Efficacy and Patient Tolerability Profiles of Probiotic Solution with Bisacodyl Versus Conventional Cleansing Solution for Bowel Preparation: A Prospective, Randomized, Controlled Trial
Abstract
:1. Introduction
2. Experimental Section
2.1. Institutional Ethics Review Board Approval of the Study Design
2.2. Study Population
2.3. Endoscopist Profiles
2.4. Bowel Cleansing Agent: Probiotic Solution with Bisacodyl vs. A Conventional 4-L PEG Solution
2.5. Outcome Variables: Efficacy, Safety, and Patient Tolerability Profiles
2.6. Statistics
3. Results
3.1. Baseline Characteristics
3.2. Efficacy Profiles of Probiotic Solution with Bisacodyl vs. Conventional 4-L PEG Solution
3.3. Safety Profiles of Probiotic Solution with Bisacodyl vs. Conventional 4-L PEG Solution
3.4. Patient Tolerability Profiles of Probiotic Solution with Bisacodyl vs. Conventional 4-L PEG Solution
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Data Sharing and Data Accessibility
References
- Doubeni, C.; Corley, D.; Quinn, V.P.; Jensen, C.D.; Zauber, A.G.; Goodman, M.; Johnson, J.R.; Mehta, S.J.; Becerra, T.; Zhao, W.K.; et al. Effectiveness of screening colonoscopy in reducing the risk of death from right and left colon cancer: A large community-based study. Gut 2016, 67, 291–298. [Google Scholar] [CrossRef] [PubMed]
- Meester, R.G.S.; Doubeni, C.A.; Lansdorp-Vogelaar, I.; Jensen, C.D.; Van Der Meulen, M.P.; Levin, T.R.; Quinn, V.P.; Schottinger, J.E.; Zauber, A.G.; Corley, U.A.; et al. Variation in Adenoma Detection Rate and the Lifetime Benefits and Cost of Colorectal Cancer Screening: A Microsimulation Model. JAMA 2015, 313, 2349–2358. [Google Scholar] [CrossRef] [PubMed]
- Young, P.E.; Womeldorph, C.M. Colonoscopy for Colorectal Cancer Screening. J. Cancer 2013, 4, 217–226. [Google Scholar] [CrossRef] [PubMed]
- Djinbachian, R.; Dubé, A.-J.; Durand, M.; Camara, L.R.; Panzini, B.; Bouchard, S.; Von Renteln, D. Adherence to post-polypectomy surveillance guidelines: A systematic review and meta-analysis. Endoscopy 2019, 51, 673–683. [Google Scholar] [CrossRef]
- Park, J.H.; Moon, H.S.; Kwon, I.S.; Kim, J.S.; Kang, S.H.; Lee, E.S.; Kim, S.H.; Sung, J.K.; Lee, B.S.; Jeong, H.Y. Quality of Preoperative Colonoscopy Affects Missed Postoperative Adenoma Detection in Colorectal Cancer Patients. Dig. Dis. Sci. 2019, 65, 2063–2070. [Google Scholar] [CrossRef]
- Hong, S.N.; Sung, I.K.; Kim, J.H.; Choe, W.H.; Kim, B.K.; Ko, S.Y.; Lee, J.H.; Seol, D.C.; Ahn, S.Y.; Lee, S.-Y.; et al. The Effect of the Bowel Preparation Status on the Risk of Missing Polyp and Adenoma during Screening Colonoscopy: A Tandem Colonoscopic Study. Clin. Endosc. 2012, 45, 404–411. [Google Scholar] [CrossRef]
- Wieszczy, P.; Regula, J.; Kaminski, M. Adenoma detection rate and risk of colorectal cancer. Best Pr. Res. Clin. Gastroenterol. 2017, 31, 441–446. [Google Scholar] [CrossRef]
- Jrebi, N.Y.; Hefty, M.; Jalouta, T.; Ogilvie, J.; Davis, A.T.; Asgeirsson, T.; Luchtefeld, M.A. High-definition colonoscopy increases adenoma detection rate. Surg. Endosc. 2016, 31, 78–84. [Google Scholar] [CrossRef]
- Harewood, G.C.; Sharma, V.K.; De Garmo, P. Impact of colonoscopy preparation quality on detection of suspected colonic neoplasia. Gastrointest. Endosc. 2003, 58, 76–79. [Google Scholar] [CrossRef]
- Clark, B.T.; Rustagi, T.; Laine, L. What Level of Bowel Prep Quality Requires Early Repeat Colonoscopy: Systematic Review and Meta-Analysis of the Impact of Preparation Quality on Adenoma Detection Rate. Am. J. Gastroenterol. 2014, 109, 1714–1723. [Google Scholar] [CrossRef] [Green Version]
- Millan, M.S.; Gross, P.; Manilich, E.; Church, J.M. Adenoma Detection Rate: The Real Indicator of Quality in Colonoscopy. Dis. Colon Rectum 2008, 51, 1217–1220. [Google Scholar] [CrossRef] [PubMed]
- Chokshi, R.V.; Hovis, C.E.; Hollander, T.; Early, D.; Wang, J. Prevalence of missed adenomas in patients with inadequate bowel preparation on screening colonoscopy. Gastrointest. Endosc. 2012, 75, 1197–1203. [Google Scholar] [CrossRef] [PubMed]
- Woo, D.H.; Kim, K.O.; Da Eun Jeong, Y.J.N.; Nam, Y.J.; Lee, S.H.; Jang, B.I.; Kim, T.N. Prospective analysis of factors associated with inadequate bowel preparation for colonoscopy in actual clinical practice. Intest. Res. 2018, 16, 293–298. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ness, R.M.; Manam, R.; Hoen, H.; Chalasani, N. Predictors of inadequate bowel preparation for colonoscopy. Am. J. Gastroenterol. 2001, 96, 1797–1802. [Google Scholar] [CrossRef] [PubMed]
- Hendry, P.; Jenkins, J.T.; Diament, R.H. The impact of poor bowel preparation on colonoscopy: A prospective single centre study of 10 571 colonoscopies. Color. Dis. 2007, 9, 745–748. [Google Scholar] [CrossRef] [PubMed]
- Shah, H.A.; Paszat, L.F.; Saskin, R.; Stukel, T.A.; Rabeneck, L. Factors Associated With Incomplete Colonoscopy: A Population-Based Study. Gastroenterology 2007, 132, 2297–2303. [Google Scholar] [CrossRef]
- Htet, H. New ultra low volume bowel preparation and overview of existing bowel preparations. Curr. Drug Metab. 2020, 21, 1–8. [Google Scholar] [CrossRef]
- Hong, K.H.; Lim, Y.J. Recent Update of Gastrointestinal Endoscope Reprocessing. Clin. Endosc. 2013, 46, 267–273. [Google Scholar] [CrossRef]
- Yoon, J.Y.; Cha, J.M.; Jeen, Y.T.; on behalf of Medical Policy Committee of Korean Association for the Study of Intestinal Diseases (KASID); Quality Improvement Committee of Korean Society of Gastrointestinal Endoscopy (KSGE). Quality is the Key for Emerging Issues of Population-Based Colonoscopy Screening. Clin. Endosc. 2018, 51, 50–55. [Google Scholar] [CrossRef] [Green Version]
- Abu Baker, F.; Mari, A.; Nafrin, S.; Suki, M.; Ovadia, B.; Gal, O.; Kopelamn, Y. Predictors and colonoscopy outcomes of inadequate bowel cleansing: A 10-year experience in 28,725 patients. Ann. Gastroenterol. 2019, 32, 457–462. [Google Scholar] [CrossRef]
- Min, J.K.; Cha, J.M.; Cho, Y.K.; Kim, J.-H.; Yoon, S.M.; Im, J.P.; Jung, Y.; Moon, J.S.; Kim, J.-O.; Jeen, Y.T. Revision of Quality Indicators for the Endoscopy Quality Improvement Program of the National Cancer Screening Program in Korea. Clin. Endosc. 2018, 51, 239–252. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Li, X.; Wang, Z.; Zhai, J.; Liu, Q.; Ding, K.; Pan, Y. Reinforced education improves the quality of bowel preparation for colonoscopy: An updated meta-analysis of randomized controlled trials. PLoS ONE 2020, 15, e0231888. [Google Scholar] [CrossRef] [PubMed]
- Sim, J.S.; Koo, J.S. Predictors of Inadequate Bowel Preparation and Salvage Options on Colonoscopy. Clin. Endosc. 2016, 49, 346–349. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moon, W. Optimal and Safe Bowel Preparation for Colonoscopy. Clin. Endosc. 2013, 46, 219–223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Atreja, A.; Nepal, S.; Lashner, B.A. Making the most of currently available bowel preparations for colonoscopy. Clevel. Clin. J. Med. 2010, 77, 317–326. [Google Scholar] [CrossRef] [Green Version]
- Sharma, P.; Burke, C.A.; Johnson, D.A.; Cash, B.D. The importance of colonoscopy bowel preparation for the detection of colorectal lesions and colorectal cancer prevention. Endosc. Int. Open 2020, 8, E673–E683. [Google Scholar] [CrossRef] [Green Version]
- Park, J.; Kim, T.O. How Can We Achieve Good Compliance for Bowel Preparation? Clin. Endosc. 2019, 52, 3–4. [Google Scholar] [CrossRef] [Green Version]
- Yeh, J.-H.; Hsu, M.-H.; Tseng, C.-M.; Chen, T.-H.; Huang, R.-Y.; Lee, C.-T.; Lin, C.-W.; Wang, W.-L. The benefit of adding oral simethicone in bowel preparation regimen for the detection of colon adenoma: A systematic review and meta-analysis. J. Gastroenterol. Hepatol. 2018, 34, 830–836. [Google Scholar] [CrossRef]
- De Leone, A.; Tamayo, D.; Fiori, G.; Ravizza, D.; Trovato, C.; De Roberto, G.; Fazzini, L.; Fante, M.D.; Crosta, C. Same-day 2-L PEG-citrate-simethicone plus bisacodylvssplit 4-L PEG: Bowel cleansing for late-morning colonoscopy. World J. Gastrointest. Endosc. 2013, 5, 433–439. [Google Scholar] [CrossRef]
- Castro, F.J.; Al-Khairi, B.; Singh, H.; Mohameden, M.; Tandon, K.; Lopez, R. Randomized Controlled Trial: Split-dose and same-day large volume bowel preparation for afternoon colonoscopy have similar quality of preparation. J. Clin. Gastroenterol. 2019, 53, 724–730. [Google Scholar] [CrossRef]
- Huh, J.-G.; Kim, Y.-S.; Park, J.-H.; Ok, K.-S.; Jang, W.-C.; Jeong, T.-Y.; Ryu, S.-H.; Lee, J.-H.; Moon, J.-S. A prospective comparison of sulfate free polyethylene glycol versus sodium phosphate solution for precolonoscopic bowel preparation. Clin. Endosc. 2009, 39, 265–270. [Google Scholar]
- Clark, R.E.; Godfrey, J.D.; Choudhary, A.; Ashraf, I.; Matteson, M.L.; Bechtold, M.L. Low-volume polyethylene glycol and bisacodyl for bowel preparation prior to colonoscopy: A meta-analysis. Ann. Gastroenterol. 2013, 26, 319–324. [Google Scholar] [CrossRef] [PubMed]
- Corporaal, S.; Kleibeuker, J.H.; Koornstra, J.J. Low-volume PEG plus ascorbic acid versus high-volume PEG as bowel preparation for colonoscopy. Scand. J. Gastroenterol. 2010, 45, 1380–1386. [Google Scholar] [CrossRef] [PubMed]
- Park, S.-W.; Shin, S.-P.; Hong, J.T. Efficacy and Tolerability of Prucalopride in Bowel Preparation for Colonoscopy: A Systematic Review and Meta-Analysis. Adv. Ther. 2020, 37, 2507–2519. [Google Scholar] [CrossRef] [PubMed]
- Allegretti, A.; Carulla, D.B.; Bertelè, A.; Franzèc, A.; Kouroumalis, E.; Mancini, M.; Paspatis, G.; Reina-Serrano, S. Efficacy and safety of bowel cleansing solutions for colonoscopy: A prospective observational study. Eur. Rev. Med. Pharmacol. Sci. 2010, 14, 979–985. [Google Scholar]
- Hookey, L.; Bertiger, G.; Johnson, K.L.; Ayala, J.; Seifu, Y.; Brogadir, S.P. Efficacy and safety of a ready-to-drink bowel preparation for colonoscopy: A randomized, controlled, non-inferiority trial. Ther. Adv. Gastroenterol. 2019, 12, 1756284819851510. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chung, K.-W.; Park, J.-I.; Park, J.-H.; Hwang, D.-Y.; Lee, H.-H.; Lee, D.-S.; Kwack, D.-H.; Seo, J.-Y.; Jung, J.-J.; Lee, Y.-H.; et al. Effectiveness of single-dose sodium phosphate on bowel preparation. Clin. Endosc. 2003, 27, 515–520. [Google Scholar]
- Rodríguez-Fragoso, L.; Sandoval-Ocampo, A.; Corbalá-Nava, M.; Arjona-Canul, C.A.; Gomez-Galicia, D.L.; Tellez, G.; Hargis, B.; Reyes-Esparza, J.; Lourdes, R.-F.; Adriana, S.-O.; et al. Evaluation Regarding the Efficacy and Safety of a Probiotic Mixture in Healthy Volunteers with Evacuation Disorders. Food Nutr. Sci. 2012, 3, 117–122. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.; Kim, Y.-H.; Kim, J.H.; Chang, N.K.; Kim, J.Y.; Son, H.J.; Rhee, P.-L.; Kim, J.J.; Rhee, J.C. A Feasibility Study of Probiotics Pretreatment as a Bowel Preparation for Colonoscopy in Constipated Patients. Dig. Dis. Sci. 2009, 55, 2344–2351. [Google Scholar] [CrossRef]
- Picard, C.; Fioramonti, J.; Francois, A.; Robinson, T.; Neant, F.; Matuchansky, C. Bifidobacteria as probiotic agents–physiological effects and clinical benefits. Aliment. Pharmacol. Ther. 2005, 22, 495–512. [Google Scholar] [CrossRef]
- Kastenberg, D.; Bertiger, G.; Brogadir, S. Bowel preparation quality scales for colonoscopy. World J. Gastroenterol. 2018, 24, 2833–2843. [Google Scholar] [CrossRef] [PubMed]
- Francis, D.L.; Rodriguez-Correa, D.T.; Buchner, A.; Harewood, G.C.; Wallace, M. Application of a conversion factor to estimate the adenoma detection rate from the polyp detection rate. Gastrointest. Endosc. 2011, 73, 493–497. [Google Scholar] [CrossRef] [PubMed]
- Corley, D.A.; Jensen, C.D.; Marks, A.R.; Zhao, W.K.; Lee, J.K.; Doubeni, C.A.; Zauber, A.G.; De Boer, J.; Fireman, B.H.; Schottinger, J.E.; et al. Adenoma Detection Rate and Risk of Colorectal Cancer and Death. N. Engl. J. Med. 2014, 370, 1298–1306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barclay, R.L.; Vicari, J.J.; Doughty, A.S.; Johanson, J.F.; Greenlaw, R.L. Colonoscopic Withdrawal Times and Adenoma Detection during Screening Colonoscopy. N. Engl. J. Med. 2006, 355, 2533–2541. [Google Scholar] [CrossRef] [Green Version]
- Chmielewska, A.; Szajewska, H. Systematic review of randomised controlled trials: Probiotics for functional constipation. World J. Gastroenterol. 2010, 16, 69–75. [Google Scholar]
- Floch, M.H. The Power of Poop. J. Clin. Gastroenterol. 2012, 46, 625–626. [Google Scholar] [CrossRef] [PubMed]
- Sanders, M.E.; Akkermans, L.M.; Haller, D.; Hammerman, C.; Heimbach, J.T.; Hörmannsperger, G.; Huys, G. Safety assessment of probiotics for human use. Gut Microbes 2010, 1, 164–185. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Yang, Z.; Chen, H.-Q.; Qin, H.-L. Effect of bowel preparation with probiotics on intestinal barrier after surgery for colorectal cancer. Zhonghua Wei Chang. Wai Ke Za Zhi Chin. J. Gastrointest. Surg. 2010, 13, 528–531. [Google Scholar]
- Adams, W.J.; Meagher, A.P.; Lubowski, D.Z.; King, D.W. Bisacodyl reduces the volume of polyethylene glycol solution required for bowel preparation. Dis. Colon Rectum 1994, 37, 229–234. [Google Scholar] [CrossRef]
- Kim, J.-W.; Choi, M.-G.; Kim, J.-O.; Jang, J.-Y.; the Quality Management Committee of Korean Society of Gastrointestinal Endoscopy. Accredited Endoscopy Unit Program of Korea: Overview and Qualification. Clin. Endosc. 2019, 52, 426–430. [Google Scholar] [CrossRef]
- Lee, T.H.; Yoon, J.Y.; Paik, C.N.; Choi, H.S.; Jang, J.Y.; The Quality Management Committee of The Korean Society of Gastrointestinal Endoscopy. Updates on the Facilities, Procedures, and Performance of the Accredited Endoscopy Unit. Clin. Endosc. 2019, 52, 431–442. [Google Scholar] [CrossRef] [PubMed]
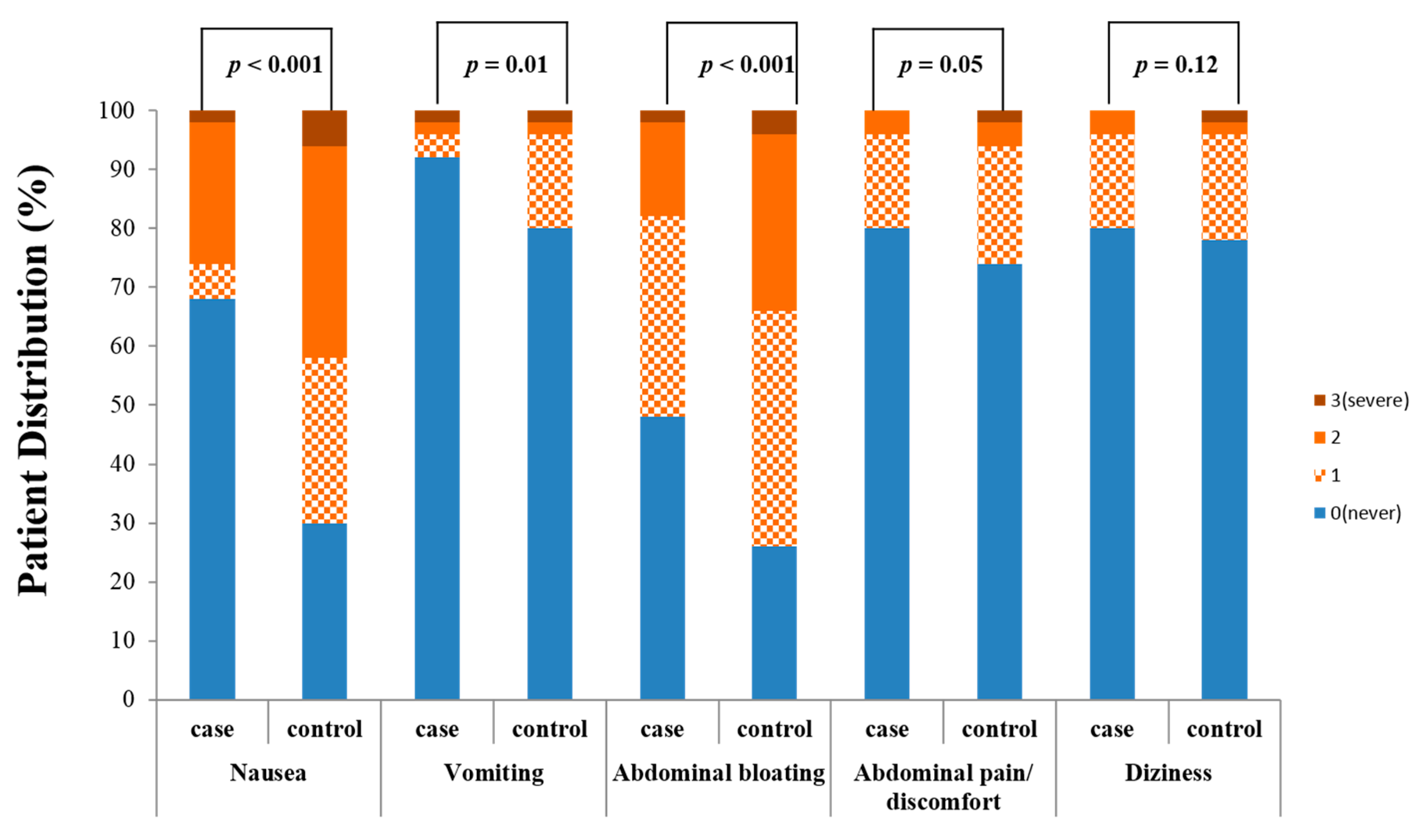
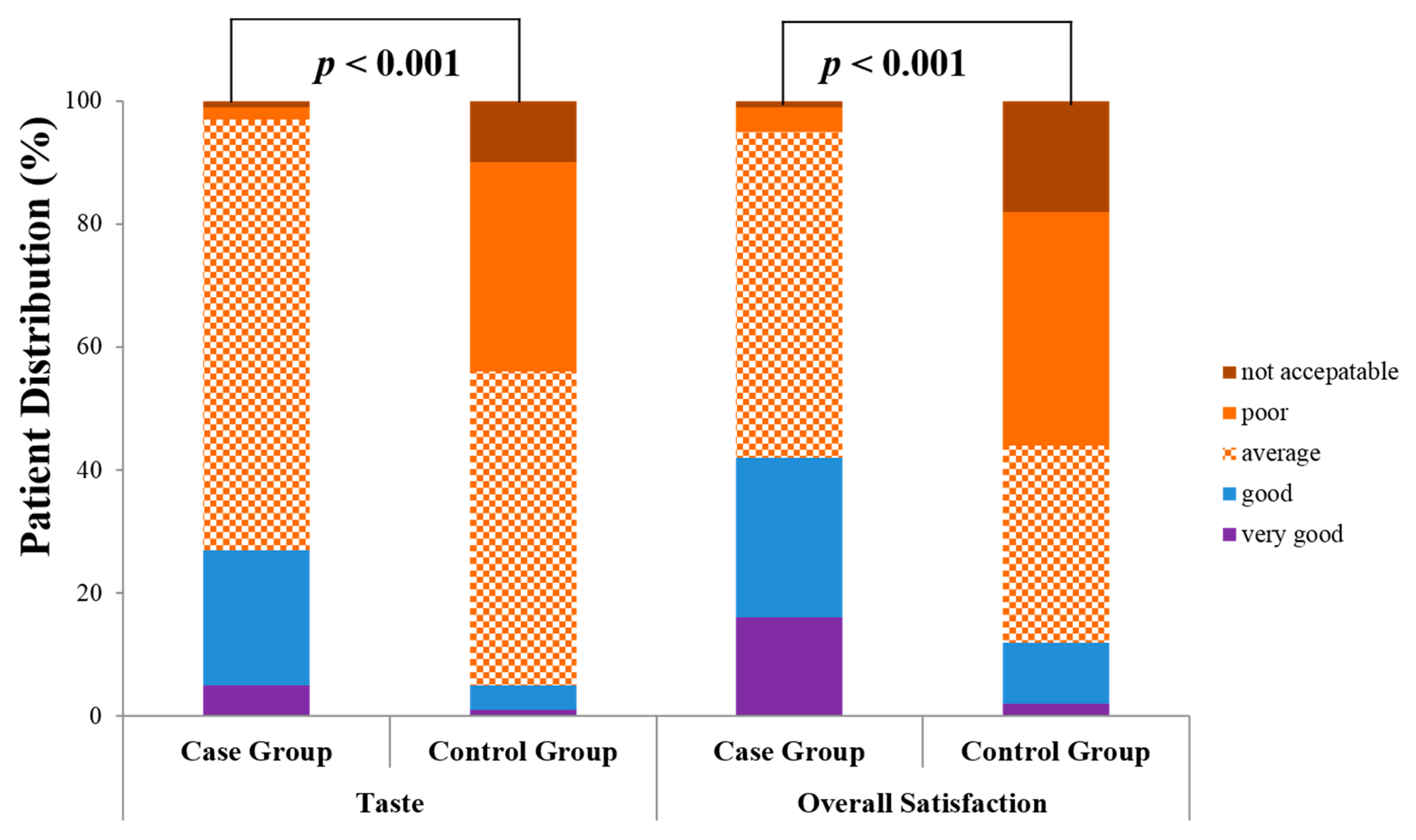
| Case Group (Bisacodyl + Probiotic Solution Group, n = 195) | Control Group (PEG Solution Group, n = 190) | p Value | |
|---|---|---|---|
| Age (year) | 47.94 ± 9.86 | 49.03 ± 9.86 | 0.28 |
| Height (cm) | 166.05 ± 8.63 | 165.78 ± 8.25 | 0.75 |
| Weight (kg) | 67.32 ± 12.01 | 66.80 ± 11.87 | 0.67 |
| BMI (kg/m2) | 24.30 ± 3.08 | 24.17 ± 2.99 | 0.68 |
| Ottawa Scale Score | Case Group (Bisacodyl with Probiotics Group, n = 195) | Control Group (PEG Group, n = 190) | p Value | |
|---|---|---|---|---|
| Total Ottawa score | 7.67 ± 2.78 | 6.42 ± 2.58 | 0.01 | |
| Right sided colon, n (%) | Adequate | 170 (87.18%) | 166 (87.37%) | 0.91 |
| poor | 25 (12.82%) | 24 (12.63%) | ||
| Descending colon, n (%) | Adequate | 175 (89.74%) | 181 (95.26%) | 0.43 |
| poor | 20 (10.26%) | 9 (4.74%) | ||
| Recto-sigmoid colon, n (%) | Adequate | 178 (91.28%) | 185 (97.37%) | 0.51 |
| poor | 17 (8.72%) | 5 (2.63%) | ||
| Fluid, n (%) | Adequate | 144 (73.68%) | 121 (63.59%) | 0.36 |
| poor | 51 (26.32%) | 69 (36.41%) |
| Case Group (Probiotics Group, n = 195) | Control Group (PEG Group, n = 190) | p Value | |
|---|---|---|---|
| Polyp detection rate, n (%) | 32.82% | 38.42% | 0.30 |
| Adenoma detection rate, n (%) | 18.97% | 25.79% | 0.11 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, Y.I.; Lee, J.-J.; Chung, J.-W.; Kim, K.O.; Kim, Y.J.; Kim, J.H.; Park, D.K.; Kwon, K.A. Efficacy and Patient Tolerability Profiles of Probiotic Solution with Bisacodyl Versus Conventional Cleansing Solution for Bowel Preparation: A Prospective, Randomized, Controlled Trial. J. Clin. Med. 2020, 9, 3286. https://doi.org/10.3390/jcm9103286
Choi YI, Lee J-J, Chung J-W, Kim KO, Kim YJ, Kim JH, Park DK, Kwon KA. Efficacy and Patient Tolerability Profiles of Probiotic Solution with Bisacodyl Versus Conventional Cleansing Solution for Bowel Preparation: A Prospective, Randomized, Controlled Trial. Journal of Clinical Medicine. 2020; 9(10):3286. https://doi.org/10.3390/jcm9103286
Chicago/Turabian StyleChoi, Youn I, Jong-Joon Lee, Jun-Won Chung, Kyoung Oh Kim, Yoon Jae Kim, Jung Ho Kim, Dong Kyun Park, and Kwang An Kwon. 2020. "Efficacy and Patient Tolerability Profiles of Probiotic Solution with Bisacodyl Versus Conventional Cleansing Solution for Bowel Preparation: A Prospective, Randomized, Controlled Trial" Journal of Clinical Medicine 9, no. 10: 3286. https://doi.org/10.3390/jcm9103286





