Skeletal Muscle Myofibrillar Protein Abundance Is Higher in Resistance-Trained Men, and Aging in the Absence of Training May Have an Opposite Effect
Abstract
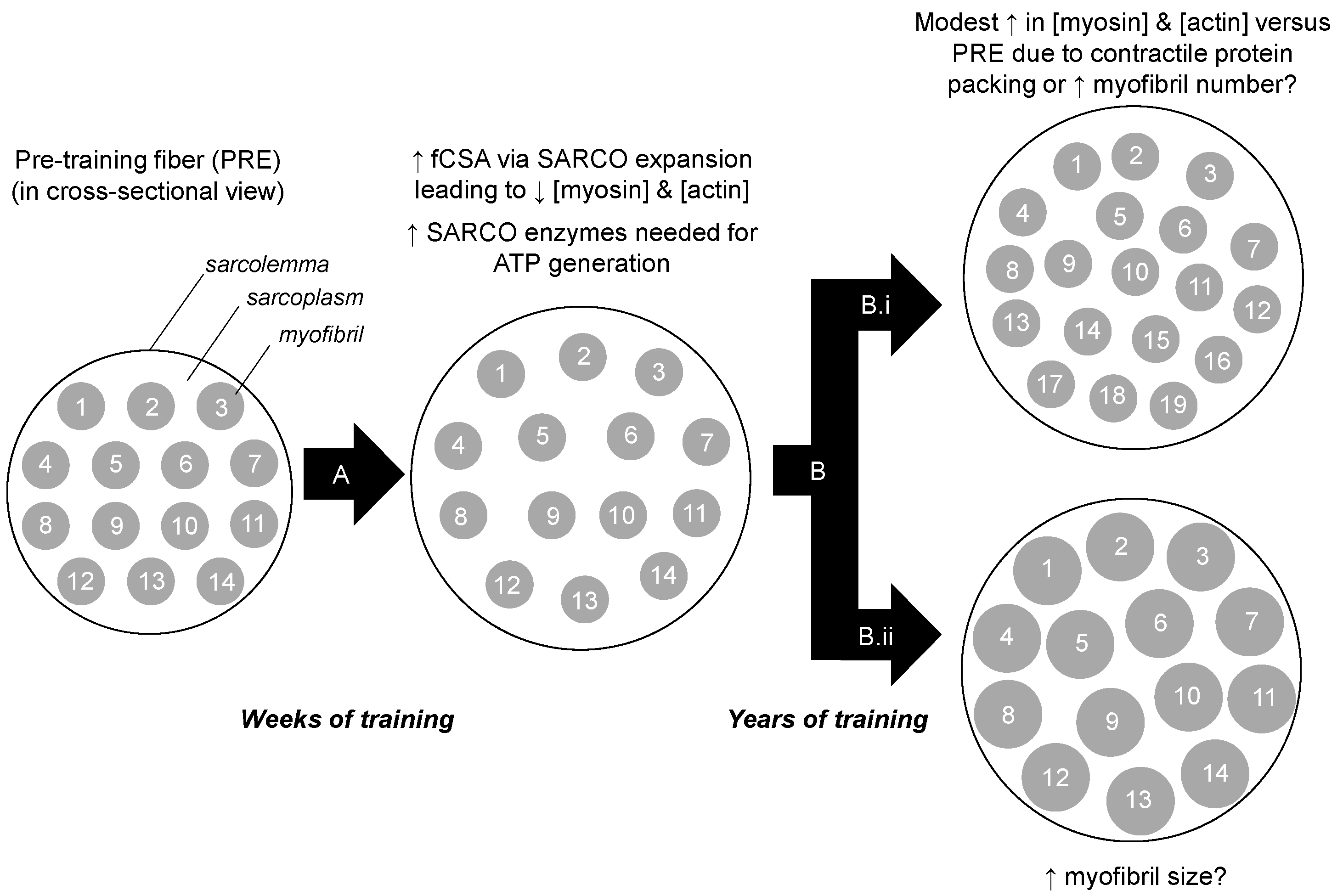
:1. Introduction
2. Materials and Methods
2.1. Ethical Approval and Study Design
2.2. Testing Session
2.2.1. Body Composition and Hydration Testing
2.2.2. Ultrasound for Vastus Lateralis (VL) Thickness
2.2.3. Leg Extensor Peak Torque Testing Using an Isokinetic Dynamometer
2.3. Biochemical Assays
2.3.1. Sarcoplasmic and Myofibrillar Protein Isolation
2.3.2. Determination of Protein Concentrations
2.3.3. SDS-PAGE and Coomassie Staining for Determination of Relative Myosin Heavy Chain and Actin Abundances
2.3.4. Proteomic Analysis of the Sarcoplasmic and Myofibrillar Fractions
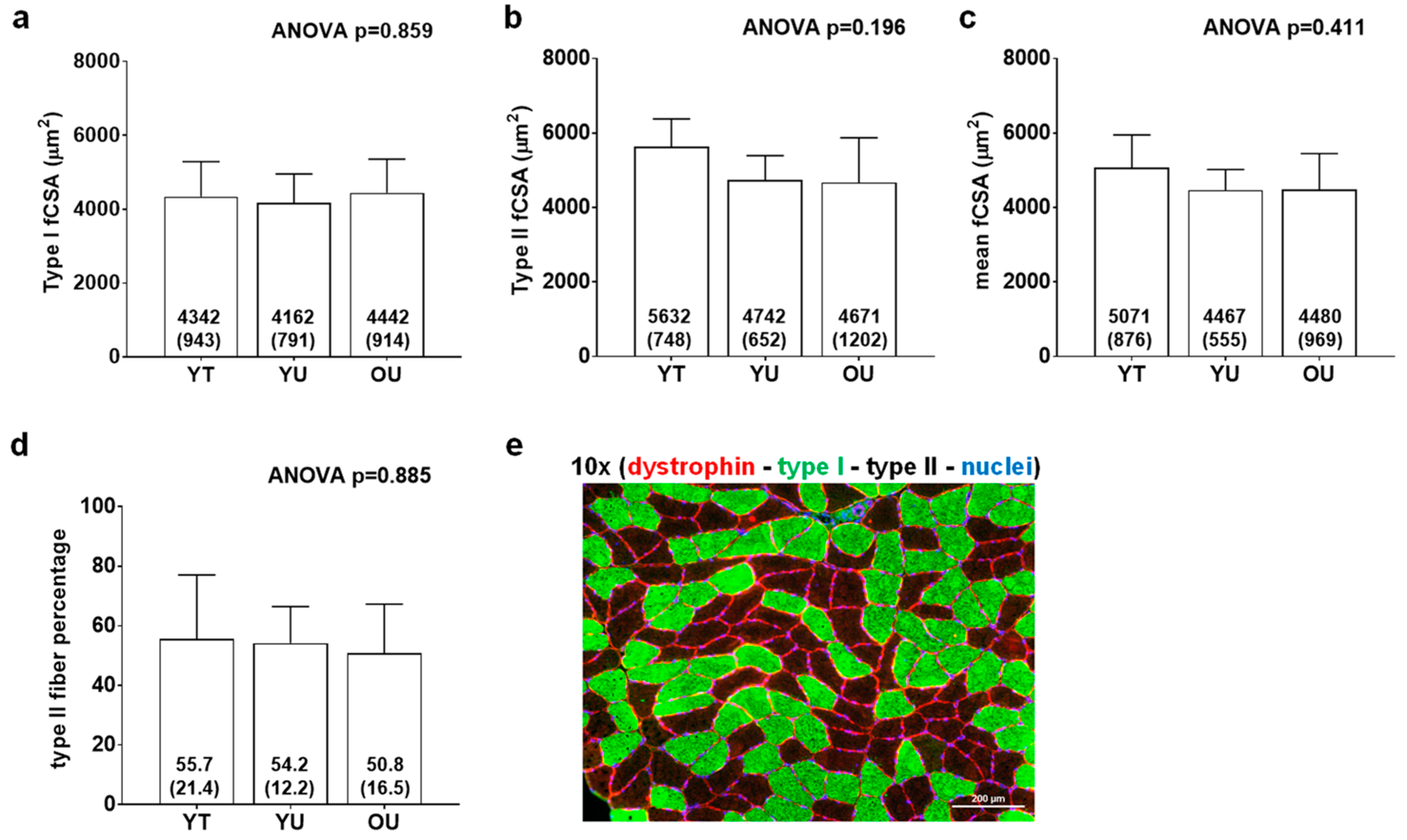
2.3.5. fCSA Analysis
2.4. Statistical Analysis
3. Results
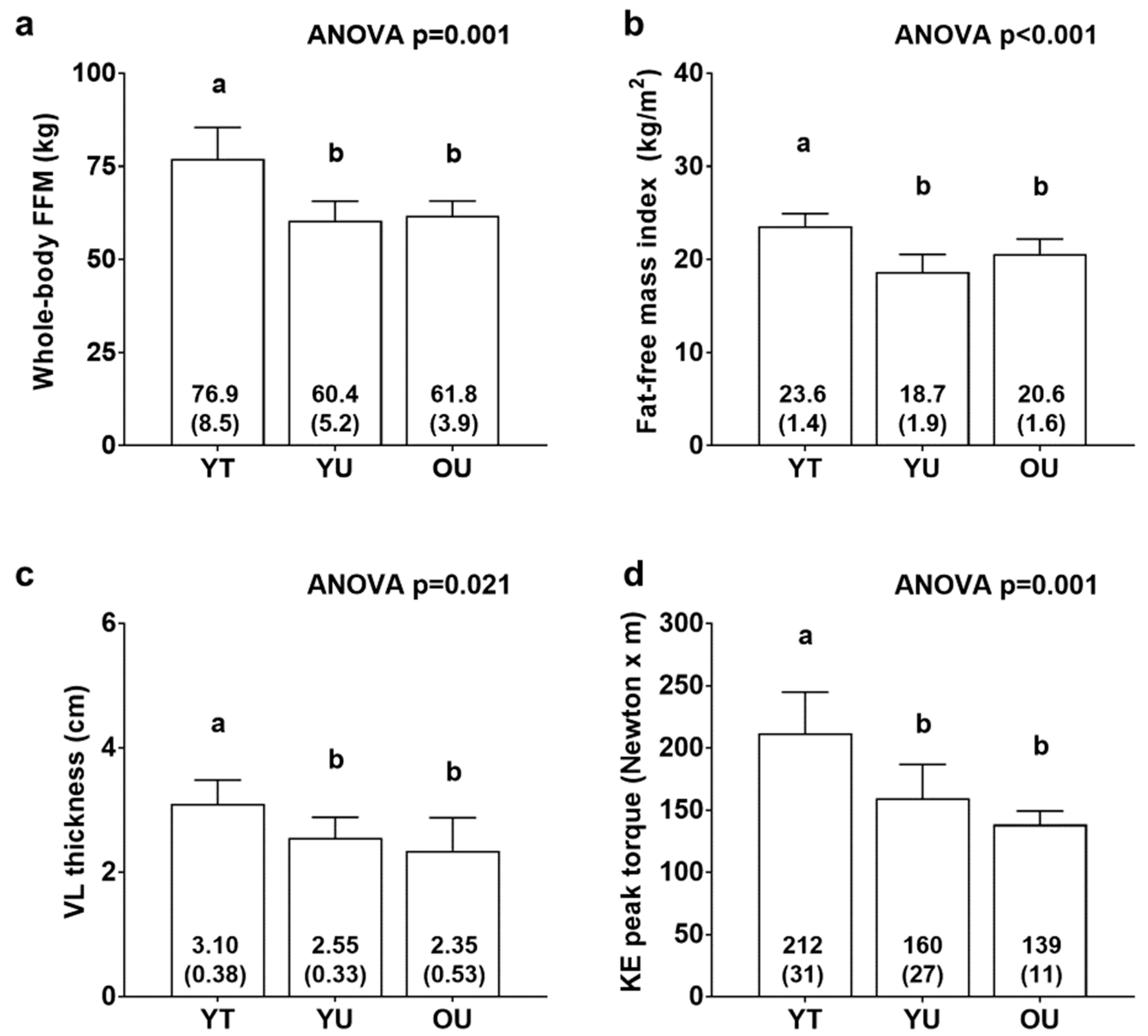
3.1. Participant Characteristics
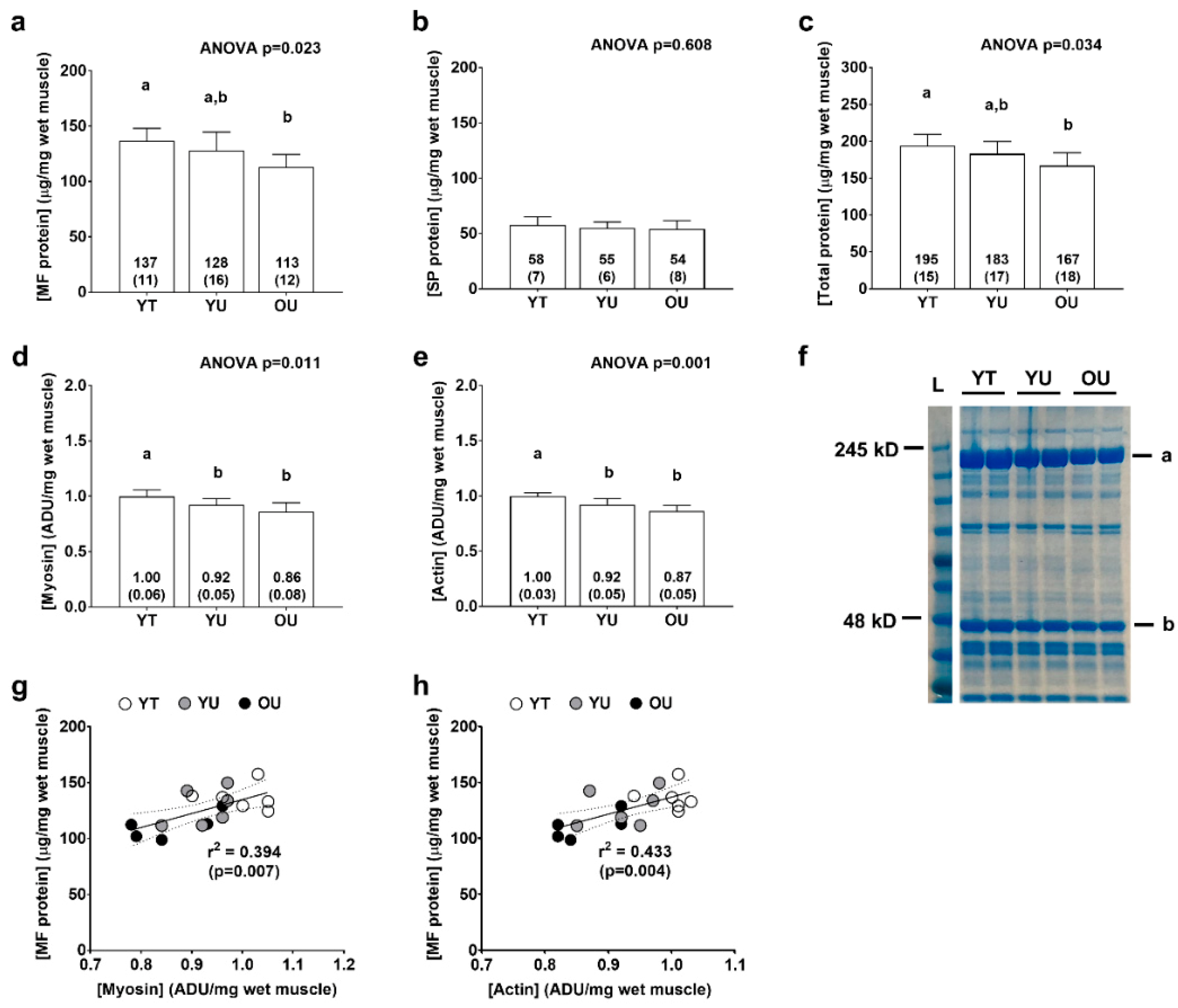
3.2. Total Myofibrillar and Sarcoplasmic Protein Concentrations
3.3. Muscle Proteome Analysis
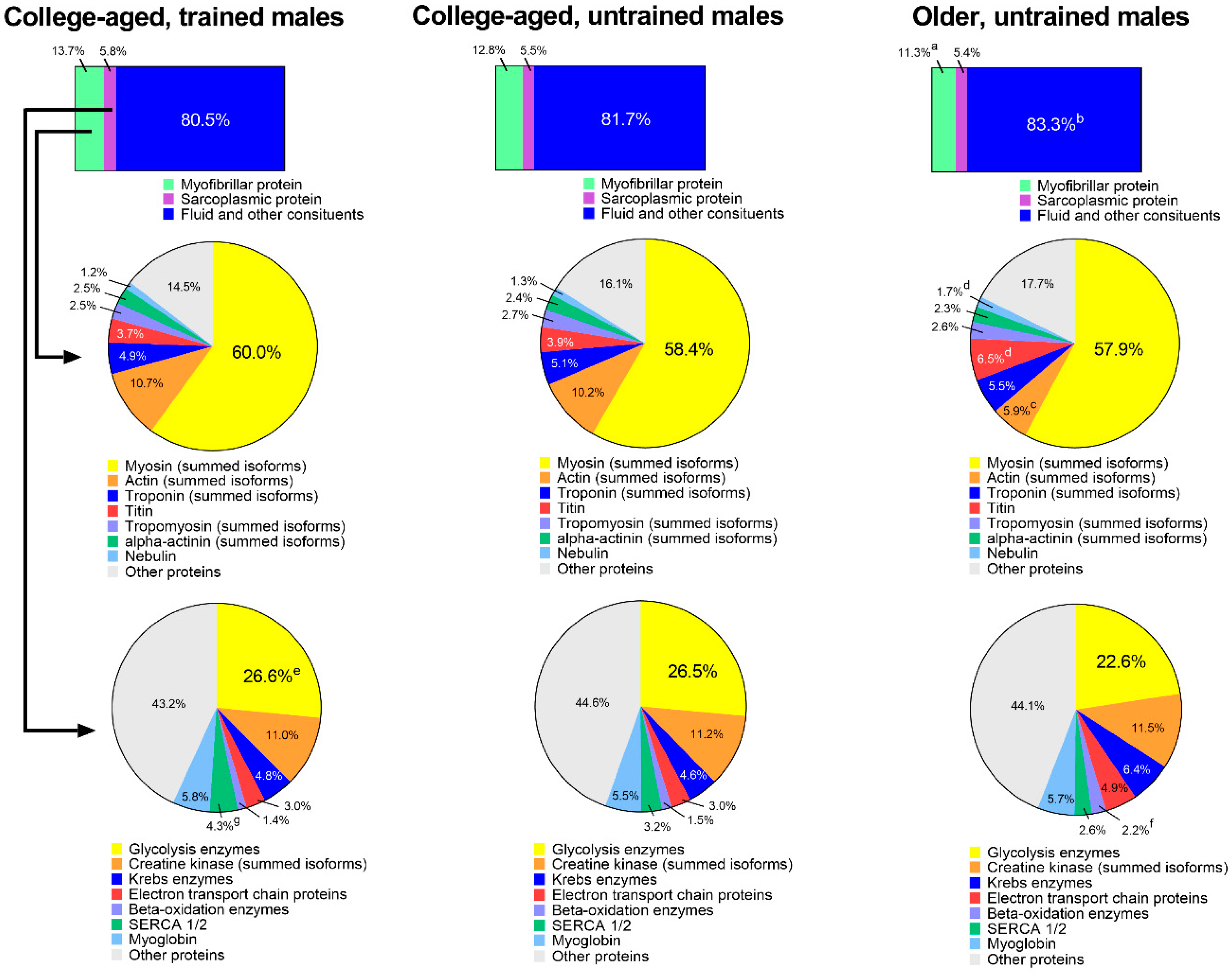
3.4. Extrapolation of Muscle Composition between Groups
4. Discussion
4.1. Proteome Differences between YT versus Other Groups
4.2. Proteome Differences between OU versus Other Groups
4.3. Experimental Considerations
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Haun, C.T.; Vann, C.G.; Roberts, B.M.; Vigotsky, A.D.; Schoenfeld, B.J.; Roberts, M.D. A Critical Evaluation of the Biological Construct Skeletal Muscle Hypertrophy: Size Matters but So Does the Measurement. Front. Physiol. 2019, 10, 247. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grgic, J.; Schoenfeld, B.J. Are the Hypertrophic Adaptations to High and Low-Load Resistance Training Muscle Fiber Type Specific? Front. Physiol. 2018, 9, 402. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Evans, J.W. Periodized Resistance Training for Enhancing Skeletal Muscle Hypertrophy and Strength: A Mini-Review. Front. Physiol. 2019, 10, 13. [Google Scholar] [CrossRef] [PubMed]
- Fry, A.C. The role of resistance exercise intensity on muscle fibre adaptations. Sports Med. 2004, 34, 663–679. [Google Scholar] [CrossRef] [PubMed]
- Marzetti, E.; Calvani, R.; Tosato, M.; Cesari, M.; Di Bari, M.; Cherubini, A.; Collamati, A.; D’Angelo, E.; Pahor, M.; Bernabei, R.; et al. Sarcopenia: An overview. Aging Clin. Exp. Res. 2017, 29, 11–17. [Google Scholar] [CrossRef]
- Trappe, S.; Gallagher, P.; Harber, M.; Carrithers, J.; Fluckey, J.; Trappe, T. Single muscle fibre contractile properties in young and old men and women. J. Physiol. 2003, 552, 47–58. [Google Scholar] [CrossRef]
- Gelfi, C.; Vigano, A.; Ripamonti, M.; Pontoglio, A.; Begum, S.; Pellegrino, M.A.; Grassi, B.; Bottinelli, R.; Wait, R.; Cerretelli, P. The human muscle proteome in aging. J. Proteome Res. 2006, 5, 1344–1353. [Google Scholar] [CrossRef]
- MacDougall, J.D.; Sale, D.G.; Elder, G.C.; Sutton, J.R. Muscle ultrastructural characteristics of elite powerlifters and bodybuilders. Eur. J. Appl. Physiol. Occup. Physiol. 1982, 48, 117–126. [Google Scholar] [CrossRef]
- Toth, M.J.; Miller, M.S.; VanBuren, P.; Bedrin, N.G.; LeWinter, M.M.; Ades, P.A.; Palmer, B.M. Resistance training alters skeletal muscle structure and function in human heart failure: Effects at the tissue, cellular and molecular levels. J. Physiol. 2012, 590, 1243–1259. [Google Scholar] [CrossRef]
- Haun, C.T.; Vann, C.G.; Osburn, S.C.; Mumford, P.W.; Roberson, P.A.; Romero, M.A.; Fox, C.D.; Johnson, C.A.; Parry, H.A.; Kavazis, A.N.; et al. Muscle fiber hypertrophy in response to 6 weeks of high-volume resistance training in trained young men is largely attributed to sarcoplasmic hypertrophy. PLoS ONE 2019, 14, e0215267. [Google Scholar] [CrossRef] [Green Version]
- Murgia, M.; Toniolo, L.; Nagaraj, N.; Ciciliot, S.; Vindigni, V.; Schiaffino, S.; Reggiani, C.; Mann, M. Single Muscle Fiber Proteomics Reveals Fiber-Type-Specific Features of Human Muscle Aging. Cell Rep. 2017, 19, 2396–2409. [Google Scholar] [CrossRef] [Green Version]
- Hody, S.; Leprince, P.; Sergeant, K.; Renaut, J.; Croisier, J.L.; Wang, F.; Rogister, B. Human muscle proteome modifications after acute or repeated eccentric exercises. Med. Sci. Sports Exerc. 2011, 43, 2281–2296. [Google Scholar] [CrossRef] [PubMed]
- Tibana, R.A.; Franco, O.L.; Cunha, G.V.; Sousa, N.M.F.; Sousa Neto, I.V.; Carvalho, M.M.; Almeida, J.A.; Durigan, J.L.Q.; Marqueti, R.C.; Navalta, J.W.; et al. The Effects of Resistance Training Volume on Skeletal Muscle Proteome. Int. J. Exerc. Sci. 2017, 10, 1051–1066. [Google Scholar] [PubMed]
- Esco, M.R.; Fedewa, M.V.; Freeborn, T.J.; Moon, J.R.; Wingo, J.E.; Cicone, Z.; Holmes, C.J.; Hornikel, B.; Welborn, B. Agreement between supine and standing bioimpedance spectroscopy devices and dual-energy X-ray absorptiometry for body composition determination. Clin Physiol Funct Imaging 2019, 39, 355–361. [Google Scholar] [CrossRef] [PubMed]
- Haun, C.T.; Vann, C.G.; Mobley, C.B.; Roberson, P.A.; Osburn, S.C.; Holmes, H.M.; Mumford, P.M.; Romero, M.A.; Young, K.C.; Moon, J.R.; et al. Effects of Graded Whey Supplementation During Extreme-Volume Resistance Training. Front. Nutr. 2018, 5, 84. [Google Scholar] [CrossRef] [PubMed]
- Mobley, C.B.; Haun, C.T.; Roberson, P.A.; Mumford, P.W.; Romero, M.A.; Kephart, W.C.; Anderson, R.G.; Vann, C.G.; Osburn, S.C.; Pledge, C.D.; et al. Effects of Whey, Soy or Leucine Supplementation with 12 Weeks of Resistance Training on Strength, Body Composition, and Skeletal Muscle and Adipose Tissue Histological Attributes in College-Aged Males. Nutrients 2017, 9, 972. [Google Scholar] [CrossRef] [PubMed]
- Roberts, M.D.; Romero, M.A.; Mobley, C.B.; Mumford, P.W.; Roberson, P.A.; Haun, C.T.; Vann, C.G.; Osburn, S.C.; Holmes, H.H.; Greer, R.A.; et al. Skeletal muscle mitochondrial volume and myozenin-1 protein differences exist between high versus low anabolic responders to resistance training. PeerJ 2018, 6, e5338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cohen, S.; Brault, J.J.; Gygi, S.P.; Glass, D.J.; Valenzuela, D.M.; Gartner, C.; Latres, E.; Goldberg, A.L. During muscle atrophy, thick, but not thin, filament components are degraded by MuRF1-dependent ubiquitylation. J. Cell Biol. 2009, 185, 1083–1095. [Google Scholar] [CrossRef] [Green Version]
- Martin, J.S.; Kephart, W.C.; Haun, C.T.; McCloskey, A.E.; Shake, J.J.; Mobley, C.B.; Goodlett, M.D.; Kavazis, A.; Pascoe, D.D.; Zhang, L.; et al. Impact of external pneumatic compression target inflation pressure on transcriptome-wide RNA expression in skeletal muscle. Physiol. Rep. 2016, 4. [Google Scholar] [CrossRef]
- Hyatt, H.W.; Toedebusch, R.G.; Ruegsegger, G.; Mobley, C.B.; Fox, C.D.; McGinnis, G.R.; Quindry, J.C.; Booth, F.W.; Roberts, M.D.; Kavazis, A.N. Comparative adaptations in oxidative and glycolytic muscle fibers in a low voluntary wheel running rat model performing three levels of physical activity. Physiol. Rep. 2015, 3. [Google Scholar] [CrossRef]
- Wen, Y.; Murach, K.A.; Vechetti, I.J., Jr.; Fry, C.S.; Vickery, C.; Peterson, C.A.; McCarthy, J.J.; Campbell, K.S. MyoVision: Software for automated high-content analysis of skeletal muscle immunohistochemistry. J. Appl. Physiol. (1985) 2018, 124, 40–51. [Google Scholar] [CrossRef] [PubMed]
- Phillips, S.M. Short-term training: When do repeated bouts of resistance exercise become training? Can. J. Appl. Physiol. 2000, 25, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Meijer, J.P.; Jaspers, R.T.; Rittweger, J.; Seynnes, O.R.; Kamandulis, S.; Brazaitis, M.; Skurvydas, A.; Pisot, R.; Simunic, B.; Narici, M.V.; et al. Single muscle fibre contractile properties differ between body-builders, power athletes and control subjects. Exp. Physiol. 2015, 100, 1331–1341. [Google Scholar] [CrossRef] [PubMed]
- Bhasin, S.; Storer, T.W.; Berman, N.; Callegari, C.; Clevenger, B.; Phillips, J.; Bunnell, T.J.; Tricker, R.; Shirazi, A.; Casaburi, R. The effects of supraphysiologic doses of testosterone on muscle size and strength in normal men. N. Engl. J. Med. 1996, 335, 1–7. [Google Scholar] [CrossRef]
- Tsukamoto, S.; Shibasaki, A.; Naka, A.; Saito, H.; Iida, K. Lactate Promotes Myoblast Differentiation and Myotube Hypertrophy via a Pathway Involving MyoD In Vitro and Enhances Muscle Regeneration In Vivo. Int. J. Mol. Sci. 2018, 19, 3649. [Google Scholar] [CrossRef] [Green Version]
- Lovering, R.M.; De Deyne, P.G. Contractile function, sarcolemma integrity, and the loss of dystrophin after skeletal muscle eccentric contraction-induced injury. Am. J. Physiol. Cell Physiol. 2004, 286, C230–C238. [Google Scholar] [CrossRef]
- Woolstenhulme, M.T.; Conlee, R.K.; Drummond, M.J.; Stites, A.W.; Parcell, A.C. Temporal response of desmin and dystrophin proteins to progressive resistance exercise in human skeletal muscle. J. Appl. Physiol. (1985) 2006, 100, 1876–1882. [Google Scholar] [CrossRef]
- Tesch, P.A.; Colliander, E.B.; Kaiser, P. Muscle metabolism during intense, heavy-resistance exercise. Eur. J. Appl. Physiol. Occup. Physiol. 1986, 55, 362–366. [Google Scholar] [CrossRef]
- Costill, D.L.; Coyle, E.F.; Fink, W.F.; Lesmes, G.R.; Witzmann, F.A. Adaptations in skeletal muscle following strength training. J. Appl. Physiol. Respir. Environ. Exerc. Physiol. 1979, 46, 96–99. [Google Scholar] [CrossRef]
- Swaggart, K.A.; Demonbreun, A.R.; Vo, A.H.; Swanson, K.E.; Kim, E.Y.; Fahrenbach, J.P.; Holley-Cuthrell, J.; Eskin, A.; Chen, Z.; Squire, K.; et al. Annexin A6 modifies muscular dystrophy by mediating sarcolemmal repair. Proc. Natl. Acad. Sci. USA 2014, 111, 6004–6009. [Google Scholar] [CrossRef] [Green Version]
- Quattrocelli, M.; Barefield, D.Y.; Warner, J.L.; Vo, A.H.; Hadhazy, M.; Earley, J.U.; Demonbreun, A.R.; McNally, E.M. Intermittent glucocorticoid steroid dosing enhances muscle repair without eliciting muscle atrophy. J. Clin. Investig. 2017, 127, 2418–2432. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gonzalez-Freire, M.; Semba, R.D.; Ubaida-Mohien, C.; Fabbri, E.; Scalzo, P.; Hojlund, K.; Dufresne, C.; Lyashkov, A.; Ferrucci, L. The Human Skeletal Muscle Proteome Project: A reappraisal of the current literature. J. Cachexia Sarcopenia Muscle 2017, 8, 5–18. [Google Scholar] [CrossRef] [PubMed]
- Piec, I.; Listrat, A.; Alliot, J.; Chambon, C.; Taylor, R.G.; Bechet, D. Differential proteome analysis of aging in rat skeletal muscle. FASEB J. 2005, 19, 1143–1145. [Google Scholar] [CrossRef] [PubMed]
- Kanehisa, M.; Goto, S. KEGG: Kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 2000, 28, 27–30. [Google Scholar] [CrossRef] [PubMed]
- Nagano, K. Alteration of cathepsin-D expression in atrophied muscles and apoptotic myofibers by hindlimb unloading in a low-temperature environment. J. Phys. Ther. Sci. 2015, 27, 3585–3591. [Google Scholar] [CrossRef] [Green Version]
- Lundholm, K.; Schersten, T. Leucine incorporation into proteins and cathepsin-D activity in human skeletal muscles. The influence of the age of the subject. Exp. Gerontol. 1975, 10, 155–159. [Google Scholar] [CrossRef]
- Wiederanders, B.; Oelke, B. Accumulation of inactive cathepsin D in old rats. Mech. Ageing Dev. 1984, 24, 265–271. [Google Scholar] [CrossRef]
| Protein Symbol | Protein Name | YT Mean ± SD | YU Mean ± SD | OU Mean ± SD |
|---|---|---|---|---|
| YT < other groups (p < 0.05) | ||||
| NDUFB8 | NADH dehydrogenase 1 beta subcomplex subunit 8 | 0.5 ± 1.3 | 4.4 ± 3.9 | 10.9 ± 7.3 |
| LAMB2 | Laminin subunit beta-2 | 3.7 ± 5.4 | 12.4 ± 6.2 | 16.2 ± 12.3 |
| PHB | Prohibitin | 6.5 ± 5.6 | 14.1 ± 5.8 | 19.6 ± 12.0 |
| CHCHD3 | MICOS complex subunit MIC19 | 13.2 ± 3.6 | 25.1 ± 12.5 | 28.6 ± 11.9 |
| DMD | Dystrophin | 16.6 ± 6.3 | 29.2 ± 11.8 | 50.3 ± 19.8 |
| YT > other groups (p < 0.05) | ||||
| MYH16 | Putative uncharacterized protein MYH16 | 487.1 ± 402.4 | 48.1 ± 117.7 | 0.0 ± 0.0 |
| Protein Symbol | Protein Name | YT Mean ± SD | YU Mean ± SD | OU Mean ± SD |
|---|---|---|---|---|
| YT < other groups (p < 0.05) | ||||
| COQ8A | Atypical kinase COQ8A | 5.5 ± 7.1 | 22.4 ± 16.9 | 36.6 ± 24.0 |
| YT > other groups (p < 0.05) | ||||
| SYPL2 | Synaptophysin-like protein 2 | 79.1 ± 17.5 | 41.5 ± 26.4 | 44.1 ± 24.4 |
| PHKG1 | Phosphorylase b kinase gamma catalytic chain | 22.8 ± 11.0 | 6.5 ± 8.6 | 7.4 ± 7.3 |
| HSPA2 | Heat shock-related 70 kDa protein | 190.4 ± 24.9 | 145.6 ± 32.7 | 89.1 ± 100.3 |
| GDI2 | Rab GDP dissociation inhibitor beta | 61.9 ± 14.4 | 37.3 ± 18.5 | 28.0 ± 14.8 |
| ANXA6 | Annexin A6 | 524.8 ± 98.6 | 409.7 ± 66.7 | 404.6 ± 68 |
| Protein Symbol | Protein Name | YT Mean ± SD | YU Mean ± SD | OU Mean ± SD |
|---|---|---|---|---|
| OU < other groups (p < 0.05) | ||||
| ACTA1 | Actin, alpha skeletal muscle | 5150.3 ± 429.5 | 5126.8 ± 765.0 | 4165.7 ± 317.9 |
| ACTC1 | Actin, alpha cardiac muscle | 4549.8 ± 360.0 | 3981.9 ± 1404.4 | 782.3 ± 1452.8 |
| GAPDH | Glyceraldehyde-3-phosphate dehydrogenase | 325.9 ± 71.2 | 318.1 ± 33.0 | 245.1 ± 41.7 |
| MB | Myoglobin | 235.3 ± 96.0 | 188.3 ± 17.6 | 136.0 ± 40.1 |
| CSRP3 | Cysteine and glycine-rich protein 3 | 27.7 ± 5.2 | 29.0 ± 10.0 | 5.8 ± 9.7 |
| AK1 | Adenylate kinase isoenzyme 1 | 13.9 ± 6.9 | 13.7 ± 7.7 | 3.1 ± 4.2 |
| MYBPH | Myosin-binding protein H | 34.3 ± 18.2 | 32.7 ± 27.8 | 1.3 ± 3.1 |
| OU > other groups (p < 0.05) | ||||
| TTN | Titin | 3491.7 ± 970.9 | 3689.1 ± 1566.7 | 6163.6 ± 611.1 |
| NEB | Nebulin | 1087.4 ± 179.1 | 1185.5 ± 280.2 | 1572.7 ± 102.0 |
| TNNT1 | Troponin T | 533.5 ± 81.6 | 615.4 ± 240.5 | 958.0 ± 245.9 |
| ATP5PD | ATP synthase subunit d | 51.3 ± 19.3 | 68.7 ± 28.6 | 115.1 ± 39.6 |
| PLEC | Plectin | 43.8 ± 13.0 | 37.9 ± 19.4 | 85.9 ± 13.4 |
| HIST3H2BB | Histone H2B type 3-B | 19.1 ± 20.0 | 26.5 ± 18.7 | 54.2 ± 13.3 |
| DMD | Dystrophin | 16.6 ± 6.3 | 29.2 ± 11.8 | 50.3 ± 19.8 |
| HIST1H4A | Histone H4 | 30.5 ± 7.5 | 33.5 ± 5.0 | 48.7 ± 12.5 |
| H2AFV | Histone H2A.V | 21.3 ± 3.7 | 24.6 ± 4.2 | 33.5 ± 4.5 |
| NDUFV2 | NADH dehydrogenase flavoprotein 2 | 17.6 ± 4.5 | 21.4 ± 4.6 | 32.4 ± 10.6 |
| DLST | Dihydrolipoyllysine-residue succinyltransferase component of 2-oxoglutarate dehydrogenase complex | 7.8 ± 3.8 | 10.1 ± 2.9 | 16.8 ± 5.0 |
| Protein Symbol | Protein Name | YT Mean ± SD | YU Mean ± SD | OU Mean ± SD |
|---|---|---|---|---|
| OU < other groups (p < 0.05) | ||||
| KRT9 | Keratin, type I cytoskeletal 9 | 430.4 ± 138.7 | 392.1 ± 24.9 | 250.6 ± 61.1 |
| FHL1 | Four and a half LIM domains protein 1 | 322.9 ± 70.9 | 278.1 ± 32.7 | 221.9 ± 32.8 |
| KRT2 | Keratin, type II cytoskeletal 2 | 202.9 ± 66.5 | 185.2 ± 41.3 | 133.0 ± 31.7 |
| DUSP3 | Dual specificity protein phosphatase 3 | 33.9 ± 11.6 | 25.3 ± 9.0 | 8.9 ± 6.9 |
| ANXA2 | Annexin A2 | 37.5 ± 21.7 | 34.3 ± 16.5 | 8.2 ± 6.7 |
| GSN | Gelsolin | 35.0 ± 20.6 | 43.7 ± 24.0 | 7.6 ± 8.6 |
| ACYP2 | Acylphosphatase-2 | 69.1 ± 18.9 | 43.2 ± 25.7 | 0.0 ± 0.0 |
| UCHL1 | Ubiquitin carboxyl-terminal hydrolase isozyme L1 | 50.8 ± 29.0 | 25.7 ± 27.1 | 0.0 ± 0.0 |
| OU > other groups (p < 0.05) | ||||
| ACO2 | Aconitate hydratase, mitochondrial | 596.0 ± 60.1 | 601.9 ± 45.4 | 764.7 ± 141.5 |
| FABP3 | Fatty acid-binding protein, heart | 474.9 ± 78.3 | 488.9 ± 84.9 | 672.2 ± 127.6 |
| MDH1 | Malate dehydrogenase, cytoplasmic | 386.0 ± 44.5 | 385.7 ± 61.4 | 493.6 ± 48.0 |
| HADHA | Trifunctional enzyme subunit alpha | 266.0 ± 55.4 | 287.6 ± 110.4 | 458.9 ± 141.5 |
| ACADVL | Very long-chain specific acyl-CoA dehydrogenase | 214.6 ± 21.5 | 208.7 ± 60.7 | 379.2 ± 149.0 |
| HADH | Hydroxyacyl-coenzyme A dehydrogenase | 229.1 ± 35.1 | 228.1 ± 33.9 | 327.0 ± 69.4 |
| CKB | Creatine kinase B-type | 59.8 ± 28.7 | 69.8 ± 46.2 | 307.0 ± 157.2 |
| ETFA | Electron transfer flavoprotein subunit alpha | 108.5 ± 28.4 | 120.8 ± 24.2 | 194.2 ± 61.1 |
| ETFB | Electron transfer flavoprotein subunit beta | 74.6 ± 27.7 | 76.6 ± 28.4 | 145.9 ± 61.9 |
| ACOT1 | Acyl-coenzyme A thioesterase 1 | 94.9 ± 17.2 | 92.7 ± 29.2 | 140.1 ± 31.2 |
| COQ9 | Ubiquinone biosynthesis protein COQ9 | 86.1 ± 16.5 | 93.3 ± 22.4 | 127.8 ± 28.1 |
| ES1 homolog | ES1 protein homolog, mitochondrial | 55.0 ± 34.0 | 73.5 ± 31.1 | 127.3 ± 46.1 |
| HSPA9 | Stress-70 protein, mitochondrial | 61.3 ± 11.4 | 62.2 ± 27.1 | 116.7 ± 47.5 |
| CYCS | Cytochrome c | 9.3 ± 10.9 | 19.1 ± 14.9 | 59.8 ± 12.7 |
| GPT | Alanine aminotransferase 1 | 34.7 ± 9.5 | 30.5 ± 10.3 | 59.2 ± 14.9 |
| ALDH2 | Aldehyde dehydrogenase, mitochondrial | 23.5 ± 25.8 | 21.0 ± 20.6 | 56.8 ± 24.9 |
| CTSD | Cathepsin D | 11.4 ± 8.5 | 7.6 ± 8.4 | 53.9 ± 15.4 |
| ALDH5A1 | Succinate-semialdehyde dehydrogenase, mitochondrial | 24.3 ± 20.6 | 20.2 ± 15.9 | 53.3 ± 23.6 |
| AIFM1 | Apoptosis-inducing factor 1, mitochondrial | 13.2 ± 6.2 | 8.7 ± 8.9 | 45.7 ± 26.8 |
| NIPSNAP2 | Protein NipSnap homolog 2 | 15.0 ± 13.6 | 16.6 ± 16.8 | 42.8 ± 13.5 |
| FABP4 | Fatty acid-binding protein, adipocyte | 4.7 ± 5.3 | 16.0 ± 17.8 | 35.9 ± 5.9 |
| L2HGDH | L-2-hydroxyglutarate dehydrogenase, mitochondrial | 4.8 ± 6.5 | 6.2 ± 7.2 | 28.7 ± 22.2 |
| IMPA1 | Inositol monophosphatase 1 | 15.6 ± 8.5 | 11.3 ± 10.4 | 26.5 ± 4.3 |
| NDUFB6 | NADH dehydrogenase 1 beta subcomplex subunit 6 | 0.0 ± 0.0 | 0.0 ± 0.0 | 25.8 ± 24.4 |
| DCXR | L-xylulose reductase | 6.6 ± 7.2 | 4.7 ± 6.2 | 24.4 ± 15.3 |
| NDUFB11 | NADH dehydrogenase 1 beta subcomplex subunit 11 | 0.5 ± 1.3 | 0.0 ± 0.0 | 22.6 ± 20.5 |
| LRPPRC | Leucine-rich PPR motif-containing protein, mitochondrial | 0.0 ± 0.0 | 0.7 ± 1.8 | 13.3 ± 12.0 |
| DGLUCY | D-glutamate cyclase, mitochondrial | 1.0 ± 1.5 | 0.0 ± 0.0 | 12.6 ± 10.3 |
| DCN | Decorin | 3.3 ± 5.2 | 3.9 ± 3.7 | 11.0 ± 3.4 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vann, C.G.; Roberson, P.A.; Osburn, S.C.; Mumford, P.W.; Romero, M.A.; Fox, C.D.; Moore, J.H.; Haun, C.T.; Beck, D.T.; Moon, J.R.; et al. Skeletal Muscle Myofibrillar Protein Abundance Is Higher in Resistance-Trained Men, and Aging in the Absence of Training May Have an Opposite Effect. Sports 2020, 8, 7. https://doi.org/10.3390/sports8010007
Vann CG, Roberson PA, Osburn SC, Mumford PW, Romero MA, Fox CD, Moore JH, Haun CT, Beck DT, Moon JR, et al. Skeletal Muscle Myofibrillar Protein Abundance Is Higher in Resistance-Trained Men, and Aging in the Absence of Training May Have an Opposite Effect. Sports. 2020; 8(1):7. https://doi.org/10.3390/sports8010007
Chicago/Turabian StyleVann, Christopher G., Paul. A. Roberson, Shelby C. Osburn, Petey W. Mumford, Matthew A. Romero, Carlton D. Fox, Johnathon H. Moore, Cody T. Haun, Darren T. Beck, Jordan R. Moon, and et al. 2020. "Skeletal Muscle Myofibrillar Protein Abundance Is Higher in Resistance-Trained Men, and Aging in the Absence of Training May Have an Opposite Effect" Sports 8, no. 1: 7. https://doi.org/10.3390/sports8010007






