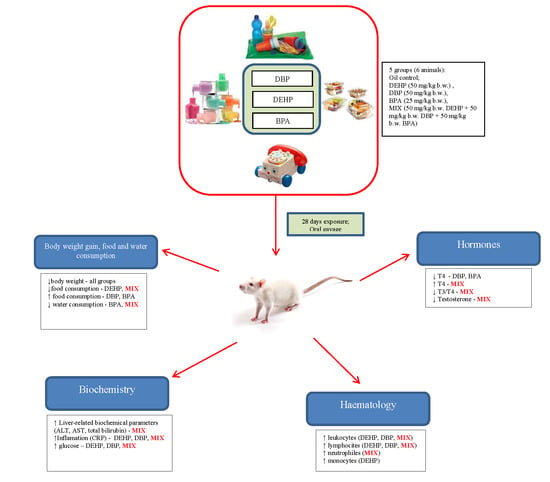
Toxic Effects of the Mixture of Phthalates and Bisphenol A—Subacute Oral Toxicity Study in Wistar Rats
Abstract
- More adverse effects induced by the mixture compared to the single substances
- Significant changes in lipid profile, liver-related biochemical parameters, and glucose level
- Opposite effects of single substances on the thyroxine level in comparison with the mixture
- More pronounced effect of the mixture on testosterone level
- Mixture toxicity studies are of primary importance for endocrine disruptors
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Animals
2.3. Study Design and Experimental Procedure
2.4. Blood Collection, Body and Organ Weights
2.5. Haematology Analysis
2.6. Biochemical Analysis
2.7. Hormone Analysis
2.8. Statistical Analysis
3. Results
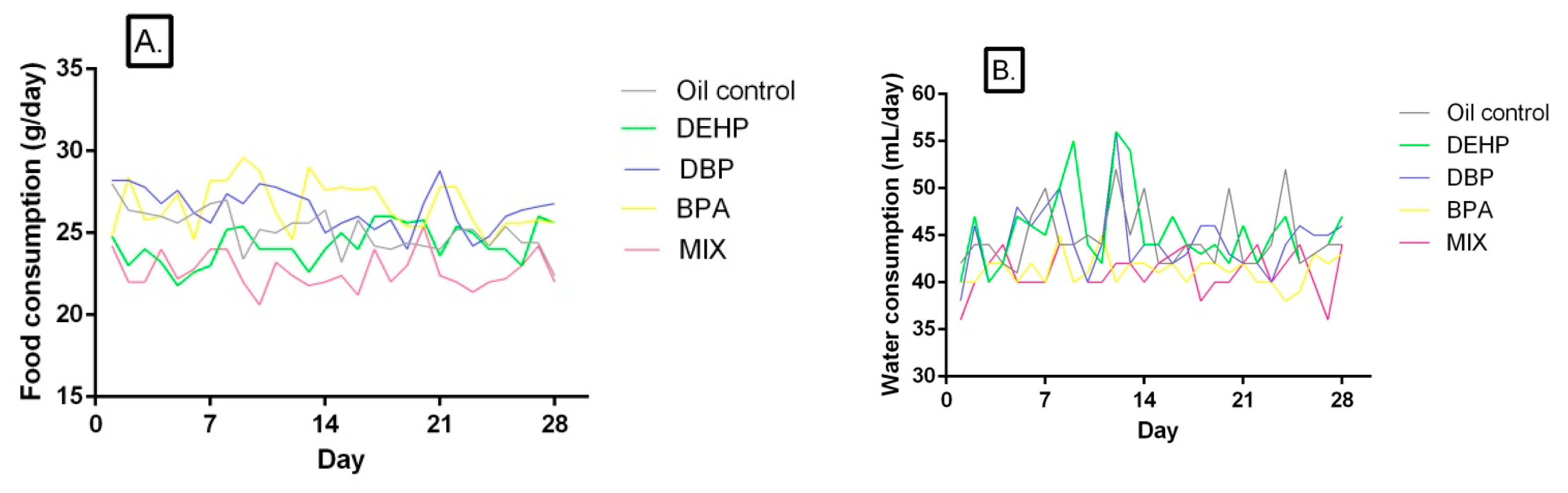
3.1. Body Weight Gain, Food and Water Consumption
3.2. Food and Water Consumption
3.3. Relative Organ weight
3.4. Haemotological Parameters
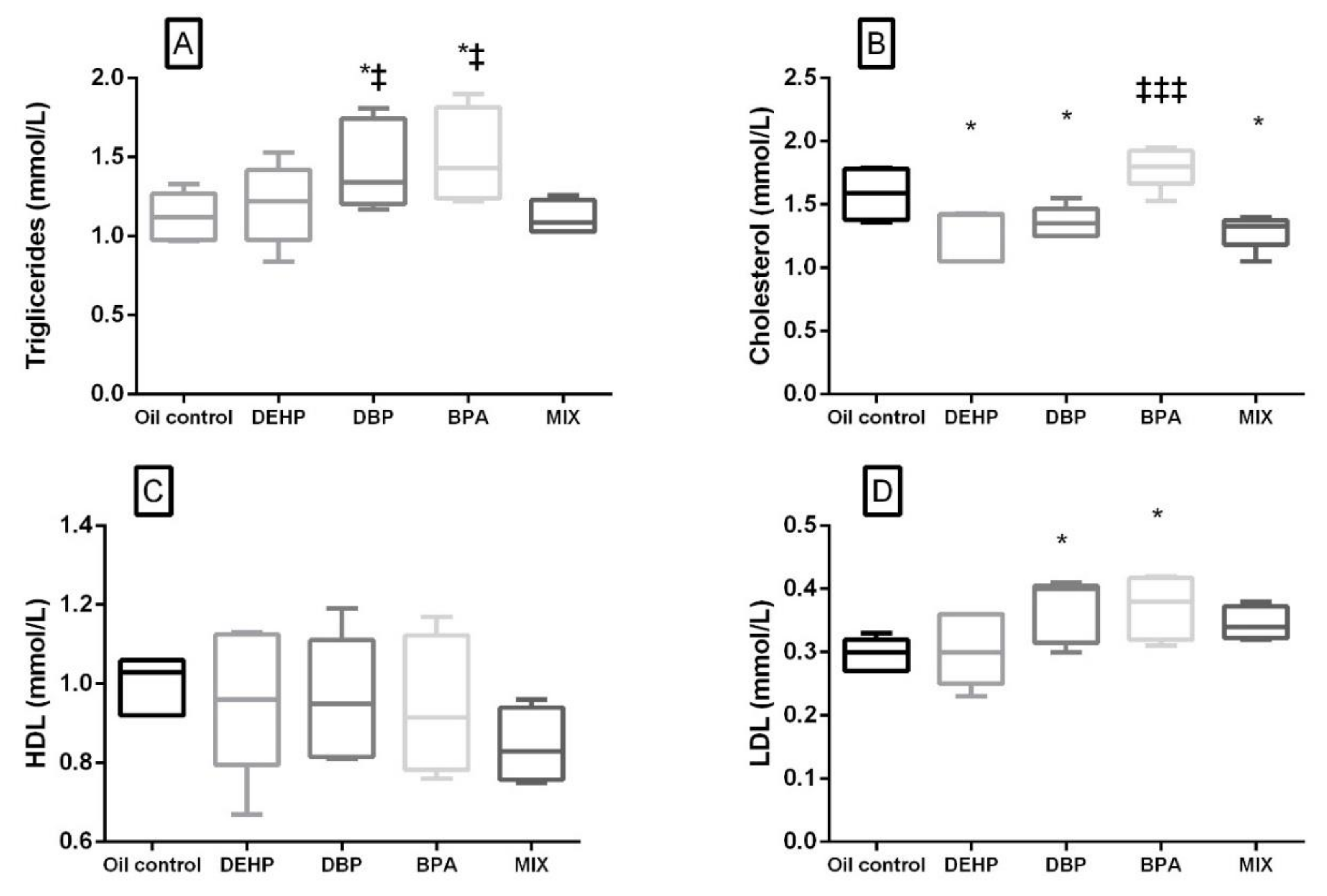
3.5. Serum Biochemistry Parameters
3.6. Hormone Parameters
4. Discussion
4.1. Body Weight Gain, Food and Water Consumption
4.2. Inflammation
4.3. Thyroid Hormones
4.4. Lipid Profile
4.5. Liver-Related Biochemical Parameters
4.6. Glucose Level
4.7. Antiandrogen Activity and Serum Testosterone Level
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hernandez, A.F.; Buha, A.; Constantin, C.; Wallace, D.R.; Sarigiannis, D.; Neagu, M.; Antonijevic, B.; Hayes, A.W.; Wilks, M.F.; Tsatsakis, A. Critical assessment and integration of separate lines of evidence for risk assessment of chemical mixtures. Arch. Toxicol. 2019, 93, 2741–2757. [Google Scholar] [CrossRef] [PubMed]
- Docea, A.O.; Gofita, E.; Goumenou, M.; Calina, D.; Rogoveanu, O.; Varut, M.; Olaru, C.; Kerasioti, E.; Fountoucidou, P.; Taitzoglou, I.; et al. Six months exposure to a real life mixture of 13 chemicals’ below individual NOAELs induced non monotonic sex-dependent biochemical and redox status changes in rats. Food Chem. Toxicol. 2018, 115, 470–481. [Google Scholar] [CrossRef] [PubMed]
- Hernández, A.F.; Tsatsakis, A.M. Human exposure to chemical mixtures: Challenges for the integration of toxicology with epidemiology data in risk assessment. Food Chem. Toxicol. 2017, 103, 188–193. [Google Scholar] [CrossRef] [PubMed]
- Tsatsakis, A.; Goumenou, M.; Liesivuori, J.; Dekant, W.; Hernández, A.F. Toxicology for real-life risk simulation—Editorial preface to this special issue. Toxicol. Lett. 2019, 309, 33–34. [Google Scholar] [CrossRef] [PubMed]
- Tsatsakis, A.M.; Docea, A.O.; Tsitsimpikou, C. New challenges in risk assessment of chemicals when simulating real exposure scenarios; simultaneous multi-chemicals’ low dose exposure. Food Chem. Toxicol. 2016, 96, 174–176. [Google Scholar] [CrossRef]
- Andjelkovic, M.; Djordjevic, A.B.; Antonijevic, E.; Antonijevic, B.; Stanic, M.; Kotur-Stevuljevic, J.; Spasojevic-Kalimanovska, V.; Jovanovic, M.; Boricic, N.; Wallace, D.; et al. Toxic Effect of Acute Cadmium and Lead Exposure in Rat Blood, Liver, and Kidney. Int. J. Environ. Res. Public Heal. 2019, 16, 274. [Google Scholar] [CrossRef]
- Buha, A.; Antonijevic, B.; Bulat, Z.; Jacevic, V.; Milovanović, V.; Matović, V. The impact of prolonged cadmium exposure and co-exposure with polychlorinated biphenyls on thyroid function in rats. Toxicol. Lett. 2013, 221, 83–90. [Google Scholar] [CrossRef]
- Ćurčić, M.; Tanasković, S.; Stanković, S.; Janković, S.; Antunović, M.; Djordjević, S.; Kilibarda, V.; Vučinić, S.; Antonijević, B. Odnos hepatotoksičnosti i doze dekabromovanog difeniletra u ciljnom tkivu kod subakutno izloženih Wistar pacova. Vojnosanit. Pregl. 2015, 72, 405–413. [Google Scholar]
- Docea, A.O.; Goumenou, M.; Calina, D.; Arsene, A.L.; Dragoi, C.M.; Gofita, E.; Pisoschi, C.G.; Zlatian, O.; Stivaktakis, P.D.; Nikolouzakis, T.K.; et al. Adverse and hormetic effects in rats exposed for 12 months to low dose mixture of 13 chemicals: RLRS part III. Toxicol. Lett. 2019, 310, 70–91. [Google Scholar] [CrossRef]
- Tsatsakis, A.M.; Kouretas, D.; Tzatzarakis, M.N.; Stivaktakis, P.; Tsarouhas, K.; Golokhvast, K.S.; Rakitskii, V.N.; Tutelyan, V.A.; Hernandez, A.F.; Rezaee, R.; et al. Simulating real-life exposures to uncover possible risks to human health: A proposed consensus for a novel methodological approach. Hum. Exp. Toxicol. 2017, 36, 554–564. [Google Scholar] [CrossRef]
- United N.E.P. WHO (World Health Organisation). State-of-the-Science of Endocrine Disrupting Chemicals, 2012; United Nations Environment Programme and the World Health Organization: Geneva, Switzerland, 2013. [Google Scholar]
- EFSA Scientific Committee Scientific. EFSA Scientific Committee Scientific Opinion on the hazard assessment of endocrine disruptors: Scientific criteria for identification of endocrine disruptors and appropriateness of existing test methods for assessing effects mediated by these substances on human health and the env. EFSA J. 2013, 11, 3132. [Google Scholar] [CrossRef]
- La Merrill, M.A.; Vandenberg, L.N.; Smith, M.T.; Goodson, W.; Browne, P.; Patisaul, H.B.; Guyton, K.Z.; Kortenkamp, A.; Cogliano, V.J.; Woodruff, T.J.; et al. Consensus on the key characteristics of endocrine-disrupting chemicals as a basis for hazard identification. Nat. Rev. Endocrinol. 2019, 16, 45–57. [Google Scholar] [CrossRef] [PubMed]
- Munn, S.; Goumenou, M. Key scientific issues relevant to the identification and characterisation of endocrine disrupting substances—Report of the Endocrine Disrupters Expert Advisory Group. Toxicol. Lett. 2013, 221. [Google Scholar] [CrossRef]
- Kortenkamp, A. Low dose mixture effects of endocrine disrupters: Implications for risk assessment and epidemiology. Int. J. Androl. 2008, 31, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Zhang, W.; Rui, B.-B.; Yang, S.-M.; Xu, W.-P.; Wei, W. Di(2-ethylhexyl) phthalate exacerbates non-alcoholic fatty liver in rats and its potential mechanisms. Environ. Toxicol. Pharmacol. 2016, 42, 38–44. [Google Scholar] [CrossRef]
- Curcic, M.; Janković, S.; Jacevic, V.; Stankovic, S.; Vucinic, S.; Durgo, K.; Bulat, Z.; Antonijevic, B. Combined effects of cadmium and decabrominated diphenyl ether on thyroid hormones in rats. Arch. Ind. Hyg. Toxicol. 2012, 63, 255–262. [Google Scholar]
- Zhu, B.; Wang, Q.; Wang, X.; Zhou, B. Impact of co-exposure with lead and decabromodiphenyl ether (BDE-209) on thyroid function in zebrafish larvae. Aquat. Toxicol. 2014, 157, 186–195. [Google Scholar] [CrossRef]
- Margina, D.; Nițulescu, G.; Ungurianu, A.; Mesnage, R.; Goumenou, M.; Sarigiannis, D.; Aschner, M.; Spandidos, D.; Renieri, E.; Hernandez, A.; et al. Overview of the effects of chemical mixtures with endocrine disrupting activity in the context of real-life risk simulation (RLRS): An integrative approach (Review). World Acad. Sci. J. 2019, 1, 157–164. [Google Scholar] [CrossRef]
- Buha, A.; Matović, V.; Antonijevic, B.; Bulat, Z.; Čurćić, M.; Renieri, E.A.; Tsatsakis, A.M.; Schweitzer, A.; Wallace, D. Overview of Cadmium Thyroid Disrupting Effects and Mechanisms. Int. J. Mol. Sci. 2018, 19, 1501. [Google Scholar] [CrossRef]
- Djordjevic, A.B.; Antonijevic, E.; Curcic, M.; Milovanovic, V.; Antonijevic, B. Endocrine disrupting mechanisms of polychlorinated biphenyls. Curr. Opin. Toxicol. 2019, 19, 42–49. [Google Scholar] [CrossRef]
- Gore, A.C.; Chappell, V.A.; Fenton, S.E.; Flaws, J.A.; Nadal, A.; Prins, G.S.; Toppari, J.; Zoeller, R.T. EDC-2: The Endocrine Society’s Second Scientific Statement on Endocrine-Disrupting Chemicals. Endocr. Rev. 2015, 36, 1–150. [Google Scholar] [CrossRef] [PubMed]
- Vandenberg, L.N.; Maffini, M.V.; Sonnenschein, C.; Rubin, B.S.; Soto, A.M. Bisphenol-a and the great divide: A review of controversies in the field of endocrine disruption. Endocr. Rev. 2009, 30, 75–95. [Google Scholar] [CrossRef] [PubMed]
- Dereumeaux, C.; Saoudi, A.; Pecheux, M.; Berat, B.; De Crouy-Chanel, P.; Zaros, C.; Brunel, S.; Delamaire, C.; Le Tertre, A.; Lefranc, A.; et al. Biomarkers of exposure to environmental contaminants in French pregnant women from the Elfe cohort in 2011. Environ. Int. 2016, 97, 56–67. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Park, M.J. Phthalate exposure and childhood obesity. Ann. Pediatr. Endocrinol. Metab. 2014, 19, 69–75. [Google Scholar] [CrossRef]
- Rochester, J.R. Bisphenol A and human health: A review of the literature. Reprod. Toxicol. 2013, 42, 132–155. [Google Scholar] [CrossRef]
- Wittassek, M.; Koch, H.M.; Angerer, J.; Brüning, T. Assessing exposure to phthalates–the human biomonitoring approach. Mol. Nutr. Food Res. 2011, 55, 7–31. [Google Scholar] [CrossRef]
- Manikkam, M.; Tracey, R.; Guerrero-Bosagna, C.; Skinner, M.K. Plastics Derived Endocrine Disruptors (BPA, DEHP and DBP) Induce Epigenetic Transgenerational Inheritance of Obesity, Reproductive Disease and Sperm Epimutations. PLoS ONE 2013, 8. [Google Scholar] [CrossRef]
- Singh, S.; Li, S.S.-L. Bisphenol A and phthalates exhibit similar toxicogenomics and health effects. Gene 2012, 494, 85–91. [Google Scholar] [CrossRef]
- Stojanoska, M.M.; Milosevic, N.; Milic, N.; Abenavoli, L. The influence of phthalates and bisphenol A on the obesity development and glucose metabolism disorders. Endocrine 2017, 55, 666–681. [Google Scholar] [CrossRef]
- Zhang, W.Z.; Yong, L.; Jia, X.D.; Li, N.; Fan, Y.X. Combined subchronic toxicity of bisphenol A and dibutyl phthalate on male rats. Biomed. Environ. Sci. 2013, 26, 63–69. [Google Scholar]
- Zhu, Y.-T.; Lai, J.-H.; Liao, X.-D.; Liu, S.-L. Screening of lactic acid bacteria strains for their ability to bind phthalate monoesters in vitro and the binding characteristics. Food Control. 2018, 90, 364–371. [Google Scholar] [CrossRef]
- Rubin, B.S. Bisphenol A: An endocrine disruptor with widespread exposure and multiple effects. J. Steroid Biochem. Mol. Boil. 2011, 127, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Li, S. Epigenetic Effects of Environmental Chemicals Bisphenol a and Phthalates. Epigenetics Pathol. 2013, 267–278. [Google Scholar]
- Kim, M.; Jeong, J.S.; Kim, H.; Hwang, S.; Park, I.-H.; Lee, B.-C.; Yoon, S.I.; Jee, S.H.; Nam, K.T.; Lim, K.-M. Low Dose Exposure to Di-2-Ethylhexylphthalate in Juvenile Rats Alters the Expression of Genes Related with Thyroid Hormone Regulation. Biomol. Ther. 2018, 26, 512–519. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-X.; Zhou, B.; Chen, Y.-J.; Liu, C.; Huang, L.-L.; Liao, J.-Q.; Hu, X.-J.; Lu, W.-Q.; Zeng, Q.; Pan, A. Thyroid function, phthalate exposure and semen quality: Exploring associations and mediation effects in reproductive-aged men. Environ. Int. 2018, 116, 278–285. [Google Scholar] [CrossRef] [PubMed]
- Howarth, A.J.; Price, S.C.; Dobrota, M.; Kentish, A.P.; Hinton, R.H. Effects on male rats of di-(2-ethylhexyl) phthalate and di-n-hexylphthalate administered alone or in combination. Toxicol. Lett. 2001, 121, 35–43. [Google Scholar] [CrossRef]
- Sun, D.; Zhou, L.; Wang, S.; Liu, T.; Zhu, J.; Jia, Y.; Xu, J.; Chen, H.; Wang, Q.; Xu, F.; et al. Effect of Di-(2-ethylhexyl) phthalate on the hypothalamus-pituitary-thyroid axis in adolescent rat. Endocr. J. 2018, 65, 261–268. [Google Scholar] [CrossRef]
- Wu, Y.; Li, J.; Yan, B.; Zhu, Y.; Liu, X.; Chen, M.; Li, D.; Lee, C.-C.; Yang, X.; Ma, P. Oral exposure to dibutyl phthalate exacerbates chronic lymphocytic thyroiditis through oxidative stress in female Wistar rats. Sci. Rep. 2017, 7, 15469. [Google Scholar] [CrossRef]
- Alkalby, J.M.A. Effect of Bisphenol a on Thyroid, Liver And Testicular Functions In Adult Male Rats. Basrah J. Veter Res. 2015, 14, 187–206. [Google Scholar] [CrossRef]
- Xu, X.; Fan, S.; Guo, Y.; Tan, R.; Zhang, J.; Zhang, W.; Pan, B.-X.; Kato, N. The effects of perinatal bisphenol A exposure on thyroid hormone homeostasis and glucose metabolism in the prefrontal cortex and hippocampus of rats. Brain Behav. 2019, 9, e01225. [Google Scholar] [CrossRef]
- Meeker, J.D.; Ferguson, K.K. Relationship between Urinary Phthalate and Bisphenol A Concentrations and Serum Thyroid Measures in U.S. Adults and Adolescents from the National Health and Nutrition Examination Survey (NHANES) 2007–2008. Environ. Health Perspect. 2011, 119, 1396–1402. [Google Scholar] [CrossRef] [PubMed]
- Christen, V.; Crettaz, P.; Oberli-Schrämmli, A.; Fent, K. Antiandrogenic activity of phthalate mixtures: Validity of concentration addition. Toxicol. Appl. Pharmacol. 2012, 259, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Howdeshell, K.L.; Hotchkiss, A.K.; Gray, L.E. Cumulative effects of antiandrogenic chemical mixtures and their relevance to human health risk assessment. Int. J. Hyg. Environ. Health 2017, 220, 179–188. [Google Scholar] [CrossRef]
- Scarano, W.R.; Bedrat, A.; Alonso-Costa, L.G.; Aquino, A.M.; Fantinatti, E.A.B.; Justulin, A.L.; Barbisan, L.F.; Freire, P.P.; Flaws, A.J.; Lemos, B. Exposure to an Environmentally Relevant Phthalate Mixture During Prostate Development Induces MicroRNA Upregulation and Transcriptome Modulation in Rats. Toxicol. Sci. 2019, 171, 84–97. [Google Scholar] [CrossRef]
- Singh, S.; Li, S.S.-L. Phthalates: Toxicogenomics and inferred human diseases. Genomics 2011, 97, 148–157. [Google Scholar] [CrossRef]
- Rider, C.V.; Wilson, V.S.; Howdeshell, K.L.; Hotchkiss, A.K.; Furr, J.R.; Lambright, C.R.; Gray, L.E. Cumulative Effects of In Utero Administration of Mixtures of “Antiandrogens” on Male Rat Reproductive Development. Toxicol. Pathol. 2009, 37, 100–113. [Google Scholar] [CrossRef] [PubMed]
- Pflieger-Bruss, S.; Schuppe, H.-C.; Schill, W.-B. The male reproductive system and its susceptibility to endocrine disrupting chemicals. Andrologia 2004, 36, 337–345. [Google Scholar] [CrossRef] [PubMed]
- Chitra, K. Induction of oxidative stress by bisphenol A in the epididymal sperm of rats. Toxicology 2003, 185, 119–127. [Google Scholar] [CrossRef]
- Mylchreest, E.; Wallace, D.G.; Cattley, R.C.; Foster, P.M.D. Dose-dependent alterations in androgen-regulated male reproductive development in rats exposed to Di(n-butyl) phthalate during late gestation. Toxicol. Sci. 2000, 55, 143–151. [Google Scholar] [CrossRef]
- EFSA Panel on Food Contact Materials; Enzymes, Flavourings and Processing Aids (CEF). Scientific Opinion on Bisphenol A: Evaluation of a study investigating its neurodevelopmental toxicity, review of recent scientific literature on its toxicity and advice on the Danish risk assessment of Bisphenol A. EFSA J. 2010, 8, 1829. [Google Scholar] [CrossRef]
- Zhou, L.; Chen, H.; Xu, Q.; Han, X.; Zhao, Y.; Song, X.; Zhao, T.; Ye, L. The effect of di-2-ethylhexyl phthalate on inflammation and lipid metabolic disorder in rats. Ecotoxicol. Environ. Saf. 2019, 170, 391–398. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Zhou, L.; Wang, S.; Zhu, J.; Liu, T.; Jia, Y.; Sun, D.; Chen, H.; Wang, Q.; Xu, F.; et al. Di-(2-ethylhexyl)-phthalate induces glucose metabolic disorder in adolescent rats. Environ. Sci. Pollut. Res. 2018, 25, 3596–3607. [Google Scholar] [CrossRef] [PubMed]
- European Food Safety Authority (EFSA). Opinion of the Scientific Panel on food additives, flavourings, processing aids and materials in contact with food (AFC) on a request from the Commission related to tertiary-Butylhydroquinone (TBHQ). EFSA J. 2004, 2. [Google Scholar] [CrossRef]
- Ha, M.; Guan, X.; Wei, L.; Li, P.; Yang, M.; Liu, C. Di-(2-ethylhexyl) phthalate inhibits testosterone level through disturbed hypothalamic–pituitary–testis axis and ERK-mediated 5α-Reductase 2. Sci. Total. Environ. 2016, 563, 566–575. [Google Scholar] [CrossRef]
- WHO. Pesticide Residues in Food: WHO Core Assessment Group on Pesticide Residues; WHO: Geneva, Switzerland, 2015; pp. 1–106. [Google Scholar]
- Miao, S.; Gao, Z.; Kou, Z.; Xu, G.; Su, C.; Liu, N. Influence of Bisphenol A on Developing Rat Estrogen Receptors and Some Cytokines in Rats: A Two-Generational Study. J. Toxicol. Environ. Health Part A 2008, 71, 1000–1008. [Google Scholar] [CrossRef]
- Hatch, E.E.; Nelson, J.W.; Stahlhut, R.W.; Webster, T.F. Association of endocrine disruptors and obesity: Perspectives from epidemiological studies. Int. J. Androl. 2010, 33, 324–332. [Google Scholar] [CrossRef]
- Rubin, B.S.; Schaeberle, C.M.; Soto, A.M. The Case for BPA as an Obesogen: Contributors to the Controversy. Front. Endocrinol. 2019, 10, 30. [Google Scholar] [CrossRef]
- Cogni, G.; Chiovato, L. An overview of the pathogenesis of thyroid autoimmunity. Hormones 2013, 12, 19–29. [Google Scholar] [CrossRef]
- Da Silva, M.M.; Gonçalves, C.F.L.; Miranda-Alves, L.; Fortunato, R.S.; Carvalho, D.P.; Ferreira, A.C.F. Inhibition of Type 1 Iodothyronine Deiodinase by Bisphenol A. Horm. Metab. Res. 2019, 51, 671–677. [Google Scholar] [CrossRef]
- Sengupta, P. The Laboratory Rat: Relating Its Age With Human’s. Int. J. Prev. Med. 2013, 4, 624–630. [Google Scholar]
- Liu, C.; Zhao, L.; Wei, L.; Li, L. DEHP reduces thyroid hormones via interacting with hormone synthesis-related proteins, deiodinases, transthyretin, receptors, and hepatic enzymes in rats. Environ. Sci. Pollut. Res. 2015, 22, 12711–12719. [Google Scholar] [CrossRef] [PubMed]
- Gassman, N.R. Induction of oxidative stress by bisphenol A and its pleiotropic effects. Environ. Mol. Mutagen. 2017, 58, 60–71. [Google Scholar] [CrossRef] [PubMed]
- Renieri, E.A.; Sfakianakis, D.G.; Alegakis, A.A.; Safenkova, I.V.; Buha, A.; Matović, V.; Tzardi, M.; Dzantiev, B.B.; Divanach, P.; Kentouri, M.; et al. Nonlinear responses to waterborne cadmium exposure in zebrafish. An in vivo study. Environ. Res. 2017, 157, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Warner, G.R.; A Flaws, J. Bisphenol A and Phthalates: How Environmental Chemicals Are Reshaping Toxicology. Toxicol. Sci. 2018, 166, 246–249. [Google Scholar] [CrossRef]
- Lin, C.-J.; Lai, C.-K.; Kao, M.-C.; Wu, L.-T.; Lo, U.-G.; Lin, L.-C.; Chen, Y.-A.; Lin, H.; Hsieh, J.-T.; Lai, C.-H.; et al. Impact of cholesterol on disease progression. BioMedicine 2015, 5, 7. [Google Scholar] [CrossRef]
- Majeed, K.A.; Rehman, H.U.; Yousaf, M.S.; Zaneb, H.; Rabbani, I.; Tahir, S.K.; Rashid, M.A. Sub-chronic exposure to low concentration of dibutyl phthalate affects anthropometric parameters and markers of obesity in rats. Environ. Sci. Pollut. Res. 2017, 24, 25462–25467. [Google Scholar] [CrossRef]
- Abdel-Wahab, W.M.; Abdel-Wahab, W.M. Thymoquinone attenuates toxicity and oxidative stress induced by bisphenol a in liver of male rats. Pak. J. Biol. Sci. 2014, 17, 1152–1160. [Google Scholar] [CrossRef][Green Version]
- Venturelli, A.C.; Fischer, S.V.; De Morais, R.N.; Grassiolli, S.; Andrade, A.J.M. Effects of exposure to Di-(2-ethylhexyl) phthalate (DEHP) during lactation and puberty on sexual maturation and glycemic homeostasis in males rats. Clin. Nutr. ESPEN 2015, 10, e5–e12. [Google Scholar] [CrossRef]
- Erkekoglu, P.; Zeybek, N.D.; Giray, B.K.; Rachidi, W.; Kizilgün, M.; Hininger-Favier, I.; Favier, A.; Asan, E.; Hincal, F. The effects of di(2-ethylhexyl)phthalate on rat liver in relation to selenium status. Int. J. Exp. Pathol. 2014, 95, 64–77. [Google Scholar] [CrossRef]
- Hasmall, S.C.; James, N.H.; Macdonald, N.; Soames, A.R.; Roberts, R.A. Species differences in response to diethylhexylphthalate: Suppression of apoptosis, induction of DNA synthesis and peroxisome proliferator activated receptor alpha-mediated gene expression. Arch. Toxicol. 2000, 74, 85–91. [Google Scholar] [CrossRef]
- James, N.H.; Soames, A.R.; Roberts, R.A. Suppression of hepatocyte apoptosis and induction of DNA synthesis by the rat and mouse hepatocarcinogen diethylhexylphlathate (DEHP) and the mouse hepatocarcinogen 1,4-dichlorobenzene (DCB). Arch. Toxicol. 1998, 72, 784–790. [Google Scholar] [CrossRef]
- Roberts, R.; James, N.; Hasmall, S.; Holden, P.; Lambe, K.; Macdonald, N.; West, D.; Woodyatt, N.; Whitcome, D. Apoptosis and proliferation in nongenotoxic carcinogenesis: Species differences and role of PPARα. Toxicol. Lett. 2000, 112, 49–57. [Google Scholar] [CrossRef]
- Srinivasan, C.; Khan, A.I.; Balaji, V.; Selvaraj, J.; Balasubramanian, K. Diethyl hexyl phthalate-induced changes in insulin signaling molecules and the protective role of antioxidant vitamins in gastrocnemius muscle of adult male rat. Toxicol. Appl. Pharmacol. 2011, 257, 155–164. [Google Scholar] [CrossRef] [PubMed]
- Saltiel, A.R.; Kahn, C.R. Insulin signalling and the regulation of glucose and lipid metabolism. Nature 2001, 414, 799–806. [Google Scholar] [CrossRef] [PubMed]
- Rajesh, P.; Sathish, S.; Srinivasan, C.; Selvaraj, J.; Balasubramanian, K. Diethyl Hexyl Phthalate (DEHP) is associated with insulin resistance in adipose tissue of male rat: Protective role of antioxidant vitamins (C & E). J. Cell. Biochem. 2013, 114, 558–569. [Google Scholar] [PubMed]
- Moghaddam, H.S.; Samarghandian, S.; Farkhondeh, T. Effect of bisphenol A on blood glucose, lipid profile and oxidative stress indices in adult male mice. Toxicol. Mech. Methods 2015, 25, 507–513. [Google Scholar] [CrossRef]
- Borman, E.D.; Vecchi, N.; Pollock, T.; Decatanzaro, D. Diethylhexyl phthalate magnifies deposition of 14 C-bisphenol A in reproductive tissues of mice. J. Appl. Toxicol. 2017, 37, 1225–1231. [Google Scholar] [CrossRef]
- Furr, J.R.; Lambright, C.S.; Wilson, V.S.; Foster, P.M.; Gray, L.E. A Short-term In Vivo Screen Using Fetal Testosterone Production, a Key Event in the Phthalate Adverse Outcome Pathway, to Predict Disruption of Sexual Differentiation. Toxicol. Sci. 2014, 140, 403–424. [Google Scholar] [CrossRef]
- Nakamura, D.; Yanagiba, Y.; Duan, Z.; Ito, Y.; Okamura, A.; Asaeda, N.; Tagawa, Y.; Li, C.; Taya, K.; Zhang, S.-Y. Bisphenol A may cause testosterone reduction by adversely affecting both testis and pituitary systems similar to estradiol. Toxicol. Lett. 2010, 194, 16–25. [Google Scholar] [CrossRef]
- Cariati, F.; D’Uonno, N.; Borrillo, F.; Iervolino, S.; Galdiero, G.; Tomaiuolo, R. Bisphenol a: An emerging threat to male fertility. Reprod. Boil. Endocrinol. 2019, 17, 6. [Google Scholar] [CrossRef]
- Jin, P.; Wang, X.; Chang, F.; Bai, Y.; Li, Y.; Zhou, R.; Chen, L. Low dose bisphenol A impairs spermatogenesis by suppressing eproductive hormone production and promoting germ cell apoptosis in adult rats. J. Biomed. Res. 2013, 27, 135–144. [Google Scholar] [PubMed]
- Howdeshell, K.L.; Rider, C.V.; Wilson, V.S.; Furr, J.R.; Lambright, C.R.; Gray, L.E. Dose Addition Models Based on Biologically Relevant Reductions in Fetal Testosterone Accurately Predict Postnatal Reproductive Tract Alterations by a Phthalate Mixture in Rats. Toxicol. Sci. 2015, 148, 488–502. [Google Scholar] [CrossRef] [PubMed]
- Ogli, S.A.; Odeh, S.O.; Egesie, G.U. Gonadotoxic Potentials of Di- (2-Ethyl Hexyl) Phthalate in the Adult Male Wistar Rats. J. Res. Basic Clin. Sci. 2019, 2, 163–171. [Google Scholar]

| Group | Parameter | 1st Week | 2nd Week | 3rd Week | 4th Week |
|---|---|---|---|---|---|
| Oil Control | BWG | 0.4344 ± 0.05996 | 0.8265 ± 0.1092 | 1.151 ± 0.1708 | 1.425 ± 0.2461 |
| DEHP | BWG | 0.3496 ± 0.02875 * | 0.7347 ± 0.09337 | 1.077 ± 0.1378 | 1.327 ± 0.2461 |
| % to control | −20% | −11% | −6% | −7% | |
| DBP | BWG | 0.2826 ± 0.05433 *** | 0.5559 ± 0.05379 *** | 0.8089 ± 0.06926 *** | 1.016 ± 0.08851 *** |
| % to control | −35% | −33% | −30% | −29% | |
| BPA | BWG | 0.3228 ± 0.03756 *** | 0.7001 ± 0.09798 * | 0.9503 ± 0.1422 * | 1.192 ± 0.1682 * |
| % to control | −26% | −15% | −17% | −16% | |
| MIX | BWG | 0.3190 ± 0.03681 *** | 0.6459 ± 0.04416 ** | 0.9237 ± 0.3505 ** | 1.147 ± 0.1048 ** |
| % to control | −26% | −22% | −20% | −19% |
| Group | Parameter | 1st Week | 2nd Week | 3rd Week | 4th Week | Overall Food Consumption |
|---|---|---|---|---|---|---|
| Oil Control | Food consumption (g) | 26.13 ± 1.451 | 25.9 ± 1.010 | 25.5 ± 0.5477 | 26.17 ± 1.169 | 25.16 ± 1.261 |
| DEHP | Food consumption (g) | 23.50 ±1.871 ** | 24.83 ± 2.483 ‡ | 24.27 ± 2.439 | 25.27 ± 0.7659 ‡‡‡ | 24.06 ± 1.796 ** ‡‡‡ |
| % to control | −10 | −4 | −5 | −3 | −4 | |
| DBP | Food consumption (g) | 25.67 ± 1.211 ‡‡ | 25.67 ± 0.0165 ‡‡ | 27.47 ± 1.840 * ‡‡‡ | 27.13 ± 0.9933 ‡‡‡ | 26.52 ± 1.243 ** ‡‡‡ |
| % to control | −2 | −1 | 8 | 4 | 5 | |
| BPA | Food consumption (g) | 26.87 ± 1.633 ‡‡‡ | 27.10 ± 1.435 ‡‡‡ | 27.97 ± 1.417 * ‡‡‡ | 25.43 ± 1.023 ‡‡‡ | 26.70 ± 1.510 ** ‡‡‡ |
| % to control | 3 | 5 | 10 | −3 | 6 | |
| MIX | Food consumption (g) | 23.17 ± 1.472 ** | 22.50 ± 1.871 ** | 22.57 ± 1.023 ** | 22.17 ± 1.169 ** | 22.66 ± 1.102 ** |
| % to control | −11 | −13 | −11 | −15 | −10 |
| Group | Parameter | 1st Week | 2nd Week | 3rd Week | 4th Week | Overall Water Consumption |
|---|---|---|---|---|---|---|
| Oil Control | Water consumption (mL) | 45.20 ± 0.8367 | 43.80 ± 0.8367 | 46.20 ± 1.924 | 44.20 ± 0.8367 | 44.68 ± 3.198 |
| DEHP | Water consumption (mL) | 44.60 ± 1.140 ‡‡‡ | 44.20 ± 0.8367 ‡‡‡ | 45.20 ± 1.304 ‡‡‡ | 46.80 ± 0.8367 ** ‡‡‡ | 45.43 ± 4.095 ‡‡‡ |
| % to control | −1 | 1 | −2 | 6 | 2 | |
| DBP | Water consumption (mL) | 44.20 ± 1.095 ‡‡‡ | 44.00± 0.7071 ‡‡‡ | 41.40 ± 1.440 *** | 45.20 ± 0.8367 ‡‡ | 44.36 ± 3.551 ‡‡ |
| % to control | −2 | 0 | −10 | 2 | −1 | |
| BPA | Water consumption (mL) | 41.60 ± 1.817 *** ‡‡ | 40.08 ± 1.183 *** ‡ | 41.50 ± 0.8944 *** | 43.00 ± 1.531 | 41.29 ± 1.607 ** |
| % to control | −8 | −8 | −10 | −3 | −8 | |
| MIX | Water consumption (mL) | 39.20 ± 1.304 *** | 39.40 ± 1.140 *** | 41.40 ± 0.8944 *** | 43.00 ± 1.551 | 41.18 ± 2.294 *** |
| % to control | −13 | −10 | −10 | −3 | −8 |
| Group | Parameter | Liver | Lungs | Heart | Kidneys | Testes | Spleen | Thymus | Brain | Thyroid Gland |
|---|---|---|---|---|---|---|---|---|---|---|
| Oil Control | ROW | 3.448 ± 0.3311 | 0.3917 ± 0.04355 | 0.2833 ± 0.03266 | 0.6067 ± 0.03615 | 0.8117 ± 0.1137 | 0.2000 ± 0.02098 | 0.1967 ± 0.03011 | 0.4950 ± 0.05822 | 0.02600 ± 0.003847 |
| DEHP | ROW | 4.266 ± 0.2100 *** | 0.3560 ± 0.0251 | 0.3160 ± 0.02302 * | 0.6500 ± 0.05701 | 0.7880 ± 0.1011 | 0.1800 ± 0.02915 | 0.2260 ± 0.04615‡ | 0.4940 ± 0.03578 | 0.02400 ± 0.002236 |
| % to control | 24 | −9 | 12 | 7 | −3 | −10 | 15 | 0 | −8 | |
| DBP | ROW | 3.560 ± 0.2754 ‡‡ | 0.3440 ± 0.05413 | 0.3160 ± 0.02302 | 0.600 ± 0.01414 ‡‡ | 0.7860 ± 0.08264 | 0.1920 ± 0.03564 ‡ | 0.1720 ± 0.01483 | 0.4580 ± 0.01643 ‡ | 0.02250 ± 0.001915 |
| % to control | 3 | −12 | 12 | −1 | −3 | −4 | −13 | −7 | −13 | |
| BPA | ROW | 3.390 ± 0.1920 ‡‡‡ | 0.3480 ± 0.04147 | 0.2720 ± 0.02588 | 0.6040 ± 0.03782‡ | 0.8120 ± 0.07120 | 0.1860 ± 0.03362 | 0.1680 ± 0.02387 | 0.4660 ± 0.03050 | 0.02260 ± 0.001517 * |
| % to control | −2 | −11 | −4 | 0 | 0 | −7 | −15 | −6 | −17 | |
| MIX | ROW | 4.032 ± 0.2513 ** | 0.4167 ± 0.03327 | 0.3017 ± 0.01602 | 0.6783 ± 0.05981 * | 0.8467 ± 0.08937 | 0.1550 ± 0.01517 ** | 0.1767 ± 0.03445 | 0.5183 ± 0.03656 | 0.02467 ± 0.001862 |
| % to control | 17 | 6 | 6 | 12 | 4 | −23 | −10 | 5 | −5 |
| Parameter | Value Presentation | Oil Control | DEHP | DBP | BPA | MIX |
|---|---|---|---|---|---|---|
| WBC (109/L) | Average | 5.400 | 9.000 ** | 9.320 ** | 5.340 ‡ | 8.700 ** |
| SD | 1.740 | 0.9850 | 1.450 | 1.419 | 0.8958 | |
| % to control | 67 | 73 | −1 | 61 | ||
| NEU (%) | Average | 6.680 | 5.280 | 6.060 | 6.975 | 5.833 |
| SD | 1.983 | 1.145 | 1.553 | 0.1500 | 0.7581 | |
| % to control | −21 | −9 | 4 | −13 | ||
| LYM (%) | Average | 86.40 | 93.20 ** | 92.76 * | 88.86 | 92.67 * |
| SD | 6.597 | 1.259 | 1.440 | 4.143 | 1.060 | |
| % to control | 8 | 7 | 3 | 7 | ||
| MON (%) | Average | 0.4333 | 0.6800 | 0.4600 | 0.7600 | 0.9200 * |
| SD | 0.1633 | 0.3768 | 0.2302 | 0.1140 | 0.3114 | |
| % to control | 57 | 6 | 75 | 112 | ||
| EOS (%) | Average | 0.4167 | 0.3000 | 0.3400 | 0.3000 | 0.2333 |
| SD | 0.1472 | 0.2000 | 0.1342 | 0.1225 | 0.1966 | |
| % to control | −28 | −18 | −28 | −44 | ||
| BAS (%) | Average | 0.6000 | 0.5400 | 0.3800 | 0.6200 | 0.5167 |
| SD | 0.2000 | 0.2881 | 0.08367 | 0.1483 | 0.2639 | |
| % to control | −10 | −37 | 3 | −14 | ||
| NEU (109/L) | Average | 0.4583 | 0.4683 | 0.5020 | 0.3700 | 0.6260* |
| SD | 0.1157 | 0.1516 | 0.1596 | 0.1089 | 0.05737 | |
| % to control | 2 | 10 | −19 | 37 | ||
| LYM (109/L) | Average | 4.810 | 8.205** | 7.634 ** | 4.958‡ | 7.440 ** |
| SD | 0.3540 | 0.9052 | 0.7413 | 1.335 | 2.180 | |
| % to control | 71 | 59 | 3 | 55 | ||
| MON (109/L) | Average | 0.03667 | 0.0780 * | 0.0600 | 0.0320‡ | 0.06333 |
| SD | 0.02503 | 0.02280 | 0.03312 | 0.01095 | 0.04274 | |
| % to control | 113 | 64 | −13 | 73 | ||
| EOS (109/L) | Average | 0.0200 | 0.0280 | 0.0180 | 0.0260 | 0.01667 |
| SD | 0.007071 | 0.01924 | 0.008367 | 0.008944 | 0.008165 | |
| % to control | 40 | −10 | 30 | −17 | ||
| BAS (109/L) | Average | 0.03333 | 0.0492 | 0.0204 | 0.0531 | 0.04317 |
| SD | 0.01325 | 0.02531 | 0.00666 | 0.0137 | 0.02740 | |
| % to control | 48 | −39 | 59 | 30 | ||
| RBC (1012/L) | Average | 7.100 | 7.582 | 7.320‡ | 7.850 ** | 7.940 ** |
| SD | 0.5571 | 0.2384 | 0.1775 | 0.3424 | 0.6403 | |
| % to control | 7 | 3 | 11 | 12 | ||
| HGB (g/L) | Average | 138.0 | 147 | 140.4 | 150.4 ** | 151.7 ** |
| SD | 2.550 | 2.702 | 2.702 | 2.333 | 2.0101 | |
| % to control | 7 | 2 | 2 | 10 | ||
| HCT (%) | Average | 0.4400 | 0.4448 ‡‡ | 0.4476 ‡‡ | 0.4816 ** | 0.4940 ** |
| SD | 0.02052 | 0.01706 | 0.0065 | 0.0101 | 0.01937 | |
| % to control | 1 | 2 | 9 | 12 | ||
| MCV (fL) | Average | 61.37 | 61.56 | 61.16 | 59.92 | 62.23 |
| SD | 1.857 | 2.137 | 1.286 | 2.204 | 1.773 | |
| % to control | 0 | 0 | −2 | 1 | ||
| MCH (pg) | Average | 19.12 | 18.98 | 19.18 | 19.40 | 19.10 |
| SD | 0.5020 | 0.7120 | 0.4324 | 0.4848 | 0.3735 | |
| % to control | −1 | 0 | 1 | 0 | ||
| MCHC (g/L) | Average | 313.2 | 313.0‡ | 313.6‡ | 317.4 * | 317.5* |
| SD | 3.114 | 2.550 | 4.159 | 3.194 | 4.215 | |
| % to control | 0 | 0 | 1 | 1 | ||
| PLT (109/L) | Average | 548.0 | 627.6 | 568.0 | 515.3 | 589.4 |
| SD | 22.64 | 46.16 | 60.55 | 44.92 | 55.89 | |
| % to control | 15 | 4 | −6 | 8 |
| Parameter | Value Presentation | Oil Control | DEHP | DBP | BPA | MIX |
|---|---|---|---|---|---|---|
| Glucose (mmol/L) | Average | 11.78 | 14.86 * | 14.78 * | 11.06 ‡ | 14.58 * |
| SD | 2.931 | 2.808 | 1.630 | 2.241 | 1.941 | |
| % to control | 26 | 25 | −6 | 24 | ||
| CRP (mg/L) | Average | 0.4500 | 0.7800 * | 0.7400 * | 0.6000 | 0.7833 * |
| SD | 0.05477 | 0.1789 | 0.1517 | 0.2449 | 0.1169 | |
| % to control | 73 | 64 | 33 | 74 | ||
| Urea (mmol/L) | Average | 7.540 | 7.300 | 7.300 | 7.550 | 8.033 * |
| SD | 0.4506 | 0.7517 | 0.7517 | 0.7937 | 0.8779 | |
| % to control | −3 | −3 | 0 | 7 | ||
| Creatinine (µmol/L) | Average | 43 | 43.80 | 44.20 | 44.25 | 42.50 |
| SD | 4.743 | 3.347 | 3.114 | 3.775 | 2.345 | |
| % to control | 2 | 3 | 3 | −1 | ||
| Uric acid (µmol/L) | Average | 337.2 | 360.8 | 373.4 | 304.0 | 355.8 |
| SD | 71.71 | 98.04 | 98.04 | 40.32 | 59.18 | |
| % to control | 7 | 11 | −10 | 6 | ||
| Total protein (g/L) | Average | 76.20 | 78.80 | 76.40 | 72.50 | 76.17 |
| SD | 4.207 | 8.408 | 2.074 | 7.506 | 2.639 | |
| % to control | 3 | 0 | −5 | 0 | ||
| Albumin (g/L) | Average | 50.75 | 53.00 | 50.080 | 49.75 | 53.00 |
| SD | 2.500 | 5.149 | 1.643 | 5.252 | 2.530 | |
| % to control | 6 | 1 | 0 | 6 | ||
| Total bilirubin (µmol/L) | Average | 1.020 | 1.260 | 1.183 | 1.325 | 1.520 * |
| SD | 0.3271 | 0.5079 | 0.3061 | 0.09574 | 0.1789 | |
| % to control | 24 | 16 | 30 | 49 | ||
| Direct bilirubin (µmol/L) | Average | 0.6500 | 0.6600 | 0.7600 | 0.8250 | 0.7500 |
| SD | 0.1291 | 0.2966 | 0.1517 | 0.1500 | 0.1378 | |
| % to control | 2 | 17 | 27 | 15 | ||
| ALT (U/L) | Average | 57.8 | 62.8‡ | 60.0 ‡‡ | 62.8 ‡ | 73.00 ** |
| SD | 6.140 | 5.933 | 8.396 | 7.463 | 2.309 | |
| % to control | 2 | 1 | 5 | 27 | ||
| AST (U/L) | Average | 134.6 | 131.4‡ | 142.0 | 137.8 | 154.5 * |
| SD | 7.733 | 8.417 | 12.17 | 18.5 | 12.26 | |
| % to control | −1 | 7 | 5 | 15 | ||
| The De Ritis ratio | Average | 2.353 | 2.111 | 2.384 | 2.220 | 2.087 |
| SD | 0.3168 | 0.2680 | 0.1841 | 0.4169 | 0.1331 | |
| % to control | −10 | 1 | −6 | −11 | ||
| ALP (U/L) | Average | 363.00 | 422.2 * | 374.7 | 422.5 * | 385.0 |
| SD | 36.98 | 57.76 | 12.88 | 38.51 | 34.29 | |
| % to control | 16 | 3 | 16 | 2 | ||
| Cholesterol (mmol/L) | Average | 1.584 | 1.274 ** | 1.356 * | 1.796 ‡‡‡ | 1.290 * |
| SD | 0.2031 | 0.2045 | 0.1232 | 0.1623 | 0.1377 | |
| % to control | 0 | 7 | 18 | 4 | ||
| HDL (mmol/L) | Average | 0.9980 | 0.9600 | 0.9600 | 0.9400 | 0.8425 |
| SD | 0.07225 | 0.1872 | 0.1581 | 0.1780 | 0.09605 | |
| % to control | −4 | −4 | −6 | −16 | ||
| LDL (mmol/L) | Average | 0.2960 | 0.3040 | 0.3680* | 0.3725 * | 0.3450 |
| SD | 0.02608 | 0.05188 | 0.0497 | 0.05188 | 0.01243 | |
| % to control | 3 | 24 | 26 | 16.5 | ||
| Triglycerides (mmol/L) | Average | 1.122 | 1.202 | 1.448 * ‡ | 1.508 * ‡ | 1.115 |
| SD | 0.1535 | 0.2545 | 0.2815 | 0.2973 | 0.1097 | |
| % to control | 7 | 29 | 34 | −1 | ||
| Serum iron (µmol/L) | Average | 46.33 | 43.05 | 44.00 | 37.75 * | 41.13 |
| SD | 3.963 | 6.717 | 2.993 | 3.148 | 5.086 | |
| % to control | −7 | −5 | −19 | −11 | ||
| Na+ (mmol/L) | Average | 157.0 | 155.8 | 153.8 | 160.3 | 159.0 |
| SD | 4.393 | 2.683 | 1.304 | 6.602 | 3.521 | |
| % to control | −1 | −2 | 1 | 1 | ||
| K+ (mmol/L) | Average | 8.920 | 9.300 | 9.100 | 8.450 | 8.365 |
| SD | 1.066 | 1.703 | 0.6782 | 1.507 | 0.3061 | |
| % to control | 4 | 2 | −5 | −6 | ||
| Cl− (mmol/L) | Average | 102.6 | 100.6 | 100.4 | 101.3 | 101.8 |
| SD | 2.191 | 1.817 | 1.140 | 2.754 | 2.041 | |
| % to control | −2 | −2 | −1 | −1 | ||
| Ca2+ (mmol/L) | Average | 3.762 | 3.843 | 3.988 | 3.890 | 3.927 |
| SD | 0.2095 | 0.2451 | 0.2895 | 0.1158 | 0.1782 | |
| % to control | 2 | 6 | 3 | 4 | ||
| PO43− (mmol/L) | Average | 4.910 | 5.520 | 4.954 | 5.410 | 5.227 |
| SD | 0.3955 | 0.9345 | 0.4149 | 0.4859 | 0.4700 | |
| % to control | 12 | 1 | 10 | 6 | ||
| Mg2+ (mmol/L) | Average | 2.198 | 2.380 | 2.478 | 2.195 | 2.277 |
| SD | 0.2869 | 0.2616 | 0.3268 | 0.2272 | 0.2045 | |
| % to control | 8 | 13 | 0 | 4 |
| Group | Value Presentation | T3 (nmol/L) | T4 (nmol/L) | T3/T4 ratio | Testosterone (ng/mL) |
|---|---|---|---|---|---|
| Oil Control | 1.980 ± 0.3114 | 70.58 ±12.85 | 0.02831 ± 0.003273 | 3.958 ± 0.6442 | |
| DEHP | 1.940 ± 0.2793 | 71.70 ± 13.46 ‡ | 0.02820 ± 0.008791 ‡ | 3.488 ± 1.001 | |
| % to control | −2 | 1 | 0 | −12 | |
| DBP | 1.780 ± 0.1304 | 49.68 ± 3.975 * ‡‡‡ | 0.03608 ± 0.004508 ‡‡‡ | 2.990 ± 0.6719 | |
| % to control | −10 | −30 | 27 | −24 | |
| BPA | 1.925 ± 0.2217 | 53.70 ± 15.09 * ‡‡‡ | 0.03585 ± 0.01310 ‡‡ | 3.113 ± 0.6540 | |
| % to control | −3 | −24 | 27 | −21 | |
| MIX | 1.740 ± 0.3209 | 90.73 ± 10.56 ** | 0.01927 ±0.001498 * | 2.680 ± 0.3492 ** | |
| % to control | −12 | 28.5 | −32 | −32 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baralić, K.; Buha Djordjevic, A.; Živančević, K.; Antonijević, E.; Anđelković, M.; Javorac, D.; Ćurčić, M.; Bulat, Z.; Antonijević, B.; Đukić-Ćosić, D. Toxic Effects of the Mixture of Phthalates and Bisphenol A—Subacute Oral Toxicity Study in Wistar Rats. Int. J. Environ. Res. Public Health 2020, 17, 746. https://doi.org/10.3390/ijerph17030746
Baralić K, Buha Djordjevic A, Živančević K, Antonijević E, Anđelković M, Javorac D, Ćurčić M, Bulat Z, Antonijević B, Đukić-Ćosić D. Toxic Effects of the Mixture of Phthalates and Bisphenol A—Subacute Oral Toxicity Study in Wistar Rats. International Journal of Environmental Research and Public Health. 2020; 17(3):746. https://doi.org/10.3390/ijerph17030746
Chicago/Turabian StyleBaralić, Katarina, Aleksandra Buha Djordjevic, Katarina Živančević, Evica Antonijević, Milena Anđelković, Dragana Javorac, Marijana Ćurčić, Zorica Bulat, Biljana Antonijević, and Danijela Đukić-Ćosić. 2020. "Toxic Effects of the Mixture of Phthalates and Bisphenol A—Subacute Oral Toxicity Study in Wistar Rats" International Journal of Environmental Research and Public Health 17, no. 3: 746. https://doi.org/10.3390/ijerph17030746
APA StyleBaralić, K., Buha Djordjevic, A., Živančević, K., Antonijević, E., Anđelković, M., Javorac, D., Ćurčić, M., Bulat, Z., Antonijević, B., & Đukić-Ćosić, D. (2020). Toxic Effects of the Mixture of Phthalates and Bisphenol A—Subacute Oral Toxicity Study in Wistar Rats. International Journal of Environmental Research and Public Health, 17(3), 746. https://doi.org/10.3390/ijerph17030746