Environment-Friendly Removal Methods for Endocrine Disrupting Chemicals
Abstract
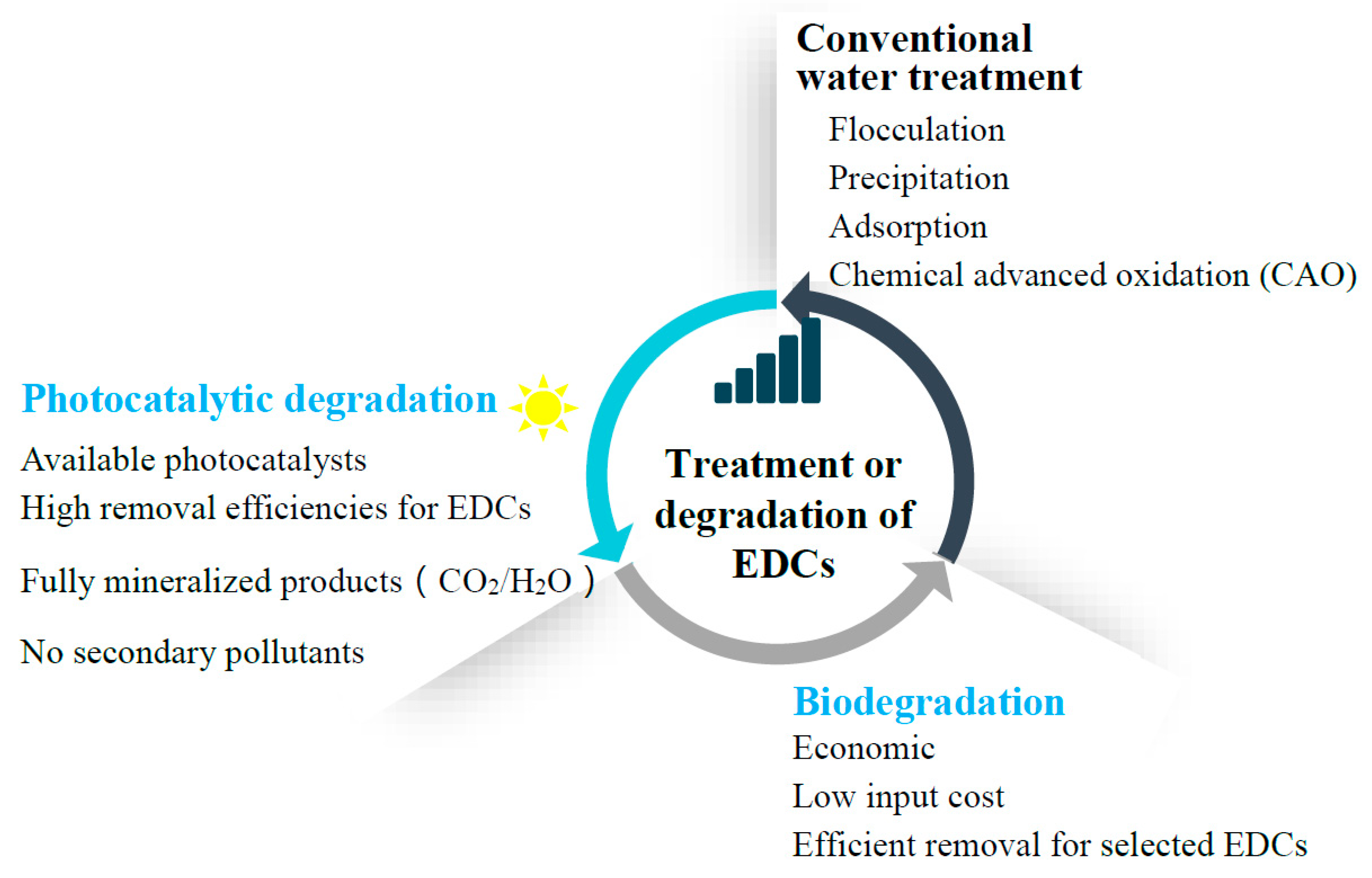
1. Introduction
2. Representatives of EDCs
2.1. Bisphenol A
2.2. Nonylphenol
2.3. Triclosan
2.4. Estrone
3. Conventional Water Treatment Technologies for EDCs
3.1. Adsorption Technology
3.2. Chemical Advanced Oxidation
4. Biodegradation of EDCs
5. Photocatalytic Degradation of EDCs
5.1. TiO2 and ZnO Photocatalysts
5.2. ZnO Nanocomposites
5.3. TiO2 Nanocomposites
5.4. Ag3PO4/LaCoO3 Composites
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Marty, M.S.; Borgert, C.; Coady, K.; Green, R.; Levine, S.L.; Mihaich, E.; Ortego, L.; Wheeler, J.R.; Yi, K.D.; Zorrilla, L.M. Distinguishing between endocrine disruption and non-specific effects on endocrine systems. Regul. Toxicol. Pharmacol. 2018, 99, 142–158. [Google Scholar] [CrossRef] [PubMed]
- Kleine, B.; Rossmanith, W.G. Hormones and the Endocrine System: Textbook of Endocrinology; Springer International Publishing: Heidelberg, Germany, 2016. [Google Scholar]
- Kabir, E.R.; Rahman, M.S.; Rahman, I. A review on endocrine disruptors and their possible impacts on human health. Environ. Toxicol. Pharmacol. 2015, 40, 241–258. [Google Scholar] [CrossRef] [PubMed]
- Yang, O.; Kim, H.L.; Weon, J.I.; Seo, Y.R. Endocrine-disrupting Chemicals: Review of Toxicological Mechanisms Using Molecular Pathway Analysis. J. Cancer Prev. 2015, 20, 12–24. [Google Scholar] [CrossRef]
- Sarangapani, C.; Danaher, M.; Tiwari, B.; Lu, P.; Bourke, P.; Cullen, P.J. Efficacy and mechanistic insights into endocrine disruptor degradation using atmospheric air plasma. Chem. Eng. J. 2017, 326, 700–714. [Google Scholar] [CrossRef]
- Li, J.; Jiang, L.; Liu, X.; Lv, J. Adsorption and aerobic biodegradation of four selected endocrine disrupting chemicals in soil–water system. Int. Biodeterior. Biodegrad. 2013, 76, 3–7. [Google Scholar] [CrossRef]
- Ternes, T.A.; Stumpf, M.; Mueller, J.; Haberer, K.; Wilken, R.D.; Servos, M. Behavior and occurrence of estrogens in municipal sewage treatment plants--I. Investigations in Germany, Canada and Brazil. Ence Total Environ. 1999, 225, 81–90. [Google Scholar] [CrossRef]
- Johnson, A.C.; Sumpter, J.P. Removal of Endocrine-Disrupting Chemicals in Activated Sludge Treatment Works. Environ. Sci. Technol. 2001, 35, 4697–4703. [Google Scholar] [CrossRef] [PubMed]
- Hhne, C.; Püttmann, W. Occurrence and temporal variations of the xenoestrogens bisphenol A, 4-tert-octylphenol, and tech. 4-nonylphenol in two German wastewater treatment plants. Environ. Ence Pollut. Res. 2008, 15, 405–416. [Google Scholar] [CrossRef]
- Zegura, B.; Klemencic, A.K.; Balabanic, D.; Filipic, M. Raw and biologically treated paper mill wastewater effluents and the recipient surface waters: Cytotoxic and genotoxic activity and the presence of endocrine disrupting compounds. Sci. Total Environ. 2017, 574, 78–89. [Google Scholar]
- Daughton, C.G. Non-regulated water contaminants: Emerging research. Environ. Impact Assess. Rev. 2004, 24, 711–732. [Google Scholar] [CrossRef]
- Papaevangelou, V.A.; Gikas, G.D.; Tsihrintzis, V.A.; Antonopoulou, M.; Konstantinou, I.K. Removal of Endocrine Disrupting Chemicals in HSF and VF pilot-scale constructed wetlands. Chem. Eng. J. 2016, 294, 146–156. [Google Scholar] [CrossRef]
- Drewes, J.E.; Heberer, T.; Rauch, T.; Reddersen, K. Fate of Pharmaceuticals During Ground Water Recharge. Ground Water Monit. Remediat. 2010, 23, 64–72. [Google Scholar] [CrossRef]
- Shemesh, M.; Shore, L.S. Topic 2.2: Naturally produced steroid hormones and their release into the environment. Pure Appl. Chem. 2003, 75, 1859–1871. [Google Scholar]
- Snyder, S.A.; Westerhoff, P.; Yoon, Y.; Sedlak, D.L. Pharmaceuticals, Personal Care Products, and Endocrine Disruptors in Water: Implications for the Water Industry. Environ. Eng. Sci. 2003, 20, 449–469. [Google Scholar] [CrossRef]
- Gimeno, S.; Gerritsen, A.; Bowmer, T.; Komen, H. Feminization of male carp. Nature 1996, 384, 221–222. [Google Scholar] [CrossRef]
- Gross-Sorokin, M.Y.; Roast, S.D.; Brighty, G.C. Assessment of Feminization of Male Fish in English Rivers by the Environment Agency of England and Wales. Environ. Health Perspect. 2006, 114, 147–151. [Google Scholar] [CrossRef]
- Balabanič, D.; Rupnik, M.; Klemenčič, A.K. Negative impact of endocrine-disrupting compounds on human reproductive health. Reprod. Fertil. Dev. 2011, 23, 403–416. [Google Scholar] [CrossRef]
- Hatch, E.E.; Nelson, J.W.; Stahlhut, R.W.; Webster, T.F. Association of endocrine disruptors and obesity: Perspectives from epidemiological studies. Int. J. Androl. 2010, 33, 324–332. [Google Scholar] [CrossRef]
- Kim, S.D.; Cho, J.; Kim, I.S.; Vanderford, B.J.; Snyder, S.A. Occurrence and removal of pharmaceuticals and endocrine disruptors in South Korean surface, drinking, and waste waters. Water Res. 2007, 41, 1013–1021. [Google Scholar] [CrossRef]
- Gómez, M.; Garralón, G.; Plaza, F.; Vílchez, R.; Hontoria, E.; Gómez, M.A. Rejection of endocrine disrupting compounds (bisphenol A, bisphenol F and triethyleneglycol dimethacrylate) by membrane technologies. Desalination 2007, 212, 79–91. [Google Scholar] [CrossRef]
- Liu, Z.H.; Kanjo, Y.; Mizutani, S. Removal mechanisms for endocrine disrupting compounds (EDCs) in wastewater treatment-physical means, biodegradation, and chemical advanced oxidation: A review. Sci. Total Environ. 2009, 407, 731–748. [Google Scholar] [CrossRef] [PubMed]
- Staples, C.A.; Dorn, P.B.; Klecka, G.M.; O’Block, S.T.; Harris, L.R. A review of the environmental fate, effects, and exposures of bisphenol A. Chemosphere 1998, 36, 2149–2173. [Google Scholar] [CrossRef]
- Crain, D.A.; Eriksen, M.; Iguchi, T.; Jobling, S.; Laufer, H.; Leblanc, G.A.; Guillette, L.J., Jr. An ecological assessment of bisphenol-A: Evidence from comparative biology. Reprod. Toxicol. 2007, 24, 225–239. [Google Scholar] [CrossRef] [PubMed]
- Hoepner, L.A.; Whyatt, R.M.; Just, A.C.; Calafat, A.M.; Perera, F.P.; Rundle, A.G. Urinary concentrations of bisphenol A in an urban minority birth cohort in New York City, prenatal through age 7 years. Environ. Res. 2013, 122, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Xiao, S.; Diao, H.; Smith, M.A.; Song, X.; Ye, X. Preimplantation exposure to bisphenol A (BPA) affects embryo transport, preimplantation embryo development, and uterine receptivity in mice. Reprod. Toxicol. 2011, 32, 434–441. [Google Scholar] [CrossRef]
- Fasano, E.; Bono-Blay, F.; Cirillo, T.; Montuori, P.; Lacorte, S. Migration of phthalates, alkylphenols, bisphenol A and di(2-ethylhexyl)adipate from food packaging. Food Control 2012, 27, 132–138. [Google Scholar] [CrossRef]
- Flint, S.; Markle, T.; Thompson, S.; Wallace, E. Bisphenol A exposure, effects, and policy: A wildlife perspective. J. Environ. Manag. 2012, 104, 19–34. [Google Scholar] [CrossRef]
- Birkett, J.W.; Lester, J.N. (Eds.) Endocrine Disrupters in Wastewater and Sludge Treatment Processes; Lewis Publishers: Boca Raton, FL, USA, 2003; p. 304. ISBN 1-56670-601-7. [Google Scholar]
- Caliman, F.A.; Gavrilescu, M. Pharmaceuticals, personal care products and endocrine disruptig agents in the environment—A review. Clean Soil Air Water 2010, 37, 277–303. [Google Scholar] [CrossRef]
- Brooke, L.; Thursby, G. Ambient aquatic life water quality criteria for nonylphenol. In Report for the United States EPA; Office of Water, Office of Science and Technology: Washington, DC, USA, 2005. [Google Scholar]
- Waltman, E.L.; Venables, B.J.; Waller, W.T. Triclosan in a North Texas wastewater treatment plant and the influent and effluent of an experimental constructed wetland. Environ. Toxicol. Chem. 2010, 25, 367–372. [Google Scholar] [CrossRef]
- D’Ascenzo, G.; Corcia, A.D.; Gentili, A.; Mancini, R.; Mastropasqua, R.; Nazzari, M.; Samperi, R. Fate of natural estrogen conjugates in municipal sewage transport and treatment facilities. Sci. Total Environ. 2003, 302, 199–209. [Google Scholar] [CrossRef]
- Benfenati, E.; Barcelò, D.; Johnson, I.; Galassi, S.; Levsen, K. Emerging organic contaminants in leachates from industrial waste landfills and industrial effluent. Trac Trends Anal. Chem. 2003, 22, 757–765. [Google Scholar] [CrossRef]
- Fine, D.D.; Breidenbach, G.P.; Price, T.L.; Hutchins, S.R. Quantitation of estrogens in ground water and swine lagoon samples using solid-phase extraction, pentafluorobenzyl/trimethylsilyl derivatizations and gas chromatography-negative ion chemical ionization tandem mass spectrometry. J. Chromatogr. A 2003, 1017, 167–185. [Google Scholar] [CrossRef] [PubMed]
- Czarczyńska-Golińska, B.; Zgoa-Grzekowiak, A.; Jeszka-Skowron, M.; Frankowski, R.; Grzekowiak, T. Detection of bisphenol A, cumylphenol and parabens in surface waters of Greater Poland Voivodeship. J. Environ. Manag. 2017, 204, 50–60. [Google Scholar] [CrossRef] [PubMed]
- Tan, B.L.L.; Hawker, D.W.; Mueller, J.F.; Leusch, F.D.L.; Tremblay, L.A.; Chapman, H.F. Comprehensive study of endocrine disrupting compounds using grab and passive sampling at selected wastewater treatment plants in South East Queensland, Australia. Environ. Int. 2007, 33, 654–669. [Google Scholar] [CrossRef] [PubMed]
- Corrales, J.; Kristofco, L.A.; Steele, W.B.; Yates, B.S.; Breed, C.S.; Williams, E.S.; Brooks, B.W. Global Assessment of Bisphenol A in the Environment. Dose-Response 2015, 13, 1–29. [Google Scholar] [CrossRef] [PubMed]
- Limmun, W.; Ito, A.; Ishikawa, N.; Momotori, J.; Umita, T. Removal of nonylphenol and nonylphenol monoethoxylate from water and anaerobically digested sewage sludge by Ferrate(VI). Chemosphere 2019, 236, 124399. [Google Scholar] [CrossRef]
- Marazuela, M.D.; García-Fresnadillo, D. An integrated photosensitizing/adsorbent material for the removal of triclosan from water samples. Sep. Purif. Technol. 2020, 251, 117392. [Google Scholar] [CrossRef]
- Yaoyu, Z.; Shikang, W.; Hao, H.; Huang, J.; Zhao, Y. Chiral pharmaceuticals: Environment sources, potential human health impacts, remediation technologies and future perspective. Environ. Int. 2018, 121, 523–537. [Google Scholar] [CrossRef]
- Benotti, M.J.; Trenholm, R.A.; Vanderford, B.J.; Holady, J.C.; Stanford, B.D.; Snyder, S.A. Pharmaceuticals and Endocrine Disrupting Compounds in U.S. Drinking Water. Environ. Sci. Technol. 2009, 43, 597–603. [Google Scholar] [CrossRef]
- Ternes, T.A.; Meisenheimer, M.; McDowell, D.; Sacher, F.; Brauch, H.-J.; Haist, F.; Preuss, G.; Wilme, U.; Zulei-Seibert, N. Removal of Pharmaceuticals during Drinking Water Treatment. Environ. Sci. Technol. 2002, 36, 3855–3863. [Google Scholar] [CrossRef]
- Sun, Y.; Chen, M.; Liu, H.; Zhu, Y.; Wang, D.; Yan, M. Adsorptive removal of dye and antibiotic from water with functionalized zirconium-based metal organic framework and graphene oxide composite nanomaterial Uio-66-(OH)2/GO. Appl. Surf. Sci. 2020, 525, 146614. [Google Scholar] [CrossRef]
- Adams, C.; Asce, M.; Wang, Y.; Loftin, K.; Meyer, M. Removal of Antibiotics from Surface and Distilled Water in Conventional Water Treatment Processes. J. Environ. Eng. 2002, 128, 253–260. [Google Scholar] [CrossRef]
- Nam, S.W.; Choi, D.J.; Kim, S.K.; Her, N.; Zoh, K.D. Adsorption characteristics of selected hydrophilic and hydrophobic micropollutants in water using activated carbon. J. Hazard. Mater. 2014, 270, 144–152. [Google Scholar] [CrossRef]
- Wu, Q.; Shi, H.; Adams, C.D.; Timmons, T.; Ma, Y. Oxidative removal of selected endocrine-disruptors and pharmaceuticals in drinking water treatment systems, and identification of degradation products of triclosan. Ence Total Environ. 2012, 439, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Olmez-Hanci, T.; Imren, C.; Arslan-Alaton, I.; Kabdaşlı, I.; Tünay, O. H2O2/UV-C oxidation of potential endocrine disrupting compounds: A case study with dimethyl phthalate. Photochem. Photobiol. 2009, 8, 620–627. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Cheng, S.; Aizawa, T.; Terao, Y.; Kunikane, S. Products of Aqueous Chlorination of 17β-Estradiol and Their Estrogenic Activities. Environ. Sci. Technol. 2003, 37, 5665–5670. [Google Scholar] [CrossRef] [PubMed]
- Ning, B.; Graham, N.; Zhang, Y.; Nakonechny, M.; Gamal El-Din, M. Degradation of Endocrine Disrupting Chemicals by Ozone/AOPs. Ozone Ence Eng. 2007, 29, 153–176. [Google Scholar] [CrossRef]
- Baig, S.; Hansmann, G.; Paolini, B. Ozone oxidation of oestrogenic active substances in wastewater and drinking water. Water Sci. Technol. 2008, 58, 451–458. [Google Scholar] [CrossRef] [PubMed]
- Irmak, S.; Erbatur, O.; Akgerman, A. Degradation of 17beta-estradiol and bisphenol A in aqueous medium by using ozone and ozone/UV techniques. J. Hazard. Mater. 2005, 126, 54–62. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.Y.; Aizawa, T.; Ookubo, S. Products of aqueous chlorination of bisphenol A and their estrogenic activity. Environ. Ence Technol. 2002, 21, 1980–1987. [Google Scholar] [CrossRef]
- Cajthaml, T.; Kresinová, Z.; Svobodová, K.; Möder, M. Biodegradation of endocrine-disrupting compounds and suppression of estrogenic activity by ligninolytic fungi. Chemosphere 2009, 75, 745–750. [Google Scholar] [CrossRef] [PubMed]
- Ming, C.; Qin, X.; Zeng, G. Biodegradation of Carbon Nanotubes, Graphene, and Their Derivatives. Trends Biotechnol. 2017, 35, 836–846. [Google Scholar] [CrossRef]
- Asgher, M.; Bhatti, H.N.; Ashraf, M.; Legge, R.L. Recent developments in biodegradation of industrial pollutants by white rot fungi and their enzyme system. Biodegradation 2008, 19, 771–783. [Google Scholar] [CrossRef] [PubMed]
- Harms, H.; Schlosser, D.; Wick, L.Y. Untapped potential: Exploiting fungi in bioremediation of hazardous chemicals. Nat. Rev. Microbiol. 2011, 9, 177–192. [Google Scholar] [CrossRef] [PubMed]
- Křesinová, Z.; Linhartová, L.; Filipová, A.; Ezechiáš, M.; Mašín, P.; Cajthaml, T. Biodegradation of endocrine disruptors in urban wastewater using Pleurotus ostreatus bioreactor. New Biotechnol. 2018, 43, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.; Kim, H. Transformation of triclosan by Trametes versicolor and Pycnoporus cinnabarinus. Appl. Environ. Microbiol. 2000, 66, 4157–4160. [Google Scholar]
- Takamiya, M.; Magan, N.; Warner, P.J. Impact assessment of bisphenol A on lignin-modifying enzymes by basidiomycete Trametes versicolor. J. Hazard. Mater. 2008, 154, 33–37. [Google Scholar] [CrossRef]
- Janicki, T.; Krupiński, M.; Długoński, J. Degradation and toxicity reduction of the endocrine disruptors nonylphenol, 4-tert-octylphenol and 4-cumylphenol by the non-ligninolytic fungus Umbelopsis isabellina. Bioresour. Technol. 2016, 200, 223. [Google Scholar] [CrossRef]
- Rajendran, R.K.; Lin, C.C.; Huang, S.L.; Kirschner, R. Enrichment, isolation, and biodegradation potential of long-branched chain alkylphenol degrading non-ligninolytic fungi from wastewater. Mar. Pollut. Bull. 2017, 125, 416–425. [Google Scholar] [CrossRef]
- Rajendran, R.K.; Huang, S.L.; Lin, C.C.; Kirschner, R. Biodegradation of the endocrine disrupter 4-tert-octylphenol by the yeast strain Candida rugopelliculosa RRKY5 via phenolic ring hydroxylation and alkyl chain oxidation pathways. Bioresour. Technol. 2016, 226, 55–64. [Google Scholar] [CrossRef]
- Hom-Diaz, A.; Llorca, M.; Rodríguez-Mozaz, S.; Vicent, T.; Barceló, D.; Blánquez, P. Microalgae cultivation on wastewater digestate: β-estradiol and 17α-ethynylestradiol degradation and transformation products identification. J. Environ. Manag. 2015, 155, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Ji, M.K.; Kabra, A.N.; Choi, J.; Hwang, J.H.; Kim, J.R.; Abou-Shanab, R.A.I.; Oh, Y.K.; Jeon, B.H. Biodegradation of bisphenol A by the freshwater microalgae Chlamydomonas mexicana and Chlorella vulgaris. Ecol. Eng. 2014, 73, 260–269. [Google Scholar] [CrossRef]
- Hirano, T.; Honda, Y.; Watanabe, T.; Kuwahara, M. Degradation of bisphenol A by the lignin-degrading enzyme, manganese peroxidase, produced by the white-rot basidiomycete, Pleurotus ostreatus. J. Agric. Chem. Soc. Jpn. 2000, 64, 1958–1962. [Google Scholar] [CrossRef] [PubMed]
- Cabana, H.; Jiwan, J.L.; Rozenberg, R.; Elisashvili, V.; Penninckx, M.; Agathos, S.N.; Jones, J.P. Elimination of endocrine disrupting chemicals nonylphenol and bisphenol A and personal care product ingredient triclosan using enzyme preparation from the white rot fungus Coriolopsis polyzona. Chemosphere 2007, 67, 770–778. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Cavazos, L.I.; Junghanns, C.; Ornelas-Soto, N.; Cárdenas-Chávez, D.L.; Hernández-Luna, C.; Demarche, P.; Enaud, E.; García-Morales, R.; Agathos, S.N.; Parra, R. Purification and characterization of two thermostable laccases from Pycnoporus sanguineus and potential role in degradation of endocrine disrupting chemicals. J. Mol. Catal. B Enzym. 2014, 108, 32–42. [Google Scholar] [CrossRef]
- Chen, M.; Zeng, G.; Xu, P.; Lai, C.; Tang, L. How Do Enzymes ‘Meet’ Nanoparticles and Nanomaterials? Trends Biochem. Sci. 2017, 42, 914. [Google Scholar] [CrossRef]
- Chen, M.; Zeng, G.; Xu, P.; Yan, M.; Xiong, W.; Zhou, S. Interaction of carbon nanotubes with microbial enzymes: Conformational transitions and potential toxicity. Environ. Sci. Nano 2017, 4, 1954–1960. [Google Scholar] [CrossRef]
- Chen, M.; Qin, X.; Li, J.; Zeng, G. Probing molecular basis of single-walled carbon nanotube degradation and nondegradation by enzymes based on manganese peroxidase and lignin peroxidase. Rsc Adv. 2016, 6, 3592–3599. [Google Scholar] [CrossRef]
- Chen, M.; Qin, X.; Zeng, G. Biodiversity change behind wide applications of nanomaterials? Nano Today 2017, 17, 11–13. [Google Scholar] [CrossRef]
- Chen, M.; Zeng, G.; Lai, C.; Zhang, C.; Xu, P.; Yan, M.; Xiong, W. Interactions of carbon nanotubes and/or graphene with manganese peroxidase during biodegradation of endocrine disruptors and triclosan. Chemosphere 2017, 184, 127. [Google Scholar] [CrossRef]
- Toyama, T.; Ojima, T.; Tanaka, Y.; Mori, K.; Morikawa, M. Sustainable biodegradation of phenolic endocrine-disrupting chemicals by Phragmites australis-rhizosphere bacteria association. Water Sci. Technol. 2013, 68, 522–529. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, H.; Peng, Y.E.; Tong, L.; Feng, L.; Ma, K. New pathways for the biodegradation of diethyl phthalate by Sphingobium yanoikuyae SHJ. Process Biochem. 2018, 71, 152–158. [Google Scholar] [CrossRef]
- Feng, N.X.; Yu, J.; Mo, C.H.; Zhao, H.M.; Li, Y.W.; Wu, B.X.; Cai, Q.Y.; Li, H.; Zhou, D.M.; Wong, M.H. Biodegradation of di-n-butyl phthalate (DBP) by a novel endophytic Bacillus megaterium strain YJB3. Sci. Total Environ. 2017, 616, 117. [Google Scholar] [CrossRef] [PubMed]
- Gouvãªa, C.A.; Wypych, F.; Moraes, S.G.; Durã, N.; Nagata, N.; Peralta-Zamora, P. Semiconductor-assisted photocatalytic degradation of reactive dyes in aqueous solution. Chemosphere 2000, 40, 433–440. [Google Scholar] [CrossRef]
- Pauporté, T.; Rathouský, J. Electrodeposited Mesoporous ZnO Thin Films as Efficient Photocatalysts for the Degradation of Dye Pollutants. J. Phys. Chem. C 2007, 111, 7639–7644. [Google Scholar] [CrossRef]
- Guo, J.; Dai, Y.Z.; Chen, X.J.; Zhou, L.L.; Liu, T.H. Synthesis and characterization of Ag3PO4/LaCoO3 nanocomposite with superior mineralization potential for bisphenol A degradation under visible light. J. Alloys Compd. 2016, 696, 226–233. [Google Scholar] [CrossRef]
- Dolat, D.; Quici, N.; Kusiak-Nejman, E.; Morawski, A.W.; Puma, G.L. One-step, hydrothermal synthesis of nitrogen, carbon co-doped titanium dioxide (N,C TiO2 ) photocatalysts. Effect of alcohol degree and chain length as carbon dopant precursors on photocatalytic activity and catalyst deactivation. Appl. Catal. B Environ. 2012, 115–116, 81–89. [Google Scholar] [CrossRef]
- Zatloukalová, K.; Obalová, L.; Kočí, K.; Čapek, L.; Matěj, Z.; Šnajdhaufová, H.; Ryczkowski, J.; Słowik, G.; Zatloukalová, K.; Obalová, L. Photocatalytic degradation of endocrine disruptor compounds in water over immobilized TiO2 photocatalysts. Iran. J. Chem. Chem. Eng. Int. Engl. Ed. 2017, 36, 29–38. [Google Scholar]
- Vela, N.; Calín, M.; Yáñez-Gascón, M.J.; Garrido, I.; Pérez-Lucas, G.; Fenoll, J.; Navarro, S. Photocatalytic oxidation of six pesticides listed as endocrine disruptor chemicals from wastewater using two different TiO2 samples at pilot plant scale under sunlight irradiation. J. Photochem. Photobiol. A Chem. 2017, 353, 271–278. [Google Scholar] [CrossRef]
- Zúñiga-Benítez, H.; Aristizábal-Ciro, C.; Peñuela, G.A. Heterogeneous photocatalytic degradation of the endocrine-disrupting chemical Benzophenone-3: Parameters optimization and by-products identification. J. Environ. Manag. 2016, 167, 246–258. [Google Scholar] [CrossRef]
- Mu, J.; Shao, C.; Guo, Z.; Zhang, Z.; Zhang, M.; Zhang, P.; Chen, B.; Liu, Y. High photocatalytic activity of ZnO-carbon nanofiber heteroarchitectures. Acs Appl. Mater. Interfaces 2011, 3, 590. [Google Scholar] [CrossRef] [PubMed]
- Morales-Flores, N.; Pal, U.; Mora, E.S. Photocatalytic behavior of ZnO and Pt-incorporated ZnO nanoparticles in phenol degradation. Appl. Catal. A Gen. 2011, 394, 269–275. [Google Scholar] [CrossRef]
- Pant, H.R.; Chan, H.P.; Pant, B.; Tijing, L.D.; Kim, H.Y.; Kim, C.S. Synthesis, Characterization, and Photocatalytic Properties of ZnO Nano-Flower Containing TiO2 NPs. Ceram. Int. 2012, 38, 2943–2950. [Google Scholar] [CrossRef]
- Meenakshi, G.; Sivasamy, A. Nanorod ZnO/SiC nanocomposite: An efficient catalyst for the degradation of an endocrine disruptor under UV and visible light irradiations. J. Environ. Chem. Eng. 2018, 6, 3757–3769. [Google Scholar] [CrossRef]
- Radhika, S.; Thomas, J. Solar light driven photocatalytic degradation of organic pollutants using ZnO nanorods coupled with photosensitive molecules. J. Environ. Chem. Eng. 2017, 5, 4239–4250. [Google Scholar] [CrossRef]
- Lam, S.M.; Sin, J.C.; Abdullah, A.Z.; Mohamed, A.R. Efficient Photodegradation of Endocrine-Disrupting Chemicals with Bi2O3 –ZnO Nanorods Under a Compact Fluorescent Lamp. Water Air Soil Pollut. 2013, 224, 1–11. [Google Scholar] [CrossRef]
- Ayati, A.; Ahmadpour, A.; Bamoharram, F.F.; Tanhaei, B.; Mänttäri, M.; Sillanpää, M. A review on catalytic applications of Au/TiO2 nanoparticles in the removal of water pollutant. Chemosphere 2014, 107, 163–174. [Google Scholar] [CrossRef]
- Sornalingam, K.; Mcdonagh, A.; Zhou, J.L.; Mah, J.; Ahmed, M.B. Photocatalysis of estrone in water and wastewater: Comparison between Au-TiO2 nanocomposite and TiO2, and degradation by-products. Sci. Total Environ. 2017, 610–611, 521. [Google Scholar] [CrossRef]
- Li, Q.; Feng, X.; Zhang, X.; Song, H.; Zhang, J.; Shang, J.; Sun, W.; Zhu, T.; Wakamura, M.; Tsukada, M. Photocatalytic degradation of bisphenol A using Ti-substituted hydroxyapatite. Chin. J. Catal. 2014, 35, 90–98. [Google Scholar] [CrossRef]
- Czecha, B.; Rubinowska, K. TiO2-assisted photocatalytic degradation of diclofenac, metoprolol,;estrone and chloramphenicol as endocrine disruptors in water. Adsorpt. J. Int. Adsorpt. Soc. 2013, 19, 619–630. [Google Scholar] [CrossRef]
- Lam, S.M.; Sin, J.C.; Abdullah, A.Z.; Mohamed, A.R. ZnO nanorods surface-decorated by WO3 nanoparticles for photocatalytic degradation of endocrine disruptors under a compact fluorescent lamp. Ceram. Int. 2013, 39, 2343–2352. [Google Scholar] [CrossRef]
- Mboula, V.M.; Héquet, V.; Andrès, Y.; Pastrana-Martínez, L.M.; Doña-Rodríguez, J.M.; Silva, A.M.T.; Falaras, P. Photocatalytic degradation of endocrine disruptor compounds under simulated solar light. Water Res. 2013, 47, 3997–4005. [Google Scholar] [CrossRef] [PubMed]
- Sin, J.C.; Lam, S.M.; Lee, K.T.; Mohamed, A.R. Self-assembly fabrication of ZnO hierarchical micro/nanospheres for enhanced photocatalytic degradation of endocrine-disrupting chemicals. Mater. Sci. Semicond. Process. 2013, 16, 1542–1550. [Google Scholar] [CrossRef]
- Lalhriatpuia, C.; Tiwari, D.; Tiwari, A.; Lee, S.M. Immobilized Nanopillars-TiO2 in the efficient removal of micro-pollutants from aqueous solutions: Physico-chemical studies. Chem. Eng. J. 2015, 281, 782–792. [Google Scholar] [CrossRef]
- Han, B.; Zhang, M.; Zhao, D. In-situ degradation of soil-sorbed 17β-estradiol using carboxymethyl cellulose stabilized manganese oxide nanoparticles: Column studies. Environ. Pollut. 2017, 223, 238–246. [Google Scholar] [CrossRef] [PubMed]
| Compounds | Bisphenol A (BPA) | Nonylphenol (NP) | Triclosan (TCS) | Estrone (E1) |
|---|---|---|---|---|
| Molecular formula | C15H16O2 | C15H24O | C12H7Cl3O2 | C18H22O2 |
| Molecular weight (g/mol) | 228.29 | 220.35 | 289.54 | 270.4 |
| Solubility in water (mg/L) | 120–300 | Insoluble | Slightly soluble in water, 10 mg/L | 30 |
| LogKow | 3.32 | 5.76 | 4.76 | 3.43 |
| Usage/origin | Food and beverage packaging, baby bottles, dental sealants, etc. | Commercial and industrial surfactants, paints, lubricants, etc. | Spectral antimicrobials; household products such as soap and toothpaste | Estrogen drugs, biochemical studies, etc. |
| Properties/environmental effects | Harmful substance; the concentration of BPA in most water systems keeps a level of nanograms per liter; bioaccumulation | Hazardous substance; negative effects on organisms | Recalcitrant micropollutant | Natural estrogen; negative effects on organisms |
| Examples | (1) BPA could accumulate in zooplankton through phytoplankton; (2) fetuses and infants are at higher risk of BPA exposure and accumulation due to their low metabolizable xenobiotics | NP was listed as a priority toxic substance by the European Union | TCS is a refractory compound and persistent in the environment due to the aromatic nature and high chlorine content | E1 could disturb sexual development or cause breast cancer in females |
| References | [38] | [39] | [40] | [41] |
| Microorganism/Enzyme | Substrate | Removal Efficiency | Optimal Conditions | Reference |
|---|---|---|---|---|
| Rhizosphere bacteria TIK1 and IT4 | Phenolic EDCs | Extensive | - | [74] |
| Purified LacI and LacII isoforms produced by P. sanguineus CS43 | NP and TCS | 95% | - | [68] |
| Nonligninolytic fungus U. isabellina | tNP,4-t-OP, 4-CP | 90% | 90% of initially applied tNP, 4-t-OP and 4-CP (25 mg/L) were eliminated | [61] |
| Fusarium falciforme RRK20 | 4-t-OP | Most effective | - | [62] |
| Yeast C. rugopelliculosa strain RRKY5 | Alkylphenols | Effective | 30 °C, pH 5.0, an initial 4-t-OP concentration of 30 mg/L | [63] |
| S. yanoikuyae SHJ | DEP | Effective | - | [75] |
| White-rot fungus Pleurotus ostreatus HK35 | EDCs | >90% | - | [58] |
| Novel endophytic strain YJB3 | DBP | 82.50% | Under the optimal conditions | [76] |
| Freshwater microalgae, Chlamydomonas mexicana and Chlorella vulgaris | BPA | 24%, 23% | - | [65] |
| Microalgae, Selenastrum capricornutum and Chlamydomonas reinhardtii | E2, EE2 | 88% to 100% of E2 was removed by S. capricornutum; E2 and EE2 were completely removed by C. reinhardtii | - | [64] |
| Photocatalyst | Synthetic Method | Contaminant | Characterization | Reference |
|---|---|---|---|---|
| Anatase TiO2 | - | Diclofenac, chloramphenicol and estrone | Efficient | [93] |
| WO3–ZNR nanocomposites | Hydrothermal technique with a chemical solution | Phenol, BPA and methylparaben | Recyclable | [94] |
| ECT-1023t, N–TiO2 and GO–TiO2 | - | BPA | ECT-1023t was the most efficient | [95] |
| Bi2O3–ZnO nanorods | Hydrothermal technique and chemical precipitation | Phenol and methylparaben | Easily recovered and reused, high ·OH generation ability | [89] |
| ZnO hierarchical micro-/nanospheres | A facile chemical solution route without any organic solvent or surfactant | - | Active species is ·OH | [96] |
| Ti-substituted hydroxyapatite | - | BPA | Large adsorption capacity for BPA | [92] |
| Nanopillars of TiO2 | Sol–gel template method | - | Efficient | [97] |
| MnO2 nanoparticles | A food-grade carboxymethyl cellulose as a stabilizer | 17β-estradiol | Promising for in situ oxidation of EDCs in groundwater | [98] |
| Ag3PO4/LaCoO3 nanocomposites | Liquid deposition | - | Enhanced photocatalytic activity and stability | [79] |
| ZnO nanorods | A simple low-temperature hydrothermal | - | PTCDA sensitized ZnO nanorods showed highest photocatalytic activity | [88] |
| Nanorod ZnO/SiC nanocomposites | A simple sol–gel method | DEP | Crystalline, nanoscale, rough and porous on surface and possessed absorption | [87] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, X.; Kang, S.; Xiong, R.; Chen, M. Environment-Friendly Removal Methods for Endocrine Disrupting Chemicals. Sustainability 2020, 12, 7615. https://doi.org/10.3390/su12187615
Gao X, Kang S, Xiong R, Chen M. Environment-Friendly Removal Methods for Endocrine Disrupting Chemicals. Sustainability. 2020; 12(18):7615. https://doi.org/10.3390/su12187615
Chicago/Turabian StyleGao, Xiufang, Shuang Kang, Rongwei Xiong, and Ming Chen. 2020. "Environment-Friendly Removal Methods for Endocrine Disrupting Chemicals" Sustainability 12, no. 18: 7615. https://doi.org/10.3390/su12187615
APA StyleGao, X., Kang, S., Xiong, R., & Chen, M. (2020). Environment-Friendly Removal Methods for Endocrine Disrupting Chemicals. Sustainability, 12(18), 7615. https://doi.org/10.3390/su12187615




