Effects and Mechanisms of Phthalates’ Action on Reproductive Processes and Reproductive Health: A Literature Review
Abstract
1. Introduction
2. Regulators of Reproduction
2.1. Hypothalamic–Pituitary–Gonadal (HPG) Axis
2.2. Steroidogenesis
2.3. Sex Hormone-Binding Protein (SHBG)
2.4. Mechanisms of Steroid Action
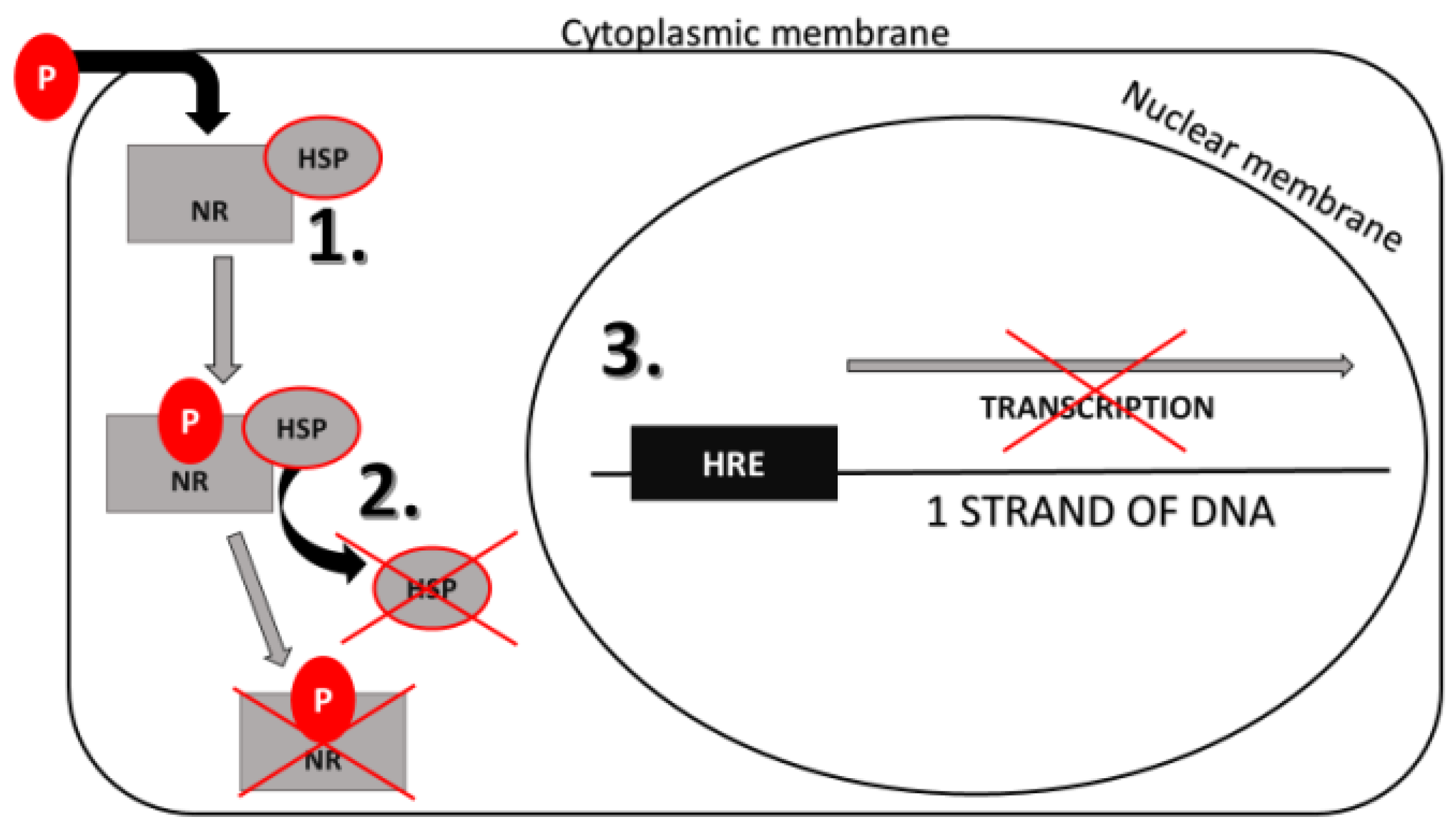
2.4.1. Genomic Action of Steroid Hormones
2.4.2. Non-Genomic Action of Steroid Hormones
2.4.3. Epigenetic Processes
3. Structure, Source, and Toxicity of Phthalates
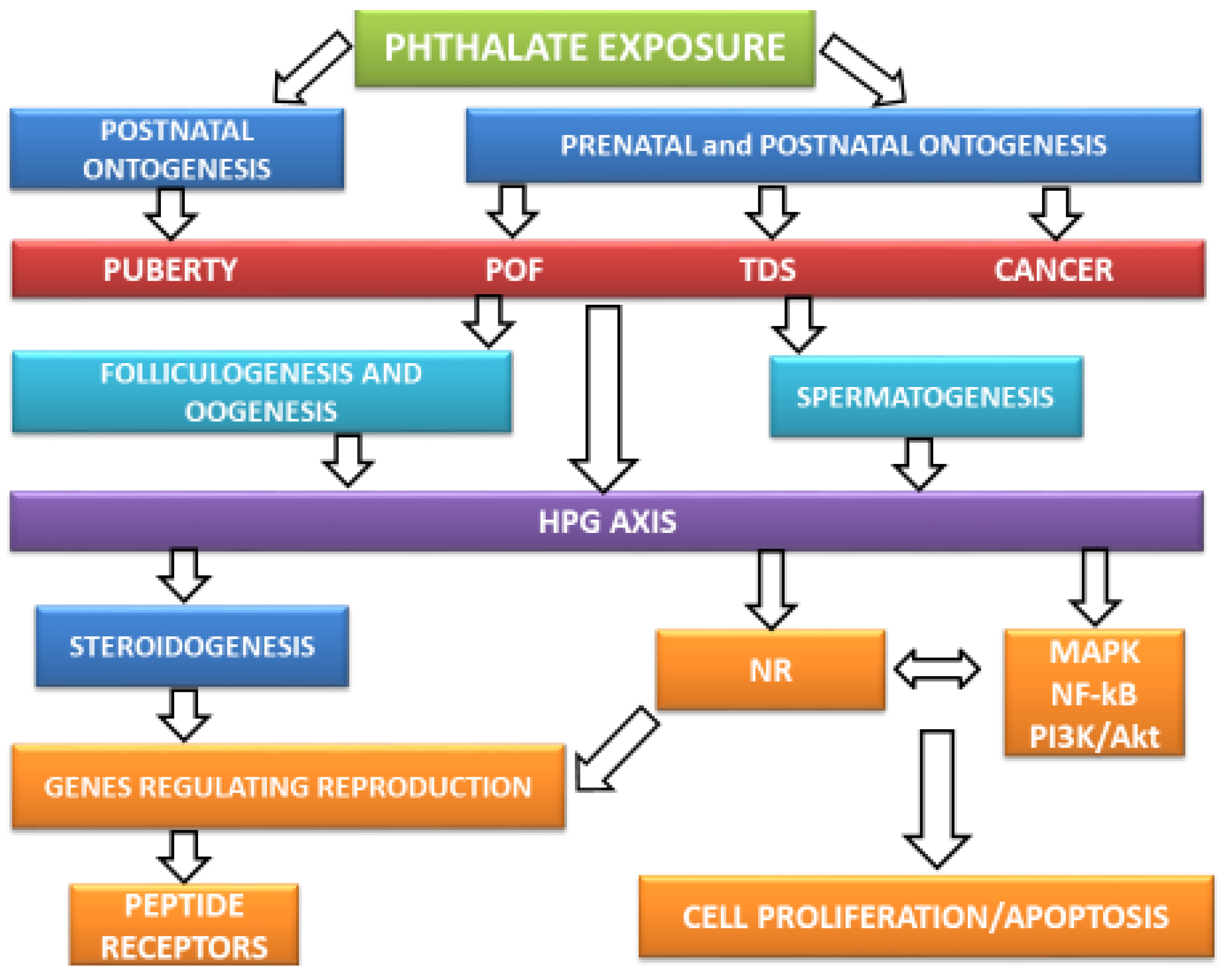
4. Phthalates’ Action on Male Reproductive Health
4.1. Phthalates Can Influence Testicular Function
4.2. Phthalates Can Induce Testicular Dysgenesis Syndrome (TDS)
4.3. Phthalates Can Influence Male Puberty and Its Dysfunctions
4.4. Phthalates Can Induce Cancer in Male Reproductive Organs
5. Phthalates’ Action on Female Reproductive Health
5.1. Phthalates Can Influence Ovarian Function
5.2. Phthalates Can Induce Premature Ovarian Failure (POF)
5.3. Phthalates Can Induce Dysfunctions of Female Puberty
5.4. Phthalates Can Induce Dysfunctions of Pregnancy
5.5. Phthalates Can Induce Cancer in Female Reproductive Organs
6. Hormonal Mechanisms of Phthalates’ Action on Reproductive Functions and Health
6.1. Phthalates’ Effect on the Hypothalamic–Pituitary–Gonadal (HPG) Axis and Steroidogenesis
6.2. Phthalates’ Effect on Sex Hormone-Binding Globulin (SHBG)
7. Intracellular Signaling Mechanisms of Phthalates’ Action on Reproductive Functions and Health
7.1. Phthalates’ Effect on Signaling Pathways of Peptide Hormones
7.2. Phthalates’ Effect on Nuclear Receptors (NRs)
Phthalates’ Effect on Gene Expression Mediated by NRs
7.3. Phthalates’ Effects on Apoptosis and Proliferation of Cells of the Reproductive System
7.4. Phthalates’ Effects on Maturation and Activation of Gonadal Cells before Fertilization
8. Conclusions
9. Future Directions of Phthalate Research
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AR | androgen receptor |
| BBzP | benzylbutyl phthalate |
| BMI | body mass index |
| cAMP | cyclic adenosine monophosphate |
| DBP | dibutyl phthalate |
| DEHP | di(2-ethylhexyl) phthalate |
| DEHT | di(2-ethylhexyl) terephthalate |
| DEP | diethyl phthalate |
| DiBP | di-iso-butyl phthalate |
| DiDP | di-iso-decyl phthalate |
| DiNP | di-iso-nonyl phthalate |
| DMP | dimethyl phthalate |
| DnBP | di-n-butyl phthalate |
| DnOP | di-n-octyl phthalate |
| DPhP | di(2-propylheptyl) phthalate |
| EDs | endocrine disruptors |
| ER | estrogen receptor |
| FSH | follicle-stimulating hormone |
| FSHR | follicle-stimulating hormone receptor |
| GnRH | gonadotropin-releasing hormone |
| GnRHR | gonadotropin-releasing hormone receptor |
| GPCR | G protein-coupled receptor |
| HMWP | high-molecular weight phthalate |
| HPG | hypothalamic–pituitary–gonadal axis |
| HRE | hormone response elements |
| HSP | heat-shock protein |
| LBD | ligand-binding domain |
| LH | luteinizing hormone |
| LHR | luteinizing hormone receptor |
| LMWP | low-molecular-weight phthalates |
| MAPK | mitogen-activated protein kinase |
| MBP | monobutyl phthalate |
| MBzP | monobenzyl phthalate |
| MCNP | monocarboxy-iso-nonyl phthalate |
| MCOP | monocarboxyoctyl phthalate |
| MCPP | mono-(3-carboxypropyl) phthalate |
| MECPP | mono-(2-ethyl-5-carboxypentyl) phthalate |
| MEHHP | mono(2-ethyl-5-hydroxyhexyl) phthalate |
| MEHP | mono-(2-ethylhexyl) phthalate |
| MEOHP | mono(2-ethyl-5-oxohexyl) phthalate |
| MEP | monoethyl phthalate |
| MiBP | mono-iso-butyl phthalate |
| MiDP | mono-iso-decyl phthalate |
| MMP | monomethyl phthalate |
| MnBP | mono-n-butyl phthalate |
| MPhP | mono-(2-propylheptyl) phthalate |
| NF-κB | nuclear factor kappa B |
| NR | nuclear receptor |
| PI3k/Akt | phosphoinositide 3-kinase |
| POF | premature ovarian failure |
| PPAR | peroxisome proliferator-activated receptor |
| PVC | polyvinyl chloride |
| SHBG | sex hormone-binding globulin |
| TDS | testicular dysgenesis syndrome |
| TGF | transforming growth factor |
References
- Benjamin, S.; Masai, E.; Kamimura, N.; Takahashi, K.; Anderson, R.C.; Faisal, P.A. Phthalates impact human health: Epidemiological evidences and plausible mechanism of action. J. Hazard. Mater. 2017, 34, 360–383. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Singh, A.K. Trends of male factor infertility, an important cause of infertility: A review of literature. J. Hum. Reprod. Sci. 2015, 8, 191–196. [Google Scholar] [CrossRef] [PubMed]
- CDC. FastStats—Infertility. Available online: https://www.cdc.gov/nchs/fastats/infertility.htm (accessed on 27 April 2019).
- Sengupta, P.; Borges, E.; Dutta, S.; Krajewska-Kulak, E. Decline in sperm count in European men during the past 50 years. Hum. Exp. Toxicol. 2018, 37, 247–255. [Google Scholar] [CrossRef] [PubMed]
- Acevedo-Rodriguez, A.; Kauffman, A.S.; Cherrington, B.D.; Borges, C.S.; Roepke, T.A.; Laconi, M. Emerging insights into hypothalamic-pituitary-gonadal axis regulation and interaction with stress signalling. J. Neuroendocrinol. 2018, 30, e12590. [Google Scholar] [CrossRef]
- Harter, C.J.L.; Kavanagh, G.S.; Smith, J.T. The role of kisspeptin neurons in reproduction and metabolism. J. Endocrinol. 2018, 238, R173–R183. [Google Scholar] [CrossRef]
- Cadagan, D.; Towlson, C. Mechanisms of Luteinising Hormone Regulation in Female Steroidogenesis. Am. J. Med. Case Rep. 2017, 5, 65–68. [Google Scholar] [CrossRef]
- Casarini, L.; Crépieux, P. Molecular mechanisms of action of FSH. Front. Endocrinol. 2019, 10, 305. [Google Scholar] [CrossRef]
- Jones, R.E.; Lopez, K.H. Endocrinology, Brain and Pituitary Gland. In Human Reproductive Biology, 3rd ed.; Maragioglio, N., Ed.; Academic Press: Cambridge, MA, USA, 2006; pp. 3–29. [Google Scholar]
- Xu, R.; Mao, B.; Li, S.; Liu, J.; Li, X.; Li, H. Structure-activity relationships of phthalates in inhibition of human placental 3β-hydroxysteroid dehydrogenase 1 and aromatase. Reprod. Toxicol. 2016, 61, 151–161. [Google Scholar] [CrossRef]
- Grynnerup, A.G.A.; Lindhard, A.; Sørensen, S. The role of anti-Müllerian hormone in female fertility and infertility—An overview. Acta Obstet. Gynecol. Scand. 2012, 91, 1252–1260. [Google Scholar] [CrossRef]
- Bay, K.; Main, K.M.; Toppari, J.; SkakkebÆk, N.E. Testicular descent: INSL3, testosterone, genes and the intrauterine milieu. Nat. Rev. Urol. 2011, 8, 187–196. [Google Scholar] [CrossRef]
- Miller, W.L.; Auchus, R.J. The Molecular Biology, Biochemistry, and Physiology of Human Steroidogenesis and Its Disorders. Endocr. Rev. 2018, 32, 81–151. [Google Scholar] [CrossRef] [PubMed]
- Walker, W.H. Testosterone signaling and the regulation of spermatogenesis. Spermatogenesis 2011, 1, 116–120. [Google Scholar] [CrossRef] [PubMed]
- Knapczyk-Stwora, K.; Grzesiak, M.; Ciereszko, R.E.; Czaja, E.; Koziorowski, M.; Slomczynska, M. The impact of sex steroid agonists and antagonists on folliculogenesis in the neonatal porcine ovary via cell proliferation and apoptosis. Theriogenology 2018, 113, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.C.; Lee, T.H.; Chang, S.D. Effects of sex hormones on cell proliferation and apoptosis in the urinary bladder muscle of ovariectomized rat. Taiwan. J. Obstet. Gynecol. 2013, 52, 335–340. [Google Scholar] [CrossRef]
- Lee, E.J.; Bajracharya, P.; Jang, E.J.; Chang, J.S.; Lee, H.J.; Hong, S.K. Effect of sex steroid hormones on bovine myogenic satellite cell proliferation, differentiation and lipid accumulation in myotube. Asian Australas. J. Anim. Sci. 2010, 23, 649–658. [Google Scholar] [CrossRef]
- Bramble, M.S.; Vashist, N.; Vilain, E. Sex steroid hormone modulation of neural stem cells: A critical review. Biol. Sex. Differ. 2019, 10. [Google Scholar] [CrossRef]
- Thompson, E.B. Apoptosis and Steroid Hormones. Mol. Endocirnol. 1994, 8, 665–673. [Google Scholar]
- Hammond, G.L. Plasma steroid-binding proteins: Primary gatekeepers of steroid hormone action. J. Endocrinol. 2016, 230, R13–R25. [Google Scholar] [CrossRef]
- Somboonporn, W.; Davis, S.R. Testosterone Effects on the Breast: Implications for Testosterone Therapy for Women. Endocr. Rev. 2004, 25, 374–388. [Google Scholar] [CrossRef]
- Sever, R.; Glass, C.K. Signaling by Nuclear Receptors. Cold Spring Harb. Perspect. Biol. 2013, 5, 1–5. [Google Scholar] [CrossRef]
- Wierman, M.E. Sex steroid effects at target tissues: Mechanisms of action. Adv. Physiol. Educ. 2007, 31, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Piferrer, F. Epigenetics of sex determination and gonadogenesis. Dev. Dyn. 2013, 242, 360–370. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Zou, C.; Qin, B. The association between nuclear receptors and ocular diseases. Oncotarget 2017, 8, 27603–27615. [Google Scholar] [CrossRef] [PubMed]
- Condon, C.J.; Hardy, D.B.; Kovaric, K.; Mendelson, C.R. Up-regulation of the progesterone receptor (PR)-C isoform in laboring myometrium by activation of nuclear factor-kappaB may contribute to the onset of labor through inhibition of PR function. Mol. Endocrinol. 2006, 20, 764–775. [Google Scholar] [CrossRef] [PubMed]
- Chan, K.K.-L.; Leung, T.H.-Y.; Chan, D.W.; Wei, N.; Lau, G.T.-Y.; Liu, S.S.; Siu, M.K.Y.; Ngan, H.Y.-S. Targeting estrogen receptor subtypes (ERα and ERβ) with selective ER modulators in ovarian cancer. J. Endocrinol. 2014, 221, 325–336. [Google Scholar] [CrossRef]
- Giguère, V. To ERR in the estrogen pathway. Trends Endocrinol. Metab. 2002, 13, 220–225. [Google Scholar] [CrossRef]
- Puzianowska-Kuznicka, M.; Pawlik-Pachucka, E.; Owczarz, M.; Polosak, J. Small-Molecule Hormones: Molecular Mechanisms of Action. Int. J. Endocrinol. 2013, 601246. [Google Scholar] [CrossRef]
- Heinlein, C.A.; Chang, C. The roles of androgen receptors and androgen-binding proteins in nongenomic androgen actions. Mol. Endocrinol. 2002, 16, 2181–2187. [Google Scholar] [CrossRef]
- Moriarty, K.; Kim, K.H.; Bender, J.R. Minireview: Estrogen receptor-mediated rapid signaling. Endocrinology 2006, 147, 5557–5563. [Google Scholar] [CrossRef]
- Simoncini, T.; Genazzani, A. Non-genomic actions of sex steroid hormones. Eur. J. Endocrinol. 2003, 281–292. [Google Scholar] [CrossRef]
- Kamińska, A.; Pardyak, L.; Marek, S.; Wróbel, K.; Kotula-Balak, M.; Bilińska, B.; Hejmej, A. Notch signaling regulates nuclear androgen receptor AR and membrane androgen receptor ZIP9 in mouse Sertoli cells. Andrology 2019, 8. [Google Scholar] [CrossRef]
- Soltysik, K.; Czekaj, P. Membrane estrogen receptors—Is it an alternative way of estrogen action? J. Physiol. Pharmacol. 2013, 64, 129–142. [Google Scholar] [PubMed]
- Valades-Cosmes, P.; Vázquez-Martínez, E.R.; Cerbón, M.; Camacho-Arroyo, I. Membrane progesterone receptors in reproduction and cancer. Mol. Cell. Endocrinol. 2016, 434, 166–175. [Google Scholar] [CrossRef] [PubMed]
- Kotula-Balak, M.; Pawlickyi, P.; Milon, A.; Tworzydlo, W.; Sekula, M.; Pacwa, A.; Gorowska-Wojtowicz, E.; Bilinska, B.; Pawlicka, B.; WIater, J.; et al. The role of G-protein-coupled membrane estrogen receptor in mouse Leydig cell function-in vivo and in vitro evaluation. Cell Tissue Res. 2018, 374, 389–412. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Liu, D. Does GPER Really Function as a G Protein-Coupled Estrogen Receptor in vivo? Front. Endocrinol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Podda, M.V.; Grassi, C. New perspectives in cyclic nucleotide-mediated functions in the CNS: The emerging role of cyclic nucleotide-gated (CNG) channels. Pflüg. Arch. 2014, 466, 1241–1257. [Google Scholar] [CrossRef]
- Williams, C.J. Signalling mechanisms of mammalian oocyte activation. Hum. Reprod. Update 2002, 8, 313–321. [Google Scholar] [CrossRef]
- Navarrete, F.A.; García-Vázquez, F.A.; Alvau, A.; Escoffier, J.; Krapf, D.; Sánchez-Cárdenas, C. Biphasic role of calcium in mouse sperm capacitation signaling pathways. J. Cell. Physiol. 2015, 230, 1758–1769. [Google Scholar] [CrossRef]
- Wakabayashi, T. Mechanism of the calcium-regulation of muscle contraction: In pursuit of its structural basis. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 2015, 91, 321–350. [Google Scholar] [CrossRef]
- Südhof, T.C. Calcium control of neurotransmitter release. Cold Spring Harb. Perspect. Biol. 2012, 4. [Google Scholar] [CrossRef]
- Momeni, H.R. Role of calpain in apoptosis. Cell J. 2011, 13, 65–72. [Google Scholar] [PubMed]
- Aksamitiene, E.; Kiyatkin, A.; Kholodenko, B.N. Cross-talk between mitogenic Ras/MAPK and survival PI3K/Akt pathways: A fine balance. Biochem. Soc. Trans. 2012, 40, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Plotnikov, A.; Zehorai, E.; Procaccia, S.; Seger, R. The MAPK cascades: Signaling components, nuclear roles and mechanisms of nuclear translocation. Biochim. Biophys. Acta Mol. Cell. Res. 2011, 1813, 1619–1633. [Google Scholar] [CrossRef] [PubMed]
- Williams, R.A.; Timmis, J.; Qwarnstrom, E.E. Computational Models of the NF-B Signalling Pathway. Computation 2014, 20, 131–158. [Google Scholar] [CrossRef]
- Kiraz, Y.; Adan, A.; Kartal-Yandim, M.; Baran, Y. Major apoptotic mechanisms and genes involved in apoptosis. Tumor Biol. 2016, 37, 8471–8486. [Google Scholar] [CrossRef] [PubMed]
- Duronio, R.J.; Xiong, Y. Signaling pathways that control cell proliferation. Cold Spring Harb. Perspect. Biol. 2013, 5, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Mason, E.; Rathmell, J. Cell metabolism: An essential link between cell growth and apoptosis. Biochim. Biophys. Acta 2011, 1813, 645–654. [Google Scholar] [CrossRef]
- Weinhold, B. Epigenetics: The Science of Change. Environ. Health Perspect. 2006, 114. [Google Scholar] [CrossRef]
- Martinez-Arguelles, D.B.; Papadopoulos, V. Epigenetic regulation of the expression of genes involved in steroid hormone biosynthesis and action. Steroids 2010, 75, 467–476. [Google Scholar] [CrossRef]
- Benjamin, S.; Pradeep, S.; Sarath, J.M.; Kumar, S.; Masai, E. A monograph on the remediation of hazardous phthalates. J. Hazard. Mater. 2015, 298, 58–72. [Google Scholar] [CrossRef]
- Mikula, P.; Svobodová, Z.; Smutná, M. Phthalates: Toxicology and food safety—A review. Czech J. Food Sci. 2005, 23, 217–223. [Google Scholar] [CrossRef]
- Staples, C.A.; Peterson, D.R.; Parkerton, T.F.; Adams, W.J. The environmental fate of phthalate esters: A literature review. Chemosphere 1997, 35, 667–749. [Google Scholar] [CrossRef]
- CDC. Phthalates Factsheet. Available online: https://www.cdc.gov/biomonitoring/Phthalates_FactSheet.html (accessed on 6 April 2019).
- Yen, T.H.; Lin-Tan, D.T.; Lin, J.L. Food safety involving ingestion of foods and beverages prepared with phthalate-plasticizer-containing clouding agents. J. Formos. Med. Assoc. 2011, 110, 671–684. [Google Scholar] [CrossRef] [PubMed]
- Petrovičová, I.; Kolena, B.; Pilka, T. The human biomonitoring of occupational exposure to phthalates. Mediterr. J. Soc. Sci. 2014, 5, 101–107. [Google Scholar] [CrossRef][Green Version]
- Przybylińska, P.A.; Wyszkowski, M. Environmental contamination with phthalates and its impact on living organisms. Ecol. Chem. Eng. 2016, 23, 347–356. [Google Scholar] [CrossRef]
- Latini, G. Monitoring phthalate exposure in humans. Clin. Chim. Acta 2005, 361. [Google Scholar] [CrossRef]
- Bonde, J.P.; Flachs, E.M.; Rimborg, S.; Glazer, C.H.; Giwercman, A.; Ramlau-Hansen, C.H.; Rylander, L. The epidemiologic evidence linking prenatal and postnatal exposure to endocrine disrupting chemicals with male reproductive disorders: A systematic review and meta-analysis. Hum. Reprod. Update 2016, 23, 104–125. [Google Scholar] [CrossRef]
- Pryor, J.L.; Hughes, C.; Foster, W.; Hales, B.F.; Robaire, B. Critical windows of exposure for children’s health: The reproductive system in animals and humans. Environ. Health Perspect. 2000, 108 (Suppl. 3), 491–503. [Google Scholar]
- Ventrice, P.; Ventrice, D.; Russo, E.; De Sarro, G. Phthalates: European regulation, chemistry, pharmacokinetic and related toxicity. Environ. Toxicol. Pharmacol. 2013, 36, 88–96. [Google Scholar] [CrossRef]
- Johns, L.E.; Cooper, G.S.; Galizia, A.; Meeker, J.D. Exposure Assessment Issues in Epidemiology Studies of Phthalates. Environ. Int. 2015. [Google Scholar] [CrossRef]
- Frederiksen, H.; Skakkebæk, N.E.; Andersson, A.-M. Metabolism of phthalates in humans. Mol. Nutr. Food. Res. 2007, 51. [Google Scholar] [CrossRef] [PubMed]
- Hoppin, J.A.; Brock, J.W.B.; Davis, B.J.; Baird, D.D. Reproducibility of urinary phthalate metabolites in first morning urine samples. Environ. Health Perpsect. 2002, 110, 515–518. [Google Scholar] [CrossRef]
- Koch, H.M.; Rüther, M.; Schütze, A.; Conrad, A.; Pälmke, C.; Apel, P.; Kolossa-Gehring, M. Phthalate metabolites in 24-h urine samples of the German Environmental Specimen Bank (ESB) from 1988 to 2015 and a comparison with US NHANES data from 1999 to 2012. Int. J. Hyg. Environ. Health 2017, 220, 130–141. [Google Scholar] [CrossRef] [PubMed]
- Meeker, J.D.; Ferguson, K.K. Urinary phthalate metabolites are associated with decreased serum testosterone in men, women, and children from NHANES 2011–2012. J. Clin. Endocrinol. Metab. 2014, 99, 4346–4352. [Google Scholar] [CrossRef] [PubMed]
- Diamanti-Kandarakis, E.; Bourguignon, J.P.; Giudice, L.C.; Hauser, R.; Prins, G.S.; Soto, A.M.; Gore, A.C. Endocrine-Disrupting Chemicals: An Endocrine Society Scientific Statement. Endocr. Rev. 2009, 30, 293–342. [Google Scholar] [CrossRef]
- Kortenkamp, A. Low dose mixture effects of endocrine disrupters and their implications for regulatory thresholds in chemical risk assessment. Curr. Opin. Pharmacol. 2014, 19, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Vandenberg, L.N.; Colborn, T.; Hayes, T.B.; Heindel, J.J.; Jacobs, D.R.; Lee, D.H. Hormones and Endocrine-Disrupting Chemicals: Low-Dose Effects and Nonmonotonic Dose Responses. Endocr. Rev. 2012, 33, 378–455. [Google Scholar] [CrossRef]
- Foster, P.M.D.; Mylchreest, E.; Gaido, K.W.; Sar, M. Effects of phthalate esters on the developing reproductive tract of male rats. Hum. Reprod. Update 2001, 7, 231–235. [Google Scholar] [CrossRef]
- Sun, Y.; Shen, J.; Zeng, L.; Yang, D.; Shao, S.; Wang, J. Role of autophagy in di-2-ethylhexyl phthalate (DEHP)-induced apoptosis in mouse Leydig cells. Environ. Pollut. 2018, 243, 563–572. [Google Scholar] [CrossRef]
- Wang, H.; Wang, J.; Zhang, J.; Jin, S.; Li, H. Role of PI3K/AKT/mTOR signaling pathway in DBP-induced apoptosis of testicular sertoli cells in vitro. Environ. Toxicol. Pharmacol. 2017, 53, 145–150. [Google Scholar] [CrossRef]
- Li, L.; Bu, T.; Su, H.; Chen, Z.; Liang, Y.; Zhang, G. Inutero exposure to diisononyl phthalate caused testicular dysgenesis of rat fetal testis. Toxicol. Lett. 2015, 232, 466–474. [Google Scholar] [CrossRef] [PubMed]
- Mahood, I.K.; Mckinnell, C.; Walker, M.; Hallmark, N.; Scott, H.; Fisher, J.S. Cellular origins of testicular dysgenesis in rats exposed in utero to di(n-butyl) phthalate. Int. J. Androl. 2006, 29, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Chen, X.; Hu, G.; Li, L.; Su, H.; Wang, Y. Effects of in utero exposure to dicyclohexyl phthalate on rat fetal leydig cells. Int. J. Environ. Res. Public Health 2016, 13, 246. [Google Scholar] [CrossRef] [PubMed]
- Aly, H.A.; Hassan, M.H.; El-Beshbishy, H.A.; Alahdal, A.M.; Moneim, A.; Osman, M. Dibutyl phthalate induces oxidative stress and impairs spermatogenesis in adult rat. Toxicol. Ind. Health 2015, 32, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Thurston, S.W.; Mendiola, J.; Bellamy, A.R.; Levine, H.; Wang, C.; Sparks, A.; Swan, S.H. Phthalate exposure and semen quality in fertile US men. Andrology 2016, 4. [Google Scholar] [CrossRef]
- Liu, L.; Bao, H.; Liu, F.; Zhang, J.; Shen, H. Phthalates exposure of Chinese reproductive age couples and its effect on male semen quality, a primary study. Environ. Int. 2012, 42, 78–83. [Google Scholar] [CrossRef]
- Jurewicz, J.; Radwan, M.; Sobala, W.; Ligocka, D.; Radwan, P.; Bochenek, M. Human urinary phthalate metabolites level and main semen parameters, sperm chromatin structure, sperm aneuploidy and reproductive hormones. Reprod. Toxicol. 2013, 42, 232–241. [Google Scholar] [CrossRef]
- Wang, Y.-X.; Zeng, Q.; Sun, Y.; Yang, P.; Wang, P.; Li, J. Semen phthalate metabolites, semen quality parameters and serum reproductive hormones: A cross-sectional study in China. Environ. Pollut. 2016, 211, 173–182. [Google Scholar] [CrossRef]
- Schiffer, C.; Müller, A.; Egeberg, D.L.; Alvarez, L.; Brenker, C.; Rehfeld, A. Direct action of endocrine disrupting chemicals on human sperm. EMBO Rep. 2014, 15, 758–765. [Google Scholar] [CrossRef]
- Sharpe, R.M.; Skakkebaek, N.E. Testicular dysgenesis syndrome: Mechanistic insights and potential new downstream effects. Fertil. Steril. 2008, 89, e33–e38. [Google Scholar] [CrossRef]
- Bay, K.; Asklund, C.; Skakkebaek, N.E.; Andersson, A.M. Testicular dysgenesis syndrome: Possible role of endocrine disrupters. Best Pract. Res. Clin. Endocrinol. Metab. 2006, 20, 77–90. [Google Scholar] [CrossRef] [PubMed]
- Sharpe, R.M. Hormones and testis development and the possible adverse effects of environmental chemicals. Toxicol. Lett. 2001, 120, 221–232. [Google Scholar] [CrossRef]
- Toppari, J.; Virtanen, H.E.; Main, K.M.; Skakkebaek, N.E. Cryptorchidism and hypospadias as a sign of testicular dysgenesis syndrome (TDS): Environmental connection. Birth Defects Res. Part A Clin. Mol. Teratol. 2010, 88, 910–919. [Google Scholar] [CrossRef] [PubMed]
- Schoeters, G.; Den, H.E.; Dhooge, W.; Van Larebeke, N.; Leijs, M. Endocrine Disruptors and Abnormalities of Pubertal Development. Basic Clin. Pharmacol. Toxicol. 2008, 102, 168–175. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Chou, Y.; Wu, Y.; Lin, C.; Lin, S.; Lee, C. Phthalates may promote female puberty by increasing kisspeptin activity. Hum. Reprod. 2013, 28, 2765–2773. [Google Scholar] [CrossRef]
- Wolff, M.S.; Teitelbaum, S.L.; Mcgovern, K.; Windham, G.C.; Pinney, S.M.; Galvez, M. Phthalate exposure and pubertal development in a longitudinal study of US girls. Hum. Reprod. 2014, 29, 1558–1566. [Google Scholar] [CrossRef]
- Ge, R.-S.; Chen, G.-R.; Dong, Q.; Akingbemi, B.; Sottas, C.M.; Santos, M. Biphasic Effects of Postnatal Exposure to Diethylhexylphthalate on the Timing of Puberty in Male Rats. J. Androl. 2007, 28, 513–520. [Google Scholar] [CrossRef]
- Shi, H.; Cao, Y.; Shen, Q.; Zhao, Y.; Zhang, Z.; Zhang, Y. Association Between Urinary Phthalates and Pubertal Timing in Chinese Adolescents. J. Epidemiol. 2015, 25, 574–582. [Google Scholar] [CrossRef]
- Xie, C.; Zhao, Y.; Gao, L.; Chen, J.; Cai, D.; Zhang, Y. Elevated phthalates’ exposure in children with constitutional delay of growth and puberty. Mol. Cell. Endocrinol. 2015, 407, 67–73. [Google Scholar] [CrossRef]
- Zhang, Y.; Cao, Y.; Shi, H.; Jiang, X.; Zhao, Y.; Fang, X. Could exposure to phthalates speed up or delay pubertal onset and development? A 1.5-year follow-up of a school-based population. Environ. Int. 2015, 83, 41–49. [Google Scholar] [CrossRef]
- Mouritsen, A.; Frederiksen, H.; Sørensen, K.; Aksglaede, L.; Hagen, C.; Skakkebaek, N.E. Urinary Phthalates From 168 Girls and Boys Measured Twice a Year During a 5-Year Period: Associations with Adrenal Androgen Levels and Puberty. J. Clin. Endocrinol. Metab. 2013, 98, 3755–3764. [Google Scholar] [CrossRef]
- Berger, K.; Eskenazi, B.; Kogut, K.; Parra, K.; Lustig, R.H.; Greenspan, L.C. Association of Prenatal Urinary Concentrations of Phthalates and Bisphenol A and Pubertal Timing in Boys and Girls. Environ. Health Perspect. 2018, 126, 097004. [Google Scholar] [CrossRef]
- Mieritz, M.G.; Frederiksen, H.; Sørensen, K.; Aksglaede, L.; Mouritsen, A.; Hagen, C.P. Urinary phthalate excretion in 555 healthy Danish boys with and without pubertal gynaecomastia. Int. J. Androl. 2012, 35, 227–235. [Google Scholar] [CrossRef] [PubMed]
- Jakobsson, J.; Ekström, L.; Inotsume, N.; Garle, M.; Lorentzon, M.; Ohlsson, C. Large Differences in Testosterone Excretion in Korean and Swedish Men Are Strongly Associated with a UDP-Glucuronosyl Transferase 2B17 Polymorphism. J. Clin. Endocrinol. Metab. 2006, 91, 687–693. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.M.; Chen, S.K.; Chen, R.M.; Zhu, C.; Xiong, F.; Li, T. Pubertal development timing in urban Chinese boys. Int. J. Androl. 2011, 34, e435–e445. [Google Scholar] [CrossRef] [PubMed]
- Litman, H.J.; Bhasin, S.; Link, C.L.; Araujo, A.B.; McKinlay, J.B. Serum Androgen Levels in Black, Hispanic, and White Men. J. Clin. Endocrinol. Metab. 2006, 91, 4326–4334. [Google Scholar] [CrossRef]
- Herman-Giddens, M.E.; Wang, L.; Koch, G. Secondary sexual characteristics in boys: Estimates from the national health and nutrition examination survey III, 1988-1994. Arch. Pediatr. Adolesc. Med. 2001, 155, 1022–1028. [Google Scholar] [CrossRef]
- Renwick, A.G. Inter-ethnic differences in xenobiotic metabolism. Environ. Toxicol. Pharmacol. 1996, 2, 165–170. [Google Scholar] [CrossRef]
- Zhou, C.; Dhall, D.; Nissen, N.N.; Chen, C.-R.; Yu, R. A homozygous P86S mutation of the human glucagon receptor is associated with hyperglucagonemia, α cell hyperplasia, and islet cell tumor. Pancreas 2009, 38, 941–946. [Google Scholar] [CrossRef]
- James-Todd, T.M.; Meeker, J.D.; Huang, T.; Hauser, R.; Seely, E.W.; Ferguson, K.K. Racial and ethnic variations in phthalate metabolite concentration changes across full-term pregnancies. J. Expo. Sci. Environ. Epidemiol. 2017, 27, 160–166. [Google Scholar] [CrossRef]
- Bu, S.; Wang, Y.; Wang, H.; Wang, F.; Tan, Y. Analysis of global commonly-used phthalates and non-dietary exposure assessment in indoor environment. Build. Environ. 2020, 177. [Google Scholar] [CrossRef]
- Perera, F.; Herbstman, J. Prenatal environmental exposures, epigenetics, and disease. Reprod. Toxicol. 2011, 31, 363–373. [Google Scholar] [CrossRef] [PubMed]
- Abreu Velez, A.M.; Howard, M.S. Tumor-suppressor genes, cell cycle regulatory checkpoints, and the skin. N. Am. J. Med. Sci. 2015, 7, 176–188. [Google Scholar] [CrossRef] [PubMed]
- Chang, W.; Tsai, Y.; Wang, J.; Chen, H.; Yang, W. Sex hormones and oxidative stress mediated phthalate-induced effects in prostatic enlargement. Environ. Int. 2019, 126, 184–192. [Google Scholar] [CrossRef] [PubMed]
- Scarano, W.R.; Toledo, F.C.; Guerra, M.T.; Campos, S.G.P.; Júnior, L.A.J.; Felisbino, S.L. Long-term effects of developmental exposure to di-n-butyl-phthalate (DBP) on rat prostate: Proliferative and inflammatory disorders and a possible role of androgens. Toxicology 2009, 262, 215–223. [Google Scholar] [CrossRef]
- Zhu, M.; Huang, C.; Ma, X.; Wu, R.; Zhu, W.; Li, X. Phthalates promote prostate cancer cell proliferation through activation of ERK5 and p38. Environ. Toxicol. Pharmacol. 2018, 63, 29–33. [Google Scholar] [CrossRef]
- Zhu, M.; Wu, J.; Ma, X.; Huang, C.; Wu, R.; Zhu, W. Butyl benzyl phthalate promotes prostate cancer cell proliferation through miR-34a downregulation. Toxicol. Vitr. 2019, 54, 82–88. [Google Scholar] [CrossRef]
- Lymperi, S.; Giwercman, A. Endocrine disruptors and testicular function. Metabolism 2018, 86, 79–90. [Google Scholar] [CrossRef]
- Sweeney, M.F.; Hasan, N.; Soto, A.M.; Sonnenschein, C. Environmental endocrine disruptors: Effects on the human male reproductive system. Rev. Endocr. Metab. Disord. 2015, 16, 341–357. [Google Scholar] [CrossRef]
- Mohan, H. The Male Reproductive System and Prostate. In Textbook of Pathology, 6th ed.; Mohan, P., Mohan, T., Mohan, S., Eds.; Jaypee Brothers Medical Publishers (P) Ltd.: New Delhi, India, 2010; pp. 703–720. [Google Scholar]
- Ohlson, C.-G.; Hardell, L. Testicular cancer and occupational exposures with a focus on xenoestrogens in polyvinyl chloride plastics. Chemosphere 2000, 40, 1277–1282. [Google Scholar] [CrossRef]
- Westberg, H.B.T.; Hardell, L.O.; Malmqvist, N.; Ohlson, C.-G.; Axelson, O. On the Use of Different Measures of Exposure—Experiences from a Case-Control Study on Testicular Cancer and PVC Exposure. J. Occup. Environ. Hyg. 2005, 2, 351–356. [Google Scholar] [CrossRef] [PubMed]
- Hardell, L.; Malmqvist, N.; Ohlson, C.-G.; Westberg, H.; Eriksson, M. Testicular cancer and occupational exposure to polyvinyl chloride plastics: A case-control study. Int. J. Cancer 2004, 109, 425–429. [Google Scholar] [CrossRef] [PubMed]
- Hardell, L.; Ohlson, C.-G.; Fredrikson, M. Occupational exposure to polyvinyl chloride as a risk factor for testicular cancer evaluated in a case-control study. Int. J. Cancer 1997, 73, 828–830. [Google Scholar] [CrossRef]
- Ghazarian, A.A.; Trabert, B.; Robien, K.; Graubard, B.I.; Mcglynn, K.A. Maternal use of personal care products during pregnancy and risk of testicular germ cell tumors in sons. Environ. Res. 2018, 164, 109–113. [Google Scholar] [CrossRef]
- Patiño-Garćia, D.; Cruz-Fernandes, L.; Buñay, J.; Palomino, J.; Moreno, R.D. Reproductive alterations in chronically exposed female mice to environmentally relevantdoses of a mixture of phthalates and alkylphenols. Endocrinology 2018, 159, 1050–1061. [Google Scholar] [CrossRef]
- Moyer, B.; Hixon, M.L. Reproductive effects in F1 adult females exposed in utero to moderate to high doses of mono-2-ethylhexylphthalate (MEHP). Reprod. Toxicol. 2012, 34, 43–50. [Google Scholar] [CrossRef]
- Hannon, P.R.; Brannick, K.E.; Wang, W.; Gupta, R.K.; Flaws, J.A. Di(2-ethylhexyl) phthalate inhibits antral follicle growth, induces atresia, and inhibits steroid hormone production in cultured mouse antral follicles. Toxicol. Appl. Pharmacol. 2015, 284, 42–53. [Google Scholar] [CrossRef]
- Carnevali, O.; Tosti, L.; Speciale, C.; Peng, C.; Zhu, Y.; Maradonna, F. DEHP impairs zebrafish reproduction by affecting critical factors in oogenesis. PLoS ONE 2010, 5, e10201. [Google Scholar] [CrossRef]
- Yin, J.; Liu, R.; Jian, Z.; Yang, D.; Pu, Y.; Yin, L.; Wang, D. Di (2-ethylhexyl) phthalate-induced reproductive toxicity involved in dna damage-dependent oocyte apoptosis and oxidative stress in Caenorhabditis elegans. Ecotoxicol. Environ. Saf. 2018, 15, 298–306. [Google Scholar] [CrossRef]
- Zhang, X.F.; Zhang, L.J.; Li, L.; Feng, Y.N.; Chen, B.; Ma, J.M.; Shen, W. Diethylhexyl phthalate exposure impairs follicular development and affects oocyte maturation in the mouse. Environ. Mol. Mutagen. 2013, 54. [Google Scholar] [CrossRef]
- Liu, J.-C.; Lai, F.-N.; Li, L.; Sun, X.-F.; Cheng, S.-F. Di (2-ethylhexyl) phthalate exposure impairs meiotic progression and DNA damage repair in fetal mouse oocytes in vitro. Cell. Death Dis. 2017, 8, e2966. [Google Scholar] [CrossRef]
- Mirihagalle, S.; You, T.; Suh, L.; Patel, C.; Gao, L.; Rattan, S.; Qiao, H. Prenatal exposure to di-(2-ethylhexyl) phthalate and high-fat diet synergistically disrupts mouse fetal oogenesis and affects folliculogenesis. Biol. Reprod. 2014, 100, 1561–1570. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Li, L.; Qin, X.S.; Zhou, Y.; Zhang, X.F.; Wang, L.Q.; De Felici, M.; Chen, H.; Qin, G.Q.; Shen, W. Di-(2-ethylhexyl) phthalate and bisphenol a exposure impairs mouse primordial follicle assembly in vitro. Environ. Mol. Mutagen. 2014, 55. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Shen, W.; De Felici, M.; Zhang, X.-F. Di(2-ethylhexyl)phthalate: Adverse effects on folliculogenesis that cannot be neglected. Environ. Mol. Mutagen. 2016, 57. [Google Scholar] [CrossRef]
- Lu, Z.; Zhang, C.; Han, C.; An, Q.; Cheng, Y.; Chen, Y. Plasticizer Bis(2-ethylhexyl) Phthalate Causes Meiosis Defects and Decreases Fertilization Ability of Mouse Oocytes in Vivo. J. Agric. Food Chem. 2019, 67, 3459–3468. [Google Scholar] [CrossRef] [PubMed]
- Ma, M.; Kondo, T.; Ban, S.; Umemura, T.; Kurahashi, N.; Takeda, M. Exposure of Prepubertal Female Rats to Inhaled Di(2-ethylhexyl)phthalate Affects the Onset of Puberty and Postpubertal Reproductive Functions. Toxicol. Sci. 2006, 93, 164–171. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Gao, L.; Flaws, J.A. Prenatal exposure to an environmentally relevant phthalate mixture disrupts reproduction in F1 female mice. Toxicol. Appl. Pharmacol. 2017, 318, 49–57. [Google Scholar] [CrossRef]
- Liu, T.; Li, N.; Zhu, J.; Yu, G.; Guo, K.; Zhou, L.; Zheng, D.; Qu, X.; Huang, J.; Chen, X.; et al. Effects of di-(2-ethylhexyl) phthalate on the hypothalamus-pituitary-ovarian axis in adult female rats. Reprod. Toxicol. 2014, 46, 141–147. [Google Scholar] [CrossRef]
- Li, S.-W.; How, C.M.; Liao, V.H.-C. Prolonged exposure of di (2-ethylhexyl) phthalate induces multigenerational toxic effects in Caenorhabditis elegans. Sci. Total Environ. 2018, 634, 260–266. [Google Scholar] [CrossRef]
- Ayesha, J.V.; Goswami, D. Premature Ovarian Failure: An Association with Autoimmune Diseases. J. Clin. Diagn. Res. 2016, 10. [Google Scholar] [CrossRef]
- Gallicchio, L.; Miller, S.; Greene, T.; Zacur, H.; Flaws, J. Premature ovarian failure among hairdressers. Hum. Reprod. 2009, 24, 2636–2641. [Google Scholar] [CrossRef] [PubMed]
- Vabre, P.; Gatimel, N.; Moreau, J.; Gayrard, V.; Picard-Hagen, N.; Parinaud, J. Environmental pollutants, a possible etiology for premature ovarian insufficiency: A narrative review of animal and human data. Environ. Health 2017, 16, 37. [Google Scholar] [CrossRef] [PubMed]
- Messerlian, C.; Souter, I.; Gaskins, A.J.; Williams, P.L.; Ford, J.B.; Chiu, Y.-H. Urinary phthalate metabolites and ovarian reserve among women seeking infertility care. Hum. Reprod. 2016, 31, 75–83. [Google Scholar] [CrossRef]
- Meltzer, D.; Martinez-Arguelles, D.B.; Campioli, E.; Lee, S.; Papadopoulos, V. In utero exposure to the endocrine disruptor di(2-ethylhexyl) phthalate targets ovarian theca cells and steroidogenesis in the adult female rat. Reprod. Toxicol. 2015, 51, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Jankowska, K. Premature ovarian failure. Prz. Menopauzalny 2017, 16, 51–56. [Google Scholar] [CrossRef]
- Liu, T.; Wang, Y.; Yang, M.; Shao, P.; Duan, L.; Li, M. Di-(2-ethylhexyl) phthalate induces precocious puberty in adolescent female rats. Iran. J. Basic Med. Sci. 2018, 21, 848. [Google Scholar] [CrossRef]
- Srilanchakon, K.; Thadsri, T.; Jantarat, C.; Thengyai, S.; Nosoognoen, W.; Supornsilchai, V. Higher phthalate concentrations are associated with precocious puberty in normal weight Thai girls. J. Pediatr. Endocrinol. Metab. 2017, 30, 1293–1298. [Google Scholar] [CrossRef]
- Hashemipour, M.; Kelishadi, R.; Amin, M.M.; Ebrahim, K. Is there any association between phthalate exposure and precocious puberty in girls? Environ. Sci. Pollut. Res. 2018, 25, 13589–13596. [Google Scholar] [CrossRef]
- Wolff, M.S.; Teitelbaum, S.L.; Pinney, S.M.; Windham, G.; Liao, L.; Biro, F. Investigation of relationships between urinary biomarkers of phytoestrogens, phthalates, and phenols and pubertal stages in girls. Environ. Health Perspect. 2010, 118, 1039–1046. [Google Scholar] [CrossRef]
- Binder, A.M.; Corvalan, C.; Calafat, A.M.; Ye, X.; Mericq, V.; Pereira, A. Childhood and adolescent phenol and phthalate exposure and the age of menarche in Latina girls. Environ. Health 2018, 17, 32. [Google Scholar] [CrossRef]
- Newbold, R.R. Impact of environmental endocrine disrupting chemicals on the development of obesity. Hormones 2010, 9, 206–217. [Google Scholar] [CrossRef] [PubMed]
- Kasper-Sonnenberg, M.; Wittsiepe, J.; Wald, K.; Koch, H.M.; Wilhelm, M. Pre-pubertal exposure with phthalates and bisphenol A and pubertal development. PLoS ONE 2017, 12, e0187922. [Google Scholar] [CrossRef] [PubMed]
- Frederiksen, H.; Sørensen, K.; Mouritsen, A.; Aksglaede, L.; Hagen, C.P.; Petersen, J.H. High urinary phthalate concentration associated with delayed pubarche in girls. Int. J. Androl. 2012, 35, 216–226. [Google Scholar] [CrossRef] [PubMed]
- Zong, T.; Lai, L.; Hu, J.; Guo, M.; Li, M.; Zhang, L. Maternal exposure to di-(2-ethylhexyl) phthalate disrupts placental growth and development in pregnant mice. J. Hazard. Mater. 2015, 297, 25–33. [Google Scholar] [CrossRef]
- Yu, Z.; Han, Y.; Shen, R.; Huang, K.; Xu, Y.; Wang, Q. Gestational di-(2-ethylhexyl) phthalate exposure causes fetal intrauterine growth restriction through disturbing placental thyroid hormone receptor signaling. Toxicol. Lett. 2018, 294, 1–10. [Google Scholar] [CrossRef]
- Toft, G.; Jönsson, B.A.G.; Lindh, C.H.; Jensen, T.K.; Hjollund, N.H.; Vested, A. Association between Pregnancy Loss and Urinary Phthalate Levels around the Time of Conception. Environ. Health Perspect. 2012, 120, 458–463. [Google Scholar] [CrossRef]
- Messerlian, C.; Wylie, B.J.; Mínguez-Alarcón, L.; Williams, P.L.; Ford, J.B.; Souter, I.C. Urinary Concentrations of Phthalate Metabolites and Pregnancy Loss Among Women Conceiving with Medically Assisted Reproduction. Epidemiology 2016, 27, 879–888. [Google Scholar] [CrossRef]
- Yi, H.; Gu, H.; Zhou, T.; Chen, Y. A pilot study on association between phthalate exposure and missed miscarriage. europeanreview.org. Eur. Rev. Med. Pharmacol. Sci. 2016, 20, 1894–1902. [Google Scholar]
- Liao, K.-W.; Kuo, P.-L.; Huang, H.-B.; Chang, J.-W.; Chiang, H.-C.; Huang, P.-C. Increased risk of phthalates exposure for recurrent pregnancy loss in reproductive-aged women. Environ. Pollut. 2018, 241, 969–977. [Google Scholar] [CrossRef]
- Adibi, J.J.; Hauser, R.; Williams, P.L.; Whyatt, R.M.; Calafat, A.M.; Nelson, H. Original Contribution Maternal Urinary Metabolites of Di-(2-Ethylhexyl) Phthalate in Relation to the Timing of Labor in a US Multicenter Pregnancy Cohort Study. Am. J. Epidemiol. 2009, 169, 1015–1024. [Google Scholar] [CrossRef]
- Ferguson, K.K.; McElrath, T.F.; Meeker, J.D. Environmental phthalate exposure and preterm birth. JAMA Pediatr. 2014, 168, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Latini, G.; Felice, C.; Presta, G.; Vecchio, A.; Paris, I.; Ruggieri, F. In Utero Exposure to Di-(2-ethylhexyl) phthalate and Duration of Human Pregnancy. Environ. Health Perspect. 2003, 111, 1783–1785. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Hu, W.; Li, Y.; Shen, H.; Hu, J. Mono-2-ethylhexyl phthalate inhibits human extravillous trophoblast invasion via the PPARγ pathway. Toxicol. Appl. Pharmacol. 2017, 327, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Shoaito, H.; Petit, J.; Chissey, A.; Auzeil, N.; Guibourdenche, J.; Gil, S. The Role of Peroxisome Proliferator–Activated Receptor Gamma (PPARγ) in Mono(2-ethylhexyl) Phthalate (MEHP)-Mediated Cytotrophoblast Differentiation. Environ. Health Perspect. 2019, 127, 027003. [Google Scholar] [CrossRef] [PubMed]
- Pinkas, A.; Gonçalves, C.L.; Aschner, M. Neurotoxicity of fragrance compounds: A review. Environ. Res. 2017, 158, 342–349. [Google Scholar] [CrossRef] [PubMed]
- Choi, K.-C.; Hwang, K.-A.; Lee, H.-R.; Yi, B.-R.; Jeung, E.-B.; Choi, K.-C. Cell growth of BG-1 ovarian cancer cells is promoted by di-n-butyl phthalate and hexabromocyclododecane via upregulation of the cyclin D and cyclin-dependent kinase-4 genes. Mol. Med. Rep. 2011, 5, 761–766. [Google Scholar] [CrossRef]
- Yang, W.; Tan, W.; Zheng, J.; Zhang, B.; Li, H.; Li, X. MEHP promotes the proliferation of cervical cancer via GPER mediated activation of Akt. Eur. J. Pharmacol. 2018, 824, 11–16. [Google Scholar] [CrossRef]
- Neamtiu, I.A.; Bloom, M.S.; Dumitrascu, I.; Roba, C.A.; Pop, C.; Ordeanu, C. Impact of exposure to tobacco smoke, arsenic, and phthalates on locally advanced cervical cancer treatment—Preliminary results. Peer J. 2016, 4, e2448. [Google Scholar] [CrossRef]
- Kim, J.; Kim, S.; Oh, Y.; Ihm, H.; Chae, H.; Kim, C.-H.; Kang, B.M. In vitro effects of phthalate esters in human myometrial and leiomyoma cells and increased urinary level of phthalate metabolite in women with uterine leiomyoma. Fertil. Steril. 2017, 107, 1061–1069. [Google Scholar] [CrossRef]
- Weuve, J.; Hauser, R.; Calafat, A.M.; Missmer, S.A.; Wise, L.A. Association of Exposure to Phthalates with Endometriosis and Uterine Leiomyomata: Findings from NHANES, 1999–2004. Environ. Health Perspect. 2010, 118, 825–832. [Google Scholar] [CrossRef]
- Pollack, A.Z.; Buck, L.G.M.; Chen, Z.; Sun, L.; Trabert, B.; Guo, Y. Bisphenol A, benzophenone-type ultraviolet filters, and phthalates in relation to uterine leiomyoma. Environ. Res. 2015, 137, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Zota, A.R.; Geller, R.J.; Calafat, A.M.; Marfori, C.Q.; Baccarelli, A.A.; Moawad, G.N. Phthalates exposure and uterine fibroid burden among women undergoing surgical treatment for fibroids: A preliminary study. Fertil. Steril. 2019, 111, 112–121. [Google Scholar] [CrossRef] [PubMed]
- Morgan, M.; Deoraj, A.; Felty, Q.; Yoo, C.; Roy, D. Association between exposure to estrogenic endocrine disruptors-polychlorinated biphenyls, phthalates, and bisphenol A and gynecologic cancers-cervical, ovarian, uterine cancers. J. Carcinog. Mutagen. 2016, 7, 6. [Google Scholar] [CrossRef]
- Fu, Z.; Zhao, F.; Chen, K.; Xu, J.; Li, P.; Xia, D. Association between urinary phthalate metabolites and risk of breast cancer and uterine leiomyoma. Reprod. Toxicol. 2017, 74, 134–142. [Google Scholar] [CrossRef] [PubMed]
- Rachoń, D. Endocrine disrupting chemicals (EDCs) and female cancer: Informing the patients. Rev. Endocr. Metab. Disord. 2015, 16, 359–364. [Google Scholar] [CrossRef]
- Rochefort, H. Endocrine disruptors (EDs) and hormone-dependent cancers: Correlation or causal relationship? Comptes Rendus Biol. 2017, 340, 439–445. [Google Scholar] [CrossRef]
- Hannon, P.R.; Flaws, J.A. The effects of phthalates on the ovary. Front. Endocrinol. 2015, 6, 1–19. [Google Scholar] [CrossRef]
- Sheikh, I.A.; Turki, R.F.; Abuzenadah, A.M.; Damanhouri, G.A.; Beg, M.A. Endocrine Disruption: Computational Perspectives on Human Sex Hormone-Binding Globulin and Phthalate Plasticizers. PLoS ONE 2016, 11, e0151444. [Google Scholar] [CrossRef]
- Martinez-Arguelles, D.B.; Guichard, T.; Culty, M.; Zirkin, B.R.; Papadopoulos, V. In Utero Exposure to the Antiandrogen Di-(2-Ethylhexyl) Phthalate Decreases Adrenal Aldosterone Production in the Adult Rat. Biol. Reprod. 2011, 85, 51–61. [Google Scholar] [CrossRef]
- Nuttall, J.R.; Kucera, H.R.; Supasai, S.; Gaikwad, N.W.; Oteiza, P.I. Combined effects of gestational phthalate exposure and zinc deficiency on steroid metabolism and growth. Toxicol. Sci. 2017, 156, 469–479. [Google Scholar] [CrossRef][Green Version]
- Carbone, S.; Samaniego, Y.A.; Cutrera, R.; Reynoso, R.; Cardoso, N.; Scacchi, P. Different effects by sex on hypothalamic-pituitary axis of prepubertal offspring rats produced by in utero and lactational exposure to di-(2-ethylhexyl) phthalate (DEHP). Neurotoxicology 2012, 33, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Jia, Y.; Zhou, L.; Wang, Q.; Sun, D.; Xu, J. Effects of Di-(2-ethylhexyl) Phthalate on the Hypothalamus–Uterus in Pubertal Female Rats. Int. J. Environ. Res. Public Health 2016, 13, 1130. [Google Scholar] [CrossRef] [PubMed]
- Sen, N.; Liu, X.; Craig, Z.R. Short term exposure to di-n-butyl phthalate (DBP) disrupts ovarian function in young CD-1 mice. Reprod. Toxicol. 2015, 53, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Brehm, E.; Rattan, S.; Gao, L.; Flaws, J.A. Prenatal exposure to Di(2-ethylhexyl) phthalate causes long-term transgenerational effects on female reproduction in mice. Endocrinology 2018, 159, 795–809. [Google Scholar] [CrossRef] [PubMed]
- Thompson, C.J.; Ross, S.M.; Hensley, J.; Liu, K.; Heinze, S.C.; Young, S.S. Differential Steroidogenic Gene Expression in the Fetal Adrenal Gland Versus the Testis and Rapid and Dynamic Response of the Fetal Testis to Di (n-butyl) Phthalate. Biol. Reprod. 2005, 73, 908–917. [Google Scholar] [CrossRef]
- Zhu, Y.-P.; Li, E.-H.; Sun, W.-L.; Xu, D.-L.; Liu, Z.-H.; Zhao, W. Maternal exposure to di-n-butyl phthalate (DBP) induces combined anorectal and urogenital malformations in male rat offspring. Oncotarget 2017, 8, 31101–31111. [Google Scholar] [CrossRef]
- Ha, M.; Guan, X.; Wei, L.; Li, P.; Yang, M.; Liu, C. Di-(2-ethylhexyl) phthalate inhibits testosterone level through disturbed hypothalamic-pituitary-testis axis and ERK-mediated 5α-Reductase 2. Sci. Total Environ. 2016, 563–564, 566–575. [Google Scholar] [CrossRef]
- Giribabu, N.; Reddy, P.S. Protection of male reproductive toxicity in rats exposed to di-n-butyl phthalate during embryonic development by testosterone. Biomed. Pharmacother. 2017, 87, 355–365. [Google Scholar] [CrossRef]
- Qin, X.; Ma, Q.; Yuan, J.; Hu, X.; Tan, Q. The effects of di-2-ethylhexyl phthalate on testicular ultrastructure and hormone-regulated gene expression in male rats. Toxicol. Res. 2018, 7, 408–414. [Google Scholar] [CrossRef]
- Abdel-Maksoud, F.M.; Leasor, K.R.; Butzen, K.; Braden, T.D.; Akingbemi, B.T. Prenatal Exposures of Male Rats to the Environmental Chemicals Bisphenol A and Di(2-Ethylhexyl) Phthalate Impact the Sexual Differentiation Process. Endocrinology 2015, 156, 4672–4683. [Google Scholar] [CrossRef]
- Lv, Y.; Dong, Y.; Wang, Y.; Zhu, Q.; Li, L.; Li, X. Benzyl butyl phthalate non-linearly affects rat Leydig cell development during puberty. Toxicol. Lett. 2019, 314, 53–62. [Google Scholar] [CrossRef]
- Yamaguchi, T.; Maeda, M.; Ogata, K.; Abe, J.; Utsumi, T.; Kimura, K. The effects on the endocrine system under hepatotoxicity induction by phenobarbital and di(2-ethylhexyl)phthalate in intact juvenile male rats. J. Toxicol. Sci. 2019, 44, 459–469. [Google Scholar] [CrossRef] [PubMed]
- Ambe, K.; Sakakibara, Y.; Sakabe, A.; Makino, H.; Ochibe, T.; Tohkin, M. Comparison of the developmental/reproductive toxicity and hepatotoxicity of phthalate esters in rats using an open toxicity data source. J. Toxicol. Sci. 2019, 44, 245–255. [Google Scholar] [CrossRef]
- Talsness, C.E.; Andrade, A.J.M.; Kuriyama, S.N.; Taylor, J.A.; Saal, F.S.V. Components of plastic: Experimental studies in animals and relevance for human health. Philosophical Transactions of the Royal Society B: Biological Sciences. R. Soc. 2009, 364, 2079–2096. [Google Scholar] [CrossRef]
- Johnson, K.J.; Heger, N.E.; Boekelheide, K. Of mice and men (and rats): Phthalate-induced fetal testis endocrine disruption is species-dependent. Toxicol. Sci. 2012, 129, 235–248. [Google Scholar] [CrossRef]
- Soldin, O.P.; Mattison, D.R. Sex differences in pharmacokinetics and pharmacodynamics. Clin. Pharmacokinet. 2009, 48, 143–157. [Google Scholar] [CrossRef]
- Repouskou, A.; Panagiotidou, E.; Panagopoulou, L.; Bisting, P.L.; Tuck, A.R.; Sjödin, M. Gestational exposure to an epidemiologically defined mixture of phthalates leads to gonadal dysfunction in mouse offspring of both sexes. Sci. Rep. 2019, 9, 6424. [Google Scholar] [CrossRef] [PubMed]
- Kelley, A.S.; Banker, M.; Goodrich, J.M.; Dolinoy, D.C.; Burant, C.; Domino, S.E. Early pregnancy exposure to endocrine disrupting chemical mixtures are associated with inflammatory changes in maternal and neonatal circulation. Sci. Rep. 2019, 9, 1–14. [Google Scholar] [CrossRef]
- Hart, R.J.; Frederiksen, H.; Doherty, D.A.; Keelan, J.A.; Skakkebaek, N.E.; Minaee, N.S. The possible impact of antenatal exposure to ubiquitous phthalates upon male reproductive function at 20 years of age. Front. Endocrinol. 2018, 9, 1–11. [Google Scholar] [CrossRef]
- Axelsson, J.; Rylander, L.; Rignell-Hydbom, A.; Jönsson, B.A.G.; Lindh, C.H.; Giwercman, A. Phthalate exposure and reproductive parameters in young men from the general Swedish population. Environ. Int. 2015, 85, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Jing, J.; Dong, F.; Yao, Q.; Zhang, W.; Zhang, H. Association between phthalate metabolites and biomarkers of reproductive function in 1066 Chinese men of reproductive age. J. Hazard. Mater. 2015, 300, 729–736. [Google Scholar] [CrossRef]
- Wen, H.-J.; Chen, C.-C.; Wu, M.-T.; Chen, M.-L.; Sun, C.-W.; Wu, W.-C. Phthalate exposure and reproductive hormones and sex-hormone binding globulin before puberty—Phthalate contaminated-foodstuff episode in Taiwan. PLoS ONE 2017, 12, e0175536. [Google Scholar] [CrossRef]
- Al-Saleh, I.; Coskun, S.; Al-Doush, I.; Al-Rajudi, T.; Abduljabbar, M.; Al-Rouqi, R. The relationships between urinary phthalate metabolites, reproductive hormones and semen parameters in men attending in vitro fertilization clinic. Sci. Total Environ. 2019, 658, 982–995. [Google Scholar] [CrossRef]
- Woodward, M.J.; Obsekov, V.; Jacobson, M.H.; Kahn, L.G.; Trasande, L. Phthalates and Sex Steroid Hormones Among Men From NHANES, 2013–2016. J. Clin. Endocrinol. Metab. 2020, 105, 2013–2016. [Google Scholar] [CrossRef]
- Mendiola, J.; Jørgensen, N.; Andersson, A.-M.; Calafat, A.M.; Silva, M.J.; Redmon, J.B. Associations between urinary metabolites of di(2-ethylhexyl) phthalate and reproductive hormones in fertile men. Int. J. Androl. 2011, 34, 369–378. [Google Scholar] [CrossRef]
- Maradonna, F.; Evangelisti, M.; Gioacchini, G.; Migliarini, B.; Olivotto, I.; Carnevali, O. Assay of vtg, ERs and PPARs as endpoint for the rapid in vitro screening of the harmful effect of Di-(2-ethylhexyl)-phthalate (DEHP) and phthalic acid (PA) in zebrafish primary hepatocyte cultures. Toxicol. In Vitro 2013, 27, 84–91. [Google Scholar] [CrossRef]
- Park, C.; Lee, J.; Kong, B.; Park, J.; Song, H.; Choi, K.O. The effects of bisphenol A, benzyl butyl phthalate, and di(2-ethylhexyl) phthalate on estrogen receptor alpha in estrogen receptor-positive cells under hypoxia. Environ. Pollut. 2019, 248, 774–781. [Google Scholar] [CrossRef]
- Lee, H.-R.; Hwang, K.-A.; Choi, K.-C. The estrogen receptor signaling pathway activated by phthalates is linked with transforming growth factor-β in the progression of LNCaP prostate cancer models. Int. J. Oncol. 2014, 45, 595–602. [Google Scholar] [CrossRef]
- Jönsson, B.A.G.; Richthoff, J.; Rylander, L.; Giwercman, A.; Hagmar, L. Urinary Phthalate Metabolites and Biomarkers of Reproductive Function in Young Men. Epidemiology 2005, 16, 487–493. [Google Scholar] [CrossRef]
- Duty, S.M.; Calafat, A.M.; Silva, M.J.; Ryan, L.; Hauser, R. Phthalate exposure and reproductive hormones in adult men. Hum. Reprod. 2005, 20, 604–610. [Google Scholar] [CrossRef]
- Cathey, A.L.; Watkins, D.; Rosario, Z.Y.; Vélez, C.; Alshawabkeh, A.N.; Cordero, J.F. Associations of Phthalates and Phthalate Replacements With CRH and Other Hormones Among Pregnant Women in Puerto Rico. J. Endocr. Soc. 2019, 3, 1127–1149. [Google Scholar] [CrossRef] [PubMed]
- Tian, M.; Liu, L.; Wang, H.; Wang, X.; Martin, F.L.; Zhang, J. Phthalates Induce Androgenic Effects at Exposure Levels That Can Be Environmentally Relevant in Humans. Environ. Sci. Technol. Lett. 2018, 5, 232–236. [Google Scholar] [CrossRef]
- Araki, A.; Mitsui, T.; Miyashita, C.; Nakajima, T.; Naito, H.; Ito, S. Association between maternal exposure to di(2-ethylhexyl) phthalate and reproductive hormone levels in fetal blood: The Hokkaido Study on environment and children’s health. PLoS ONE 2014, 9, e109039. [Google Scholar] [CrossRef] [PubMed]
- Specht, I.; Toft, G.; Hougaard, K.S.; Lindh, C.H.; Lenters, V.; Jönsson, B.A.G. Associations between serum phthalates and biomarkers of reproductive function in 589 adult men. Environ. Int. 2014, 66, 146–156. [Google Scholar] [CrossRef] [PubMed]
- Joensen, U.N.; Frederiksen, H.; Jensen, M.B.; Lauritsen, M.P.; Olesen, I.A.; Lassen, T.H. Phthalate Excretion Pattern and Testicular Function: A Study of 881 Healthy Danish Men. Environ. Health Perspect. 2012, 120, 1397–1403. [Google Scholar] [CrossRef]
- Pan, G.; Hanaoka, T.; Yu, L.; Na, J.; Yamano, Y.; Hara, K. Associations between hazard indices of di-n-butylphthalate- and di-2-ethylhexylphthalate exposure and serum reproductive hormone levels among occupationally exposed and unexposed Chinese men. Int. J. Androl. 2011, 34, 397–406. [Google Scholar] [CrossRef] [PubMed]
- Cao, M.; Pan, W.; Shen, X.; Li, C.; Zhou, J.; Liu, J. Urinary levels of phthalate metabolites in women associated with risk of premature ovarian failure and reproductive hormones. Chemosphere 2020, 242, 125206. [Google Scholar] [CrossRef]
- Wen, H.-J.; Sie, L.; Su, P.-H.; Chuang, C.-J.; Chen, H.-Y.; Sun, C.-W. Prenatal and childhood exposure to phthalate diesters and sex steroid hormones in 2-, 5-, 8-, and 11-year-old children: A pilot study of the Taiwan Maternal and Infant Cohort Study. J. Epidemiol. 2017, 27, 516–523. [Google Scholar] [CrossRef]
- Lin, L.; Wang, S.; Chang, Y.; Huang, P.; Cheng, J.; Su, P. Associations between maternal phthalate exposure and cord sex hormones in human infants. Chemosphere 2011, 83, 1192–1199. [Google Scholar] [CrossRef]
- Sheikh, I.A.; Yasir, M.; Abu-Elmagd, M.; Dar, T.A.; Abuzenadah, A.M.; Damanhouri, G.A. Human sex hormone-binding globulin as a potential target of alternate plasticizers: An in silico study. BMC Struct. Biol. 2016, 16, 11–20. [Google Scholar] [CrossRef]
- Lottrup, G.; Andersson, A.-M.; Leffers, H.; Mortensen, G.K.; Toppari, J.; Skakkebaek, N.E. Possible impact of phthalates on infant reproductive health. Int. J. Androl. 2006, 29, 172–180. [Google Scholar] [CrossRef]
- Somasundaram, D.B.; Selvanesan, B.C.; Ramachandran, I.; Bhaskaran, R.S. Lactational Exposure to di (2-ethylhexyl) Phthalate Impairs the Ovarian and Uterine Function of Adult Offspring Rat. Reprod. Sci. 2016, 23, 549–559. [Google Scholar] [CrossRef]
- Pocar, P.; Fiandanese, N.; Secchi, C.; Berrini, A.; Fischer, B.; Schmidt, J.S. Exposure to di(2-ethyl-hexyl) phthalate (DEHP) in Utero and during lactation causes long-term pituitary-gonadal axis disruption in male and female mouse offspring. Endocrinology 2012, 153, 937–948. [Google Scholar] [CrossRef]
- Asai, D.; Tahara, Y.; Nakai, M.; Yakabe, Y.; Takatsuki, M.; Nose, T. Structural essentials of xenoestrogen dialkyl phthalates to bind to the estrogen receptors. Toxicol. Lett. 2000, 118, 1–8. [Google Scholar] [CrossRef]
- Brzozowski, A.M.; Pike, A.C.W.; Dauter, Z.; Hubbard, R.E.; Bonn, T.; Engström, O. Molecular basis of agonism and antagonism in the oestrogen receptor. Nature 1997, 389, 753–758. [Google Scholar] [CrossRef]
- Okamoto, Y.; Ueda, K.; Kojima, N. Potential Risks of Phthalate Esters: Acquisition of Endocrine-disrupting Activity during Environmental and Metabolic Processing. J. Health Sci. 2011, 57, 497–503. [Google Scholar] [CrossRef]
- Takeuchi, S.; Iida, M.; Kobayashi, S.; Jin, K.; Matsuda, T.; Kojima, H. Differential effects of phthalate esters on transcriptional activities via human estrogen receptors α and β, and androgen receptor. Toxicology 2005, 210, 223–233. [Google Scholar] [CrossRef]
- ESR2 Estrogen Receptor 2 (Homo Sapiens (Human)) NCBI. Available online: https://www.ncbi.nlm.nih.gov/gene/2100#gene-expression (accessed on 18 March 2020).
- ESR1 Estrogen Receptor 1 (Homo Sapiens (Human)) NCBI. Available online: https://www.ncbi.nlm.nih.gov/gene/2099#gene-expression (accessed on 18 March 2020).
- AR Androgen Receptor (Homo Sapiens (Human)) NCBI. Available online: https://www.ncbi.nlm.nih.gov/gene/367 (accessed on 18 March 2020).
- Josh, M.K.S.; Pradeep, S.; Vijayalekshmy, K.S.A.; Sudha, R.D.; Balachandran, S.; Sreejith, M.N. Human ketosteroid receptors interact with hazardous phthalate plasticizers and their metabolites: An in silico study. J. Appl. Toxicol. 2016, 36, 836–843. [Google Scholar] [CrossRef]
- Josh, M.K.S.; Pradeep, S.; Adarsh, V.K.; Vijayalekshmi, K.S.A.; Sudha, R.D.; Balachandran, S. In silico evidences for the binding of phthalates onto human estrogen receptor α, β subtypes and human estrogen-related receptor γ. Mol. Simul. 2014, 40, 408–417. [Google Scholar] [CrossRef]
- Josh, M.K.S.; Pradeep, S.; Vijayalekshmy, K.S.A.; Balachandran, S.; Jaleel, U.C.A.; Doble, M. Phthalates effi ciently bind to human peroxisome proliferator activated receptor and retinoid X receptor α, β, γ subtypes: An in silico approach. J. Appl. Toxicol. 2014, 34, 754–765. [Google Scholar] [CrossRef]
- Kambia, N.K.; Séverin, I.; Farce, A.; Moreau, E.; Dahbi, I.; Duval, C. In vitro and in silico hormonal activity studies of di-(2-ethylhexyl)terephthalate, a di-(2-ethylhexyl)phthalate substitute used in medical devices, and its metabolites. J. Appl. Toxicol. 2019, 39, 1043–1056. [Google Scholar] [CrossRef]
- Engel, A.; Buhrke, T.; Imber, F.; Jessel, S.; Seidel, A.; Völkel, W. Agonistic and antagonistic effects of phthalates and their urinary metabolites on the steroid hormone receptors ERα, ERβ, and AR. Toxicol. Lett. 2017, 277, 54–63. [Google Scholar] [CrossRef]
- Dvořáková, M.; Kejlová, K.; Rucki, M.; Jírová, D. Selected bisphenols and phthalates screened for estrogen and androgen disruption by in silico and in vitro methods. Neuroendocrinol. Lett. 2018, 39, 409–416. [Google Scholar]
- Simon, C.; Onghena, M.; Covaci, A.; Van Hoeck, E.; Van Loco, J.; Vandermarken, T. Screening of endocrine activity of compounds migrating from plastic baby bottles using a multi-receptor panel of in vitro bioassays. Toxicol. In Vitro 2016, 37, 121–133. [Google Scholar] [CrossRef]
- Georget, V.; Térouanne, B.; Nicolas, J.C.; Sultan, C. Mechanism of Antiandrogen Action: Key Role of Hsp90 in Conformational Change and Transcriptional Activity of the Androgen Receptor. Biochemistry 2002, 41, 11824–11831. [Google Scholar] [CrossRef]
- Bisson, W.H.; Abagyan, R.; Cavasotto, C.N. Molecular basis of agonicity and antagonicity in the androgen receptor studied by molecular dynamics simulations. J. Mol. Graph. Model. 2008, 27, 452–458. [Google Scholar] [CrossRef]
- Shanle, E.K.; Xu, W. Endocrine Disrupting Chemicals Targeting Estrogen Receptor Signaling: Identification and Mechanisms of Action. Chem. Res. Toxicol. 2011, 24, 6–19. [Google Scholar] [CrossRef]
- Lee, H.; Lee, J.; Choi, K.; Kim, K.-T. Comparative analysis of endocrine disrupting effects of major phthalates in employed two cell lines (MVLN and H295R) and embryonic zebrafish assay. Environ. Res. 2019, 172, 319–325. [Google Scholar] [CrossRef]
- Fang, H.; Fang, W.; Cao, H.; Luo, S.; Cong, J.; Liu, S. Di-(2-ethylhexyl)-phthalate induces apoptosis via the PPARγ/PTEN/AKT pathway in differentiated human embryonic stem cells. Food Chem. Toxicol. 2019, 131, 110552. [Google Scholar] [CrossRef]
- Kwon, B.; Ji, K. Estrogenic and Androgenic Potential of Phthalates and Their Alternatives. Korean J. Environ. Health Sci. 2016, 42, 169–188. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, W.; Yang, Q.; Yu, M.; Zhang, Z. Di (2-ethylhexyl) phthalate exposure during pregnancy disturbs temporal sex determination regulation in mice offspring. Toxicology 2015, 336, 10–16. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, Q.; Liu, W.; Yu, M.; Zhang, Z.; Cui, X. DEHP exposure in utero disturbs sex determination and is potentially linked with precocious puberty in female mice. Toxicol. Appl. Pharmacol. 2016, 307, 123–129. [Google Scholar] [CrossRef]
- NURSA. NURSA—Transcriptomine|Discovering Relationships between Nuclear Receptor Signaling Pathways, Genes and Tissues. Available online: https://www.nursa.org/nursa/transcriptomine/index.jsf (accessed on 19 March 2020).
- Sekaran, S.; Jagadeesan, A. In utero exposure to phthalate downregulates critical genes in Leydig cells of F1 male progeny. J. Cell. Biochem. 2015, 116, 1466–1477. [Google Scholar] [CrossRef]
- Wilson, C.A.; Davies, D.C. The control of sexual differentiation of the reproductive system and brain. Reproduction 2007, 133, 331–359. [Google Scholar] [CrossRef]
- Franco, H.L.; Yao, H.H.-C. Sex and hedgehog: Roles of genes in the hedgehog signaling pathway in mammalian sexual differentiation. Chromosom. Res. 2012, 20, 247–258. [Google Scholar] [CrossRef]
- Boyer, A.; Goff, A.K.; Boerboom, D. WNT signaling in ovarian follicle biology and tumorigenesis. Trends Endocrinol. Metab. 2010, 21, 25–32. [Google Scholar] [CrossRef]
- Jiang, J.; Ma, L.; Yuan, L.; Wang, X.; Zhang, W. Study on developmental abnormalities in hypospadiac male rats induced by maternal exposure to di-n-butyl phthalate (DBP) steroidogenic acute regulatory protein The experiments were carried out in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Toxicology 2007, 232, 286–293. [Google Scholar] [CrossRef]
- Ríos, O.; Frias, S.; Rodríguez, A.; Kofman, S.; Merchant, H.; Torres, L. A Boolean network model of human gonadal sex determination. Theor. Biol. Med. Model. 2015, 12, 26. [Google Scholar] [CrossRef]
- Yong, W.; Jiao, C.; Jianhui, W.; Yan, Z.; Qi, P.; Xiu, W. Mono-2-ethyhexyl phthalate advancing the progression of prostate cancer through activating the hedgehog pathway in LNCaP cells. Toxicol. In Vitro 2016, 32, 86–91. [Google Scholar] [CrossRef]
- Xin, F.; Susiarjo, M.; Bartolomei, M.S. Multigenerational and transgenerational effects of endocrine disrupting chemicals: A role for altered epigenetic regulation? Semin. Cell. Dev. Biol. 2015, 43, 66–75. [Google Scholar] [CrossRef]
- Kang, C.S.; Lee, M.B. DNA Methylation of Estrogen Receptor α Gene by Phthalates. J. Toxicol. Environ. Health A 2005, 68, 1995–2003. [Google Scholar] [CrossRef]
- Li, L.; Zhang, T.; Qin, X.S.; Ge, W.; Ma, H.G.; Sun, L.L. Exposure to diethylhexyl phthalate (DEHP) results in a heritable modification of imprint genes DNA methylation in mouse oocytes. Mol. Biol. Rep. 2014, 41, 1227–1235. [Google Scholar] [CrossRef]
- Rasoulpour, R.J.; Boekelheide, K. NF-κB Is Activated in the Rat Testis Following Exposure to Mono-(2-Ethylhexyl) Phthalate. Biol. Reprod. 2005, 72, 479–486. [Google Scholar] [CrossRef]
- Giammona, C.J.; Sawhney, P.; Chandrasekaran, Y.; Richburg, J.H. Death receptor response in rodent testis after mono-(2-ethylhexyl) phthalate exposure. Toxicol. Appl. Pharmacol. 2002, 185, 119–127. [Google Scholar] [CrossRef]
- Rogers, R.; Ouellet, G.; Brown, C.; Moyer, B.; Rasoulpour, T.; Hixon, M. Cross-talk between the Akt and NF-κB Signaling Pathways Inhibits MEHP-Induced Germ Cell Apoptosis. Toxicol. Sci. 2008, 106, 497–508. [Google Scholar] [CrossRef]
- Lucas, B.E.G.; Fields, C.; Joshi, N.; Hofmann, M.C. Mono-(2-ethylhexyl)-phthalate (MEHP) affects ERK-dependent GDNF signalling in mouse stem-progenitor spermatogonia. Toxicology 2012, 299, 10–19. [Google Scholar] [CrossRef][Green Version]
- Mlynarčíková, A.; Nagyová, E.; Ficková, M.; Scsuková, S. Effects of selected endocrine disruptors on meiotic maturation, cumulus expansion, synthesis of hyaluronan and progesterone by porcine oocyte-cumulus complexes. Toxicol. In Vitro 2009, 23, 371–377. [Google Scholar] [CrossRef]

| Inducers | Effectors | |
|---|---|---|
| Cell Proliferation | mitogens (growth factors—EGF, BDNF), survival factors (Bcl-2), steroids (testosterone) | cyclin-dependent kinases |
| Apoptosis | DNA damage, lack of nutrients, toxins, growth factors (TGF) | caspases, Ca2+ -dependent proteases (calpain) |
| Phthalate Diester | Primary Metabolite | Secondary Metabolite |
|---|---|---|
| DMP | MMP | - |
| DEP | MEP | - |
| DnBP | MnBP | MHnBP |
| DiBP | MiBP | MHiBP |
| BBzP | MBzP | - |
| DEHP | MEHP | MEHHP |
| MEOHP | ||
| MECPP | ||
| DPeP | MPeP | - |
| DCHP | MCHP | - |
| DiDP | MiDP | MHiDP |
| MOiDP | ||
| MCiDP | ||
| DiNP | MiNP | MHiNP |
| MOiNP | ||
| MCiNP | ||
| DnOP | MnOP | MCPP |
| Females | |||||
| Phthalates | Dose Effect (mg/kg/day) | Animal/Cell Model | Time of Exposure | Effect | References |
| DEHP | 300 | Sprague-Dawley rats | prenatal | ↓ estradiol | Martinez-Arguelles et al. [173] |
| DEHP | 1, 50, or 300 | Sprague-Dawley rats | prenatal | ↑ FSH | Meltzer et al. [138] |
| 300 | ↓ estradiol | ||||
| DEHP | 300 | Sprague-Dawley rats | postnatal | ↓ pregnenolone, progesterone | Nuttall et al. [174] |
| DEHP | 30 | Wistar rats | prepubertal | ↑ LH | Carbone et al. [175] |
| DEHP | 1000, and 3000 | Wistar rats | postnatal | ↑ GnRH | Liu et al. [132] |
| DEHP | 3000 | ↓ FSH, LH, estradiol, progesterone, testosterone | |||
| DEHP | 1000, and 500 | Wistar rats | postnatal | ↑ GnRH | Liu et al. [176] |
| DEHP | 1, 10, 100 μg/mL | Mouse antral follicles (CD-1 mice) | postnatal | ↓ progesterone, dehydroepiandrosterone, androstendione, testosterone, estradiol | Hannon et al. [121] |
| 1, 10, 100 μg/mL | ↓ Cyp11a1 | ||||
| 100 μg/mL | ↓ Cyp17a1 | ||||
| 10 μg/mL | ↑ Cyp19a1, Hsd17b1 | ||||
| 100 μg/mL | ↓ Cyp19a1, Hsd17b1 | ||||
| 100 μg/mL | ↑ Hsd3b1 | ||||
| DBP | 0.01, 0.1 and 1000 | CD-1 mice | postnatal | ↓ estradiol, ↑ FSH | Sen, Liu and Craig [177] |
| 1000 | ↓ Star, Hsd3b | ||||
| 0.01, 0.1 and 1000 | ↓ P450scc, Cyp17a1, Hsd17b, Cyp19a1 | ||||
| 0.01 | ↑ Star, Hsd17b | ||||
| DEHP | 500 and 750 | CD-1 mice | prenatal | ↑ estradiol (F1 generation) | Brehm et al. [178] |
| 20 | ↑ estradiol (F3) | ||||
| 500 | ↓ testosterone (F1) | ||||
| 20 | ↓ testosterone (F2) | ||||
| 20 and 500 | ↓ testosterone (F3) | ||||
| 200 | ↓ progesterone (F2) | ||||
| 500 | ↓ FSH (F1) | ||||
| 500 | ↑ FSH (F3) | ||||
| 20 | ↑ LH (F1) | ||||
| Males | |||||
| Phthalates | Dose Effect (mg/kg/day) | Animal | Time of Exposure | Effect | References |
| DnBP | 500 | Sprague-Dawley outbred CD rats | prenatal | ↓ Star, Cyp11a1, Cyp17a1 | Thompson et al. [179] |
| DEHP | 100, 300, 750 | Sprague-Dawley rats | prenatal | ↓ testosterone | Martinez-Arguelles et al. [173] |
| DEHP | 3 and 30 | Wistar rats | prepubertal | ↑ GnRH | Carbone et al. [175] |
| DnBP | 850 | Sprague-Dawley rats | prenatal | ↓ Cyp11a1, Hsd3b, Star | Zhu et al. [180] |
| DEHP | 250, 500, or 750 | Sprague-Dawley rats | postnatal | ↓ testosterone, FSH, LH | Ha et al. [181] |
| DnBP | 100 and 500 | Wistar rats | postnatal | ↓ Hsd17b, Hsd13b | Giribabu et al [182] |
| ↓ testosterone, FSH, LH | |||||
| DEHP | 500 and 1500 | Sprague-Dawley rats | postnatal | ↓ GnRH | Qin et al. [183] |
| 100, 500, 1500 | ↑ Star, Hsd3b | ||||
| 1500 | ↑ Hsd17b | ||||
| DEHP | 5 and 50 µg/kg/d | Long Evans rats | prenatal | ↓ Hsd17b | Abdel-Maksoud, Ali and Akingbemi [184] |
| BBzP | 10 | Sprague-Dawley rats | pubertal | ↑ testosterone | Lv et al. [185] |
| 1000 | ↓ testosterone | ||||
| 100 | ↑ Cyp11a1 | ||||
| 10, 100 and 1000 | ↑ Hsd3b | ||||
| DEHP | 500 | Crl:CD rats | postnatal | ↑ Cyp4a | Yamaguchi et al. [186] |
| Men | |||
| Phthalate | Time of Exposure | Effect | References |
| ΣDEHP | postnatal (more than 60 years) | ↓ free testosterone, total testosterone | Woodward et al. [198] |
| ΣLMWP | postnatal (20–30 years) | ↓ free testosterone, total testosterone | |
| MEHP | postnatal | ↑ DHT, estradiol, P450AROM, SRD5A | Chang et al. [107] |
| MEHHP | |||
| MEOHP | |||
| MECPP | |||
| MiBP | postnatal | ↓ testosterone | Al-Saleh et al. [197] |
| MEHHP | ↓ FSH | ||
| MEP | ↑ estradiol | ||
| MEHP | ↑ FSH, LH | ||
| ↓ testosterone/LH, testosterone/estradiol | |||
| MEHP | prenatal | ↑ total testosterone (postnatal, 20 years old) | Hart et al. [193] |
| MiNP | |||
| ΣDEHP | |||
| ΣDiNP | |||
| ΣHMWP | |||
| ΣMEHP | postnatal (boys) | ↓ testosterone | Wen et al. [196] |
| MBP | postnatal | ↓ total testosterone, free testosterone, LH | Pan et al. [195] |
| MiBP | |||
| MHiNP | postnatal | ↑ FSH, LH | Axelsson et al. [194] |
| MOiNP | ↑ FSH, LH | ||
| MEHP | prenatal (boys) | ↓ progesterone, INSL3, inhibin | Araki et al. [207] |
| ΣDEHP | postnatal | ↓ testosterone | Specht et al. [208] |
| ΣDiNP | |||
| MEHP | postnatal | ↓ testosterone | Jurewicz et al. [80] |
| MEHP | postnatal | ↓ testosterone/LH, testosterone/FSH, total testosterone, free testosterone, testosterone/estradiol | Joensen et al. [209] |
| MiNP | ↓ testosterone/LH, testosterone/FSH, ↑SHBG | ||
| ΣDEHP | postnatal | ↓ testosterone, LH, FSH | Pan et al. [210] |
| ΣDBP | |||
| MEHP | postnatal | ↓ free testosterone, estradiol | Mendiola et al. [199] |
| MEHHP | ↓ free testosterone, ↑ SHBG | ||
| MEOHP | ↓ free testosterone, ↑ SHBG | ||
| MEP | postnatal | ↓ LH | Jonsson et al. [203] |
| MBzP | postnatal | ↓ FSH | Duty et al. [204] |
| Women | |||
| Phthalate | Time of Exposure | Effect | References |
| MiBP | postnatal | ↑FSH, ↓estradiol/FSH | Cao et al. [211] |
| MnBP | ↓estradiol, ↑FSH, ↓estradiol/FSH | ||
| MMP | ↓estradiol, ↑FSH, ↓estradiol/FSH | ||
| MEOHP | ↑FSH | ||
| MEHHP | ↑FSH, ↓estradiol/FSH | ||
| ΣLMWP | ↓estradiol, ↓estradiol/FSH | ||
| ΣHMWP | ↓estradiol/FSH | ||
| MHBP | postnatal | ↑testosterone | Cathey et al. [205] |
| MEP | ↓testosterone | ||
| MEHHTP | ↓ progesterone | ||
| ∑MEHP | prenatal | ↓ progesterone | Wen et al. [212] |
| postnatal (girls) | |||
| MEHP | prenatal (girls and boys) | ↓ testosterone/estradiol, progesterone, inhibin, INSL3 | Araki et al. [207] |
| MEHP | prenatal | ↓ free testosterone, free testosterone/estradiol (cord serum in newborns) | Lin et al. [213] |
| MEHHP | |||
| ΣDEHP | |||
| Estrogenic Affinity | Anti-Estrogenic Affinity | Androgenic Affinity | Anti-Androgenic Affinity | |
|---|---|---|---|---|
| DEP | Yes | ND | ND | ND |
| DnBP | Yes | Yes | Yes | Yes |
| DiBP | Yes | ND | ND | Yes |
| BBzP | Yes | Yes | ND | Yes |
| DiNP | Yes | ND | ND | ND |
| DEHP | Yes | Yes | Yes | Yes |
| DCHP | Yes | Yes | ND | Yes |
| Gene | Function in Male Reproductive Development |
| FGF9 | proliferation and differentiation of Sertoli cells, formation of testicle tubules and Leydig cells |
| GATA4 | triggers anti-Müllerian hormone secretion in Sertoli cells and regulates secretion of testosterone by Leydig cells |
| PTCH | expression is activated by Hh signaling pathway, which regulates the process of genital tubercle growth and differentiation in a masculine way [243] |
| SF1 | anti-Müllerian hormone secretion in Sertoli cells and regulation of the secretion of testosterone by Leydig cells; secretion of insulin-like peptide 3 |
| SOX9 | differentiation of indifferent gonads to the testes; stimulation of anti-Müllerian hormone secretion in Sertoli cells |
| SRY | necessary gene for male sexual development |
| WT1 | anti-Müllerian hormone secretion in Sertoli cells |
| Gene | Function in Female Reproductive Development |
| DAX1 | testis development inhibition by acting antagonistically to SRY |
| FOXL2 | ovarian development and function |
| RSPO1 | positive regulation of WNT signaling pathway [244] |
| WNT4 | ovary development; cell proliferation, apoptosis and differentiation within the female reproductive system |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hlisníková, H.; Petrovičová, I.; Kolena, B.; Šidlovská, M.; Sirotkin, A. Effects and Mechanisms of Phthalates’ Action on Reproductive Processes and Reproductive Health: A Literature Review. Int. J. Environ. Res. Public Health 2020, 17, 6811. https://doi.org/10.3390/ijerph17186811
Hlisníková H, Petrovičová I, Kolena B, Šidlovská M, Sirotkin A. Effects and Mechanisms of Phthalates’ Action on Reproductive Processes and Reproductive Health: A Literature Review. International Journal of Environmental Research and Public Health. 2020; 17(18):6811. https://doi.org/10.3390/ijerph17186811
Chicago/Turabian StyleHlisníková, Henrieta, Ida Petrovičová, Branislav Kolena, Miroslava Šidlovská, and Alexander Sirotkin. 2020. "Effects and Mechanisms of Phthalates’ Action on Reproductive Processes and Reproductive Health: A Literature Review" International Journal of Environmental Research and Public Health 17, no. 18: 6811. https://doi.org/10.3390/ijerph17186811
APA StyleHlisníková, H., Petrovičová, I., Kolena, B., Šidlovská, M., & Sirotkin, A. (2020). Effects and Mechanisms of Phthalates’ Action on Reproductive Processes and Reproductive Health: A Literature Review. International Journal of Environmental Research and Public Health, 17(18), 6811. https://doi.org/10.3390/ijerph17186811






