Abstract
Selenium is an essential micronutrient required for the health of humans and lower plants, but its importance for higher plants is still being investigated. The biological functions of Se related to human health revolve around its presence in 25 known selenoproteins (e.g., selenocysteine or the 21st amino acid). Humans may receive their required Se through plant uptake of soil Se, foods enriched in Se, or Se dietary supplements. Selenium nanoparticles (Se-NPs) have been applied to biofortified foods and feeds. Due to low toxicity and high efficiency, Se-NPs are used in applications such as cancer therapy and nano-medicines. Selenium and nano-selenium may be able to support and enhance the productivity of cultivated plants and animals under stressful conditions because they are antimicrobial and anti-carcinogenic agents, with antioxidant capacity and immune-modulatory efficacy. Thus, nano-selenium could be inserted in the feeds of fish and livestock to improvise stress resilience and productivity. This review offers new insights in Se and Se-NPs biofortification for edible plants and farm animals under stressful environments. Further, extensive research on Se-NPs is required to identify possible adverse effects on humans and their cytotoxicity.
1. Introduction
The discovery of selenium in 1817 triggered a huge amount of innovative scientific inquiry into human health. Selenium is an essential micronutrient for humans and animals as well as some lower plants, but it still needs more investigation to establish whether or not it is essential for higher plants [1,2]. Selenium (Se) plays a vital role in the metabolism of humans, animals, and many prokaryotes as well as some algae [1]. This micronutrient is the only metalloid that is incorporated into specific proteins, called “selenoproteins”, and genetically encoded as well as forming a constitutive part of selenocysteine (SeCys), “the 21st amino acid” [3]. In total, 25 selenoproteins have been identified in the human proteome and are often oxido-reductases, including SeCys as a catalytic residue [4,5]. These selenoproteins mainly have wide redox functions that are vital for regulating human immunity [6], mediating thyroid disorders [7], and for the health of the reproductive system [1,3,8,9]. The essential role of selenium in human health has been confirmed by several researchers [10,11,12,13,14,15,16]. A major distinguishing feature of Se is the narrow margin between Se-deficiency (<40 μg day−1) and toxicity (˃400 μg day−1) [17]. The recommended daily dose for adults is 55 μg day−1 in the USA and 55 to 70 μg day−1 in Europe [18]. Selenium is called the “the essential poison” and characterized as “the double-edged sword” due to its biological effects under deficiency and toxicity [13].
Selenium and nano-selenium (nano-Se) or (Se-NPs) have been used in the maintenance of human health [19]. They can be applied in biomedical and drug delivery [20] dietary supplements, therapeutic agents [18], and nano-medicine applications [21]. The antimicrobial and anticancer properties of both Se and Se-NPs have been confirmed [19,22]. The biofortification of edible foods [23,24] and feeds [25,26] with Se and Se-NPs is an important approach to support human and livestock health.
The primary natural source of Se in foods is crop uptake from soil [13,27]. There are wide geographic variations in the Se content of soils, meaning some regions face Se deficiencies while others have Se toxicity issues based on the Se content in their crops, with both situations having negative impacts on human health [28]. Crops grown in Se deficient soils can be biofortified, including the use of both soil-based and foliar fertilizers to correct the deficiency [27]. Food crops that are commonly biofortified include cereals [29], leafy vegetables like spinach [30] and lettuce [31], and fruits like strawberry [24,32,33] and pomegranate [23]. Due to their lower toxicity, strong capacity to scavenge free radicals, higher bioavailability, and stimulation effect, Se-NPs have been recently used in the production of plants [23,24,34,35,36], fish [37,38,39,40], livestock [41], and poultry [42,43,44,45,46,47,48].
Therefore, this review explored available information on the use of Se and Se-nanoparticles in biofortification. The use of selenium and nano-selenium to promote human health is discussed, including the biofortification of crops through soil and other amendments. We also investigated the role of Se and nano-Se to support crops under stress.
2. Selenium and Nano Selenium: General Information
Although Selenium and its nanoparticles share some common and general properties, they important differences based on their unique chemical, physical, and biological properties (Table 1). For example, bulk elemental Se is not water soluble, but Se-NPs are partially water soluble (Figure 1). The behavior and biological features of Se and Se-NPs in the nutrition of higher plants and humans may differ. The role of Se in human nutrition has been confirmed, whereas the biological effects, recommended daily intake and toxicity/deficiency levels of Se-NPs still need more investigations [3,8,10,11,19,49]. Indications of the general role of Se-NPs on human nutrition have been distinguished through studies on fertilization of crops [50,51,52,53], poultry [42,43,44,46,47,48,54], and human health [18,19,21,55,56,57].

Table 1.
The biological features of selenium and nano-selenium and the possible roles in plant and human nutrition.
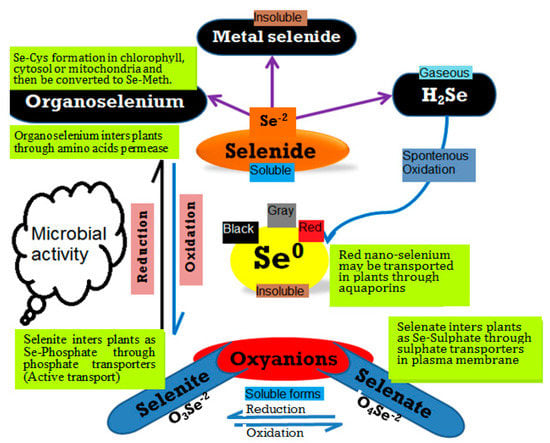
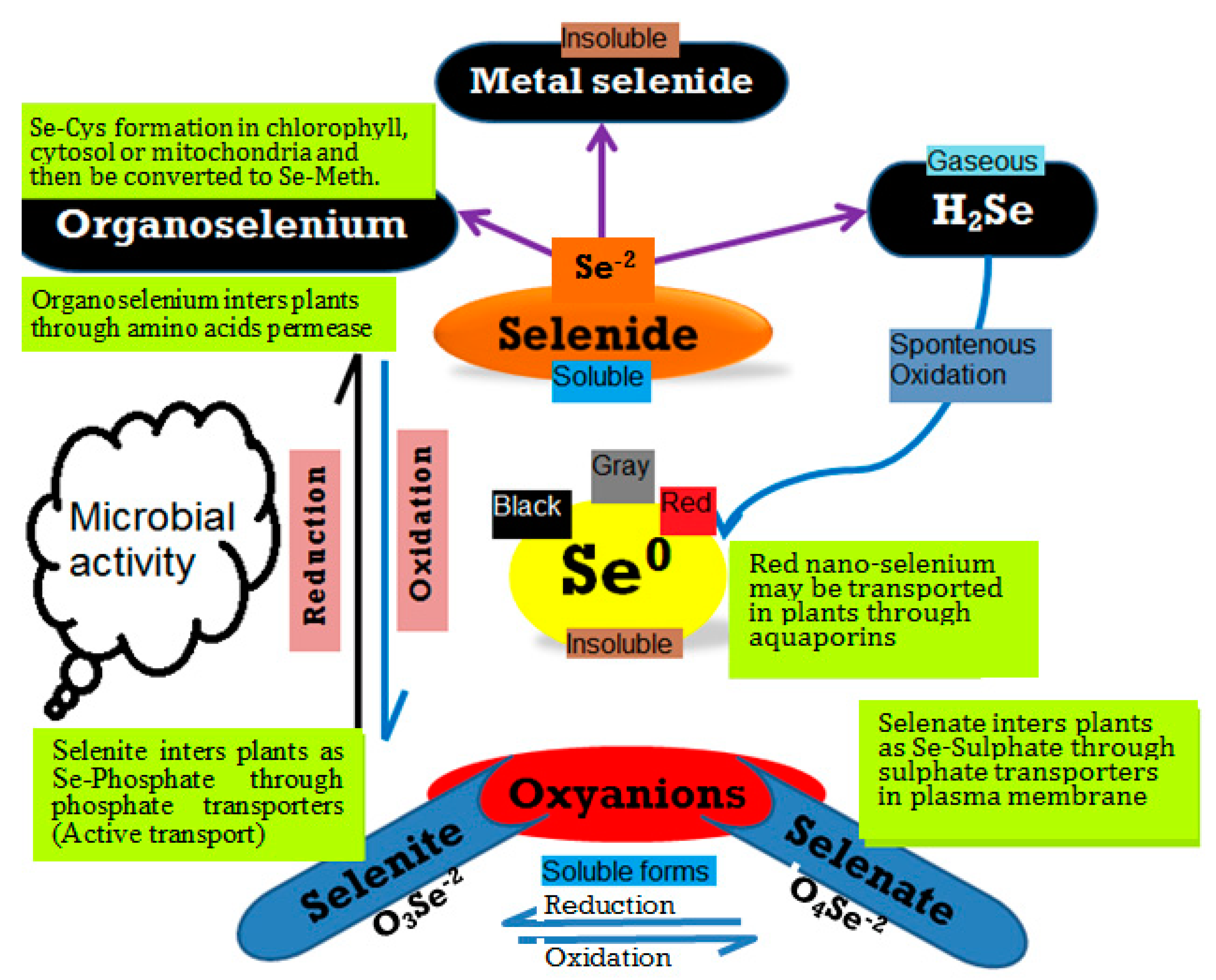
Figure 1.
An overview of selenium and its transformations in the soil environment. Different pathways for the fate and transformation of Se and its forms in the environment can be distinguished, including selenate, selenite, and elemental nano-Se.
There are many studies on Se and Se-NPs concerning their potential impact on human health, but the situation is different for higher plants, where much effort is still needed. Uptake from the soil and translocation as well as transformation of Se-NPs in higher plants needs more research. Will these nanoparticles be transformed into toxic forms? What will happen if Se nanoparticles are added or co-applied with another nanoparticle? What are the conditions that control Se-NPs transformations in the rhizosphere? What are the expected ecotoxicological effects of applied Se-NPs in the rhizosphere? At present, there are limited studies of the role of Se-NPs in plant nutrition [23,24,58,59,60], but the biogeochemistry of Se and Se-NPs in agroecosystems and their speciation in cultivated plants are important issues in terms of biofortification of crops for human health [13,61].
Major questions exist regarding Se and Se-NPs biofortification. Do biological Se-NPs have the ability to replace mineral Se-fertilizer in the framework of sustainable agriculture [62,63]? Will it be possible to find standard levels for deficiency and toxicity of Se-NPs as has been done for Se for humans, animals and higher plants? It is important to understand the different forms of Se, including inorganic (i.e., selenate, selenite, selenide, and elemental nano-Se) and organic (i.e., selenomethionine and selenocysteine), as these are important for Se behavior, especially in soil environments (Figure 1). These forms might control Se availability for plant uptake with contributions to the biofortification process [64,65,66].
The biofortification of cultivated crops using nano-Se may be an important strategy [30] that could be adapted to minimize environmental problems, in particular problems that resulted from the over-use of mineral fertilizers. This is particularly true because Se is rare in the Earth’s crust. Nano-Se and Se biofortified edible crops still need more research from different points of view, such as environmental, economic, human health, and animal health perspectives [81,82,83]. Nano-Se has the potential to protect animals against oxidative stress [84], ameliorate heavy metal stress [85], or function as an effective cancer therapy [86]. The use of nano-Se or Se to support cultivated plants under different stresses is an important strategy due to the ameliorative effects of Se and nano-Se in enhancing the productivity of cultivated crops under harsh conditions such as heat stress [34], nitrate stress [87], pathogen (like Alternaria solani) stress [59], NaCl stress [60], and soil salinity stress [24].
3. Biofortification of Selenium and Nano-Selenium for Human Health
Realization of the relationship between Se as a nutrient and human health started with the discovery of the essentiality of this micronutrient in 1817. Many studies have confirmed that Se is essential for human health due to its role in preventing many chronic diseases such as cancer, neurodegenerative diseases, and cardiovascular disease as an essential component of more than 25 enzymes in humans [53]. The level of Se or nano-Se can be increased in foods through the biofortification approach [29,36,52,88,89,90,91,92]. Products from farm animals can also be enriched with Se [41,45,93]. The biofortification of cereal crops including wheat, rice and maize as well as some main vegetable crops including tomato, potato and lettuce will be reviewed in this section.
3.1. Biofortification of Cereal Crops: Wheat, Rice and Maize
The biofortification process is a method in which selected nutrients (e.g., Ca, Cu, Fe, I, Se, and Zn) or nutritional materials are inserted into the food chain [94]. These materials might include folate [95,96,97], riboflavin [98,99], lysine [100,101,102], and pro-vitamin A [103]. This can be achieved using the agronomic approach, traditional breeding, and transgenic approaches to reduce nutrient deficiencies for humans [104,105]. The most important nutrients that have been investigated in several biofortification studies include calcium [106], iron [92,107], copper [108], zinc [109,110,111], iodine [29,92], potassium [112], and selenium [66,113]. The use of Se fertilizers is one of the most common methods for Se-biofortification of several crops [105], such as rice [113,114,115], maize [116,117], wheat [92,111,118], cowpea [119], potato [85,120], carrot [90,121], turnip [122,123], shallot [124], beans [125], lettuce [91,126], basil [127], strawberry [32,33], and apple [128,129]. Edible plants that have been biofortified with Se [105] or livestock fed selenium-enriched alfalfa [25,130] are used to support human health as reported by the Finnish experience in biofortification with Se through fertilizers. This Finnish experience started in 1984, when the Finnish authorities decided to improve the Se content of foods and feeds by applying synthetic fertilizers containing Na2SeO4. The applied doses of Se to soil reached 10 mg ha−1 in 2012 with an optimal level of 70–80 µg in the daily Se intake of the Finnish people.
Malnutrition and micronutrient deficiencies have become a global issue and improving the nutrition of millions of people around the world may be achieved using staple crops and appropriate agronomic practices [105,131]. In the past biofortification mainly involved the main cereal crops (e.g., rice, maize, and wheat) and then moved to include pulse crops as well as some animal-based foods such as milk and cheese [132], meat [133], and eggs [134]. The Se-biofortification of cereal crops depends on Se forms, method of application, the efficacy of Se-fertilizers [118], the time of application, and plant growth stage [83,135]. It also depends on soil properties, in particular soil pH, salinity content, redox potential, organic matter content, and the soil microbial community [13,69,116,136,137,138,139]. The Se- biofortification of cereal crops including wheat, rice, and maize could be evaluated under different applied Se-forms and different growth conditions (Table 2). A review of the literature led to the following conclusions:

Table 2.
Selenium biofortification results of some selected cereal crops (wheat, rice, and maize) under different growth conditions.
- The main Se-forms applied to cereal crops for biofortification include selenate, selenite, selenomethionine (SeMet), methio-seleno-cysteine (MeSeCys), and nano-Se.
- The recommended Se-dose for biofortification of cereal crops mainly depends on the plant species and its variety or cultivar, the application method (seed coating and priming, foliar, or soil application), the growth media (e.g., soil, hydroponics, artificial growth media), the growth conditions (open field, controlled greenhouse, or in vitro experiment), the Se-form (inorganic, organic, or nano form), nano-Se characterization (the method of preparing, the size and color of nanoparticles), the background Se content in the soil, and the agricultural management practices [69,85].
- For wheat crops, the recommended Se-dose under field experiments was 21 g Se ha−1 as a foliar application [89], while an applied dose of up 120 g Se ha−1 did not cause any visible phytotoxicity symptoms [140]. Under pot experiments, an applied Se-dose of 2.5 mg Se kg −1 soil was a suitable dose for Se-fortification of grain wheat [136].
- For rice crops, Se-foliar fertilization up to 100 g Se ha−1 as sodium selenite produces safe and high converting levels of Se into general rice proteins under field experiments when there was an initial low total soil Se content up to 0.1 mg Se kg−1 soil [141]. The best method to fortify the rice plants was to use 6 mg Se L−1 under NaCl stress as a combination of foliar spraying and seed priming [73]. The recommended applied Se-dose for the growth of rice clearly depends on the growth stage (the seedling, tillering, booting, full heading, and mature stage). Foliar application of sodium selenite (10 mg L−1) at the booting and full heading stages enhanced the accumulation of SeMet, confirming that the previous Se rate is the ideal level for Se-biofortification of rice [115].
- For maize crops, biofortification with Se could be achieved under field conditions through a fertigation system at an application rate of 100–200 g of Se ha−1 as sodium selenite. The applied Se might enhance the nutraceutical value and antioxidant content of maize grains without any leaching of Se into groundwater [142,143]. Ngigi et al. [125] reported that the Se biofortification level (0.3 mg kg−1) could be achieved in three field locations in Kenya using a foliar Se-dose of 20 g ha−1 as sodium selenate, whereas Wang et al. [64] indicated that the Se-level may be up to 30 g Se ha−1 in China.
3.2. Biofortification of Vegetable Crops: Tomato, Potato and Lettuce
Vegetables are a major source of nutrients and phytochemicals that support human health and nutritional sustainability [148]. Vegetables routinely grown for human consumption include allium, cruciferous, legumes, yellow-orange-red, and leafy green vegetables [149]. These vegetables are important in biofortification programs due to their importance for human health and short growth period (Table 3). Several vegetable crops have already been used in biofortification programs, including vegetables enriched in Se such as tomato [150,151], potato [85,94,120], lettuce [91,126,152], onion [153], garlic [154,155], cabbage [139], carrot, broccoli [156,157], asparagus [158], radish [66,159,160], and spinach [30,158].

Table 3.
Some selected vegetable crops (tomato, potato, and lettuce) that have been investigated for selenium biofortification.
For tomato crops, the Se-biofortification program may differ depending on the growth media. A Se concentration of 0.05 mg L−1 with selenate and selenite as dual Se sources may be optimum for tomato fruits grown under the hydroponic technique [69], but under drip irrigation this dose may be up 1.5 mg Se L−1 [151]. For potato crops, foliar applied Se at 100 g ha−1 at the tuber bulking stage led to the highest tuber Se-content [85], whereas a low Se dose (0.75 mg kg−1 as selenate) for pots filled with tropical soil (pH 4.8; clay 71%; total Se content 0.065 mg kg−1) was the most efficient source for potato biofortification under tropical conditions [94]. The hydroponic system is a common technique for producing lettuce under greenhouse conditions and the Se-bioavailability in a hydroponic system was higher for selenite (40 µmol L−1 Se) compared with selenate due to its fast bio-transformation into organic forms in plant cells [126]. Under field conditions, a foliar of rate of 100 mg kg−1 may be the proper Se dose for lettuce biofortification in salt-affected soils (pH: 8.65; EC: 4.49 dS m−1; [152]).
4. Interaction of Selenium and Nano-Selenium with Environmental Conditions
Plant growth and development are mainly controlled by environmental conditions (e.g., water, nutrients, air, light, etc.). These conditions include both normal and stressful environments (i.e., biotic and abiotic stresses). Plants have the ability to alleviate these stresses using exogenous and endogenous anti-stressors or tools (through their defense system) such as plant growth-promoting rhizobacteria [167], silicon [168,169,170], nanoparticles of selenium, or silicon [171] and selenium [2]. Several studies have confirmed the identified role of Se in promoting cultivated plant growth under a variety of stresses [24,83,86,172,173]. There is a growing body of literature that shows the potential of Se-nanoparticles to promote plant growth under different conditions (Table 4), but this still needs more investigation, particularly under stressful conditions. Comparing the potential of Se-NPs as revealed in human and animal studies with plant investigations, much more progress has been achieved in the human and animal fields.

Table 4.
The role of selenium-nanoparticles (Se-NPs) in the growth of selected plants under different growth conditions.
It is well documented that biological nano-Se has desirable characteristics such as high biosafety and bioactivity properties, low toxicity, high solubility, and high mobility due to their large surface/volume ratio compared with soluble inorganic Se salts, mainly selenate and selenite [24,34]. The uptake of Se-NPs by plants primarily depends on the synthesis method (bio-synthesis or chemo-synthesis) and the size of the nanoparticles [141].
5. Selenium and Nano-Selenium Reduce the Toxicity of As, Cd, and Hg
Selenium has long been of great interest in a wide range of fields including the medical, pharmaceutical, agricultural, and industrial sectors. Recently, a considerable literature has developed around the potential of Se and Se-NPs to help humans and animals deal with environmental stresses, but much less research has been done regarding Se, Se-NPs, and environmental stress in plants [175]. Plant related soil and environmental stresses include salinity, drought, waterlogging, heat, and heavy metal stress, which represent serious constraints for global crop productivity [109]. Heavy metals and potential toxic trace metals that may exist in soil include arsenic (As), cadmium (Cd), chromium (Cr), mercury (Hg), lead (Pb), and selenium, which can be introduced into the soil through human activities such as mining [176], industrialization [177], urbanization [33], and agricultural practices [178,179]. These metals can also naturally occur in soils at levels that may cause problems [28,105].
Several investigations suggest that Se can play an important role for plants growing under stressful conditions, although further work on Se is required to confirm its essentiality for higher plants. Many studies conducted on Se and its potential in higher plants have investigated its uptake, translocation, metabolism, and toxicity [17,160,180,181]. Biofortification studies with Se and Se-NPs are considered promising because Se is essential for human and animal health [53]. Selenium biofortification and phytoremediation are at the same level of importance from the environmental protection point of view [81]. Based on the work done to date, some general comments can be made concerning the role of Se and nano-Se in higher plants under different stresses:
- The ameliorative role of Se when plants are stressed by soil heavy metal content has been investigated in several studies, including how Se protects plants against heavy metal stress, whereas there is little work investigating the use of Se-NPs in this context [34,78].
- Selenium has already been investigated by many researchers as a way to ameliorate As stress on rice plants [2,92,182,183,184,185,186,187,188,189,190,191]. These studies primed rice seed with Se during germination under As-stress. The ameliorative role of Se under As-oxidative stress occurred through the modulation of thiol (R-SH) and antioxidant enzymes in rice or Se-modulating the level of phenolics and nutrients alleviating the toxicity of arsenic in the rice plants.
- The most important studies investigating Se and its role under Cd stress on rice plants include the application of Se to mitigate Cd accumulation in high-Cd-contaminated soils [192], the behavior of Se at different planting times [115], the effects of Se-forms and application methods on modulation of rice growth [83], how Se reduces the uptake and translocation of Cd from contaminated soils [193], and reducing oxidative stress induced by Cd [33,194,195,196,197,198].
- Selenium has been shown to moderate the impacts of mercury (Hg) stress on cultivated rice [78,138,199,200,201]. Chapman et al. [202] studied how native plants in a mined field were able to grow under Hg and As soil pollution as well as how selenium promoted the growth of the plant seedlings by decreasing Hg and As bioaccumulation in these plants. Selenium also has the ability to decrease rice plant uptake of methylmercury in Hg-contaminated soils while increasing the uptake of other nutrient elements [78].
- Selenium can alleviate chromium (Cr) stress in many crops by regulating the Cr uptake. Research into this relationship has included Chinese cabbage [203], pak choi (Brassica campestris L. ssp. Chinensis Makino) [204], and mitigating Cr-toxicity in Brassicca napus L. [205], Brassica juncea seedlings [206], and cabbage (Brassica campestris L. ssp. Pekinensis) [207].
- Studies have demonstrated the mitigation of lead (Pb) toxicity by Se-application in ginger (Zingiber officinale Roscoe.) [208] and oilseed rape (Brassica napus L.) plants [209].
- Selenium nanoparticles are also thought to behave like Se in protecting cultivated plants under heavy metals stress but only a few studies have been published. Investigations that have been conducted regarding Se-NPs and stressful environments include the role of Se-NPs in enhancing the growth of some cultivated plants under stress such as sorghum under high temperature stress [34], strawberry under salinity stress [24] and rice under Cd and Pb toxicity [171].
6. Are Selenium and Nano-Selenium Emerging Pollutants?
With widespread interest in Se biofortification, it is possible that the environment (i.e., soil, water, air, and plants) might become contaminated with Se due to extensive Se applications, which in turn could create a risk for human health [28,210] (Figure 2). The main anthropogenic sources of Se-pollution include agricultural and industrial activities [211,212,213]. The most serious problem resulting from Se-pollution is contamination of drinking water sources; hence, remediation requires effective techniques to remove Se from natural waters [214,215]. Environmental Se-contamination may be bio-remediated using proper microbial adaptations like alkylation [216] or Se-transformation, bioavailability, mobility and volatilization into the atmosphere [15].
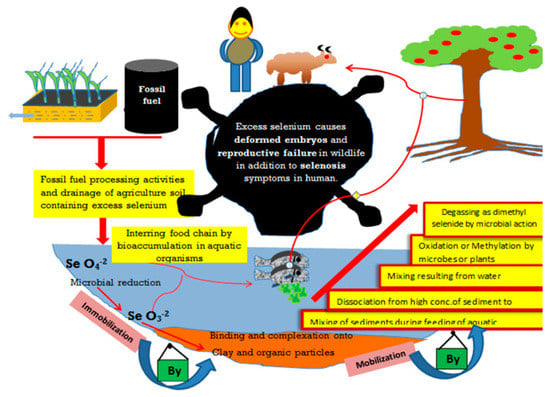
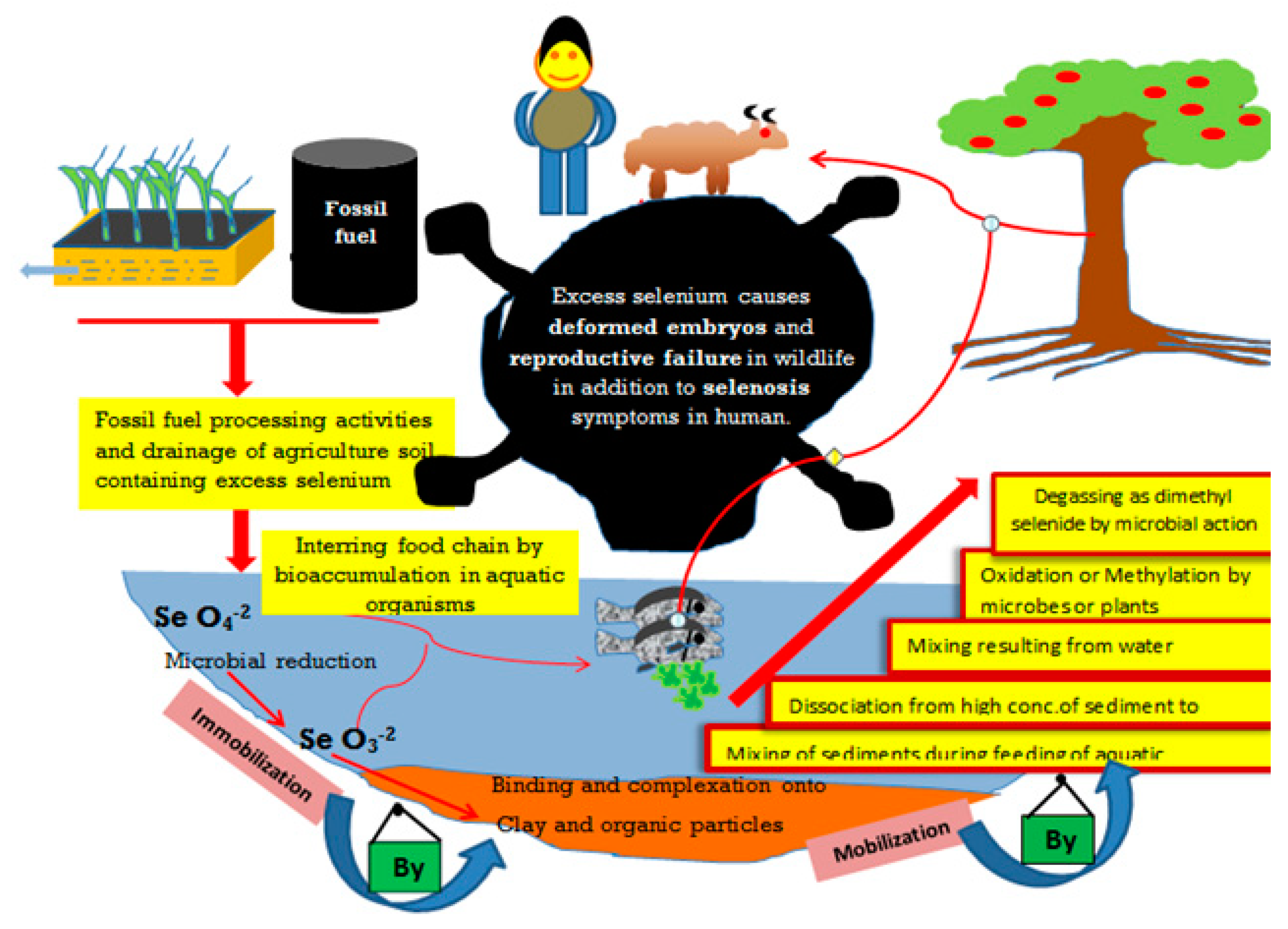
Figure 2.
Possible fates of selenium in the environment. Selenium could be released into the aquatic environment from industrial and agricultural activities. The excessive application of Se as fertilizer may lead to environmental hazards, which may be controlled by immobilization and mobilization reactions in soil, driven by soil clay and organic matter content as well as microbial activities. These Se-pathways may threaten human and animal health.
In addition to anthropogenic Se contamination problems, soils that are naturally high in Se content (seleniferous soils) are found in several places worldwide such as Punjab, India [217,218,219], Pine Ridge, Fort Collins, Colorado, USA [220], Western Colorado, USA [221], Enshi in China [222,223], Saskatchewan in Canada, Queensland in Australia, Irapuato in Mexico, the State of Boyaca in Colombia, and the Deog-Pyoung area in Korea [224]. Therefore, to address both anthropogenic and natural Se problems in soil, there is an urgent need to study Se-biofortification and phytoremediation through hyper-accumulating plants, which have the ability to uptake huge amounts of Se from soils and transfer it into the atmosphere by volatilization [67]. The agronomic Se biofortification of edible plants, pasture and forage crops should be improved through judicious biofortification programs without excessive applications or applications of Se in places that do not experience Se deficiency in the soil. Over-biofortification of products with selenium should be avoided, even in areas with Se-deficiencies, to prevent the build-up of Se to toxic levels in food and related products.
Further investigations are needed to monitor the behavior of Se-NPs in different environments including agricultural soils, waters, and farm animals. Se-NPs have already been used in the remediation of soils and waters that were contaminated with heavy metals like mercury [225,226,227]. This work confirmed that soils and groundwater contaminated with elemental mercury could be remediated via biogenic Se-NPs based on mechanisms like the immobilization of elemental mercury [226,227], applied hetero-aggregation of soil particulate organic matter [225], applied biofilm-coated quartz sand [228], and applied goethite colloids [138] in the presence of Se-NPs [64].
7. Conclusions
Selenium is an essential micronutrient for the nutrition of humans, animals, and lower plants, but whether or not it is essential for higher plants has yet to be confirmed. Nano-selenium is one of the most potentially useful Se-forms and has fascinating properties that lead to its use in nanomedicine applications, drug delivery, therapeutic applications, biomedicalue applications, and cancer prevention. Several places worldwide have a Se-deficiency problem due to low Se soils and should implement programs that guarantee safe and proper Se-supplementation levels for human health. The biofortification approach has shown particular promise for dietary supplementation of Se so that humans and/or animals have Se present in the right form, place, dose, and time. Due to the changing world and environmental stressors, there is an urgent need to document how and when Se helps plants and animals overcome stresses, including the identification of suitable Se and Se-NP fertilizers, application timing, and application rates. Several studies have demonstrated the ameliorative effects of Se and nano-Se on plants and farm animals under stress, but the use of Se-NPs in biofortification programs still needs more research. Despite all the documented benefits of Se, the difference between Se deficiency and Se toxicity is very small. We also need to understand the possible adverse effects and cytotoxicity of these Se-NPs on humans and effects on crops. It is also important to understand Se and Se-NP accumulation, transformation, and transport through soil. Due to the intensive use of Se and its forms in many fields, Se and nano-Se have become potential emerging pollutants in agroecosystems. This issue has increasingly been recognized as a potentially serious, worldwide public health concern.
Author Contributions
This project was conceived and led by H.E.R. All authors contributed to writing the paper, interpreting information presented, and have read and agreed to the published version of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research was financed by the Higher Education Institutional Excellence Program (NKFIH-1150-6/2019) of the Ministry of Innovation and Technology in Hungary, within the framework of the Biotechnology thematic program of the University of Debrecen. E.C. Brevik was partially supported by the National Science Foundation, Established Program to Stimulate Competitive Research (EPSCoR), under Grant Number IIA-1355466 during the writing of this paper.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Pilon-Smits, E.A.H. On the Ecology of Selenium Accumulation in Plants. Plants 2019, 8, 197. [Google Scholar] [CrossRef] [PubMed]
- Pokhrel, G.R.; Wang, K.T.; Zhuang, H.; Wu, Y.; Chen, W.; Lan, Y.; Zhu, X.; Li, Z.; Fu, F.F.; Yang, G. Effect of selenium in soil on the toxicity and uptake of arsenic in rice plant. Chemosphere 2020, 239, 124712. [Google Scholar] [CrossRef]
- Roman, M.; Jitaru, P.; Barbante, C. Selenium biochemistry and its role for human health. Metallomics 2014, 6, 25–54. [Google Scholar] [CrossRef] [PubMed]
- Baclaocos, J.; Santesmasses, D.; Mariotti, M.; Bierła, K.; Vetick, M.B.; Lynch, S.; McAllen, R.; Mackrill, J.J.; Loughran, G.; Guigó, R.; et al. Processive recoding and metazoan evolution of selenoprotein P: Up to 132 UGAs in Molluscs. J. Mol. Biol. 2019, 431, 4381–4407. [Google Scholar] [CrossRef] [PubMed]
- Shu, N.; Cheng, Q.; Arnér, E.S.J.; Davies, M.J. Inhibition and crosslinking of the selenoprotein thioredoxin reductase-1 by p-benzoquinone. Redox Biol. 2020, 28, 101335. [Google Scholar] [CrossRef] [PubMed]
- Avery, J.C.; Hoffmann, P.R. Selenium, Selenoproteins, and Immunity. Nutrients 2018, 10, 1203. [Google Scholar] [CrossRef]
- Santos, L.R.; Neves, C.; Melo, M.; Soares, P. Selenium and selenoproteins in immune mediated thyroid disorders. Diagnostics 2018, 8, 70. [Google Scholar] [CrossRef]
- Rayman, M.P. Selenium and human health. Lancet 2012, 379, 1256–1268. [Google Scholar] [CrossRef]
- Qazi, I.H.; Angel, C.; Yang, H.; Zoidis, E.; Pan, B.; Wu, Z.; Ming, Z.; Zeng, C.J.; Meng, Q.; Han, H.; et al. Role of selenium and selenoproteins in male reproductive function: A review of past and present evidences. Antioxidants 2019, 8, 268. [Google Scholar] [CrossRef]
- Schomburg, L. Dietary selenium and human health. Nutrients 2016, 9, 22. [Google Scholar] [CrossRef]
- Vinceti, M.; Filippini, T.; Cilloni, S.; Bargellini, A.; Vergoni, A.V.; Tsatsakis, A.; Ferrante, M. Health risk assessment of environmental selenium: Emerging evidence and challenges: Review. Mol. Med. Rep. 2017, 15, 3323–3335. [Google Scholar] [CrossRef] [PubMed]
- Brigelius-Flohé, R. Selenium in human health and disease: An overview. In Selenium: Its Molecular and Integrative Toxicology; Michalke, B., Ed.; Springer International Publishing AG: Cham, Switzerland, 2018; pp. 3–26. [Google Scholar] [CrossRef]
- Shahid, M.; Niazi, N.K.; Khalid, S.; Murtaza, B.; Bibi, I.; Rashid, M.I. A critical review of selenium biogeochemical behavior in soil-plant system with an inference to human health. Environ. Pollut. 2018, 234, 915–934. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, S.A.Z.; Kerkadi, A.; Agouni, A. Selenium and health: An update on the situation in the Middle East and North Africa. Nutrients 2019, 11, 1457. [Google Scholar] [CrossRef] [PubMed]
- Ullah, H.; Liu, G.; Yousaf, B.; Ali, M.U.; Irshad, S.; Abbas, Q.; Ahmad, R. A comprehensive review on environmental transformation of selenium: Recent advances and research perspectives. Environ. Geochem. Health 2019, 41, 1003–1035. [Google Scholar] [CrossRef]
- Schomburg, L.; Orho-Melander, M.; Struck, J.; Bergmann, A.; Melander, O. Selenoprotein-P deficiency predicts cardiovascular disease and death. Nutrients 2019, 11, 1852. [Google Scholar] [CrossRef]
- Gupta, M.; Gupta, S. An Overview of Selenium Uptake, Metabolism, and Toxicity in Plants. Front. Plant Sci. 2017, 7, 2074. [Google Scholar] [CrossRef]
- Skalickova, S.; Milosavljevic, V.; Cihalova, K.; Horky, P.; Richtera, L.; Adam, V. Selenium nanoparticles as a nutritional supplement. Nutrition 2017, 33, 83–90. [Google Scholar] [CrossRef]
- Tan, H.W.; Mo, H.Y.; Lau, A.T.Y.; Xu, Y.M. Selenium species: Current status and potentials in cancer prevention and therapy. Int. J. Mol. Sci. 2019, 20, 75. [Google Scholar] [CrossRef]
- Guan, B.; Yan, R.; Li, R.; Zhang, X. Selenium as a pleiotropic agent for medical discovery and drug delivery. Int. J. Nanomed. 2018, 13, 7473–7490. [Google Scholar] [CrossRef]
- Hosnedlova, B.; Kepinska, M.; Skalickova, S.; Fernandez, C.; Ruttkay-Nedecky, B.; Peng, Q.; Baron, M.; Melcova, M.; Opatrilova, R.; Zidkova, J.; et al. Nano-selenium and its nanomedicine applications: A critical review. Int. J. Nanomed. 2018, 13, 2107–2128. [Google Scholar] [CrossRef]
- Kamal, A.; Nazari, V.M.; Yaseen, M.; Iqbal, M.A.; Ahamed, M.B.K.; Majid, A.S.A.; Bhatti, H.N. Green synthesis of selenium-N-heterocyclic carbene compounds: Evaluation of antimicrobial and anticancer potential. Bioorg. Chem. 2019, 90, 103042. [Google Scholar] [CrossRef] [PubMed]
- Zahedi, S.M.; Hosseini, M.S.; Meybodi, N.; da Silva, J.A.T. Foliar application of selenium and nano-selenium affects pomegranate (Punica granatum cv. Malase Saveh) fruit yield and quality. S. Afr. J. Bot. 2019, 124, 350–358. [Google Scholar] [CrossRef]
- Zahedi, S.M.; Abdelrahman, M.; Hosseini, M.S.; Hoveizeh, N.F.; Tran, L.P. Alleviation of the effect of salinity on growth and yield of strawberry by foliar spray of selenium-nanoparticles. Environ. Pollut. 2019, 253, 246–258. [Google Scholar] [CrossRef] [PubMed]
- Hall, J.A.; Isaiah, A.; Estill, C.T.; Pirelli, G.J.; Suchodolski, J.S. Weaned beef calves fed selenium-biofortified alfalfa hay have an enriched nasal microbiota compared with healthy controls. PLoS ONE 2017, 12, 0179215. [Google Scholar] [CrossRef]
- Saleh, A.A.; Ebeid, T.A. Feeding sodium selenite and nano-selenium stimulates growth and oxidation resistance in broilers. S. Afr. J. Anim. Sci. 2019, 49. [Google Scholar] [CrossRef]
- Lopes, G.; Ávila, F.W.; Guilherme, L.R.G. Selenium behavior in the soil environment and its implication for human health. Ciência Agrotecnologia 2017, 41, 605–615. [Google Scholar] [CrossRef]
- Steffan, J.J.; Brevik, E.C.; Burgess, L.C.; Cerdà, A. The effect of soil on human health: An overview. Eur. J. Soil Sci. 2018, 69, 159–171. [Google Scholar] [CrossRef]
- Lyons, G. Biofortification of cereals with foliar selenium and iodine could reduce hypothyroidism. Front. Plant Sci. 2018, 9, 730. [Google Scholar] [CrossRef]
- Golubkina, N.A.; Koshelev, O.V.; Krivenkova, L.V.; Dobrutskaya, H.G.; Nadezhkin, S.; Caruso, G. Intersexual differences in plant growth, yield, mineral composition and antioxidants of spinach (Spinacia oleracea L.) as affected by selenium form. Sci. Hortic. 2017, 225, 350–358. [Google Scholar] [CrossRef]
- Leija-Martínez, P.; Benavides-Mendoza, A.; La Fuente, M.C.; Robledo-Olivo, A.; Ortega-Ortíz, H.; Sandoval-Rangel, A.; González-Morales, S. Lettuce biofortification with selenium in chitosan-polyacrylic acid complexes. Agronomy 2018, 8, 275. [Google Scholar] [CrossRef]
- Mimmo, T.; Tiziani, R.; Valentinuzzi, F.; Lucini, L.; Nicoletto, C.; Sambo, P.; Scampicchio, M.; Pii, Y.; Cesco, S. Selenium biofortification in Fragaria × ananassa: Implications on strawberry fruits quality, content of bioactive health beneficial compounds and metabolomic profile. Front. Plant Sci. 2017, 8, 1887. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Qin, N.; Sun, L.; Yu, M.; Hu, W.; Qi, Z. Selenium Improves Physiological Parameters and Alleviates Oxidative Stress in Strawberry Seedlings under Low-Temperature Stress. Int. J. Mol. Sci. 2018, 19, 1913. [Google Scholar] [CrossRef] [PubMed]
- Djanaguiraman, M.; Belliraj, N.; Bossmann, S.H.; Vara Prasad, P.V. High-Temperature stress alleviation by selenium nanoparticle treatment in grain sorghum. ACS Omega 2018, 3, 2479–2491. [Google Scholar] [CrossRef] [PubMed]
- Hussein, H.A.; Darwesh, O.M.; Mekki, B.B. Environmentally friendly nano-selenium to improve antioxidant system and growth of groundnut cultivars under sandy soil conditions. Biocatal. Agric. Biotechnol. 2019, 18, 101080. [Google Scholar] [CrossRef]
- Hussein, H.A.; Darwesh, O.M.; Mekki, B.B.; El-Hallouty, S.M. Evaluation of cytotoxicity, biochemical profile and yield components of groundnut plants treated with nano-selenium. Biotechnol. Rep. 2019, 24, e00377. [Google Scholar] [CrossRef]
- Kumar, N.; Krishnani, K.K.; Gupta, S.K.; Singh, N.P. Selenium nanoparticles enhanced thermal tolerance and maintain cellular stress protection of Pangasius hypophthalmus reared under lead and high temperature. Respir. Physiol. Neurobiol. 2017, 246, 107–116. [Google Scholar] [CrossRef]
- Kumar, N.; Krishnani, K.K.; Singh, N.P. Comparative study of selenium and selenium nanoparticles with reference to acute toxicity, biochemical attributes, and histopathological response in fish. Environ. Sci. Pollut. Res. Int. 2018, 25, 8914–8927. [Google Scholar] [CrossRef]
- Kumar, N.; Krishnani, K.K.; Gupta, S.K.; Sharma, R.; Baitha, R.; Singh, D.K.; Singh, N.P. Immuno-protective role of biologically synthesized dietary selenium nanoparticles against multiple stressors in Pangasinodon hypophthalmus. Fish Shellfish Immunol. 2018, 78, 289–298. [Google Scholar] [CrossRef]
- Kumar, N.; Brahmchari, R.K.; Bhushan, S.; Thorat, S.T.; Kumar, P.; Chandan, N.K.; Kumar, M.; Singh, N.P. Synergistic effect of dietary selenium nanoparticles and riboflavin on the enhanced thermal efficiency of fish against multiple stress factors. J. Therm. Biol. 2019, 85, 102417. [Google Scholar] [CrossRef]
- Sarkar, B.; Bhattacharjee, S.; Daware, A.; Tribedi, P.; Krishnani, K.K.; Minhas, P.S. Selenium nanoparticles for stress-resilient fish and livestock. Nanoscale Res. Lett. 2015, 10, 371. [Google Scholar] [CrossRef]
- Gulyas, G.; Csosz, E.; Prokisch, J.; Javor, A.; Mezes, M.; Erdelyi, M.; Balogh, K.; Janaky, T.; Szabo, Z.; Simon, A.; et al. Effect of nano-sized, elemental selenium supplement on the proteome of chicken liver. J. Anim. Physiol. Anim. Nutr. 2017, 101, 502–510. [Google Scholar] [CrossRef] [PubMed]
- Xueting, L.; Rehman, M.U.; Mehmood, K.; Huang, S.; Tian, X.; Wu, X.; Zhou, D. Ameliorative effects of nano-elemental selenium against hexavalent chromium-induced apoptosis in broiler liver. Environ. Sci. Pollut. Res. Int. 2018, 25, 15609–15615. [Google Scholar] [CrossRef] [PubMed]
- Xueting, L.; Rehman, M.U.; Zhang, H.; Tian, X.; Wu, X.; Shixue, M.K.; Zhou, D. Protective effects of Nano-elemental selenium against chromium-vi-induced oxidative stress in broiler liver. J. Biol. Regul. Homeost. Agents. 2018, 32, 47–54. [Google Scholar] [PubMed]
- Bakhshalinejad, R.; Hassanabadi, A.; Swick, R.A. Dietary sources and levels of selenium supplements affect growth performance, carcass yield, meat quality and tissue selenium deposition in broilers. Anim. Nutr. 2019, 5, 256–263. [Google Scholar] [CrossRef]
- Jalali, S.S.; Talebi, J.; Allymehr, M.; Soleimanzadeh, A.; Razi, M. Effects of nano-selenium on mRNA expression of markers for spermatogonial stem cells in the testis of broiler breeder males. Vet. Res. Forum. 2019, 10, 139–144. [Google Scholar] [CrossRef]
- Lee, J.; Hosseindoust, A.; Kim, M.; Kim, K.; Choi, Y.; Lee, S.; Cho, H.; Kang, W.S.; Chae, B. Biological evaluation of hot-melt extruded nano-selenium and the role of selenium on the expression profiles of selenium-dependent antioxidant enzymes in chickens. Biol. Trace Elem. Res. 2019, 194, 536–544. [Google Scholar] [CrossRef]
- Meng, T.; Liu, Y.L.; Xie, C.Y.; Zhang, B.; Huang, Y.Q.; Zhang, Y.W.; Yao, Y.; Huang, R.; Wu, X. Effects of different selenium sources on laying performance, egg selenium concentration, and antioxidant capacity in laying hens. Biol. Trace Elem. Res. 2019, 189, 548–555. [Google Scholar] [CrossRef] [PubMed]
- Dinh, Q.T.; Cui, Z.; Huang, J.; Tran, T.A.T.; Wang, D.; Yang, W.; Zhou, F.; Wang, M.; Yu, D.; Liang, D. Selenium distribution in the Chinese environment and its relationship with human health: A review. Environ. Int. 2018, 112, 294–309. [Google Scholar] [CrossRef]
- Alfthan, G.; Eurola, M.; Ekholm, P.; Venäläinen, E.R.; Root, T.; Korkalainen, K.; Hartikainen, H.; Salminen, P.; Hietaniemi, V.; Aspila, P.; et al. Effects of nationwide addition of selenium to fertilizers on foods, and animal and human health in Finland: From deficiency to optimal selenium status of the population. J. Trace Elem. Med. Biol. 2015, 31, 142–147. [Google Scholar] [CrossRef]
- Malagoli, M.; Schiavon, M.; dall’Acqua, S.; Pilon-Smits, E.A.H. Effects of selenium biofortification on crop nutritional quality. Front. Plant Sci. 2015, 6, 280. [Google Scholar] [CrossRef]
- Puccinelli, M.; Malorgio, F.; Pezzarossa, B. Selenium enrichment of horticultural crops. Molecules 2017, 22, 933. [Google Scholar] [CrossRef] [PubMed]
- Newman, R.; Waterland, N.; Moon, Y.; Tou, J.C. Selenium biofortification of agricultural crops and effects on plant nutrients and bioactive compounds important for human health and disease prevention—A review. Plant Foods Hum. Nutr. 2019, 74, 449–460. [Google Scholar] [CrossRef] [PubMed]
- Bakhshalinejad, R.; Kakhki, R.A.M.; Zoidis, E. Effects of different dietary sources and levels of selenium supplements on growth performance, antioxidant status and immune parameters in Ross 308 broiler chickens. Br. Poult. Sci. 2018, 59, 81–91. [Google Scholar] [CrossRef]
- Maiyo, F.; Singh, M. Selenium nanoparticles: Potential in cancer gene and drug delivery. Nanomedicine 2017, 12, 1075–1089. [Google Scholar] [CrossRef] [PubMed]
- Khurana, A.; Tekula, S.; Saifi, M.A.; Venkatesh, P.; Godugu, C. Therapeutic applications of selenium nanoparticles. Biomed. Pharmacother. 2019, 111, 802–812. [Google Scholar] [CrossRef]
- Vahidi, H.; Barabadi, H.; Saravanan, M. Emerging selenium nanoparticles to combat cancer: A systematic review. J. Clust. Sci. 2020, 31, 301–309. [Google Scholar] [CrossRef]
- Hernández-Hernández, H.; Quiterio-Gutiérrez, T.; Cadenas-Pliego, G.; Ortega-Ortiz, H.; Hernández-Fuentes, A.D.; de la Fuente, M.C.; Valdés-Reyna, J.; Juárez-Maldonado, A. Impact of selenium and copper nanoparticles on yield, antioxidant system, and fruit quality of tomato plants. Plants 2019, 8, 355. [Google Scholar] [CrossRef]
- Quiterio-Gutiérrez, T.; Ortega-Ortiz, H.; Cadenas-Pliego, G.; Hernández-Fuentes, A.D.; Sandoval-Rangel, A.; Benavides-Mendoza, A.; la Fuente, M.; Juárez-Maldonado, A. The application of selenium and copper nanoparticles modifies the biochemical responses of tomato plants under stress by Alternaria solani. Int. J. Mol. Sci. 2019, 20, 1950. [Google Scholar] [CrossRef]
- Morales-Espinoza, M.C.; Cadenas-Pliego, G.; Pérez-Alvarez, M.; Hernández-Fuentes, A.D.; de la Fuente, M.C.; Benavides-Mendoza, A.; Valdés-Reyna, J.; Juárez-Maldonado, A. Se nanoparticles induce changes in the growth, antioxidant responses, and fruit quality of tomato developed under NaCl stress. Molecules 2019, 24, 3030. [Google Scholar] [CrossRef]
- El-Ramady, H.R.; Domokos-Szabolcsy, É.; Abdalla, N.A.; Alshaal, T.A.; Shalaby, T.A.; Sztrik, A.; Prokisch, J.; Fári, M. Selenium and nano-selenium in agroecosystems. Environ. Chem. Lett. 2014, 12, 495–510. [Google Scholar] [CrossRef]
- El-Ramady, H.; Abdalla, N.; Alshaal, T.; El-Henawy, A.; Elmahrouk, M.; Bayoumi, Y.; Shalaby, T.; Amer, M.; Shehata, S.; Fári, M.; et al. Plant nano-nutrition: Perspectives and challenges. In Nanotechnology, Food Security and Water Treatment; Gothandam, K.M., Ranjan, S., Dasgupta, N., Ramalingam, C., Lichtfouse, E., Eds.; Springer International Publishing AG: Cham, Switzerland, 2018; pp. 129–161. [Google Scholar] [CrossRef]
- Sharma, D.; Dhuriya, Y.K.; Dhuriya, Y.K.; Sharma, J.; Gupta, M. Nanoelements: An agricultural paradigm for targeted plant nutrition therapeutic approach. In Nanotechnology for Agriculture: Crop Production & Protection; Panpatte, D.G., Jhala, Y.K., Eds.; Springer International Publishing AG: Cham, Switzerland, 2019; pp. 73–83. [Google Scholar] [CrossRef]
- Wang, Q.; Yu, Y.; Li, J.; Wan, Y.; Huang, Q.; Guo, Y.; Li, H. Effects of different forms of selenium fertilizers on Se accumulation, distribution, and residual effect in winter wheat-summer maize rotation system. J. Agric. Food Chem. 2017, 65, 1116–1123. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, R.J.B.; Pilon-Smits, E.A. Plant selenium hyperaccumulation- Ecological effects and potential implications for selenium cycling and community structure. Biochim. Biophys. Acta Gen. Subj. 2018, 1862, 2372–2382. [Google Scholar] [CrossRef] [PubMed]
- Hu, T.; Liang, Y.; Zhao, G.; Wu, W.; Li, H.; Guo, Y. Selenium Biofortification and Antioxidant Activity in Cordyceps militaris Supplied with Selenate, Selenite, or Selenomethionine. Biol. Trace Elem. Res. 2019, 187, 553–561. [Google Scholar] [CrossRef] [PubMed]
- Schiavon, M.; Pilon-Smits, E.A. The fascinating facets of plant selenium accumulation—Biochemistry, physiology, evolution and ecology. New Phytol. 2017, 213, 1582–1596. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Fan, H.; Wu, D.; Wan, J.; Wang, X.; Huang, R.; Liu, W.; Shen, F. Assessing bioaccessibility of Se and I in dual biofortified radish seedlings using simulated in vitro digestion. Food Res. Int. 2019, 119, 701–708. [Google Scholar] [CrossRef]
- Wang, M.; Peng, Q.; Zhou, F.; Yang, W.; Dinh, Q.T.; Liang, D. Uptake kinetics and interaction of selenium species in tomato (Solanum lycopersicum L.) seedlings. Environ. Sci. Pollut. Res. Int. 2019, 26, 9730–9738. [Google Scholar] [CrossRef]
- White, P.J. Selenium accumulation by plants. Ann. Bot. 2016, 117, 217–235. [Google Scholar] [CrossRef]
- Zsiros, O.; Nagy, V.; Párducz, Á.; Nagy, G.; Ünnep, R.; El-Ramady, H.; Prokisch, J.; Lisztes-Szabó, Z.; Fári, M.; Csajbók, J.; et al. Effects of selenate and red Se-nanoparticles on the photosynthetic apparatus of Nicotiana tabacum. Photosynth. Res. 2019, 139, 449–460. [Google Scholar] [CrossRef]
- Kabata-Pendias, A. Trace Elements in Soils and Plants, 4th ed.; CRC Press: Boca Raton, FL, USA, 2011. [Google Scholar]
- Subramanyam, K.; Du Laing, G.; Van Damme, E.J.M. Sodium selenate treatment using a combination of seed priming and foliar spray alleviates salinity stress in rice. Front. Plant Sci. 2019, 10, 116. [Google Scholar] [CrossRef]
- Kim, Y.; Oh, J.-M.; Roh, Y. Microbial precipitation of Cr(III)-hydroxide and Se(0) nanoparticles during anoxic bioreduction of Cr(VI)- and Se(VI)-contaminated water. J. Nanosci. Nanotechnol. 2017, 17, 2302–2304. [Google Scholar] [CrossRef]
- Brevik, E. Soil, food security, and human health. In Soils, Plant Growth and Crop Production; Verheye, W., Ed.; EOLSS Publishers: Oxford, UK, 2009. [Google Scholar]
- Wiesner-Reinhold, M.; Schreiner, M.; Baldermann, S.; Schwarz, D.; Hanschen, F.S.; Kipp, A.P.; Rowan, D.D.; Bentley-Hewitt, K.L.; McKenzie, M.J. Mechanisms of selenium enrichment and measurement in brassicaceous vegetables, and their application to human health. Front. Plant Sci. 2017, 8, 1365. [Google Scholar] [CrossRef] [PubMed]
- López-Bellido, F.J.; Sanchez, V.; Rivas, I.; López-Bellido, R.J.; López-Bellido, L. Wheat grain selenium content as affected by year and tillage system in a rainfed Mediterranean Vertisol. Field Crop. Res. 2019, 233, 41–48. [Google Scholar] [CrossRef]
- Li, Y.; Ge, Y.; Wang, R.; Zhao, J.; Jing, H.; Lin, X.; Ma, S.; Gao, Y.; Li, B.; Chen, C.; et al. Nanoelemental selenium alleviated the mercury load and promoted the formation of high-molecular-weight mercury- and selenium-containing proteins in serum samples from methylmercury-poisoned rats. Ecotoxicol. Environ. Saf. 2019, 169, 128–133. [Google Scholar] [CrossRef]
- Hadrup, N.; Ravn-Haren, G. Acute human toxicity and mortality after selenium ingestion: A review. J. Trace Elem. Med. Biol. 2020, 58, 126435. [Google Scholar] [CrossRef] [PubMed]
- Ren, B.; Liu, M.; Ni, J.; Tian, J. Role of selenoprotein F in protein folding and secretion: Potential involvement in human disease. Nutrients 2018, 10, 1619. [Google Scholar] [CrossRef] [PubMed]
- Schiavon, M.; Pilon-Smits, E.A. Selenium biofortification and phytoremediation phytotechnologies: A review. J. Environ. Qual. 2017, 46, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Germ, M.; Stibilj, V.; Šircelj, H.; Jerše, A.; Kroflič, A.; Golob, A.; Maršić, N.K. Biofortification of common buckwheat microgreens and seeds with different forms of selenium and iodine. J. Sci. Food Agric. 2019, 99, 4353–4362. [Google Scholar] [CrossRef]
- Yin, H.; Qi, Z.; Li, M.; Ahammed, G.J.; Chu, X.; Zhou, J. Selenium forms and methods of application differentially modulate plant growth, photosynthesis, stress tolerance, selenium content and speciation in Oryza sativa L. Ecotoxicol. Environ. Saf. 2019, 169, 911–917. [Google Scholar] [CrossRef]
- Tavoosi, S.; Baghsheikhi, A.H.; Shetab-Boushehri, S.V.; Navaei-Nigjeh, M.; Sarvestani, N.N.; Karimi, M.Y.; Ranjbar, A.; Ebadollahi-Natanzi, A.; Hosseini, A. Cerium and yttrium oxide nanoparticles and nano-selenium produce protective effects against H2O2-induced oxidative stress in pancreatic beta cells by modulating mitochondrial dysfunction. Pharm. Nanotechnol. 2019, 8, 63–75. [Google Scholar] [CrossRef]
- Zhang, X.; Gan, X.E.Q.; Zhang, Q.; Ye, Y.; Cai, Y.; Han, A.; Tian, M.; Wang, Y.; Wang, C.; Su, L.; et al. Ameliorative effects of nano-selenium against NiSO4-induced apoptosis in rat testes. Toxicol. Mech. Methods 2019, 29, 467–477. [Google Scholar] [CrossRef]
- Zhang, X.; He, C.; Yan, R.; Chen, Y.; Zhao, P.; Li, M.; Fan, T.; Yang, T.; Lu, Y.; Luo, J.; et al. HIF-1 dependent reversal of cisplatin resistance via anti-oxidative nano selenium for effective cancer therapy. Chem. Eng. J. 2020, 380, 122540. [Google Scholar] [CrossRef]
- Safari, M.; Oraghi Ardebili, Z.; Iranbakhsh, A. Selenium nano-particle induced alterations in expression patterns of heat shock factor A4A (HSFA4A), and high molecular weight glutenin subunit 1Bx (Glu-1Bx) and enhanced nitrate reductase activity in wheat (Triticum aestivum L.). Acta Physiol. Plant. 2018, 40, 117. [Google Scholar] [CrossRef]
- Boldrin, P.F.; Faquin, V.; Clemente, A.D.C.S.; de Andrade, T.; Guilherme, L.R.G. Genotypic Variation and Biofortification with Selenium in Brazilian Wheat Cultivars. J. Environ. Qual. 2018, 47, 1371–1379. [Google Scholar] [CrossRef] [PubMed]
- Lara, T.S.; de Lima Lessa, J.H.; de Souza, K.R.D.; Corguinha, A.P.B.; Martins, F.A.D.; Lopes, G.; Guilherme, L.R.G. Selenium biofortification of wheat grain via foliar application and its effect on plant metabolism. J. Food Compos. Anal. 2019, 81, 10–18. [Google Scholar] [CrossRef]
- Smoleń, S.; Baranski, R.; Ledwożyw-Smoleń, I.; Skoczylas, Ł.; Sady, W. Combined biofortification of carrot with iodine and selenium. Food Chem. 2019, 300, 125202. [Google Scholar] [CrossRef] [PubMed]
- Smoleń, S.; Kowalska, I.; Kováčik, P.; Halka, M.; Sady, W. Biofortification of six varieties of lettuce (Lactuca sativa L.) with iodine and selenium in combination with the application of salicylic acid. Front. Plant Sci. 2019, 10, 143. [Google Scholar] [CrossRef]
- Zou, C.; Du, Y.; Rashid, A.; Ram, H.; Savasli, E.; Pieterse, P.J.; Ortiz-Monasterio, I.; Yazici, A.; Kaur, C.; Mahmood, K.; et al. Simultaneous biofortification of wheat with zinc, iodine, selenium, and iron through foliar treatment of a micronutrient cocktail in six countries. J. Agric. Food Chem. 2019, 67, 8096–8106. [Google Scholar] [CrossRef]
- Ramírez Bribiesca, J.E.; Casas, R.L.; Cruz Monterrosa, R.G.; Pérez, A.R. Supplementing selenium and zinc nanoparticles in ruminants for improving their bioavailability meat. In Nutrient Delivery: A volume in Nanotechnology in the Agri-Food Industry; Grumezescu, A.M., Ed.; Academic Press: Cambridge, MA, USA, 2017; pp. 713–747. [Google Scholar] [CrossRef]
- De Oliveira, V.C.; Faquin, V.; Andrade, F.R.; Carneiro, J.P.; da Silva Júnior, E.C.; de Souza, K.R.D.; Pereira, J.; Guilherme, L.R.G. Physiological and physicochemical responses of potato to selenium biofortification in tropical soil. Potato Res. 2019, 62, 315–331. [Google Scholar] [CrossRef]
- Strobbe, S.; Van Der Straeten, D. Folate biofortification in food crops. Curr. Opin. Biotechnol. 2017, 44, 202–211. [Google Scholar] [CrossRef]
- De Lepeleire, J.; Strobbe, S.; Verstraete, J.; Blancquaert, D.; Ambach, L.; Visser, R.G.F.; Stove, C.; Van Der Straeten, D. Folate biofortification of potato by tuber-specific expression of four folate biosynthesis genes. Mol. Plant. 2018, 11, 175–188. [Google Scholar] [CrossRef]
- Liang, Q.; Wang, K.; Liu, X.; Riaz, B.; Jiang, L.; Wan, X.; Ye, X.; Zhang, C. Improved folate accumulation in genetically modified maize and wheat. J. Exp. Bot. 2019, 70, 1539–1551. [Google Scholar] [CrossRef] [PubMed]
- Rajendran, S.C.C.; Chamlagain, B.; Kariluoto, S.; Piironen, V.; Saris, P.E.J. Biofortification of riboflavin and folate in idli batter, based on fermented cereal and pulse, by Lactococcus lactis N8 and Saccharomyces boulardii SAA655. J. Appl. Microbiol. 2017, 122, 1663–1671. [Google Scholar] [CrossRef] [PubMed]
- Yépez, A.; Russo, P.; Spano, G.; Khomenko, I.; Biasioli, F.; Capozzi, V.; Aznar, R. In situ riboflavin fortification of different kefir-like cereal-based beverages using selected Andean LAB strains. Food Microbiol. 2019, 77, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Wong, H.W.; Liu, Q.; Sun, S.S. Biofortification of rice with lysine using endogenous histones. Plant Mol. Biol. 2015, 87, 235–248. [Google Scholar] [CrossRef]
- Wang, W.; Galili, G. Transgenic high-lysine rice–A realistic solution to malnutrition. J. Exp. Bot. 2016, 67, 4009–4011. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.Q.; Suen, P.K.; Zhang, C.Q.; Mak, W.S.; Gu, M.H.; Liu, Q.Q.; Sun, S.S. Improved growth performance, food efficiency, and lysine availability in growing rats fed with lysine-biofortified rice. Sci. Rep. 2017, 7, 1389. [Google Scholar] [CrossRef]
- Sestili, F.; Garcia-Molina, M.D.; Gambacorta, G.; Beleggia, R.; Botticella, E.; De Vita, P.; Savatin, D.V.; Masci, S.; Lafiandra, D. Provitamin A biofortification of durum wheat through a TILLING approach. Int. J. Mol. Sci. 2019, 20, 5703. [Google Scholar] [CrossRef]
- Garg, M.; Sharma, N.; Sharma, S.; Kapoor, P.; Kumar, A.; Chunduri, V.; Arora, P. Biofortified crops generated by breeding, agronomy, and transgenic approaches are improving lives of millions of people around the world. Front. Nutr. 2018, 5, 12. [Google Scholar] [CrossRef]
- Brevik, E.C.; Slaughter, L.; Singh, B.R.; Steffan, J.J.; Collier, D.; Barnhart, P.; Pereira, P. Soil and human health: Current status and future needs. Air Soil Water Res. 2020, 13, 1–23. [Google Scholar] [CrossRef]
- D’Imperio, M.; Renna, M.; Cardinali, A.; Buttaro, D.; Serio, F.; Santamaria, P. Calcium biofortification and bioaccessibility in soilless “baby leaf” vegetable production. Food Chem. 2016, 213, 149–156. [Google Scholar] [CrossRef]
- Ludwig, Y.; Slamet-Loedin, I.H. Genetic biofortification to enrich rice and wheat grain iron: From genes to product. Front. Plant Sci. 2019, 10, 833. [Google Scholar] [CrossRef] [PubMed]
- Rzymski, P.; Mleczek, M.; Niedzielski, P.; Siwulski, M.; Gąsecka, M. Cultivation of Agaricus bisporus enriched with selenium, zinc and copper. J. Sci. Food Agric. 2017, 97, 923–928. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.M.; Zhao, W.Y.; Gao, A.X.; Su, T.T.; Wang, Y.K.; Zhang, Y.Q.; Zhou, X.B.; He, X.H. How could agronomic biofortification of rice be an alternative strategy with higher cost-effectiveness for human iron and zinc deficiency in China? Food Nutr. Bull. 2018, 39, 246–259. [Google Scholar] [CrossRef]
- Akhtar, M.; Yousaf, S.; Sarwar, N.; Hussain, S. Zinc biofortification of cereals-role of phosphorus and other impediments in alkaline calcareous soils. Environ. Geochem. Health 2019, 41, 2365–2379. [Google Scholar] [CrossRef]
- Du, W.; Yang, J.; Peng, Q.; Liang, X.; Mao, H. Comparison study of zinc nanoparticles and zinc sulphate on wheat growth: From toxicity and zinc biofortification. Chemosphere 2019, 227, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Adu, M.O.; Asare, P.A.; Yawson, D.O.; Nyarko, M.A.; Osei-Agyeman, K. Agronomic biofortification of selected underutilised solanaceae vegetables for improved dietary intake of potassium (K) in Ghana. Heliyon 2020, 4, e00750. [Google Scholar] [CrossRef] [PubMed]
- Reis, H.P.G.; Barcelos, J.P.Q.; Silva, V.M.; Santos, E.F.; Tavanti, R.F.R.; Putti, F.F.; Young, S.D.; Broadley, M.R.; White, P.J.; Dos Reis, A.R. Agronomic biofortification with selenium impacts storage proteins in grains of upland rice. J. Sci. Food Agric. 2019, 100, 1990–1997. [Google Scholar] [CrossRef]
- D’Amato, R.; Fontanella, M.C.; Falcinelli, B.; Beone, G.M.; Bravi, E.; Marconi, O.; Benincasa, P.; Businelli, D. Selenium Biofortification in Rice (Oryza sativa L.) Sprouting: Effects on Se Yield and Nutritional Traits with Focus on Phenolic Acid Profile. J. Agric. Food Chem. 2018, 66, 4082–4090. [Google Scholar] [CrossRef]
- Farooq, M.U.; Tang, Z.; Zeng, R.; Liang, Y.; Zhang, Y.; Zheng, T.; Ei, H.H.; Ye, X.; Jia, X.; Zhu, J.; et al. Accumulation, mobilization, and transformation of selenium in rice grain provided with foliar sodium selenite. J. Sci. Food Agric. 2019, 99, 2892–2900. [Google Scholar] [CrossRef]
- Bocchini, M.; D’Amato, R.; Ciancaleoni, S.; Fontanella, M.C.; Palmerini, C.A.; Beone, G.M.; Onofri, A.; Negri, V.; Marconi, G.; Albertini, E.; et al. Soil selenium (Se) biofortification changes the physiological, biochemical and epigenetic. by inducing a higher drought tolerance. Front. Plant Sci. 2018, 9, 389. [Google Scholar] [CrossRef]
- Joy, E.J.M.; Kalimbira, A.A.; Gashu, D.; Ferguson, E.L.; Sturgess, J.; Dangour, A.D.; Banda, L.; Chiutsi-Phiri, G.; Bailey, E.H.; Langley-Evans, S.C.; et al. Can selenium deficiency in Malawi be alleviated through consumption of agro-biofortified maize flour? Study protocol for a randomised, double-blind, controlled trial. Trials 2019, 20, 795. [Google Scholar] [CrossRef] [PubMed]
- Ramkissoon, C.; Degryse, F.; da Silva, R.C.; Baird, R.; Young, S.D.; Bailey, E.H.; McLaughlin, M.J. Improving the efficacy of selenium fertilizers for wheat biofortification. Sci. Rep. 2019, 9, 19520. [Google Scholar] [CrossRef] [PubMed]
- Silva, V.M.; Boleta, E.H.M.; Martins, J.T.; dos Santos, F.L.M.; da Rocha Silva, A.C.; Alcock, T.D.; Wilson, L.; de Sá, M.E.; Young, S.D.; Broadley, M.R.; et al. Agronomic biofortification of cowpea with selenium: Effects of selenate and selenite applications on selenium and phytate concentrations in seeds. J. Sci. Food Agric. 2020, 99, 5969–5983. [Google Scholar] [CrossRef] [PubMed]
- Smoleń, S.; Kowalska, I.; Skoczylas, Ł.; Liszka-Skoczylas, M.; Grzanka, M.; Halka, M.; Sady, W. The effect of salicylic acid on biofortification with iodine and selenium and the quality of potato cultivated in the NFT system. Sci. Hortic. 2018, 240, 530–543. [Google Scholar] [CrossRef]
- Smoleń, S.; Skoczylas, Ł.; Ledwożyw-Smoleń, I.; Rakoczy, R.; Kopeć, A.; Piątkowska, E.; Bieżanowska-Kopeć, R.; Koronowicz, A.; Kapusta-Duch, J. Biofortification of carrot (Daucus carota L.) with iodine and selenium in a field experiment. Front. Plant Sci. 2016, 7, 730. [Google Scholar] [CrossRef]
- Li, X.; Wu, Y.; Li, B.; Yang, Y.; Yang, Y. Selenium accumulation characteristics and biofortification potentiality in turnip (Brassica rapa var. rapa) supplied with selenite or selenate. Front. Plant Sci. 2018, 8, 2207. [Google Scholar] [CrossRef]
- Li, X.; Li, B.; Yang, Y. Effects of foliar selenite on the nutrient components of turnip (Brassica rapa var. rapa Linn.). Front. Chem. 2018, 6, 42. [Google Scholar] [CrossRef]
- Golubkina, N.; Zamana, S.; Seredin, T.; Poluboyarinov, P.; Sokolov, S.; Baranova, H.; Krivenkov, L.; Pietrantonio, L.; Caruso, G. Effect of selenium biofortification and beneficial microorganism inoculation on yield, quality and antioxidant properties of shallot bulbs. Plants 2019, 8, 102. [Google Scholar] [CrossRef]
- Ngigi, P.B.; Lachat, C.; Masinde, P.W.; Du Laing, G. Agronomic biofortification of maize and beans in Kenya through selenium fertilization. Environ. Geochem. Health 2019, 41, 2577–2591. [Google Scholar] [CrossRef]
- Do Nascimento da Silva, E.; Cadore, S. Bioavailability assessment of copper, iron, manganese, molybdenum, selenium, and zinc from selenium-enriched lettuce. J. Food Sci. 2019, 84, 2840–2846. [Google Scholar] [CrossRef]
- Puccinelli, M.; Malorgio, F.; Rosellini, I.; Pezzarossa, B. Production of selenium-biofortified microgreens from selenium-enriched seeds of basil. J. Sci. Food Agric. 2019, 99, 5601–5605. [Google Scholar] [CrossRef] [PubMed]
- Wortmann, L.; Enneking, U.; Daum, D. German consumers’ attitude towards selenium-biofortified apples and acceptance of related nutrition and health claims. Nutrients 2018, 10, 190. [Google Scholar] [CrossRef] [PubMed]
- Babalar, M.; Mohebbi, S.; Zamani, Z.; Askari, M.A. Effect of foliar application with sodium selenate on selenium biofortification and fruit quality maintenance of ‘Starking Delicious’ apple during storage. J. Sci. Food Agric. 2019, 99, 5149–5156. [Google Scholar] [CrossRef] [PubMed]
- Wallace, L.G.; Bobe, G.; Vorachek, W.R.; Dolan, B.P.; Estill, C.T.; Pirelli, G.J.; Hall, J.A. Effects of feeding pregnant beef cows selenium-enriched alfalfa hay on selenium status and antibody titers in their newborn calves. J. Anim. Sci. 2017, 95, 2408–2420. [Google Scholar] [CrossRef]
- Kihara, J.; Bolo, P.; Kinyua, M.; Rurinda, J.; Piikki, K. Micronutrient deficiencies in African soils and the human nutritional nexus: Opportunities with staple crops. Environ. Geochem. Health 2020. [Google Scholar] [CrossRef]
- Witkowska, Z.; Michalak, I.; Korczyński, M.; Szołtysik, M.; Świniarska, M.; Dobrzański, Z.; Tuhy, Ł.; Samoraj, M.; Chojnacka, K. Biofortification of milk and cheese with microelements by dietary feed bio-preparations. J. Food Sci. Technol. 2015, 52, 6484–6492. [Google Scholar] [CrossRef]
- Mattioli, S.; Machado Duarte, J.M.; Castellini, C.; D’Amato, R.; Regni, L.; Proietti, P.; Businelli, D.; Cotozzolo, E.; Rodrigues, M.; Dal Bosco, A. Use of olive leaves (whether or not fortified with sodium selenate) in rabbit feeding: Effect on performance, carcass and meat characteristics, and estimated indexes of fatty acid metabolism. Meat Sci. 2018, 143, 230–236. [Google Scholar] [CrossRef]
- Witkowska, Z.; Świniarska, M.; Korczyński, M.; Opaliński, S.; Konkol, D.; Michalak, I.; Saeid, A.; Mironiuk, M.; Chojnacka, K. Biofortification of hens’ eggs with microelements by innovative bio-based dietary supplement. J. Anim. Physiol. Anim. Nutr. 2019, 103, 485–492. [Google Scholar] [CrossRef]
- Filek, M.; Sieprawska, A.; Telk, A.; Łabanowska, M.; Kurdziel, M.; Walas, S.; Hartikainen, H. Translocation of elements and sugars in wheat genotypes at vegetative and generative stages under continuous selenium exposure. J. Sci. Food Agric. 2019, 99, 6364–6371. [Google Scholar] [CrossRef]
- Ali, F.; Peng, Q.; Wang, D.; Cui, Z.; Huang, J.; Fu, D.; Liang, D. Effects of selenite and selenate application on distribution and transformation of selenium fractions in soil and its bioavailability for wheat (Triticum aestivum L.). Environ. Sci. Pollut. Res. Int. 2017, 24, 8315–8325. [Google Scholar] [CrossRef]
- Liu, K.; Cai, M.; Hu, C.; Sun, X.; Cheng, Q.; Jia, W.; Yang, T.; Nie, M.; Zhao, X. Selenium (Se) reduces Sclerotinia stem rot disease incidence of oilseed rape by increasing plant Se concentration and shifting soil microbial community and functional profiles. Environ. Pollut. 2019, 254, 113051. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, D.; Qian, H.; Liang, Y.; Pan, X.; Gadd, G.M. Interactions between biogenic selenium nanoparticles and goethite colloids and consequence for remediation of elemental mercury contaminated groundwater. Sci. Total Environ. 2018, 613–614, 672–678. [Google Scholar] [CrossRef] [PubMed]
- Bañuelos, G.S.; Freeman, J.; Arroyo, I. Accumulation and speciation of selenium in biofortified vegetables grown under high boron and saline field conditions. Food Chem. 2020, 5, 100073. [Google Scholar] [CrossRef] [PubMed]
- De Vita, P.; Platani, C.; Fragasso, M.; Ficco, D.B.M.; Colecchia, S.A.; Del Nobile, M.A.; Padalino, L.; Di Gennaro, S.; Petrozza, A. Selenium-enriched durum wheat improves the nutritional profile of pasta without altering its organoleptic properties. Food Chem. 2017, 214, 374–382. [Google Scholar] [CrossRef]
- Hu, T.; Li, H.; Li, J.; Zhao, G.; Wu, W.; Liu, L.; Wang, Q.; Guo, Y. Absorption and bio-transformation of selenium nanoparticles by wheat seedlings (Triticum aestivum L.). Front. Plant Sci. 2018, 9, 597. [Google Scholar] [CrossRef]
- D’Amato, R.; De Feudis, M.; Guiducci, M.; Businelli, D. Zea mays L. grain: Increase in nutraceutical and antioxidant properties due to se fortification in low and high water regimes. J. Agric Food Chem. 2019, 67, 7050–7059. [Google Scholar] [CrossRef]
- De Feudis, M.; D’Amato, R.; Businelli, D.; Guiducci, M. Fate of selenium in soil: A case study in a maize (Zea mays L.) field under two irrigation regimes and fertilized with sodium selenite. Sci. Total Environ. 2019, 659, 131–139. [Google Scholar] [CrossRef]
- Yasin, M.; El-Mehdawi, A.F.; Pilon-Smits, E.A.; Faisal, M. Selenium-fortified wheat: Potential of microbes for biofortification of selenium and other essential nutrients. Int. J. Phytoremediat. 2015, 17, 777–786. [Google Scholar] [CrossRef]
- Gong, R.; Ai, C.; Zhang, B.; Cheng, X. Effect of selenite on organic selenium speciation and selenium bioaccessibility in rice grains of two Se-enriched rice cultivars. Food Chem. 2018, 264, 443–448. [Google Scholar] [CrossRef]
- Ashraf, M.A.; Akbar, A.; Parveen, A.; Rasheed, R.; Hussain, I.; Iqbal, M. Phenological application of selenium differentially improves growth, oxidative defense and ion homeostasis in maize under salinity stress. Plant Physiol. Biochem. 2018, 123, 268–280. [Google Scholar] [CrossRef]
- Jiang, C.; Zu, C.; Lu, D.; Zheng, Q.; Shen, J.; Wang, H.; Li, D. Effect of exogenous selenium supply on photosynthesis, Na+ accumulation and antioxidative capacity of maize (Zea mays L.) under salinity stress. Sci. Rep. 2017, 7, 42039. [Google Scholar] [CrossRef] [PubMed]
- Finley, J.W.; Dimick, D.; Marshall, E.; Nelson, G.C.; Mein, J.R.; Gustafson, D.I. Nutritional sustainability: Aligning priorities in nutrition and public health with agricultural production. Adv. Nutr. 2017, 8, 780–788. [Google Scholar] [CrossRef] [PubMed]
- Blekkenhorst, L.C.; Sim, M.; Bondonno, C.P.; Bondonno, N.P.; Ward, N.C.; Prince, R.L.; Devine, A.; Lewis, J.R.; Hodgson, J.M. Cardiovascular Health Benefits of Specific Vegetable Types: A Narrative Review. Nutrients 2018, 10, 595. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Zhang, Y.; Liu, J.; Chen, Y.; Zhang, X. Exploring the effects of selenium treatment on the nutritional quality of tomato fruit. Food Chem. 2018, 252, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Puccinelli, M.; Malorgio, F.; Terry, L.A.; Tosetti, R.; Rosellini, I.; Pezzarossa, B. Effect of selenium enrichment on metabolism of tomato (Solanum lycopersicum) fruit during postharvest ripening. J. Sci. Food Agric. 2019, 99, 2463–2472. [Google Scholar] [CrossRef]
- Shalaby, T.; Bayoumi, Y.; Alshaal, T.; Elhawat, N.; Sztrik, A.; El-Ramady, H. Selenium fortification induces growth, antioxidant activity, yield and nutritional quality of lettuce in salt-affected soil using foliar and soil applications. Plant Soil. 2017, 421, 245–258. [Google Scholar] [CrossRef]
- Castro Grijalba, A.; Martinis, E.M.; Wuilloud, R.G. Inorganic selenium speciation analysis in Allium and Brassica vegetables by ionic liquid assisted liquid-liquid microextraction with multivariate optimization. Food Chem. 2017, 219, 102–108. [Google Scholar] [CrossRef]
- Ogra, Y.; Ogihara, Y.; Anan, Y. Comparison of the metabolism of inorganic and organic selenium species between two selenium accumulator plants, garlic and Indian mustard. Metallomics 2017, 9, 61–68. [Google Scholar] [CrossRef]
- Astaneh, R.K.; Bolandnazar, S.; Nahandi, F.Z.; Oustan, S. Effects of selenium on enzymatic changes and productivity of garlic under salinity stress. S. Afr. J. Bot. 2019, 121, 447–455. [Google Scholar] [CrossRef]
- Bañuelos, G.S.; Arroyo, I.; Pickering, I.J.; Yang, S.I.; Freeman, J.L. Selenium biofortification of broccoli and carrots grown in soil amended with Se-enriched hyperaccumulator Stanleya pinnata. Food Chem. 2015, 166, 603–608. [Google Scholar] [CrossRef]
- Bañuelos, G.S.; Arroyo, I.S.; Dangi, S.R.; Zambrano, M.C. Continued Selenium Biofortification of Carrots and Broccoli Grown in Soils Once Amended with Se-enriched S. pinnata. Front. Plant Sci. 2016, 7, 1251. [Google Scholar] [CrossRef] [PubMed]
- Conversa, G.; Lazzizera, C.; Chiaravalle, A.E.; Miedico, O.; Bonasia, A.; La Rotonda, P.; Elia, A. Selenium fern application and arbuscular mycorrhizal fungi soil inoculation enhance Se content and antioxidant properties of green asparagus (Asparagus officinalis L.) spears. Sci. Hortic. 2019, 252, 176–191. [Google Scholar] [CrossRef]
- Da Silva, D.F.; Cipriano, P.E.; de Souza, R.R.; Júnior, M.S.; Faquin, V.; Silva, M.L.S.; Guilherme, L.R.G. Biofortification with selenium and implications in the absorption of macronutrients in Raphanus sativus L. J. Food Compos. Anal. 2020, 86, 103382. [Google Scholar] [CrossRef]
- Da Silva, D.F.; Cipriano, P.E.; de Souza, R.R.; Júnior, M.S.; da Silva, R.F.; Faquin, V.; Silva, M.L.S.; Guilherme, L.R.G. Anatomical and physiological characteristics of Raphanus sativus L. submitted to different selenium sources and forms application. Sci. Hortic. 2020, 260, 108839. [Google Scholar] [CrossRef]
- Lima, L.W.; Checchio, M.V.; dos Reis, A.R.; de Cássia Alves, R.; Tezzoto, T.; Gratão, P.L. Selenium restricts cadmium uptake and improve micronutrients and proline concentration in tomato fruits. Biocatal. Agric. Biotechnol. 2019, 18, 101057. [Google Scholar] [CrossRef]
- Zhu, Z.; Chen, Y.L.; Zhang, X.J.; Li, M. Effect of foliar treatment of sodium selenate on postharvest decay and quality of tomato fruits. Sci. Hortic. 2016, 198, 304–310. [Google Scholar] [CrossRef]
- Zhu, Z.; Chen, Y.; Shi, G.; Zhang, X. Selenium delays tomato fruit ripening by inhibiting ethylene biosynthesis and enhancing the antioxidant defense system. Food Chem. 2017, 219, 179–184. [Google Scholar] [CrossRef]
- Shahid, M.A.; Balal, R.M.; Khan, N.; Zotarelli, L.; Liu, G.D.; Sarkhosh, A.; Fernández-Zapata, J.C.; Martínez Nicolás, J.J.; Garcia-Sanchez, F. Selenium impedes cadmium and arsenic toxicity in potato by modulating carbohydrate and nitrogen metabolism. Ecotoxicol. Environ. Saf. 2019, 180, 588–599. [Google Scholar] [CrossRef]
- Lei, B.; Bian, Z.; Yang, Q.; Wang, J.; Cheng, R.; Li, K.; Liu, W.-K.; Zhang, Y.; Fang, H.; Tong, Y. The positive function of selenium supplementation on reducing nitrate accumulation in hydroponic lettuce (Lactuca sativa L.). J. Integr. Agric. 2018, 17, 837–846. [Google Scholar] [CrossRef]
- Do Nascimento da Silva, E.; Aureli, F.; D’Amato, M.; Raggi, A.; Cadore, S.; Cubadda, F. Selenium Bioaccessibility and Speciation in Selenium-Enriched Lettuce: Investigation of the Selenocompounds Liberated after in Vitro Simulated Human Digestion Using Two-Dimensional HPLC-ICP-MS. J. Agric. Food Chem. 2017, 65, 3031–3038. [Google Scholar] [CrossRef]
- Goswami, M.; Deka, S. Plant growth-promoting rhizobacteria—Alleviators of abiotic stresses in soil: A review. Pedosphere 2020, 30, 40–61. [Google Scholar] [CrossRef]
- Debona, D.; Rodrigues, F.A.; Datnoff, L.E. Silicon’s Role in Abiotic and Biotic Plant Stresses. Annu. Rev. Phytopathol. 2017, 55, 85–107. [Google Scholar] [CrossRef] [PubMed]
- Etesami, H.; Jeong, B.R. Silicon (Si): Review and future prospects on the action mechanisms in alleviating biotic and abiotic stresses in plants. Ecotoxicol. Environ. Saf. 2018, 147, 881–896. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Bilal, S.; Khan, A.L.; Imran, M.; Al-Harrasi, A.; Al-Rawahi, A.; Lee, I.J. Silicon-mediated alleviation of combined salinity and cadmium stress in date palm (Phoenix dactylifera L.) by regulating physio-hormonal alteration. Ecotoxicol. Environ. Saf. 2020, 188, 109885. [Google Scholar] [CrossRef] [PubMed]
- Hussain, B.; Lin, Q.; Hamid, Y.; Sanaullah, M.; Di, L.; Hashmi, M.L.R.; Khan, M.B.; He, Z.; Yang, X. Foliage application of selenium and silicon nanoparticles alleviates Cd and Pb toxicity in rice (Oryza sativa L.). Sci. Total Environ. 2020, 712, 136497. [Google Scholar] [CrossRef] [PubMed]
- Kavčič, A.; Budič, B.; Vogel-Mikuš, K. The effects of selenium biofortification on mercury bioavailability and toxicity in the lettuce-slug food chain. Food Chem. Toxicol. 2020, 135, 110939. [Google Scholar] [CrossRef]
- Wu, C.; Dun, Y.; Zhang, Z.; Li, M.; Wu, G. Foliar application of selenium and zinc to alleviate wheat (Triticum aestivum L.) cadmium toxicity and uptake from cadmium-contaminated soil. Ecotoxicol. Environ. Saf. 2020, 190, 110091. [Google Scholar] [CrossRef]
- Domokos-Szabolcsy, E.; Marton, L.; Sztrik, A.; Babka, B.; Prokisch, J.; Fari, M. Accumulation of red elemental selenium nanoparticles and their biological effects in Nicotinia tabacum. Plant Growth Regul. 2012, 68, 525–531. [Google Scholar] [CrossRef]
- Astaneh, R.K.; Bolandnazar, S.; Zaare Nahandi, F.; Oustan, S. Effect of selenium application on phenylalanine ammonia-lyase (PAL) activity, phenol leakage and total phenolic content in garlic (Allium sativum L.) under NaCl stress. Inf. Process. Agric. 2018, 5, 339–344. [Google Scholar] [CrossRef]
- Tepanosyan, G.; Sahakyan, L.; Belyaeva, O.; Asmaryan, S.; Saghatelyan, A. Continuous impact of mining activities on soil heavy metals levels and human health. Sci. Total Environ. 2018, 639, 900–909. [Google Scholar] [CrossRef]
- Jiang, Y.; Shi, L.; Guang, A.L.; Mu, Z.; Zhan, H.; Wu, Y. Contamination levels and human health risk assessment of toxic heavy metals in street dust in an industrial city in Northwest China. Environ. Geochem. Health 2018, 40, 2007–2020. [Google Scholar] [CrossRef] [PubMed]
- Minari, G.D.; Rosalen, D.L.; da Cruz, M.C.P.; de Melo, W.J.; Alves, L.M.C.; Saran, L.M. Agricultural management of an Oxisol affects accumulation of heavy metals. Chemosphere 2017, 185, 344–350. [Google Scholar] [CrossRef] [PubMed]
- Cai, M.; Wang, Q.S.; Wen, H.H.; Luo, J.; Wang, S. Heavy metals in agricultural soils from a typical township in Guangdong Province, China: Occurrences and spatial distribution. Ecotoxicol. Environ. Saf. 2019, 168, 184–191. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, A.P.; Nomura, C.S.; Naozuka, J. Evaluation of selenium enrichment of adzuki bean (Vigna angularis) sprouts: Translocation, bioaccessibility and Se-protein speciation. Microchem. J. 2017, 134, 19–26. [Google Scholar] [CrossRef]
- Kolbert, Z.; Molnár, Á.; Feigl, G.; Van Hoewyk, D. Plant selenium toxicity: Proteome in the crosshairs. J. Plant Physiol. 2019, 232, 291–300. [Google Scholar] [CrossRef] [PubMed]
- Kaur, S.; Singh, D.; Singh, K. Effect of selenium application on arsenic uptake in rice (Oryza sativa L.). Environ. Monit. Assess. 2017, 189, 430. [Google Scholar] [CrossRef] [PubMed]
- Moulick, D.; Ghosh, D.; Chandra Santra, S. Evaluation of effectiveness of seed priming with selenium in rice during germination under arsenic stress. Plant Physiol Biochem. 2016, 109, 571–578. [Google Scholar] [CrossRef]
- Chauhan, R.; Awasthi, S.; Tripathi, P.; Mishra, S.; Dwivedi, S.; Niranjan, A.; Mallick, S.; Tripathi, P.; Pande, V.; Tripathi, R.D. Selenite modulates the level of phenolics and nutrient element to alleviate the toxicity of arsenite in rice (Oryza sativa L.). Ecotoxicol. Environ. Saf. 2017, 138, 47–55. [Google Scholar] [CrossRef]
- Biswas, A.; Biswas, S.; Das, A.; Roychowdhury, T. Spatial variability and competing dynamics of arsenic, selenium, iron and bioavailable phosphate from ground water and soil to paddy plant parts. Groundw. Sustain. Dev. 2018, 7, 328–335. [Google Scholar] [CrossRef]
- Pandey, C.; Gupta, M. Selenium amelioration of arsenic toxicity in rice shows genotypic variation: A transcriptomic and biochemical analysis. J. Plant Physiol. 2018, 231, 168–181. [Google Scholar] [CrossRef]
- Moulick, D.; Santra, S.C.; Ghosh, D. Effect of selenium induced seed priming on arsenic accumulation in rice plant and subsequent transmission in human food chain. Ecotoxicol. Environ. Saf. 2018, 152, 67–77. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Upadhyay, A.K.; Singh, D.P. Regulation of oxidative stress and mineral nutrient status by selenium in arsenic treated crop plant Oryza sativa. Ecotoxicol. Environ. Saf. 2018, 148, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Wan, Y.; Camara, A.Y.; Huang, Q.; Yu, Y.; Wang, Q.; Li, H. Arsenic uptake and accumulation in rice (Oryza sativa L.) with selenite fertilization and water management. Ecotoxicol. Environ. Saf. 2018, 156, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Camara, A.Y.; Wan, Y.; Yu, Y.; Wang, Q.; Li, H. Effect of selenium on uptake and translocation of arsenic in rice seedlings (Oryza sativa L.). Ecotoxicol. Environ. Saf. 2018, 148, 869–875. [Google Scholar] [CrossRef]
- Camara, A.Y.; Wan, Y.; Yu, Y.; Wang, Q.; Wang, K.; Li, H. Effect of endogenous selenium on arsenic uptake and antioxidative enzymes in as-exposed rice seedlings. Int. J. Environ. Res. Public Health 2019, 16, 3350. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Jiang, Z.; Li, X.; Liu, H.; Li, N.; Wei, S. Mitigation of rice cadmium (Cd) accumulation by joint application of organic amendments and selenium (Se) in high-Cd-contaminated soils. Chemosphere 2020, 241, 125106. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.B.; Yang, C.; Shao, Z.Y.; Wang, H.; Zan, S.T.; Zhu, M.; Zhou, S.B.; Yang, R.Y. Selenium (Se) does not reduce cadmium (Cd) uptake and translocation in rice (Oryza sativa L.) in naturally occurred Se-rich paddy fields with a high geological background of Cd. Bull. Environ. Contam. Toxicol. 2019, 103, 127–132. [Google Scholar] [CrossRef]
- Huang, B.; Xin, J.; Dai, H.; Zhou, W. Effects of interaction between cadmium (Cd) and selenium (Se) on grain yield and Cd and Se accumulation in a hybrid rice (Oryza sativa L.) system. J. Agric. Food Chem. 2017, 65, 9537–9546. [Google Scholar] [CrossRef]
- Huang, G.; Ding, C.; Guo, F.; Li, X.; Zhang, T.; Wang, X. Underlying mechanisms and effects of hydrated lime and selenium application on cadmium uptake by rice (Oryza sativa L.) seedlings. Environ. Sci. Pollut. Res. Int. 2017, 24, 18926–18935. [Google Scholar] [CrossRef]
- Cui, J.; Liu, T.; Li, Y.; Li, F. Selenium reduces cadmium uptake into rice suspension cells by regulating the expression of lignin synthesis and cadmium-related genes. Sci. Total Environ. 2018, 644, 602–610. [Google Scholar] [CrossRef]
- Gao, M.; Zhou, J.; Liu, H.; Zhang, W.; Hu, Y.; Liang, J.; Zhou, J. Foliar spraying with silicon and selenium reduces cadmium uptake and mitigates cadmium toxicity in rice. Sci. Total Environ. 2018, 631–632, 1100–1108. [Google Scholar] [CrossRef] [PubMed]
- Wan, Y.; Wang, K.; Liu, Z.; Yu, Y.; Wang, Q.; Li, H. Effect of selenium on the subcellular distribution of cadmium and oxidative stress induced by cadmium in rice (Oryza sativa L.). Environ. Sci. Pollut. Res. Int. 2019, 26, 16220–16228. [Google Scholar] [CrossRef]
- Li, Y.; Li, H.; Li, Y.-F.; Zhao, J.; Guo, J.; Wang, R.; Li, B.; Zhang, Z.; Gao, Y. Evidence for molecular antagonistic mechanism between mercury and selenium in rice (Oryza sativa L.): A combined study using 1, 2-dimensional electrophoresis and SR-XRF techniques. J. Trace Elem. Med. Biol. 2017, 50, 435–440. [Google Scholar] [CrossRef]
- Tang, W.; Dang, F.; Evans, D.; Zhong, H.; Xiao, L. Understanding reduced inorganic mercury accumulation in rice following selenium application: Selenium application routes, speciation and doses. Chemosphere 2017, 169, 369–376. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Yan, M.; Liang, L.; Lu, Q.; Han, J.; Liu, L.; Feng, X.; Guo, J.; Wang, Y.; Qiu, G. Impacts of selenium supplementation on soil mercury speciation, and inorganic mercury and methylmercury uptake in rice (Oryza sativa L.). Environ. Pollut. 2019, 249, 647–654. [Google Scholar] [CrossRef] [PubMed]
- Chapman, E.E.V.; Moore, C.; Campbell, L.M. Native plants for revegetation of mercury- and arsenic-contaminated historical mining waste—Can a low-dose selenium additive improve seedling growth and decrease contaminant bioaccumulation? Water Air Soil Pollut. 2019, 230, 225. [Google Scholar] [CrossRef]
- Zhao, Y.; Hu, C.; Wang, X.; Qing, X.; Wang, P.; Zhang, Y.; Zhang, X.; Zhao, X. Selenium alleviated chromium stress in Chinese cabbage (Brassica campestris L. ssp. Pekinensis) by regulating root morphology and metal element uptake. Ecotoxicol. Environ. Saf. 2019, 173, 314–321. [Google Scholar] [CrossRef]
- Cai, M.; Hu, C.; Wang, X.; Zhao, Y.; Jia, W.; Sun, X.; Elyamine, A.M.; Zhao, X. Selenium induces changes of rhizosphere bacterial characteristics and enzyme activities affecting chromium/selenium uptake by pak choi (Brassica campestris L. ssp. Chinensis Makino) in chromium contaminated soil. Environ. Pollut. 2019, 249, 716–727. [Google Scholar] [CrossRef]
- Ulhassan, Z.; Gill, R.A.; Huang, H.; Ali, S.; Mwamba, M.; Ali, B.; Huang, Q.; Hamid, Y.; Khan, A.R.; Wang, J.; et al. Selenium mitigates the chromium toxicity in Brassicca napus L. by ameliorating nutrients uptake, amino acids metabolism and antioxidant defense system. Plant Physiol. Biochem. 2019, 145, 142–152. [Google Scholar] [CrossRef]
- Handa, N.; Kohli, S.K.; Sharma, A.; Thukral, A.K.; Bhardwaj, R.; Alyemeni, M.N.; Wijaya, L.; Ahmad, P. Selenium ameliorates chromium toxicity through modifications in pigment system, antioxidative capacity, osmotic system, and metal chelators in Brassica juncea seedlings. S. Afr. J. Bot. 2018, 119, 1–10. [Google Scholar] [CrossRef]
- Qing, X.; Zhao, X.; Hu, C.; Wang, P.; Zhang, Y.; Zhang, X.; Wang, P.; Shi, H.; Jia, F.; Qu, C. Selenium alleviates chromium toxicity by preventing oxidative stress in cabbage (Brassica campestris L. ssp. Pekinensis) leaves. Ecotoxicol. Environ. Saf. 2015, 114, 179–189. [Google Scholar] [CrossRef]
- Chen, Z.; Xu, J.; Xu, Y.; Wang, K.; Cao, B.; Xu, K. Alleviating effects of silicate, selenium, and microorganism fertilization on lead toxicity in ginger (Zingiber officinale Roscoe.). Plant Physiol. Biochem. 2019, 145, 153–163. [Google Scholar] [CrossRef]
- Wu, Z.; Yin, X.; Bañuelos, G.S.; Lin, Z.Q.; Liu, Y.; Li, M.; Yuan, L. Indications of selenium protection against cadmium and lead toxicity in oilseed rape (Brassica napus L.). Front. Plant Sci. 2016, 7, 1875. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Xiang, Y.; Zhou, Y.; Yang, Y.; Zhang, J.; Huang, H.; Shang, C.; Luo, L.; Gao, J.; Tang, L. Selenium contamination, consequences and remediation techniques in water and soils: A review. Environ Res. 2018, 164, 288–301. [Google Scholar] [CrossRef]
- Tan, L.C.; Nancharaiah, Y.V.; van Hullebusch, E.D.; Lens, P.N.L. Selenium: Environmental significance, pollution, and biological treatment technologies. Biotechnol. Adv. 2016, 34, 886–907. [Google Scholar] [CrossRef] [PubMed]
- Henry, B.L.; Wesner, J.S.; Kerby, J.L. Cross-ecosystem effects of agricultural tile drainage, surface runoff, and selenium in the Prairie Pothole Region. Wetlands 2019. [Google Scholar] [CrossRef]
- Etteieb, S.; Magdouli, S.; Zolfaghari, M.; Brar, S.K. Monitoring and analysis of selenium as an emerging contaminant in mining industry: A critical review. Sci. Total Environ. 2020, 698, 134339. [Google Scholar] [CrossRef]
- Sharma, V.K.; Sohn, M.; McDonald, T.J. Remediation of selenium in water: A review. In Advances in Water Purification Techniques, Meeting the Needs of Developed and Developing Countries; Ahuja, S., Ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2019; pp. 203–218. [Google Scholar] [CrossRef]
- Malhotra, M.; Pal, M.; Pal, P. A response surface optimized nanofiltration-based system for efficient removal of selenium from drinking water. J. Water Process Eng. 2020, 33, 101007. [Google Scholar] [CrossRef]
- Paul, T.; Saha, N.C. Environmental arsenic and selenium contamination and approaches towards its bioremediation through the exploration of microbial adaptations: A review. Pedosphere 2019, 29, 554–568. [Google Scholar] [CrossRef]
- Solovyev, N.; Prakash, N.T.; Bhatia, P.; Prakash, R.; Drobyshev, E.; Michalke, B. Selenium-rich mushrooms cultivation on a wheat straw substrate from seleniferous area in Punjab, India. J. Trace Elem. Med. Biol. 2018, 50, 362–366. [Google Scholar] [CrossRef]
- Chawla, R.; Filippini, T.; Loomba, R.; Cilloni, S.; Dhillon, K.S.; Vinceti, M. Exposure to a high selenium environment in Punjab, India: Biomarkers and health conditions. Sci. Total Environ. 2020, 719, 134541. [Google Scholar] [CrossRef] [PubMed]
- Loomba, R.; Filippini, T.; Chawla, R.; Chaudhary, R.; Cilloni, S.; Datt, C.; Singh, S.; Dhillon, K.S.; Vinceti, M. Exposure to a high selenium environment in Punjab, India: Effects on blood chemistry. Sci. Total Environ. 2019, 716, 135347. [Google Scholar] [CrossRef] [PubMed]
- Yasin, M.; El Mehdawi, A.F.; Jahn, C.E.; Anwar, A.; Turner, M.F.S.; Faisal, M.; Pilon-Smits, E.A.H. Seleniferous soils as a source for production of selenium-enriched foods and potential of bacteria to enhance plant selenium uptake. Plant Soil 2015, 386, 385–394. [Google Scholar] [CrossRef]
- Statwick, J.; Sher, A.A. Selenium in soils of western Colorado. J. Arid Environ. 2017, 137, 1–6. [Google Scholar] [CrossRef]
- Chang, C.; Yin, R.; Wang, X.; Shao, S.; Chen, C.; Zhang, H. Selenium translocation in the soil-rice system in the Enshi seleniferous area, Central China. Sci. Total Environ. 2019, 669, 83–90. [Google Scholar] [CrossRef]
- Chang, C.; Yin, R.; Zhang, H.; Yao, L. Bioaccumulation and health risk assessment of heavy metals in the soil-rice system in a typical seleniferous area, central China. Environ. Toxicol. Chem. 2019, 38, 1577–1584. [Google Scholar] [CrossRef]
- Dhillon, K.S.; Dhillon, S.K.; Singh, B. Genesis of seleniferous soils and associated animal and human health problems. Adv. Agron. 2019, 154, 1–80. [Google Scholar] [CrossRef]
- Wang, X.; Wang, S.; Pan, X.; Gadd, G.M. Heteroaggregation of soil particulate organic matter and biogenic selenium nanoparticles for remediation of elemental mercury contamination. Chemosphere 2019, 221, 486–492. [Google Scholar] [CrossRef]
- Wang, X.; Pan, X.; Gadd, G.M. Immobilization of elemental mercury by biogenic Se nanoparticles in soils of varying salinity. Sci. Total Environ. 2019, 668, 303–309. [Google Scholar] [CrossRef]
- Wang, X.; Pan, X.; Gadd, G.M. Soil dissolved organic matter affects mercury immobilization by biogenic selenium nanoparticles. Sci. Total Environ. 2019, 658, 8–15. [Google Scholar] [CrossRef]
- Wang, X.; Liu, B.; Pan, X.; Gadd, G.M. Transport and retention of biogenic selenium nanoparticles in biofilm-coated quartz sand porous media and consequence for elemental mercury immobilization. Sci. Total Environ. 2019, 692, 1116–1124. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).