Impact of Cyanidin-3-Glucoside on Gut Microbiota and Relationship with Metabolism and Inflammation in High Fat-High Sucrose Diet-Induced Insulin Resistant Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Dietary Intervention
2.3. Animal Monitoring and Sample Collection
2.4. Analyses of Metabolic Variables
2.5. Measurement of Circulating Inflammatory Markers
2.6. Fecal Sample Collection
2.7. Extraction and Sequencing of Bacteria DNA
2.8. Bioinformatic Analysis and Statistics
3. Results
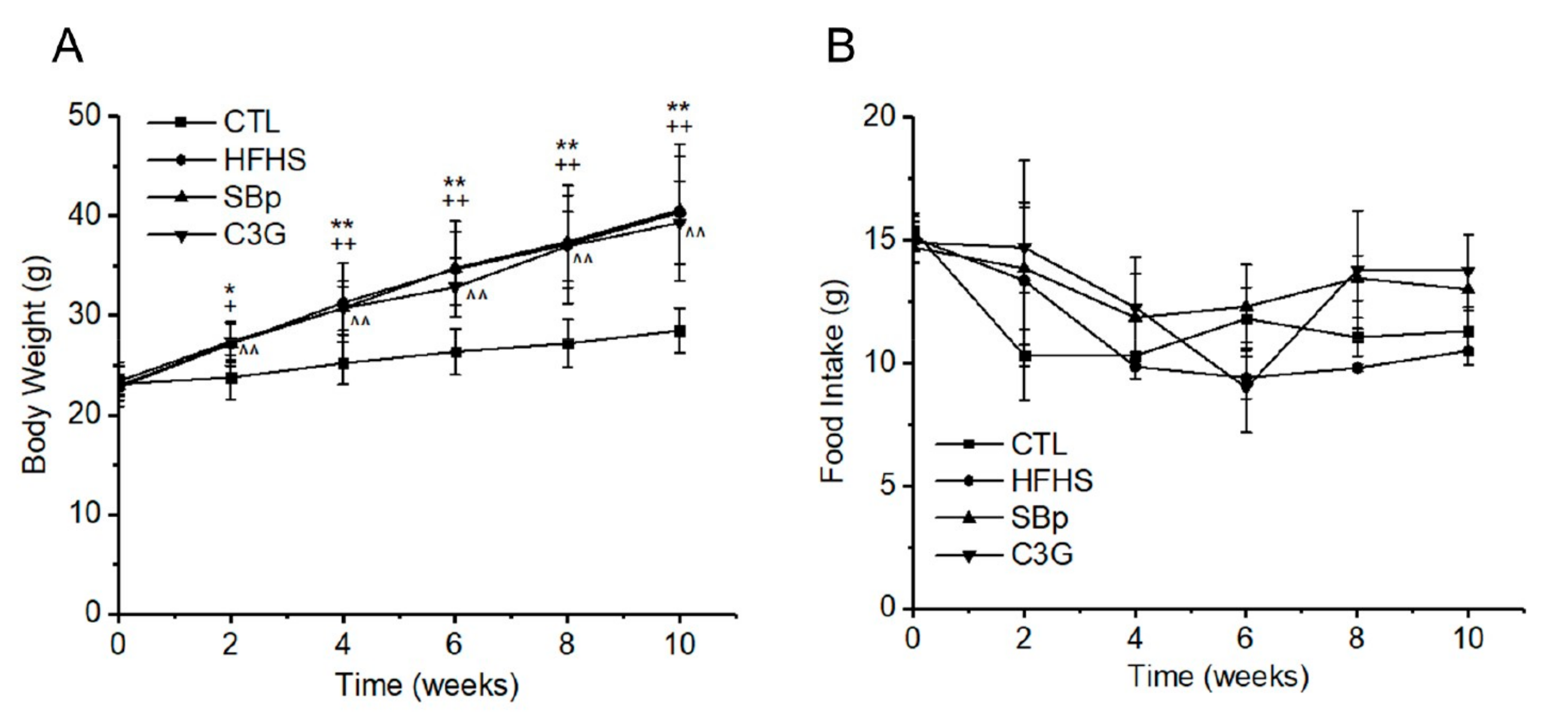
3.1. Effects of HFHS Diet and Supplementation of C3G on Body Weights and Food Intake of Mice
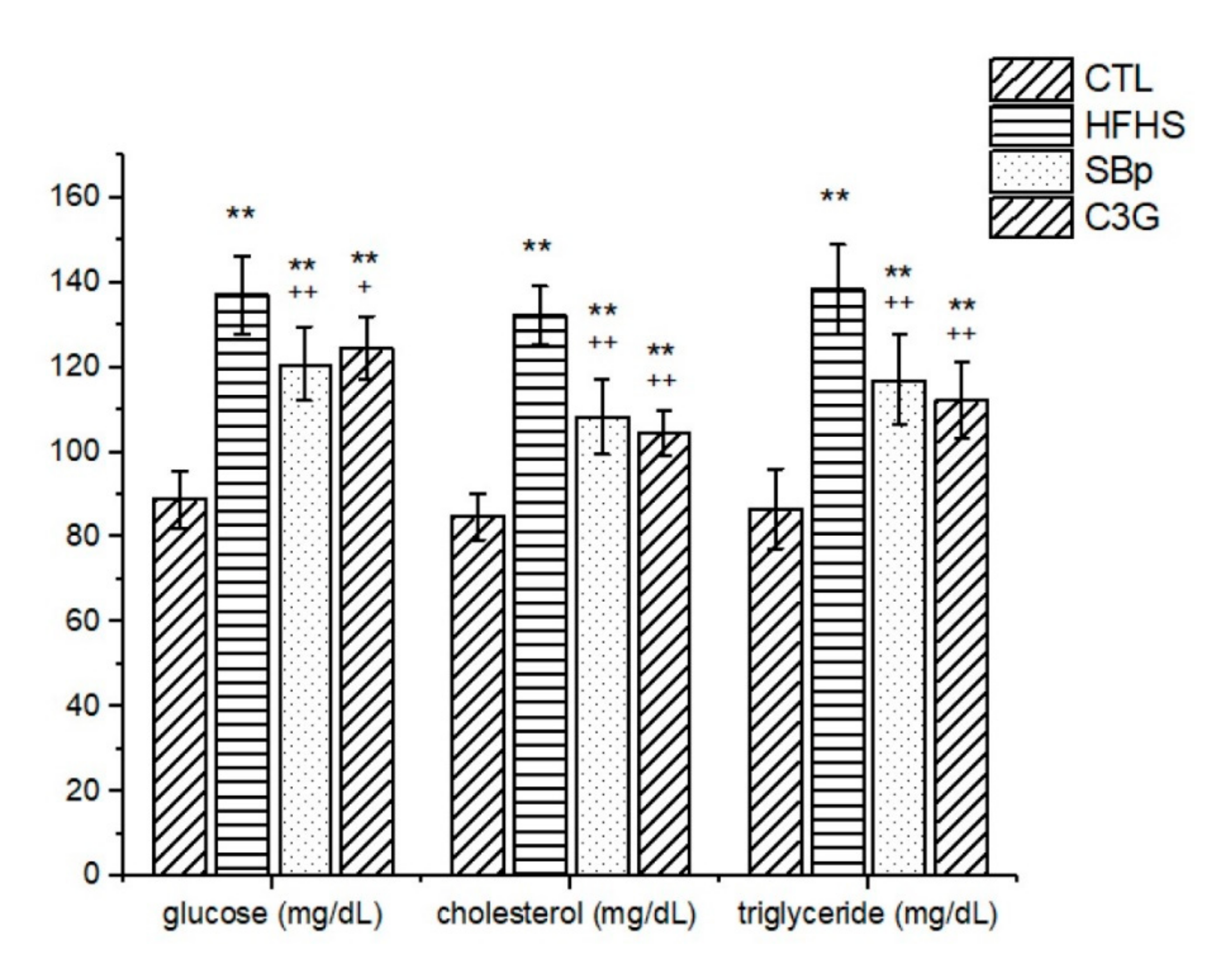
3.2. Effects of C3G on Glucose and Lipid Metabolism in HFHS Diet-Induced Obese Mice
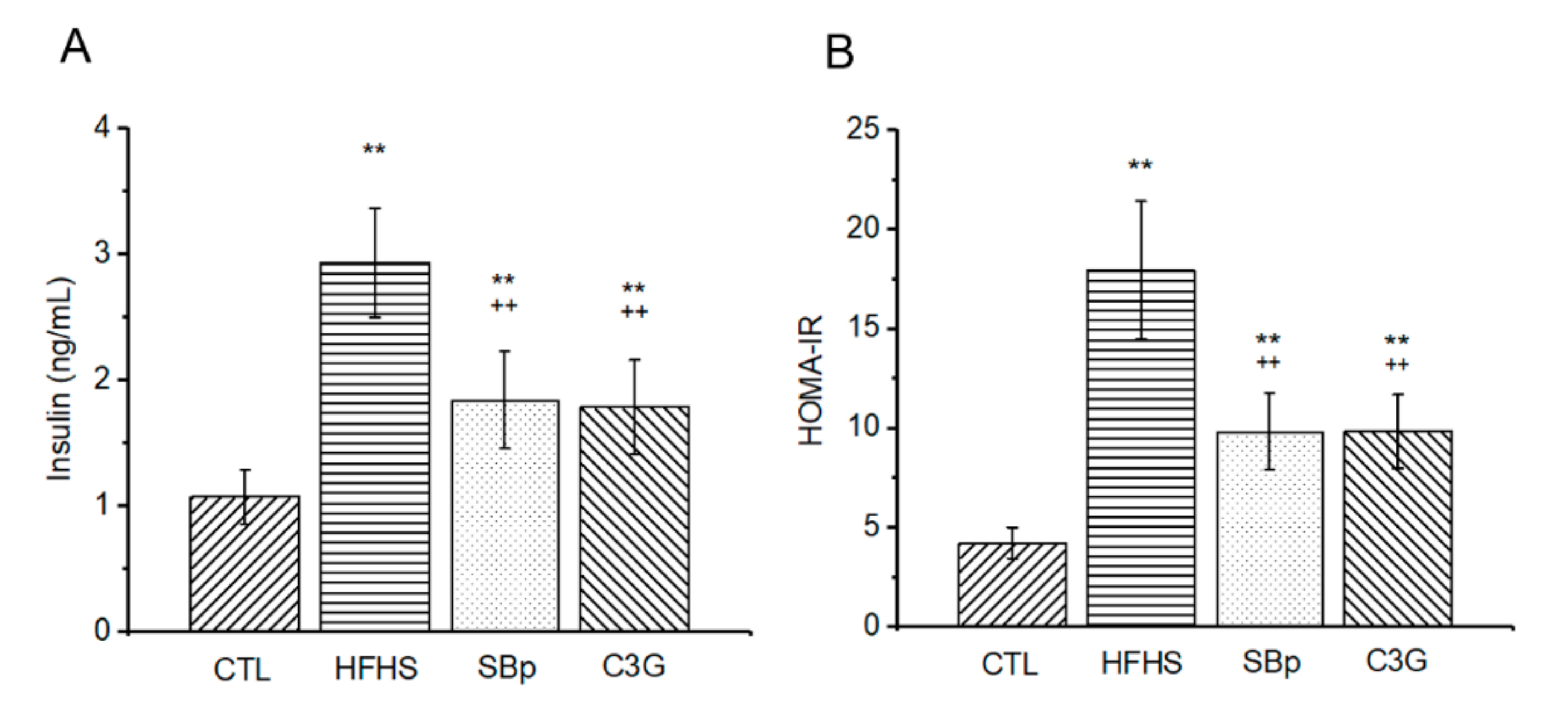
3.3. Effects of C3G on Fasting Plasma Insulin and Insulin Resistance in Mice Receiving the HFHS Diet
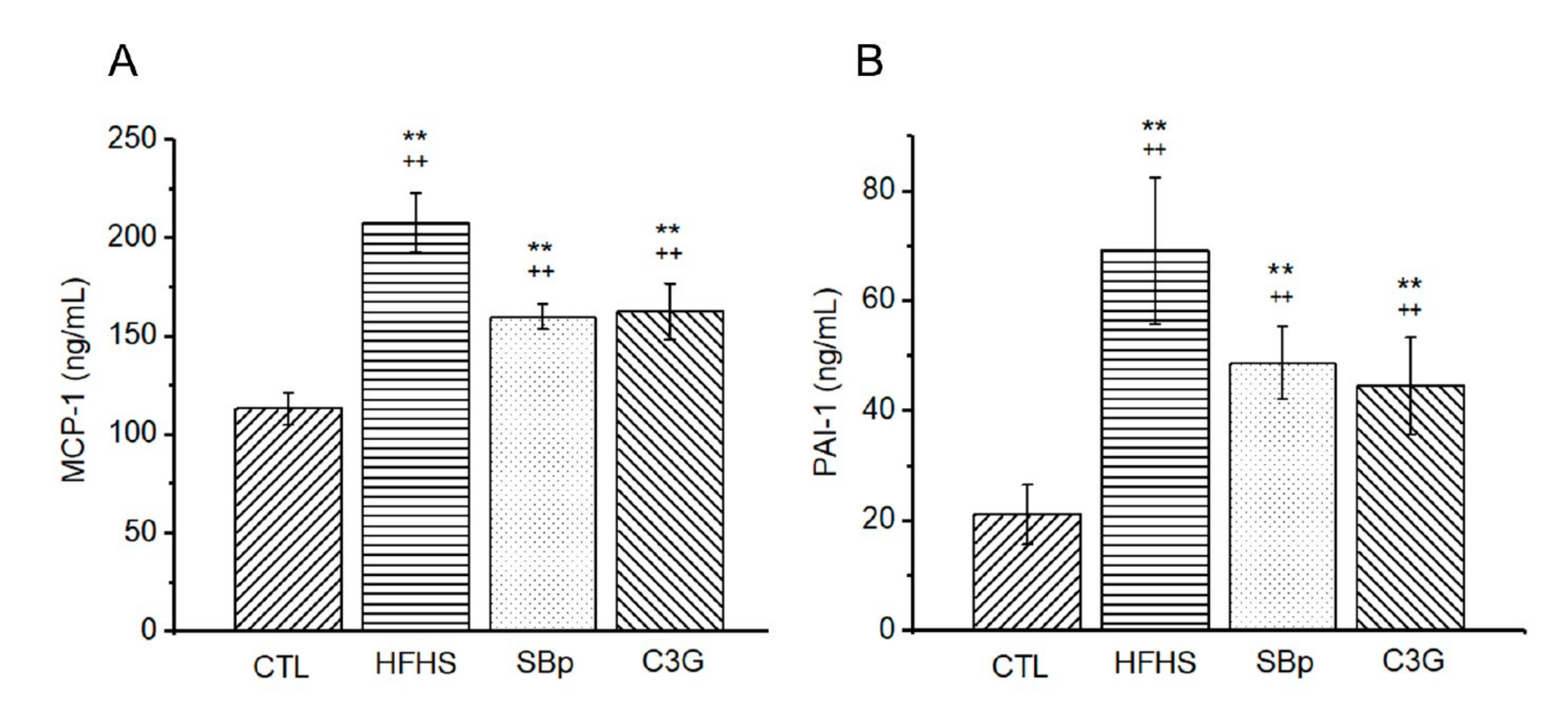
3.4. Effects of HFHS Diet and the Supplementation of C3G on Circulating Inflammatory Markers in Mice
3.5. Impact of C3G Supplementation on Gut Microbiota in HFHS Diet-Fed Mice
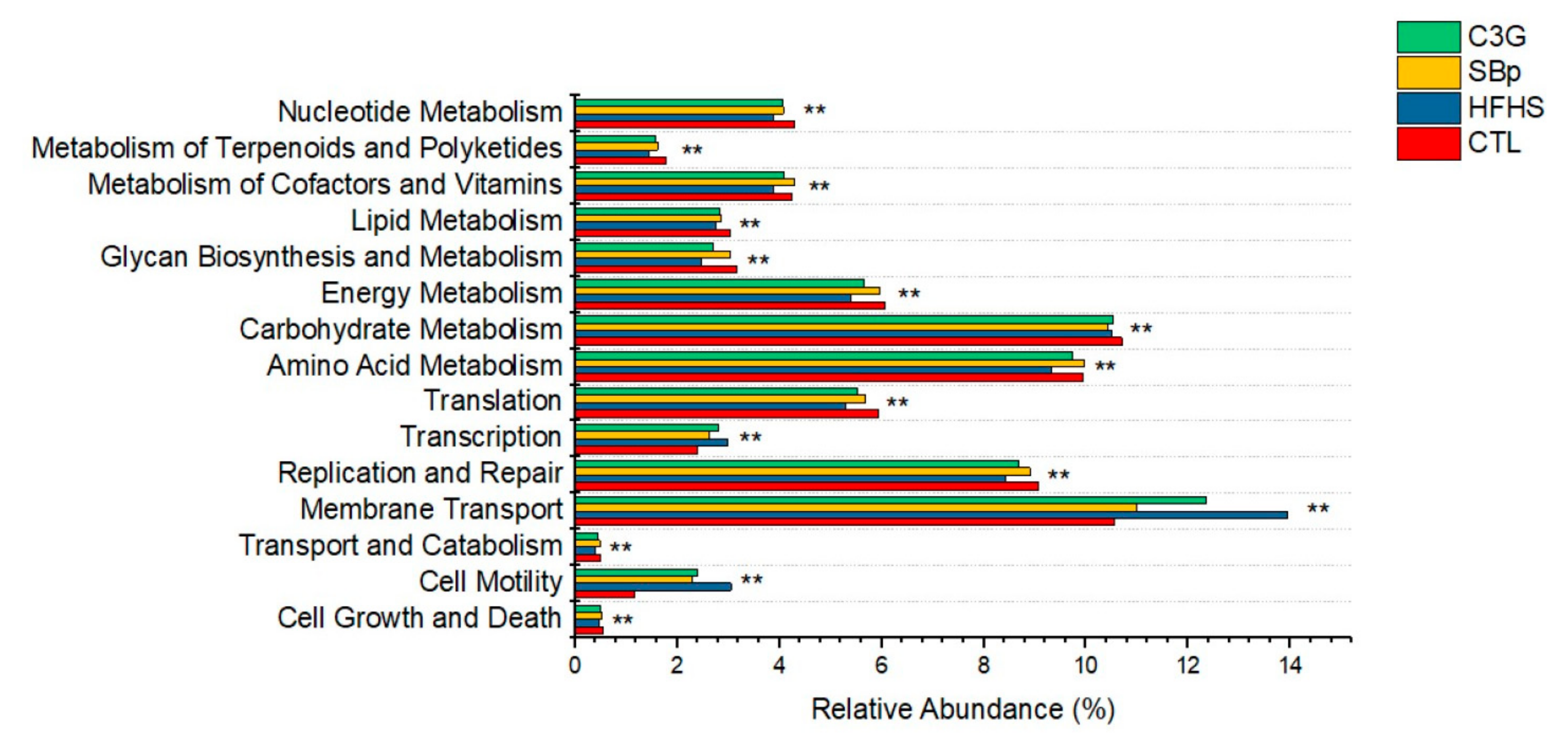
3.6. Functional Predication of the Changes in Gut Microbial Genes Induced by the HFHS Diet with and without C3G Supplementation
4. Discussion
5. Conclusions
Declarations
Author's Information
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ANOVA | analysis of variance |
| ASVs | amplicon sequence variants |
| B/F | Bacteroidetes/Firmicutes |
| C3G | cyanidin-3-glucoside |
| db/db | leptin receptor knockout (mice) |
| ELISA | enzyme-linked immunosorbent assay |
| FPG | fasting plasma glucose |
| HFHS | high fat-high sucrose |
| HOMA-IR | homeostatic model assessment of insulin resistance |
| MCP-1 | monocyte chemotactic protein-1 |
| PAI-1 | plasminogen activator inhibitor-1 |
| PCR | polymerase chain reaction |
| PICRUSt | Phylogenetic Investigation of Communities by Reconstruction of Unobserved States |
| QIIME2 | Quantitative Insights Into Microbial Ecology 2 |
| rRNA | ribosomal ribonucleic acid |
| SBp | Saskatoon berry powder |
| T2D | type 2 diabetes |
References
- Henning, R.J. Type-2 diabetes mellitus and cardiovascular disease. Future Cardiol. 2018, 14, 491–509. [Google Scholar] [CrossRef] [PubMed]
- Skyler, J.S.; Bakris, G.L.; Bonifacio, E.; Darsow, T.; Eckel, R.H.; Groop, L.; Groop, P.-H.; Handelsman, Y.; Insel, R.A.; Mathieu, C. Differentiation of diabetes by pathophysiology, natural history, and prognosis. Diabetes 2017, 66, 241–255. [Google Scholar] [CrossRef] [PubMed]
- Bradley, B.H.R. Dietary fat and risk for type 2 diabetes: A review of recent research. Cur. Nutr. Rep. 2018, 7, 214–226. [Google Scholar] [CrossRef] [PubMed]
- Kopp, W. How western diet and lifestyle drive the pandemic of obesity and civilization diseases. Diab. Met. Syn. Obes. Targets Ther. 2019, 12, 2221. [Google Scholar] [CrossRef] [PubMed]
- Sonnenburg, J.L.; Bäckhed, F. Diet–microbiota interactions as moderators of human metabolism. Nature 2016, 535, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Wen, L.; Duffy, A. Factors influencing the gut microbiota, inflammation, and type 2 diabetes. J. Nutr. 2017, 147, 1468S–1475S. [Google Scholar] [CrossRef]
- Mazza, G. Compositional and functional properties of saskatoon berry and blueberry. Int. J. Fruit. Sci. 2005, 5, 101–120. [Google Scholar] [CrossRef]
- Mazza, G. Anthocyanins and Other Phenolic Compounds of Saskatoon Berries Amelanchier alnifolia Nutt. J. Food Sci. 1986, 51, 1260–1264. [Google Scholar] [CrossRef]
- Gould, K.; Smreciu, A.; Wood, S. Amelanchier Alnifolia: Saskatoon, Service Berry, June Berry, Shadbush, Shadblow, Indian Pear, May Cherry. Available online: https://era.library.ualberta.ca/items/d3680caa-0ad3-43cc-abe4-cf398296ae9b (accessed on 13 August 2020).
- Hosseinian, F.S.; Beta, T. Saskatoon and wild blueberries have higher anthocyanin contents than other Manitoba berries. J. Agric. Food Chem. 2007, 55, 10832–10838. [Google Scholar] [CrossRef]
- Zhao, R.; Le, K.; Li, W.; Ren, S.; Moghadasian, M.H.; Beta, T.; Shen, G.X. Effects of Saskatoon berry powder on monocyte adhesion to vascular wall of leptin receptor-deficient diabetic mice. J. Nutr. Biochem. 2014, 25, 851–857. [Google Scholar] [CrossRef]
- De Souza, D.R.; Willems, J.L.; Low, N.H. Phenolic composition and antioxidant activities of saskatoon berry fruit and pomace. Food Chem. 2019, 290, 168–177. [Google Scholar] [CrossRef] [PubMed]
- Lachowicz, S.; Oszmiański, J.; Seliga, Ł.; Pluta, S. Phytochemical composition and antioxidant capacity of seven saskatoon berry (Amelanchier alnifolia Nutt.) Genotypes Grown in Poland. Molecules 2017, 22, 853. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Xie, X.; Le, K.; Li, W.; Moghadasian, M.H.; Beta, T.; Shen, G.X. Endoplasmic reticulum stress in diabetic mouse or glycated LDL-treated endothelial cells: Protective effect of Saskatoon berry powder and cyanidin glycans. J. Nutr. Biochem. 2015, 26, 1248–1253. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Khafipour, E.; Sepehri, S.; Huang, F.; Beta, T.; Shen, G.X. Impact of Saskatoon berry powder on insulin resistance and relationship with intestinal microbiota in high fat–high sucrose diet-induced obese mice. J. Nutr. Biochem. 2019, 69, 130–138. [Google Scholar] [CrossRef]
- Miranda, C.L.; Johnson, L.A.; De Montgolfier, O.; Elias, V.D.; Ullrich, L.S.; Hay, J.J.; Paraiso, I.L.; Choi, J.; Reed, R.L.; Revel, J.S. Non-estrogenic xanthohumol derivatives mitigate insulin resistance and cognitive impairment in high-fat diet-induced obese mice. Sci. Rep. 2018, 8, 1–17. [Google Scholar] [CrossRef]
- Walters, W.; Hyde, E.R.; Berg-Lyons, D.; Ackermann, G.; Humphrey, G.; Parada, A.; Gilbert, J.A.; Jansson, J.K.; Caporaso, J.G.; Fuhrman, J.A. Improved bacterial 16S rRNA gene (V4 and V4–5) and fungal internal transcribed spacer marker gene primers for microbial community surveys. Msystems 2016, 1, e00009–e00015. [Google Scholar] [CrossRef]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotech. 2019, 37, 852–857. [Google Scholar] [CrossRef]
- Langille, M.G.; Zaneveld, J.; Caporaso, J.G.; McDonald, D.; Knights, D.; Reyes, J.A.; Clemente, J.C.; Burkepile, D.E.; Thurber, R.L.V.; Knight, R. Predictive functional profiling of microbial communities using 16S rRNA marker gene sequences. Nat. Biotech. 2013, 31, 814. [Google Scholar] [CrossRef]
- Yamashita, Y.; Wang, L.; Nanba, F.; Ito, C.; Toda, T.; Ashida, H. Procyanidin promotes translocation of glucose transporter 4 in muscle of mice through activation of insulin and AMPK signaling pathways. PLoS ONE 2016, 11, e0161704. [Google Scholar] [CrossRef]
- Luna-Vital, D.A.; de Mejia, E.G. Anthocyanins from purple corn activate free fatty acid-receptor 1 and glucokinase enhancing in vitro insulin secretion and hepatic glucose uptake. PLoS ONE 2018, 13, e0200449. [Google Scholar] [CrossRef]
- Du, C.; Shi, Y.; Ren, Y.; Wu, H.; Yao, F.; Wei, J.; Wu, M.; Hou, Y.; Duan, H. Anthocyanins inhibit high-glucose-induced cholesterol accumulation and inflammation by activating LXRα pathway in HK-2 cells. Drug Des. Dev. Ther. 2015, 9, 5099. [Google Scholar]
- Sun, C.-D.; Zhang, B.; Zhang, J.-K.; Xu, C.-J.; Wu, Y.-L.; Li, X.; Chen, K.-S. Cyanidin-3-glucoside-rich extract from Chinese bayberry fruit protects pancreatic β cells and ameliorates hyperglycemia in streptozotocin-induced diabetic mice. J. Med. Food 2012, 15, 288–298. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Zhao, R.; Shen, G.X. Influence of delphinidin-3-glucoside on oxidized low-density lipoprotein-induced oxidative stress and apoptosis in cultured endothelial cells. J. Agric. Food Chem. 2012, 60, 1850–1856. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Wang, G.; Zhu, C.; Jiang, X.; Sun, J.; Tian, L.; Bai, W. Effects of cyanidin-3-O-glucoside on 3-chloro-1, 2-propanediol induced intestinal microbiota dysbiosis in rats. Food Chem. Toxicol. 2019, 133, 110767. [Google Scholar] [CrossRef]
- Pradipta, I.; Kong, D.; Tan, J.B.L. Natural organic antioxidants from green tea form a protective layer to inhibit corrosion of steel reinforcing bars embedded in mortar. Constr. Build. Mater. 2019, 221, 351–362. [Google Scholar] [CrossRef]
- Chen, Y.; Li, Q.; Zhao, T.; Zhang, Z.; Mao, G.; Feng, W.; Wu, X.; Yang, L. Biotransformation and metabolism of three mulberry anthocyanin monomers by rat gut microflora. Food Chem. 2017, 237, 887–894. [Google Scholar] [CrossRef]
- Lagkouvardos, I.; Lesker, T.R.; Hitch, T.C.A.; Gálvez, E.J.C.; Smit, N.; Neuhaus, K.; Wang, J.; Baines, J.F.; Abt, B.; Stecher, B.; et al. Sequence and cultivation study of Muribaculaceae reveals novel species, host preference, and functional potential of this yet undescribed family. Microbiome 2019, 7, 28. [Google Scholar] [CrossRef]
- Volk, J.K.; Nyström, E.E.; van der Post, S.; Abad, B.M.; Schroeder, B.O.; Johansson, Å.; Svensson, F.; Jäverfelt, S.; Johansson, M.E.; Hansson, G.C. The Nlrp6 inflammasome is not required for baseline colonic inner mucus layer formation or function. J. Exp. Med. 2019, 216, 2602–2618. [Google Scholar] [CrossRef]
- Smith, B.J.; Miller, R.A.; Ericsson, A.C.; Harrison, D.C.; Strong, R.; Schmidt, T.M. Changes in the gut microbiome and fermentation products concurrent with enhanced longevity in acarbose-treated mice. BMC Microbiol. 2019, 19, 130. [Google Scholar] [CrossRef]
- Zhao, L.; Zhang, F.; Ding, X.; Wu, G.; Lam, Y.Y.; Wang, X.; Fu, H.; Xue, X.; Lu, C.; Ma, J.; et al. Gut bacteria selectively promoted by dietary fibers alleviate type 2 diabetes. Science 2018, 359, 1151–1156. [Google Scholar] [CrossRef]
- Zhang, L.; Xie, C.; Nichols, R.G.; Chan, S.H.; Jiang, C.; Hao, R.; Smith, P.B.; Cai, J.; Simons, M.N.; Hatzakis, E. Farnesoid X receptor signaling shapes the gut microbiota and controls hepatic lipid metabolism. MSystems 2016, 1, e00016–e00070. [Google Scholar] [CrossRef] [PubMed]
- Petersen, C.; Wankhade, U.D.; Bharat, D.; Wong, K.; Mueller, J.E.; Chintapalli, S.V.; Piccolo, B.D.; Jalili, T.; Jia, Z.; Symons, J.D. Dietary supplementation with strawberry induces marked changes in the composition and functional potential of the gut microbiome in diabetic mice. J. Nutr. Biochem. 2019, 66, 63–69. [Google Scholar] [CrossRef] [PubMed]
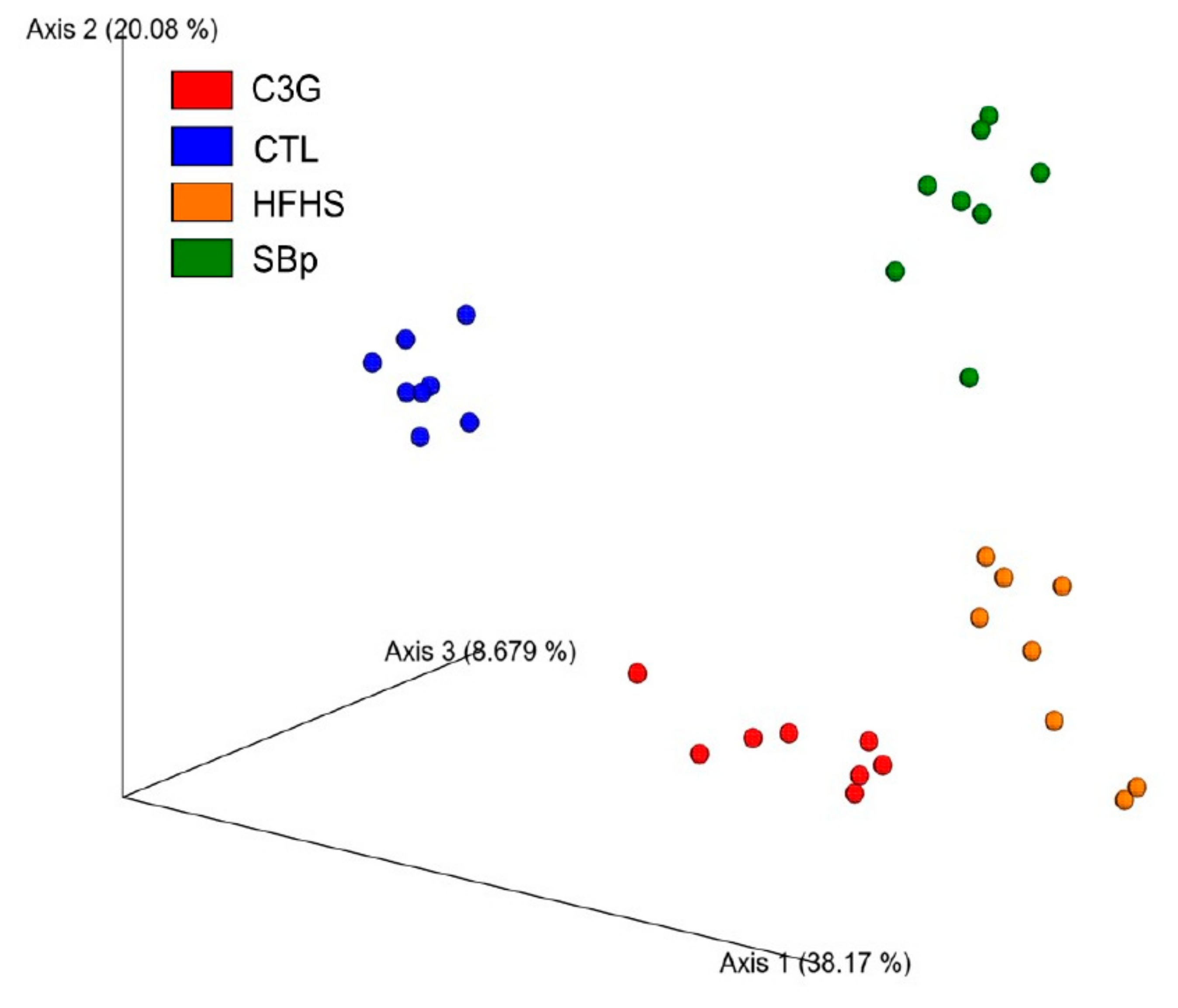
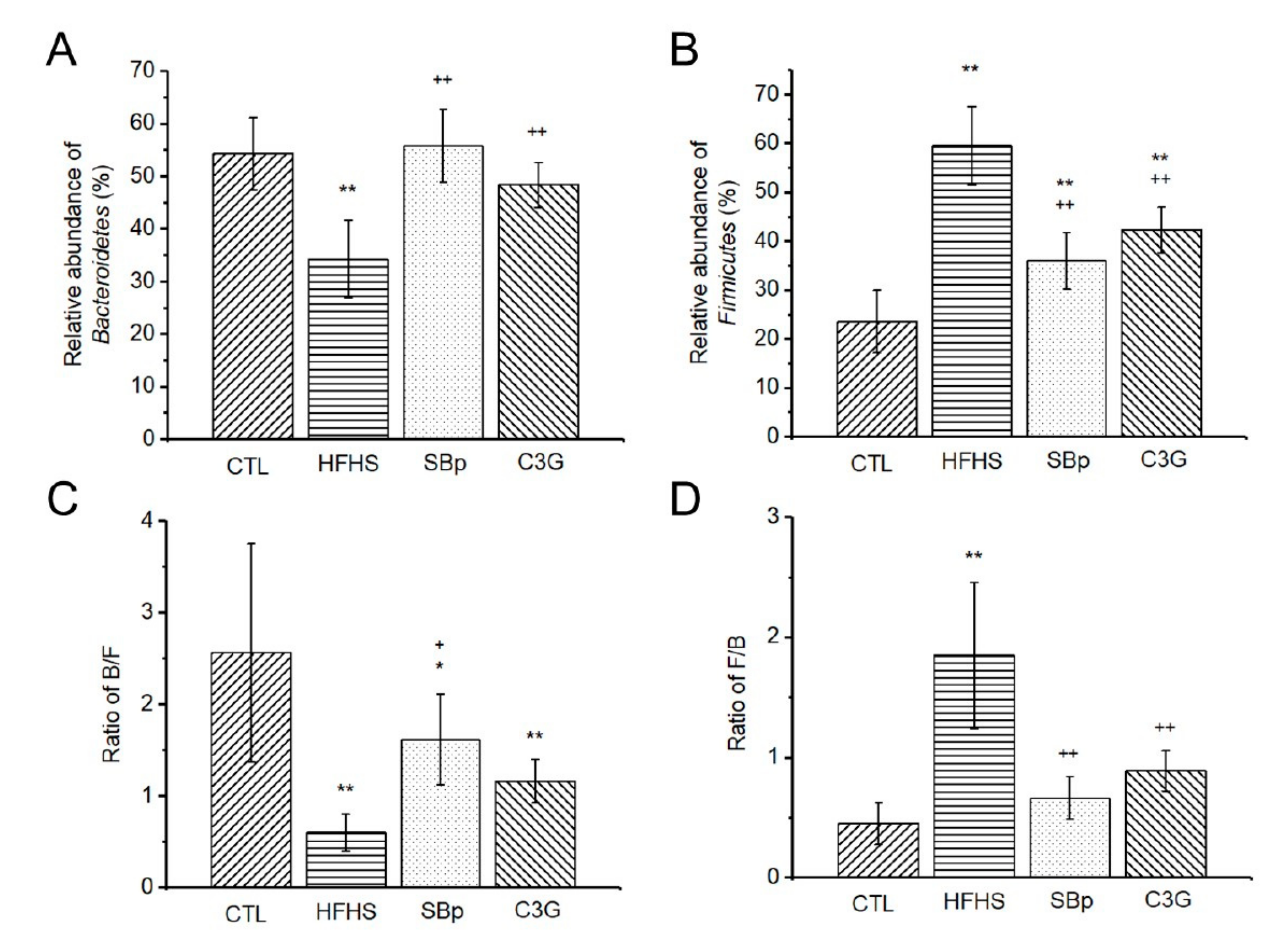
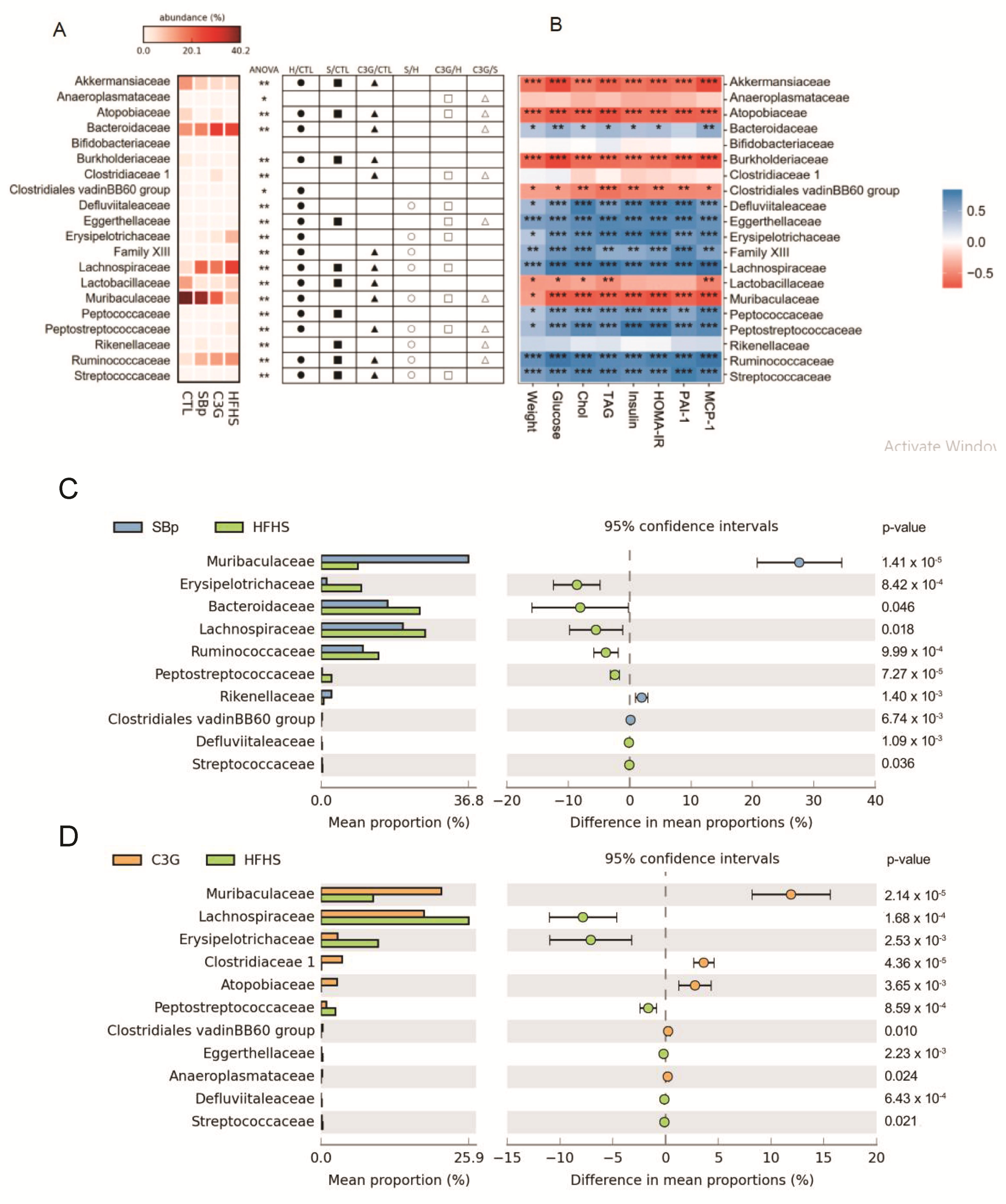
| Phylum Bacteria | CTL (%) | HFHS (%) | SBp (%) | C3G (%) |
|---|---|---|---|---|
| Actinobacteria | 5.66 ± 2.75 | 0.26 ± 0.11 ** | 0.24 ± 0.15** | 2.85 ± 1.83 *,+,^ |
| Bacteroidetes | 54.28 ± 6.91 | 34.25 ± 7.36 ** | 55.80 ± 6.95 ++ | 48.36 ± 4.31 ++ |
| Firmicutes | 23.59 ± 6.41 | 59.53 ± 8.00 ** | 36.01 ± 5.73 **,++ | 42.34 ± 4.63 **,++ |
| Proteobacteria | 2.36 ± 0.71 | 0.68 ± 0.20 ** | 0.81 ± 0.21 ** | 0.63 ± 0.26 ** |
| Tenericutes | 0.21 ± 0.23 | 0.20 ± 0.14 | 0.24 ± 0.16 | 0.46 ± 0.34 |
| Verrucomicrobia | 13.88 ± 3.81 | 4.96 ± 3.23 ** | 6.78 ± 2.27 ** | 5.29 ± 1.81 ** |
| Others | 0.02 ± 0.02 | 0.12 ± 0.09 * | 0.11 ± 0.09 | 0.08 ± 0.03 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, F.; Zhao, R.; Xia, M.; Shen, G.X. Impact of Cyanidin-3-Glucoside on Gut Microbiota and Relationship with Metabolism and Inflammation in High Fat-High Sucrose Diet-Induced Insulin Resistant Mice. Microorganisms 2020, 8, 1238. https://doi.org/10.3390/microorganisms8081238
Huang F, Zhao R, Xia M, Shen GX. Impact of Cyanidin-3-Glucoside on Gut Microbiota and Relationship with Metabolism and Inflammation in High Fat-High Sucrose Diet-Induced Insulin Resistant Mice. Microorganisms. 2020; 8(8):1238. https://doi.org/10.3390/microorganisms8081238
Chicago/Turabian StyleHuang, Fei, Ruozhi Zhao, Min Xia, and Garry X. Shen. 2020. "Impact of Cyanidin-3-Glucoside on Gut Microbiota and Relationship with Metabolism and Inflammation in High Fat-High Sucrose Diet-Induced Insulin Resistant Mice" Microorganisms 8, no. 8: 1238. https://doi.org/10.3390/microorganisms8081238
APA StyleHuang, F., Zhao, R., Xia, M., & Shen, G. X. (2020). Impact of Cyanidin-3-Glucoside on Gut Microbiota and Relationship with Metabolism and Inflammation in High Fat-High Sucrose Diet-Induced Insulin Resistant Mice. Microorganisms, 8(8), 1238. https://doi.org/10.3390/microorganisms8081238





