S.; Liu, B.; Sun, L. Synthesis, properties, and applications of graphene oxide/reduced graphene oxide and their nanocomposites. Nano Mater. Sci. 2019, 1, 31–47. [Google Scholar] [CrossRef]
- Brodie, B.C. XIII. On the atomic weight of graphite. Philos. Trans. R. Soc. Lond. 1859, 149, 249–259. [Google Scholar]
- Staudenmaier, L. Verfahren zur darstellung der graphitsäure. Ber. Dtsch. Chem. Ges. 1898, 31, 1481–1487. [Google Scholar] [CrossRef] [Green Version]
- Hummers, W.S., Jr.; Offeman, R.E. Preparation of graphitic oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
- Chen, J.; Yao, B.; Li, C.; Shi, G. An improved Hummers method for eco-friendly synthesis of graphene oxide. Carbon 2013, 64, 225–229. [Google Scholar] [CrossRef]
- McCoy, T.M.; Turpin, G.; Teo, B.M.; Tabor, R.F. Graphene oxide: A surfactant or particle? Curr. Opin. Colloid Interface Sci. 2019, 39, 98–109. [Google Scholar] [CrossRef]
- Dong, Y.; Shao, J.; Chen, C.; Li, H.; Wang, R.; Chi, Y.; Lin, X.; Chen, G. Blue luminescent graphene quantum dots and graphene oxide prepared by tuning the carbonization degree of citric acid. Carbon 2012, 50, 4738–4743. [Google Scholar] [CrossRef]
- Masha, S.; Oluwafemi, S.O. Synthesis of blue and green emitting carbon-based quantum dots (CBQDs) and their cell viability against colon and bladder cancer cell lines. Mater. Lett. 2020, 283, 128790. [Google Scholar] [CrossRef]
- Saravanan, A.; Krishna, V.M.; Somanathan, T.; Prasad, K.; Ostrikov, K.K. Graphene Oxide Synthesis from Agro Waste. Nanomaterials 2015, 5, 826–834. [Google Scholar]
- Wu, Z.; Li, W.; Chen, J.; Yu, C. A graphene quantum dot-based method for the highly sensitive and selective fluorescence turn on detection of biothiols. Talanta 2014, 119, 538–543. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Chen, Z.-G.; Cole, I.; Li, Q. Structural evolution of graphene quantum dots during thermal decomposition of citric acid and the corresponding photoluminescence. Carbon 2015, 82, 304–313. [Google Scholar] [CrossRef] [Green Version]
- Choi, Y.-J.; Kim, E.; Han, J.; Kim, J.-H.; Gurunathan, S. A novel biomolecule-mediated reduction of graphene oxide: A multifunctional anti-cancer agent. Molecules 2016, 21, 375. [Google Scholar] [CrossRef]
- Fernández-Merino, M.J.; Guardia, L.; Paredes, J.; Villar-Rodil, S.; Solís-Fernández, P.; Martínez-Alonso, A.; Tascón, J. Vitamin C is an ideal substitute for hydrazine in the reduction of graphene oxide suspensions. J. Phys. Chem. C 2010, 114, 6426–6432. [Google Scholar] [CrossRef]
- Khosroshahi, Z.; Kharaziha, M.; Karimzadeh, F.; Allafchian, A. Green reduction of graphene oxide by ascorbic acid. AIP Conf. Proc. 2018, 1920, 020009. [Google Scholar] [CrossRef]
- Wang, J.; Salihi, E.C.; Šiller, L. Green reduction of graphene oxide using alanine. Mater. Sci. Eng. C 2017, 72, 1–6. [Google Scholar] [CrossRef]
- Chen, D.; Li, L.; Guo, L. An environment-friendly preparation of reduced graphene oxide nanosheets via amino acid. Nanotechnology 2011, 22, 325601. [Google Scholar] [CrossRef]
- Moosa, A.A.; Jaafar, J.N. Green reduction of graphene oxide using tea leaves extract with applications to lead ions removal from water. Nanosci. Nanotechnol. 2017, 7, 38–47. [Google Scholar]
- Chandu, B.; Mosali, V.S.S.; Mullamuri, B.; Bollikolla, H.B. A facile green reduction of graphene oxide using Annona squamosa leaf extract. Carbon Lett. 2017, 21, 74–80. [Google Scholar] [CrossRef] [Green Version]
- Wijaya, R.; Andersan, G.; Santoso, S.P.; Irawaty, W. Green Reduction of Graphene Oxide using Kaffir Lime Peel Extract (Citrus hystrix) and Its Application as Adsorbent for Methylene Blue. Sci. Rep. 2020, 10, 667. [Google Scholar] [CrossRef] [PubMed]
- Mascarenhas, F.C.; Sykam, N.; Selvakumar, M.; Mahesha, M. Green reduction of graphene oxide using Indian gooseberry (Amla) extract for gas sensing applications. J. Environ. Chem. Eng. 2020, 8, 103712. [Google Scholar] [CrossRef]
- Hu, C.; Rong, J.; Cui, J.; Yang, Y.; Yang, L.; Wang, Y.; Liu, Y. Fabrication of a graphene oxide–gold nanorod hybrid material by electrostatic self-assembly for surface-enhanced Raman scattering. Carbon 2013, 51, 255–264. [Google Scholar] [CrossRef]
- Xue, C.; Kung, C.-C.; Gao, M.; Liu, C.-C.; Dai, L.; Urbas, A.; Li, Q. Facile fabrication of 3D layer-by-layer graphene-gold nanorod hybrid architecture for hydrogen peroxide based electrochemical biosensor. Sens. Bio-Sens. Res. 2015, 3, 7–11. [Google Scholar] [CrossRef] [Green Version]
- Xu, C.; Yang, D.; Mei, L.; Lu, B.; Chen, L.; Li, Q.; Zhu, H.; Wang, T. Encapsulating gold nanoparticles or nanorods in graphene oxide shells as a novel gene vector. ACS Appl. Mater. Interfaces 2013, 5, 2715–2724. [Google Scholar] [CrossRef]
- Wei, Q.; Ni, H.; Jin, X.; Yuan, J. Graphene oxide wrapped gold nanorods for enhanced photo-thermal stability. RSC Adv. 2015, 5, 54971–54977. [Google Scholar] [CrossRef]
- Caires, A.; Alves, D.; Fantini, C.; Ferlauto, A.; Ladeira, L. One-pot in situ photochemical synthesis of graphene oxide/gold nanorod nanocomposites for surface-enhanced Raman spectroscopy. RSC Adv. 2015, 5, 46552–46557. [Google Scholar] [CrossRef]
- Tsolekile, N.; Parani, S.; Matoetoe, M.C.; Songca, S.P.; Oluwafemi, O.S. Evolution of ternary I–III–VI QDs: Synthesis, characterization and application. Nano-Struct. Nano-Objects 2017, 12, 46–56. [Google Scholar] [CrossRef]
- Fard, J.K.; Jafari, S.; Eghbal, M.A. A review of molecular mechanisms involved in toxicity of nanoparticles. Adv. Pharm. Bull. 2015, 5, 447. [Google Scholar] [CrossRef] [PubMed]
- Frohlich, E. Cellular targets and mechanisms in the cytotoxic action of non-biodegradable engineered nanoparticles. Curr. Drug Metab. 2013, 14, 976–988. [Google Scholar] [CrossRef] [PubMed]
- Jones, C.F.; Grainger, D.W. In vitro assessments of nanomaterial toxicity. Adv. Drug Deliv. Rev. 2009, 61, 438–456. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tomasella, P.; Sanfilippo, V.; Bonaccorso, C.; Cucci, L.M.; Consiglio, G.; Nicosia, A.; Mineo, P.G.; Forte, G.; Satriano, C. Theranostic Nanoplatforms of Thiolated Reduced Graphene Oxide Nanosheets and Gold Nanoparticles. Appl. Sci. 2020, 10, 5529. [Google Scholar] [CrossRef]
- Turcheniuk, K.; Hage, C.-H.; Spadavecchia, J.; Serrano, A.Y.; Larroulet, I.; Pesquera, A.; Zurutuza, A.; Pisfil, M.G.; Héliot, L.; Boukaert, J. Plasmonic photothermal destruction of uropathogenic E. coli with reduced graphene oxide and core/shell nanocomposites of gold nanorods/reduced graphene oxide. J. Mater. Chem. B 2015, 3, 375–386. [Google Scholar] [CrossRef]
- Austin, L.A.; Mackey, M.A.; Dreaden, E.C.; El-Sayed, M.A. The optical, photothermal, and facile surface chemical properties of gold and silver nanoparticles in biodiagnostics, therapy, and drug delivery. Arch. Toxicol. 2014, 88, 1391–1417. [Google Scholar] [CrossRef] [Green Version]
- Choi, J.; Kim, S.Y. Photothermally enhanced photodynamic therapy based on glutathione-responsive pheophorbide a-conjugated gold nanorod formulations for cancer theranostic applications. J. Ind. Eng. Chem. 2020, 85, 66–74. [Google Scholar] [CrossRef]
- Cong, B. Gold nanorods: Near-infrared plasmonic photothermal conversion and surface coating. J. Mater. Sci. Chem. Eng. 2014, 2, 20. [Google Scholar] [CrossRef] [Green Version]
- De Freitas, L.; Zanelatto, L.; Mantovani, M.; Silva, P.; Ceccini, R.; Grecco, C.; Moriyama, L.; Kurachi, C.; Martins, V.; Plepis, A. In vivo photothermal tumour ablation using gold nanorods. Laser Phys. 2013, 23, 066003. [Google Scholar] [CrossRef]
- Khot, M.I.; Andrew, H.; Svavarsdottir, H.S.; Armstrong, G.; Quyn, A.J.; Jayne, D.G. A Review on the Scope of Photothermal Therapy–Based Nanomedicines in Preclinical Models of Colorectal Cancer. Clin. Colorectal Cancer 2019, 18, e200–e209. [Google Scholar] [CrossRef]
- Lim, E.-K.; Kim, T.; Paik, S.; Haam, S.; Huh, Y.-M.; Lee, K. Nanomaterials for theranostics: Recent advances and future challenges. Chem. Rev. 2015, 115, 327–394. [Google Scholar] [CrossRef]
- Zhang, J.; Ning, L.; Huang, J.; Zhang, C.; Pu, K. Activatable molecular agents for cancer theranostics. Chem. Sci. 2020, 11, 618–630. [Google Scholar] [CrossRef] [Green Version]
- Qi, Z.; Shi, J.; Zhu, B.; Li, J.; Cao, S. Gold nanorods/graphene oxide nanosheets immobilized by polydopamine for efficient remotely triggered drug delivery. J. Mater. Sci. 2020, 55, 14530–14543. [Google Scholar] [CrossRef]
- Robinson, J.T.; Perkins, F.K.; Snow, E.S.; Wei, Z.; Sheehan, P.E. Reduced Graphene Oxide Molecular Sensors. Nano Lett. 2008, 8, 3137–3140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deng, K.; Li, C.; Li, X.; Huang, H. Simultaneous detection of sunset yellow and tartrazine using the nanohybrid of gold nanorods decorated graphene oxide. J. Electroanal. Chem. 2016, 780, 296–302. [Google Scholar] [CrossRef]
- Nirala, N.R.; Abraham, S.; Kumar, V.; Pandey, S.A.; Yadav, U.; Srivastava, M.; Srivastava, S.K.; Singh, V.N.; Kayastha, A.M.; Srivastava, A.; et al. Partially reduced graphene oxide–gold nanorods composite based bioelectrode of improved sensing performance. Talanta 2015, 144, 745–754. [Google Scholar] [CrossRef]
- Shi, A.; Wang, J.; Han, X.; Fang, X.; Zhang, Y. A sensitive electrochemical DNA biosensor based on gold nanomaterial and graphene amplified signal. Sens. Actuators B 2014, 200, 206–212. [Google Scholar] [CrossRef]
- Zhang, J.; Sun, Y.; Xu, B.; Zhang, H.; Gao, Y.; Zhang, H.; Song, D. A novel surface plasmon resonance biosensor based on graphene oxide decorated with gold nanorod–antibody conjugates for determination of transferrin. Biosens. Bioelectron. 2013, 45, 230–236. [Google Scholar] [CrossRef]
- Liu, M.; Zheng, C.; Cui, M.; Zhang, X.; Yang, D.-P.; Wang, X.; Cui, D. Graphene oxide wrapped with gold nanorods as a tag in a SERS based immunoassay for the hepatitis B surface antigen. Microchim. Acta 2018, 185, 458. [Google Scholar] [CrossRef]
- Li, Y.; Li, Z.; Ye, W.; Zhao, S.; Yang, Q.; Ma, S.; Xiao, G.; Liu, G.; Wang, Y.; Yue, Z. Gold nanorods and graphene oxide enhanced BSA-AgInS2 quantum dot-based photoelectrochemical sensors for detection of dopamine. Electrochim. Acta 2019, 295, 1006–1016. [Google Scholar] [CrossRef]











Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lebepe, T.C.; Parani, S.; Oluwafemi, O.S. Graphene Oxide-Coated Gold Nanorods: Synthesis and Applications. Nanomaterials 2020, 10, 2149. https://doi.org/10.3390/nano10112149
Lebepe TC, Parani S, Oluwafemi OS. Graphene Oxide-Coated Gold Nanorods: Synthesis and Applications. Nanomaterials. 2020; 10(11):2149. https://doi.org/10.3390/nano10112149
Chicago/Turabian StyleLebepe, Thabang C., Sundararajan Parani, and Oluwatobi S. Oluwafemi. 2020. "Graphene Oxide-Coated Gold Nanorods: Synthesis and Applications" Nanomaterials 10, no. 11: 2149. https://doi.org/10.3390/nano10112149
APA StyleLebepe, T. C., Parani, S., & Oluwafemi, O. S. (2020). Graphene Oxide-Coated Gold Nanorods: Synthesis and Applications. Nanomaterials, 10(11), 2149. https://doi.org/10.3390/nano10112149