Risk of Adverse Outcomes in Females Taking Oral Creatine Monohydrate: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Methods
2.1. Study Selection
2.2. Search Strategy
2.3. Data Extraction
2.4. Data Analysis and Statistical Methods
2.5. Risk of Bias
3. Results
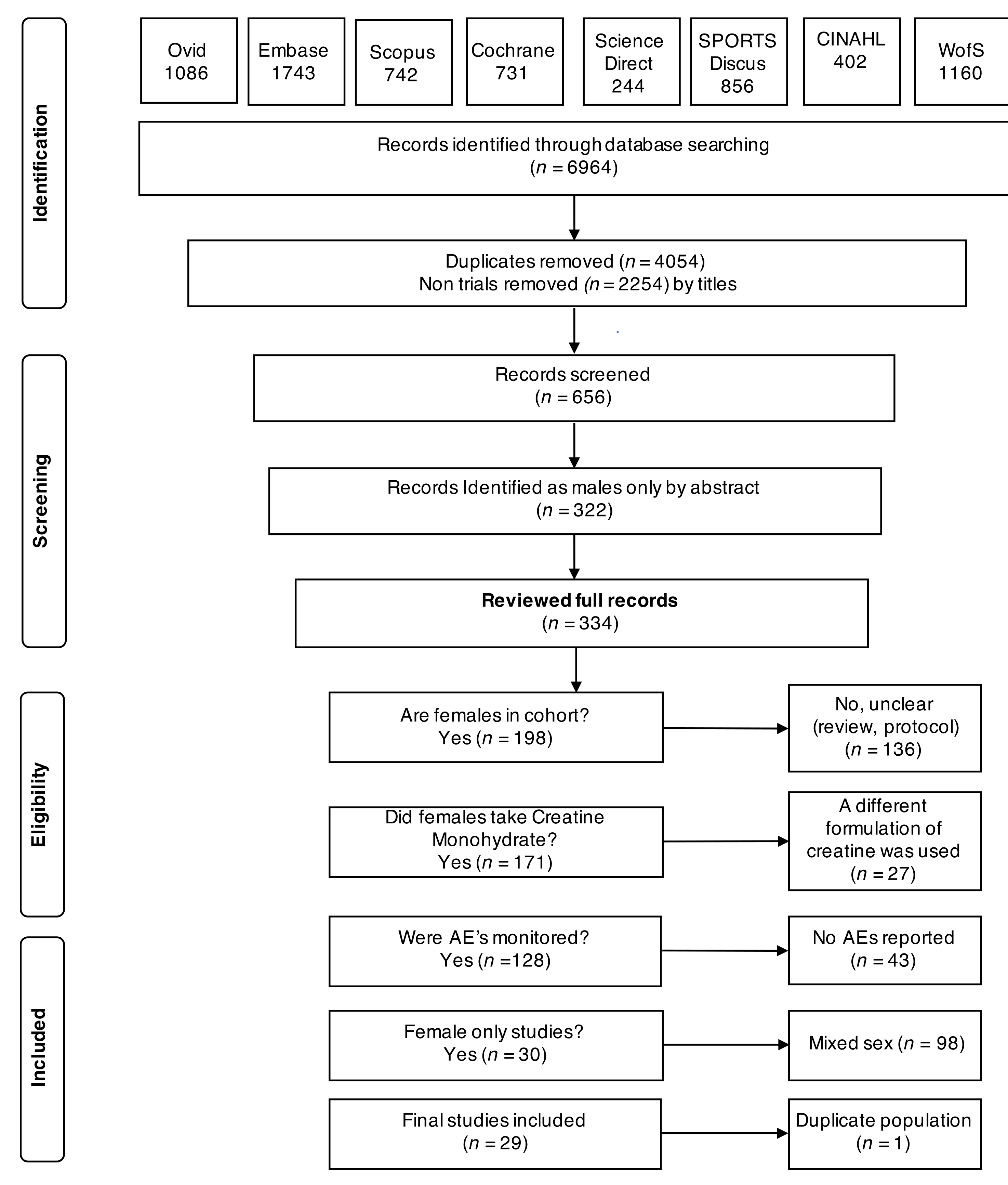
3.1. Search Characteristics
3.2. Study and Participant Characteristics
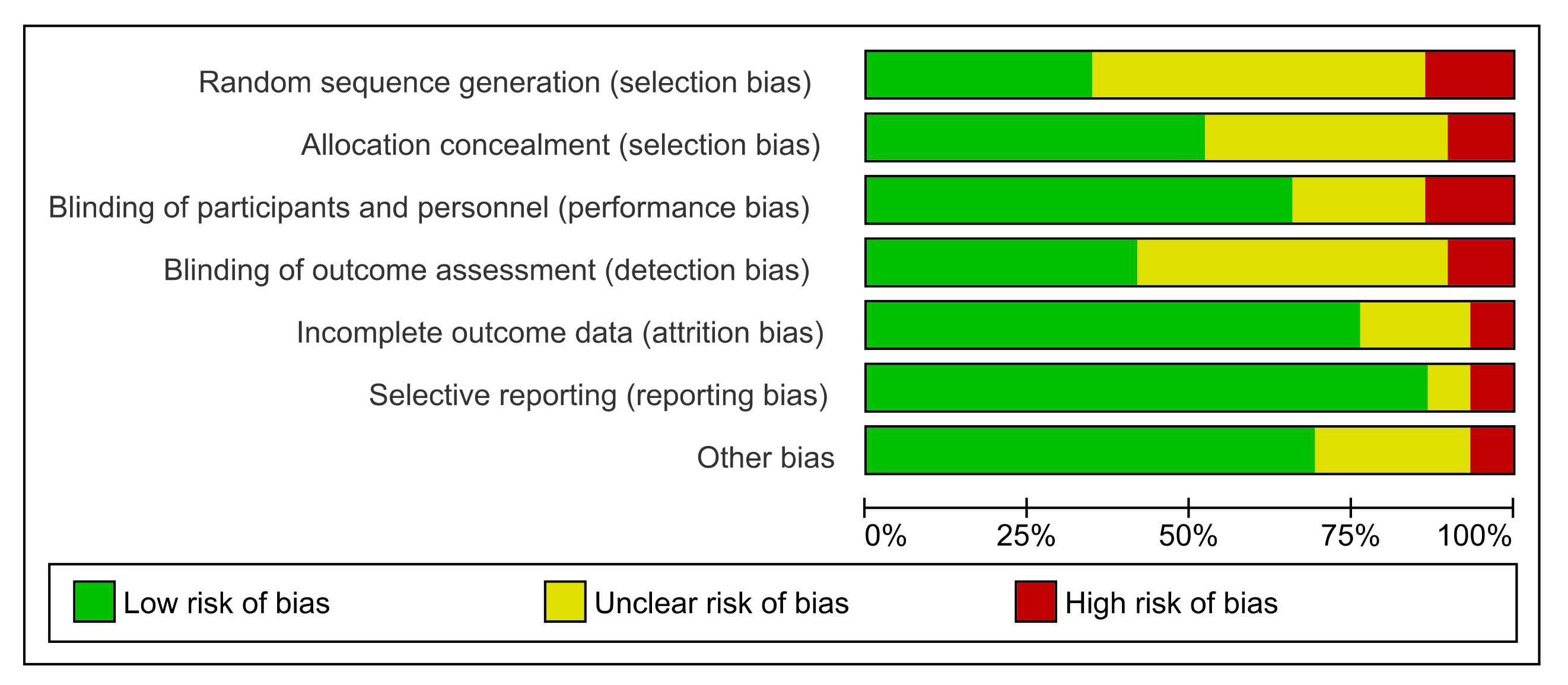
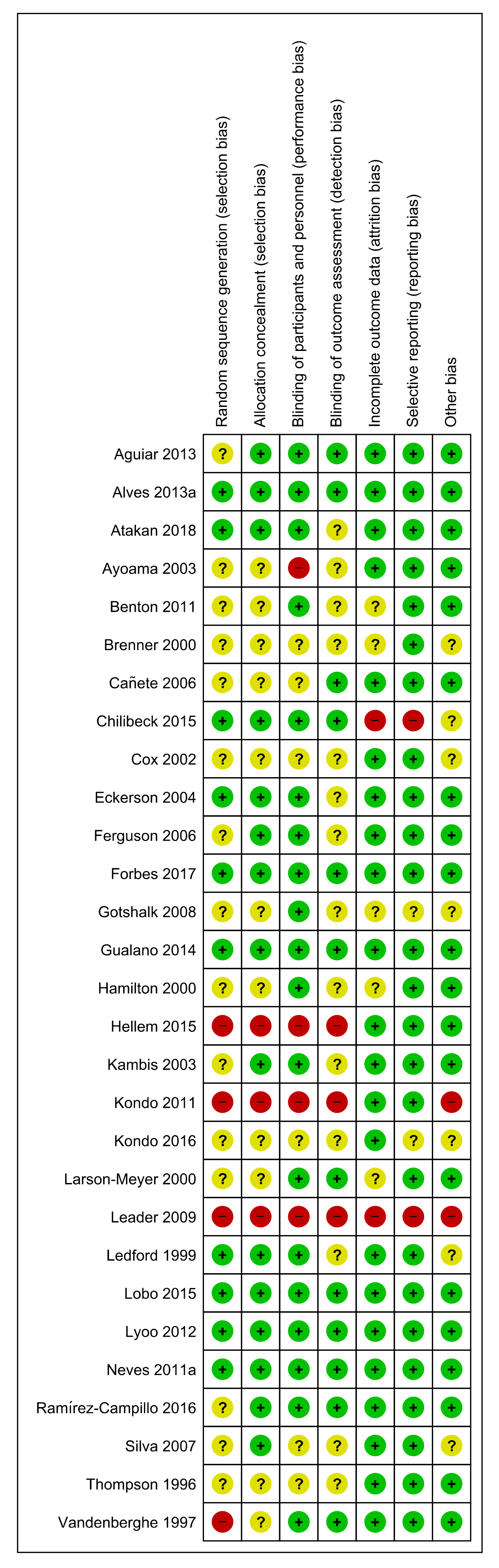
3.3. Risk of Bias
3.4. Deaths
3.5. Adverse Outcomes (Symptoms and Signs)
3.6. GIT Events
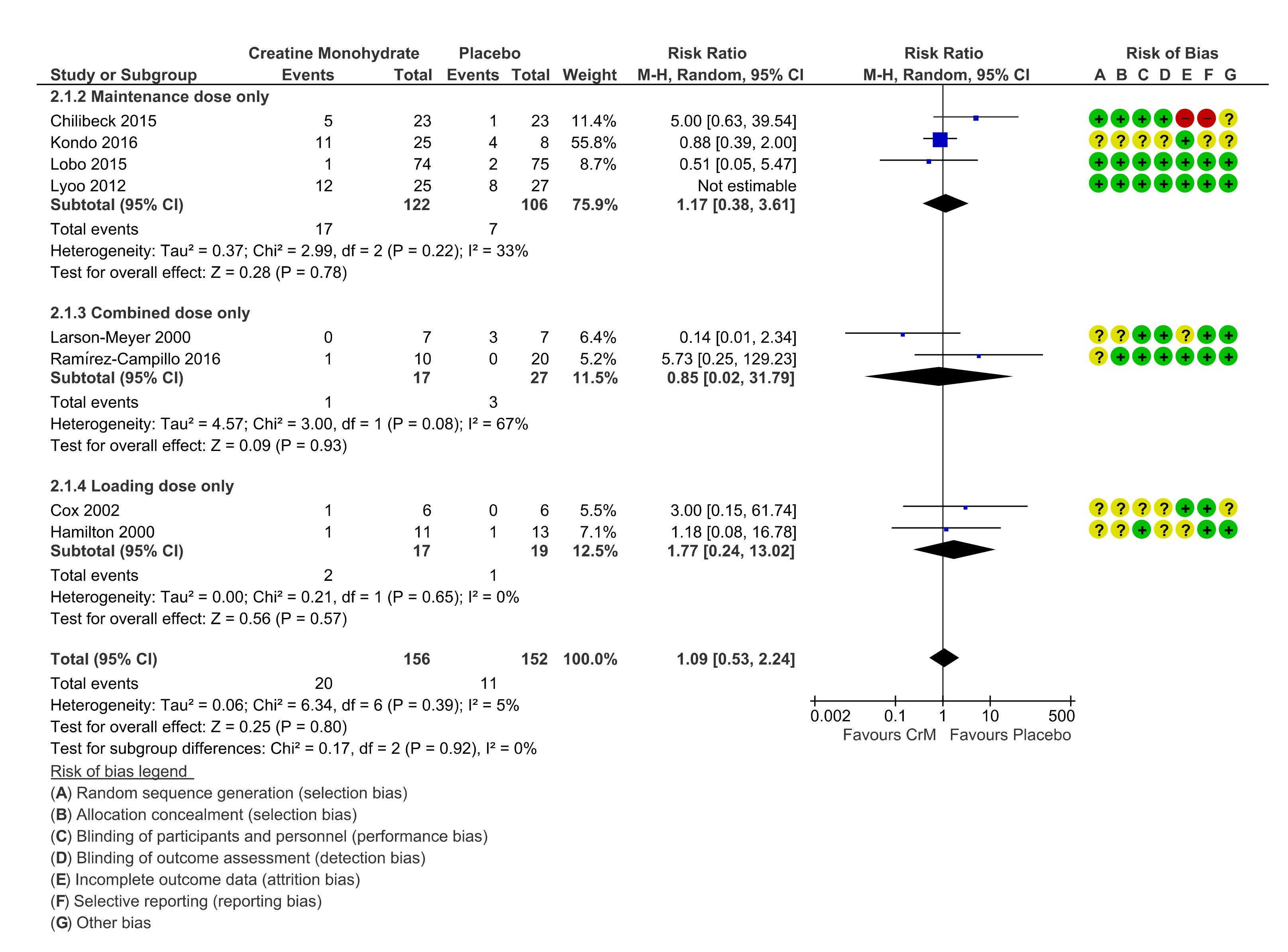
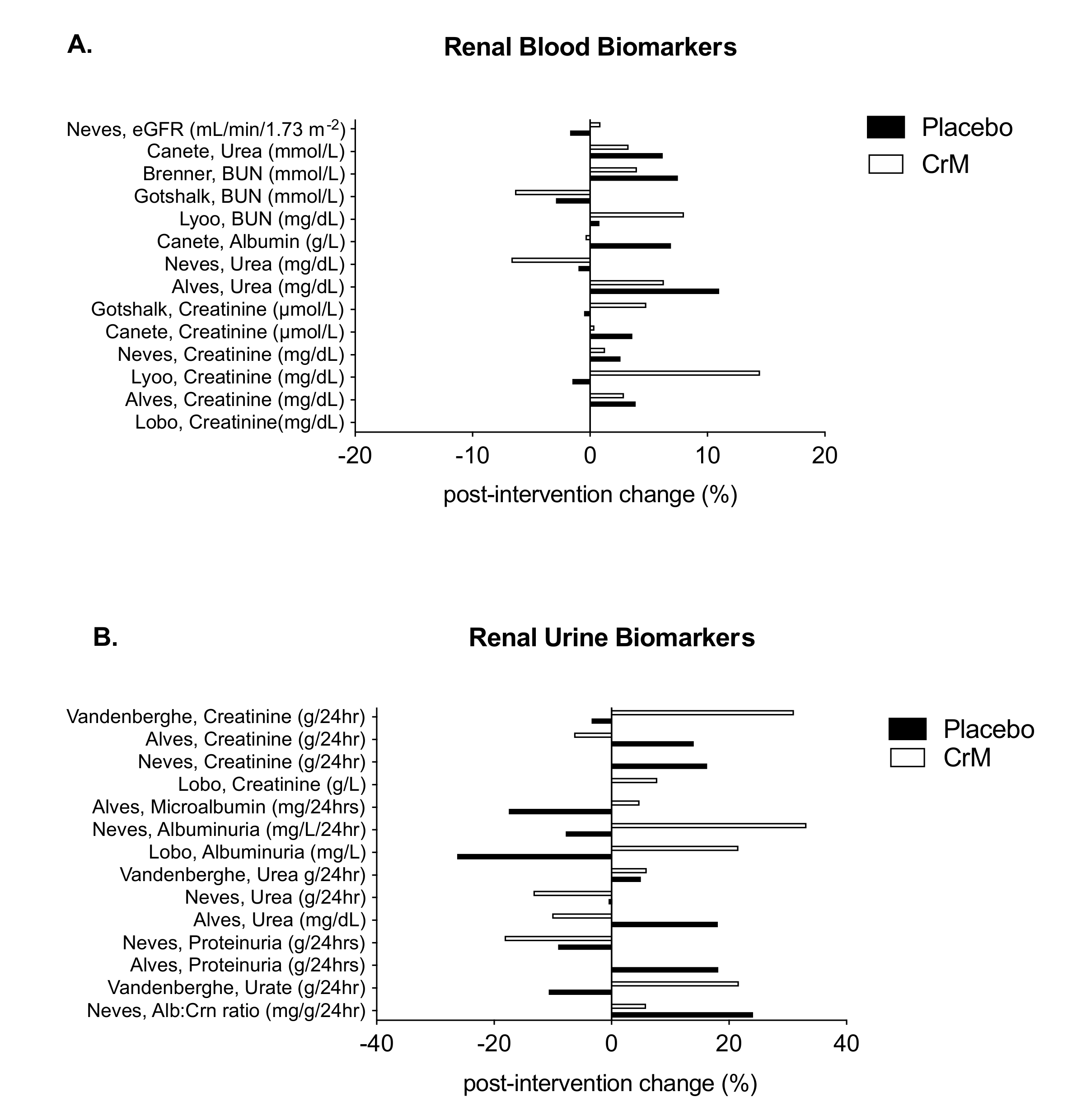
3.7. Renal System
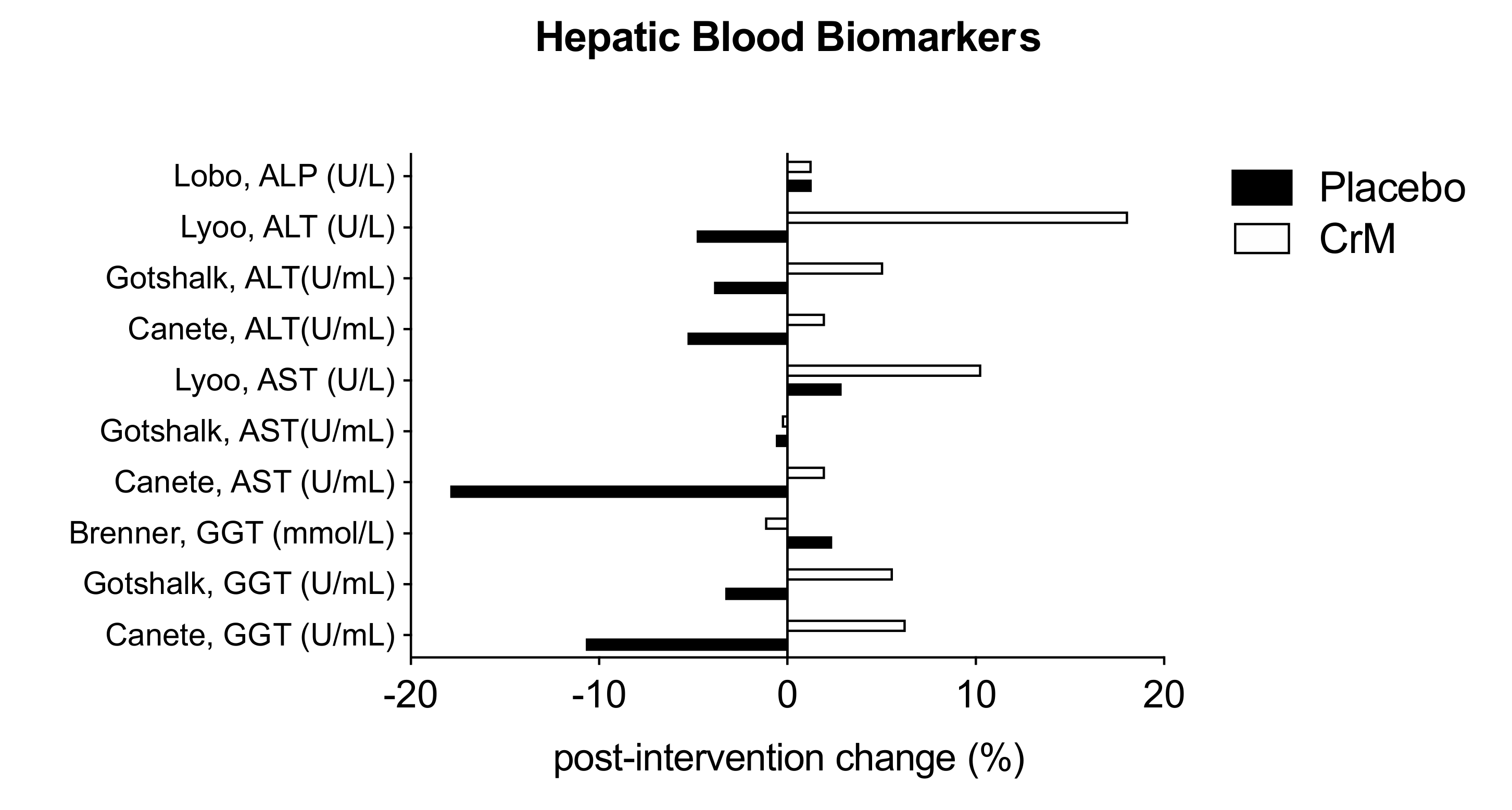
3.8. Hepatic System
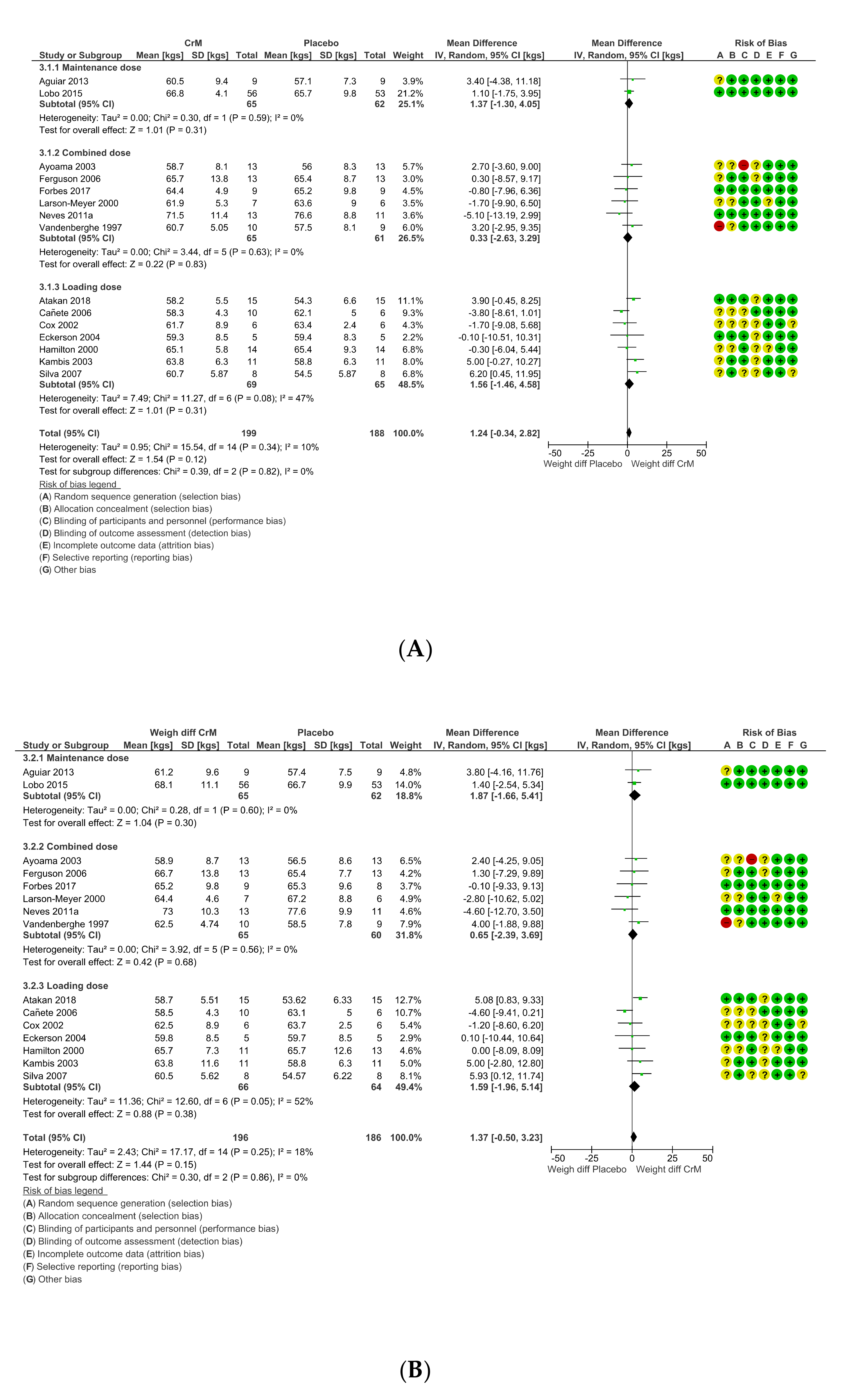
3.9. Body Composition Effects
3.10. A Comment on Cardiovascular Effects or Events
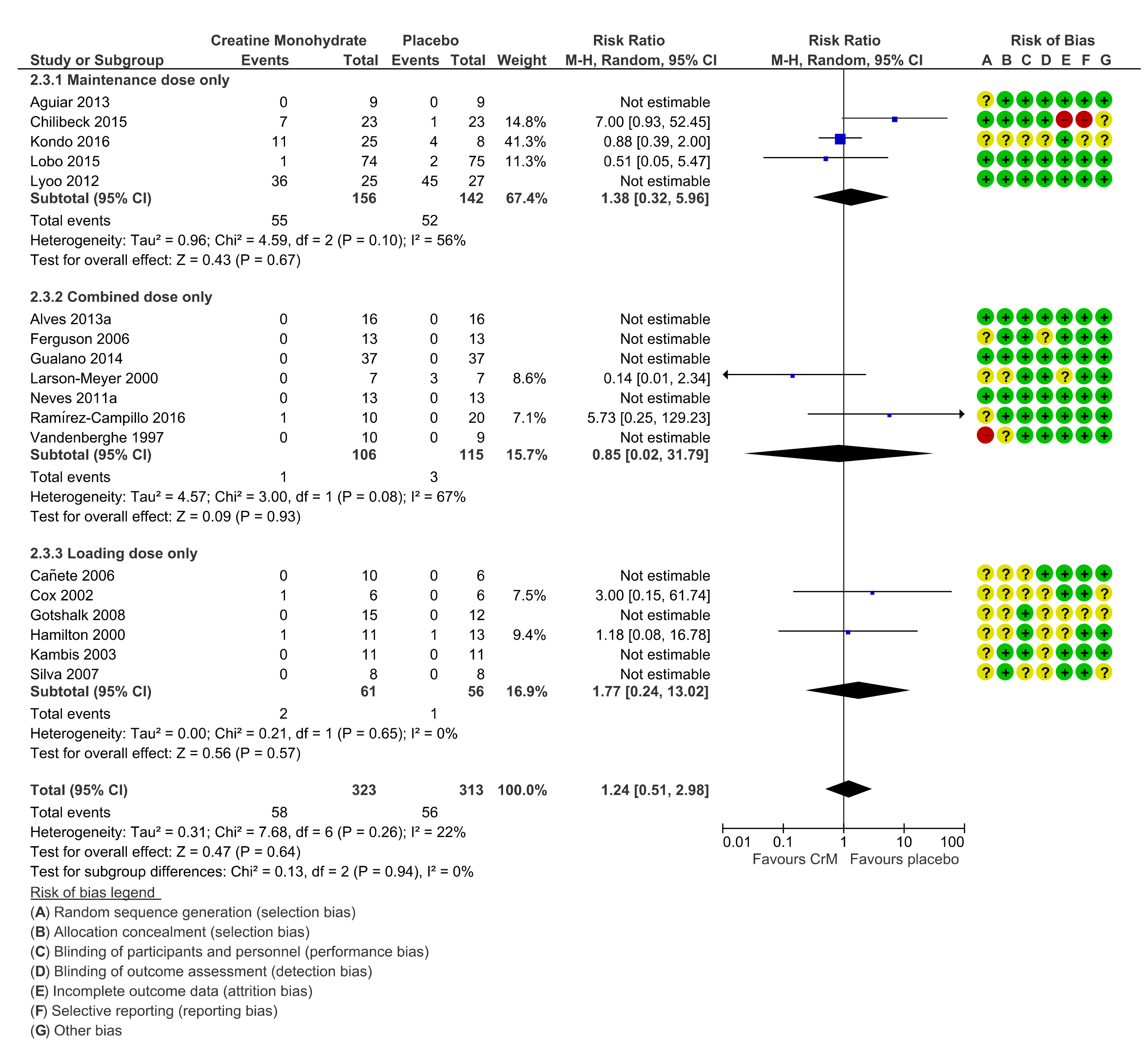
3.11. Dosing Regimens
3.12. Withdrawals, Loss to Follow Up, Cessation of Intervention
4. Discussion
5. Strengths and Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Sources of Support
Short Running Head
Abbreviations
| ATP | Adenosine triphosphate |
| CI | Confidence Interval |
| CrM | Creatine Monohydrate |
| I² | Statistical measure of heterogeneity |
| MD | Mean Difference |
| Pl | Placebo |
| RR | Risk Ratio |
References
- Wallimann, T.; Wyss, M.; Brdiczka, D.; Nicolay, K.; Eppenberger, H.M. Intracellular compartmentation, structure and function of creatine kinase isoenzymes in tissues with high and fluctuating energy demands: The ‘phosphocreatine circuit’ for cellular energy homeostasis. Biochem. J. 1992, 281, 21–40. [Google Scholar] [CrossRef] [PubMed]
- Wyss, M.; Kaddurah-Daouk, R. Creatine and creatinine metabolism. Physiol. Rev. 2000, 80, 1107–1213. [Google Scholar] [CrossRef] [PubMed]
- Stöckler, S.; Hanefeld, F.; Frahm, J. Creatine replacement therapy in guanidineoacetate methyltransferase deficiency, a novel inborn error of metabolism. Lancet 1996, 348, 789–790. [Google Scholar] [CrossRef]
- Schulze, A. Creatine deficiency syndromes. Mol. Cell. Biochem. 2003, 244, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Braissant, O.; Henry, H.; Beard, E.; Uldry, J. Creatine deficiency syndromes and the importance of creatine synthesis in the brain. Amino Acids 2011, 14, 1315–1324. [Google Scholar] [CrossRef] [PubMed]
- Rosas, H.D.; Doros, G.; Gevorkian, S.; Malarick, K.; Reuter, M.; Coutu, J.P.; Triggs, T.D.; Wilkens, P.J.; Matson, W.; Salat, D.H.; et al. PRECREST: A phase II prevention and biomarker trial of creatine in at-risk huntington disease. Neurology 2014, 82, 850–857. [Google Scholar] [CrossRef]
- Atassi, N.; Ratai, E.M.; Greenblatt, D.J.; Pulley, D.; Zhao, Y.; Bombardier, J.; Wallace, S.; Eckenrode, J.; Cudkowicz, M.; Dibernardo, A. A phase I, pharmacokinetic, dosage escalation study of creatine monohydrate in subjects with amyotrophic lateral sclerosis. Amyotroph. Lateral Scler. 2010, 11, 508–513. [Google Scholar] [CrossRef][Green Version]
- Pastula, D.M.; Moore, D.H.; Bedlack, R.S. Creatine for amyotrophic lateral sclerosis/motor neuron disease. Cochrane Database Syst. Rev. 2012, 12, CD005225. [Google Scholar] [CrossRef]
- Tarnopolsky, M.A.; Mahoney, D.J.; Vajsar, J.; Rodriguez, C.; Doherty, T.J.; Roy, B.D.; Biggar, D. Creatine monohydrate enhances strength and body composition in Duchenne muscular dystrophy. Neurology 2004, 62, 1771–1777. [Google Scholar] [CrossRef]
- Kley, R.A.; Tarnopolsky, M.A.; Vorgerd, M. Creatine for treating muscle disorders. Cochrane Database Syst. Rev. 2011, 2, CD004760. [Google Scholar] [CrossRef]
- Bender, A.; Klopstock, T. Creatine for neuroprotection in neurodegenerative disease: End of story? Amino Acids 2016, 48, 1929–1940. [Google Scholar] [CrossRef] [PubMed]
- Harris, R.C.; Soderlund, K.; Hultman, E. Elevation of creatine in resting and exercised muscle of normal subjects by creatine supplementation. Clin. Sci. 1992, 83, 367–374. [Google Scholar] [CrossRef] [PubMed]
- Sipila, I.; Rapola, J.; Simell, O.; Vannas, A. Supplementary creatine as a treatment for gyrate atrophy of the choroid and retina. N. Engl. J. Med. 1981, 304, 867–870. [Google Scholar] [CrossRef]
- Kreider, R.B.; Kalman, D.S.; Antonio, J.; Ziegenfuss, T.N.; Wildman, R.; Collins, R.; Candow, D.G.; Kleiner, S.M.; Almada, A.L.; Lopez, H.L. International Society of Sports Nutrition position stand: Safety and efficacy of creatine supplementation in exercise, sport, and medicine. J. Int. Soc. Sports Nutr. 2017, 14, C18. [Google Scholar] [CrossRef] [PubMed]
- Yoshizumi, W.M.; Tsourounis, C. Effects of creatine supplementation on renal function. J. Herb. Pharmacother. 2004, 4, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Dalbo, V.J.; Roberts, M.D.; Stout, J.R.; Kerksick, C.M. Putting to rest the myth of creatine supplementation leading to muscle cramps and dehydration. Br. J. Sports Med. 2008, 42, 567–573. [Google Scholar] [CrossRef]
- Balestrino, M.; Adriano, E. Beyond sports: Efficacy and safety of creatine supplementation in pathological or paraphysiological conditions of brain and muscle. Med. Res. Rev. 2019, 39, 2427–2459. [Google Scholar] [CrossRef]
- Kley, R.A.; Tarnopolsky, M.A.; Vorgerd, M. Creatine for treating muscle disorders: Meta-analysis of randomised controlled trials. Neuromuscul. Disord. 2010, 20, 657–658. [Google Scholar] [CrossRef]
- E Silva, A.D.S.; Pertille, A.; Barbosa, C.G.R.; de Oliveira Silva, J.A.; de Jesus, D.V.; Ribeiro, A.G.S.V.; Baganha, R.J.; de Oliveira, J.J. Effects of Creatine Supplementation on Renal Function: A Systematic Review and Meta-Analysis. J. Ren. Nutr. 2019, 29, 480–489. [Google Scholar] [CrossRef]
- Lopez, R.M.; Casa, D.J.; McDermott, B.P.; Ganio, M.S.; Armstrong, L.E.; Maresh, C.M. Does creatine supplementation hinder exercise heat tolerance or hydration status? A systematic review with meta-analyses. J. Athl. Train. 2009, 44, 215–223. [Google Scholar] [CrossRef]
- Bolotte, C.P. Creatine supplementation in athletes: Benefits and potential risks. J. La. State Med Soc. 1998, 150, 325–327. [Google Scholar] [PubMed]
- Graham, A.S.; Hatton, R.C. Creatine: A Review of Efficacy and Safety. J. Am. Pharm. Assoc. 1996 1999, 39, 803–810. [Google Scholar] [CrossRef]
- Poortmans, J.R.; Francaux, M. Adverse effects of creatine supplementation—Fact or fiction? Sports Med. 2000, 30, 155–170. [Google Scholar] [CrossRef] [PubMed]
- Juhn, M.S.; Tarnopolsky, M. Potential side effects of oral creatine supplementation: A critical review. Clin. J. Sport Med. 1998, 8, 298–304. [Google Scholar] [CrossRef]
- Kim, H.J.; Kim, C.K.; Carpentier, A.; Poortmans, J.R. Studies on the safety of creatine supplementation. Amino Acids 2011, 40, 1409–1418. [Google Scholar] [CrossRef]
- Sobolewski, E.J.; Thompson, B.J.; Smith, A.E.; Ryan, E.D. The Physiological Effects of Creatine Supplementation on Hydration: A Review. Am. J. Lifestyle Med. 2011, 5, 320–327. [Google Scholar] [CrossRef]
- Gualano, B.; Roschel, H.; Lancha, A.H., Jr.; Brightbill, C.E.; Rawson, E.S. In sickness and in health: The widespread application of creatine supplementation. Amino Acids 2012, 43, 519–529. [Google Scholar] [CrossRef]
- Glenn, J.M.; Gray, M.; Gualano, B.; Roschel, H. The Ergogenic Effects of Supplemental Nutritional Aids on Anaerobic Performance in Female Athletes. Strength Cond. J. 2016, 38, 105–120. [Google Scholar] [CrossRef]
- Wilkinson, T.J.; Lemmey, A.B.; Jones, J.G.; Sheikh, F.; Ahmad, Y.A.; Chitale, S.; Maddison, P.J.; O’brien, T.D. Can Creatine Supplementation Improve Body Composition and Objective Physical Function in Rheumatoid Arthritis Patients? A Randomized Controlled Trial. Arthritis Care Res. 2016, 68, 729–737. [Google Scholar] [CrossRef]
- Malin, S.K.; Cotugna, N.; Fang, C.S. Effect of creatine supplementation on muscle capacity in individuals with multiple sclerosis. J. Diet. Suppl. 2008, 5, 20–32. [Google Scholar] [CrossRef]
- Fairweather, D.; Rose, N.R. Women and autoimmune diseases. Emerg. Infect. Dis. 2004, 10, 2005–2011. [Google Scholar] [CrossRef] [PubMed]
- Lyoo, I.K.; Yoon, S.; Kim, T.S.; Hwang, J.; Kim, J.E.; Won, W.; Bae, S.; Renshaw, P.F. A randomized, double-blind placebo-controlled trial of oral creatine monohydrate augmentation for enhanced response to a selective serotonin reuptake inhibitor in women with major depressive disorder. Am. J. Psychiatry 2012, 169, 937–945. [Google Scholar] [CrossRef] [PubMed]
- Kondo, D.G.; Forrest, L.N.; Shi, X.; Sung, Y.H.; Hellem, T.L.; Huber, R.S.; Renshaw, P.F. Creatine target engagement with brain bioenergetics: A dose-ranging phosphorus-31 magnetic resonance spectroscopy study of adolescent females with SSRI-resistant depression. Amino Acids 2016, 48, 1941–1954. [Google Scholar] [CrossRef]
- Kondo, D.G.; Sung, Y.H.; Hellem, T.L.; Fiedler, K.K.; Shi, X.; Jeong, E.K.; Renshaw, P.F. Open-label adjunctive creatine for female adolescents with SSRI-resistant major depressive disorder: A 31-phosphorus magnetic resonance spectroscopy study. J. Affect. Disord. 2011, 135, 354–361. [Google Scholar] [CrossRef] [PubMed]
- Hellem, T.L.; Sung, Y.H.; Shi, X.F.; Pett, M.A.; Latendresse, G.; Morgan, J.; Huber, R.S.; Kuykendall, D.; Lundberg, K.J.; Renshaw, P.F. Creatine as a novel treatment for depression in females using methamphetamine: A pilot study. J. Dual Diagn. 2015, 11, 189–202. [Google Scholar] [CrossRef]
- Accortt, E.E.; Freeman, M.P.; Allen, J.J. Women and major depressive disorder: Clinical perspectives on causal pathways. J. Womens Health 2008, 17, 1583–1590. [Google Scholar] [CrossRef]
- Ireland, Z.; Castillo-Melendez, M.; Dickinson, H..; Snow, R.; Walker, D.W. Amaternal diet supplemented with creatine from mid-pregnancy protects the newborn spiny mouse brain from birth hypoxia. Neuroscience 2011, 194, 372–379. [Google Scholar] [CrossRef]
- LaRosa, D.A.; Ellery, S.J.; Snow, R.J.; Walker, D.W.; Dickinson, H. Maternal creatine supplementation during pregnancy prevents acute and long-term deficits in skeletal muscle after birth asphyxia: A study of structure and function of hind limb muscle in the spiny mouse. Pediatric Res. 2016, 80, 852–860. [Google Scholar] [CrossRef]
- Ellery, S.J.; Dickinson, H.; McKenzie, M.; Walker, D.W. Dietary interventions designed to protect the perinatal brain from hypoxic-ischemic encephalopathy—Creatine prophylaxis and the need for multi-organ protection. Neurochem. Int. 2016, 95, 15–23. [Google Scholar] [CrossRef]
- Sartini, S.; Lattanzi, D.; Di Palma, M.; Savelli, D.; Eusebi, S.; Sestili, S.; Cuppini, R.; Ambrogini, P. Maternal Creatine Supplementation Positively Affects Male Rat Hippocampal Synaptic Plasticity in Adult Offspring. Nutrients 2019, 11, 2014. [Google Scholar] [CrossRef]
- Sartini, S.; Sestili, P.; Colombo, E.; Martinelli, C.; Bartolini, F.; Ciuffoli, S.; Lattanzi, D.; Sisti, D.; Cuppini, R. Creatine affects in vitro electrophysiological maturation of neuroblasts and protects them from oxidative stress. J. Neurosci. Res. 2012, 90, 435–446. [Google Scholar] [CrossRef] [PubMed]
- De Guingand, D.L.; Ellery, S.J.; Davies-Tuck, M.L.; Dickinson, H. Creatine and pregnancy outcomes, a prospective cohort study in low-risk pregnant women: Study protocol. BMJ Open 2019, 9, e026756. [Google Scholar] [CrossRef] [PubMed]
- Wallimann, T.; Harris, R. Creatine: A miserable life without it. Amino Acids 2016, 48, 1739–1750. [Google Scholar] [CrossRef] [PubMed]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: Explanation and elaboration. BMJ 2009, 339, b2700. [Google Scholar] [CrossRef]
- Golder, S.; Loke, Y.K.; Bland, M. Unpublished data can be of value in systematic reviews of adverse effects: methodological overview. J. Clin. Epidemiol. 2010, 63, 1071–1081. [Google Scholar] [CrossRef]
- Golder, S.; Loke, Y.; McIntosh, H.M. Poor reporting and inadequate searches were apparent in systematic reviews of adverse effects. J. Clin. Epidemiol. 2008, 61, 440–448. [Google Scholar] [CrossRef]
- Loke, Y.K.; Price, D.; Herxheimer, A. Systematic reviews of adverse effects: framework for a structured approach. BMC Med Res. Methodol. 2007, 7, 32. [Google Scholar] [CrossRef]
- Golder, S.; Loke, Y.K.; Zorzela, L. Comparison of search strategies in systematic reviews of adverse effects to other systematic reviews. Heal. Inf. Libr. J. 2014, 31, 92–105. [Google Scholar] [CrossRef]
- Van Bavel, D.; Moraes, R.; Tibirica, E. Effects of dietary supplementation with creatine on homocysteinemia and systemic microvascular endothelial function in individuals adhering to vegan diets. Fundam. Clin. Pharmacol. 2019, 33, 428–440. [Google Scholar] [CrossRef]
- Ostojic, S.M.; Vranes, M.; Loncar, D.; Zenic, N.; Sekulic, D. Guanidinoacetic Acid and Creatine are Associated with Cardiometabolic Risk Factors in Healthy Men and Women: A Cross-Sectional Study. Nutrients 2018, 10, 87. [Google Scholar] [CrossRef]
- Pereira, R.T.D.S.; Dörr, F.A.; Pinto, E.; Solis, M.Y.; Artioli, G.G.; Fernandes, A.L.; Murai, I.H.; Dantas, W.S.; Seguro, A.C.; Santinho, M.A.R.; et al. Can creatine supplementation form carcinogenic heterocyclic amines in humans? J. Physiol. 2015, 593, 3959–3971. [Google Scholar] [CrossRef] [PubMed]
- Oladazimi, S.; Dabidiroshan, V.; Asadi, S. The effects of short time monohydrate creatine supplementation on systemic stress homeostasis following repeated maximum swimming in young women. J. Kermanshah Univ. Med. Sci. 2017, 20, 130–134. [Google Scholar]
- Gualano, B.; Painneli, V.D.S.; Roschel, H.; Artioli, G.G.; Neves, M.; Pinto, A.L.D.S.; Da Silva, M.E.R.; Cunha, M.R.; Otaduy, M.C.G.; Leite, C.D.C.; et al. Creatine in Type 2 Diabetes. Med. Sci. Sports Exerc. 2011, 43, 770–778. [Google Scholar] [CrossRef] [PubMed]
- Lee, N.; Kim, I.; Park, S.; Han, D.; Ha, S.; Kwon, M.; Kim, J.; Byun, S.-H.; Oh, W.; Jeon, H.B.; et al. Creatine Inhibits Adipogenesis by Downregulating Insulin-Induced Activation of the Phosphatidylinositol 3-Kinase Signaling Pathway. Stem Cells Dev. 2015, 24, 983–994. [Google Scholar] [CrossRef]
- Pinto, C.L.; Botelho, P.B.; Pimentel, G.D.; Campos-Ferraz, P.L.; Mota, J.F. Creatine supplementation and glycemic control: A systematic review. Amino Acids 2016, 48, 2103–2129. [Google Scholar] [CrossRef]
- Rees, M. The age of menarche. ORGYN Organon’s Mag. Women Health 1995, 4, 2–4. [Google Scholar]
- The Nordic Cochrane Centre: The Cochrane Collaboration. ReviewManager, 5.3 ed.; The Nordic Cochrane Centre, The Cochrane Collaboration: Copenhagen, Denmark, 2014. [Google Scholar]
- Higgins, J.P.T.; Green, S. (Eds.) Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 [updated March 2010]. The Cochrane Collaboration. 2011. Available online: www.handbook.cochrane.org (accessed on 1 June 2020).
- Schunemann, H.; Brozek, J.; Guyatt, G.; Oxman, A. Handbook for Grading the Quality of Evidence and the Strength of Recommendations Using the GRADE Approach. Available online: https://gdt.gradepro.org/app/handbook/handbook.html#h.m9385o5z3li7 (accessed on 12 March 2019).
- Sterne, J.A.; Hernán, M.; Reeves, B.C.; Savović, J.; Berkman, N.D.; Viswanathan, M.; Henry, D.; Altman, U.G.; Ansari, M.T.; Boutron, I.; et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016, 355, i4919. [Google Scholar] [CrossRef] [PubMed]
- Leader, A.; Amital, D.; Rubinow, A.; Amital, H. An Open-label Study Adding Creatine Monohydrate to Ongoing Medical Regimens in Patients with the Fibromyalgia Syndrome. Ann. N. Y. Acad. Sci. 2009, 1173, 829–836. [Google Scholar] [CrossRef]
- Eckerson, J.M.; Stout, J.R.; Moore, G.A.; Stone, N.J.; Nishimura, K.; Tamura, K. Effect of two and five days of creatine loading on anaerobic working capacity in women. J. Strength Cond. Res. 2004, 18, 168–173. [Google Scholar]
- Ledford, A.; Branch, J.D. Creatine supplementation does not increase peak power production and work capacity during repetitive wingate testing in women. J. Strength Cond. Res. 1999, 13, 394–399. [Google Scholar]
- Aguiar, A.F.; Januário, R.S.B.; Junior, R.P.; Gerage, A.M.; Pina, F.L.C.; Nascimento, M.A.D.; Padovani, C.; Cyrino, E.S. Long-term creatine supplementation improves muscular performance during resistance training in older women. Graefe’s Arch. Clin. Exp. Ophthalmol. 2012, 113, 987–996. [Google Scholar] [CrossRef] [PubMed]
- Alves, C.R.; Santiago, B.M.; Lima, F.R.; Otaduy, M.C.G.; Calich, A.L.; Tritto, A.C.C.; Pinto, A.L.D.S.; Roschel, H.; Leite, C.C.; Benatti, F.B.; et al. Creatine Supplementation in Fibromyalgia: A Randomized, Double-Blind, Placebo-Controlled Trial. Arthritis Care Res. 2013, 65, 1449–1459. [Google Scholar] [CrossRef] [PubMed]
- Atakan, M.M.; Karavelioğlu, M.B.; Harmancı, H.; Cook, M.; Bulut, S. Short term creatine loading without weight gain improves sprint, agility and leg strength performance in female futsal players. Sci. Sports 2019, 34, 321–327. [Google Scholar] [CrossRef]
- Ayoama, R.; Hiruma, E.; Sasaki, H. Effects of creatine loading on muscular strength and endurance of female softball players. J. Sports Med. Phys. Fit. 2003, 43, 481–487. [Google Scholar]
- Benton, D.; Donohoe, R. The influence of creatine supplementation on the cognitive functioning of vegetarians and omnivores. Br. J. Nutr. 2011, 105, 1100–1105. [Google Scholar] [CrossRef]
- Brenner, M.; Rankin, J.W.; Sebolt, D. The Effect of Creatine Supplementation during Resistance Training in Women. J. Strength Cond. Res. 2000, 14, 207–213. [Google Scholar]
- Cañete, S.; San Juan, A.F.; Pérez, M.; Gómez-Gallego, F.; López-Mojares, L.M.; Earnest, C.P.; Fleck, S.J.; Lucia, A. Does creatine supplementation improve functional capacity in elderly women? J. Strength Cond. Res. 2006, 20, 22–28. [Google Scholar]
- Chilibeck, P.D.; Candow, D.G.; Landeryou, T.; Kaviani, M.; Paus-Jenssen, L. Effects of Creatine and Resistance Training on Bone Health in Postmenopausal Women. Med. Sci. Sports Exerc. 2015, 47, 1587–1595. [Google Scholar] [CrossRef]
- Cox, G.; Mujika, I.; Tumilty, D.; Burke, L.M. Acute creatine supplementation and performance during a field test simulating match play in elite female soccer players. Int. J. Sport Nutr. Exerc. Metab. 2002, 12, 33–46. [Google Scholar] [CrossRef]
- Ferguson, T.B.; Syrotuik, D.G. Effects of creatine monohydrate supplementation on body composition and strength indices in experienced resistance trained women. J. Strength Cond. Res. 2006, 20, 939–946. [Google Scholar]
- Forbes, S.C.; Sletten, N.; Durrer, C.; Myette-Cote, É.; Candow, D.; Little, J.P. Creatine Monohydrate Supplementation Does Not Augment Fitness, Performance, or Body Composition Adaptations in Response to Four Weeks of High-Intensity Interval Training in Young Females. Int. J. Sport Nutr. Exerc. Metab. 2017, 27, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Gotshalk, L.A.; Kraemer, W.J.; Mendonca, M.A.G.; Vingren, J.L.; Kenny, A.M.; Spiering, B.A.; Hatfield, D.L.; Fragala, M.S.; Volek, J.S. Creatine supplementation improves muscular performance in older women. Graefe’s Arch. Clin. Exp. Ophthalmol. 2007, 102, 223–231. [Google Scholar] [CrossRef] [PubMed]
- Gualano, B.; Macedo, A.R.; Alves, C.R.; Roschel, H.; Benatti, F.B.; Takayama, L.; Pinto, A.L.D.S.; Lima, F.R.; Pereira, R. Creatine supplementation and resistance training in vulnerable older women: A randomized double-blind placebo-controlled clinical trial. Exp. Gerontol. 2014, 53, 7–15. [Google Scholar] [CrossRef]
- Hamilton, K.L.; Meyers, M.C.; Skelly, W.A.; Marley, R.J. Oral creatine supplementation and upper extremity anaerobic response in females. Int. J. Sport Nutr. Exerc. Metab. 2000, 10, 277–289. [Google Scholar] [CrossRef] [PubMed]
- Kambis, K.W.; Pizzedaz, S.K. Short-term creatine supplementation improves maximum quadriceps contraction in women. Int. J. Sport Nutr. Exerc. Metab. 2003, 13, 87–96. [Google Scholar] [CrossRef]
- Larson-Meyer, D.E.; Hunter, G.R.; Trowbridge, C.A.; Turk, J.C.; Ernest, J.M.; Torman, S.L.; Harbin, P.A. The Effect of Creatine Supplementation on Muscle Strength and Body Composition during Off-Season Training in Female Soccer Players. J. Strength Cond. Res. 2000, 14, 434–442. [Google Scholar]
- Lobo, D.M.; Tritto, A.C.; Da Silva, L.R.; De Oliveira, P.B.; Benatti, F.B.; Roschel, H.; Nies, B.; Gualano, B.; Pereira, R. Effects of long-term low-dose dietary creatine supplementation in older women. Exp. Gerontol. 2015, 70, 97–104. [Google Scholar] [CrossRef]
- Neves, M.; Gualano, B.; Roschel, H.; Lima, F.R.; De Sá-Pinto, A.L.; Seguro, A.C.; Shimizu, M.H.; Sapienza, M.T.; Fuller, R.; Lancha, A.H.; et al. Effect of creatine supplementation on measured glomerular filtration rate in postmenopausal women. Appl. Physiol. Nutr. Metab. 2011, 36, 419–422. [Google Scholar] [CrossRef]
- Ramirez-Campillo, R.; González-Jurado, J.A.; Martínez, C.; Nakamura, F.Y.; Peñailillo, L.; Meylan, C.M.; Caniuqueo, A.; Cañas-Jamet, R.; Moran, J.; Martínez-Salazar, C.; et al. Effects of plyometric training and creatine supplementation on maximal-intensity exercise and endurance in female soccer players. J. Sci. Med. Sport 2016, 19, 682–687. [Google Scholar] [CrossRef]
- Silva, A.J.; Reis, V.M.; Guidetti, L.; Alves, F.B.; Mota, P.; Freitas, J.; Baldari, C. Effect of creatine on swimming velocity, body composition and hydrodynamic variables. J. Sports Med. Phys. Fit. 2007, 47, 58–64. [Google Scholar]
- Thompson, C.H.; Kemp, G.J.; Sanderson, A.L.; Dixon, R.M.; Styles, P.; Taylor, D.J.; Radda, G.K. Effect of creatine on aerobic and anaerobic metabolism in skeletal muscle in swimmers. Br. J. Sports Med. 1996, 30, 222–225. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Vandenberghe, K.; Goris, M.; Van Hecke, P.; Van Leemputte, M.; Vangerven, L.; Hespel, P. Long-term creatine intake is beneficial to muscle performance during resistance training. J. Appl. Physiol. 1997, 83, 2055–2063. [Google Scholar] [CrossRef] [PubMed]
- Bizzarini, E.; De Angelis, L. Is the use of oral creatine supplementation safe? J. sports Med. Phys. Fit. 2004, 4, 411–416. [Google Scholar]
- Persky, A.M.; Rawson, E.S. Safety of creatine supplementation. Membr. Biog. 2007, 46, 275–289. [Google Scholar] [CrossRef]
- Jager, R.; Purpura, M.; Shao, A.; Inoue, T.; Kreider, R.B. Analysis of the efficacy, safety, and regulatory status of novel forms of creatine. Amino Acids 2011, 40, 1369–1383. [Google Scholar] [CrossRef]
- Avgerinos, K.I.; Spyrou, N.; Bougioukas, K.I.; Kapogiannis, D. Effects of creatine supplementation on cognitive function of healthy individuals: A systematic review of randomized controlled trials. Exp. Gerontol. 2018, 108, 166–173. [Google Scholar] [CrossRef]
- Terjung, R.L.; Clarkson, P.; Eichner, E.R.; Greenhaff, P.L.; Hespel, P.J.; Israel, R.G.; Kraemer, W.J.; Meyer, R.A.; Spriet, L.L.; Tarnopolsky, M.A.; et al. American College of Sports Medicine roundtable. The physiological and health effects of oral creatine supplementation. Med. Sci. Sports Exerc. 2000, 32, 706–717. [Google Scholar]
- Andres, S.; Ziegenhagen, R.; Trefflich, I.; Pevny, S.; Schultrich, K.; Braun, H.; Schänzer, W.; Hirsch-Ernst, K.; Schäfer, B.; Lampen, A. Creatine and creatine forms intended for sports nutrition. Mol. Nutr. Food Res. 2017, 61, 1600772. [Google Scholar] [CrossRef]
- Francaux, M.; Poortmans, J.R. Side Effects of Creatine Supplementation in Athletes. Int. J. Sports Physiol. Perform. 2006, 1, 311–323. [Google Scholar] [CrossRef]
- Rosenfeld, J.; King, R.M.; Jackson, C.E.; Bedlack, R.S.; Barohn, R.J.; Dick, A.; Phillips, L.H.; Chapin, J.; Gelinas, D.F.; Lou, J.-S. Creatine monohydrate in ALS: Effects on strength, fatigue, respiratory status and ALSFRS. Amyotroph. Lateral Scler. 2009, 9, 266–272. [Google Scholar] [CrossRef]
- Groeneveld, G.J.; Beijer, C.; Veldink, J.H.; Kalmijn, S.; Wokke, J.H.J.; Berg, L.H.V.D. Few Adverse Effects of Long-Term Creatine Supplementation in a Placebo-Controlled Trial. Int. J. Sports Med. 2005, 26, 307–313. [Google Scholar] [CrossRef] [PubMed]
- Deacon, S.J.; Vincent, E.E.; Greenhaff, P.L.; Fox, J.; Steiner, M.C.; Singh, S.J.; Morgan, M.D. Randomized Controlled Trial of Dietary Creatine as an Adjunct Therapy to Physical Training in Chronic Obstructive Pulmonary Disease. Am. J. Respir. Crit. Care Med. 2008, 178, 233–239. [Google Scholar] [CrossRef] [PubMed]
- Hersch, S.M.; Schifitto, G.; Oakes, D.; Bredlau, A.-L.; Meyers, C.M.; Nahin, R.; Rosas, H.D. The CREST-E study of creatine for Huntington disease: A randomized controlled trial. Neurology 2017, 89, 594–601. [Google Scholar] [CrossRef] [PubMed]
- Taes, Y.; Delanghe, J.R.; De Bacquer, D.; Langlois, M.; Stevens, L.; Geerolf, I.; Lameire, N.H.; De Vriese, A.S. Creatine supplementation does not decrease total plasma homocysteine in chronic hemodialysis patients. Kidney Int. 2004, 66, 2422–2428. [Google Scholar] [CrossRef]
- Shefner, J.M.; Cudkowicz, M.E.; Schoenfeld, D.; Conrad, T.; Taft, J.; Chilton, M.; Urbinelli, L.; Qureshi, M.; Zhang, H.; Pestronk, A.; et al. A clinical trial of creatine in ALS. Neurology 2004, 63, 1656–1661. [Google Scholar] [CrossRef]
- Norman, K.; Stübler, D.; Baier, P.; Schütz, T.; Ocran, K.; Holm, E.; Lochs, H.; Pirlich, M. Effects of creatine supplementation on nutritional status, muscle function and quality of life in patients with colorectal cancer—A double blind randomised controlled trial. Clin. Nutr. 2006, 25, 596–605. [Google Scholar] [CrossRef]
- Fuld, J.P.; Kilduff, L.P.; Neder, J.A.; Pitsiladis, Y.; Lean, M.E.J.; Ward, S.A.; Cotton, M.M. Creatine supplementation during pulmonary rehabilitation in chronic obstructive pulmonary disease. Thorax 2005, 60, 531–537. [Google Scholar] [CrossRef]
- Kuethe, F.; Krack, A.; Richartz, B.M.; Figulla, H.R. Creatine supplementation improves muscle strength in patients with congestive heart failure. Pharmazie 2006, 61, 218–222. [Google Scholar]
- Sakellaris, G.; Nasis, G.; Kotsiou, M.; Tamiolaki, M.; Charissis, G.; Evangeliou, A. Prevention of traumatic headache, dizziness and fatigue with creatine administration. A pilot study. Acta Paediatr. 2007, 97, 31–34. [Google Scholar] [CrossRef]
- Drory, V.E.; Gross, D. No effect of creatine on respiratory distress in amyotrophic lateral sclerosis. Amyotroph. Lateral Scler. Other Mot. Neuron Disord. 2002, 3, 43–46. [Google Scholar] [CrossRef]
- Kuklo, T.R.; Tis, J.E.; Moores, L.K.; Schaefer, R.A. Fatal Rhabdomyolysis with Bilateral Gluteal, Thigh, and Leg Compartment Syndrome after the Army Physical Fitness Test. Am. J. Sports Med. 2000, 28, 112–116. [Google Scholar] [CrossRef] [PubMed]
- Saidi, H.; Mani, M. Severe metabolic acidosis secondary to coadministration of creatine and metformin, a case report. Am. J. Emerg. Med. 2010, 28, e5–e6. [Google Scholar] [CrossRef] [PubMed]
- Roukens, M.P.M.; Brugman, E.S.; Niessen, H.W.; Girbes, A.R.J. A case of fatal asthma after use of a dietary supplement containing creatine. Neth. J. Crit. Care 2013, 17, 32–33. [Google Scholar]
- Pritchard, N.R.; Kalra, P.A. Renal dysfunction accompanying oral creatine supplements. Lancet 1998, 351, 1252–1253. [Google Scholar] [CrossRef]
- Koshy, K.M.; Griswold, E.; Schneeberger, E.E. Interstitial Nephritis in a Patient Taking Creatine. N. Engl. J. Med. 1999, 340, 814–815. [Google Scholar] [CrossRef] [PubMed]
- Sandhu, R.S.; Como, J.J.; Scalea, T.S. Renal Failure and Exercise-Induced Rhabdomyolysis in Patients Taking Performance-Enhancing Compounds. J. Trauma: Inj. Infect. Crit. Care 2002, 53, 761–763. [Google Scholar] [CrossRef]
- Revai, T.; Sapi, Z.; Benedek, S.; Kovács, A.; Kaszás, I.; Virányi, M.; Winkler, G. Severe nephrotic syndrome in a young man taking anabolic steroid and creatine long term. Orv. Hetil. 2003, 144, 2425–2427. [Google Scholar]
- Sheth, N.; Sennett, B.; Berns, J.S. Rhabdomyolysis and acute renal failure following arthroscopic knee surgery in a college football player taking creatine supplements. Clin. Nephrol. 2006, 65, 134–137. [Google Scholar] [CrossRef]
- Thorsteinsdottir, B.; Grande, J.P.; Garovic, V.D. Acute Renal Failure in a Young Weight Lifter Taking Multiple Food Supplements, Including Creatine Monohydrate. J. Ren. Nutr. 2006, 16, 341–345. [Google Scholar] [CrossRef]
- Barisic, N.; Bernert, G.; Ipsiroglu, O.; Stromberger, C.; Müller, T.; Gruber, S.; Prayer, D.; Moser, E.; Bittner, R.E.; Stöckler-Ipsiroglu, S. Effects of oral creatine supplementation in a patient with MELAS phenotype and associated nephropathy. Neuropediatrics 2002, 33, 157–161. [Google Scholar] [CrossRef]
- Basturk, T.; Aysim, O.; Abdulkadir, U. The effects of the recommended dose of creatine monohydrate on kidney function. NDT Plus 2010, 4, 23–24. [Google Scholar] [CrossRef]
- Soltani, Z. When “creatine” increases creatinine. J. Investig. Med. 2011, 59, 459–460. [Google Scholar]
- Robinson, S.J. Acute quadriceps compartment syndrome and rhabdomyolysis in a weight lifter using high-dose creatine supplementation. J. Am. Board Fam. Pract. 2000, 13, 134–137. [Google Scholar] [CrossRef][Green Version]
- Do, K.D.; Bellabarba, C.; Bhananker, S.M. Exertional Rhabdomyolysis in a Bodybuilder Following Overexertion: A Possible Link to Creatine Overconsumption. Clin. J. Sport Med. 2007, 17, 78–79. [Google Scholar] [CrossRef] [PubMed]
- Pourmand, A.; Li, A.; Yiu, A.; Mazer-Amirshahi, M.; Shokoohi, H. Survival after profound acidosis and rhabdomyolysis due to dietary supplement use. Am. J. Emerg. Med. 2016, 34, e1–e3. [Google Scholar] [CrossRef]
- Vahedi, K.; Domigo, V.; Amarenco, P.; Bousser, M. Ischaemic stroke in a sportsman who consumed MaHuang extract and creatine monohydrate for body building. J. Neurol. Neurosurg. Psychiatry 2000, 68, 112–113. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Harris, B.F.; Winn, C.; Ableman, T.B. Hemorrhagic stroke in a young healthy male following use of pre-workout supplement animal rage XL. Mil. Med. 2017, 182, e2030–e2033. [Google Scholar] [CrossRef]
- Waintraub, D.J.; Serouya, S.; Madrigal, E.; Benias, P.; Min, A.; Carr-Locke, D.L. A Rare Case of Severe Liver Injury Related to Whey Protein Supplementation. Am. J. Gastroenterol. 2016, 111, S945. [Google Scholar] [CrossRef]
- Timcheh-Hariri, A.; Balali-Mood, M.; Aryan, E.; Sadeghi, M.; Riahi-Zanjani, B. Toxic hepatitis in a group of 20 male body-builders taking dietary supplements. Food Chem. Toxicol. 2012, 50, 3826–3832. [Google Scholar] [CrossRef]
- Kammer, R.T. Lone atrial fibrillation associated with creatine monohydrate supplementation. Pharmacother. J. Hum. Pharmacol. Drug Ther. 2005, 25, 762–764. [Google Scholar] [CrossRef]
- Whitt, K.; Ward, S.; Deniz, K.; Liu, L.; Odin, J.; Qin, L. Cholestatic Liver Injury Associated with Whey Protein and Creatine Supplements. Semin. Liver Dis. 2008, 28, 226–231. [Google Scholar] [CrossRef] [PubMed]
- Greenhaff, P.; Pritchard, N.; Kalra, P.; Poortmans, J.R.; Francaux, M. Renal dysfunction accompanying oral creatine supplements. Lancet 1998, 352, 233–234. [Google Scholar] [CrossRef]
- Kreider, R.B. 6 Creatine Fears: Real Concern or False Alarm? Joe Weider’s Muscle Fit. 1999, 60, 160. [Google Scholar]
- Poortmans, J.R.; Francaux, M. Renal dysfunction accompanying oral creatine supplements—reply. Lancet 1998, 352, 234. [Google Scholar] [CrossRef]
- Wyss, M.; Schulze, A. Health implications of creatine: Can oral creatine supplementation protect against neurological and atherosclerotic disease? Neuroscience 2002, 112, 243–260. [Google Scholar] [CrossRef]
- Shao, A.; Hathcock, J.N. Risk assessment for creatine monohydrate. Regul. Toxicol. Pharmacol. 2006, 45, 242–251. [Google Scholar] [CrossRef]
- Rawson, E.S.; Clarkson, P.M.; Tarnopolsky, M.A. Perspectives on Exertional Rhabdomyolysis. Sports Med. 2017, 47, S33–S49. [Google Scholar] [CrossRef]
- VKM; Panel on Nutrition, Dietetic Products. Novel Food and Allergy of the Norwegian Scientific Committee for Food Safety. In Risk Assessment of "other Substances"—Creatine; Opinion of the Panel on Nutrition, dietetic products, Novel Food an Allergy of the Norwegian Scientific Committee for Food Safety: Oslo, Norway, 2016. [Google Scholar]
- Brudnak, M.A. Creatine: Are the benefits worth the risk? Toxicol. Lett. 2004, 150, 123–130. [Google Scholar] [CrossRef]
- Ostojic, S.M.; Ahmetovic, Z. Gastrointestinal Distress After Creatine Supplementation in Athletes: Are Side Effects Dose Dependent? Res. Sports Med. 2008, 16, 15–22. [Google Scholar] [CrossRef]
- Greenwood, M.; Farris, J.; Kreider, R.; Greenwood, L.; Byars, A. Creatine Supplementation Patterns and Perceived Effects in Select Division I Collegiate Athletes. Clin. J. Sport Med. 2000, 10, 191–194. [Google Scholar] [CrossRef]
- Kreider, R.B.; Melton, C.; Rasmussen, C.; Greenwood, M.; Lancaster, S.; Cantler, E.C.; Milnor, P.; Almada, A. Long-term creatine supplementation does not significantly affect clinical markers of health in athletes. Mol. Cell. Biochem. 2003, 244, 95–104. [Google Scholar] [CrossRef]
- Greenwood, M.; Kreider, R.B.; Melton, C.; Rasmussen, C.; Lancaster, S.; Cantler, E.; Milnor, P.; Almada, A. Creatine supplementation during college football training does not increase the incidence of cramping or injury. Mol. Cell. Biochem. 2003, 244, 83–88. [Google Scholar] [CrossRef]
- Grindstaff, P.D.; Kreider, R.; Bishop, R.; Wilson, M.; Wood, L.; Alexander, C.; Almada, A. Effects of creatine supplementation on repetitive sprint performance and body composition in competitive swimmers. Int. J. Sport Nutr. 1997, 7, 330–346. [Google Scholar] [CrossRef]
- Bashiri, J.; Gaeini, A.; Nikbakht, H.; Hadi, H.; Bashiri, M. Effect of concurrent creatine monohydrate ingestion and resistance training on hepatic enzymes activity levels in non-athlete males. Iran. J. Endocrinol. Metab. 2010, 12, 21–47,83. [Google Scholar]
- Earnest, C.; Almada, A.; Mitchell, T.L. High-Performance Capillary Electrophoresis-Pure Creatine Monohydrate Reduces Blood Lipids in Men and Women. Clin. Sci. 1996, 91, 113–118. [Google Scholar] [CrossRef]
- Bender, A.; Samtleben, W.; Elstner, M.; Klopstock, T. Long-term creatine supplementation is safe in aged patients with Parkinson disease. Nutr. Res. 2008, 28, 172–178. [Google Scholar] [CrossRef]
- Cornelissen, V.; DeFoor, J.; Stevens, A.L.; Schepers, D.; Hespel, P.; Decramer, M.; Mortelmans, L.; Dobbels, F.; Vanhaecke, J.; Fagard, R.; et al. Effect of creatine supplementation as a potential adjuvant therapy to exercise training in cardiac patients: a randomized controlled trial. Clin. Rehabil. 2010, 24, 988–999. [Google Scholar] [CrossRef]
- Gualano, B.; Ugrinowitsch, C.; Novaes, R.B.; Artioli, G.G.; Shimizu, M.H.; Seguro, A.C.; Harris, R.C.; Lancha, A.H. Effects of creatine supplementation on renal function: a randomized, double-blind, placebo-controlled clinical trial. Graefe’s Arch. Clin. Exp. Ophthalmol. 2008, 103, 33–40. [Google Scholar] [CrossRef]
- Earnest, C.; Almada, A.; Milchell, T. Influence of chronic creatine supplementation on hepatorenal function. FASEB J. 1996, 10, 3–A790. [Google Scholar]
- Mihic, S.; Macdonald, J.R.; McKenzie, S.; Tarnopolsky, M.A. Acute creatine loading increases fat-free mass, but does not affect blood pressure, plasma creatinine, or CK activity in men and women. Med. Sci. Sports Exerc. 2000, 32, 291–296. [Google Scholar] [CrossRef]
- Robinson, T.M.; Sewell, D.; Casey, A.; Steenge, G.; Greenhaff, P.L. Dietary creatine supplementation does not affect some haematological indices, or indices of muscle damage and hepatic and renal function. Br. J. Sports Med. 2000, 34, 284–288. [Google Scholar] [CrossRef]
- Poortmans, J.R.; Francaux, M. Long-term oral creatine supplementation does not impair renal function in healthy athletes. / Une supplementation orale en creatine sur le long terme ne deteriore pas la fonction renale chez des athletes en bonne sante. Med. Sci. Sports Exerc. 1999, 31, 1108–1110. [Google Scholar] [CrossRef]
- Harris, R.; Almada, A.; Harris, D.B.; Dunnett, M.; Hespel, P. The creatine content of Creatine Serum (TM) and the change in the plasma concentration with ingestion of a single dose. J. Sports Sci. 2004, 22, 851–857. [Google Scholar] [CrossRef]
- Schroder, H.; Terrados, N.; Tramullas, A. Risk assessment of the potential side effects of long-term creatine supplementation in team sport athletes. Eur. J. Nutr. 2005, 44, 255–261. [Google Scholar] [CrossRef]
- Mayhew, D.L.; Mayhew, J.L.; Ware, J.S. Effects of long-term creatine supplementation on liver and kidney functions in American college football players. Int. J. Sport Nutr. Exerc. Metab. 2002, 12, 453–460. [Google Scholar] [CrossRef]
- Schedel, J.M.; Tanaka, M.; Tanaka, H.; Kiyonaga, A.; Shindo, M.; Terrier, P.; Schutz, Y. Consequences of one-week creatine supplementation on creatine and creatinine levels in athletes serum and urine. Schweiz. Z. fur Sportmed. und Sporttraumatologie 2000, 48, 111–116. [Google Scholar]
- Schilling, B.; Stone, M.H.; Utter, A.; Kearney, J.T.; Johnson, M.; Coglianese, R.; Smith, L.; O’Bryant, H.S.; Fry, A.C.; Starks, M.; et al. Creatine supplementation and health variables: a retrospective study. Med. Sci. Sports Exerc. 2001, 33, 183–188. [Google Scholar] [CrossRef]
- Kamber, M.; Koster, M.; Kreis, R.; Walker, G.; Boesch, C.; Hoppeler, H. Creatine supplementation—Part I: Performance, clinical chemistry, and muscle volume. Med. Sci. Sports Exerc. 1999, 31, 1763–1769. [Google Scholar] [CrossRef]
- Williamson, L.; New, D. How the use of creatine supplements can elevate serum creatinine in the absence of underlying kidney pathology. BMJ Case Rep. 2014, 2014. [Google Scholar] [CrossRef]
- Willis, J.; Jones, R.; Nwokolo, N.; Levy, J. Protein and creatine supplements and misdiagnosis of kidney disease. BMJ 2010, 340, b5027. [Google Scholar] [CrossRef]
- Kreider, R.B. Effects of creatine supplementation on performance and training adaptations. Mol. Cell. Biochem. 2003, 244, 89–94. [Google Scholar] [CrossRef]
- Ransone, J.; Park, D.H. Physiological effects of creatine supplementation: A meta-analysis. Int. J. Appl. Sports Sci. 2002, 14, 1–26. [Google Scholar]
- Waldron, J.E.; Pendlay, G.W.; Kilgore, T.G.; Haff, G.G.; Reeves, J.S.; Kilgore, J.L. Concurrent creatine monohydrate supplementation and resistance training does not affect markers of hepatic function in trained weightlifters. J. Exerc. Physiol. Online 2002, 5, 57–64. [Google Scholar]
- Lazo, M.; Selvin, E.; Clark, J.M. Brief communication: clinical implications of short-term variability in liver function test results. Ann. Intern. Med. 2008, 148, 348–352. [Google Scholar] [CrossRef]
- Hall, P.; Cash, J. What is the Real Function of the Liver ‘Function’ Tests? Ulst. Med J. 2012, 81, 30–36. [Google Scholar]
- Murphy, A.; Watsford, M.; Coutts, A.; Richards, D. Effects of creatine supplementation on aerobic power and cardiovascular structure and function. J. Sci. Med. Sport 2005, 8, 305–313. [Google Scholar] [CrossRef]
- Arazi, H.; Rahmaninia, F.; Hosseini, K.; Asadi, A. Effects of short term creatine supplementation and resistance exercises on resting hormonal and cardiovascular responses. Sci. Sports 2015, 30, 105–109. [Google Scholar] [CrossRef]
- Karamat, F.A.; Horjus, D.L.; Haan, Y.C.; van der Woude, L.; Schaap, M.C.; Oudman, I.; Van Montfrans, G.A.; Nieuwland, R.; Salomons, G.S.; Clark, J.F.; et al. The acute effect of beta-guanidinopropionic acid versus creatine or placebo in healthy men (ABC-Trial): A randomized controlled first-in-human trial. Br. J. Clin. Pharmacol. 2017, 83, 2626–2635. [Google Scholar] [CrossRef]
- Horjus, D.L.; Oudman, I.; van Montfrans, G.A.; Brewster, L.M. Creatine and creatine analogues in hypertension and cardiovascular disease. Cochrane Database Syst. Rev. 2011, 2011. [Google Scholar] [CrossRef]
- Branch, J.D. Effect of creatine supplementation on body composition and performance: A meta-analysis. Int. J. Sport Nutr. Exerc. Metab. 2003, 13, 198–226. [Google Scholar] [CrossRef]
- Duffy, J.M.; Hirsch, M.; Pealing, L.; Showell, M.; Khan, K.S.; Ziebland, S.; McManus, R.; Hooft, J.; Gale, C.; Brown, M.; et al. Inadequate safety reporting in pre-eclampsia trials: a systematic evaluation. BJOG Int. J. Obstet. Gynaecol. 2018, 125, 795–803. [Google Scholar] [CrossRef] [PubMed]
- Bent, S.; Padula, A.; Avins, A.L. Brief Communication: Better Ways To Question Patients about Adverse Medical Events. Ann. Intern. Med. 2006, 144, 257–261. [Google Scholar] [CrossRef] [PubMed]
- Raglin, J.S.; Szabo, A.; Lindheimer, J.B.; Beedie, C. Understanding placebo and nocebo effects in the context of sport: A psychological perspective. Eur. J. Sport Sci. 2020, 20, 293–301. [Google Scholar] [CrossRef]
- Planès, S.; Villier, C.; Mallaret, M. The nocebo effect of drugs. Pharmacol. Res. Perspect. 2016, 4. [Google Scholar] [CrossRef]
- Vadeboncoeur, C.; Townsend, N.; Foster, C. A meta-analysis of weight gain in first year university students: Is freshman 15 a myth? BMC Obes. 2015, 2, 22. [Google Scholar] [CrossRef] [PubMed]
- Tobias, D.K.; Chen, M.; Manson, J.E.; Ludwig, D.S.; Willett, W.; Hu, F.B. Effect of low-fat diet interventions versus other diet interventions on long-term weight change in adults: A systematic review and meta-analysis. Lancet Diabetes Endocrinol. 2015, 3, 968–979. [Google Scholar] [CrossRef]
| Study Characteristics | Adverse Outcomes | |||||||
|---|---|---|---|---|---|---|---|---|
| Author (Year) | Study Design (Study Type) | Number Treated (Cr:Pl) | Population Type (Mean Age ± SD) (Cr:Pl) | Dosing Regimen M, C or L (Total Days Duration) | General Symptoms (R, S, T.) | Renal System | Hepatic System | Body Comp |
| AGUIAR (2013) | DBRPCT (E) | 18 (9:9) | Post-menopausal (64 ± 4:65 ± 6) | M 5 g/day (84) | No AE’s reported (R) | - | - | No effect |
| ALVES (2013) | DBRPCT (T) | 32 (16:16) | Pre-menopausal (48.7 ± 10.1:49 ± 8.4) | C 20 g/day (5) then 5 g/day (107) | No AE’s reported (S) | No difference between pre-and post-intervention | - | - |
| ATAKAN (2018) | DBRPCT (E) | 30 (15:15) | Pre-menopausal (19.8 ± 1.1, all) | L 0.25 g/kg/day (7) | - | - | - | No effect |
| AYOAMA (2003) | DBRPCPT (E) | 26 (13:13) | Pre-menopausal (19.4 ± 0.8:19.3 ± 0.7) | C 20 g/day (7) then 3 g/day (14) | - | No difference between pre-and post-intervention | No difference between pre-and post-intervention | No effect |
| BENTON (2011) | DBRPCT (T) | 121 (61:60) | Pre-menopausal (20.3 ± 2.1, all) | L 20 g/day (5) | AE’s reported across study (R) | - | - | - |
| BRENNER (2000) | DBRPCT (E) | 20 (10:10) | Pre-menopausal (18.1 ± 7.6:19.5 ± 8.5) | C 20 g/day, (7) then 2 g/day (28) | One AE reported (R) | No difference between pre-and post-intervention | No difference between pre-and post-intervention | No effect |
| CANETE (2006) | SBRPCT (E) | 16 (10:6) | Post-menopausal (67 ± 6:68 ± 4) | L 0.3 g/kg/day (7) | No AE’s reported (S) | No difference between pre-and post-intervention | No difference between pre-and post-intervention | No effect |
| CHILIBECK (2015) | DBRPCPT (T) | 47 (23:24) | Post-menopausal (57 ± 4:57 ± 7) | M 0.1 g/kg/day (365) | AE’s reported across study (R) | No difference between pre-and post-intervention | No difference between pre-and post-intervention | No effect |
| COX (2002) | DBPCMT (E) | 12 (6:6) | Pre-menopausal (22.1 ± 5.4, all) | L 20 g/day (6) | AE’s reported across study (S) | - | - | Effect |
| ECKERSON (2006) | DBRPCMXT (E) | 10 (All) | Pre-menopausal (22 ± 5, all) | L 20 g/day (5) | - | - | - | No effect |
| FERGUSON (2006) | DBRPCMT (E) | 26 (13:13) | Pre-menopausal (24.6 ± 3.4, all) | C 0.3 g/kg/day (7) then 0.03 g/kg/day (63) | No AE’s reported (S) | - | - | No effect |
| FORBES (2017) | DBRPCT (E) | 18 (9:8) | Pre-menopausal (23.8 ± 4.7:22.4 ± 3) | C 0.3 g/kg/day (5) then 0.1 g/kg/day (28) | - | - | - | No effect |
| GOTSHALK (2008) | DBRPCMT (E) | 30 (15:12) | Post-menopausal (63.3 ± 4.6:63 ± 3.8) | L 0.3 g/kg/day (7) | No AE’s reported (S) | No difference between pre-and post-intervention | No difference between pre-and post-intervention | Effect |
| GUALANO (2014) | DBRPCPT (T) | 74 (37:37) | Post-menopausal (66.1 ± 4.8:66.3 ± 6) | C 20 g/day (5) then 5 g/day (161) | No AE’s reported (S) | No difference between pre-and post-intervention | No difference between pre-and post-intervention | Effect |
| HAMILTON (2000) | SBRCMT (E) | 28 (11:13) | Pre-menopausal (22.5 ± 4.23:23.9 ± 4.76) | L 30 g/day (9) | AE’s reported across study (S) | - | - | No effect |
| HELLEM (2015) | PSOL (T) | 14 (All) | Pre-menopausal (37.4 ± 9.9) | M 5 g/day (56) | AE’s reported across study (R) | Increase in serum creatinine reported | - | |
| KAMBIS (2003) | DBRPCMT (E) | 22 (11:11) | Pre-menopausal (63.2 ± 6.68:63 ± 6.08) | L 20 g/day (5) | No AE’s reported (S) | - | - | No effect |
| KONDO (2011) | PSOL (T) | 5 (All) | Pre-menopausal 14–18) | M 4 g/day (56) | AE’s reported across study (R) | No abnormal levels reported | - | |
| KONDO * (2016) | DBRPCDRT (T) | 33 (25:8) | Pre-menopausal (13–20) | M 2, 4 or 10 g/day (56) | AE’s reported across study (R) | No difference between pre-and post-intervention | - | No effect |
| LARSON-MEYER (2000) | DBRPCT (E) | 14 (7:7) | Pre-menopausal (19 ± 1.5:19.3 ± 1.4) | C 15 g/day (5) then 5 g/day (86) | AE’s reported across study (R) | - | - | No effect |
| LEADER (2009) | OLT (T) | 30 (All) | Pre-menopausal (over 18) | M 3 g/day (7) then 5 g/day (49) | No AE’s reported (S) | - | - | |
| LEDFORD (1999) | DBRPCMXT (E) | 10 (All) | Pre-menopausal (26 ± 4:28 ± 7) | L 20 g/day (5) | One AE reported (U) | - | - | No effect |
| LOBO (2015) | DBRPCPT (T) | 149 (74:75) | Post-menopausal (58 ± 5:58 ± 6) | M 1 g/day (365) | AE’s reported across study (S) | No difference between pre-and post-intervention | No difference between pre-and post-intervention | No effect |
| LYOO (2012) | DBRPCT (T) | 52 (25:27) | Pre-menopausal (45.7 ± 12.7:47.5 ± 9.5) | M 3 g/day (7) then 5 g/day for (49) | AE’s reported across study (R) | No abnormal levels reported | No difference between pre-and post-intervention 3 showed mild increase in liver enzymes (2Cr,1Pl) | - |
| NEVES (2011) | DBRPCT (T) | 26 (13:13) | Post-menopausal (59 ± 3:57 ± 3) | C 20 g/day (7) then 5 g/day (77) | No AE’s reported (R) | No difference between pre-and post-intervention | - | No effect |
| RAMIREZ-CAMPILLOᵆ (2016) | DBRPCT (E) | 33 (10:10:10) | Pre-menopausal (23.1 ± 3.4:22.9 ± 1.7:22.5 ± 2.1) | C 20 g/day (7) then 5 g/day (35) | AE’s reported across study (S) | - | - | Effect |
| SILVA (1996) | DBRPCT (E) | 16 (8:8) | Pre-menopausal (16.3 ± 1.8:15.7 ± 1.2) | L 20 g/day (21) | No AE’s reported (S) | - | No effect | |
| THOMPSON (1996) | RPCT (E) | 10 (−) | Pre-menopausal (university students) | M 2 g/day (42) | - | - | - | No effect |
| VANDENBERGHE (1997) | DBPCT (E) | 19 (10:9) | Pre-menopausal (19–22) | C 20 g/day (4) then 5 g/day (73) | No AE’s reported (S) | No abnormal levels reported | Effect | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Guingand, D.L.; Palmer, K.R.; Snow, R.J.; Davies-Tuck, M.L.; Ellery, S.J. Risk of Adverse Outcomes in Females Taking Oral Creatine Monohydrate: A Systematic Review and Meta-Analysis. Nutrients 2020, 12, 1780. https://doi.org/10.3390/nu12061780
de Guingand DL, Palmer KR, Snow RJ, Davies-Tuck ML, Ellery SJ. Risk of Adverse Outcomes in Females Taking Oral Creatine Monohydrate: A Systematic Review and Meta-Analysis. Nutrients. 2020; 12(6):1780. https://doi.org/10.3390/nu12061780
Chicago/Turabian Stylede Guingand, Deborah L., Kirsten R. Palmer, Rodney J. Snow, Miranda L. Davies-Tuck, and Stacey J. Ellery. 2020. "Risk of Adverse Outcomes in Females Taking Oral Creatine Monohydrate: A Systematic Review and Meta-Analysis" Nutrients 12, no. 6: 1780. https://doi.org/10.3390/nu12061780
APA Stylede Guingand, D. L., Palmer, K. R., Snow, R. J., Davies-Tuck, M. L., & Ellery, S. J. (2020). Risk of Adverse Outcomes in Females Taking Oral Creatine Monohydrate: A Systematic Review and Meta-Analysis. Nutrients, 12(6), 1780. https://doi.org/10.3390/nu12061780






