Pro-Aging Effects of Xanthine Oxidoreductase Products
Abstract
:1. Introduction
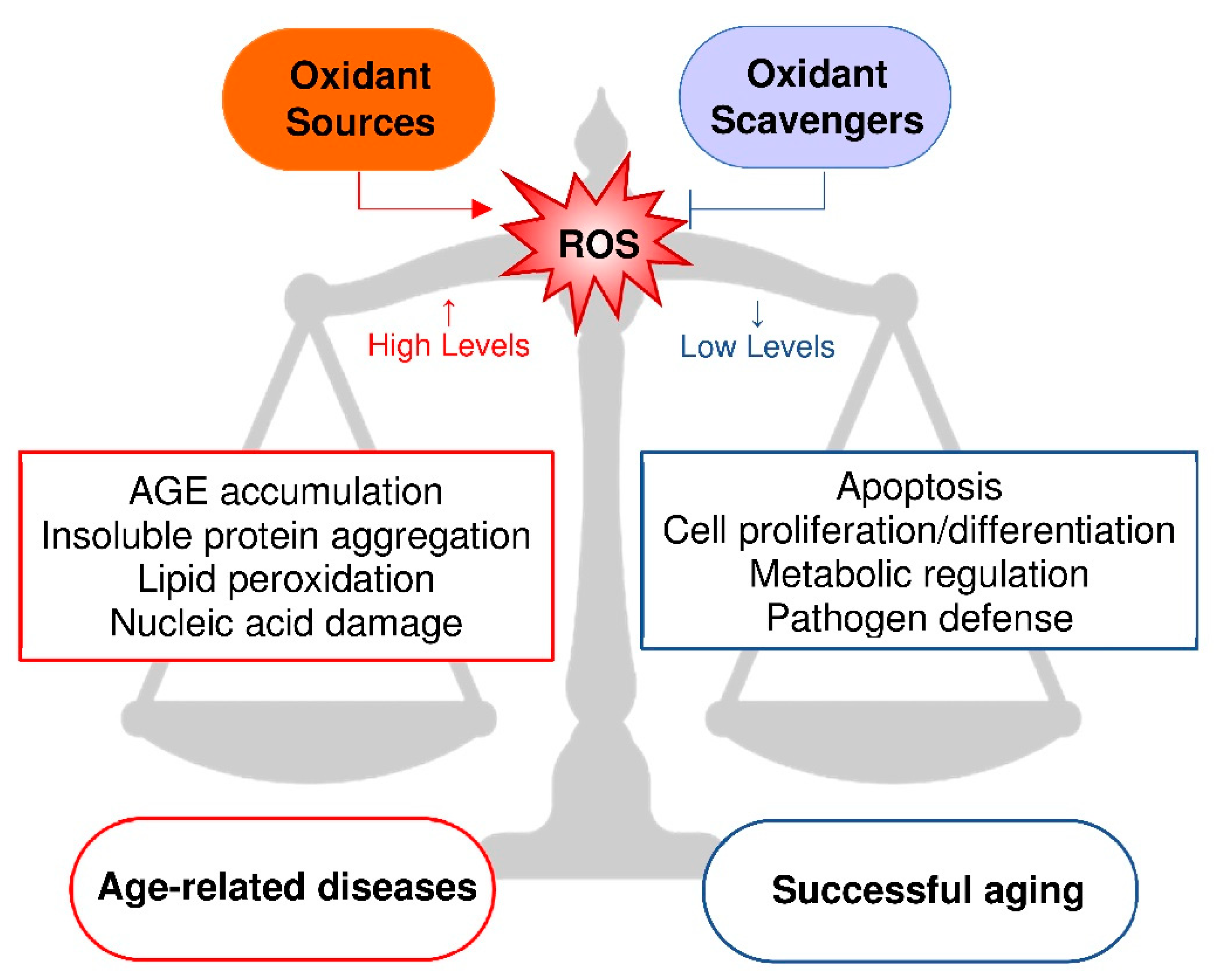
2. Oxidative Stress and Aging
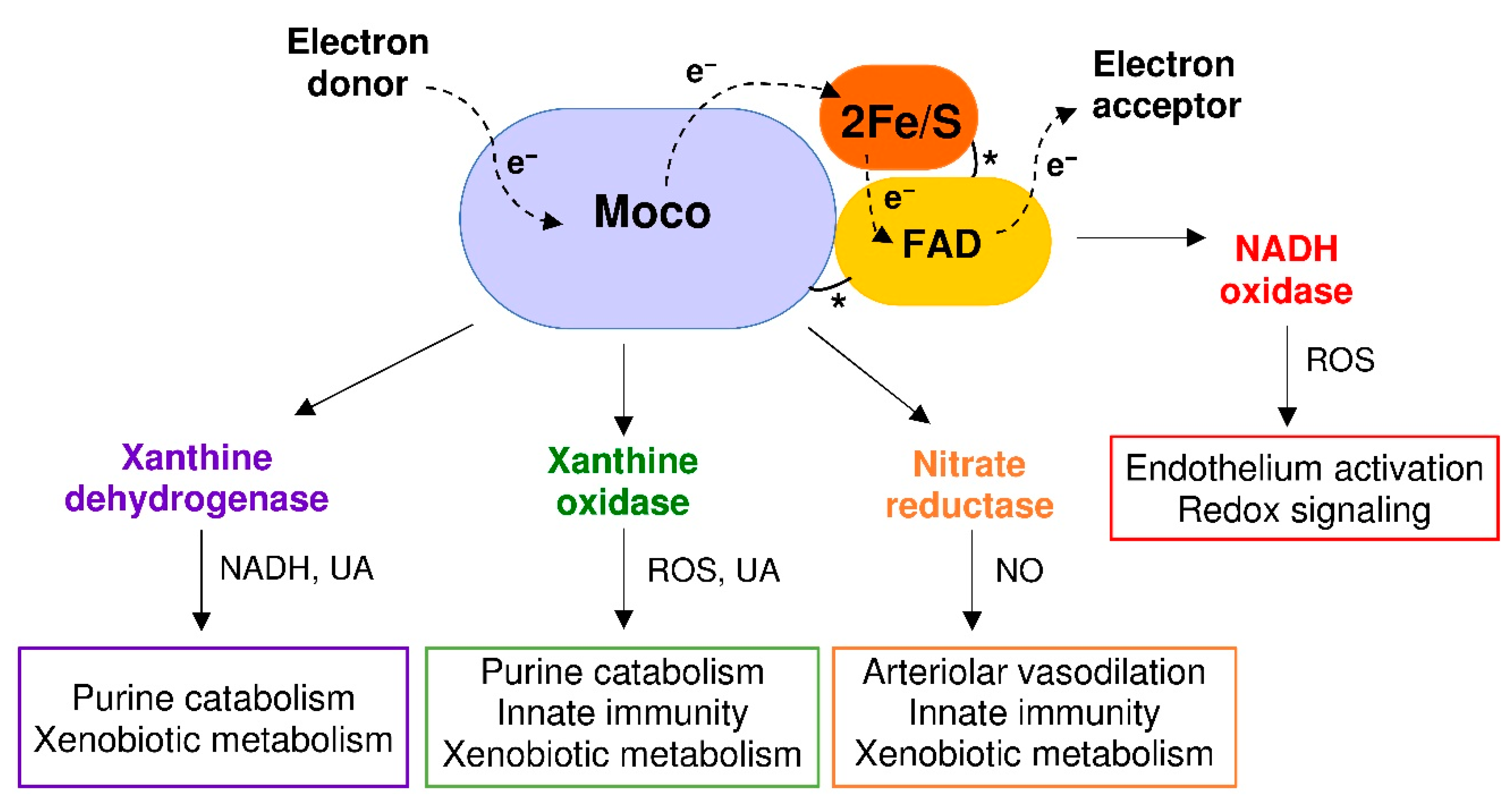
3. Reactive Species Produced by XOR Activity
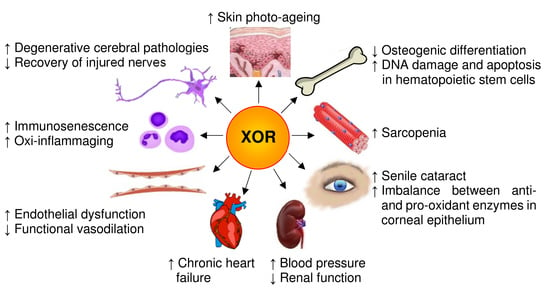
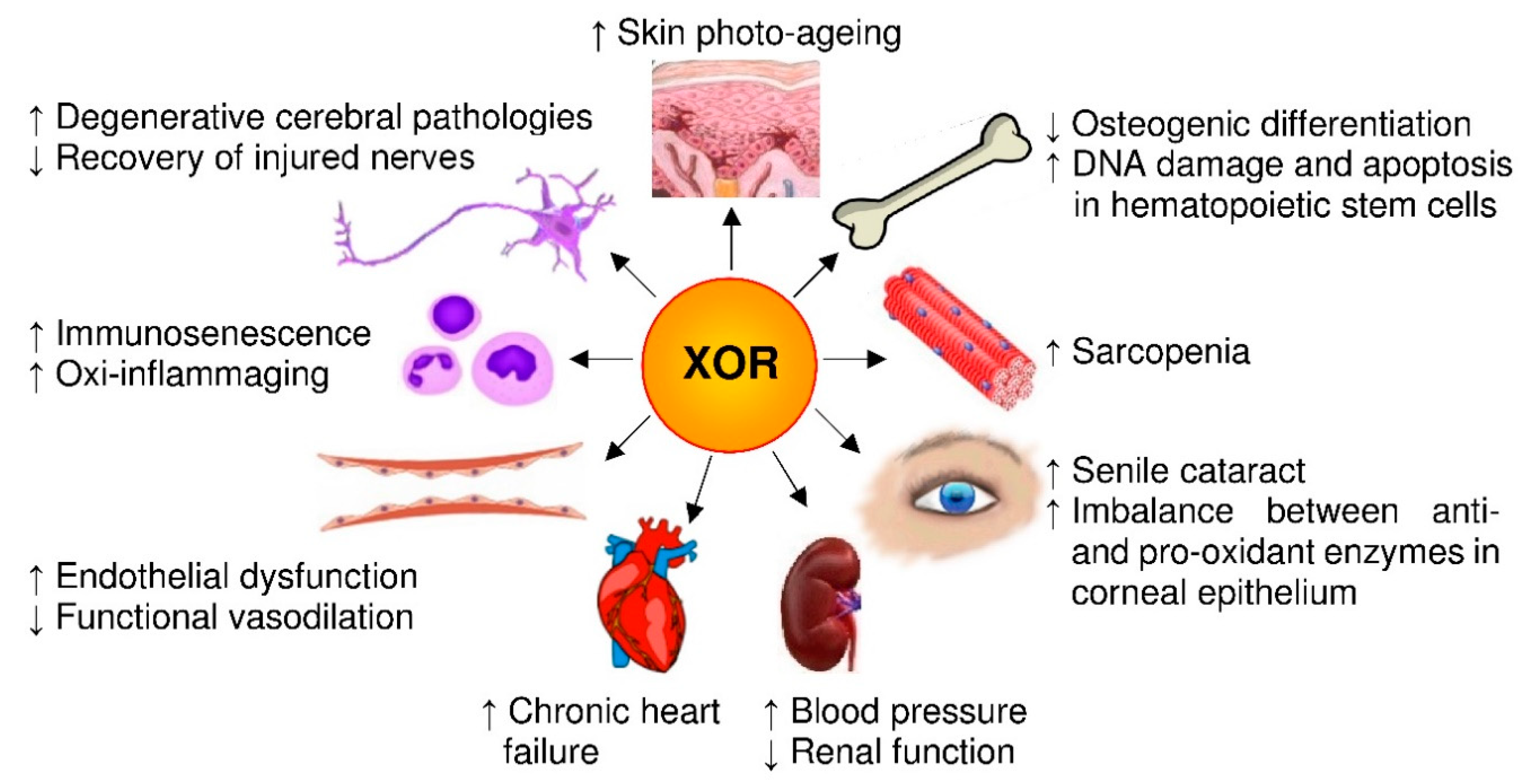
4. XOR-Deriving Reactive Species and Aging
4.1. Molecular Pathways and Pharmacological Agents
4.2. In Vitro Experiments
4.3. Animal Studies
4.4. Clinical Reports
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Barrera, G.; Pizzimenti, S.; Daga, M.; Dianzani, C.; Arcaro, A.; Cetrangolo, G.P.; Giordano, G.; Cucci, M.A.; Graf, M.; Gentile, F. Lipid Peroxidation-Derived Aldehydes, 4-Hydroxynonenal and Malondialdehyde in Aging-Related Disorders. Antioxidants 2018, 7, 102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santoro, A.; Ostan, R.; Candela, M.; Biagi, E.; Brigidi, P.; Capri, M.; Franceschi, C. Gut microbiota changes in the extreme decades of human life: A focus on centenarians. Cell. Mol. Life Sci. 2018, 75, 129–148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jones, D.P. Redox theory of aging. Redox Biol. 2015, 5, 71–79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anderson, R.M.; Weindruch, R. Metabolic reprogramming, caloric restriction and aging. Trends Endocrinol. Metab. 2010, 21, 134–141. [Google Scholar] [CrossRef] [Green Version]
- Liochev, S.I. Reactive oxygen species and the free radical theory of aging. Free Radic. Biol. Med. 2013, 60, 1–4. [Google Scholar] [CrossRef]
- Ristow, M.; Schmeisser, S. Extending life span by increasing oxidative stress. Free Radic. Biol. Med. 2011, 51, 327–336. [Google Scholar] [CrossRef] [Green Version]
- Franceschi, C.; Zaikin, A.; Gordleeva, S.; Ivanchenko, M.; Bonifazi, F.; Storci, G.; Bonafè, M. Inflammaging 2018: An update and a model. Semin. Immunol. 2018, 40, 1–5. [Google Scholar] [CrossRef]
- Prattichizzo, F.; De Nigris, V.; Spiga, R.; Mancuso, E.; La Sala, L.; Antonicelli, R.; Testa, R.; Procopio, A.D.; Olivieri, F.; Ceriello, A. Inflammageing and metaflammation: The yin and yang of type 2 diabetes. Ageing Res. Rev. 2018, 41, 1–17. [Google Scholar] [CrossRef]
- Latz, E.; Duewell, P. NLRP3 inflammasome activation in inflammaging. Semin. Immunol. 2018, 40, 61–73. [Google Scholar] [CrossRef]
- Bullone, M.; Lavoie, J.P. The Contribution of Oxidative Stress and Inflamm-Aging in Human and Equine Asthma. Int. J. Mol. Sci. 2017, 18, 2612. [Google Scholar] [CrossRef] [Green Version]
- De la Fuente, M.; Miquel, J. An update of the oxidation-inflammation theory of aging: The involvement of the immune system in oxi-inflamm-aging. Curr. Pharm. Des. 2009, 15, 3003–3026. [Google Scholar] [CrossRef] [PubMed]
- Kudryavtseva, A.V.; Krasnov, G.S.; Dmitriev, A.A.; Alekseev, B.Y.; Kardymon, O.L.; Sadritdinova, A.F.; Fedorova, M.S.; Pokrovsky, A.V.; Melnikova, N.V.; Kaprin, A.D.; et al. Mitochondrial dysfunction and oxidative stress in aging and cancer. Oncotarget 2016, 7, 44879–44905. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Battelli, M.G.; Polito, L.; Bortolotti, M.; Bolognesi, A. Xanthine Oxidoreductase-Derived Reactive Species: Physiological and Pathological Effects. Oxid. Med. Cell. Longev. 2016, 2016, 3527579. [Google Scholar] [CrossRef] [Green Version]
- Forman, H.J. Redox signaling: An evolution from free radicals to aging. Free Radic. Biol. Med. 2016, 97, 398–407. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schulz, E.; Wenzel, P.; Münzel, T.; Daiber, A. Mitochondrial redox signaling: Interaction of mitochondrial reactive oxygen species with other sources of oxidative stress. Antioxid. Redox Signal. 2014, 20, 308–324. [Google Scholar] [CrossRef]
- Wen, X.; Wu, J.; Wang, F.; Liu, B.; Huang, C.; Wei, Y. Deconvoluting the role of reactive oxygen species and autophagy in human diseases. Free Radic. Biol. Med. 2013, 65, 402–410. [Google Scholar] [CrossRef]
- Donato, A.J.; Morgan, R.G.; Walker, A.E.; Lesniewski, L.A. Cellular and molecular biology of aging endothelial cells. J. Mol. Cell. Cardiol. 2015, 89, 122–135. [Google Scholar] [CrossRef] [Green Version]
- Kim, W.K.; Meliton, V.; Bourquard, N.; Hahn, T.J.; Parhami, F. Hedgehog signaling and osteogenic differentiation in multipotent bone marrow stromal cells are inhibited by oxidative stress. J. Cell. Biochem. 2010, 111, 1199–1209. [Google Scholar] [CrossRef]
- Chen, F.; Liu, Y.; Wong, N.K.; Xiao, J.; So, K.F. Oxidative Stress in Stem Cell Aging. Cell Transplant. 2017, 26, 1483–1495. [Google Scholar] [CrossRef]
- Rowan, S.; Bejarano, E.; Taylor, A. Mechanistic targeting of advanced glycation end-products in age-related diseases. Biochim. Biophys. Acta Mol. Basis Dis. 2018, 1864, 3631–3643. [Google Scholar] [CrossRef]
- Currais, A.; Fischer, W.; Maher, P.; Schubert, D. Intraneuronal protein aggregation as a trigger for inflammation and neurodegeneration in the aging brain. FASEB J. 2017, 31, 5–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kao, Y.C.; Ho, P.C.; Tu, Y.K.; Jou, I.M.; Tsai, K.J. Lipids and Alzheimer’s Disease. Int. J. Mol. Sci. 2020, 21, 1505. [Google Scholar] [CrossRef]
- Lin, X.; Kapoor, A.; Gu, Y.; Chow, M.J.; Peng, J.; Zhao, K.; Tang, D. Contributions of DNA Damage to Alzheimer’s Disease. Int. J. Mol. Sci. 2020, 21, 1666. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davalli, P.; Mitic, T.; Caporali, A.; Lauriola, A.; D’Arca, D. ROS, Cell Senescence, and Novel Molecular Mechanisms in Aging and Age-Related Diseases. Oxid. Med. Cell. Longev. 2016, 2016, 3565127. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.; Cho, Y.S.; Jung, H.; Choi, I. Pharmacological Regulation of Oxidative Stress in Stem Cells. Oxid. Med. Cell. Longev. 2018, 2018, 4081890. [Google Scholar] [CrossRef] [PubMed]
- Forrester, S.J.; Kikuchi, D.S.; Hernandes, M.S.; Xu, Q.; Griendling, K.K. Reactive Oxygen Species in Metabolic and Inflammatory Signaling. Circ. Res. 2018, 122, 877–902. [Google Scholar] [CrossRef] [PubMed]
- Roy, J.; Galano, J.M.; Durand, T.; Le Guennec, J.Y.; Lee, J.C. Physiological role of reactive oxygen species as promoters of natural defenses. FASEB J. 2017, 31, 3729–3745. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vasilaki, A.; Richardson, A.; Van Remmen, H.; Brooks, S.V.; Larkin, L.; McArdle, A.; Jackson, M.J. Role of nerve-muscle interactions and reactive oxygen species in regulation of muscle proteostasis with ageing. J. Physiol. 2017, 595, 6409–6415. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Damiano, S.; Muscariello, E.; La Rosa, G.; Di Maro, M.; Mondola, P.; Santillo, M. Dual role of reactive oxygen species in muscle function: Can antioxidant dietary supplements counteract age-related sarcopenia? Int. J. Mol. Sci. 2019, 20, 3815. [Google Scholar] [CrossRef] [Green Version]
- Terao, M.; Romão, M.J.; Leimkühler, S.; Bolis, M.; Fratelli, M.; Coelho, C.; Santos-Silva, T.; Garattini, E. Structure and function of mammalian aldehyde oxidases. Arch. Toxicol. 2016, 90, 753–780. [Google Scholar] [CrossRef]
- Nishino, T.; Okamoto, K.; Kawaguchi, Y.; Matsumura, T.; Eger, B.T.; Pai, E.F.; Nishino, T. The C-terminal peptide plays a role in the formation of an intermediate form during the transition between xanthine dehydrogenase and xanthine oxidase. FEBS J. 2015, 282, 3075–3090. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cortese, F.; Giordano, P.; Scicchitano, P.; Faienza, M.F.; De Pergola, G.; Calculli, G.; Meliota, G.; Ciccone, M.M. Uric acid: From a biological advantage to a potential danger. A focus on cardiovascular effects. Vasc. Pharmacol. 2019, 120, 106565. [Google Scholar] [CrossRef] [PubMed]
- Battelli, M.G.; Polito, L.; Bortolotti, M.; Bolognesi, A. Xanthine oxidoreductase in drug metabolism: Beyond a role as a detoxifying enzyme. Curr. Med. Chem. 2016, 23, 4027–4036. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Battelli, M.G.; Bortolotti, M.; Polito, L.; Bolognesi, A. The role of xanthine oxidoreductase and uric acid in metabolic syndrome. Biochim. Biophys. Acta Mol. Basis Dis. 2018, 1864, 2557–2565. [Google Scholar] [CrossRef]
- Battelli, M.G.; Bolognesi, A.; Polito, L. Pathophysiology of circulating xanthine oxidoreductase: New emerging roles for a multi-tasking enzyme. Biochim. Biophys. Acta Mol. Basis Dis. 2014, 1842, 1502–1517. [Google Scholar] [CrossRef] [Green Version]
- Battelli, M.G.; Bortolotti, M.; Polito, L.; Bolognesi, A. Metabolic syndrome and cancer risk: The role of xanthine oxidoreductase. Redox Biol. 2019, 21, 101070. [Google Scholar] [CrossRef]
- Battelli, M.G.; Polito, L.; Bortolotti, M.; Bolognesi, A. Xanthine oxidoreductase in cancer: More than a differentiation marker. Cancer Med. 2016, 5, 546–557. [Google Scholar] [CrossRef]
- Ives, A.; Nomura, J.; Martinon, F.; Roger, T.; LeRoy, D.; Miner, J.N.; Simon, G.; Busso, N.; So, A. Xanthine oxidoreductase regulates macrophage IL1β secretion upon NLRP3 inflammasome activation. Nat. Commun. 2015, 6, 6555. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Wang, X.; Vikash, V.; Ye, Q.; Wu, D.; Liu, Y.; Dong, W. ROS and ROS-Mediated Cellular Signaling. Oxid. Med. Cell. Longev. 2016, 2016, 4350965. [Google Scholar] [CrossRef] [Green Version]
- Pan, H.; Finkel, T. Key proteins and pathways that regulate lifespan. J. Biol. Chem. 2017, 292, 6452–6460. [Google Scholar] [CrossRef] [Green Version]
- López-Otín, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. The hallmarks of aging. Cell 2013, 153, 1194–1217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Papadopoli, D.; Boulay, K.; Kazak, L.; Pollak, M.; Mallette, F.; Topisirovic, I.; Hulea, L. mTOR as a central regulator of lifespan and aging. F1000Research 2019, 8, 998. [Google Scholar] [CrossRef] [PubMed]
- Lanaspa, M.A.; Sanchez-Lozada, L.G.; Choi, Y.J.; Cicerchi, C.; Kanbay, M.; Roncal-Jimenez, C.A.; Ishimoto, T.; Li, N.; Marek, G.; Duranay, M.; et al. Uric acid induces hepatic steatosis by generation of mitochondrial oxidative stress: Potential role in fructose-dependent and -independent fatty liver. J. Biol. Chem. 2012, 287, 40732–40744. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cicerchi, C.; Li, N.; Kratzer, J.; Garcia, G.; Roncal-Jimenez, C.A.; Tanabe, K.; Hunter, B.; Rivard, C.J.; Sautin, Y.Y.; Gaucher, E.A.; et al. Uric acid-dependent inhibition of AMP kinase induces hepatic glucose production in diabetes and starvation: Evolutionary implications of the uricase loss in hominids. FASEB J. 2014, 28, 3339–3350. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cordero-Herrera, I.; Kozyra, M.; Zhuge, Z.; McCann Haworth, S.; Moretti, C.; Peleli, M.; Caldeira-Dias, M.; Jahandideh, A.; Huirong, H.; de Campos Cruz, J.; et al. AMP-activated protein kinase activation and NADPH oxidase inhibition by inorganic nitrate and nitrite prevent liver steatosis. Proc. Natl. Acad. Sci. USA 2019, 116, 217–226. [Google Scholar] [CrossRef] [Green Version]
- He, L.; Chen, Y.; Feng, J.; Sun, W.; Li, S.; Ou, M.; Tang, L. Cellular senescence regulated by SWI/SNF complex subunits through p53/p21 and p16/pRB pathway. Int. J. Biochem. Cell Biol. 2017, 90, 29–37. [Google Scholar] [CrossRef]
- Alef, M.J.; Vallabhaneni, R.; Carchman, E.; Morris, S.M., Jr.; Shiva, S.; Wang, Y.; Kelley, E.E.; Tarpey, M.M.; Gladwin, M.T.; Tzeng, E.; et al. Nitrite-generated NO circumvents dysregulated arginine/NOS signaling to protect against intimal hyperplasia in Sprague-Dawley rats. J. Clin. Investig. 2011, 121, 1646–1656. [Google Scholar] [CrossRef]
- Kim, B.S.; Serebreni, L.; Hamdan, O.; Wang, L.; Parniani, A.; Sussan, T.; Scott Stephens, R.; Boyer, L.; Damarla, M.; Hassoun, P.M.; et al. Xanthine oxidoreductase is a critical mediator of cigarette smoke-induced endothelial cell DNA damage and apoptosis. Free Radic. Biol. Med. 2013, 60, 336–346. [Google Scholar] [CrossRef]
- Vaiserman, A.M.; Lushchak, O.V.; Koliada, A.K. Anti-aging pharmacology: Promises and pitfalls. Ageing Res. Rev. 2016, 31, 9–35. [Google Scholar] [CrossRef]
- Cosic, V.; Antic, S.; Pesic, M.; Jovanovic, O.; Kundalic, S.; Djordjevic, V.B. Monotherapy with metformin: Does it improve hypoxia in type 2 diabetic patients? Clin. Chem. Lab. Med. 2001, 39, 818–821. [Google Scholar] [CrossRef]
- Gungor, O.; Tanrisev, M.; Kircelli, F.; Turan, M.N.; Tugmen, C.; Tatar, E.; Toz, H. The effects of mammalian target of rapamycin inhibitors on serum uric acid levels in renal transplant patients. Int. Urol. Nephrol. 2013, 45, 547–552. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.X.; Kong, L.D.; Ye, W.C.; Cheng, C.H.; Tan, R.X. Inhibition of xanthine and monoamine oxidases by stilbenoids from Veratrum taliense. Planta Med. 2001, 67, 158–161. [Google Scholar] [CrossRef] [PubMed]
- Løvaas, E.; Carlin, G. Spermine: An anti-oxidant and anti-inflammatory agent. Free Radic. Biol. Med. 1991, 11, 455–461. [Google Scholar] [CrossRef]
- Kawaguchi, Y.; Tanaka, H.; Okada, T.; Konishi, H.; Takahashi, M.; Ito, M.; Asai, J. Effect of reactive oxygen species on the elastin mRNA expression in cultured human dermal fibroblasts. Free Radic. Biol. Med. 1997, 23, 162–165. [Google Scholar] [CrossRef]
- Rinnerthaler, M.; Bischof, J.; Streubel, M.K.; Trost, A.; Richter, K. Oxidative stress in aging human skin. Biomolecules 2015, 5, 545–589. [Google Scholar] [CrossRef] [Green Version]
- Recuero, M.; Vicente, M.C.; Martínez-García, A.; Ramos, M.C.; Carmona-Saez, P.; Sastre, I.; Aldudo, J.; Vilella, E.; Frank, A.; Bullido, M.J.; et al. A free radical-generating system induces the cholesterol biosynthesis pathway: A role in Alzheimer’s disease. Aging Cell 2019, 8, 128–139. [Google Scholar] [CrossRef]
- Recuero, M.; Muñoz, T.; Aldudo, J.; Subías, M.; Bullido, M.J.; Valdivieso, F. A free radical-generating system regulates APP metabolism/processing. FEBS Lett. 2010, 584, 4611–4618. [Google Scholar] [CrossRef] [Green Version]
- Llorente, P.; Kristen, H.; Sastre, I.; Toledano-Zaragoza, A.; Aldudo, J.; Recuero, M.; Bullido, M.J. A Free Radical-Generating System Regulates Amyloid Oligomers: Involvement of Cathepsin B. J. Alzheimers Dis. 2018, 66, 1397–1408. [Google Scholar] [CrossRef]
- Marques, S.; Trevisan, T.; Maia, C.; Breuer, A.; Owen, R.W. Improved Methods for the Rapid Formation and Prevention of Advanced Glycation End Products (AGEs) In Vitro by Coupling to the Hypoxanthine/Xanthine Oxidase Assay System. Biomedicines 2018, 6, 88. [Google Scholar] [CrossRef] [Green Version]
- Miric, D.J.; Kisic, B.M.; Filipovic-Danic, S.; Grbic, R.; Dragojevic, I.; Miric, M.B.; Puhalo-Sladoje, D. Xanthine Oxidase Activity in Type 2 Diabetes Mellitus Patients with and without Diabetic Peripheral Neuropathy. J. Diabetes Res. 2016, 2016, 4370490. [Google Scholar] [CrossRef] [Green Version]
- Khalil, Z.; Khodr, B. A role for free radicals and nitric oxide in delayed recovery in aged rats with chronic constriction nerve injury. Free Radic. Biol. Med. 2001, 31, 430–439. [Google Scholar] [CrossRef]
- Schoutsen, B.; de Jong, J.W. Age-dependent increase in xanthine oxidoreductase differs in various heart cell types. Circ. Res. 1987, 61, 604–607. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chung, H.Y.; Song, S.H.; Kim, H.J.; Ikeno, Y.; Yu, B.P. Modulation of renal xanthine oxidoreductase in aging: Gene expression and reactive oxygen species generation. J. Nutr. Health Aging 1999, 3, 19–23. [Google Scholar] [PubMed]
- Csiszar, A.; Ungvari, Z.; Edwards, J.G.; Kaminski, P.; Wolin, M.S.; Koller, A.; Kaley, G. Aging-induced phenotypic changes and oxidative stress impair coronary arteriolar function. Circ. Res. 2002, 90, 1159–1166. [Google Scholar] [CrossRef] [Green Version]
- Newaz, M.A.; Yousefipour, Z.; Oyekan, A. Oxidative stress-associated vascular aging is xanthine oxidase-dependent but not NAD(P)H oxidase-dependent. J. Cardiovasc. Pharmacol. 2006, 48, 88–94. [Google Scholar] [CrossRef]
- Aranda, R.; Doménech, E.; Rus, A.D.; Real, J.T.; Sastre, J.; Viña, J.; Pallardó, F.V. Age-related increase in xanthine oxidase activity in human plasma and rat tissues. Free Radic. Res. 2007, 41, 1195–1200. [Google Scholar] [CrossRef]
- Jacobson, A.; Yan, C.; Gao, Q.; Rincon-Skinner, T.; Rivera, A.; Edwards, J.; Huang, A.; Kaley, G.; Sun, D. Aging enhances pressure-induced arterial superoxide formation. Am. J. Physiol. Heart Circ. Physiol. 2007, 293, H1344–H1350. [Google Scholar] [CrossRef] [Green Version]
- Cejková, J.; Vejrazka, M.; Pláteník, J.; Stípek, S. Age-related changes in superoxide dismutase, glutathione peroxidase, catalase and xanthine oxidoreductase/xanthine oxidase activities in the rabbit cornea. Exp. Gerontol. 2004, 39, 1537–1543. [Google Scholar] [CrossRef]
- Ryan, M.J.; Jackson, J.R.; Hao, Y.; Leonard, S.S.; Always, S.E. Inhibition of xanthine oxidase reduces oxidative stress and improves skeletal muscle function in response to electrically stimulated isometric contractions in aged mice. Free Radic. Biol. Med. 2011, 51, 38–52. [Google Scholar] [CrossRef] [Green Version]
- Porto, M.L.; Rodrigues, B.P.; Menezes, T.N.; Ceschim, S.L.; Casarini, D.E.; Gava, A.L.; Pereira, T.M.; Vasquez, E.C.; Campagnaro, B.P.; Meyrelles, S.S. Reactive oxygen species contribute to dysfunction of bone marrow hematopoietic stem cells in aged C57BL/6 J mice. J. Biomed. Sci. 2015, 22, 97. [Google Scholar] [CrossRef] [Green Version]
- Vida, C.; Rodríguez-Terés, S.; Heras, V.; Corpas, I.; De la Fuente, M.; González, E. The aged-related increase in xanthine oxidase expression and activity in several tissues from mice is not shown in long-lived animals. Biogerontology 2011, 12, 551–564. [Google Scholar] [CrossRef] [PubMed]
- Vida, C.; Corpas, I.; De la Fuente, M.; González, E.M. Age-related changes in xanthine oxidase activity and lipid peroxidation, as well as in the correlation between both parameters, in plasma and several organs from female mice. J. Physiol. Biochem. 2011, 67, 551–558. [Google Scholar] [CrossRef] [PubMed]
- Vida, C.; de Toda, I.M.; Cruces, J.; Garrido, A.; Gonzalez-Sanchez, M.; De la Fuente, M. Role of macrophages in age-related oxidative stress and lipofuscin accumulation in mice. Redox Biol. 2017, 12, 423–437. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gheorghe, A.; Pérez de Heredia, F.; Hunsche, C.; Redondo, N.; Díaz, L.E.; Hernández, O.; Marcos, A.; De la Fuente, M. Oxidative stress and immunosenescence in spleen of obese mice can be reversed by 2-hydroxyoleic acid. Exp. Physiol. 2017, 102, 533–544. [Google Scholar] [CrossRef]
- Garrido, A.; Cruces, J.; Ceprián, N.; Hernández-Sánchez, C.; De la Fuente, M. Premature aging in behavior and immune functions in tyrosine hydroxylase haploinsufficient female mice. A longitudinal study. Brain Behav. Immun. 2018, 69, 440–455. [Google Scholar] [CrossRef] [Green Version]
- Jia, G.; Aroor, A.R.; Jia, C.; Sowers, J.R. Endothelial cell senescence in aging-related vascular dysfunction. Biochim. Biophys. Acta Mol. Basis Dis. 2019, 1865, 1802–1809. [Google Scholar] [CrossRef]
- Ungvári, Z.; Gupte, S.A.; Recchia, F.A.; Bátkai, S.; Pacher, P. Role of oxidative-nitrosative stress and downstream pathways in various forms of cardiomyopathy and heart failure. Curr. Vasc. Pharmacol. 2005, 3, 221–229. [Google Scholar] [CrossRef]
- Eskurza, I.; Kahn, Z.D.; Seals, D.R. Xanthine oxidase does not contribute to impaired peripheral conduit artery endothelium-dependent dilatation with ageing. J. Physiol. 2006, 571, 661–668. [Google Scholar] [CrossRef]
- Liu, L.; Liu, Y.; Cui, J.; Liu, H.; Liu, Y.B.; Qiao, W.L.; Sun, H.; Yan, C.D. Oxidative stress induces gastric submucosal arteriolar dysfunction in the elderly. World J. Gastroenterol. 2013, 19, 9439–9446. [Google Scholar] [CrossRef]
- Miric, D.J.; Kisic, B.B.; Zoric, L.D.; Mitic, R.V.; Miric, B.M.; Dragojevic, I.M. Xanthine oxidase and lens oxidative stress markers in diabetic and senile cataract patients. J. Diabetes Complicat. 2013, 27, 171–176. [Google Scholar] [CrossRef]
- Griffiths, H.R.; Dias, I.H.; Willetts, R.S.; Devitt, A. Redox regulation of protein damage in plasma. Redox Biol. 2014, 2, 430–435. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beveridge, L.A.; Ramage, L.; McMurdo, M.E.; George, J.; Witham, M.D. Allopurinol use is associated with greater functional gains in older rehabilitation patients. Age Ageing 2013, 42, 400–404. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Küçükdiler, A.H.E.; Varlı, M.; Yavuz, Ö.; Yalçın, A.; Selvi Öztorun, H.; Devrim, E.; Aras, S. Evaluation of Oxidative Stress Parameters and Antioxidant Status in Plasma and Erythrocytes of Elderly Diabetic Patients with Sarcopenia. J. Nutr. Health Aging 2019, 23, 239–245. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.; Wu, C.; Liu, L.; Chou, R.; Kuo, C.; Huang, P.; Chen, L.; Lin, S. Association between serum uric acid and cardiovascular risk in nonhypertensive and nondiabetic individuals: The Taiwan I-Lan Longitudinal Aging Study. Sci. Rep. 2018, 8, 5234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brombo, G.; Bonetti, F.; Volpato, S.; Morieri, M.L.; Napoli, E.; Bandinelli, S.; Cherubini, A.; Maggio, M.; Guralnik, J.; Ferrucci, L.; et al. Uric acid within the “normal” range predict 9-year cardiovascular mortality in older individuals. The InCHIANTI study. Nutr. Metab. Cardiovasc. Dis. 2019, 29, 1061–1067. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.A.; Quinn, T.J.; Hewitt, J.; Fan, Y.; Dawson, J. Serum uric acid level and association with cognitive impairment and dementia: Systematic review and meta-analysis. AGE 2016, 38, 16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tana, C.; Ticinesi, A.; Prati, B.; Nouvenne, A.; Meschi, T. Uric Acid and Cognitive Function in Older Individuals. Nutrients 2018, 10, 975. [Google Scholar] [CrossRef] [Green Version]
- Labat-Robert, J.; Robert, L. Longevity and aging. Role of free radicals and xanthine oxidase. A review. Pathol. Biol. (Paris) 2014, 62, 61–66. [Google Scholar] [CrossRef]
- Levy, G.; Shi, J.M.; Cheetham, T.C.; Rashid, N. Urate-Lowering Therapy in Moderate to Severe Chronic Kidney Disease. Perm. J. 2018, 22, 17–142. [Google Scholar] [CrossRef]
- Gupta, M.K.; Singh, J.A. Cardiovascular Disease in Gout and the Protective Effect of Treatments Including Urate-Lowering Therapy. Drugs 2019, 79, 531–541. [Google Scholar] [CrossRef]
- Perez-Gomez, M.V.; Bartsch, L.A.; Castillo-Rodriguez, E.; Fernandez-Prado, R.; Kanbay, M.; Ortiz, A. Potential Dangers of Serum Urate-Lowering Therapy. Am. J. Med. 2019, 132, 457–467. [Google Scholar] [CrossRef] [PubMed]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Battelli, M.G.; Bortolotti, M.; Bolognesi, A.; Polito, L. Pro-Aging Effects of Xanthine Oxidoreductase Products. Antioxidants 2020, 9, 839. https://doi.org/10.3390/antiox9090839
Battelli MG, Bortolotti M, Bolognesi A, Polito L. Pro-Aging Effects of Xanthine Oxidoreductase Products. Antioxidants. 2020; 9(9):839. https://doi.org/10.3390/antiox9090839
Chicago/Turabian StyleBattelli, Maria Giulia, Massimo Bortolotti, Andrea Bolognesi, and Letizia Polito. 2020. "Pro-Aging Effects of Xanthine Oxidoreductase Products" Antioxidants 9, no. 9: 839. https://doi.org/10.3390/antiox9090839