An Update on the Role of Dietary Phytochemicals in Human Skin Cancer: New Insights into Molecular Mechanisms
Abstract
:1. Introduction
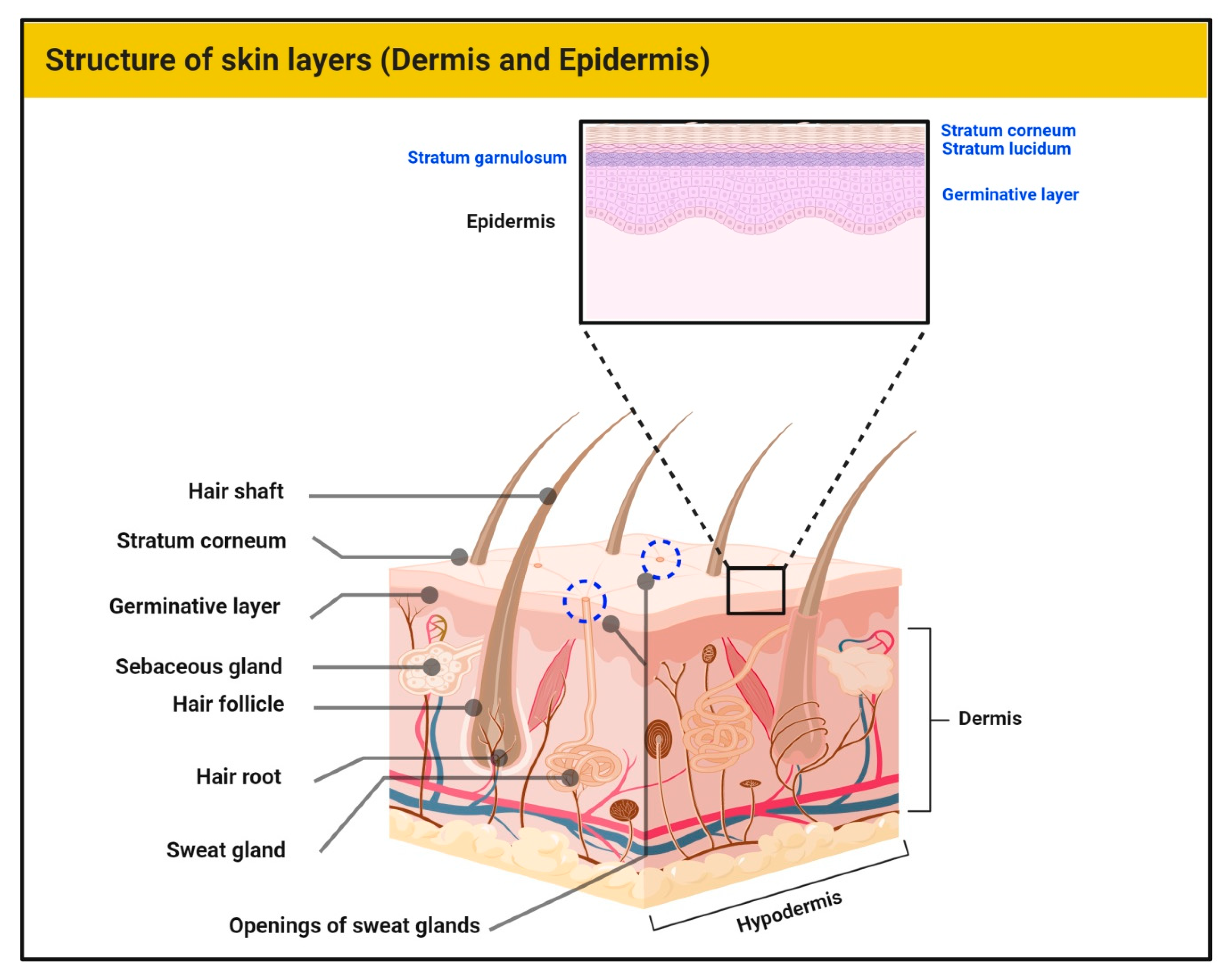
1.1. Structure of Skin
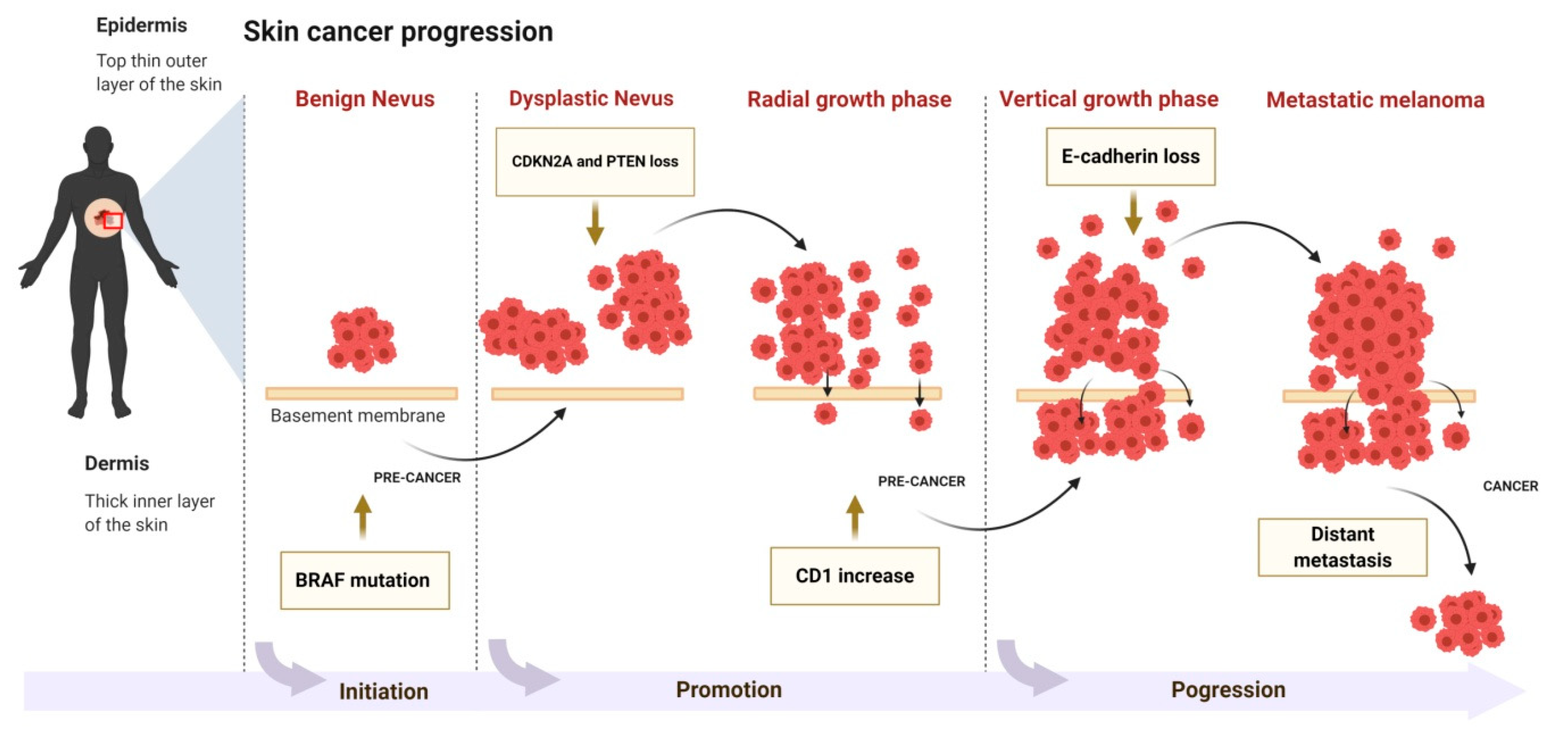
1.2. Skin Carcinogenesis
2. Dietary Phytochemicals for Skin Cancer Therapy
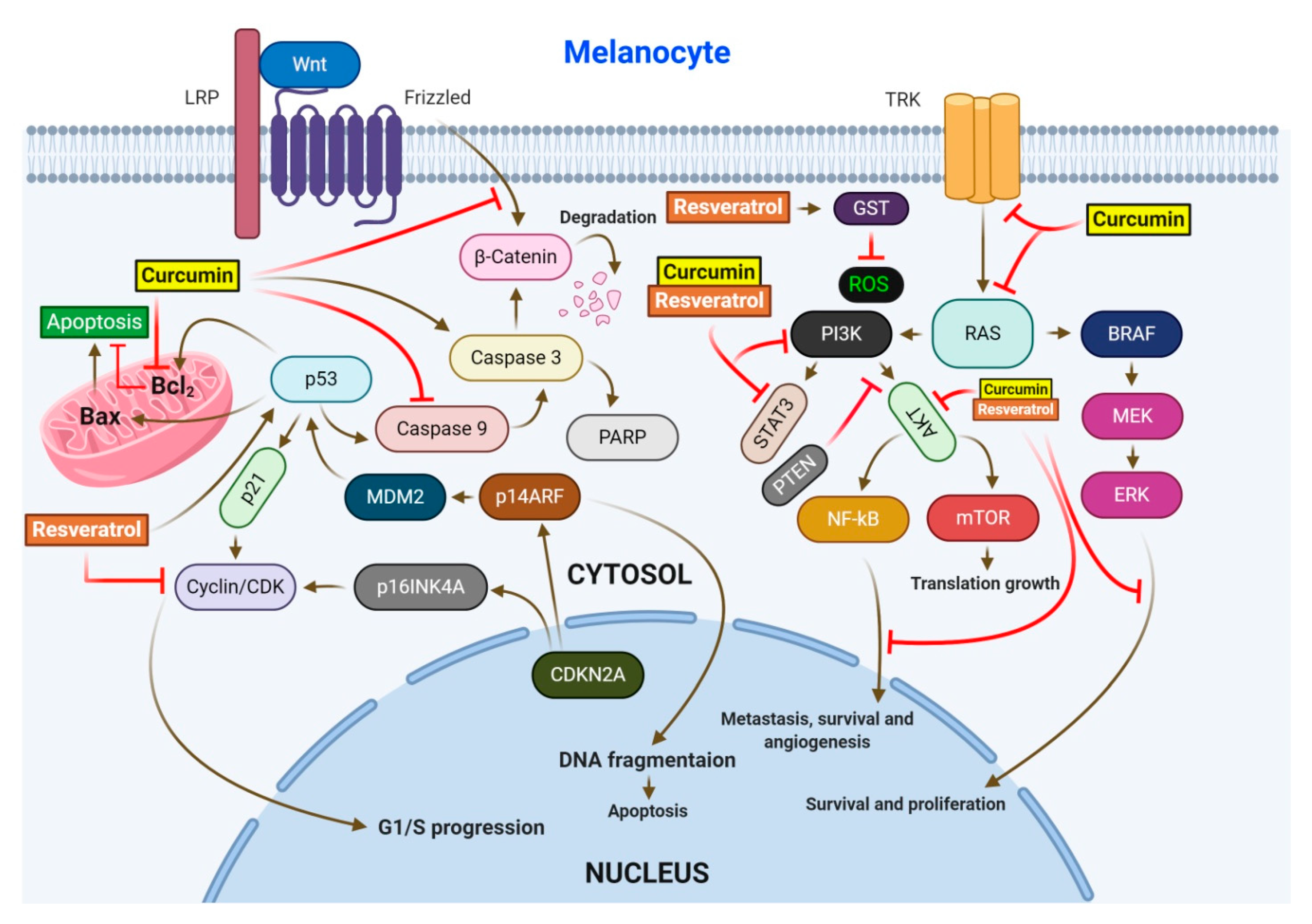
2.1. Resveratrol (RV)
2.2. Curcumin
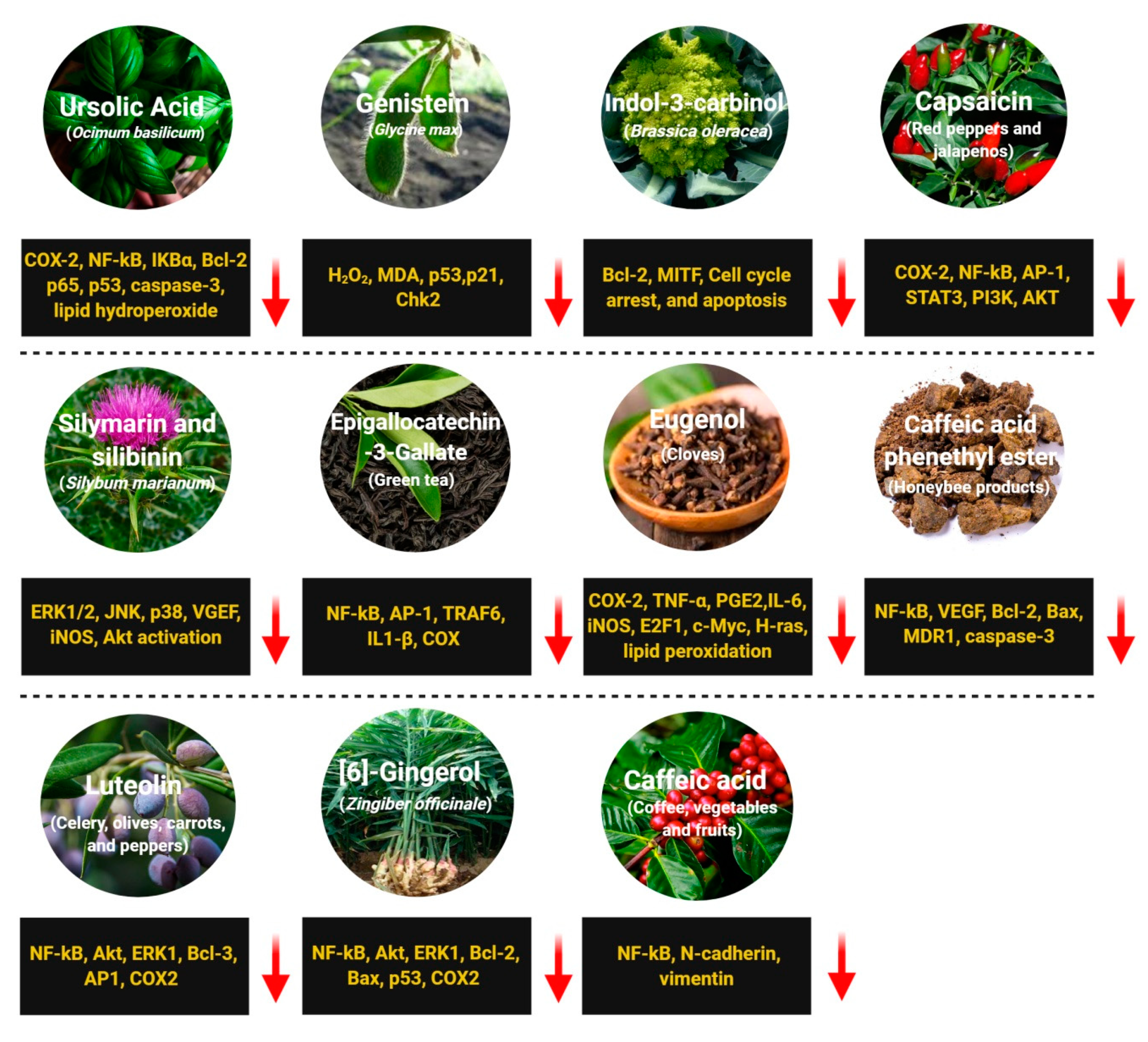
2.3. Ursolic Acid
2.4. Genistein
2.5. Indole-3-Carbinol (I3C)
2.6. Capsaicin
2.7. Silymarin and Silibinin
2.8. Epigallocatechin-3-Gallate (EGCG)
2.9. Eugenol
2.10. Caffeic Acid Phenethyl Ester (CAPE)
2.11. Luteolin
2.12. [6]-Gingerol
2.13. Caffeic Acid (CA)
3. Role of Whole Fruits and Vegetables in Skin Cancer Prevention
3.1. Apple (Malus pumila)
3.2. Pomegranate (Punica granatum)
3.3. Tomato (Solanum lycopersicum)
3.4. Grape (Vitis vinifera)
4. Limitation, Safety Consideration, and Future Prospects of Dietary Phytochemicals
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| 4EBP | Eukaryotic initiation factor 4E (eIF4E)–binding proteins |
| 5LOX | 5-lipoxygenase |
| 8-oxodG | 8-Oxo-2′-deoxyguanosine |
| A375 | Human melanoma cell line |
| A431 | Human epidermoid carcinoma cell line |
| AIN-93G | Mice diet |
| AK | Actinic keratosis |
| Akt | Protein kinase B |
| AP-1 | Activator protein 1 |
| AQP3 | Aquaporin 3 |
| Bax | Bcl-2-associated X protein |
| BCC | Basal cell carcinoma |
| Bcl2 | B-cell lymphoma 2protein |
| Bcl-3 | B-cell lymphoma 3-encoded protein |
| CAPE | Caffeic acid phenethyl ester |
| CD4 | Cluster of differentiation 4 |
| CDK4 | Cyclin-dependent kinase 4 |
| CDKN2 | Cyclin-dependent kinase inhibitor 2A |
| CDKs | Cyclin-dependent kinases |
| COX-1 | Cyclooxygenase 1 |
| COX-2 | Cyclooxygenase 2 |
| CPD | Carboxypeptidase D |
| CRP | C-Reactive Protein |
| CycD1 | Cyclin D1 |
| DMBA | 7,12-Dimethylbenz[a]anthracene |
| E2F1 | Transcription factor E2F1 |
| E-cadherin | Epithelial cadherin |
| EGCG | Epigallocatechin gallate |
| EGFR | Epidermal growth factor receptor |
| ERK | Extracellular signal-regulated kinase |
| GIT | Gastrointestinal tract |
| GP | Grape powder |
| GPx | Glutathione peroxidase |
| GST | Glutathione S-transferase |
| HA14-1 | Ethyl-2-amino-6-bromo-4-(1-cyano-2-ethoxy-2-oxoethyl)-4H-chromene-3-carboxylate |
| HaCaT | Aneuploid immortal keratinocyte cell line |
| H-ras | Transforming protein p21 |
| I3C | Indole-3-carbinol |
| IL-1β | Interleukin-1 beta |
| IL-6 | Interleukin-6 |
| ILGF-1 | Insulin-like growth factor 1 |
| iNOS | Inducible nitric oxide synthase |
| IRS-1 | Insulin receptor substrate 1 |
| J/m2 | Joule per square metre |
| JAK-2 | Janus Kinase 2 |
| JNK | c-Jun N-terminal kinase |
| LRP | Lipoprotein receptor-related proteins |
| MAPK | Mitogen-activated protein kinase |
| MDA | 3,4-Methylene dioxy amphetamine |
| MDR-1 | Multidrug resistance protein 1 |
| MEK | Mitogen-activated protein kinase kinase |
| miRNA | microRNA |
| MITF | Microphthalmia-associated transcription factor |
| MMP-9 | Matrix metallopeptidase 9 |
| MPO | Myeloperoxidase |
| mPTP | Mitochondrial permeability transition pore |
| mTOR | The mammalian target of rapamycin |
| N-cadherin | Neural cadherin |
| NER | Nucleotide excision repair |
| NF-κB | Nuclear factor kappa B |
| NHEK | Normal human epidermal keratinocytes |
| NMSCs | Nonmelanoma skin cancers |
| ODC | Ornithine decarboxylase |
| p21 | Cyclin-dependent kinase inhibitor 1 or CDK-interacting protein 1 |
| p38 | p38 mitogen-activated protein kinase |
| p53 | TP53 or tumor protein |
| p65 | nuclear factor NF-kappa-B p65 subunit |
| PARP | Poly (ADP-ribose) polymerase |
| PCNA | Proliferating cell nuclear antigen |
| PFE | Pomegranate fruit extract |
| PGE2 | Prostaglandin E2 |
| PI3-K | Phosphoinositide 3-kinase |
| PKB | Protein kinase B, also known as Akt |
| ROS | Reactive oxygen species |
| RV | Resvertrol |
| SCC | Squamous cell carcinoma |
| SOD | Superoxide dismutase |
| STAT3 | Signal transducer and activator of transcription 3 |
| TGF-β | Transforming growth factor beta 1 |
| TPA | 12-O-tetradecanoylphorbol-13-acetate |
| TRAF6 | Tumor necrosis factor receptor (TNFR)-associated factor 6 |
| TRK | Tyrosine kinases |
| UA | Ursolic acid |
| UV | Ultraviolet |
| UVA | Ultraviolet A |
| UVB | Ultraviolet B |
| VEGF | Vascular endothelial growth factor |
| WNT | Wingless-related integration site |
References
- Brohem, C.A.; da Silva Cardeal, L.B.; Tiago, M.; Soengas, M.S.; de Moraes Barros, S.B.; Maria-Engler, S.S. Artificial skin in perspective: Concepts and applications. Pigment. Cell Melanoma Res. 2011, 24, 35–50. [Google Scholar] [CrossRef] [Green Version]
- Kumari, S.; Pasparakis, M. Epithelial cell death and inflammation in skin. In Apoptotic and Non-Apoptotic Cell Death; Springer: Cham, Switzerland, 2015; pp. 77–93. [Google Scholar]
- Fuchs, E.; Raghavan, S. Getting under the skin of epidermal morphogenesis. Nat. Rev. Genet. 2002, 3, 199–209. [Google Scholar] [CrossRef]
- Apalla, Z.; Nashan, D.; Weller, R.B.; Castellsagué, X. Skin cancer: Epidemiology, disease burden, pathophysiology, diagnosis, and therapeutic approaches. Dermatol. Ther. 2017, 7, 5–19. [Google Scholar] [CrossRef] [Green Version]
- Bickers, D.R.; Athar, M. Oxidative stress in the pathogenesis of skin disease. J. Investig. Dermatol. 2006, 126, 2565–2575. [Google Scholar] [CrossRef] [Green Version]
- Pitot, H.C.; Dragan, Y.P. Facts and theories concerning the mechanisms of carcinogenesis. FASEB J. 1991, 5, 2280–2286. [Google Scholar] [CrossRef]
- Benjamin, C.L.; Ananthaswamy, H.N. p53 and the pathogenesis of skin cancer. Toxicol. Appl. Pharmacol. 2007, 224, 241–248. [Google Scholar] [CrossRef] [Green Version]
- Agar, N.S.; Halliday, G.M.; Barnetson, R.S.; Ananthaswamy, H.N.; Wheeler, M.; Jones, A.M. The basal layer in human squamous tumors harbors more UVA than UVB fingerprint mutations: A role for UVA in human skin carcinogenesis. Proc. Natl. Acad. Sci. USA 2004, 101, 4954–4959. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rebel, H.; Mosnier, L.O.; Berg, R.J.; Westerman-de Vries, A.; van Steeg, H.; van Kranen, H.J.; de Gruijl, F.R. Early p53-positive foci as indicators of tumor risk in ultraviolet-exposed hairless mice: Kinetics of induction, effects of DNA repair deficiency, and p53 heterozygosity. Cancer Res. 2001, 61, 977–983. [Google Scholar] [PubMed]
- Ra, S.H.; Li, X.; Binder, S. Molecular discrimination of cutaneous squamous cell carcinoma from actinic keratosis and normal skin. Mod. Pathol. 2011, 24, 963–973. [Google Scholar] [CrossRef] [PubMed]
- Rogers, H.W.; Weinstock, M.A.; Feldman, S.R.; Coldiron, B.M. Incidence estimate of nonmelanoma skin cancer (keratinocyte carcinomas) in the US population, 2012. JAMA Dermatol. 2015, 151, 1081–1086. [Google Scholar] [CrossRef]
- Christenson, L.J.; Borrowman, T.A.; Vachon, C.M.; Tollefson, M.M.; Otley, C.C.; Weaver, A.L.; Roenigk, R.K. Incidence of basal cell and squamous cell carcinomas in a population younger than 40 years. JAMA 2005, 294, 681–690. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, S.; Han, J.; Li, W.-Q.; Li, T.; Qureshi, A.A. Basal-cell carcinoma incidence and associated risk factors in US women and men. Am. J. Epidemiol. 2013, 178, 890–897. [Google Scholar] [CrossRef] [Green Version]
- Guy, G.P., Jr.; Machlin, S.R.; Ekwueme, D.U.; Yabroff, K.R. Prevalence and costs of skin cancer treatment in the US, 2002−2006 and 2007−2011. Am. J. Prev. Med. 2015, 48, 183–187. [Google Scholar] [CrossRef] [PubMed]
- Rees, J.R.; Zens, M.S.; Celaya, M.O.; Riddle, B.L.; Karagas, M.R.; Peacock, J.L. Survival after squamous cell and basal cell carcinoma of the skin: A retrospective cohort analysis. Int. J. Cancer 2015, 137, 878–884. [Google Scholar] [CrossRef] [PubMed]
- Jensen, A.Ø.; Lamberg, A.L.; Jacobsen, J.B.; Olesen, A.B.; Sørensen, H.T. Non-melanoma skin cancer and ten-year all-cause mortality: A population-based cohort study. Acta Derm. Venereol. 2010, 90, 362–367. [Google Scholar] [CrossRef] [Green Version]
- Epstein, E.H. Basal cell carcinomas: Attack of the hedgehog. Nat. Rev. Cancer 2008, 8, 743–754. [Google Scholar] [CrossRef]
- Alam, M.; Armstrong, A.; Baum, C.; Bordeaux, J.S.; Brown, M.; Busam, K.J.; Eisen, D.B.; Iyengar, V.; Lober, C.; Margolis, D.J. Guidelines of care for the management of cutaneous squamous cell carcinoma. J. Am. Acad. Dermatol. 2018, 78, 560–578. [Google Scholar]
- Brantsch, K.D.; Meisner, C.; Schönfisch, B.; Trilling, B.; Wehner-Caroli, J.; Röcken, M.; Breuninger, H. Analysis of risk factors determining prognosis of cutaneous squamous-cell carcinoma: A prospective study. Lancet Oncol. 2008, 9, 713–720. [Google Scholar] [CrossRef]
- Cooper, J.Z.; Brown, M.D. Special concern about squamous cell carcinoma of the scalp in organ transplant recipients. Arch. Dermatol. 2006, 142, 755–758. [Google Scholar] [CrossRef] [Green Version]
- Madan, V.; Lear, J.T.; Szeimies, R.-M. Non-melanoma skin cancer. Lancet 2010, 375, 673–685. [Google Scholar] [CrossRef] [Green Version]
- Werlinger, K.D.; Upton, G.; Moore, A.Y. Recurrence rates of primary nonmelanoma skin cancers treated by surgical excision compared to electrodesiccation-curettage in a private dermatological practice. Dermatol. Surg. 2002, 28, 1138–1142. [Google Scholar]
- Krzyzanowska, J.; Czubacka, A.; Oleszek, W. Dietary phytochemicals and human health. In Bio-Farms for Nutraceuticals; Springer: Boston, MA, USA, 2010; pp. 74–98. [Google Scholar]
- Probst, Y.C.; Guan, V.X.; Kent, K. Dietary phytochemical intake from foods and health outcomes: A systematic review protocol and preliminary scoping. BMJ Open 2017, 7. [Google Scholar] [CrossRef] [PubMed]
- Bansal, M.; Singh, N.; Pal, S.; Dev, I.; Ansari, K.M. Chemopreventive role of dietary phytochemicals in colorectal cancer. In Advances in Molecular Toxicology; Fishbein, J.C., Heilman, J.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; Volume 12, pp. 69–121. [Google Scholar]
- Forni, C.; Facchiano, F.; Bartoli, M.; Pieretti, S.; Facchiano, A.; D’Arcangelo, D.; Norelli, S.; Valle, G.; Nisini, R.; Beninati, S. Beneficial role of phytochemicals on oxidative stress and age-related diseases. BioMed Res. Int. 2019, 2019. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aggarwal, B.B.; Bhardwaj, A.; Aggarwal, R.S.; Seeram, N.P.; Shishodia, S.; Takada, Y. Role of resveratrol in prevention and therapy of cancer: Preclinical and clinical studies. Anticancer. Res. 2004, 24, 2783–2840. [Google Scholar] [PubMed]
- Islam, S.U.; Ahmed, M.B.; Ul-Islam, M.; Shehzad, A.; Lee, Y.S. Switching from Conventional to Nano-natural Phytochemicals to Prevent and Treat Cancers: Special Emphasis on Resveratrol. Curr. Pharm. Des. 2019, 25, 3620–3632. [Google Scholar] [CrossRef]
- Dong, Z. Molecular mechanism of the chemopreventive effect of resveratrol. Mutat. Res. Fundam. Mol. Mech. Mutagenesis 2003, 523, 145–150. [Google Scholar] [CrossRef]
- Brisdelli, F.; D’Andrea, G.; Bozzi, A. Resveratrol: A natural polyphenol with multiple chemopreventive properties. Curr. Drug Metab. 2009, 10, 530–546. [Google Scholar] [CrossRef]
- Aziz, M.H.; Reagan-Shaw, S.; Wu, J.; Longley, B.J.; Ahmad, N. Chemoprevention of skin cancer by grape constituent resveratrol: Relevance to human disease? FASEB J. 2005, 19, 1193–1195. [Google Scholar] [CrossRef] [Green Version]
- Ndiaye, M.; Philippe, C.; Mukhtar, H.; Ahmad, N. The grape antioxidant resveratrol for skin disorders: Promise, prospects, and challenges. Arch. Biochem. Biophys. 2011, 508, 164–170. [Google Scholar] [CrossRef] [Green Version]
- Kowalczyk, M.C.; Walaszek, Z.; Kowalczyk, P.; Kinjo, T.; Hanausek, M.; Slaga, T.J. Differential effects of several phytochemicals and their derivatives on murine keratinocytes in vitro and in vivo: Implications for skin cancer prevention. Carcinogenesis 2009, 30, 1008–1015. [Google Scholar] [CrossRef] [Green Version]
- Jagdeo, J.; Adams, L.; Lev-Tov, H.; Sieminska, J.; Michl, J.; Brody, N. Dose-dependent antioxidant function of resveratrol demonstrated via modulation of reactive oxygen species in normal human skin fibroblasts in vitro. J. Drugs Dermatol. 2010, 9, 1523–1526. [Google Scholar] [PubMed]
- Kundu, J.K.; Shin, Y.K.; Kim, S.H.; Surh, Y.-J. Resveratrol inhibits phorbol ester-induced expression of COX-2 and activation of NF-κB in mouse skin by blocking IκB kinase activity. Carcinogenesis 2006, 27, 1465–1474. [Google Scholar] [CrossRef] [PubMed]
- Niles, R.M.; Cook, C.P.; Meadows, G.G.; Fu, Y.-M.; McLaughlin, J.L.; Rankin, G.O. Resveratrol is rapidly metabolized in athymic (nu/nu) mice and does not inhibit human melanoma xenograft tumor growth. J. Nutr. 2006, 136, 2542–2546. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reagan-Shaw, S.; Afaq, F.; Aziz, M.H.; Ahmad, N. Modulations of critical cell cycle regulatory events during chemoprevention of ultraviolet B-mediated responses by resveratrol in SKH-1 hairless mouse skin. Oncogene 2004, 23, 5151–5160. [Google Scholar] [CrossRef] [Green Version]
- Wu, Z.; Uchi, H.; Morino-Koga, S.; Shi, W.; Furue, M. Resveratrol inhibition of human keratinocyte proliferation via SIRT1/ARNT/ERK dependent downregulation of aquaporin 3. J. Dermatol. Sci. 2014, 75, 16–23. [Google Scholar] [CrossRef]
- Osmond, G.W.; Augustine, C.K.; Zipfel, P.A.; Padussis, J.; Tyler, D.S. Enhancing melanoma treatment with resveratrol. J. Surg. Res. 2012, 172, 109–115. [Google Scholar] [CrossRef]
- Bhattacharya, S.; Darjatmoko, S.R.; Polans, A.S. Resveratrol modulates the malignant properties of cutaneous melanoma via changes in the activation and attenuation of the anti-apoptotic proto-oncogenic protein Akt/PKB. Melanoma Res. 2011, 21, 180. [Google Scholar] [CrossRef] [Green Version]
- Farris, P.; Yatskayer, M.; Chen, N.; Krol, Y.; Oresajo, C. Evaluation of efficacy and tolerance of a nighttime topical antioxidant containing resveratrol, baicalin, and vitamin e for treatment of mild to moderately photodamaged skin. J. Drugs Dermatol. JDD 2014, 13, 1467. [Google Scholar]
- Boo, Y.C. Human Skin Lightening Efficacy of Resveratrol and Its Analogs: From in Vitro Studies to Cosmetic Applications. Antioxidants 2019, 8, 332. [Google Scholar] [CrossRef] [Green Version]
- Islam, S.U.; Shehzad, A.; Sonn, J.K.; Lee, Y.S. PRPF overexpression induces drug resistance through actin cytoskeleton rearrangement and epithelial-mesenchymal transition. Oncotarget 2017, 8, 56659. [Google Scholar] [CrossRef] [Green Version]
- Gupta, S.C.; Patchva, S.; Koh, W.; Aggarwal, B.B. Discovery of curcumin, a component of golden spice, and its miraculous biological activities. Clin. Exp. Pharmacol. Physiol. 2012, 39, 283–299. [Google Scholar] [CrossRef] [PubMed]
- Dahmke, I.N.; Backes, C.; Rudzitis-Auth, J.; Laschke, M.W.; Leidinger, P.; Menger, M.D.; Meese, E.; Mahlknecht, U. Curcumin intake affects miRNA signature in murine melanoma with mmu-miR-205-5p most significantly altered. PLoS ONE 2013, 8, e81122. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.C.; Patchva, S.; Aggarwal, B.B. Therapeutic roles of curcumin: Lessons learned from clinical trials. AAPS J. 2013, 15, 195–218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santana-Martínez, R.A.; Silva-Islas, C.A.; Fernández-Orihuela, Y.Y.; Barrera-Oviedo, D.; Pedraza-Chaverri, J.; Hernández-Pando, R.; Maldonado, P.D. The therapeutic effect of curcumin in quinolinic acid-induced neurotoxicity in rats is associated with BDNF, ERK1/2, Nrf2, and antioxidant enzymes. Antioxidants 2019, 8, 388. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paunkov, A.; Chartoumpekis, D.V.; Ziros, P.G.; Sykiotis, G.P. A bibliometric review of the Keap1/Nrf2 pathway and its related antioxidant compounds. Antioxidants 2019, 8, 353. [Google Scholar] [CrossRef] [Green Version]
- Harmand, P.-O.; Duval, R.; Liagre, B.; Jayat-Vignoles, C.; Beneytout, J.-L.; Delage, C.; Simon, A. Ursolic acid induces apoptosis through caspase-3 activation and cell cycle arrest in HaCat cells. Int. J. Oncol. 2003, 23, 105–112. [Google Scholar] [CrossRef]
- Shishodia, S.; Majumdar, S.; Banerjee, S.; Aggarwal, B.B. Ursolic acid inhibits nuclear factor-κB activation induced by carcinogenic agents through suppression of IκBα kinase and p65 phosphorylation: Correlation with down-regulation of cyclooxygenase 2, matrix metalloproteinase 9, and cyclin D1. Cancer Res. 2003, 63, 4375–4383. [Google Scholar]
- Harmand, P.O.; Duval, R.; Delage, C.; Simon, A. Ursolic acid induces apoptosis through mitochondrial intrinsic pathway and caspase-3 activation in M4Beu melanoma cells. Int. J. Cancer 2005, 114, 1–11. [Google Scholar] [CrossRef]
- Manu, K.; Kuttan, G. Ursolic acid induces apoptosis by activating p53 and caspase-3 gene expressions and suppressing NF-κB mediated activation of bcl-2 in B16F-10 melanoma cells. Int. Immunopharmacol. 2008, 8, 974–981. [Google Scholar] [CrossRef]
- Prasad, N.R.; Ramachandran, S.; Pugalendi, K.; Menon, V.P. Modulation of UVB-induced Oxidative Stress by Ursolic Acid in Human Blood Lymphocytes. Asian J. Biochem. 2008, 3, 11–18. [Google Scholar] [CrossRef] [Green Version]
- Both, D.M.; Goodtzova, K.; Yarosh, D.B.; Brown, D.A. Liposome-encapsulated ursolic acid increases ceramides and collagen in human skin cells. Arch. Dermatol. Res. 2002, 293, 569–575. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Suman, S.; Shukla, Y. New enlightenment of skin cancer chemoprevention through phytochemicals: In vitro and in vivo studies and the underlying mechanisms. BioMed Res. Int. 2014, 2014. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wei, H.; Saladi, R.; Lu, Y.; Wang, Y.; Palep, S.R.; Moore, J.; Phelps, R.; Shyong, E.; Lebwohl, M.G. Isoflavone genistein: Photoprotection and clinical implications in dermatology. J. Nutr. 2003, 133, 3811S–3819S. [Google Scholar] [CrossRef] [Green Version]
- Rusin, A.; Krawczyk, Z.; Grynkiewicz, G.; Gogler, A.; Zawisza-Puchałka, J.; Szeja, W. Synthetic derivatives of genistein, their properties and possible applications. Acta Biochim. Pol. 2010, 57. [Google Scholar] [CrossRef] [Green Version]
- Sarkar, F.H.; Li, Y. Mechanisms of cancer chemoprevention by soy isoflavone genistein. Cancer Metastasis Rev. 2002, 21, 265–280. [Google Scholar] [CrossRef]
- Li, Q.-S.; Li, C.-Y.; Li, Z.-L.; Zhu, H.-L. Genistein and its synthetic analogs as anticancer agents. Anti-Cancer Agents Med. Chem. 2012, 12, 271–281. [Google Scholar] [CrossRef]
- Rusin, A.; Zawisza-Puchałka, J.; Kujawa, K.; Gogler-Pigłowska, A.; Wietrzyk, J.; Świtalska, M.; Głowala-Kosińska, M.; Gruca, A.; Szeja, W.; Krawczyk, Z. Synthetic conjugates of genistein affecting proliferation and mitosis of cancer cells. Bioorganic Med. Chem. 2011, 19, 295–305. [Google Scholar] [CrossRef]
- Chandra Pal, H.; Marchiony Hunt, K.; Diamond, A.; Elmets, C.A.; Afaq, F. Phytochemicals for the management of melanoma. Mini Rev. Med. Chem. 2016, 16, 953–979. [Google Scholar] [CrossRef]
- Wei, H.; Zhang, X.; Wang, Y.; Lebwohl, M. Inhibition of ultraviolet light-induced oxidative events in the skin and internal organs of hairless mice by isoflavone genistein. Cancer Lett. 2002, 185, 21–29. [Google Scholar] [CrossRef]
- Danciu, C.; Borcan, F.; Bojin, F.; Zupko, I.; Dehelean, C. Effect of the isoflavone genistein on tumor size, metastasis potential and melanization in a B16 mouse model of murine melanoma. Nat. Prod. Commun. 2013, 8. [Google Scholar] [CrossRef] [Green Version]
- Casagrande, F.; Darbon, J.-M. p21CIP1 is dispensable for the G2 arrest caused by genistein in human melanoma cells. Exp. Cell Res. 2000, 258, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Darbon, J.-M.; Penary, M.; Escalas, N.; Casagrande, F.; Goubin-Gramatica, F.; Baudouin, C.; Ducommun, B. Distinct Chk2 activation pathways are triggered by genistein and DNA-damaging agents in human melanoma cells. J. Biol. Chem. 2000, 275, 15363–15369. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, C.; Cha, H.-J.; Lee, H.; Hwang-Bo, H.; Ji, S.Y.; Kim, M.Y.; Hong, S.H.; Jeong, J.-W.; Han, M.H.; Choi, S.H. Induction of G2/M cell cycle arrest and apoptosis by genistein in human bladder cancer T24 cells through inhibition of the ROS-dependent PI3k/Akt signal transduction pathway. Antioxidants 2019, 8, 327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aggarwal, B.B.; Ichikawa, H. Molecular targets and anticancer potential of indole-3-carbinol and its derivatives. Cell Cycle 2005, 4, 1201–1215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Safe, S.; Papineni, S.; Chintharlapalli, S. Cancer chemotherapy with indole-3-carbinol, bis (3′-indolyl) methane and synthetic analogs. Cancer Lett. 2008, 269, 326–338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weng, J.-R.; Tsai, C.-H.; Kulp, S.K.; Wang, D.; Lin, C.-H.; Yang, H.-C.; Ma, Y.; Sargeant, A.; Chiu, C.-F.; Tsai, M.-H. A potent indole-3-carbinol–derived antitumor agent with pleiotropic effects on multiple signaling pathways in prostate cancer cells. Cancer Res. 2007, 67, 7815–7824. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, D.-S.; Jeong, Y.-M.; Moon, S.-I.; Kim, S.-Y.; Kwon, S.-B.; Park, E.-S.; Youn, S.-W.; Park, K.-C. Indole-3-carbinol enhances ultraviolet B-induced apoptosis by sensitizing human melanoma cells. Cell. Mol. Life Sci. CMLS 2006, 63, 2661–2668. [Google Scholar] [CrossRef]
- Kim, S.-Y.; Kim, D.-S.; Jeong, Y.-M.; Moon, S.-I.; Kwon, S.-B.; Park, K.-C. Indole-3-carbinol and ultraviolet B induce apoptosis of human melanoma cells via down-regulation of MITF. Pharm. Int. J. Pharm. Sci. 2011, 66, 982–987. [Google Scholar]
- Aronchik, I.; Kundu, A.; Quirit, J.G.; Firestone, G.L. The antiproliferative response of indole-3-carbinol in human melanoma cells is triggered by an interaction with NEDD4-1 and disruption of wild-type PTEN degradation. Mol. Cancer Res. 2014, 12, 1621–1634. [Google Scholar] [CrossRef] [Green Version]
- Bode, A.M.; Dong, Z. The two faces of capsaicin. Cancer Res. 2011, 71, 2809–2814. [Google Scholar] [CrossRef] [Green Version]
- Hwang, M.K.; Bode, A.M.; Byun, S.; Song, N.R.; Lee, H.J.; Lee, K.W.; Dong, Z. Cocarcinogenic effect of capsaicin involves activation of EGFR signaling but not TRPV1. Cancer Res. 2010, 70, 6859–6869. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hail, N., Jr.; Lotan, R. Examining the role of mitochondrial respiration in vanilloid-induced apoptosis. J. Natl. Cancer Inst. 2002, 94, 1281–1292. [Google Scholar] [CrossRef] [PubMed]
- Shin, D.-H.; Kim, O.-H.; Jun, H.-S.; Kang, M.-K. Inhibitory effect of capsaicin on B16-F10 melanoma cell migration via the phosphatidylinositol 3-kinase/Akt/Rac1 signal pathway. Exp. Mol. Med. 2008, 40, 486–494. [Google Scholar] [CrossRef] [PubMed]
- Marques, C.M.; Dibden, C.; Danson, S.; Haycock, J.W.; MacNeil, S. Combined effects of capsaicin and HA14-1 in inducing apoptosis in melanoma cells. J. Cosmet. Dermatol. Sci. Appl. 2013, 3, 175. [Google Scholar] [CrossRef] [Green Version]
- Mason, L.; Moore, R.A.; Derry, S.; Edwards, J.E.; McQuay, H.J. Systematic review of topical capsaicin for the treatment of chronic pain. BMJ 2004, 328, 991. [Google Scholar] [CrossRef] [Green Version]
- Hamed, M.; Kalita, D.; Bartolo, M.E.; Jayanty, S.S. Capsaicinoids, polyphenols and antioxidant activities of capsicum annuum: Comparative study of the effect of ripening stage and cooking methods. Antioxidants 2019, 8, 364. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Zhang, D.; Liu, Z.; Liu, G.; Duan, C.; Jia, L.; Feng, F.; Zhang, X.; Shi, Y.; Zhang, Q. In vitro and in vivo evaluation of silybin nanosuspensions for oral and intravenous delivery. Nanotechnology 2010, 21, 155104. [Google Scholar] [CrossRef]
- Deep, G.; Agarwal, R. Antimetastatic efficacy of silibinin: Molecular mechanisms and therapeutic potential against cancer. Cancer Metastasis Rev. 2010, 29, 447–463. [Google Scholar] [CrossRef] [Green Version]
- Deep, G.; Singh, R.; Agarwal, C.; Kroll, D.; Agarwal, R. Silymarin and silibinin cause G1 and G2–M cell cycle arrest via distinct circuitries in human prostate cancer PC3 cells: A comparison of flavanone silibinin with flavanolignan mixture silymarin. Oncogene 2006, 25, 1053–1069. [Google Scholar] [CrossRef] [Green Version]
- Singh, R.P.; Dhanalakshmi, S.; Agarwal, C.; Agarwal, R. Silibinin strongly inhibits growth and survival of human endothelial cells via cell cycle arrest and downregulation of survivin, Akt and NF-κ B: Implications for angioprevention and antiangiogenic therapy. Oncogene 2005, 24, 1188–1202. [Google Scholar] [CrossRef] [Green Version]
- Singh, R.P.; Deep, G.; Chittezhath, M.; Kaur, M.; Dwyer-Nield, L.D.; Malkinson, A.M.; Agarwal, R. Effect of silibinin on the growth and progression of primary lung tumors in mice. J. Natl. Cancer Inst. 2006, 98, 846–855. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaur, M.; Velmurugan, B.; Tyagi, A.; Deep, G.; Katiyar, S.; Agarwal, C.; Agarwal, R. Silibinin suppresses growth and induces apoptotic death of human colorectal carcinoma LoVo cells in culture and tumor xenograft. Mol. Cancer Ther. 2009, 8, 2366–2374. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Son, Y.-g.; Kim, E.H.; Kim, J.Y.; Kim, S.U.; Kwon, T.K.; Yoon, A.-R.; Yun, C.-O.; Choi, K.S. Silibinin sensitizes human glioma cells to TRAIL-mediated apoptosis via DR5 up-regulation and down-regulation of c-FLIP and survivin. Cancer Res. 2007, 67, 8274–8284. [Google Scholar] [CrossRef] [Green Version]
- Vaid, M.; Katiyar, S.K. Molecular mechanisms of inhibition of photocarcinogenesis by silymarin, a phytochemical from milk thistle (Silybum marianum L. Gaertn.). Int. J. Oncol. 2010, 36, 1053–1060. [Google Scholar]
- Dhanalakshmi, S.; Mallikarjuna, G.; Singh, R.P.; Agarwal, R. Dual efficacy of silibinin in protecting or enhancing ultraviolet B radiation-caused apoptosis in HaCaT human immortalized keratinocytes. Carcinogenesis 2004, 25, 99–106. [Google Scholar] [CrossRef] [Green Version]
- Singh, R.P.; Dhanalakshmi, S.; Mohan, S.; Agarwal, C.; Agarwal, R. Silibinin inhibits UVB-and epidermal growth factor–induced mitogenic and cell survival signaling involving activator protein-1 and nuclear factor-κB in mouse epidermal JB6 cells. Mol. Cancer Ther. 2006, 5, 1145–1153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mallikarjuna, G.; Dhanalakshmi, S.; Singh, R.P.; Agarwal, C.; Agarwal, R. Silibinin protects against photocarcinogenesis via modulation of cell cycle regulators, mitogen-activated protein kinases, and Akt signaling. Cancer Res. 2004, 64, 6349–6356. [Google Scholar] [CrossRef] [Green Version]
- Singh, R.P.; Raina, K.; Deep, G.; Chan, D.; Agarwal, R. Silibinin suppresses growth of human prostate carcinoma PC-3 orthotopic xenograft via activation of extracellular signal-regulated kinase 1/2 and inhibition of signal transducers and activators of transcription signaling. Clin. Cancer Res. 2009, 15, 613–621. [Google Scholar] [CrossRef] [Green Version]
- Chen, P.-N.; Hsieh, Y.-S.; Chiou, H.-L.; Chu, S.-C. Silibinin inhibits cell invasion through inactivation of both PI3K-Akt and MAPK signaling pathways. Chem. Biol. Interact. 2005, 156, 141–150. [Google Scholar] [CrossRef]
- Vaid, M.; Prasad, R.; Sun, Q.; Katiyar, S.K. Silymarin targets β-catenin signaling in blocking migration/invasion of human melanoma cells. PLoS ONE 2011, 6, e23000. [Google Scholar] [CrossRef]
- Linden, K.G.; Carpenter, P.M.; McLaren, C.E.; Barr, R.J.; Rite, P.; Sun, J.D.; Li, K.-T.; Viner, J.L.; Meyskens, F.L. Chemoprevention of nonmelanoma skin cancer: Experience with a polyphenol from green tea. In Tumor Prevention and Genetics; Springer: Berlin/Heidelberg, Germany, 2003; pp. 165–171. [Google Scholar]
- Farrar, M.D.; Nicolaou, A.; Clarke, K.A.; Mason, S.; Massey, K.A.; Dew, T.P.; Watson, R.E.; Williamson, G.; Rhodes, L.E. A randomized controlled trial of green tea catechins in protection against ultraviolet radiation–induced cutaneous inflammation, 2. Am. J. Clin. Nutr. 2015, 102, 608–615. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, J.; Lei, Z.; Huang, Z.; Zhang, X.; Zhou, Y.; Luo, Z.; Zeng, W.; Su, J.; Peng, C.; Chen, X. Epigallocatechin-3-gallate (EGCG) suppresses melanoma cell growth and metastasis by targeting TRAF6 activity. Oncotarget 2016, 7, 79557. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ellis, L.Z.; Liu, W.; Luo, Y.; Okamoto, M.; Qu, D.; Dunn, J.H.; Fujita, M. Green tea polyphenol epigallocatechin-3-gallate suppresses melanoma growth by inhibiting inflammasome and IL-1β secretion. Biochem. Biophys. Res. Commun. 2011, 414, 551–556. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nihal, M.; Ahsan, H.; Siddiqui, I.A.; Mukhtar, H.; Ahmad, N.; Wood, G.S. (−)-Epigallocatechin-3-gallate (EGCG) sensitizes melanoma cells to interferon induced growth inhibition in a mouse model of human melanoma. Cell Cycle 2009, 8, 2057–2063. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mantena, S.K.; Meeran, S.M.; Elmets, C.A.; Katiyar, S.K. Orally administered green tea polyphenols prevent ultraviolet radiation-induced skin cancer in mice through activation of cytotoxic T cells and inhibition of angiogenesis in tumors. J. Nutr. 2005, 135, 2871–2877. [Google Scholar] [CrossRef]
- Afaq, F.; Adhami, V.M.; Ahmad, N.; Mukhtar, H. Inhibition of ultraviolet B-mediated activation of nuclear factor κ B in normal human epidermal keratinocytes by green tea Constituent (−)-epigallocatechin-3-gallate. Oncogene 2003, 22, 1035–1044. [Google Scholar] [CrossRef] [Green Version]
- Katiyar, S.K.; Afaq, F.; Perez, A.; Mukhtar, H. Green tea polyphenol (−)-epigallocatechin-3-gallate treatment of human skin inhibits ultraviolet radiation-induced oxidative stress. Carcinogenesis 2001, 22, 287–294. [Google Scholar] [CrossRef]
- Kaur, G.; Athar, M.; Alam, M.S. Eugenol precludes cutaneous chemical carcinogenesis in mouse by preventing oxidative stress and inflammation and by inducing apoptosis. Mol. Carcinog. 2010, 49, 290–301. [Google Scholar] [CrossRef]
- Ghosh, R.; Nadiminty, N.; Fitzpatrick, J.E.; Alworth, W.L.; Slaga, T.J.; Kumar, A.P. Eugenol causes melanoma growth suppression through inhibition of E2F1 transcriptional activity. J. Biol. Chem. 2005, 280, 5812–5819. [Google Scholar] [CrossRef] [Green Version]
- Pal, D.; Banerjee, S.; Mukherjee, S.; Roy, A.; Panda, C.K.; Das, S. Eugenol restricts DMBA croton oil induced skin carcinogenesis in mice: Downregulation of c-Myc and H-ras, and activation of p53 dependent apoptotic pathway. J. Dermatol. Sci. 2010, 59, 31–39. [Google Scholar] [CrossRef]
- Esmaeili, F.; Rajabnejhad, S.; Partoazar, A.R.; Mehr, S.E.; Faridi-Majidi, R.; Sahebgharani, M.; Syedmoradi, L.; Rajabnejhad, M.R.; Amani, A. Anti-inflammatory effects of eugenol nanoemulsion as a topical delivery system. Pharm. Dev. Technol. 2016, 21, 887–893. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Omene, C.; Karkoszka, J.; Bosland, M.; Eckard, J.; Klein, C.B.; Frenkel, K. Caffeic acid phenethyl ester (CAPE), derived from a honeybee product propolis, exhibits a diversity of anti-tumor effects in pre-clinical models of human breast cancer. Cancer Lett. 2011, 308, 43–53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, K.W.; Kang, N.J.; Kim, J.H.; Lee, K.M.; Lee, D.E.; Hur, H.J.; Lee, H.J. Caffeic acid phenethyl ester inhibits invasion and expression of matrix metalloproteinase in SK-Hep1 human hepatocellular carcinoma cells by targeting nuclear factor kappa B. Genes Nutr. 2008, 2, 319–322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Onori, P.; DeMorrow, S.; Gaudio, E.; Franchitto, A.; Mancinelli, R.; Venter, J.; Kopriva, S.; Ueno, Y.; Alvaro, D.; Savage, J. Caffeic acid phenethyl ester decreases cholangiocarcinoma growth by inhibition of NF-κB and induction of apoptosis. Int. J. Cancer 2009, 125, 565–576. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, C.-S.; Chen, M.-F.; Lee, I.-L.; Tung, S.-Y. Predictive role of nuclear factor-κB activity in gastric cancer: A promising adjuvant approach with caffeic acid phenethyl ester. J. Clin. Gastroenterol. 2007, 41, 894–900. [Google Scholar] [CrossRef] [PubMed]
- Nakashima, S.; Matsuda, H.; Oda, Y.; Nakamura, S.; Xu, F.; Yoshikawa, M. Melanogenesis inhibitors from the desert plant Anastatica hierochuntica in B16 melanoma cells. Bioorg. Med. Chem. 2010, 18, 2337–2345. [Google Scholar] [CrossRef] [PubMed]
- Iwashita, K.; Kobori, M.; Yamaki, K.; Tsushida, T. Flavonoids inhibit cell growth and induce apoptosis in B16 melanoma 4A5 cells. Biosci. Biotechnol. Biochem. 2000, 64, 1813–1820. [Google Scholar] [CrossRef]
- Horváthová, K.; Chalupa, I.; Sebová, L.; Tóthová, D.; Vachálková, A. Protective effect of quercetin and luteolin in human melanoma HMB-2 cells. Mutat. Res. 2005, 565, 105–112. [Google Scholar] [CrossRef]
- Horibe, I.; Satoh, Y.; Shiota, Y.; Kumagai, A.; Horike, N.; Takemori, H.; Uesato, S.; Sugie, S.; Obata, K.; Kawahara, H.; et al. Induction of melanogenesis by 4′-O-methylated flavonoids in B16F10 melanoma cells. J. Nat. Med. 2013, 67, 705–710. [Google Scholar] [CrossRef]
- López-Lázaro, M. Distribution and biological activities of the flavonoid luteolin. Mini Rev. Med. Chem. 2009, 9, 31–59. [Google Scholar] [CrossRef]
- Kim, S.O.; Chun, K.S.; Kundu, J.K.; Surh, Y.J. Inhibitory effects of [6]-gingerol on PMA-induced COX-2 expression and activation of NF-κB and p38 MAPK in mouse skin. Biofactors 2004, 21, 27–31. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-K.; Kim, Y.; Na, K.-M.; Surh, Y.-J.; Kim, T.-Y. [6]-Gingerol prevents UVB-induced ROS production and COX-2 expression in vitro and in vivo. Free Radic. Res. 2007, 41, 603–614. [Google Scholar] [CrossRef] [PubMed]
- Bode, A.M.; Ma, W.-Y.; Surh, Y.-J.; Dong, Z. Inhibition of epidermal growth factor-induced cell transformation and activator protein 1 activation by [6]-gingerol. Cancer Res. 2001, 61, 850–853. [Google Scholar] [PubMed]
- Nigam, N.; George, J.; Srivastava, S.; Roy, P.; Bhui, K.; Singh, M.; Shukla, Y. Induction of apoptosis by [6]-gingerol associated with the modulation of p53 and involvement of mitochondrial signaling pathway in B [a] P-induced mouse skin tumorigenesis. Cancer Chemother. Pharmacol. 2010, 65, 687–696. [Google Scholar] [CrossRef]
- Ratcharin, N.; Wongtrakul, P.; Indranupakorn, R. Preparation of Zingiber officinale extract loaded solid lipid nanoparticles. In Proceedings of Advanced Materials Research; Trans. Tech. Publications Ltd.: Bäch, Switzerland, 2012; pp. 389–392. [Google Scholar]
- Moon, M.K.; Lee, Y.J.; Kim, J.S.; Kang, D.G.; Lee, H.S. Effect of caffeic acid on tumor necrosis factor-alpha-induced vascular inflammation in human umbilical vein endothelial cells. Biol. Pharm. Bull. 2009, 32, 1371–1377. [Google Scholar] [CrossRef] [Green Version]
- Tsai, S.-j.; Chao, C.-y.; Yin, M.-c. Preventive and therapeutic effects of caffeic acid against inflammatory injury in striatum of MPTP-treated mice. Eur. J. Pharmacol. 2011, 670, 441–447. [Google Scholar] [CrossRef]
- Kang, N.J.; Lee, K.W.; Kim, B.H.; Bode, A.M.; Lee, H.-J.; Heo, Y.-S.; Boardman, L.; Limburg, P.; Lee, H.J.; Dong, Z. Coffee phenolic phytochemicals suppress colon cancer metastasis by targeting MEK and TOPK. Carcinogenesis 2011, 32, 921–928. [Google Scholar] [CrossRef] [Green Version]
- Jung, J.E.; Kim, H.S.; Lee, C.S.; Park, D.-H.; Kim, Y.-N.; Lee, M.-J.; Lee, J.W.; Park, J.-W.; Kim, M.-S.; Ye, S.K. Caffeic acid and its synthetic derivative CADPE suppress tumor angiogenesis by blocking STAT3-mediated VEGF expression in human renal carcinoma cells. Carcinogenesis 2007, 28, 1780–1787. [Google Scholar] [CrossRef]
- Yang, Y.; Li, Y.; Wang, K.; Wang, Y.; Yin, W.; Li, L. P38/NF-κB/snail pathway is involved in caffeic acid-induced inhibition of cancer stem cells-like properties and migratory capacity in malignant human keratinocyte. PLoS ONE 2013, 8, e58915. [Google Scholar] [CrossRef] [Green Version]
- Kang, N.J.; Lee, K.W.; Shin, B.J.; Jung, S.K.; Hwang, M.K.; Bode, A.M.; Heo, Y.-S.; Lee, H.J.; Dong, Z. Caffeic acid, a phenolic phytochemical in coffee, directly inhibits Fyn kinase activity and UVB-induced COX-2 expression. Carcinogenesis 2009, 30, 321–330. [Google Scholar] [CrossRef] [Green Version]
- Chao, P.-c.; Hsu, C.-c.; Yin, M.-c. Anti-inflammatory and anti-coagulatory activities of caffeic acid and ellagic acid in cardiac tissue of diabetic mice. Nutr. Metab. 2009, 6, 33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khan, A.Q.; Khan, R.; Qamar, W.; Lateef, A.; Ali, F.; Tahir, M.; Sultana, S. Caffeic acid attenuates 12-O-tetradecanoyl-phorbol-13-acetate (TPA)-induced NF-κB and COX-2 expression in mouse skin: Abrogation of oxidative stress, inflammatory responses and proinflammatory cytokine production. Food Chem. Toxicol. 2012, 50, 175–183. [Google Scholar] [CrossRef] [PubMed]
- Ghani, M.A.; Barril, C.; Bedgood, D.R.; Prenzler, P.D. Development of a method suitable for high-throughput screening to measure antioxidant activity in a linoleic acid emulsion. Antioxidants 2019, 8, 366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guthrie, A.R.; Chow, H.H.S.; Martinez, J.A. Effects of resveratrol on drug-and carcinogen-metabolizing enzymes, implications for cancer prevention. Pharmacol. Res. Perspect. 2017, 5, e00294. [Google Scholar] [CrossRef] [PubMed]
- Kowalczyk, M.C.; Kowalczyk, P.; Tolstykh, O.; Hanausek, M.; Walaszek, Z.; Slaga, T.J. Synergistic effects of combined phytochemicals and skin cancer prevention in SENCAR mice. Cancer Prev. Res. 2010, 3, 170–178. [Google Scholar] [CrossRef] [Green Version]
- Yang, S.; Irani, K.; Heffron, S.E.; Jurnak, F.; Meyskens, F.L. Alterations in the expression of the apurinic/apyrimidinic endonuclease-1/redox factor-1 (APE/Ref-1) in human melanoma and identification of the therapeutic potential of resveratrol as an APE/Ref-1 inhibitor. Mol. Cancer Ther. 2005, 4, 1923–1935. [Google Scholar] [CrossRef] [Green Version]
- Fuggetta, M.P.; D’Atri, S.; Lanzilli, G.; Tricarico, M.; Cannavò, E.; Zambruno, G.; Falchetti, R.; Ravagnan, G. In vitro antitumour activity of resveratrol in human melanoma cells sensitive or resistant to temozolomide. Melanoma Res. 2004, 14, 189–196. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, X.; Kang, S.; Liu, C.; Hao, Y. Resveratrol enhances the effects of ALA-PDT on skin squamous cells A431 through p38/MAPK signaling pathway. Cancer Biomark. 2018, 21, 797–803. [Google Scholar] [CrossRef]
- Tyagi, A.; Gu, M.; Takahata, T.; Frederick, B.; Agarwal, C.; Siriwardana, S.; Agarwal, R.; Sclafani, R.A. Resveratrol selectively induces DNA Damage, independent of Smad4 expression, in its efficacy against human head and neck squamous cell carcinoma. Clin. Cancer Res. 2011, 17, 5402–5411. [Google Scholar] [CrossRef] [Green Version]
- Kim, A.L.; Zhu, Y.; Zhu, H.; Han, L.; Kopelovich, L.; Bickers, D.R.; Athar, M. Resveratrol inhibits proliferation of human epidermoid carcinoma A431 cells by modulating MEK1 and AP-1 signalling pathways. Exp. Dermatol. 2006, 15, 538–546. [Google Scholar] [CrossRef]
- Moyano-Mendez, J.R.; Fabbrocini, G.; De Stefano, D.; Mazzella, C.; Mayol, L.; Scognamiglio, I.; Carnuccio, R.; Ayala, F.; La Rotonda, M.I.; De Rosa, G. Enhanced antioxidant effect of trans-resveratrol: Potential of binary systems with polyethylene glycol and cyclodextrin. Drug Dev. Ind. Pharm. 2014, 40, 1300–1307. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shehzad, A.; Qureshi, M.; Anwar, M.N.; Lee, Y.S. Multifunctional curcumin mediate multitherapeutic effects. J. Food Sci. 2017, 82, 2006–2015. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lelli, D.; Sahebkar, A.; Johnston, T.P.; Pedone, C. Curcumin use in pulmonary diseases: State of the art and future perspectives. Pharmacol. Res. 2017, 115, 133–148. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Park, J.; Tak, K.-H.; Bu, S.Y.; Kim, E. Chemopreventive effects of curcumin on chemically induced mouse skin carcinogenesis in BK5. insulin-like growth factor-1 transgenic mice. In Vitro Cell. Dev. Biol. Anim. 2014, 50, 883–892. [Google Scholar] [CrossRef]
- Zhao, G.; Han, X.; Zheng, S.; Li, Z.; Sha, Y.; Ni, J.; Sun, Z.; Qiao, S.; Song, Z. Curcumin induces autophagy, inhibits proliferation and invasion by downregulating AKT/mTOR signaling pathway in human melanoma cells. Oncol. Rep. 2016, 35, 1065–1074. [Google Scholar] [CrossRef] [Green Version]
- Wu, J.; Lu, W.-Y.; Cui, L.-L. Inhibitory effect of curcumin on invasion of skin squamous cell carcinoma A431 cells. Asian Pac. J. Cancer Prev. 2015, 16, 2813–2818. [Google Scholar] [CrossRef]
- Qiu, Y.; Yu, T.; Wang, W.; Pan, K.; Shi, D.; Sun, H. Curcumin-induced melanoma cell death is associated with mitochondrial permeability transition pore (mPTP) opening. Biochem. Biophys. Res. Commun. 2014, 448, 15–21. [Google Scholar] [CrossRef]
- Chinembiri, T.N.; Du Plessis, L.H.; Gerber, M.; Hamman, J.H.; Du Plessis, J. Review of natural compounds for potential skin cancer treatment. Molecules 2014, 19, 11679–11721. [Google Scholar] [CrossRef] [Green Version]
- Iqbal, J.; Abbasi, B.A.; Ahmad, R.; Mahmood, T.; Kanwal, S.; Ali, B.; Khalil, A.T.; Shah, S.A.; Alam, M.M.; Badshah, H. Ursolic acid a promising candidate in the therapeutics of breast cancer: Current status and future implications. Biomed. Pharmacother. 2018, 108, 752–756. [Google Scholar] [CrossRef]
- Shanmugam, M.K.; Dai, X.; Kumar, A.P.; Tan, B.K.; Sethi, G.; Bishayee, A. Ursolic acid in cancer prevention and treatment: Molecular targets, pharmacokinetics and clinical studies. Biochem. Pharmacol. 2013, 85, 1579–1587. [Google Scholar] [CrossRef] [Green Version]
- Hsu, Y.-L.; Kuo, P.-L.; Lin, C.-C. Proliferative inhibition, cell-cycle dysregulation, and induction of apoptosis by ursolic acid in human non-small cell lung cancer A549 cells. Life Sci. 2004, 75, 2303–2316. [Google Scholar] [CrossRef] [PubMed]
- Checker, R.; Sandur, S.K.; Sharma, D.; Patwardhan, R.S.; Jayakumar, S.; Kohli, V.; Sethi, G.; Aggarwal, B.B.; Sainis, K.B. Potent anti-inflammatory activity of ursolic acid, a triterpenoid antioxidant, is mediated through suppression of NF-κB, AP-1 and NF-AT. PLoS ONE 2012, 7, e31318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramachandran, S.; Prasad, N.R.; Pugalendi, K.; Menon, V. Modulation of UVB-induced oxidative stress by ursolic acid in human blood lymphocytes. Asian J. Biochem. 2010, 5, 173–180. [Google Scholar]
- Moore, J.O.; Wang, Y.; Stebbins, W.G.; Gao, D.; Zhou, X.; Phelps, R.; Lebwohl, M.; Wei, H. Photoprotective effect of isoflavone genistein on ultraviolet B-induced pyrimidine dimer formation and PCNA expression in human reconstituted skin and its implications in dermatology and prevention of cutaneous carcinogenesis. Carcinogenesis 2006, 27, 1627–1635. [Google Scholar] [CrossRef] [PubMed]
- Ji, C.; Yang, Y.-l.; He, L.; Gu, B.; Xia, J.-p.; Sun, W.-l.; Su, Z.-l.; Chen, B.; Bi, Z.-g. Increasing ceramides sensitizes genistein-induced melanoma cell apoptosis and growth inhibition. Biochem. Biophys. Res. Commun. 2012, 421, 462–467. [Google Scholar] [CrossRef]
- Mazumder, M.A.R.; Hongsprabhas, P. Genistein as antioxidant and antibrowning agents in in vivo and in vitro: A review. Biomed. Pharmacother. 2016, 82, 379–392. [Google Scholar] [CrossRef]
- Ng, C.Y.; Yen, H.; Hsiao, H.-Y.; Su, S.-C. Phytochemicals in skin cancer prevention and treatment: An updated review. Int. J. Mol. Sci. 2018, 19, 941. [Google Scholar] [CrossRef] [Green Version]
- Iqbal, J.; Abbasi, B.A.; Ahmad, R.; Batool, R.; Mahmood, T.; Ali, B.; Khalil, A.T.; Kanwal, S.; Shah, S.A.; Alam, M.M. Potential phytochemicals in the fight against skin cancer: Current landscape and future perspectives. Biomed. Pharmacother. 2019, 109, 1381–1393. [Google Scholar] [CrossRef]
- Sarkar, F.H.; Li, Y. Indole-3-carbinol and prostate cancer. J. Nutr. 2004, 134, 3493S–3498S. [Google Scholar] [CrossRef]
- Rahman, K.W.; Aranha, O.; Glazyrin, A.; Chinni, S.R.; Sarkar, F.H. Translocation of Bax to mitochondria induces apoptotic cell death in indole-3-carbinol (I3C) treated breast cancer cells. Oncogene 2000, 19, 5764–5771. [Google Scholar] [CrossRef] [Green Version]
- Choi, H.-S.; Cho, M.-C.; Lee, H.G.; Yoon, D.-Y. Indole-3-carbinol induces apoptosis through p53 and activation of caspase-8 pathway in lung cancer A549 cells. Food Chem. Toxicol. 2010, 48, 883–890. [Google Scholar] [CrossRef] [PubMed]
- Christensen, J.G.; LeBlanc, G.A. Reversal of multidrug resistance in vivo by dietary administration of the phytochemical indole-3-carbinol. Cancer Res. 1996, 56, 574–581. [Google Scholar] [PubMed]
- Wang, S.; Shen, P.; Zhou, J.; Lu, Y. Diet phytochemicals and cutaneous carcinoma chemoprevention: A review. Pharmacol. Res. 2017, 119, 327–346. [Google Scholar] [CrossRef] [PubMed]
- Oyagbemi, A.; Saba, A.; Azeez, O. Capsaicin: A novel chemopreventive molecule and its underlying molecular mechanisms of action. Indian J. Cancer 2010, 47, 53. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, J.; Abbasi, B.A.; Mahmood, T.; Kanwal, S.; Ali, B.; Shah, S.A.; Khalil, A.T. Plant-derived anticancer agents: A green anticancer approach. Asian Pac. J. Trop. Biomed. 2017, 7, 1129–1150. [Google Scholar] [CrossRef]
- Montes de Oca, M.K.; Pearlman, R.L.; McClees, S.F.; Strickland, R.; Afaq, F. Phytochemicals for the prevention of photocarcinogenesis. Photochem. Photobiol. 2017, 93, 956–974. [Google Scholar] [CrossRef]
- Tong, W.; Zhang, C.; Hong, T.; Liu, D.; Wang, C.; Li, J.; He, X.; Xu, W. Silibinin alleviates inflammation and induces apoptosis in human rheumatoid arthritis fibroblast-like synoviocytes and has a therapeutic effect on arthritis in rats. Sci. Rep. 2018, 8, 1–12. [Google Scholar] [CrossRef]
- Gu, M.; Singh, R.P.; Dhanalakshmi, S.; Agarwal, C.; Agarwal, R. Silibinin inhibits inflammatory and angiogenic attributes in photocarcinogenesis in SKH-1 hairless mice. Cancer Res. 2007, 67, 3483–3491. [Google Scholar] [CrossRef] [Green Version]
- Katiyar, S.K.; Agarwal, R.; Wood, G.S.; Mukhtar, H. Inhibition of 12-O-tetradecanoylphorbol-13-acetate-caused tumor promotion in 7,12-dimethylbenz [a] anthracene-initiated SENCAR mouse skin by a polyphenolic fraction isolated from green tea. Cancer Res. 1992, 52, 6890–6897. [Google Scholar]
- Katiyar, S.K.; Afaq, F.; Azizuddin, K.; Mukhtar, H. Inhibition of UVB-induced oxidative stress-mediated phosphorylation of mitogen-activated protein kinase signaling pathways in cultured human epidermal keratinocytes by green tea polyphenol (−)-epigallocatechin-3-gallate. Toxicol. Appl. Pharmacol. 2001, 176, 110–117. [Google Scholar] [CrossRef]
- Ahmad, N.; Gupta, S.; Mukhtar, H. Green tea polyphenol epigallocatechin-3-gallate differentially modulates nuclear factor κB in cancer cells versus normal cells. Arch. Biochem. Biophys. 2000, 376, 338–346. [Google Scholar] [CrossRef] [PubMed]
- Fujiki, H.; Watanabe, T.; Sueoka, E.; Rawangkan, A.; Suganuma, M. Cancer prevention with green tea and its principal constituent, EGCG: From early investigations to current focus on human cancer stem cells. Mol. Cells 2018, 41, 73. [Google Scholar]
- Kim, E.; Hwang, K.; Lee, J.; Han, S.Y.; Kim, E.-M.; Park, J.; Cho, J.Y. Skin protective effect of epigallocatechin gallate. Int. J. Mol. Sci. 2018, 19, 173. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miyata, Y.; Shida, Y.; Hakariya, T.; Sakai, H. Anti-cancer effects of green tea polyphenols against prostate cancer. Molecules 2019, 24, 193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farrar, M.D.; Huq, R.; Mason, S.; Nicolaou, A.; Clarke, K.A.; Dew, T.P.; Williamson, G.; Watson, R.E.; Rhodes, L.E. Oral green tea catechins do not provide photoprotection from direct DNA damage induced by higher dose solar simulated radiation: A randomized controlled trial. J. Am. Acad. Dermatol. 2018, 78, 414–416. [Google Scholar] [CrossRef] [Green Version]
- Bezerra, D.P.; Militão, G.C.G.; De Morais, M.C.; De Sousa, D.P. The dual antioxidant/prooxidant effect of eugenol and its action in cancer development and treatment. Nutrients 2017, 9, 1367. [Google Scholar] [CrossRef] [Green Version]
- Xiang, D.; Wang, D.; He, Y.; Xie, J.; Zhong, Z.; Li, Z.; Xie, J. Caffeic acid phenethyl ester induces growth arrest and apoptosis of colon cancer cells via the β-catenin/T-cell factor signaling. Anti-Cancer Drugs 2006, 17, 753–762. [Google Scholar] [CrossRef]
- Chen, M.-F.; Wu, C.-T.; Chen, Y.-J.; Keng, P.C.; Chen, W.-C. Cell killing and radiosensitization by caffeic acid phenethyl ester (CAPE) in lung cancer cells. J. Radiat. Res. 2004, 45, 253–260. [Google Scholar] [CrossRef]
- Chen, M.-J.; Chang, W.-H.; Lin, C.-C.; Liu, C.-Y.; Wang, T.-E.; Chu, C.-H.; Shih, S.-C.; Chen, Y.-J. Caffeic acid phenethyl ester induces apoptosis of human pancreatic cancer cells involving caspase and mitochondrial dysfunction. Pancreatology 2008, 8, 566–576. [Google Scholar] [CrossRef]
- Kuo, H.-C.; Kuo, W.-H.; Lee, Y.-J.; Lin, W.-L.; Chou, F.-P.; Tseng, T.-H. Inhibitory effect of caffeic acid phenethyl ester on the growth of C6 glioma cells in vitro and in vivo. Cancer Lett. 2006, 234, 199–208. [Google Scholar] [CrossRef]
- Kudugunti, S.K.; Vad, N.M.; Ekogbo, E.; Moridani, M.Y. Efficacy of caffeic acid phenethyl ester (CAPE) in skin B16-F0 melanoma tumor bearing C57BL/6 mice. Investig. New Drugs 2011, 29, 52–62. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Horton, L.; Bosland, M.; Karkoszka, J.; Frenkel, K. Caffeic acid phenethyl ester (CAPE) as a preventive agent in preclinical model of breast cancer. In Proceedings of the AACR Annual Meeting, Los Angeles, CA, USA, 14–18 April 2007. [Google Scholar]
- Chen, Y.-J.; Shiao, M.-S.; Hsu, M.-L.; Tsai, T.-H.; Wang, S.-Y. Effect of caffeic acid phenethyl ester, an antioxidant from propolis, on inducing apoptosis in human leukemic HL-60 cells. J. Agric. Food Chem. 2001, 49, 5615–5619. [Google Scholar] [CrossRef] [PubMed]
- Yordanov, Y. Caffeic acid phenethyl ester (CAPE): Pharmacodynamics and potential for therapeutic application. Pharmacia 2019, 66, 107. [Google Scholar] [CrossRef] [Green Version]
- Ruan, J.-s.; Liu, Y.-p.; Zhang, L.; Yan, L.-g.; Fan, F.-t.; Shen, C.-s.; Wang, A.-y.; Zheng, S.-z.; Wang, S.-m.; Lu, Y. Luteolin reduces the invasive potential of malignant melanoma cells by targeting β3 integrin and the epithelial-mesenchymal transition. Acta Pharmacol. Sin. 2012, 33, 1325–1331. [Google Scholar] [CrossRef] [Green Version]
- Park, K.-K.; Chun, K.-S.; Lee, J.-M.; Lee, S.S.; Surh, Y.-J. Inhibitory effects of [6]-gingerol, a major pungent principle of ginger, on phorbol ester-induced inflammation, epidermal ornithine decarboxylase activity and skin tumor promotion in ICR mice. Cancer Lett. 1998, 129, 139–144. [Google Scholar] [CrossRef]
- DeSalvo, K.B.; Olson, R.; Casavale, K.O. Dietary guidelines for Americans. JAMA 2016, 315, 457–458. [Google Scholar] [CrossRef] [Green Version]
- Hussain, F.; Rana, Z.; Shafique, H.; Malik, A.; Hussain, Z. Phytopharmacological potential of different species of Morus alba and their bioactive phytochemicals: A review. Asian Pac. J. Trop. Biomed. 2017, 7, 950–956. [Google Scholar] [CrossRef] [Green Version]
- Chaudhary, P.; Sharma, A.; Singh, B.; Nagpal, A.K. Bioactivities of phytochemicals present in tomato. J. Food Sci. Technol. 2018, 55, 2833–2849. [Google Scholar] [CrossRef]
- Cavazos-Garduño, A.; Serrano-Niño, J.C.; García-Varela, R.; García, H.S. Anticarcinogenic phytochemicals. Fruit Veg. Phytochem. Chem. Hum. Health 2017, 2, 53–66. [Google Scholar]
- Singh, C.K.; Siddiqui, I.A.; El-Abd, S.; Mukhtar, H.; Ahmad, N. Combination chemoprevention with grape antioxidants. Mol. Nutr. Food Res. 2016, 60, 1406–1415. [Google Scholar] [CrossRef] [Green Version]
- Vinson, J.A.; Su, X.; Zubik, L.; Bose, P. Phenol antioxidant quantity and quality in foods: Fruits. J. Agric. Food Chem. 2001, 49, 5315–5321. [Google Scholar] [CrossRef] [PubMed]
- Wolfe, K.; Wu, X.; Liu, R.H. Antioxidant activity of apple peels. J. Agric. Food Chem. 2003, 51, 609–614. [Google Scholar] [CrossRef] [PubMed]
- Ding, M.; Lu, Y.; Bowman, L.; Huang, C.; Leonard, S.; Wang, L.; Vallyathan, V.; Castranova, V.; Shi, X. Inhibition of AP-1 and neoplastic transformation by fresh apple peel extract. J. Biol. Chem. 2004, 279, 10670–10676. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- George, V.C.; Rupasinghe, H. Apple flavonoids suppress carcinogen-induced DNA damage in normal human bronchial epithelial cells. Oxidative Med. Cell. Longev. 2017, 2017, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Li, C.X.; Zhao, X.H.; Zuo, W.F.; Zhang, T.L.; Zhang, Z.Y.; Chen, X.S. Phytochemical profiles, antioxidant, and antiproliferative activities of four red-fleshed apple varieties in China. J. Food Sci. 2020, 85, 718–726. [Google Scholar] [CrossRef]
- Zaid, M.A.; Afaq, F.; Syed, D.N.; Dreher, M.; Mukhtar, H. Inhibition of UVB-mediated oxidative stress and markers of photoaging in immortalized HaCaT keratinocytes by pomegranate polyphenol extract POMx. Photochem. Photobiol. 2007, 83, 882–888. [Google Scholar] [CrossRef]
- Türkyılmaz, M.; Hamzaoğlu, F.; Özkan, M. Combined use of hydrocolloids in pomegranate juice and their effects on clarification and copigmentation. Int. J. Food Sci. Technol. 2020, 55, 1426–1436. [Google Scholar] [CrossRef]
- Afaq, F.; Zaid, M.A.; Khan, N.; Dreher, M.; Mukhtar, H. Protective effect of pomegranate-derived products on UVB-mediated damage in human reconstituted skin. Exp. Dermatol. 2009, 18, 553–561. [Google Scholar] [CrossRef] [Green Version]
- Perde-Schrepler, M.; Chereches, G.; Brie, I.; Tatomir, C.; Postescu, I.D.; Soran, L.; Filip, A. Grape seed extract as photochemopreventive agent against UVB-induced skin cancer. J. Photochem. Photobiol. B Biol. 2013, 118, 16–21. [Google Scholar] [CrossRef]
- Pedro, A.C.; Maciel, G.M.; Rampazzo Ribeiro, V.; Haminiuk, C.W.I. Fundamental and applied aspects of catechins from different sources: A review. Int. J. Food Sci. Technol. 2020, 55, 429–442. [Google Scholar] [CrossRef]
- Kalekhan, F.; Bala, N.; Rao, S.; Pais, M.L.; Adnan, M.; Sajan, S.; Baliga, M.S. Usefulness of grape seed polyphenols in the prevention of skin cancer: A mini review. In Functional Foods in Cancer Prevention and Therapy; Elsevier: Amsterdam, The Netherlands, 2020; pp. 159–167. [Google Scholar]
- Afaq, F.; Saleem, M.; Krueger, C.G.; Reed, J.D.; Mukhtar, H. Anthocyanin-and hydrolyzable tannin-rich pomegranate fruit extract modulates MAPK and NF-κB pathways and inhibits skin tumorigenesis in CD-1 mice. Int. J. Cancer 2005, 113, 423–433. [Google Scholar] [CrossRef] [PubMed]
- Hora, J.J.; Maydew, E.R.; Lansky, E.P.; Dwivedi, C. Chemopreventive effects of pomegranate seed oil on skin tumor development in CD1 mice. J. Med. Food 2003, 6, 157–161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Afaq, F.; Khan, N.; Syed, D.N.; Mukhtar, H. Oral feeding of pomegranate fruit extract inhibits early biomarkers of UVB radiation-induced carcinogenesis in SKH-1 hairless mouse epidermis. Photochem. Photobiol. 2010, 86, 1318–1326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khan, N.; Syed, D.N.; Pal, H.C.; Mukhtar, H.; Afaq, F. Pomegranate fruit extract inhibits UVB-induced inflammation and proliferation by modulating NF-κB and MAPK signaling pathways in mouse skin. Photochem. Photobiol. 2012, 88, 1126–1134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kopec, R.E.; Schick, J.; Tober, K.L.; Riedl, K.M.; Francis, D.M.; Young, G.S.; Schwartz, S.J.; Oberyszyn, T.M. Sex differences in skin carotenoid deposition and acute UVB-induced skin damage in SKH-1 hairless mice after consumption of tangerine tomatoes. Mol. Nutr. Food Res. 2015, 59, 2491–2501. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tanambell, H.; Bishop, K.S.; Quek, S.Y. Tangerine tomatoes: Origin, biochemistry, potential health benefits and future prospects. Crit. Rev. Food Sci. Nutr. 2020, 1–12. [Google Scholar] [CrossRef]
- Guerra, K.C.; Crane, J.S. Skin cancer prevention. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2020. [Google Scholar]
- Cooperstone, J.L.; Tober, K.L.; Riedl, K.M.; Teegarden, M.D.; Cichon, M.J.; Francis, D.M.; Schwartz, S.J.; Oberyszyn, T.M. Tomatoes protect against development of UV-induced keratinocyte carcinoma via metabolomic alterations. Sci. Rep. 2017, 7, 1–9. [Google Scholar] [CrossRef]
- Cho, B.O.; Che, D.N.; Shin, J.Y.; Kang, H.J.; Jang, S.I. Ameliorative effects of fruit stem extract from Muscat Bailey A against chronic UV-induced skin damage in BALB/c mice. Biomed. Pharmacother. 2018, 97, 1680–1688. [Google Scholar] [CrossRef]
- Nieto, J.A.; Santoyo, S.; Prodanov, M.; Reglero, G.; Jaime, L. Valorisation of Grape Stems as a Source of Phenolic Antioxidants by Using a Sustainable Extraction Methodology. Foods 2020, 9, 604. [Google Scholar] [CrossRef]
- Lucarini, M.; Durazzo, A.; Kiefer, J.; Santini, A.; Lombardi-Boccia, G.; Souto, E.B.; Romani, A.; Lampe, A.; Ferrari Nicoli, S.; Gabrielli, P. Grape seeds: Chromatographic profile of fatty acids and phenolic compounds and qualitative analysis by FTIR-ATR spectroscopy. Foods 2020, 9, 10. [Google Scholar] [CrossRef] [Green Version]
- Armstrong, B.K.; Kricker, A. The epidemiology of UV induced skin cancer. J. Photochem. Photobiol. B Biol. 2001, 63, 8–18. [Google Scholar] [CrossRef]
- Hanausek, M.; Spears, E.; Walaszek, Z.; Kowalczyk, M.C.; Kowalczyk, P.; Wendel, C.; Slaga, T.J. Inhibition of murine skin carcinogenesis by freeze-dried grape powder and other grape-derived major antioxidants. Nutr. Cancer 2011, 63, 28–38. [Google Scholar] [CrossRef] [PubMed]
- Gil, M.I.; Tomás-Barberán, F.A.; Hess-Pierce, B.; Holcroft, D.M.; Kader, A.A. Antioxidant activity of pomegranate juice and its relationship with phenolic composition and processing. J. Agric. Food Chem. 2000, 48, 4581–4589. [Google Scholar] [CrossRef] [PubMed]
- Afaq, F.; Malik, A.; Syed, D.; Maes, D.; Matsui, M.S.; Mukhtar, H. Pomegranate Fruit Extract Modulates UV-B–mediated Phosphorylation of Mitogen-activated Protein Kinases and Activation of Nuclear Factor Kappa B in Normal Human Epidermal Keratinocytes. Photochem. Photobiol. 2005, 81, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Di Mascio, P.; Kaiser, S.; Sies, H. Lycopene as the most efficient biological carotenoid singlet oxygen quencher. Arch. Biochem. Biophys. 1989, 274, 532–538. [Google Scholar] [CrossRef]
- Stahl, W.; Heinrich, U.; Aust, O.; Tronnier, H.; Sies, H. Lycopene-rich products and dietary photoprotection. Photochem. Photobiol. Sci. 2006, 5, 238–242. [Google Scholar] [CrossRef]
- Filip, A.; Daicoviciu, D.; Clichici, S.; Mocan, T.; Muresan, A.; Postescu, I.D. Photoprotective Effects of Two Natural Products on Ultraviolet B–Induced Oxidative Stress and Apoptosis in SKH-1 Mouse Skin. J. Med. Food 2011, 14, 761–766. [Google Scholar] [CrossRef] [PubMed]
- Filip, A.; Daicoviciu, D.; Clichici, S.; Bolfa, P.; Catoi, C.; Baldea, I.; Bolojan, L.; Olteanu, D.; Muresan, A.; Postescu, I. The effects of grape seeds polyphenols on SKH-1 mice skin irradiated with multiple doses of UV-B. J. Photochem. Photobiol. B Biol. 2011, 105, 133–142. [Google Scholar] [CrossRef]
- Che, D.N.; Xie, G.H.; Cho, B.O.; Shin, J.Y.; Kang, H.J.; Jang, S.I. Protective effects of grape stem extract against UVB-induced damage in C57BL mice skin. J. Photochem. Photobiol. B Biol. 2017, 173, 551–559. [Google Scholar] [CrossRef]
- Arimoto-Kobayashi, S.; Zhang, X.; Yuhara, Y.; Kamiya, T.; Negishi, T.; Okamoto, G. Chemopreventive effects of the juice of Vitis coignetiae Pulliat on two-stage mouse skin carcinogenesis. Nutr. Cancer 2013, 65, 440–450. [Google Scholar] [CrossRef]
- Sharma, S.D.; Katiyar, S.K. Dietary grape seed proanthocyanidins inhibit UVB-induced cyclooxygenase-2 expression and other inflammatory mediators in UVB-exposed skin and skin tumors of SKH-1 hairless mice. Pharm. Res. 2010, 27, 1092–1102. [Google Scholar] [CrossRef] [PubMed]
- Singh, C.K.; Mintie, C.A.; Ndiaye, M.A.; Chhabra, G.; Dakup, P.P.; Ye, T.; Yu, M.; Ahmad, N. Chemoprotective effects of dietary grape powder on UVB radiation-mediated skin carcinogenesis in SKH-1 hairless mice. J. Investig. Dermatol. 2019, 139, 552–561. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stylos, E.; Chatziathanasiadou, M.V.; Syriopoulou, A.; Tzakos, A.G. Liquid chromatography coupled with tandem mass spectrometry (LC–MS/MS) based bioavailability determination of the major classes of phytochemicals. J. Chromatogr. B 2017, 1047, 15–38. [Google Scholar] [CrossRef] [PubMed]
- Krupkova, O.; Ferguson, S.J.; Wuertz-Kozak, K. Stability of (−)-epigallocatechin gallate and its activity in liquid formulations and delivery systems. J. Nutr. Biochem. 2016, 37, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, J.; Abbasi, B.A.; Ahmad, R.; Mahmood, T.; Ali, B.; Khalil, A.T.; Kanwal, S.; Shah, S.A.; Alam, M.M.; Badshah, H. Nanomedicines for developing cancer nanotherapeutics: From benchtop to bedside and beyond. Appl. Microbiol. Biotechnol. 2018, 102, 9449–9470. [Google Scholar] [CrossRef]
- Yadav, R.; Kumar, D.; Kumari, A.; Yadav, S.K. Encapsulation of catechin and epicatechin on BSA NPs improved their stability and antioxidant potential. EXCLI J. 2014, 13, 331. [Google Scholar]
- Ahmad, A.; Li, Y.; Sarkar, F.H. The bounty of nature for changing the cancer landscape. Mol. Nutr. Food Res. 2016, 60, 1251–1263. [Google Scholar] [CrossRef]
- Subramanian, A.; Jaganathan, S.; Manikandan, A.; Pandiaraj, K.; Gomathi, N.; Supriyanto, E. Recent trends in nano-based drug delivery systems for efficient delivery of phytochemicals in chemotherapy. RSC Adv. 2016, 6, 48294–48314. [Google Scholar] [CrossRef]
- Chikara, S.; Nagaprashantha, L.D.; Singhal, J.; Horne, D.; Awasthi, S.; Singhal, S.S. Oxidative stress and dietary phytochemicals: Role in cancer chemoprevention and treatment. Cancer Lett. 2018, 413, 122–134. [Google Scholar] [CrossRef]
- Afrin, S.; Giampieri, F.; Gasparrini, M.; Forbes-Hernández, T.Y.; Cianciosi, D.; Reboredo-Rodriguez, P.; Zhang, J.; Manna, P.P.; Daglia, M.; Atanasov, A.G. Dietary phytochemicals in colorectal cancer prevention and treatment: A focus on the molecular mechanisms involved. Biotechnol. Adv. 2020, 38, 107322. [Google Scholar] [CrossRef]

| Dietary Phytochemical | Source | Molecular and Structural Formula | Actions/Targets | Reference |
|---|---|---|---|---|
| Resveratrol | Grapes, peanuts, Japanese knotweed, blueberry, Scots pine and Reynoutria japonica | C14H12O3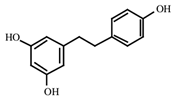 | Anticancer; anti-inflammatory; anti angiogenic; Reactive oxygen species (Ros) scavenger; anti proliferative | [31,32,33,34,35,36,37,38,39,40,41,42,43] |
| Curcumin | Turmeric | C21H20O6 | Anti-oxidant; anti-inflammatory; anticancer; anti proliferative; cell cycle regulator | [44,45,46,47,48] |
| Ursolic acid | Basil, rosemary, thyme, apples, berries, oregano, peppermint, prunes | C30H48O3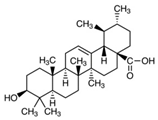 | Anti-inflammatory; antioxidant; chemopreventive; anti-proliferative | [49,50,51,52,53,54] |
| Genistein | Soybean | C15H10O5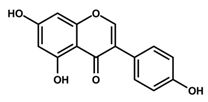 | Cell cycle arrest; anti-inflammatory; anti-oxidant; anticancer | [55,56,57,58,59,60,61,62,63,64,65,66] |
| Indole-3-carbinol | Brussels, broccoli, cauliflower, sprouts | C9H9NO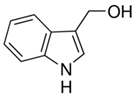 | Anticancer | [67,68,69,70,71,72] |
| Capsaicin | Pepperoni, jalapeno, piri-piri, habanero peppers | C18H27NO3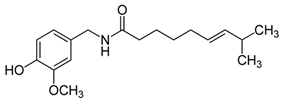 | Anticancer; chemopreventive; anti-inflammatory; anti-oxidant; pro-carcinogenic | [73,74,75,76,77,78,79] |
| Silymarin and silibinin | Milk thistle | Silymarine:C25H22O10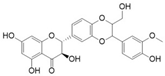 Silibinin: C25H22O10 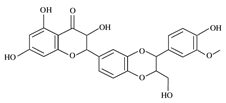 | Liver diseases; chemotherapeutic; anticancer; anti angiogenic; apoptosis inducer | [80,81,82,83,84,85,86,87,88,89,90,91,92,93] |
| Epigallocatechin-3-Gallate | Green tea | C22H18O11 | Anti-inflammatory; anti-proliferative; anticancer; anti-oxidant; anti-erythema | [94,95,96,97,98,99,100,101] |
| Eugenol | Cinnamon, basil, bay leaves, cloves, nutmeg | C10H12O2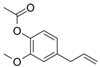 | Antioxidant; anti-proliferative; Ros scavenger; anti-inflammatory; cell death inducer | [102,103,104,105] |
| Caffeic acid phenethyl ester | Propolis | C17H16O4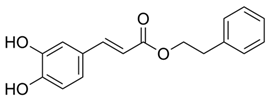 | Anticancer; anti-inflammatory; immunomodulatory; anti-oxidant | [106,107,108,109] |
| Luteolin | Celery, olives, carrots, peppers | C15H10O6 | Anti-angiogenic; Apoptosis inducer; cancer cells sensitizer; stimulates melanogenesis; cell growth inhibitor | [110,111,112,113,114] |
| [6]-Gingerol | Zingiber officinale | C17H26O4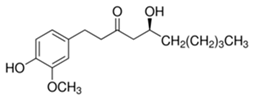 | Anti-inflammatory; anti-oxidant; Ros scavenger | [115,116,117,118,119] |
| Caffeic acid | Coffee, vegetables, fruits | C9H8O4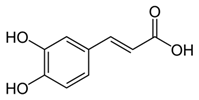 | Antioxidant; anti-inflammatory; anticancer; anti-migratory; inhibits EMT | [120,121,122,123,124,125,126,127,128] |
| In Vitro Approaches | ||||
|---|---|---|---|---|
| Constituent | Major Constituents and Their Chemical and Structural Formula | Dose | Experimental Approach and Results | Reference |
| Apple peel extract | Quercetin-3-O-β-d-glucopyranoside (C21H20O12) 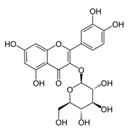 | 1:10–1:640 | JB6/AP/kB (50% inhibition in the number of papillomas, accompanied by a decrease in tumor volume) | [189,191] |
| Pomegranate extract | Delphinidin-3,5-diglucoside (C29H35ClO17)  | 10–40 µg/ml | HaCaT cell lines (photoprotective activity against UVB-induced skin damage) | [192,193] |
| Pomegranate extract, oil, or juice | Delphinidin-3,5-diglucoside (C29H35ClO17) 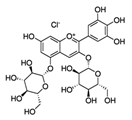 | 5–10 μg (extract), 0.1 mL/well (oil), 1–2 μL (juice) | Reconstituted human skin (epidermTM FT-200) (protective effect on UVB-mediated damage) | [193,194] |
| Grape seed extract | Catechin (C15H14O6)  | 10–40 µg/ml | HaCaT cell lines (photochemopreventive action against UVB-induced skin cancer) | [195,196,197] |
| In Vivo Approaches | ||||
| Constituent | Major Constituents and Their Chemical and Structural Formula | Dose | Experimental Approach and Results | Reference |
| Apple peel extract | Quercetin-3-O-β-d-glucopyranoside (C21H20O12) 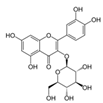 | oral, in lieu of drinking water and topical application, 6 × daily | AP-1 luciferase reporter transgenic mice (C57BL/6 × DBA2) (50% inhibition in the number of papillomas, accompanied by a decrease in tumor volume) | [189,191] |
| oral, in lieu of drinking water, 48 h prior | AP-1 luciferase reporter transgenic mice (C57BL/6 × DBA2) (50% inhibition in the number of papillomas, accompanied by a decrease in tumor volume) | |||
| Pomegranate fruit extract | Delphinidin-3,5-diglucoside (C29H35ClO17)  | 2 mg (topical), 30 min prior | CD-1 (anti-tumor; anti proliferative; anti-migratory effects) | [193,198,199,200,201] |
| 2 mg (topical), prior to TPA | ||||
| 0.2% in drinking water, 14 days prior | SKH-1 hairless mice (anti-tumor; anti proliferative; anti-migratory effects) | |||
| 0.2% in drinking water, 14 days prior up to the end of treatment | ||||
| Pomegranate seed oil | Delphinidin-3,5-diglucoside (C29H35ClO17) 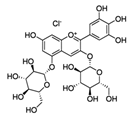 | 5% (topical), 1 h prior | CD-1 (anti-tumor; anti proliferative; anti-migratory) | [193,199] |
| Tangerine tomato powder | Vitamin C (C6H8O6) 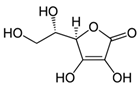 | 10% per os in AIN-93GA diet, 10 weeks prior | SKH-1 hairless mice (photoprotective; anti-inflammatory; anti-tumor) | [202,203,204] |
| Tangerine or red tomato powder | Vitamin C (C6H8O6) 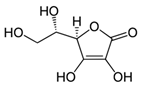 | 10% per os in AIN-93GA diet, through 35 weeks | [203,204,205] | |
| Grape stem extract | Gallic acid (C7H6O5) 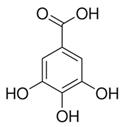 | 1 mg in 0.2 mL propylene glycol (Topical), daily | BALB/c mice (protection against chronic UV-induced skin damage) | [206,207,208] |
| Grape seed proanthocyandins | Catechin (C15H14O6)  | 0.2–0.5% per os in AIN-76A diet | SKH-1 hairless mice (protection against UV induced skin cancer) | [196,197,209] |
| Grape powder | Catechin (C15H14O6) 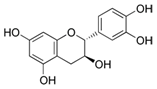 | 1–4 mg (Topical), 30 min post | SENCAR (inhibition of skin carcinogenesis) | [196,197,210] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Islam, S.U.; Ahmed, M.B.; Ahsan, H.; Islam, M.; Shehzad, A.; Sonn, J.K.; Lee, Y.S. An Update on the Role of Dietary Phytochemicals in Human Skin Cancer: New Insights into Molecular Mechanisms. Antioxidants 2020, 9, 916. https://doi.org/10.3390/antiox9100916
Islam SU, Ahmed MB, Ahsan H, Islam M, Shehzad A, Sonn JK, Lee YS. An Update on the Role of Dietary Phytochemicals in Human Skin Cancer: New Insights into Molecular Mechanisms. Antioxidants. 2020; 9(10):916. https://doi.org/10.3390/antiox9100916
Chicago/Turabian StyleIslam, Salman Ul, Muhammad Bilal Ahmed, Haseeb Ahsan, Mazharul Islam, Adeeb Shehzad, Jong Kyung Sonn, and Young Sup Lee. 2020. "An Update on the Role of Dietary Phytochemicals in Human Skin Cancer: New Insights into Molecular Mechanisms" Antioxidants 9, no. 10: 916. https://doi.org/10.3390/antiox9100916





