Separation and HPLC Characterization of Active Natural Steroids in a Standardized Extract from the Serratula coronata Herb with Antiseborrheic Dermatitis Activity
Abstract
1. Introduction
2. Material and Methods
2.1. Plant Material
2.2. Extractionand Identification of Ecdysteroids
2.3. Ecdysteroids Characterization
2.4. Quantification of Ecdysteroids Using HPLC-DAD Analysis
2.5. Study Group and Antiseborrheic Activities
2.6. Data Processing and Statistical Analysis
3. Results and Discussion
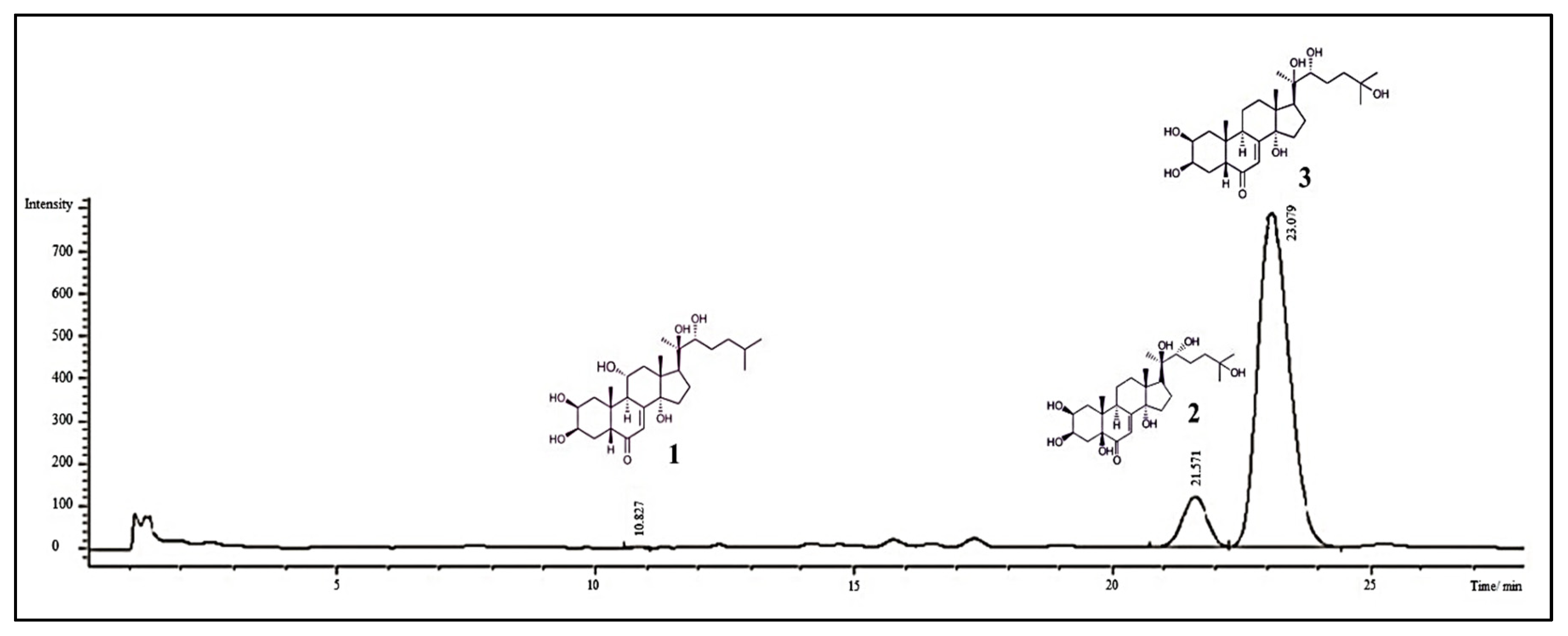
3.1. Isolation of Dominant Phytoecdysones from the S. coronata Herb
3.2. Thin Layer Chromatography (TLC) of the Isolated Compounds
3.3. HPLC Analysis
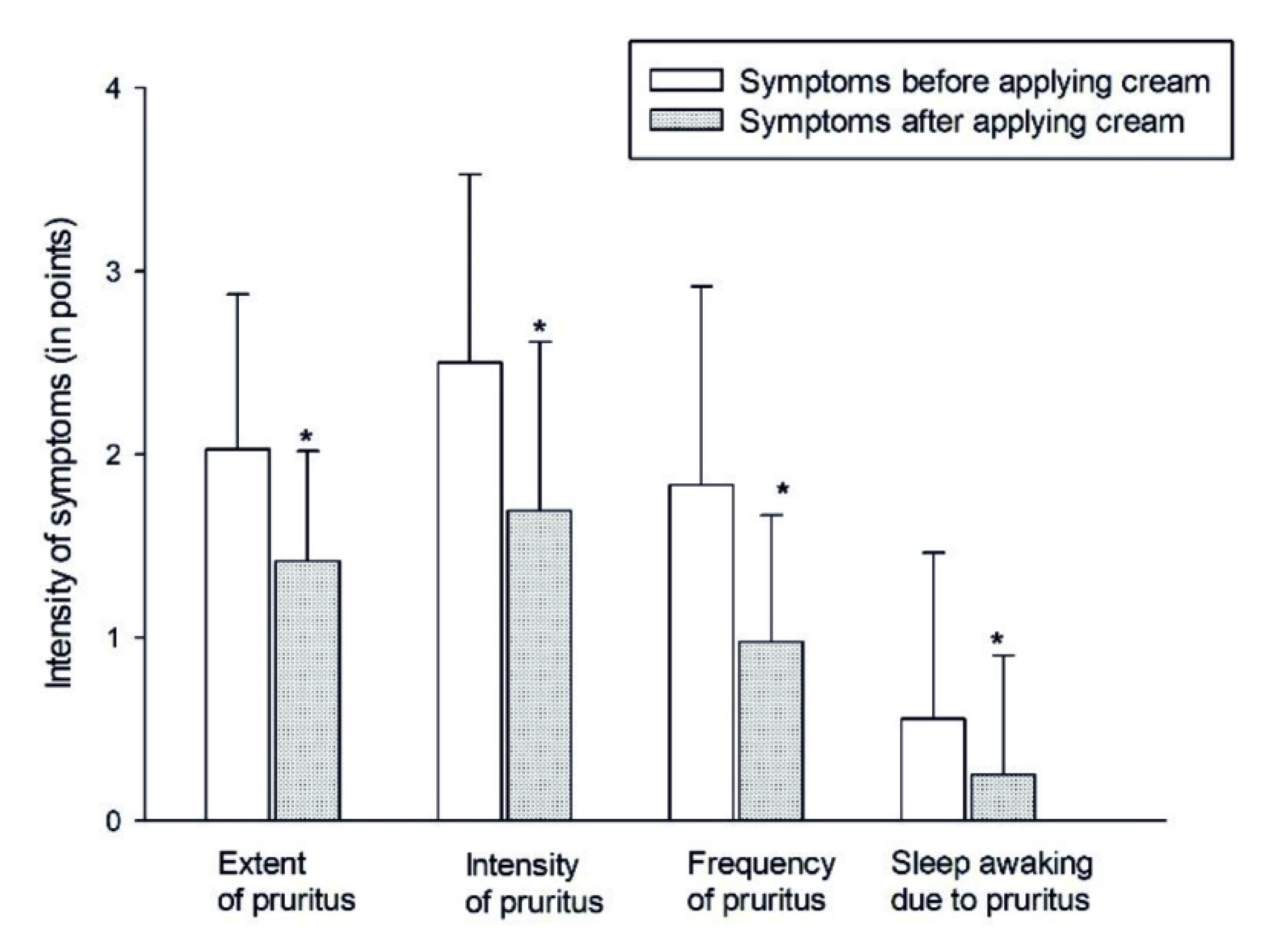
3.4. Examination of 2% Phytoecdysteroids Cream for Seborrheic Dermatitis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dinan, L. Phytoecdysteroids: Biological aspects. Phytochem 2001, 57, 325–339. [Google Scholar] [CrossRef]
- Santagati, N.; Tropea, S.; Ronsisvalle, G. Analysis of ecdysteroids by micellar electrokinetic chromatography with on-line preconcentration. J. Chromatogr. A 2005, 1081, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Lapenna, S.; Dinan, L. HPLC and TLC characterization of ecdysteroid alkyl ethers. J. Chromatogr. B 2009, 877, 2996–3002. [Google Scholar] [CrossRef] [PubMed]
- Takács, M.; Simon, A.; Liktor-Busa, E.; Báthori, M.; Zsila, F.; Bikádi, Z.; Horváth, P.; Veress, G.; Gergely, A.; Tóth, G. Structure and stereochemistry of novel ecdysteroids from the roots of Serratulawolffii. Magn. Reson. Chem. 2010, 48, 386–391. [Google Scholar] [CrossRef] [PubMed]
- Simon, L.A.; Ványolós, A.; Béni, Z.; Dékány, M.; Tóth, G.; Báthori, M. Ecdysteroids from Polypodium vulgare L. Steroids 2011, 76, 1419–1424. [Google Scholar] [CrossRef]
- Thiem, B.; Kikowska, M.; Maliński, M.P.; Kruszka, D.; Napierała, M.; Florek, E. Ecdysteroids: Production in plant in vitro cultures. Phytochem. Rev. 2017, 16, 603–622. [Google Scholar] [CrossRef]
- Báthori, M.; Pongrácz, Z. Phytoecdysteroids—from isolation to their effects on humans. Curr. Med. Chem. 2005, 12, 153–172. [Google Scholar] [CrossRef]
- Chen, Q.; Xia, Y.; Qiu, Z. Effect of ecdysterone on glucose metabolism in vitro. Life Sci. 2006, 78, 1108–1113. [Google Scholar] [CrossRef]
- Festucci-Buselli, R.A.; Contim, L.A.; Barbosa, L.C.; Stuart, J.; Otoni, W.C. Biosynthesis and potential functions of the ecdysteroid 20-hydroxyecdysone—a review. Botany 2008, 86, 978–987. [Google Scholar] [CrossRef]
- Dinan, L. The Karlson Lecture. Phytoecdysteroids: What use are they? Arch. Insect Biochem. Physiol. 2009, 72, 126–141. [Google Scholar] [CrossRef]
- Lafont, R.; Dinan, L. Practical uses for ecdysteroids in mammals including humans: An update. J. Insect Sci. 2003, 3, 1–30. [Google Scholar] [CrossRef]
- Wilborn, C.D.; Taylor, L.W.; Campbell, B.I.; Kerksick, C.; Rasmussen, C.J.; Greenwood, M.; Kreider, R.B. Effects of methoxy isoflavone ecdysterone and sulfo-polysaccharide supplementation on training adaptations in resistance-trained males. J. Inter. Soc. Sports Nutr. 2006, 3, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Báthori, M.; Tóth, N.; Hunyadi, A.; Márki, A.; Zádor, E. Phytoecdysteroids and anabolic-androgenic steroids—structure and effects on humans. Curr.Med. Chem. 2008, 15, 75–91. [Google Scholar] [CrossRef] [PubMed]
- Punegov, V.V.; Sychov, R.L.; Zainullin, V.G.; Fedorov, V.N.; Pangeova, N.V. Extraction of ecdysteron-80 from Serratulacoronata L. and assessment of its pharmacological action. Part I. Adaptogenic, gastroprotective, thermo protective, and antihypoxic activity. Pharm. Chem.J. 2008, 42, 446–451. [Google Scholar] [CrossRef]
- Kumpun, S.; Girault, J.P.; Dinan, L. The metabolism of 20-hydroxyecdysone in mice: Relevance to pharmacological effects and gene switch applications of ecdysteroids. J. Steroid Biochem. Mol. Biol. 2011, 126, 1–9. [Google Scholar] [CrossRef]
- Mamadalieva, N.Z.; El-Readi, M.Z.; Janibekov, A.A.; Tahrani, A.; Wink, M. Phytoecdysteroids of Silene guntensis and their in vitro cytotoxic and antioxidant activity. Z. Naturforsch. C 2011, 66, 215–224. [Google Scholar] [CrossRef]
- Syrov, V.N.; Iudasheva, N.K.; Egarnova, F.R.; Ismailova, G.I.; Abdulaeev, N.D.; Khusbaktova, Z.A. Estimation of the hypoglycemic effect of phytoecdysteroids. Eksp Klin Farm. 2012, 75, 28–31. [Google Scholar]
- Ochieng, C.O.; Ishola, I.O.; Opiyo, S.A.; Manguro, L.A.; Owuor, P.O.; Wong, K.C. Phytoecdysteroids from the stem bark of Vitex doniana and their anti-inflammatory effects. Planta Med. 2013, 791, 52–59. [Google Scholar] [CrossRef]
- Puri, P.; Wuttke, W.; Seidlova-Wuttke, D. 20-OH-ecdysone prevents hot flushes in ovariectomized rats. Planta Med. 2012, 78, 109–114. [Google Scholar] [CrossRef]
- Dessinioti, C.; Katsambas, A. Seborrheic dermatitis: Etiology, risk factors, and treatments: Facts and controversies. Clin.Dermatol. 2013, 31, 343–351. [Google Scholar] [CrossRef]
- Borda, L.J.; Perper, M.; Keri, J.E. Treatment of seborrheic dermatitis: A comprehensive review. J. Dermatolog. Treat. 2019, 30, 158–169. [Google Scholar] [CrossRef]
- Clark, G.W.; Pope, S.M.; Jaboori, K.A. Diagnosis and treatment of seborrheic dermatitis. Am. Fam. Phys. 2015, 91, 185–190. [Google Scholar]
- Elgash, M.; Dlova, N.; Ogunleye, T.; Taylor, S.C. Seborrheic Dermatitis in Skin of Color: Clinical Considerations. J. Drugs Dermatol. 2019, 18, 24–27. [Google Scholar] [PubMed]
- Wikramanayake, T.C.; Borda, L.J.; Miteva, M.; Paus, R. Seborrheic dermatitis—Looking beyond Malassezia. Exp. Dermatol. 2019, 28, 991–1001. [Google Scholar] [CrossRef] [PubMed]
- Hajar, T.; Leshem, Y.A.; Hanifin, J.M.; Nedorost, S.T.; Lio, P.A.; Paller, A.S.; Block, J.; Simpson, E.L. A systematic review of topical corticosteroid withdrawal (“steroid addiction”) in patients with atopic dermatitis and other dermatoses. J.Am. Acad. Dermatol. 2015, 72, 541–549.e2. [Google Scholar] [CrossRef] [PubMed]
- Ramazanov, N.S.; Bobayev, I.D.; Yusupova, U.Y.; Aliyeva, N.K.; Egamova, F.R.; Yuldasheva, N.K.; Syrov, V.N. Phytoecdysteroids-containing extract from Stachys hissarica plant and its wound-healing activity. Nat. Prod. Res. 2017, 31, 593–597. [Google Scholar] [CrossRef]
- U.S. Food and Drug Administration (FDA). Q2B Validation of Analytical Procedures: Methodology, Guidance for Industry. 1996. Available online: http://www.fda.gov/cder/Guidance/1320fnl.pdf (accessed on 1 September 2020).
- Siemiradzka, W.; Dolińska, B.; Ryszka, F. Influence of concentration on release and permeation process of model peptide substance-corticotropin-from semisolid formulations. Molecules 2020, 25, 2767. [Google Scholar] [CrossRef]
- Reich, A.; Mędrek, K.; Szepietowski, J. Four-item itch questionnaire—validation of questionnaire. Dermatol. Rev. (Przegląd Dermatol.) 2012, 99, 600–604. [Google Scholar]
- Nowak, G.; Urbańska, M.; Nawrot, J.; Bernard, M.K.; Dawid-Pać, R. Color and chemical reactions of selected sesquiterpene lactones and ecdysones from Asteraceae on TLC plates. J. Planar Chromatogr. 2013, 26, 289–293. [Google Scholar] [CrossRef]
- Gorelick-Feldman, J.; MacLean, D.; Ilic, N.; Poulev, A.; Lila, M.A.; Cheng, D.; Raskin, I. Phytoecdysteroids increase protein synthesis in skeletal muscle cells. J. Agric. Food Chem. 2008, 56, 3532–3537. [Google Scholar] [CrossRef]
- Rosselli, S.; Maggio, A.; Bruno, M.; Spadaro, V.; Formisano, C.; Irace, C.; Maffettone, C.; Mascolo, N. Furostanol saponins and ecdysones with cytotoxic activity from Helleborus bocconei ssp. Intermedius. Phytother. Res. 2009, 23, 1243–1249. [Google Scholar] [CrossRef] [PubMed]
- Detmar, M.; Dumas, M.; Bonté, F.; Meybeck, A.; Orfanos, C.E. Effects of ecdysterone on the differentiation of normal human keratinocytes in vitro. Eur. J. Derm. 1994, 4, 558–562. [Google Scholar]
- Naggar, Y.A.; Ghorab, M.; Mohamed, K. Phytoecdysteroids: Isolation and biological applications. A J. Life Sci. 2017, 5, 7–10. [Google Scholar] [CrossRef][Green Version]
- Patel, S.S.; Savjani, J.K. Systematic review of plant steroids as potential anti-inflammatory agents: Current status and future perspectives. J. Phytopharm. 2015, 4, 121–125. [Google Scholar]
- Meybeck, A.; Bonté, F. Ecdysteroid-containing liposomes for wound healing and skin regeneration. Chem. Abstr. 1990, 133, 30138r. [Google Scholar]
- Dinan, L.; Lafont, R. Effects and applications of arthropod steroid hormones (ecdysteroids) in mammals. J. Endocrinol. 2006, 191, 1–8. [Google Scholar] [CrossRef]
- Ali, S.; Khan, F.I.; Mohammad, T.; Lan, D.; Hassan, M.I.; Wang, Y. Identification and evaluation of inhibitors of lipase from Malassezia restricta using virtual high-throughput screening and molecular dynamics studies. Int. J. Mol. Sci. 2019, 20, 884. [Google Scholar] [CrossRef]
- Han, P.; Han, J.; Zhang, M.; Fan, J.; Gong, Q.; Ma, E.; Zhang, J. 20-Hydroxyecdysone enhances Immulectin-1 mediated immune response against entomogenous fungus in Locusta migratoria. Pest Manag. Sci. 2020, 76, 304–313. [Google Scholar] [CrossRef]


| Source | Active Molecule/Ecdysteroids | Structure |
|---|---|---|
| Serratula coronata Herb | Ajugasterone C (1) |  |
| Polypodine B (2) | 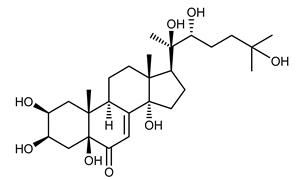 | |
| 20-hydroxyecdysone (3) |  |
| Nominal Concentration of QC Samples (µg/mL) | Predicted Concentration, Mean ± SD (µg/mL) | RSD (%) | Accuracy (%) |
|---|---|---|---|
| A. Intra-day assays; n = 6 | |||
| Ajugasterone C | |||
| QC low 300 | 291 ± 1.95 | 0.67 | 97 |
| QC middle 500 | 483 ± 3.39 | 0.70 | 97 |
| QC high 1000 | 967 ± 12.23 | 1.26 | 97 |
| Polypodine B | |||
| QC low 300 | 318 ± 1.91 | 0.60 | 106 |
| QC middle 500 | 491 ± 3.69 | 0.75 | 98 |
| QC high 1000 | 997 ± 9.50 | 0.95 | 100 |
| 20-hydroxyecdysone | |||
| QC low 300 | 288 ± 1.61 | 0.57 | 96 |
| QC middle 500 | 510 ± 3.40 | 0.51 | 102 |
| QC high 1000 | 991 ± 7.58 | 1.10 | 99 |
| B. Inter-day assays; n = 6 | |||
| Ajugasterone C | |||
| QC low 300 | 287 ± 3.02 | 1.05 | 96 |
| QC middle 500 | 481 ± 4.71 | 0.98 | 96 |
| QC high 1000 | 962 ± 12.85 | 1.34 | 96 |
| Polypodine B | |||
| QC low 300 | 317 ± 2.29 | 0.72 | 106 |
| QC middle 500 | 489 ± 3.78 | 0.77 | 98 |
| QC high 1000 | 995 ± 9.21 | 0.93 | 100 |
| 20-hydroxyecdysone | |||
| QC low 300 | 287 ± 1.46 | 0.51 | 96 |
| QC middle 500 | 511 ± 2.62 | 0.51 | 102 |
| QC high 1000 | 991 ± 8.15 | 0.82 | 99 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Napierała, M.; Nawrot, J.; Gornowicz-Porowska, J.; Florek, E.; Moroch, A.; Adamski, Z.; Kroma, A.; Miechowicz, I.; Nowak, G. Separation and HPLC Characterization of Active Natural Steroids in a Standardized Extract from the Serratula coronata Herb with Antiseborrheic Dermatitis Activity. Int. J. Environ. Res. Public Health 2020, 17, 6453. https://doi.org/10.3390/ijerph17186453
Napierała M, Nawrot J, Gornowicz-Porowska J, Florek E, Moroch A, Adamski Z, Kroma A, Miechowicz I, Nowak G. Separation and HPLC Characterization of Active Natural Steroids in a Standardized Extract from the Serratula coronata Herb with Antiseborrheic Dermatitis Activity. International Journal of Environmental Research and Public Health. 2020; 17(18):6453. https://doi.org/10.3390/ijerph17186453
Chicago/Turabian StyleNapierała, Marta, Joanna Nawrot, Justyna Gornowicz-Porowska, Ewa Florek, Arletta Moroch, Zygmunt Adamski, Anna Kroma, Izabela Miechowicz, and Gerard Nowak. 2020. "Separation and HPLC Characterization of Active Natural Steroids in a Standardized Extract from the Serratula coronata Herb with Antiseborrheic Dermatitis Activity" International Journal of Environmental Research and Public Health 17, no. 18: 6453. https://doi.org/10.3390/ijerph17186453
APA StyleNapierała, M., Nawrot, J., Gornowicz-Porowska, J., Florek, E., Moroch, A., Adamski, Z., Kroma, A., Miechowicz, I., & Nowak, G. (2020). Separation and HPLC Characterization of Active Natural Steroids in a Standardized Extract from the Serratula coronata Herb with Antiseborrheic Dermatitis Activity. International Journal of Environmental Research and Public Health, 17(18), 6453. https://doi.org/10.3390/ijerph17186453






