Adverse Effects of Methylglyoxal on Transcriptome and Metabolic Changes in Visceral Adipose Tissue in a Prediabetic Rat Model
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals and Diet
2.2. Biochemical Analysis
2.3. Basal and Insulin-Stimulated Glucose Utilisation in Adipose Tissue and Lipolysis
2.4. Fatty Acid Composition
2.5. Transcriptome and Gene Expression Profiling
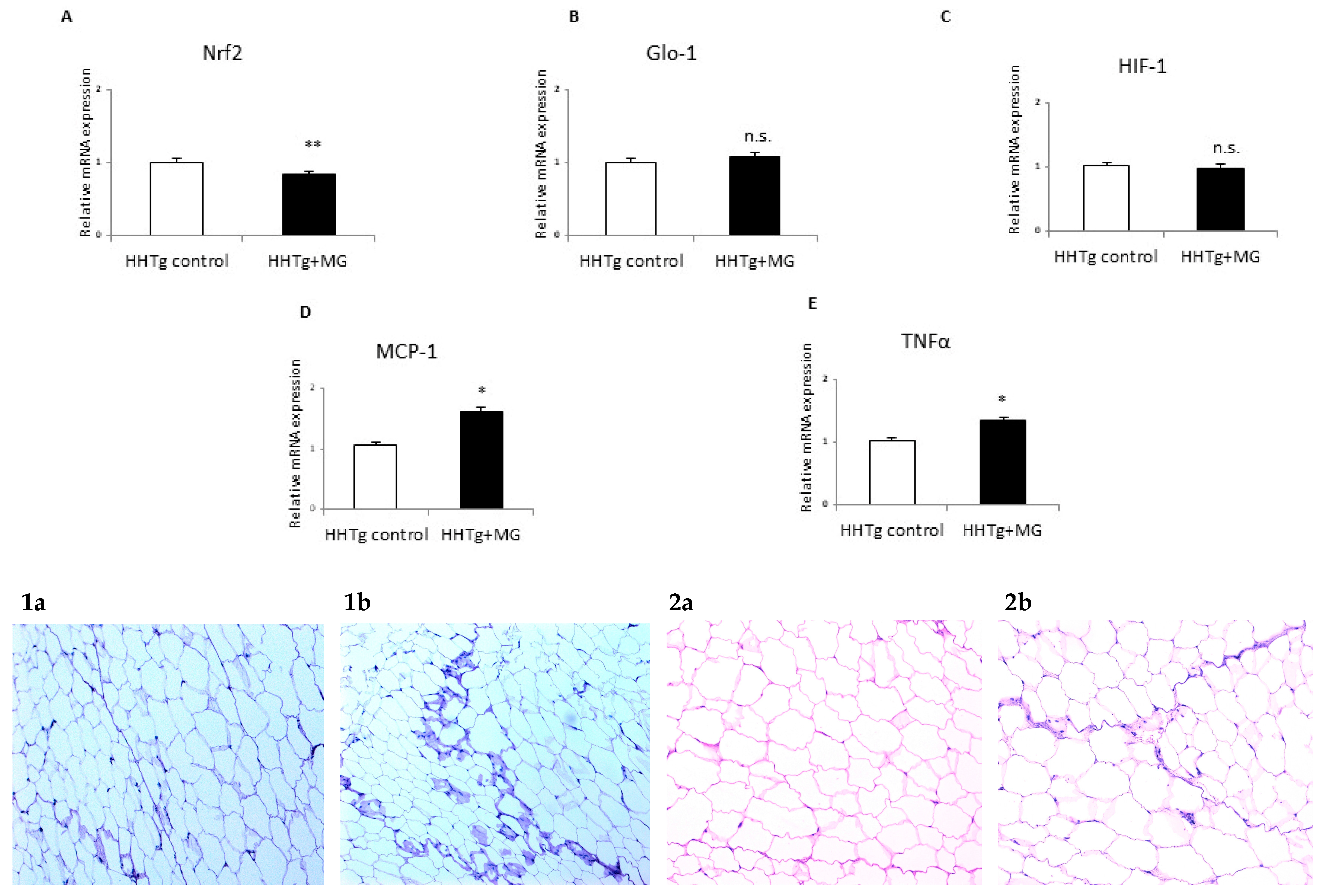
2.6. Histological Methods
2.7. Statistical and Pathway Analysis
3. Results
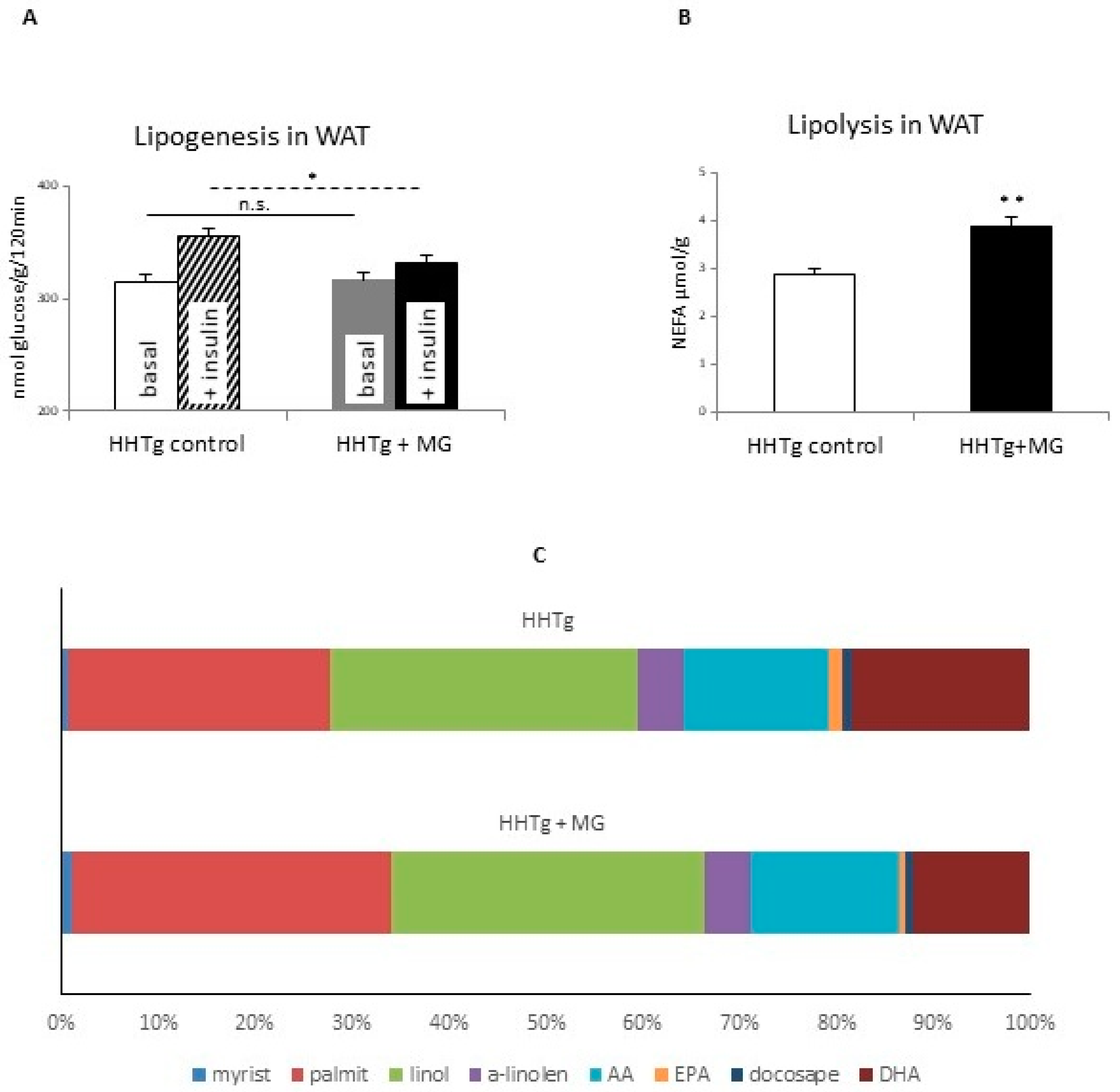
3.1. Effects of Methylglyoxal on Insulin Sensitivity and Lipolysis
3.2. Effects of Methylglyoxal on Fatty Acid Composition of Phospholipids in Visceral Adipose Tissue
3.3. Effects of meThylglyoxal on Dicarbonyl Stress and Inflammatory and Hypoxic Parameters
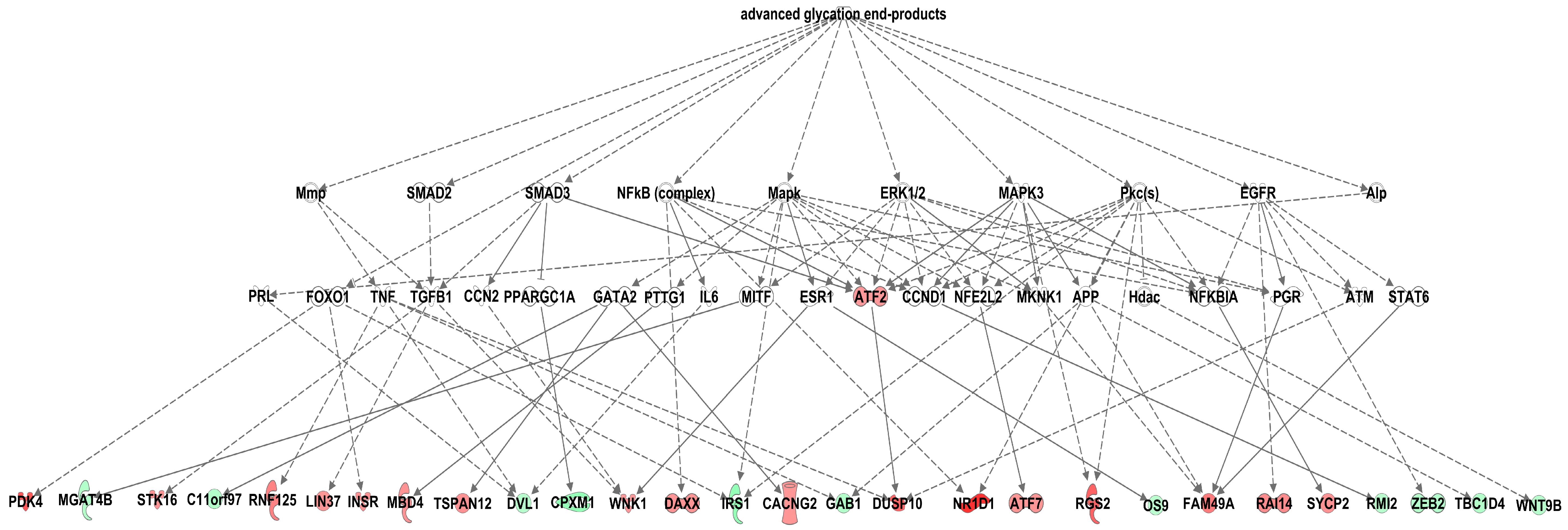
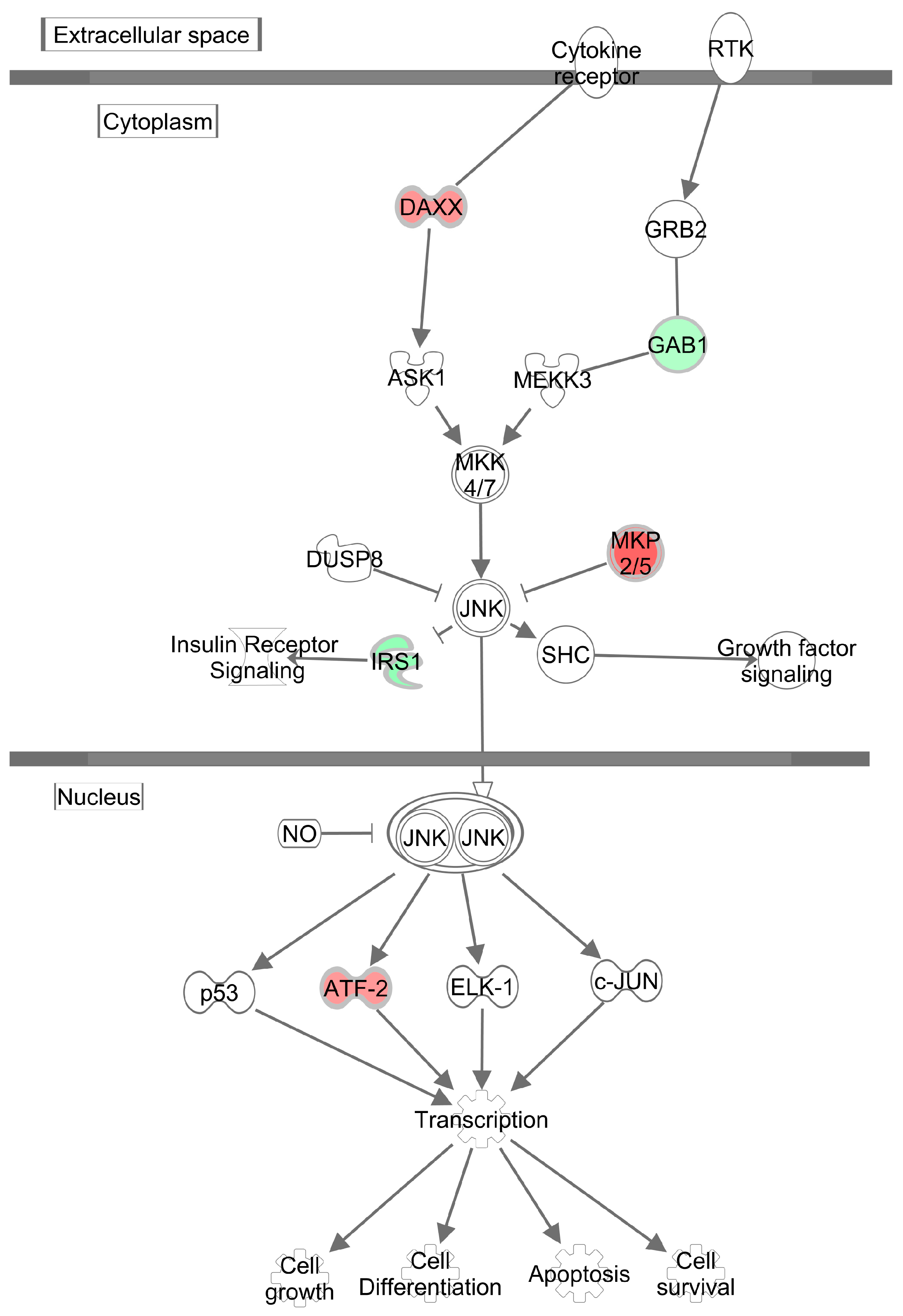
3.4. Effects of Methylglyoxal on the Visceral Adipose Tissue Transcriptome
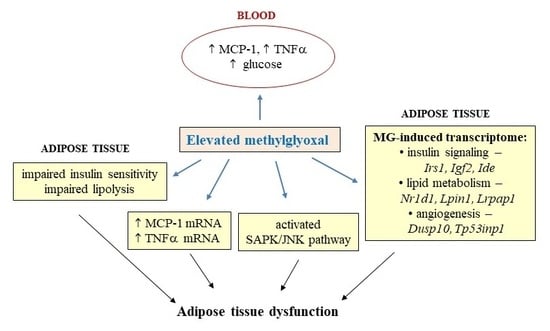
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AGEs: | advanced glycation end products; |
| DHA: | docosahexaenoic acid; |
| EPA: | eicosapentaenoic acid; |
| ERK: | extracellular signal-regulated kinase; |
| Glo-1: | glyoxalase 1; |
| HIF1: | hypoxia-inducible factor 1; |
| IL-6: | interleukin-6; |
| IRS-1: | insulin receptor substrate 1; |
| JNK: | c-Jun terminal kinases; |
| MAPK: | mitogen-activated protein kinase; |
| MCP-1: | monocyte chemoattractant protein-1; |
| MG: | methylglyoxal; |
| NFκB: | nuclear factor κB; |
| NRF2: | nuclear factor-erythroid 2-related factor-2; |
| PAS: | period acid-Schiff; |
| PKC: | protein kinase C; |
| RAGE: | receptor for advanced glycation endproducts; |
| ROS: | reactive oxygen species; |
| SAPK: | stress-activated protein kinases; |
| SREBP: | sterol regulatory element-binding protein; |
| T2D: | type 2 diabetes; |
| TLR4: | tool-like receptor 4; |
| TNFα: | tumor necrosis factor α; |
| WAT: | white adipose tissue |
References
- Bodis, K.; Roden, M. Energy metabolism of white adipose tissue and insulin resistance in humans. Eur. J. Clin. Investig. 2018, 48, e13017. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lopes, H.F.; Correa-Giannella, M.L.; Consolim-Colombo, F.M.; Egan, B.M. Visceral adiposity syndrome. Diabetol. Metab. Syndr. 2016, 8, 40. [Google Scholar] [CrossRef] [Green Version]
- Matafome, P.; Rodrigues, T.; Seica, R. Glycation and hypoxia: Two key factors for adipose tissue dysfunction. Curr. Med. Chem. 2015, 22, 2417–2437. [Google Scholar] [CrossRef]
- Matafome, P.; Santos-Silva, D.; Crisostomo, J.; Rodrigues, T.; Rodrigues, L.; Sena, C.M.; Pereira, P.; Seica, R. Methylglyoxal causes structural and functional alterations in adipose tissue independently of obesity. Arch. Physiol. Biochem. 2012, 118, 58–68. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, T.; Matafome, P.; Sereno, J.; Almeida, J.; Castelhano, J.; Gamas, L.; Neves, C.; Goncalves, S.; Carvalho, C.; Arslanagic, A.; et al. Methylglyoxal-induced glycation changes adipose tissue vascular architecture, flow and expansion, leading to insulin resistance. Sci. Rep. 2017, 7, 1698. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nigro, C.; Leone, A.; Raciti, G.A.; Longo, M.; Mirra, P.; Formisano, P.; Beguinot, F.; Miele, C. Methylglyoxal-glyoxalase 1 balance: The root of vascular damage. Int. J. Mol. Sci. 2017, 18, 188. [Google Scholar] [CrossRef] [Green Version]
- Schalkwijk, C.G.; Stehouwer, C.D.A. Methylglyoxal, a highly reactive dicarbonyl compound, in diabetes, its vascular complications, and other age-related diseases. Physiol. Rev. 2020, 100, 407–461. [Google Scholar] [CrossRef]
- Tikellis, C.; Pickering, R.J.; Tsorotes, D.; Huet, O.; Cooper, M.E.; Jandeleit-Dahm, K.; Thomas, M.C. Dicarbonyl stress in the absence of hyperglycemia increases endothelial inflammation and atherogenesis similar to that observed in diabetes. Diabetes 2014, 63, 3915–3925. [Google Scholar] [CrossRef] [Green Version]
- Fiory, F.; Lombardi, A.; Miele, C.; Giudicelli, J.; Beguinot, F.; Van Obberghen, E. Methylglyoxal impairs insulin signalling and insulin action on glucose-induced insulin secretion in the pancreatic beta cell line INS-1E. Diabetologia 2011, 54, 2941–2952. [Google Scholar] [CrossRef] [Green Version]
- Peng, Z.; Yang, X.; Qin, J.; Ye, K.; Wang, X.; Shi, H.; Jiang, M.; Liu, X.; Lu, X. Glyoxalase-1 overexpression reverses defective proangiogenic function of diabetic adipose-derived stem cells in streptozotocin-induced diabetic mice model of critical limb ischemia. Stem Cells Transl. Med. 2017, 6, 261–271. [Google Scholar] [CrossRef] [Green Version]
- Masania, J.; Malczewska-Malec, M.; Razny, U.; Goralska, J.; Zdzienicka, A.; Kiec-Wilk, B.; Gruca, A.; Stancel-Mozwillo, J.; Dembinska-Kiec, A.; Rabbani, N.; et al. Dicarbonyl stress in clinical obesity. Glycoconj. J. 2016, 33, 581–589. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zemva, J.; Pfaff, D.; Groener, J.B.; Fleming, T.; Herzig, S.; Teleman, A.; Nawroth, P.P.; Tyedmers, J. Effects of the reactive metabolite methylglyoxal on cellular signalling, insulin action and metabolism—What we know in mammals and what we can learn from yeast. Exp. Clin. Endocrinol. Diabetes 2019, 127, 203–214. [Google Scholar] [CrossRef] [PubMed]
- Vrana, A.; Kazdova, L. The hereditary hypertriglyceridemic nonobese rat: An experimental model of human hypertriglyceridemia. Transplant. Proc. 1990, 22, 2579. [Google Scholar] [PubMed]
- Malinska, H.; Skop, V.; Trnovska, J.; Markova, I.; Svoboda, P.; Kazdova, L.; Haluzik, M. Metformin attenuates myocardium dicarbonyl stress induced by chronic hypertriglyceridemia. Physiol. Res. 2018, 67, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Thornalley, P.J.; Langborg, A.; Minhas, H.S. Formation of glyoxal, methylglyoxal and 3-deoxyglucosone in the glycation of proteins by glucose. Biochem. J. 1999, 344, 109–116. [Google Scholar] [CrossRef]
- Malinska, H.; Huttl, M.; Oliyarnyk, O.; Bratova, M.; Kazdova, L. Conjugated linoleic acid reduces visceral and ectopic lipid accumulation and insulin resistance in chronic severe hypertriacylglycerolemia. Nutrition 2015, 31, 1045–1051. [Google Scholar] [CrossRef]
- Eder, K. Gas chromatographic analysis of fatty acid methyl esters. J. Chromatogr. B Biomed. Appl. 1995, 671, 113–131. [Google Scholar] [CrossRef]
- Markova, I.; Huttl, M.; Oliyarnyk, O.; Kacerova, T.; Haluzik, M.; Kacer, P.; Seda, O.; Malinska, H. The effect of dicarbonyl stress on the development of kidney dysfunction in metabolic syndrome—A transcriptomic and proteomic approach. Nutr. Metab. 2019, 16, 51. [Google Scholar] [CrossRef] [Green Version]
- Kramer, A.; Green, J.; Pollard, J., Jr.; Tugendreich, S. Causal analysis approaches in ingenuity pathway analysis. Bioinformatics 2014, 30, 523–530. [Google Scholar] [CrossRef]
- Yamamoto, M.; Kensler, T.W.; Motohashi, H. The KEAP1-NRF2 system: A thiol-based sensor-effector apparatus for maintaining redox homeostasis. Physiol. Rev. 2018, 98, 1169–1203. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Zuo, Z.; Li, L.; Ren, S.; Gao, T.; Fu, J.; Hou, Y.; Chen, Y.; Pi, J. Nrf2 in adipocytes. Arch. Pharm. Res. 2020, 43, 350–360. [Google Scholar] [CrossRef] [PubMed]
- Wen, J.; Cai, X.; Zhang, J.; Jiang, J.; Li, W.; Liu, G.; Wang, M.; Gaisano, H.Y.; Pan, Y.; He, Y. Relation of adipose tissue insulin resistance to prediabetes. Endocrine 2020, 68, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Schneider, K.S.; Chan, J.Y. Emerging role of Nrf2 in adipocytes and adipose biology. Adv. Nutr. 2013, 4, 62–66. [Google Scholar] [CrossRef] [Green Version]
- Pelikanova, T.; Kazdova, L.; Chvojkova, S.; Base, J. Serum phospholipid fatty acid composition and insulin action in type 2 diabetic patients. Metabolism 2001, 50, 1472–1478. [Google Scholar] [PubMed]
- Iggman, D.; Arnlov, J.; Vessby, B.; Cederholm, T.; Sjogren, P.; Riserus, U. Adipose tissue fatty acids and insulin sensitivity in elderly men. Diabetologia 2010, 53, 850–857. [Google Scholar] [CrossRef] [Green Version]
- Poledne, R.; Malinska, H.; Kubatova, H.; Fronek, J.; Thieme, F.; Kauerova, S.; Lesna, I.K. Polarization of macrophages in human adipose tissue is related to the fatty acid spectrum in membrane phospholipids. Nutrients 2019, 12, 8. [Google Scholar] [CrossRef] [Green Version]
- Kim, B.R.; Lee, G.Y.; Yu, H.; Maeng, H.J.; Oh, T.J.; Kim, K.M.; Moon, J.H.; Lim, S.; Jang, H.C.; Choi, S.H. Suppression of Nrf2 attenuates adipogenesis and decreases FGF21 expression through PPAR gamma in 3T3-L1 cells. Biochem. Biophys. Res. Commun. 2018, 497, 1149–1153. [Google Scholar] [CrossRef]
- Amano, S.U.; Cohen, J.L.; Vangala, P.; Tencerova, M.; Nicoloro, S.M.; Yawe, J.C.; Shen, Y.; Czech, M.P.; Aouadi, M. Local proliferation of macrophages contributes to obesity-associated adipose tissue inflammation. Cell Metab. 2014, 19, 162–171. [Google Scholar] [CrossRef] [Green Version]
- Yaribeygi, H.; Farrokhi, F.R.; Butler, A.E.; Sahebkar, A. Insulin resistance: Review of the underlying molecular mechanisms. J. Cell Physiol. 2019, 234, 8152–8161. [Google Scholar] [CrossRef]
- Borst, S.E. The role of TNF-alpha in insulin resistance. Endocrine 2004, 23, 177–182. [Google Scholar] [CrossRef]
- Ryden, M.; Dicker, A.; van Harmelen, V.; Hauner, H.; Brunnberg, M.; Perbeck, L.; Lonnqvist, F.; Arner, P. Mapping of early signaling events in tumor necrosis factor-alpha -mediated lipolysis in human fat cells. J. Biol. Chem. 2002, 277, 1085–1091. [Google Scholar] [CrossRef] [Green Version]
- Hong, F.Y.; Bao, J.F.; Hao, J.; Yu, Q.; Liu, J. Methylglyoxal and advanced glycation end-products promote cytokines expression in peritoneal mesothelial cells via MAPK signaling. Am. J. Med. Sci. 2015, 349, 105–109. [Google Scholar] [CrossRef]
- Lee, K.M.; Lee, C.Y.; Zhang, G.; Lyu, A.; Yue, K.K.M. The dataset of methylglyoxal activating p38 and p44/42 pathway in osteoclast. Data Brief 2019, 26, 104500. [Google Scholar] [CrossRef]
- Vlassara, H.; Uribarri, J. Advanced glycation end products (AGE) and diabetes: Cause, effect, or both? Curr. Diabetes Rep. 2014, 14, 453. [Google Scholar] [CrossRef] [Green Version]
- Cartier, A.; Lemieux, I.; Almeras, N.; Tremblay, A.; Bergeron, J.; Despres, J.P. Visceral obesity and plasma glucose-insulin homeostasis: Contributions of interleukin-6 and tumor necrosis factor-alpha in men. J. Clin. Endocrinol. Metab. 2008, 93, 1931–1938. [Google Scholar] [CrossRef]
- Halberg, N.; Wernstedt-Asterholm, I.; Scherer, P.E. The adipocyte as an endocrine cell. Endocrinol. Metab. Clin. N. Am. 2008, 37, 753–768. [Google Scholar] [CrossRef] [Green Version]
- Engin, A.B. Adipocyte-macrophage cross-talk in obesity. Adv. Exp. Med. Biol. 2017, 960, 327–343. [Google Scholar]
- Lorente-Cebrian, S.; Gonzalez-Muniesa, P.; Milagro, F.I.; Martinez, J.A. MicroRNAs and other non-coding RNAs in adipose tissue and obesity: Emerging roles as biomarkers and therapeutic targets. Clin. Sci. 2019, 133, 23–40. [Google Scholar] [CrossRef]
- Dong, H.; Cui, B.; Hao, X. MicroRNA22 alleviates inflammation in ischemic stroke via p38 MAPK pathways. Mol. Med. Rep. 2019, 20, 735–744. [Google Scholar]
- Phan, H.T.N.; Sjogren, B.; Neubig, R.R. Human missense mutations in regulator of G protein signaling 2 affect the protein function through multiple mechanisms. Mol. Pharmacol. 2017, 92, 451–458. [Google Scholar] [CrossRef]

| HHTg | HHTg + MG | p ˂ | |
|---|---|---|---|
| body weight (g) | 402 ± 22 | 381 ± 8 | n.s. |
| adiposity (g/100g bwt) | 1.85 ± 0.29 | 1.87 ± 0.35 | n.s. |
| non-fasting glucose (mmol/L) | 7.7 ± 0.3 | 8.6 ± 0.24 | 0.01 |
| insulin (nmol/L) | 0.24 ± 0.08 | 0.42 ± 0.05 | 0.05 |
| serum triglycerides (mmol/l) | 4.34 ± 0.74 | 3.21 ± 1.24 | n.s. |
| methylglyoxal in serum (nmol/mL) | 0.25 ± 0.03 | 0.37 ± 0.03 | 0.01 |
| methylglyoxal in adipose tissue (nmol/mg) | 0.63 ± 0.09 | 0.86 ± 0.13 | 0.05 |
| HMW adiponectin (μg/mL) | 2.90 ± 0.96 | 2.78 ± 1.14 | n.s. |
| leptin (ng/mL) | 2.15 ± 1.07 | 2.13 ± 1.42 | n.s. |
| IL-6 (pg/mL) | 87.2 ± 21.78 | 99.9 ± 11.78 | n.s. |
| MCP-1 (ng/mL) | 85.25 ± 40.20 | 124.88 ± 29.65 | 0.05 |
| TNFα (pg/mL) | 0.78 ± 0.04 | 2.11 ± 0.33 | 0.05 |
| HHTg | HHTg + MG | p ˂ | |
|---|---|---|---|
| % of total fatty acids | % of total fatty acids | ||
| 14:00 | 0.40 ± 0.18 | 0.76 ± 0.29 | 0.02 |
| 16:00 | 16.68 ± 5.14 | 24.05 ± 4.01 | 0.02 |
| 18:2n6 | 19.60 ± 1.85 | 23.17 ± 1.51 | 0.01 |
| 20:4n6 | 9.36 ± 2.39 | 10.76 ± 2.08 | n.s. |
| 18:3n3 | 3.24 ± 0.96 | 2.12 ± 0.40 | 0.05 |
| 20:5n3 | 0.91 ± 0.21 | 0.44 ± 0.14 | 0.01 |
| 22:6n3 | 13.15 ± 2.57 | 5.57 ± 1.35 | 0.001 |
| SFA | 33.35 ± 3.82 | 39.92 ± 2.96 | 0.01 |
| PUFA n6 | 31.44 ± 3.89 | 35.59 ± 3.08 | n.s. |
| PUFA n3 | 18.42 ± 2.37 | 8.80 ± 1.50 | 0.001 |
| Anti-inflammatory index | 171.20 ± 32.54 | 65.87 ± 14.46 | 0.001 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hüttl, M.; Markova, I.; Miklankova, D.; Makovicky, P.; Pelikanova, T.; Šeda, O.; Šedová, L.; Malinska, H. Adverse Effects of Methylglyoxal on Transcriptome and Metabolic Changes in Visceral Adipose Tissue in a Prediabetic Rat Model. Antioxidants 2020, 9, 803. https://doi.org/10.3390/antiox9090803
Hüttl M, Markova I, Miklankova D, Makovicky P, Pelikanova T, Šeda O, Šedová L, Malinska H. Adverse Effects of Methylglyoxal on Transcriptome and Metabolic Changes in Visceral Adipose Tissue in a Prediabetic Rat Model. Antioxidants. 2020; 9(9):803. https://doi.org/10.3390/antiox9090803
Chicago/Turabian StyleHüttl, Martina, Irena Markova, Denisa Miklankova, Pavol Makovicky, Terezie Pelikanova, Ondrej Šeda, Lucie Šedová, and Hana Malinska. 2020. "Adverse Effects of Methylglyoxal on Transcriptome and Metabolic Changes in Visceral Adipose Tissue in a Prediabetic Rat Model" Antioxidants 9, no. 9: 803. https://doi.org/10.3390/antiox9090803