Continuous-Flow Synthesis of β-Amino Acid Esters by Lipase-Catalyzed Michael Addition of Aromatic Amines
Abstract
1. Introduction
2. Results and Discussion
2.1. Effect of Reaction Media
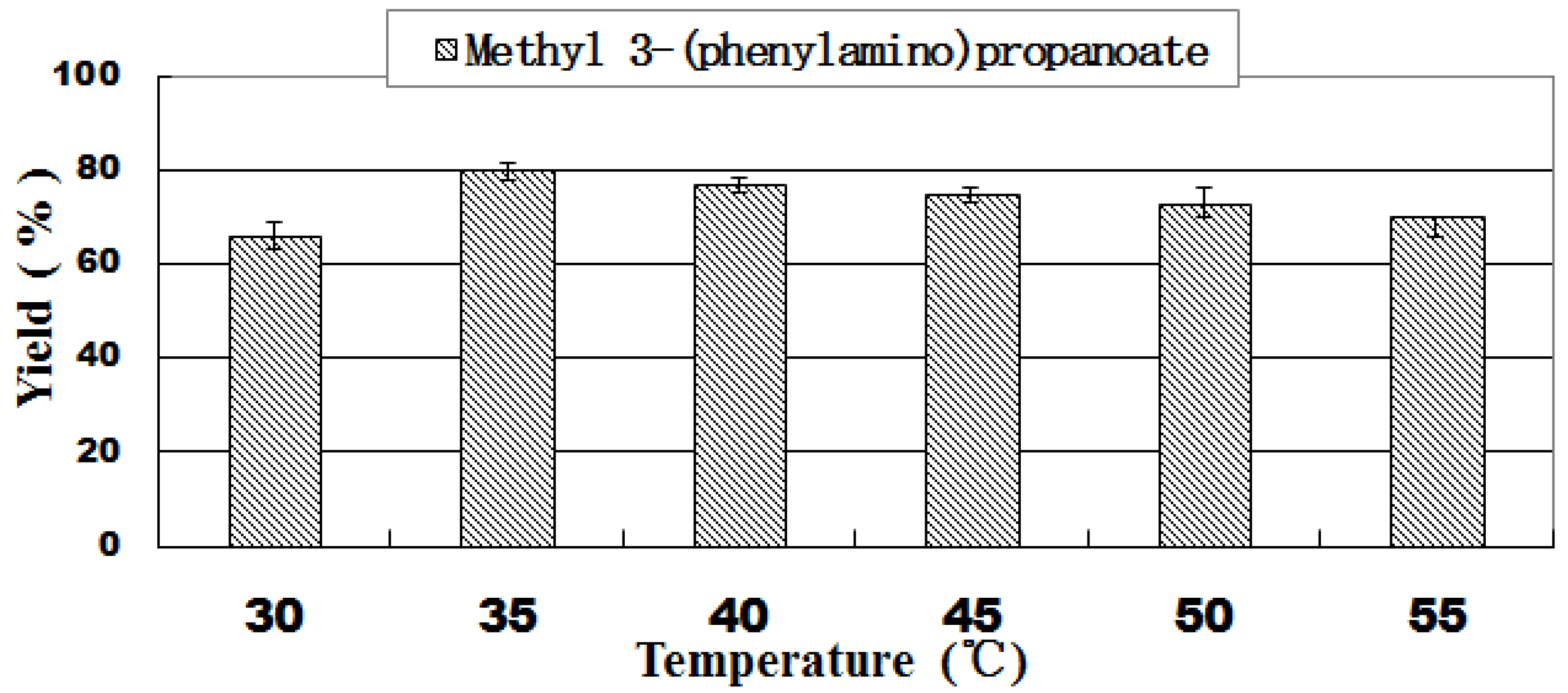
2.2. Effect of Reaction Temperature
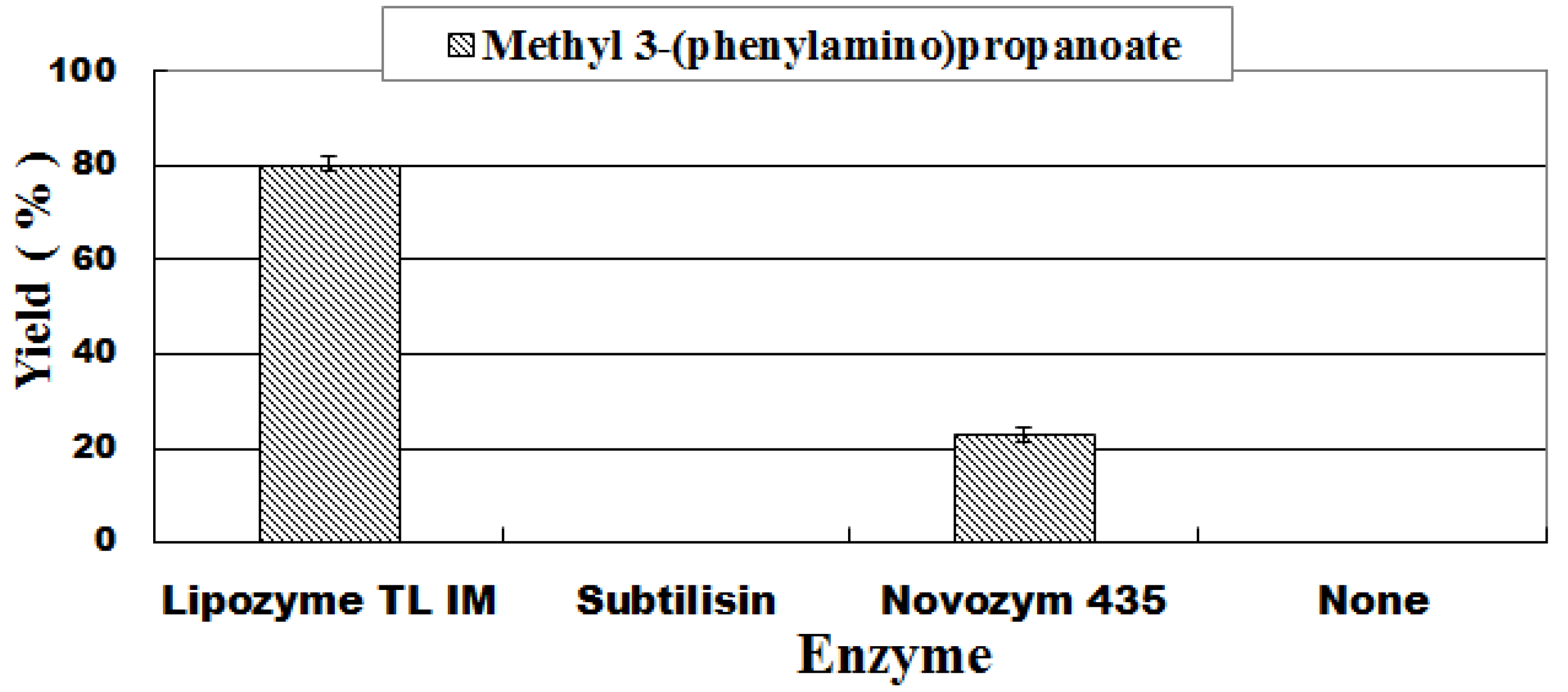
2.3. Catalyst Screening
2.4. Effect of Substrate Ratio
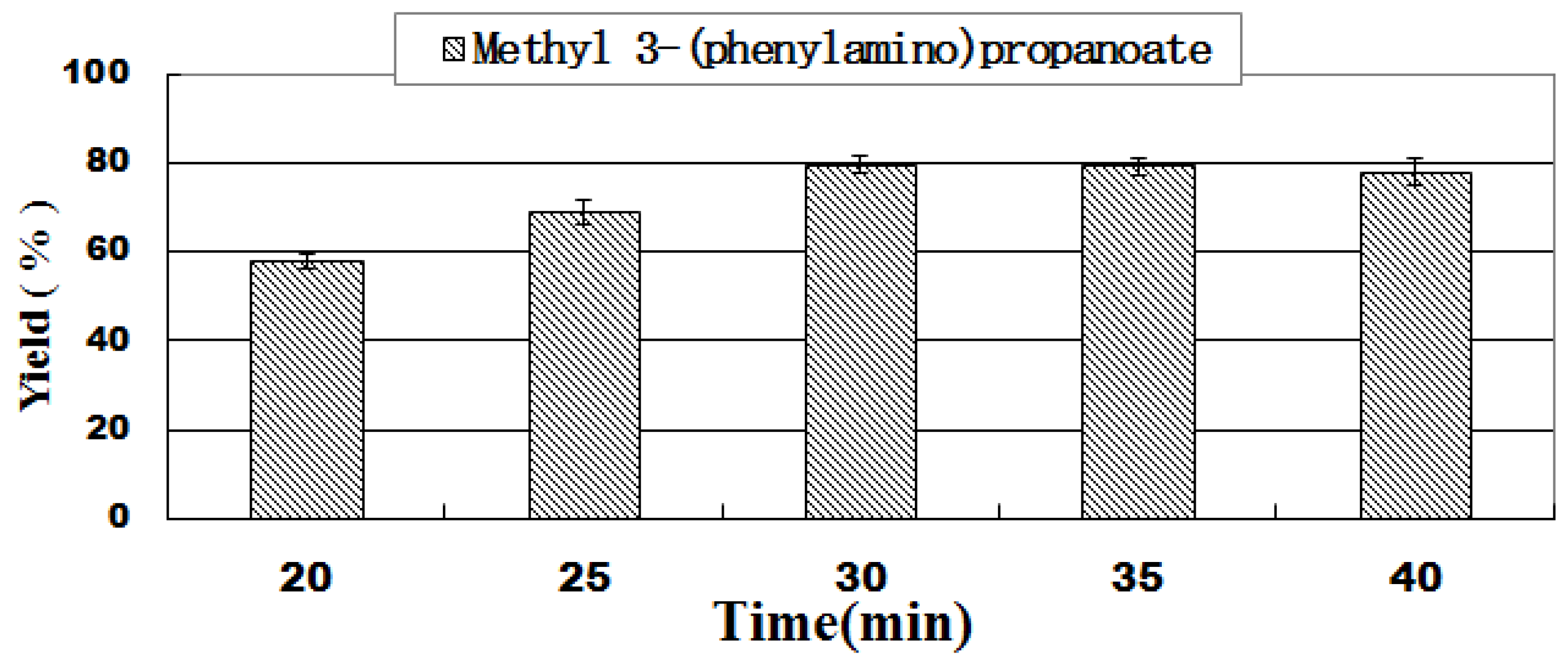
2.5. Effect of Residence Time
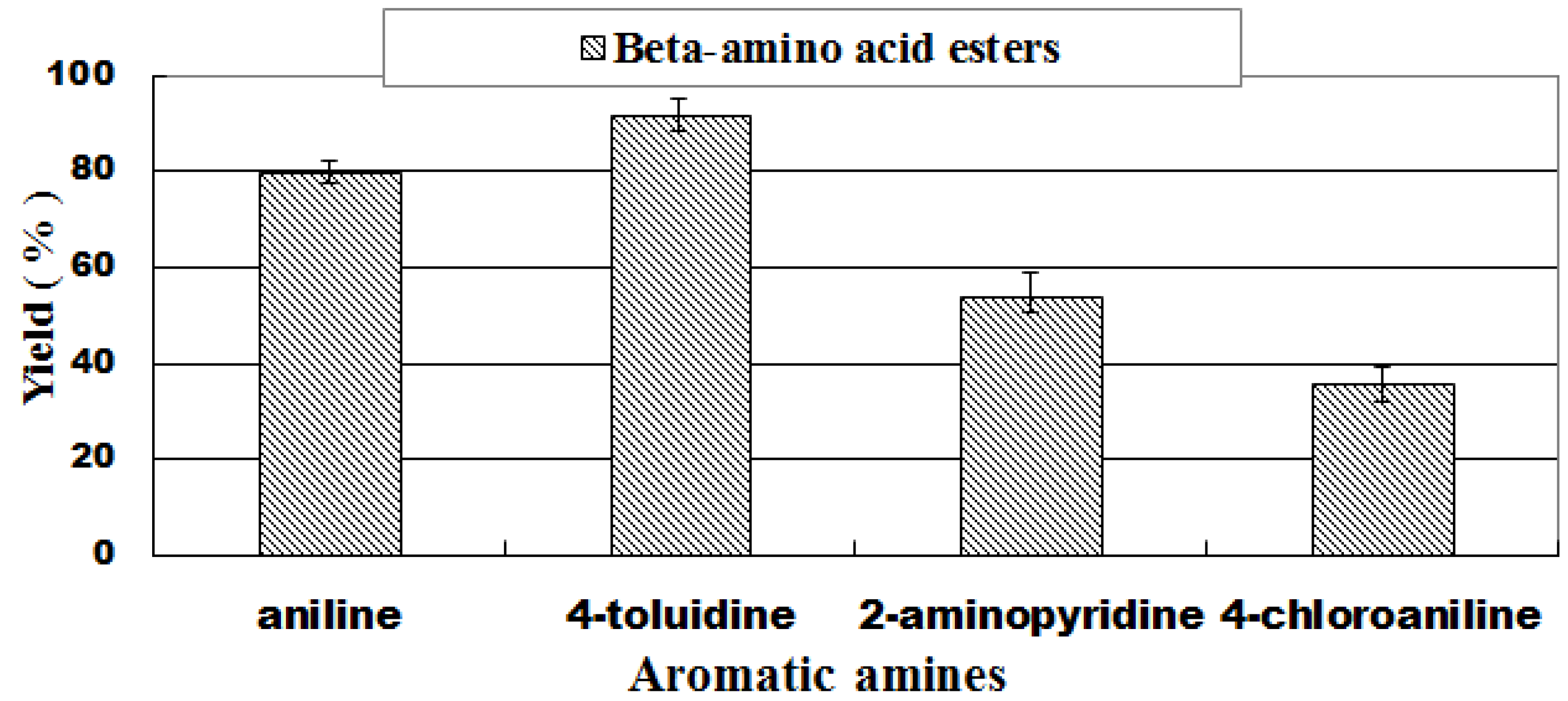
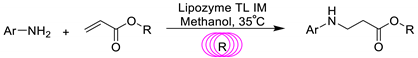
2.6. The Effect of Aromatic Amine Structure on the Reaction
2.7. Enzymatic Synthesis of β-Amino Acid Ester in Continuous-Flow Microreactor and Batch Bioreactor
2.8. Combined Effects of Process Parameters
2.9. The Scope and Limitation for the β-Amino Acid Ester Synthesis Methodology
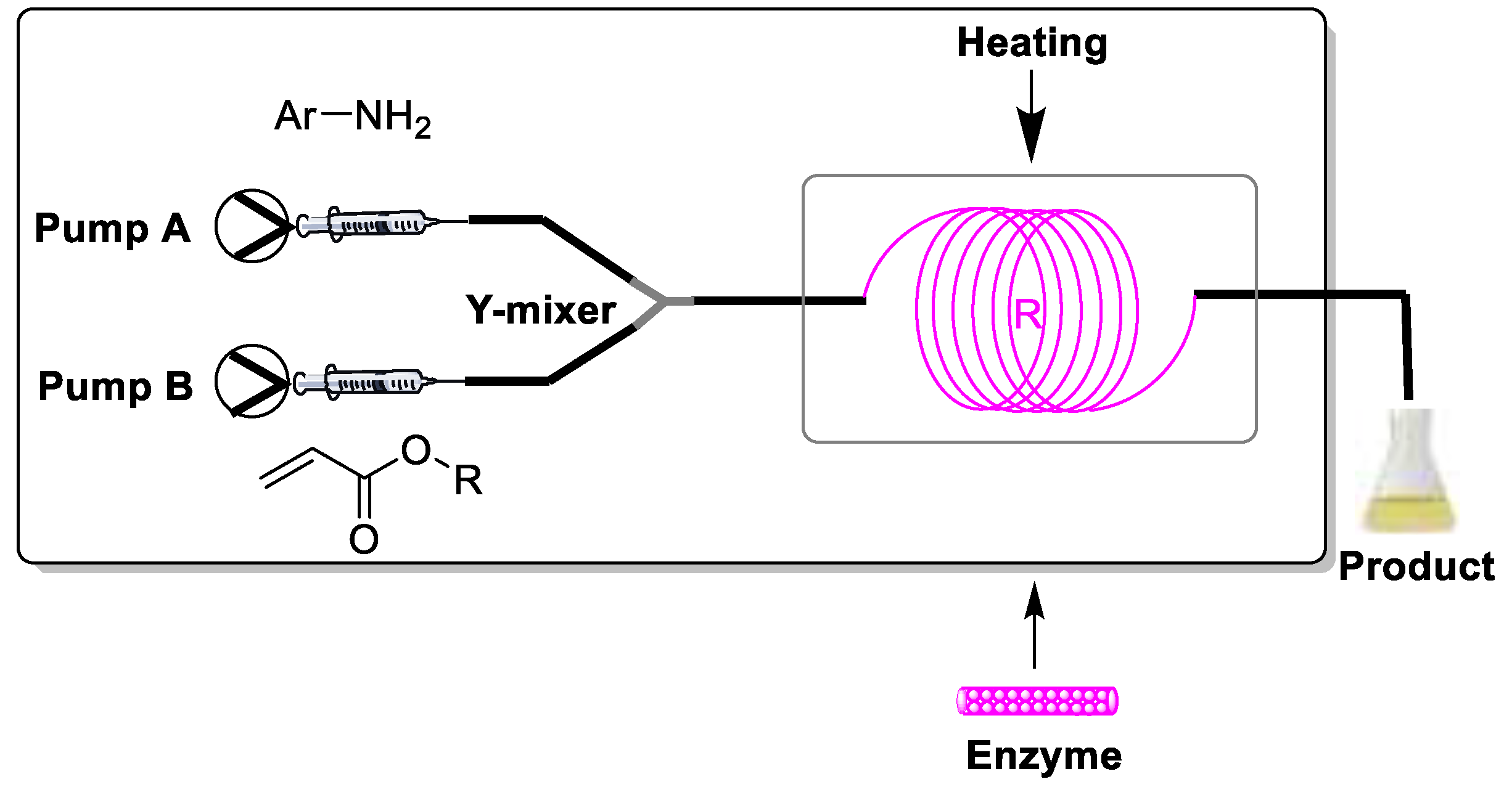
3. Materials and Methods
3.1. Materials
3.2. β-Amino Acid Ester Synthesis Operating Conditions
3.2.1. Experimental Setup
3.2.2. General Procedure for β-Amino Acid Ester Synthesis in Continuous Flow Microreactor
3.3. Analytical Methods
3.3.1. Thin-Layer Chromatography
3.3.2. High-Performance Liquid Chromatography (HPLC)
3.3.3. Nuclear Magnetic Resonance (NMR) and Electrospray Ionization Mass Spectrometry (ESI/MS) Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cardillo, G.; Tomasini, C. Asymmetric synthesis of β-amino acids and α-substituted β-amino acids. Chem. Soc. Rev. 1996, 25, 117. [Google Scholar] [CrossRef]
- Wang, W.B.; Roskamp, E.J. Conversion of β-amino esters to β-lactams via tin (II) amides. J. Am. Chem. Soc. 1993, 115, 9417–9420. [Google Scholar] [CrossRef]
- Xiao, H.; Li, P.; Hu, D.; Song, B. Synthesis and anti-TMV activity of novel β-amino acid ester derivatives containing quinazoline and benzothiazole moieties. Bioorg. Med. Chem. Lett. 2014, 24, 3452–3454. [Google Scholar] [CrossRef]
- Villalba, M.L.; Enrique, A.V.; Higgs, J.; Castaño, R.A.; Goicoechea, S.; Taborda, F.D.; Gavernet, L.; Lick, I.D.; Marder, M.; Blanch, L.E.B. Novel sulfamides and sulfamates derived from amino esters: Synthetic studies and anticonvulsant activity. Eur. J. Pharmacol. 2016, 774, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Karypidou, K.; Ribone, S.R.; Quevedo, M.A.; Persoons, L.; Pannecouque, C.; Helsen, C.; Claessens, F.; Dehaen, W. Synthesis, biological evaluation and molecular modeling of a novel series of fused 1,2,3-triazoles as potential anti-coronavirus agents. Bioorg. Med. Chem. Lett. 2018, 28, 3472–3476. [Google Scholar] [CrossRef] [PubMed]
- Ling, F.; Xiao, L.; Fang, L.; Lv, Y.; Zhong, W. Copper catalysis for nicotinate synthesis through β- alkenylation/cyclization of saturated ketones with β -enamino esters. Adv. Synth. Catal. 2017, 360, 444–448. [Google Scholar] [CrossRef]
- Gong, J.-H.; Wang, Y.; Xing, L.; Cui, P.-F.; Qiao, J.-B.; He, Y.-J.; Jiang, H.-L. Biocompatible fluorinated poly (β-amino ester)s for safe and efficient gene therapy. Int. J. Pharm. 2018, 535, 180–193. [Google Scholar] [CrossRef]
- Liu, Y.; Li, Y.; Keskin, D.; Shi, L. Poly (β-amino esters): Synthesis, formulations, and their biomedical applications. Adv. Heal. Mater. 2018, 8, 1801359. [Google Scholar] [CrossRef]
- Liu, Q.; Shi, W.-K.; Ren, S.-Z.; Ni, W.-W.; Li, W.-Y.; Chen, H.-M.; Liu, P.; Yuan, J.; He, X.-S.; Liu, J.-J.; et al. Arylamino containing hydroxamic acids as potent urease inhibitors for the treatment of Helicobacter pylori infection. Eur. J. Med. Chem. 2018, 156, 126–136. [Google Scholar] [CrossRef]
- A Alsharif, M.; Khan, D.; Mukhtar, S.; Alahmdi, M.I.; Naseem, A. KOt bu-mediated aza-Michael addition of aromatic amines or N-phenylurea to 3-nitro-2-phenyl-2H-chromenes and sequential aerobic dehydrogenation. Eur. J. Org. Chem. 2018, 2018, 3454–3463. [Google Scholar] [CrossRef]
- Xu, X.; Zhang, X.; Wang, Z.; Kong, M. HOTf-catalyzed intermolecular hydroamination reactions of alkenes and alkynes with anilines. RSC Adv. 2015, 5, 40950–40952. [Google Scholar] [CrossRef]
- Rani, P.; Srivastava, R.K. Nucleophilic addition of amines, alcohols, and thiophenol with epoxide/olefin using highly efficient zirconium metal organic framework heterogeneous catalyst. RSC Adv. 2015, 5, 28270–28280. [Google Scholar] [CrossRef]
- Payra, S.; Saha, A.; Banerjee, S. On-water magnetic NiFe 2 O 4 nanoparticle-catalyzed Michael additions of active methylene compounds, aromatic/aliphatic amines, alcohols and thiols to conjugated alkenes. RSC Adv. 2016, 6, 95951–95956. [Google Scholar] [CrossRef]
- Fedotova, A.; Crousse, B.; Chataigner, I.; Maddaluno, J.; Rulev, A.Y.; Legros, J. Benefits of a dual chemical and physical activation: Direct aza-Michael addition of anilines promoted by solvent effect under high pressure. J. Org. Chem. 2015, 80, 10375–10379. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.-C.; Xu, K.; Zeng, C.-C. Transition metal- and base-free electrochemical aza-Michael addition of aromatic aza-heterocycles or Ts-protected amines to α,β-unsaturated alkenes mediated by NaI. ACS Sustain. Chem. Eng. 2018, 7, 2255–2261. [Google Scholar] [CrossRef]
- Kim, S.; Kang, S.; Kim, G.; Lee, Y. Copper-catalyzed aza-Michael addition of aromatic amines or aromatic aza-heterocycles to α,β-unsaturated olefins. J. Org. Chem. 2016, 81, 4048–4057. [Google Scholar] [CrossRef]
- Rostamnia, S.; Alamgholiloo, H. Synthesis and catalytic application of mixed valence iron (FeII/FeIII)-based OMS-MIL-100(Fe) as an efficient green catalyst for the aza-Michael reaction. Catal. Lett. 2018, 148, 2918–2928. [Google Scholar] [CrossRef]
- Torrelo, G.; Hanefeld, U.; Hollmann, F. Biocatalysis. Catal. Lett. 2014, 145, 309–345. [Google Scholar] [CrossRef]
- Lee, C.; Sandig, B.; Buchmeiser, M.R.; Haumann, M. Supported ionic liquid phase (SILP) facilitated gas-phase enzyme catalysis – CALB catalyzed transesterification of vinyl propionate. Catal. Sci. Technol. 2018, 8, 2460–2466. [Google Scholar] [CrossRef]
- Bilal, M.; Zhao, Y.; Noreen, S.; Shah, S.Z.H.; Bharagava, R.N.; Iqbal, H.M. Modifying bio-catalytic properties of enzymes for efficient biocatalysis: A review from immobilization strategies viewpoint. Biocatal. Biotransformation 2019, 37, 159–182. [Google Scholar] [CrossRef]
- Steunenberg, P.; Sijm, M.; Zuilhof, H.; Sanders, J.P.M.; Scott, E.L.; Franssen, M.C.R. Lipase-catalyzed aza-Michael reaction on acrylate derivatives. J. Org. Chem. 2013, 78, 3802–3813. [Google Scholar] [CrossRef] [PubMed]
- Strompen, S.; Weis, M.; Gröger, H.; Hilterhaus, L.; Liese, A. Development of a continuously operating process for the enantioselective synthesis of a β-amino acid esterviaa solvent-free chemoenzymatic reaction sequence. Adv. Synth. Catal. 2013, 355, 2391–2399. [Google Scholar] [CrossRef]
- Xu, F.; Wu, Q.; Chen, X.; Lin, X.; Wu, Q. A single lipase-catalysed one-pot protocol combining aminolysis resolution and aza-Michael addition: An easy and efficient way to synthesise β-amino acid esters. Eur. J. Org. Chem. 2015, 2015, 5393–5401. [Google Scholar] [CrossRef]
- Badgujar, K.; Bhanage, B.M. Lipase immobilization on hyroxypropyl methyl cellulose support and its applications for chemo-selective synthesis of β-amino ester compounds. Process. Biochem. 2016, 51, 1420–1433. [Google Scholar] [CrossRef]
- Gu, B.; Hu, Z.-E.; Yang, Z.; Li, J.; Zhou, Z.; Wang, N.; Yu, X. Probing the mechanism of CAL-B-catalyzed aza-Michael addition of aniline compounds with acrylates using mutation and molecular docking simulations. ChemistrySelect 2019, 4, 3848–3854. [Google Scholar] [CrossRef]
- Planchestainer, M.; Contente, M.L.; Cassidy, J.; Molinari, F.; Tamborini, L.; Paradisi, F. Continuous flow biocatalysis: Production and in-line purification of amines by immobilised transaminase from Halomonas elongata. Green Chem. 2017, 19, 372–375. [Google Scholar] [CrossRef]
- Britton, J.; Majumdar, S.; Weiss, G.A. Continuous flow biocatalysis. Chem. Soc. Rev. 2018, 47, 5891–5918. [Google Scholar] [CrossRef]
- Van der Helm, M.P.; Bracco, P.; Busch, H.; Szymańska, K.; Jarzębski, A.B.; Hanefeld, U. Hydroxynitrile lyases covalently immobilized in continuous flow microreactors. Catal. Sci. Technol. 2019, 9, 1189–1200. [Google Scholar] [CrossRef]
- Movsisyan, M.; Delbeke, E.I.P.; Berton, J.K.E.T.; Battilocchio, C.; Ley, S.V.; Stevens, C.V. Taming hazardous chemistry by continuous flow technology. Chem. Soc. Rev. 2016, 45, 4892–4928. [Google Scholar] [CrossRef]
- Plutschack, M.B.; Pieber, B.; Gilmore, K.; Seeberger, P.H. The hitchhiker’s guide to flow chemistry. Chem. Rev. 2017, 117, 11796–11893. [Google Scholar] [CrossRef]
- Baumeister, T.; Zikeli, S.; Kitzler, H.; Aigner, P.; Wieczorek, P.P.; Röder, T. Continuous flow synthesis of amine oxides by oxidation of tertiary amines. React. Chem. Eng. 2019, 4, 1270–1276. [Google Scholar] [CrossRef]
- Priego, J.; Ortíz-Nava, C.; Carrillo-Morales, M.; López-Munguía, A.; Escalante, J.; Castillo, E. Solvent engineering: An effective tool to direct chemoselectivity in a lipase-catalyzed Michael addition. Tetrahedron 2009, 65, 536–539. [Google Scholar] [CrossRef]
- De, K.; Legros, J.; Crousse, B.; Bonnet-Delpon, D. Solvent-promoted and -controlled aza-Michael reaction with aromatic amines. J. Org. Chem. 2009, 74, 6260–6265. [Google Scholar] [CrossRef] [PubMed]
- Svedendahl, M.; Hult, K.; Berglund, P. Fast carbon−carbon bond formation by a promiscuous lipase. J. Am. Chem. Soc. 2005, 127, 17988–17989. [Google Scholar] [CrossRef] [PubMed]



 | ||
|---|---|---|
| Entry | Method | Yield b (%) |
| 1 | A | 80.3 ± 1.5 |
| 2 | B | 62.3 ± 2.5 |
 | ||||
|---|---|---|---|---|
| Entry | Catalyst | Solvent | T (°C) | Yield b (%) |
| 1 | Lipozyme TL IM | Tert-amyl alcohol | 45 | 5.2 ± 0.7 |
| 2 | Lipozyme TL IM | Acetonitrile | 45 | n.d. |
| 3 | Lipozyme TL IM | Toluene | 45 | 62.0 ± 1.2 |
| 4 | Lipozyme TL IM | DCM | 45 | 68.3 ± 1.9 |
| 5 | Lipozyme TL IM | Ethanol | 45 | 58.2 ± 2.3 |
| 6 | Lipozyme TL IM | Methanol | 45 | 75.1 ± 0.9 |
| 7 | Lipozyme TL IM | Methanol | 30 | 66.5 ± 2.1 |
| 8 | Lipozyme TL IM | Methanol | 35 | 80.3 ± 1.5 |
| 9 | Lipozyme TL IM | Methanol | 40 | 77.4 ± 2.5 |
| 10 | Lipozyme TL IM | Methanol | 50 | 73.1 ± 2.6 |
| 11 | Lipozyme TL IM | Methanol | 55 | 70.0 ± 1.7 |
| 12 | Subtilisin | Methanol | 35 | n.d. |
| 13 | Novozym 435 | Methanol | 35 | 23.2 ± 0.9 |
| 14 | None | Methanol | 35 | n.d. |
| Entry | n1a:n2a | Time/Min | Flow Rate/µL min−1 | Yield b (%) |
|---|---|---|---|---|
| 1 | 1:1 | 30 | 20.8 | 62.6 ± 2.3 |
| 2 | 1:2 | 30 | 20.8 | 68.1 ± 1.6 |
| 3 | 1:4 | 30 | 20.8 | 80.3 ± 1.5 |
| 4 | 1:6 | 30 | 20.8 | 77.4 ± 2.8 |
| 5 | 1:4 | 20 | 31.2 | 58.7 ± 2.2 |
| 6 | 1:4 | 25 | 25.0 | 69.0 ± 0.9 |
| 7 | 1:4 | 35 | 17.8 | 80.1 ± 1.6 |
| 8 | 1:4 | 40 | 15.6 | 78.0 ± 1.2 |
 | |||
|---|---|---|---|
| Entry | Aromatic Amine | Product | Yield b (%) |
| 1 | aniline (1a) | 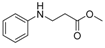 3a 3a | 80.3 ± 1.5 |
| 2 | aniline (1a) |  3b 3b | 75.1 ± 1.9 |
| 3 | 4-toluidine (1b) |  3c 3c | 92.4 ± 0.8 |
| 4 | 4-toluidine (1b) | 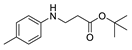 3d 3d | 81.2 ± 1.7 |
| 5 | 4-tert-butylaniline (1c) | 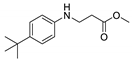 3e 3e | 83.6 ± 2.1 |
| 6 | 4-tert-butylaniline (1c) | 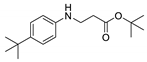 3f 3f | 87.7 ± 2.9 |
| 7 | 4-methoxyaniline (1d) |  3g 3g | 85.0 ± 2.7 |
| 8 | 4-methoxyaniline (1d) | 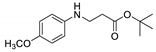 3h 3h | 82.1 ± 2.6 |
| 9 | 4-chloroaniline (1e) | 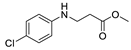 3i 3i | 36.3 ± 1.2 |
| 10 | 4-chloroaniline (1e) | 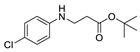 3j 3j | 48.5 ± 3.1 |
| 11 | 4-bromoaniline (1f) |  3k 3k | 42.4 ± 1.5 |
| 12 | 4-bromoaniline (1f) | 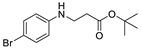 3l 3l | 47.3 ± 1.1 |
| 13 | 2-aminopyridine (1g) | 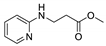 3m 3m | 43.7 ± 2.4 |
| 14 | 2-aminopyridine (1g) | 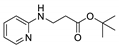 3n 3n | trace |
| 15 | 3,4-(methylenedioxy)aniline (1h) | 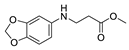 3o 3o | 88.1 ± 2.2 |
| 16 | 3,4-(methylenedioxy)aniline (1h) |  3p 3p | 85.4 ± 1.7 |
| 17 | 2-naphthylamine (1i) | 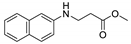 3q 3q | 74.0 ± 1.4 |
| 18 | 2-naphthylamine (1i) |  3r 3r | 72.1 ± 1.2 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Du, L.-H.; Long, R.-J.; Xue, M.; Chen, P.-F.; Yang, M.-J.; Luo, X.-P. Continuous-Flow Synthesis of β-Amino Acid Esters by Lipase-Catalyzed Michael Addition of Aromatic Amines. Catalysts 2020, 10, 432. https://doi.org/10.3390/catal10040432
Du L-H, Long R-J, Xue M, Chen P-F, Yang M-J, Luo X-P. Continuous-Flow Synthesis of β-Amino Acid Esters by Lipase-Catalyzed Michael Addition of Aromatic Amines. Catalysts. 2020; 10(4):432. https://doi.org/10.3390/catal10040432
Chicago/Turabian StyleDu, Li-Hua, Rui-Jie Long, Miao Xue, Ping-Feng Chen, Meng-Jie Yang, and Xi-Ping Luo. 2020. "Continuous-Flow Synthesis of β-Amino Acid Esters by Lipase-Catalyzed Michael Addition of Aromatic Amines" Catalysts 10, no. 4: 432. https://doi.org/10.3390/catal10040432
APA StyleDu, L.-H., Long, R.-J., Xue, M., Chen, P.-F., Yang, M.-J., & Luo, X.-P. (2020). Continuous-Flow Synthesis of β-Amino Acid Esters by Lipase-Catalyzed Michael Addition of Aromatic Amines. Catalysts, 10(4), 432. https://doi.org/10.3390/catal10040432




