Cross-Sectional Analysis of the Association between Periodontitis and Cardiovascular Disease Using the Korean Genome and Epidemiology Study Data
Abstract
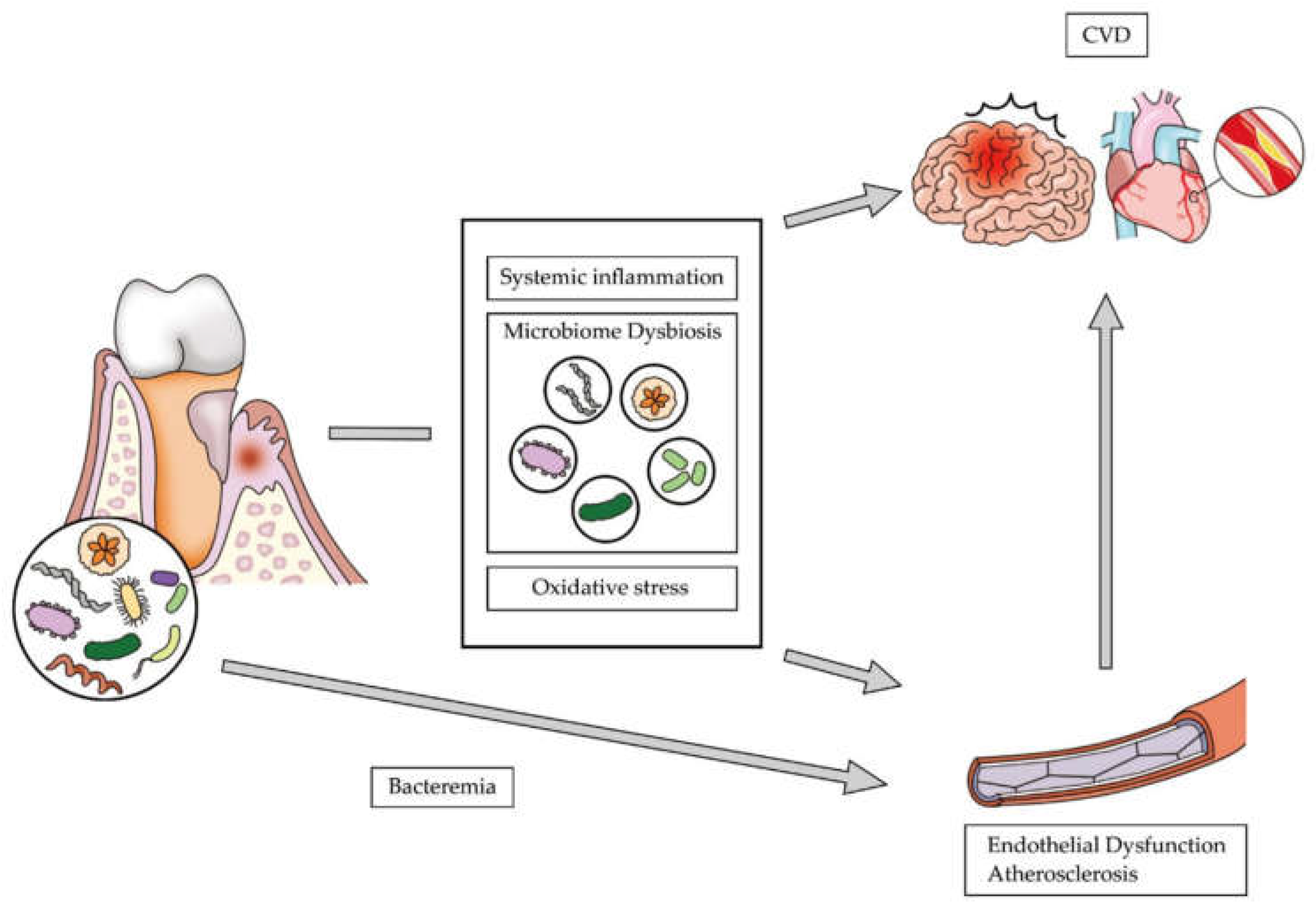
1. Introduction
2. Materials and Methods
2.1. Study Population and Data Collection
2.2. Participant Selection
2.3. Survey
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lloyd-Price, J.; Mahurkar, A.; Rahnavard, G.; Crabtree, J.; Orvis, J.; Hall, A.B.; Brady, A.; Creasy, H.H.; McCracken, C.; Giglio, M.G.; et al. Strains, functions and dynamics in the expanded Human Microbiome Project. Nature 2017, 550, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.K.; Mazmanian, S.K. Has the microbiota played a critical role in the evolution of the adaptive immune system? Science 2010, 330, 1768–1773. [Google Scholar] [CrossRef] [PubMed]
- Ramakrishnan, V.R.; Hauser, L.J.; Frank, D.N. The sinonasal bacterial microbiome in health and disease. Curr. Opin. Otolaryngol. Head Neck Surg. 2016, 24, 20–25. [Google Scholar] [CrossRef] [PubMed]
- Brown, E.M.; Sadarangani, M.; Finlay, B.B. The role of the immune system in governing host-microbe interactions in the intestine. Nat. Immunol. 2013, 14, 660–667. [Google Scholar] [CrossRef] [PubMed]
- Ott, S.J.; El Mokhtari, N.E.; Musfeldt, M.; Hellmig, S.; Freitag, S.; Rehman, A.; Kuhbacher, T.; Nikolaus, S.; Namsolleck, P.; Blaut, M.; et al. Detection of diverse bacterial signatures in atherosclerotic lesions of patients with coronary heart disease. Circulation 2006, 113, 929–937. [Google Scholar] [CrossRef] [PubMed]
- Chung, K.F. Airway microbial dysbiosis in asthmatic patients: A target for prevention and treatment? J. Allergy Clin. Immunol 2017, 139, 1071–1081. [Google Scholar] [CrossRef]
- Halfvarson, J.; Brislawn, C.J.; Lamendella, R.; Vazquez-Baeza, Y.; Walters, W.A.; Bramer, L.M.; D′Amato, M.; Bonfiglio, F.; McDonald, D.; Gonzalez, A.; et al. Dynamics of the human gut microbiome in inflammatory bowel disease. Nat. Microbiol. 2017, 2, 17004. [Google Scholar] [CrossRef]
- Roth, G.A.; Johnson, C.; Abajobir, A.; Abd-Allah, F.; Abera, S.F.; Abyu, G.; Ahmed, M.; Aksut, B.; Alam, T.; Alam, K.; et al. Global, Regional, and National Burden of Cardiovascular Diseases for 10 Causes, 1990 to 2015. J. Am. Coll. Cardiol. 2017, 70, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Timmis, A.; Townsend, N.; Gale, C.; Grobbee, R.; Maniadakis, N.; Flather, M.; Wilkins, E.; Wright, L.; Vos, R.; Bax, J.; et al. European Society of Cardiology: Cardiovascular Disease Statistics 2017. Eur. Heart J. 2018, 39, 508–579. [Google Scholar] [CrossRef]
- Scott, J. Pathophysiology and biochemistry of cardiovascular disease. Curr. Opin. Genet. Dev. 2004, 14, 271–279. [Google Scholar] [CrossRef]
- Mercurio, V.; Lobasso, A.; Barbieri, L.; Parrella, P.; Ciervo, D.; Liccardo, B.; Bonaduce, D.; Tocchetti, C.G.; De Paulis, A.; Rossi, F.W. Inflammatory, Serological and Vascular Determinants of Cardiovascular Disease in Systemic Lupus Erythematosus Patients. Int. J. Mol. Sci. 2019, 20, 2154. [Google Scholar] [CrossRef]
- Ahlehoff, O.; Skov, L.; Gislason, G.; Lindhardsen, J.; Kristensen, S.L.; Iversen, L.; Lasthein, S.; Gniadecki, R.; Dam, T.N.; Torp-Pedersen, C.; et al. Cardiovascular disease event rates in patients with severe psoriasis treated with systemic anti-inflammatory drugs: A Danish real-world cohort study. J. Intern. Med. 2013, 273, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Cairo, F.; Castellani, S.; Gori, A.M.; Nieri, M.; Baldelli, G.; Abbate, R.; Pini-Prato, G.P. Severe periodontitis in young adults is associated with sub-clinical atherosclerosis. J. Clin. Periodontol. 2008, 35, 465–472. [Google Scholar] [CrossRef]
- Byun, S.H.; Min, C.; Park, I.S.; Kim, H.; Kim, S.K.; Park, B.J.; Choi, H.G.; Hong, S.J. Increased Risk of Chronic Periodontitis in Chronic Rhinosinusitis Patients: A Longitudinal Follow-Up Study Using a National Health-Screening Cohort. J. Clin. Med. 2020, 9, 1170. [Google Scholar] [CrossRef] [PubMed]
- Dye, B.A. Global periodontal disease epidemiology. Periodontol 2000 2012, 58, 10–25. [Google Scholar] [CrossRef]
- Kassebaum, N.J.; Smith, A.G.C.; Bernabe, E.; Fleming, T.D.; Reynolds, A.E.; Vos, T.; Murray, C.J.L.; Marcenes, W.; Collaborators, G.B.D.O.H. Global, Regional, and National Prevalence, Incidence, and Disability-Adjusted Life Years for Oral Conditions for 195 Countries, 1990–2015: A Systematic Analysis for the Global Burden of Diseases, Injuries, and Risk Factors. J. Dent. Res. 2017, 96, 380–387. [Google Scholar] [CrossRef] [PubMed]
- Lockhart, P.B.; Bolger, A.F.; Papapanou, P.N.; Osinbowale, O.; Trevisan, M.; Levison, M.E.; Taubert, K.A.; Newburger, J.W.; Gornik, H.L.; Gewitz, M.H.; et al. Periodontal disease and atherosclerotic vascular disease: Does the evidence support an independent association?: A scientific statement from the American Heart Association. Circulation 2012, 125, 2520–2544. [Google Scholar] [CrossRef]
- Vidal, F.; Figueredo, C.M.; Cordovil, I.; Fischer, R.G. Periodontal therapy reduces plasma levels of interleukin-6, C-reactive protein, and fibrinogen in patients with severe periodontitis and refractory arterial hypertension. J. Periodontol. 2009, 80, 786–791. [Google Scholar] [CrossRef]
- Skrzypkowska, M.W.; Ryba-Stanislawowska, M.E.; Slominski, B.; Gutknecht, P.G.; Siebert, J.; Mysliwska, J.M. Association of circulating progenitor cells with angiotensin II in newly diagnosed hypertensive patients. J. Hum. Hypertens 2017, 32, 46–53. [Google Scholar] [CrossRef]
- Hajishengallis, G. Immunomicrobial pathogenesis of periodontitis: Keystones, pathobionts, and host response. Trends Immunol. 2014, 35, 3–11. [Google Scholar] [CrossRef]
- Schulz, S.; Schlitt, A.; Hofmann, B.; Schaller, H.G.; Reichert, S. Periodontal pathogens and their role in cardiovascular outcome. J. Clin. Periodontol. 2020, 47, 173–181. [Google Scholar] [CrossRef]
- Mattila, K.J. Dental infections as a risk factor for acute myocardial infarction. Eur. Heart J. 1993, 14, 51–53. [Google Scholar]
- Mattila, K.J.; Nieminen, M.S.; Valtonen, V.V.; Rasi, V.P.; Kesaniemi, Y.A.; Syrjala, S.L.; Jungell, P.S.; Isoluoma, M.; Hietaniemi, K.; Jokinen, M.J. Association between dental health and acute myocardial infarction. BMJ 1989, 298, 779–781. [Google Scholar] [CrossRef]
- Friedewald, V.E.; Kornman, K.S.; Beck, J.D.; Genco, R.; Goldfine, A.; Libby, P.; Offenbacher, S.; Ridker, P.M.; Van Dyke, T.E.; Roberts, W.C.; et al. The American Journal of Cardiology and Journal of Periodontology Editors’ Consensus: Periodontitis and atherosclerotic cardiovascular disease. Am. J. Cardiol 2009, 104, 59–68. [Google Scholar] [CrossRef]
- Seymour, G.J.; Palmer, J.E.; Leishman, S.J.; Do, H.L.; Westerman, B.; Carle, A.D.; Faddy, M.J.; West, M.J.; Cullinan, M.P. Influence of a triclosan toothpaste on periodontopathic bacteria and periodontitis progression in cardiovascular patients: A randomized controlled trial. J. Periodontal Res. 2017, 52, 61–73. [Google Scholar] [CrossRef]
- Holmlund, A.; Holm, G.; Lind, L. Number of teeth as a predictor of cardiovascular mortality in a cohort of 7,674 subjects followed for 12 years. J. Periodontol. 2010, 81, 870–876. [Google Scholar] [CrossRef]
- Isola, G.; Alibrandi, A.; Curro, M.; Matarese, M.; Ricca, S.; Matarese, G.; Ientile, R.; Kocher, T. Evaluation of salivary and serum ADMA levels in patients with periodontal and cardiovascular disease as subclinical marker of cardiovascular risk. J. Periodontol. 2020. [Google Scholar] [CrossRef]
- Kebschull, M.; Demmer, R.T.; Papapanou, P.N. “Gum bug, leave my heart alone!”—Epidemiologic and mechanistic evidence linking periodontal infections and atherosclerosis. J. Dent. Res. 2010, 89, 879–902. [Google Scholar] [CrossRef]
- Cui, D.; Li, H.; Lei, L.; Chen, C.; Yan, F. Nonsurgical periodontal treatment reduced aortic inflammation in ApoE(-/-) mice with periodontitis. Springerplus 2016, 5, 940. [Google Scholar] [CrossRef]
- Piepoli, M.F.; Hoes, A.W.; Agewall, S.; Albus, C.; Brotons, C.; Catapano, A.L.; Cooney, M.T.; Corra, U.; Cosyns, B.; Deaton, C.; et al. 2016 European guidelines on cardiovascular disease prevention in clinical practice. The Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of 10 societies and by invited experts. Developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation. Cardiovasc. Ital. Cardiol. 2017, 18, 547. [Google Scholar] [CrossRef]
- Kim, Y.; Han, B.G.; Ko, G.E.S.g. Cohort Profile: The Korean Genome and Epidemiology Study (KoGES) Consortium. Int J. Epidemiol. 2017, 46, 1350. [Google Scholar] [CrossRef] [PubMed]
- Byun, S.H.; Min, C.; Hong, S.J.; Choi, H.G.; Koh, D.H. Analysis of the Relation between Periodontitis and Chronic Gastritis/Peptic Ulcer: A Cross-Sectional Study Using KoGES HEXA Data. Int. J. Environ. Res. Public Health 2020, 17, 4387. [Google Scholar] [CrossRef] [PubMed]
- Byun, S.-H.; Yoo, D.M.; Lee, J.W.; Choi, H.G. Analyzing the Association between Hyperuricemia and Periodontitis: A Cross-Sectional Study Using KoGES HEXA Data. Int. J. Environ. Res. Public Health 2020, 17, 4777. [Google Scholar] [CrossRef] [PubMed]
- Ahn, Y.; Kwon, E.; Shim, J.E.; Park, M.K.; Joo, Y.; Kimm, K.; Park, C.; Kim, D.H. Validation and reproducibility of food frequency questionnaire for Korean genome epidemiologic study. Eur. J. Clin. Nutr. 2007, 61, 1435–1441. [Google Scholar] [CrossRef]
- Slocum, C.; Kramer, C.; Genco, C.A. Immune dysregulation mediated by the oral microbiome: Potential link to chronic inflammation and atherosclerosis. J. Intern. Med. 2016, 280, 114–128. [Google Scholar] [CrossRef]
- Kramer, C.D.; Genco, C.A. Microbiota, Immune Subversion, and Chronic Inflammation. Front. Immunol 2017, 8, 255. [Google Scholar] [CrossRef]
- Pietiainen, M.; Liljestrand, J.M.; Kopra, E.; Pussinen, P.J. Mediators between oral dysbiosis and cardiovascular diseases. Eur. J. Oral Sci. 2018, 126, 26–36. [Google Scholar] [CrossRef]
- Tomas, I.; Diz, P.; Tobias, A.; Scully, C.; Donos, N. Periodontal health status and bacteraemia from daily oral activities: Systematic review/meta-analysis. J. Clin. Periodontol. 2012, 39, 213–228. [Google Scholar] [CrossRef]
- Armingohar, Z.; Jorgensen, J.J.; Kristoffersen, A.K.; Abesha-Belay, E.; Olsen, I. Bacteria and bacterial DNA in atherosclerotic plaque and aneurysmal wall biopsies from patients with and without periodontitis. J. Oral Microbiol. 2014, 6. [Google Scholar] [CrossRef]
- Mahendra, J.; Mahendra, L.; Felix, J.; Romanos, G. Prevelance of periodontopathogenic bacteria in subgingival biofilm and atherosclerotic plaques of patients undergoing coronary revascularization surgery. J. Indian Soc. Periodontol. 2013, 17, 719–724. [Google Scholar] [CrossRef]
- Chukkapalli, S.S.; Velsko, I.M.; Rivera-Kweh, M.F.; Zheng, D.; Lucas, A.R.; Kesavalu, L. Polymicrobial Oral Infection with Four Periodontal Bacteria Orchestrates a Distinct Inflammatory Response and Atherosclerosis in ApoE null Mice. PloS ONE 2015, 10, e0143291. [Google Scholar] [CrossRef] [PubMed]
- Velsko, I.M.; Chukkapalli, S.S.; Rivera-Kweh, M.F.; Zheng, D.; Aukhil, I.; Lucas, A.R.; Larjava, H.; Kesavalu, L. Periodontal pathogens invade gingiva and aortic adventitia and elicit inflammasome activation in alphavbeta6 integrin-deficient mice. Infect. Immun. 2015, 83, 4582–4593. [Google Scholar] [CrossRef] [PubMed]
- Reyes, L.; Herrera, D.; Kozarov, E.; Rolda, S.; Progulske-Fox, A. Periodontal bacterial invasion and infection: Contribution to atherosclerotic pathology. J. Periodontol. 2013, 84, S30–S50. [Google Scholar] [CrossRef] [PubMed]
- Belanger, M.; Kozarov, E.; Song, H.; Whitlock, J.; Progulske-Fox, A. Both the unique and repeat regions of the Porphyromonas gingivalis hemagglutin A are involved in adhesion and invasion of host cells. Anaerobe 2012, 18, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Wu, J.; Liu, Y.; Huang, J.; Lu, Z.; Xie, L.; Sun, W.; Ji, Y. Porphyromonas gingivalis infection reduces regulatory T cells in infected atherosclerosis patients. PLoS ONE 2014, 9, e86599. [Google Scholar] [CrossRef] [PubMed]
- Kozarov, E.; Sweier, D.; Shelburne, C.; Progulske-Fox, A.; Lopatin, D. Detection of bacterial DNA in atheromatous plaques by quantitative PCR. Microbes Infect. 2006, 8, 687–693. [Google Scholar] [CrossRef]
- Schenkein, H.A.; Loos, B.G. Inflammatory mechanisms linking periodontal diseases to cardiovascular diseases. J. Periodontol. 2013, 84, S51–S69. [Google Scholar] [CrossRef]
- Mascitti, M.; Togni, L.; Troiano, G.; Caponio, V.C.A.; Gissi, D.B.; Montebugnoli, L.; Procaccini, M.; Lo Muzio, L.; Santarelli, A. Beyond Head and Neck Cancer: The Relationship Between Oral Microbiota and Tumour Development in Distant Organs. Front. Cell Infect. Microbiol. 2019, 9, 232. [Google Scholar] [CrossRef]
- Gao, L.; Xu, T.; Huang, G.; Jiang, S.; Gu, Y.; Chen, F. Oral microbiomes: More and more importance in oral cavity and whole body. Protein Cell 2018, 9, 488–500. [Google Scholar] [CrossRef]
- Chandy, S.; Joseph, K.; Sankaranarayanan, A.; Issac, A.; Babu, G.; Wilson, B.; Joseph, J. Evaluation of C-Reactive Protein and Fibrinogen in Patients with Chronic and Aggressive Periodontitis: A Clinico-Biochemical Study. J. Clin. Diagn. Res. 2017, 11, ZC41–ZC45. [Google Scholar] [CrossRef]
- Lopez, N.J.; Quintero, A.; Casanova, P.A.; Ibieta, C.I.; Baelum, V.; Lopez, R. Effects of periodontal therapy on systemic markers of inflammation in patients with metabolic syndrome: A controlled clinical trial. J. Periodontol. 2012, 83, 267–278. [Google Scholar] [CrossRef] [PubMed]
- Vidal, F.; Cordovil, I.; Figueredo, C.M.; Fischer, R.G. Non-surgical periodontal treatment reduces cardiovascular risk in refractory hypertensive patients: A pilot study. J. Clin. Periodontol. 2013, 40, 681–687. [Google Scholar] [CrossRef]
- Arvanitidis, E.; Bizzarro, S.; Alvarez Rodriguez, E.; Loos, B.G.; Nicu, E.A. Reduced platelet hyper-reactivity and platelet-leukocyte aggregation after periodontal therapy. Thromb. J. 2017, 15, 5. [Google Scholar] [CrossRef]
- He, F.; Zuo, L. Redox Roles of Reactive Oxygen Species in Cardiovascular Diseases. Int. J. Mol. Sci. 2015, 16, 27770–27780. [Google Scholar] [CrossRef] [PubMed]
- Seals, D.R.; Monahan, K.D.; Bell, C.; Tanaka, H.; Jones, P.P. The aging cardiovascular system: Changes in autonomic function at rest and in response to exercise. Int. J. Sport Nutr. Exerc. Metab. 2001, 11 (Suppl 1), S189–S195. [Google Scholar] [CrossRef]
- Bullon, P.; Cordero, M.D.; Quiles, J.L.; Morillo, J.M.; del Carmen Ramirez-Tortosa, M.; Battino, M. Mitochondrial dysfunction promoted by Porphyromonas gingivalis lipopolysaccharide as a possible link between cardiovascular disease and periodontitis. Free Radic. Biol. Med. 2011, 50, 1336–1343. [Google Scholar] [CrossRef] [PubMed]

| Characteristics | Total Participants | p-Value | |
|---|---|---|---|
| Periodontitis | Control | ||
| Age (mean, SD, y) | 54.8 (7.9) | 53.0 (8.3) | <0.001 † |
| Age group (n, %, y) | <0.001 * | ||
| 40–49 | 2650 (26.6) | 46,132 (36.8) | |
| 50–59 | 4332 (43.4) | 48,796 (38.9) | |
| 60–69 | 2772 (27.8) | 28,215 (22.5) | |
| 70–79 | 219 (2.2) | 2161 (1.7) | |
| Sex (n, %) | <0.001 * | ||
| Men | 3851 (38.6) | 43,400 (34.6) | |
| Women | 6122 (61.4) | 81,904 (65.4) | |
| BMI (mean, SD, kg/m2) | 24.0 (2.9) | 23.9 (2.9) | <0.001 † |
| Income (n, %) | <0.001 * | ||
| Missing, no response | 765 (7.7) | 10,849 (8.7) | |
| Lowest | 3437 (34.5) | 35,580 (28.4) | |
| Middle | 3675 (36.8) | 49,421 (39.4) | |
| Highest | 2096 (21.0) | 29,454 (23.5) | |
| Smoking status (n, %) | <0.001 * | ||
| Nonsmoker | 6689 (67.1) | 91,108 (72.7) | |
| Past smoker | 1795 (18.0) | 18,591 (14.8) | |
| Current smoker | 1489 (14.9) | 15,605 (12.5) | |
| Alcohol consumption (n, %) | <0.001 * | ||
| Non-drinker | 4787 (48.0) | 64,024 (51.1) | |
| Past drinker | 479 (4.8) | 4536 (3.6) | |
| Current drinker | 4707 (47.2) | 56,744 (45.3) | |
| Hypertension (n, %) | 2771 (27.8) | 28,068 (22.4) | <0.001 * |
| Diabetes mellitus (n, %) | 1176 (11.8) | 9812 (7.8) | <0.001 * |
| Hyperlipidemia (n, %) | 2204 (22.1) | 17,485 (14.0) | <0.001 * |
| Nutritional intake (mean, SD) | |||
| Total calories (kcal/d) | 1760.2 (580.5) | 1749.5 (569.4) | 0.069 |
| Protein (g/d) | 58.9 (26.6) | 59.8 (26.4) | 0.002 † |
| Fat (g/d) | 27.5 (18.5) | 28.3 (18.2) | <0.001 † |
| Carbohydrate (g/d) | 315.0 (95.2) | 309.8 (92.8) | <0.001 † |
| Stroke (n, %) | 207 (2.1) | 1523 (1.2) | <0.001 * |
| Ischemic heart disease (n, %) | 494 (5.0) | 3756 (3.0) | <0.001 * |
| Characteristics | Odd Ratios for Stroke | |||
|---|---|---|---|---|
| Crude | p-Value | Adjusted † | p-Value | |
| Total participants (n = 135,277) | ||||
| Periodontitis | 1.72 (1.49–2.00) | <0.001 * | 1.35 (1.16–1.57) | <0.001 * |
| Control | 1.00 | 1.00 | ||
| Age 40–49 years old (n = 48,782) | ||||
| Periodontitis | 1.47 (0.85–2.54) | 0.167 | 1.20 (0.69–2.08) | 0.528 |
| Control | 1.00 | 1.00 | ||
| Age 50–59 years old (n = 53,128) | ||||
| Periodontitis | 1.57 (1.23–2.01) | <0.001 * | 1.39 (1.09–1.78) | 0.009 * |
| Control | 1.00 | 1.00 | ||
| Age ≥ 60 years old (n = 33,367) | ||||
| Periodontitis | 1.49 (1.22–1.81) | <0.001 * | 1.34 (1.10–1.64) | 0.004 * |
| Control | 1.00 | 1.00 | ||
| Men (n = 47,251) | ||||
| Periodontitis | 1.53 (1.25–1.88) | <0.001 * | 1.26 (1.02–1.55) | 0.031 * |
| Control | 1.00 | 1.00 | ||
| Women (n = 88,026) | ||||
| Periodontitis | 1.85 (1.50–2.29) | <0.001 * | 1.47 (1.19–1.82) | <0.001 * |
| Control | 1.00 | 1.00 | ||
| Characteristics | Odd Ratios for Ischemic Heart Disease | |||
|---|---|---|---|---|
| Crude | p-Value | Adjusted † | p-Value | |
| Total participants (n = 135,277) | ||||
| Periodontitis | 1.69 (1.53–1.86) | <0.001 * | 1.34 (1.22–1.48) | <0.001 * |
| Control | 1.00 | 1.00 | ||
| Age 40–49 years old (n = 48,782) | ||||
| Periodontitis | 1.87 (1.37–2.56) | <0.001 * | 1.46 (1.06–2.01) | 0.020 * |
| Control | 1.00 | 1.00 | ||
| Age 50–59 years old (n = 53,128) | ||||
| Periodontitis | 1.51 (1.29–1.78) | <0.001 * | 1.34 (1.13–1.57) | 0.001 * |
| Control | 1.00 | 1.00 | ||
| Age ≥ 60 years old (n = 33,367) | ||||
| Periodontitis | 1.42 (1.25–1.62) | <0.001 * | 1.32 (1.15–1.51) | <0.001 * |
| Control | 1.00 | 1.00 | ||
| Men (n = 47,251) | ||||
| Periodontitis | 1.53 (1.34–1.75) | <0.001 * | 1.29 (1.12–1.48) | <0.001 * |
| Control | 1.00 | 1.00 | ||
| Women (n = 88,026) | ||||
| Periodontitis | 1.77 (1.55–2.03) | <0.001 * | 1.41 (1.22–1.62) | <0.001 * |
| Control | 1.00 | 1.00 | ||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Byun, S.H.; Lee, S.; Kang, S.H.; Choi, H.G.; Hong, S.J. Cross-Sectional Analysis of the Association between Periodontitis and Cardiovascular Disease Using the Korean Genome and Epidemiology Study Data. Int. J. Environ. Res. Public Health 2020, 17, 5237. https://doi.org/10.3390/ijerph17145237
Byun SH, Lee S, Kang SH, Choi HG, Hong SJ. Cross-Sectional Analysis of the Association between Periodontitis and Cardiovascular Disease Using the Korean Genome and Epidemiology Study Data. International Journal of Environmental Research and Public Health. 2020; 17(14):5237. https://doi.org/10.3390/ijerph17145237
Chicago/Turabian StyleByun, Soo Hwan, Sunki Lee, Sung Hun Kang, Hyo Geun Choi, and Seok Jin Hong. 2020. "Cross-Sectional Analysis of the Association between Periodontitis and Cardiovascular Disease Using the Korean Genome and Epidemiology Study Data" International Journal of Environmental Research and Public Health 17, no. 14: 5237. https://doi.org/10.3390/ijerph17145237
APA StyleByun, S. H., Lee, S., Kang, S. H., Choi, H. G., & Hong, S. J. (2020). Cross-Sectional Analysis of the Association between Periodontitis and Cardiovascular Disease Using the Korean Genome and Epidemiology Study Data. International Journal of Environmental Research and Public Health, 17(14), 5237. https://doi.org/10.3390/ijerph17145237






