Association of Stress-Induced Hyperglycemia and Diabetic Hyperglycemia with Mortality in Patients with Traumatic Brain Injury: Analysis of a Propensity Score-Matched Population
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Inclusion Criteria for Patient Groups: Diagnostic Methods
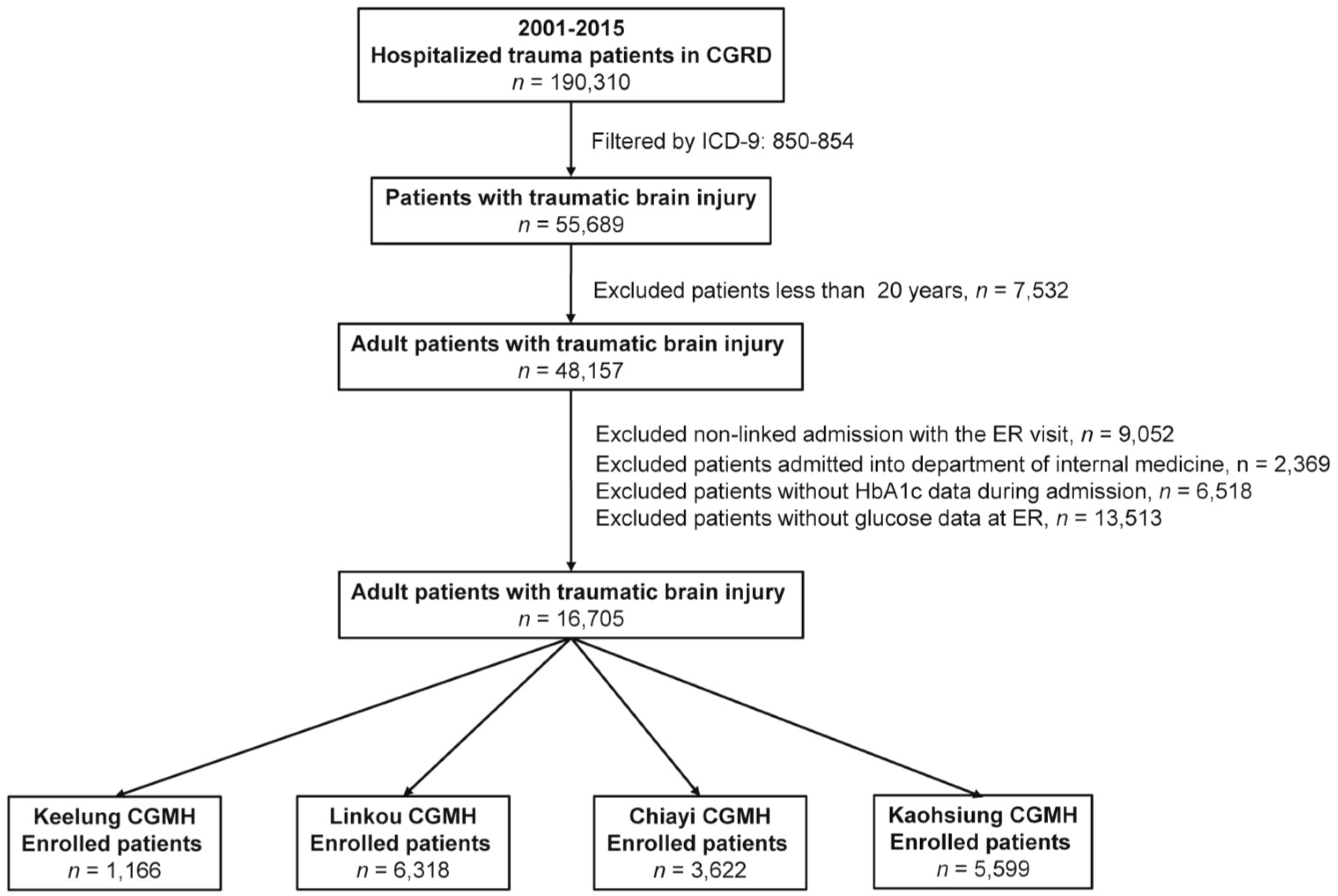
2.3. Study Population
2.4. Statistical Analysis
3. Results
3.1. Demographics and Patient Outcomes
3.2. Outcomes of the Matched Patients
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| (CGMH) | Chang Gung Memorial Hospital |
| (CGRD) | Chang Gung Research Database |
| (DH) | Diabetic hyperglycemia |
| (ICD-9) | International Classification of Diseases, Ninth Revision |
| (DM) | Diabetes mellitus |
| (DN) | Diabetic normoglycemia |
| (HbA1c) | Glycated hemoglobin |
| (NDN) | Nondiabetic normoglycemia |
| (SIH) | Stress-induced hyperglycemia |
| (TBI) | Traumatic brain injury |
References
- Oddo, M.; Schmidt, J.M.; Mayer, S.A.; Chiolero, R.L. Glucose control after severe brain injury. Curr. Opin. Clin. Nutr. Metab. Care 2008, 11, 134–139. [Google Scholar] [CrossRef]
- Rostami, E.; Bellander, B.M. Monitoring of glucose in brain, adipose tissue, and peripheral blood in patients with traumatic brain injury: A microdialysis study. J. Diabetes Sci. Technol. 2011, 5, 596–604. [Google Scholar] [CrossRef] [Green Version]
- Alexiou, G.A.; Lianos, G.; Fotakopoulos, G.; Michos, E.; Pachatouridis, D.; Voulgaris, S. Admission glucose and coagulopathy occurrence in patients with traumatic brain injury. Brain Inj. 2014, 28, 438–441. [Google Scholar] [CrossRef]
- Prisco, L.; Iscra, F.; Ganau, M.; Berlot, G. Early predictive factors on mortality in head injured patients: A retrospective analysis of 112 traumatic brain injured patients. J. Neurosurg. Sci. 2012, 56, 131–136. [Google Scholar]
- Diagnosis and classification of diabetes mellitus. Diabetes Care 2012, 35 (Suppl. 1), S64–S71. [CrossRef] [Green Version]
- Khajavikhan, J.; Vasigh, A.; Kokhazade, T.; Khani, A. Association between Hyperglycaemia with Neurological Outcomes Following Severe Head Trauma. J. Clin. Diagn. Res. 2016, 10, PC11. [Google Scholar] [CrossRef]
- Bosarge, P.L.; Shoultz, T.H.; Griffin, R.L.; Kerby, J.D. Stress-induced hyperglycemia is associated with higher mortality in severe traumatic brain injury. J. Trauma Acute Care Surg. 2015, 79, 289–294. [Google Scholar] [CrossRef]
- Kinoshita, K. Traumatic brain injury: Pathophysiology for neurocritical care. J. Intensive Care 2016, 4, 29. [Google Scholar] [CrossRef] [Green Version]
- Smit, J.W.; Romijn, J.A. Acute insulin resistance in myocardial ischemia: Causes and consequences. Semin. Cardiothorac. Vasc. Anesth. 2006, 10, 215–219. [Google Scholar] [CrossRef]
- Rau, C.S.; Wu, S.C.; Chen, Y.C.; Chien, P.C.; Hsieh, H.Y.; Kuo, P.J.; Hsieh, C.H. Mortality Rate Associated with Admission Hyperglycemia in Traumatic Femoral Fracture Patients Is Greater Than Non-Diabetic Normoglycemic Patients but Not Diabetic Normoglycemic Patients. Int. J. Environ. Res. Public Health 2017, 15, 28. [Google Scholar] [CrossRef] [Green Version]
- Richards, J.E.; Kauffmann, R.M.; Zuckerman, S.L.; Obremskey, W.T.; May, A.K. Relationship of hyperglycemia and surgical-site infection in orthopaedic surgery. J. Bone Jt. Surg. Am. 2012, 94, 1181–1186. [Google Scholar] [CrossRef] [Green Version]
- Richards, J.E.; Kauffmann, R.M.; Obremskey, W.T.; May, A.K. Stress-induced hyperglycemia as a risk factor for surgical-site infection in nondiabetic orthopedic trauma patients admitted to the intensive care unit. J. Orthop. Trauma 2013, 27, 16–21. [Google Scholar] [CrossRef] [Green Version]
- Mraovic, B.; Suh, D.; Jacovides, C.; Parvizi, J. Perioperative hyperglycemia and postoperative infection after lower limb arthroplasty. J. Diabetes Sci. Technol. 2011, 5, 412–418. [Google Scholar] [CrossRef] [Green Version]
- Leto, R.; Desruelles, D.; Gillet, J.B.; Sabbe, M.B. Admission hyperglycaemia is associated with higher mortality in patients with hip fracture. Eur. J. Emerg. Med. 2015, 22, 99–102. [Google Scholar] [CrossRef]
- Bonizzoli, M.; Zagli, G.; Lazzeri, C.; Degl’Innocenti, S.; Gensini, G.; Peris, A. Early insulin resistance in severe trauma without head injury as outcome predictor? A prospective, monocentric pilot study. Scand. J. Trauma Resusc. Emerg. Med. 2012, 20, 69. [Google Scholar] [CrossRef] [Green Version]
- Rau, C.S.; Wu, S.C.; Chen, Y.C.; Chien, P.C.; Hsieh, H.Y.; Kuo, P.J.; Hsieh, C.H. Stress-Induced Hyperglycemia, but Not Diabetic Hyperglycemia, Is Associated with Higher Mortality in Patients with Isolated Moderate and Severe Traumatic Brain Injury: Analysis of a Propensity Score-Matched Population. Int. J. Environ. Res. Public Health 2017, 14, 1340. [Google Scholar] [CrossRef] [Green Version]
- Tsai, M.S.; Lin, M.H.; Lee, C.P.; Yang, Y.H.; Chen, W.C.; Chang, G.H.; Tsai, Y.T.; Chen, P.C.; Tsai, Y.H. Chang Gung Research Database: A multi-institutional database consisting of original medical records. Biomed. J. 2017, 40, 263–269. [Google Scholar] [CrossRef]
- Hsieh, C.H.; Hsu, S.Y.; Hsieh, H.Y.; Chen, Y.C. Differences between the sexes in motorcycle-related injuries and fatalities at a Taiwanese level I trauma center. Biomed. J. 2017, 40, 113–120. [Google Scholar] [CrossRef]
- Hsieh, C.H.; Liu, H.T.; Hsu, S.Y.; Hsieh, H.Y.; Chen, Y.C. Motorcycle-related hospitalizations of the elderly. Biomed. J. 2017, 40, 121–128. [Google Scholar] [CrossRef]
- Hsieh, C.H.; Chen, Y.C.; Hsu, S.Y.; Hsieh, H.Y.; Chien, P.C. Defining polytrauma by abbreviated injury scale >/= 3 for a least two body regions is insufficient in terms of short-term outcome: A cross-sectional study at a level I trauma center. Biomed. J. 2018, 41, 321–327. [Google Scholar] [CrossRef]
- Ho Chan, W.S. Taiwan’s healthcare report 2010. EPMA J. 2010, 1, 563–585. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Christiansen, C.; Johansen, M.; Christensen, S.; O’Brien, J.M.; Tønnesen, E.; Sørensen, H. Preadmission metformin use and mortality among intensive care patients with diabetes: A cohort study. Crit. Care 2013, 17, R192. [Google Scholar] [CrossRef] [Green Version]
- Shao, S.C.; Chan, Y.Y.; Kao Yang, Y.H.; Lin, S.J.; Hung, M.J.; Chien, R.N.; Lai, C.C.; Lai, E.C. The Chang Gung Research Database-A multi-institutional electronic medical records database for real-world epidemiological studies in Taiwan. Pharmacoepidemiol. Drug Saf. 2019, 28, 593–600. [Google Scholar] [CrossRef] [PubMed]
- Tsai, M.S.; Lai, C.H.; Lee, C.P.; Yang, Y.H.; Chen, P.C.; Kang, C.J.; Chang, G.H.; Tsai, Y.T.; Lu, C.H.; Chien, C.Y.; et al. Mortality in tongue cancer patients treated by curative surgery: A retrospective cohort study from CGRD. PeerJ 2016, 4, e2794. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Keelung CGMH | NDN (n = 950) | DN (n = 56) | SIH (n = 104) | DH (n = 56) |
| Female, n (%) | 319(33.6) | 23(41.1) | 41(39.4) | 22(39.3) |
| Age (years) | 49.6 ± 20.3 | 72.8 ± 9.1 | 51.7 ± 19.4 | 62.4 ± 14.1 |
| GCS | 13.9 ± 2.8 | 13.9 ± 2.8 | 11.0 ± 4.6 | 12.3 ± 3.8 |
| Mortality, n (%) | 44(4.6) | 2(3.6) | 20(19.2) | 6(10.7) |
| Linkou CGMH | NDN (n = 4729) | DN (n = 279) | SIH (n = 811) | DH (n = 499) |
| Female, n (%) | 1276(27.0) | 102(36.6) | 262(32.3) | 179(35.9) |
| Age (years) | 47.9 ± 20.1 | 69.4 ± 12.4 | 49.7 ± 20.4 | 64.8 ± 13.4 |
| GCS | 11.8 ± 4.2 | 12.3 ± 3.8 | 8.2 ± 4.6 | 11.0 ± 4.6 |
| Mortality, n (%) | 257(5.4) | 15(5.4) | 172(21.2) | 44(8.8) |
| Chiayi CGMH | NDN (n = 2884) | DN (n = 180) | SIH (n = 305) | DH (n = 253) |
| Female, n (%) | 1022(35.4) | 63(35.0) | 104(34.1) | 91(36.0) |
| Age (years) | 51.6 ± 20.5 | 68.0 ± 12.0 | 53.8 ± 19.8 | 64.5 ± 12.3 |
| GCS | 13.7 ± 2.9 | 13.9 ± 2.7 | 10.5 ± 5.0 | 13.5 ± 3.1 |
| Mortality, n (%) | 60(2.1) | 5(2.8) | 33(10.8) | 9(3.6) |
| Kaohsiung CGMH | NDN (n = 4275) | DN (n = 352) | SIH (n = 496) | DH (n = 476) |
| Female, n (%) | 1412(33.0) | 154(43.8) | 165(33.3) | 202(42.4) |
| Age (years) | 48.4 ± 20.1 | 68.3 ± 11.3 | 50.2 ± 19.6 | 62.5 ± 12.9 |
| GCS | 12.7 ± 3.7 | 13.3 ± 3.2 | 8.5 ± 4.9 | 12.1 ± 4.1 |
| Mortality, n (%) | 157(3.7) | 12(3.4) | 90(18.1) | 36(7.6) |
| DN vs. NDN | SIH vs. NDN | DH vs. NDN | SIH vs. DH | |||||
|---|---|---|---|---|---|---|---|---|
| OR (95% CI) | p | OR (95% CI) | p | OR (95% CI) | p | OR (95% CI) | p | |
| Keelung CGMH | ||||||||
| Female | 1.38 (0.80–2.39) | 0.250 | 1.29 (0.85–1.95) | 0.233 | 1.28 (0.74–2.26) | 0.381 | 1.01 (0.52–1.96) | 0.986 |
| Age | ― | <0.001 | ― | 0.301 | ― | <0.001 | ― | <0.001 |
| GCS | ― | 0.855 | ― | <0.001 | ― | 0.005 | ― | 0.064 |
| Mortality | 0.76 (0.18–3.23) | 1.000 | 4.90 (2.76–8.70) | <0.001 | 2.47 (1.01–6.07) | 0.053 | 1.98 (0.75–5.27) | 0.164 |
| Linkou CGMH | ||||||||
| Female | 1.56 (1.21–2.01) | 0.001 | 1.29 (1.10–1.52) | 0.002 | 1.51 (1.25–1.84) | <0.001 | 0.85 (0.68–1.08) | 0.185 |
| Age | ― | <0.001 | ― | 0.017 | ― | <0.001 | ― | <0.001 |
| GCS | ― | 0.040 | ― | <0.001 | ― | <0.001 | ― | <0.001 |
| Mortality | 0.99(0.58-1.69) | 0.967 | 4.68 (3.80–5.78) | <0.001 | 1.68 (1.21–2.35) | 0.002 | 2.78 (1.96–3.96) | <0.001 |
| Chiayi CGMH | ||||||||
| Female | 0.98 (0.72–1.35) | 0.905 | 0.94 (0.74–1.21) | 0.642 | 1.02 (0.78–1.34) | 0.865 | 0.92 (0.65–1.31) | 0.645 |
| Age | ― | <0.001 | ― | 0.067 | ― | <0.001 | ― | <0.001 |
| GCS | ― | 0.158 | ― | <0.001 | ― | 0.326 | ― | <0.001 |
| Mortality | 1.35 (0.53–3.39) | 0.430 | 5.71 (3.67–8.89) | <0.001 | 1.74 (0.85–3.54) | 0.125 | 3.29 (1.54–7.01) | 0.001 |
| Kaohsiung CGMH | ||||||||
| Female | 1.58 (1.27–1.97) | <0.001 | 1.01 (0.83–1.23) | 0.915 | 1.50 (1.23–1.81) | <0.001 | 0.68 (0.52–0.88) | 0.003 |
| Age | ― | <0.001 | ― | 0.059 | ― | <0.001 | ― | <0.001 |
| GCS | ― | 0.002 | ― | <0.001 | ― | <0.001 | ― | <0.001 |
| Mortality | 0.93 (0.51–1.68) | 0.800 | 5.81 (4.40–7.68) | <0.001 | 2.15 (1.48–3.12) | <0.001 | 2.71 (1.80–4.08) | <0.001 |
| Propensity Score–Matched Cohorts | |||||
|---|---|---|---|---|---|
| Keelung CGMH | |||||
| DN vs. NDN | DN (n = 56) | NDN (n = 56) | OR (95% CI) | p | SD |
| Sex | 1.00 (0.47–2.12) | 1.000 | 0.00% | ||
| Male | 33(58.9) | 33(58.9) | |||
| Female | 23(41.1) | 23(41.1) | |||
| Age | 72.8 ± 9.1 | 73.0 ± 9.1 | ― | 0.942 | −0.68% |
| GCS | 13.9 ± 2.8 | 14.1 ± 2.4 | ― | 0.668 | −3.42% |
| SIH vs. NDN | SIH (n = 102) | NDN (n = 102) | OR (95% CI) | p | SD |
| Sex | 1.00 (0.57–1.75) | 1.000 | 0.00% | ||
| Male | 62(60.8) | 62(60.8) | |||
| Female | 40(39.2) | 40(39.2) | |||
| Age | 51.4 ± 19.4 | 50.8 ± 18.5 | ― | 0.831 | 1.38% |
| GCS | 11.2 ± 4.5 | 11.2 ± 4.6 | ― | 0.939 | −0.72% |
| DH vs. NDN | DH (n = 53) | NDN (n = 53) | OR (95% CI) | p | SD |
| Sex | 1.00 (0.45–2.21) | 1.000 | 0.00% | ||
| Male | 34(64.2) | 34(64.2) | |||
| Female | 19(35.8) | 19(35.8) | |||
| Age | 61.5 ± 13.8 | 62.3 ± 14.8 | ― | 0.766 | −3.84% |
| GCS | 12.8 ± 3.3 | 13.0 ± 3.1 | ― | 0.760 | −3.92% |
| SIH vs. DH | SIH (n = 47) | DH (n = 47) | OR (95% CI) | p | SD |
| Sex | 1.00 (0.43–2.32) | 1.000 | 0.00% | ||
| Male | 30(63.8) | 30(63.8) | |||
| Female | 17(36.2) | 17(36.2) | |||
| Age | 61.1 ± 14.3 | 60.7 ± 13.3 | ― | 0.870 | 1.23% |
| GCS | 12.0 ± 4.1 | 12.2 ± 3.8 | ― | 0.797 | −3.02% |
| Linkou CGMH | |||||
| DN vs. NDN | DN (n = 279) | NDN (n = 279) | OR (95% CI) | p | SD |
| Sex | 1.00 (0.71–1.41) | 1.000 | 0.00% | ||
| Male | 177(63.4) | 177(63.4) | |||
| Female | 102(36.6) | 102(36.6) | |||
| Age | 69.4 ± 12.4 | 69.3 ± 12.5 | ― | 0.970 | 0.24% |
| GCS | 12.3 ± 3.8 | 12.3 ± 3.8 | ― | 0.956 | 0.44% |
| SIH vs. NDN | SIH (n = 810) | NDN (n = 810) | OR (95% CI) | p | SD |
| Sex | 1.00 (0.81–1.23) | 1.000 | 0.00% | ||
| Male | 549(67.8) | 549(67.8) | |||
| Female | 261(32.2) | 261(32.2) | |||
| Age | 49.7 ± 20.3 | 49.9 ± 20.0 | ― | 0.868 | −1.42% |
| GCS | 8.3 ± 4.6 | 8.3 ± 4.6 | ― | 0.961 | 0.12% |
| DH vs. NDN | DH (n = 499) | NDN (n = 499) | OR (95% CI) | p | SD |
| Sex | 1.00 (0.77–1.30) | 1.000 | 0.00% | ||
| Male | 320(64.1) | 320(64.1) | |||
| Female | 179(35.9) | 179(35.9) | |||
| Age | 64.8 ± 13.4 | 64.8 ± 13.4 | ― | 0.964 | 0.30% |
| GCS | 11.0 ± 4.6 | 11.0 ± 4.6 | ― | 0.972 | 0.26% |
| SIH vs. DH | SIH (n = 399) | DH (n = 399) | OR (95% CI) | p | SD |
| Sex | 1.00 (0.74–1.34) | 1.000 | 0.00% | ||
| Male | 268(67.2) | 268(67.2) | |||
| Female | 131(32.8) | 131(32.8) | |||
| Age | 63.4 ± 14.0 | 62.7 ± 13.5 | ― | 0.513 | 4.82% |
| GCS | 9.9 ± 4.7 | 10.2 ± 4.7 | ― | 0.308 | −5.67% |
| Chiayi CGMH | |||||
| DN vs. NDN | DN (n = 180) | NDN (n = 180) | OR (95% CI) | p | SD |
| Sex | 1.00 (0.65–1.54) | 1.000 | 0.00% | ||
| Male | 117(65.0) | 117(65.0) | |||
| Female | 63(35.0) | 63(35.0) | |||
| Age | 68.0 ± 12.0 | 68.0 ± 12.0 | ― | 0.993 | 0.07% |
| GCS | 13.9 ± 2.7 | 13.9 ± 2.7 | ― | 0.984 | 0.12% |
| SIH vs. NDN | SIH (n = 304) | NDN (n = 304) | OR (95% CI) | p | SD |
| Sex | 1.00 (0.72–1.40) | 1.000 | 0.00% | ||
| Male | 201(66.1) | 201(66.1) | |||
| Female | 103(33.9) | 103(33.9) | |||
| Age | 53.7 ± 19.8 | 54.0 ± 18.6 | ― | 0.868 | −1.32% |
| GCS | 10.5 ± 4.9 | 10.6 ± 4.9 | ― | 0.934 | −0.71% |
| DH vs. NDN | DH (n = 253) | NDN (n = 253) | OR (95% CI) | p | SD |
| Sex | 1.00 (0.70–1.44) | 1.000 | 0.00% | ||
| Male | 162(64.0) | 162(64.0) | |||
| Female | 91(36.0) | 91(36.0) | |||
| Age | 64.5 ± 12.3 | 64.5 ± 12.3 | ― | 0.986 | 0.14% |
| GCS | 13.5 ± 3.1 | 13.5 ± 3.1 | ― | 0.977 | 0.07% |
| SIH vs. DH | SIH (n = 178) | DH (n = 178) | OR (95% CI) | p | SD |
| Sex | 1.00 (0.65–1.55) | 1.000 | 0.00% | ||
| Male | 117(65.7) | 117(65.7) | |||
| Female | 61(34.3) | 61(34.3) | |||
| Age | 62.5 ± 14.3 | 62.8 ± 13.7 | ― | 0.839 | −1.71% |
| GCS | 12.9 ± 3.6 | 13.0 ± 3.5 | ― | 0.893 | −1.12% |
| Kaohsiung CGMH | |||||
| DN vs. NDN | DN (n = 352) | NDN (n = 352) | OR (95% CI) | p | SD |
| Sex | 1.00 (0.74–1.35) | 1.000 | 0.00% | ||
| Male | 198(56.3) | 198(56.3) | |||
| Female | 154(43.8) | 154(43.8) | |||
| Age | 68.3 ± 11.3 | 68.3 ± 11.2 | ― | 0.989 | 0.02% |
| GCS | 13.3 ± 3.2 | 13.3 ± 3.2 | ― | 0.944 | 0.06% |
| SIH vs. NDN | SIH (n = 496) | NDN (n = 496) | OR (95% CI) | p | SD |
| Sex | 1.00 (0.77–1.30) | 1.000 | 0.00% | ||
| Male | 331(66.7) | 331(66.7) | |||
| Female | 165(33.3) | 165(33.3) | |||
| Age | 50.2 ± 19.6 | 50.5 ± 19.0 | ― | 0.838 | −1.77% |
| GCS | 8.5 ± 4.9 | 8.5 ± 4.9 | ― | 0.954 | 0.05% |
| DH vs. NDN | DH (n = 476) | NDN (n = 476) | OR (95% CI) | p | SD |
| Sex | 1.11 (0.77–1.29) | 1.000 | 0.00% | ||
| Male | 274(57.6) | 274(57.6) | |||
| Female | 202(42.4) | 202(42.4) | |||
| Age | 62.5 ± 12.9 | 62.5 ± 13.0 | ― | 0.984 | −0.02% |
| GCS | 12.1 ± 4.1 | 12.1 ± 4.1 | ― | 0.975 | −0.07% |
| SIH vs. DH | SIH (n = 301) | DH (n = 301) | OR (95% CI) | p | SD |
| Sex | 1.00 (0.72–1.38) | 1.000 | 0.00% | ||
| Male | 177(58.8) | 177(58.8) | |||
| Female | 124(41.2) | 124(41.2) | |||
| Age | 59.8 ± 14.4 | 58.8 ± 13.5 | ― | 0.389 | 4.78% |
| GCS | 10.4 ± 4.8 | 10.7 ± 4.6 | ― | 0.362 | −5.92% |
| Propensity Score-Matched Populations | Mortality, Group: n (%) | OR (95% CI) | p | |
|---|---|---|---|---|
| Keelung CGMH | ||||
| DN (n = 56) vs. NDN (n = 56) | DN: 2(3.6) | NDN: 2(3.6) | 1.00 (0.06–15.99) | 1.000 |
| SIH (n = 102) vs. NDN (n = 102) | SIH: 18(17.6) | NDN: 9(8.8) | 3.25 (1.06–9.97) | 0.039 |
| DH (n = 53) vs. NDN (n = 53) | DH: 4(7.5) | NDN: 2(3.8) | 3.00 (0.31–28.84) | 0.341 |
| SIH (n = 47) vs. DH (n = 47) | SIH: 6(12.8) | DH: 5(10.6) | 1.25 (0.34–4.66) | 0.739 |
| Linkou CGMH | ||||
| DN (n = 279) vs. NDN (n = 279) | DN: 15(5.4) | NDN: 12(4.3) | 1.33 (0.56–3.16) | 0.514 |
| SIH (n = 810) vs. NDN (n = 810) | SIH: 171(21.1) | NDN: 106(13.1) | 2.08 (1.53–2.83) | <0.001 |
| DH (n = 499) vs. NDN (n = 499) | DH: 44(8.8) | NDN: 50(10.0) | 0.83 (0.51–1.35) | 0.461 |
| SIH (n = 399) vs. DH (n = 399) | SIH: 79(19.8) | DH: 39(9.8) | 2.82 (1.73–4.58) | <0.001 |
| Chiayi CGMH | ||||
| DN (n = 180) vs. NDN (n = 180) | DN: 5(2.8) | NDN: 4(2.2) | 1.25 (0.34–4.66) | 0.739 |
| SIH (n = 304) vs. NDN (n = 304) | SIH: 33(10.9) | NDN: 25(8.2) | 1.50 (0.80–2.82) | 0.209 |
| DH (n = 253) vs. NDN (n = 253) | DH: 9(3.6) | NDN: 5(2.0) | 2.33 (0.60–9.02) | 0.220 |
| SIH (n = 178) vs. DH (n = 178) | SIH: 14(7.9) | DH: 8(4.5) | 1.86 (0.74–4.66) | 0.187 |
| Kaohsiung CGMH | ||||
| DN (n = 352) vs. NDN (n = 352) | DN: 12(3.4) | NDN: 13(3.7) | 0.91 (0.39–2.14) | 0.827 |
| SIH (n = 496) vs. NDN (n = 496) | SIH: 90(18.1) | NDN: 66(13.3) | 1.63 (1.09–2.44) | 0.017 |
| DH (n = 476) vs. NDN (n = 476) | DH: 36(7.6) | NDN: 33(6.9) | 1.11 (0.66–1.87) | 0.691 |
| SIH (n = 301) vs. DH (n = 301) | SIH: 51(16.9) | DH: 32(10.6) | 1.91 (1.12–3.23) | 0.017 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsai, Y.-C.; Wu, S.-C.; Hsieh, T.-M.; Liu, H.-T.; Huang, C.-Y.; Chou, S.-E.; Su, W.-T.; Hsu, S.-Y.; Hsieh, C.-H. Association of Stress-Induced Hyperglycemia and Diabetic Hyperglycemia with Mortality in Patients with Traumatic Brain Injury: Analysis of a Propensity Score-Matched Population. Int. J. Environ. Res. Public Health 2020, 17, 4266. https://doi.org/10.3390/ijerph17124266
Tsai Y-C, Wu S-C, Hsieh T-M, Liu H-T, Huang C-Y, Chou S-E, Su W-T, Hsu S-Y, Hsieh C-H. Association of Stress-Induced Hyperglycemia and Diabetic Hyperglycemia with Mortality in Patients with Traumatic Brain Injury: Analysis of a Propensity Score-Matched Population. International Journal of Environmental Research and Public Health. 2020; 17(12):4266. https://doi.org/10.3390/ijerph17124266
Chicago/Turabian StyleTsai, Yu-Chin, Shao-Chun Wu, Ting-Min Hsieh, Hang-Tsung Liu, Chun-Ying Huang, Sheng-En Chou, Wei-Ti Su, Shiun-Yuan Hsu, and Ching-Hua Hsieh. 2020. "Association of Stress-Induced Hyperglycemia and Diabetic Hyperglycemia with Mortality in Patients with Traumatic Brain Injury: Analysis of a Propensity Score-Matched Population" International Journal of Environmental Research and Public Health 17, no. 12: 4266. https://doi.org/10.3390/ijerph17124266






