Ustilago Rabenhorstiana—An Alternative Natural Itaconic Acid Producer
Abstract
1. Introduction
2. Materials and Methods
2.1. Microorganism
2.2. Media Compositions
2.3. Cultivation
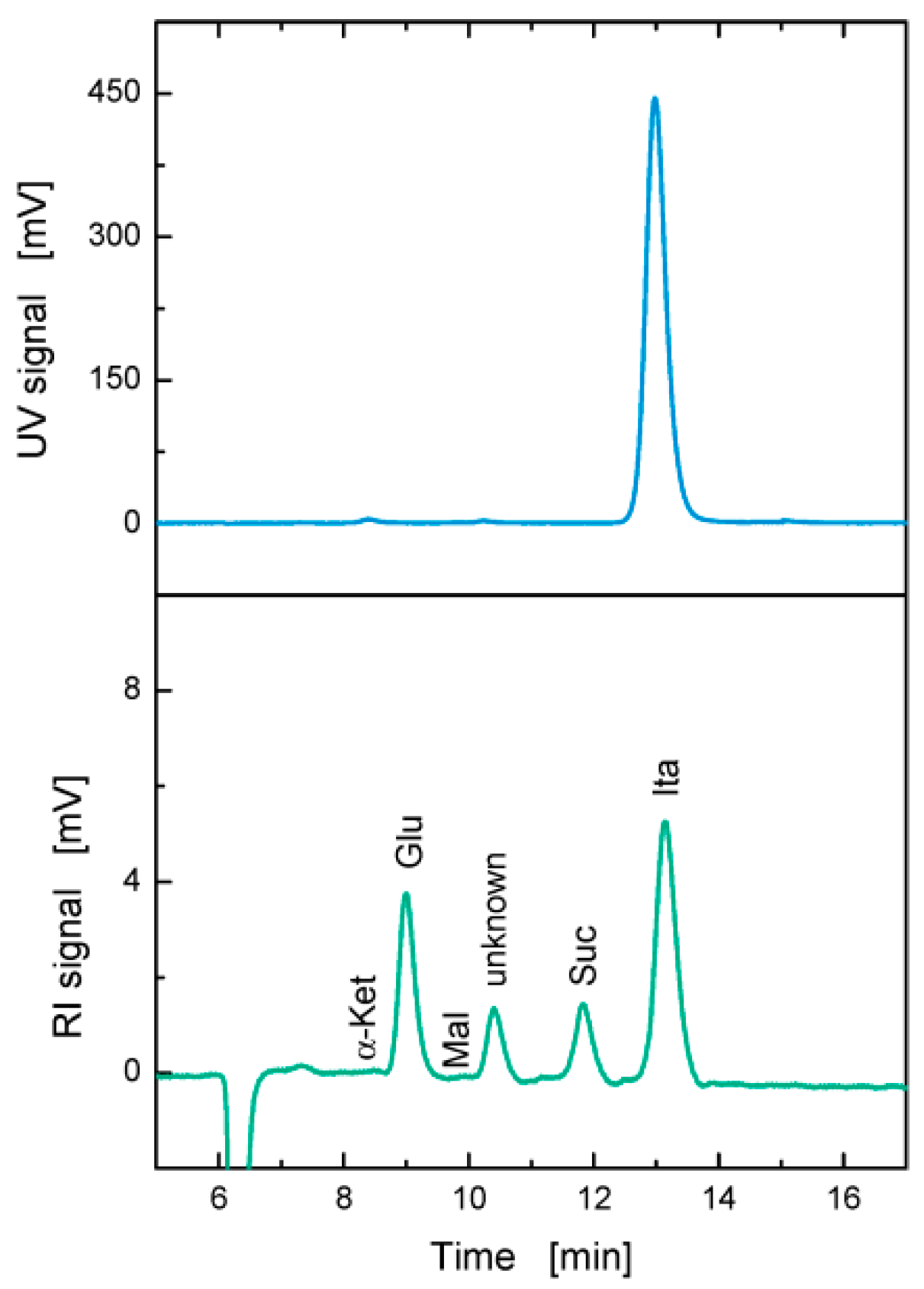
2.4. Analytical Methods
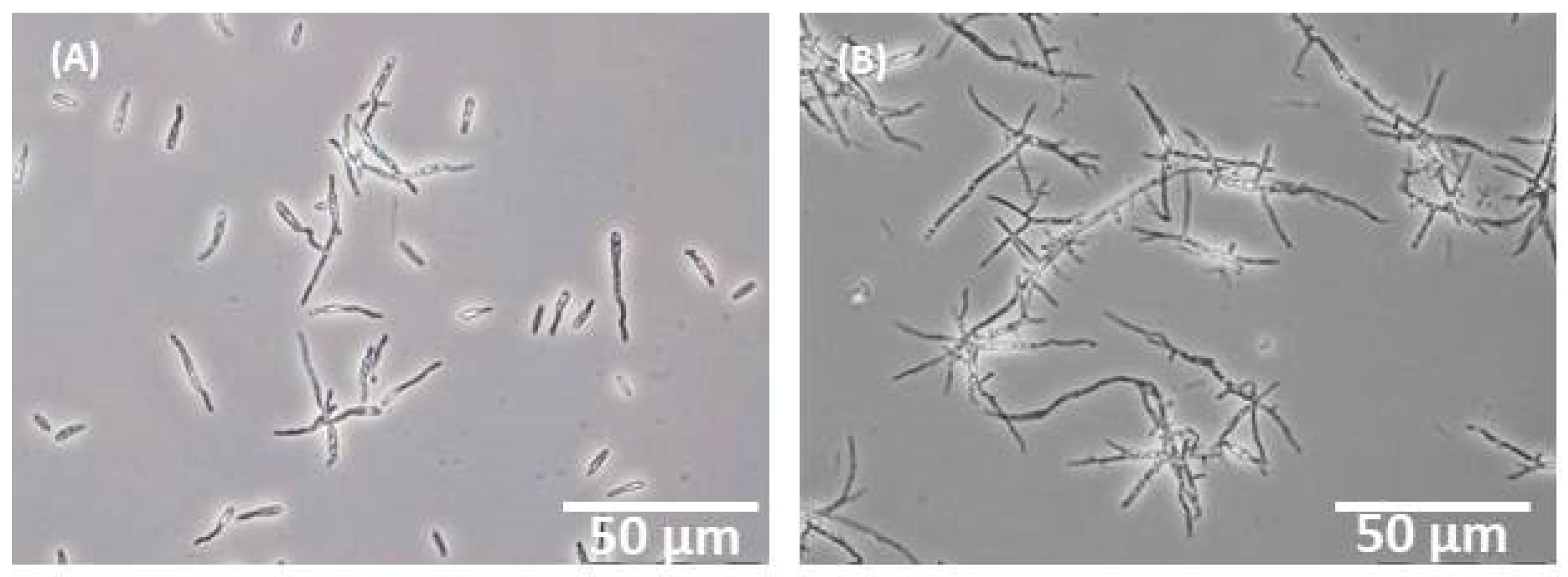
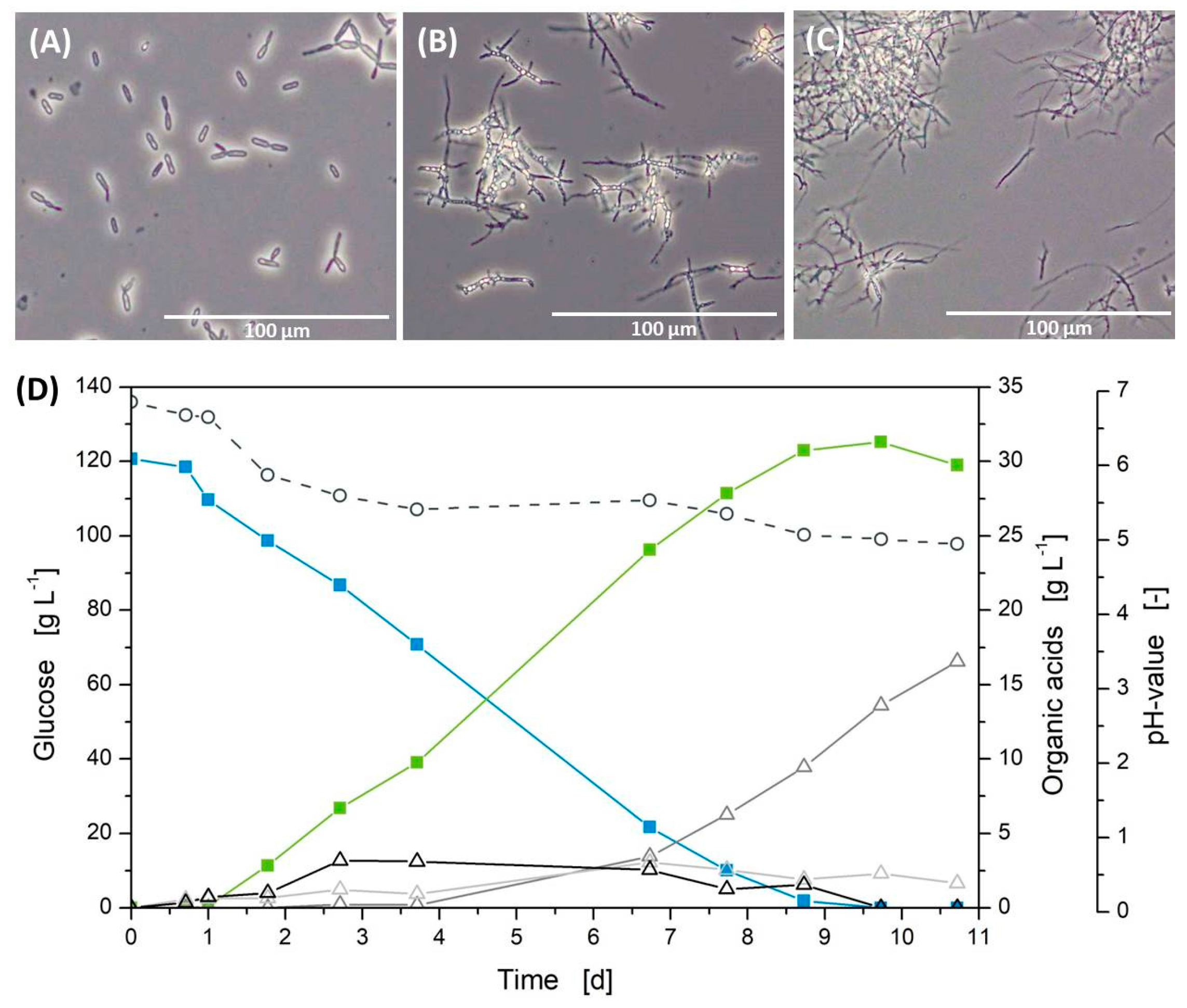
2.5. Microscopy
3. Results
3.1. Standard Cultivation in Shake Flasks
3.2. Influence of Media Components
3.3. Monosaccharide Utilization
3.4. Influence of Sugar Degradation Products
3.5. Influence of the pH-Value in 1 L-Bioreactor
3.6. Influence of Aeration in 1 L-Bioreactor
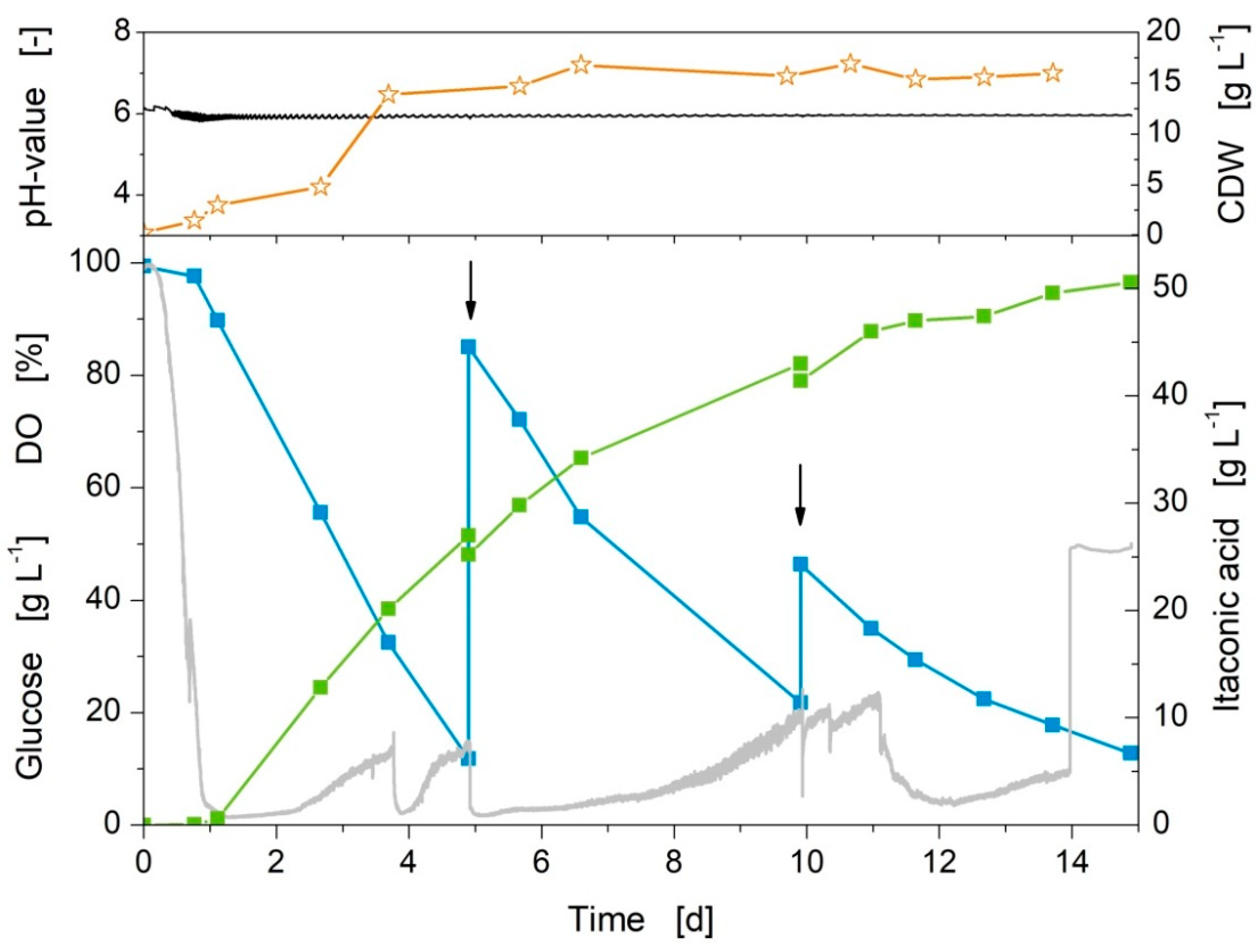
3.7. Fed-Batch Mode in 1 L-Bioreactor with Glucose
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Appendix A
| Fatty Acid | Concentration [mg·g−1CDW] |
|---|---|
| C14:0 | 1.46 |
| C16:0 | 30.04 |
| C16:1 | 1.55 |
| C18:0 | 16.94 |
| C18:1 | 1.83 |
| C18:2 | 48.38 |
| C22:0 | 4.42 |
| C24:0 | 2.45 |
References
- Werpy, T.; Petersen, G. Top Value Added Chemicals from Biomass: Volume I—Results of Screening for Potential Candidates from Sugars and Synthesis Gas; National Renewable Energy Lab.: Golden, CO, USA, 2004. [Google Scholar]
- Magalhaes, A.I.; de Carvalho, J.C.; Medina, J.D.C.; Soccol, C.R. Downstream process development in biotechnological itaconic acid manufacturing. Appl. Microbiol. Biotechnol. 2017, 101, 1–12. [Google Scholar] [CrossRef]
- Robert, T.; Friebel, S. Itaconic acid—A versatile building block for renewable polyesters with enhanced functionality. Green Chem. 2016, 18, 2922–2934. [Google Scholar] [CrossRef]
- Okabe, M.; Lies, D.; Kanamasa, S.; Park, E.Y. Biotechnological production of itaconic acid and its biosynthesis in Aspergillus terreus. Appl. Microbiol. Biotechnol. 2009, 84, 597–606. [Google Scholar] [CrossRef]
- Klement, T.; Milker, S.; Jäger, G.; Grande, P.M.; de Maria, P.D.; Büchs, J. Biomass pretreatment affects Ustilago maydis in producing itaconic acid. Microb. Cell Factories 2012, 11, 43. [Google Scholar] [CrossRef]
- Willke, T.; Vorlop, K.D. Biotechnological production of itaconic acid. Appl. Microbiol. Biotechnol. 2001, 56, 289–295. [Google Scholar] [CrossRef]
- Hevekerl, A.; Kuenz, A.; Vorlop, K.D. Influence of the pH on the itaconic acid production with Aspergillus terreus. Appl. Microbiol. Biotechnol. 2014, 98, 10005–10012. [Google Scholar] [CrossRef]
- Karaffa, L.; Diaz, R.; Papp, B.; Fekete, E.; Sandor, E.; Kubicek, C.P. A deficiency of manganese ions in the presence of high sugar concentrations is the critical parameter for achieving high yields of itaconic acid by Aspergillus terreus. Appl. Microbiol. Biotechnol. 2015, 99, 7937–7944. [Google Scholar] [CrossRef]
- Krull, S.; Hevekerl, A.; Kuenz, A.; Prüße, U. Process development of itaconic acid production by a natural wild type strain of Aspergillus terreus to reach industrially relevant final titers. Appl. Microbiol. Biotechnol. 2017, 101, 4063–4072. [Google Scholar] [CrossRef]
- Kuenz, A.; Krull, S. Biotechnological production of itaconic acid-things you have to know. Appl. Microbiol. Biotechnol. 2018, 102, 3901–3914. [Google Scholar] [CrossRef]
- Kobayashi, T. Production of itaconic acid from wood waste. Process Biochem. 1978, 13, 15–22. [Google Scholar]
- Krull, S.; Eidt, L.; Hevekerl, A.; Kuenz, A.; Prüße, U. Itaconic acid production from wheat chaff by Aspergillus terreus. Process Biochem. 2017, 63, 169–176. [Google Scholar] [CrossRef]
- Wu, X.F.; Liu, Q.; Deng, Y.D.; Li, J.H.; Chen, X.J.; Gu, Y.Z.; Lv, X.J.; Zheng, Z.; Jiang, S.T.; Li, X.J. Production of itaconic acid by biotransformation of wheat bran hydrolysate with Aspergillus terreus CICC40205 mutant. Bioresour. Technol. 2017, 241, 25–34. [Google Scholar] [CrossRef]
- Levinson, W.E.; Kurtzman, C.P.; Kuo, T.M. Production of itaconic acid by Pseudozyma antarctica NRRL Y-7808 under nitrogen-limited growth conditions. Enzym. Microb. Technol. 2006, 39, 824–827. [Google Scholar] [CrossRef]
- Specht, R.; Andreas, A.; Kreyß, E.; Barth, G.; Bodinus, C. Verfahren zur Biotechnologischen Herstellung von Itaconsäure. DE Patent 102008011854 B4, 20 February 2014. [Google Scholar]
- Geiser, E.; Wiebach, V.; Wierckx, N.; Blank, L.M. Prospecting the biodiversity of the fungal family Ustilaginaceae for the production of value-added chemicals. Fungal Biol. Biotechnol. 2014, 1, 2. [Google Scholar] [CrossRef]
- Tabuchi, T.; Sugisawa, T.; Ishidori, T.; Nakahara, T.; Sugiyama, J. Itaconic Acid Fermentation by a Yeast Belonging to the Genus Candida. Agric. Biol. Chem. 1981, 45, 475–479. [Google Scholar] [CrossRef]
- Guevarra, E.D.; Tabuchi, T. Accumulation of itaconic, 2-hydroxyparaconic, itatartaric, and malic-acids by strains of the genus Ustilago. Agric. Biol. Chem. 1990, 54, 2353–2358. [Google Scholar] [CrossRef][Green Version]
- Maassen, N.; Panakova, M.; Wierckx, N.; Geiser, E.; Zimmermann, M.; Bolker, M.; Klinner, U.; Blank, L.M. Influence of carbon and nitrogen concentration on itaconic acid production by the smut fungus Ustilago maydis. Eng. Life Sci. 2014, 14, 129–134. [Google Scholar] [CrossRef]
- Spoeckner, S.; Wray, V.; Nimtz, M.; Lang, S. Glycolipids of the smut fungus Ustilago maydis from cultivation on renewable resources. Appl. Microbiol. Biotechnol. 1999, 51, 33–39. [Google Scholar] [CrossRef]
- Aguilar, L.R.; Pardo, J.P.; Lomeli, M.M.; Bocardo, O.I.L.; Oropeza, M.A.J.; Sanchez, G.G. Lipid droplets accumulation and other biochemical changes induced in the fungal pathogen Ustilago maydis under nitrogen-starvation. Arch. Microbiol. 2017, 199, 1195–1209. [Google Scholar] [CrossRef]
- Regestein, L.; Klement, T.; Grande, P.; Kreyenschulte, D.; Heyman, B.; Maßmann, T.; Eggert, A.; Sengpiel, R.; Wang, Y.; Wierckx, N. From beech wood to itaconic acid: Case study on biorefinery process integration. Biotechnol. Biofuels 2018, 11, 1–11. [Google Scholar] [CrossRef]
- Geiser, E.; Przybilla, S.K.; Engel, M.; Kleineberg, W.; Büttner, L.; Sarikaya, E.; Den Hartog, T.; Klankermayer, J.; Leitner, W.; Bölker, M. Genetic and biochemical insights into the itaconate pathway of Ustilago maydis enable enhanced production. Metab. Eng. 2016, 38, 427–435. [Google Scholar] [CrossRef]
- Tehrani, H.H.; Tharmasothirajan, A.; Track, E.; Blank, L.M.; Wierckx, N. Engineering the morphology and metabolism of pH tolerant Ustilago cynodontis for efficient itaconic acid production. Metab. Eng. 2019, 54, 293–300. [Google Scholar] [CrossRef]
- Geiser, E.; Przybilla, S.K.; Friedrich, A.; Buckel, W.; Wierckx, N.; Blank, L.M.; Bolker, M. Ustilago maydis produces itaconic acid via the unusual intermediate trans-aconitate. Microb. Biotechnol. 2016, 9, 116–126. [Google Scholar] [CrossRef]
- Zambanini, T.; Hartmann, S.K.; Schmitz, L.M.; Buttner, L.; Hosseinpour Tehrani, H.; Geiser, E.; Beudels, M.; Venc, D.; Wandrey, G.; Buchs, J.; et al. Promoters from the itaconate cluster of Ustilago maydis are induced by nitrogen depletion. Fungal Biol. Biotechnol. 2017, 4, 11. [Google Scholar] [CrossRef]
- Wierckx, N.; Agrimi, G.; Lubeck, P.S.; Steiger, M.G.; Mira, N.P.; Punt, P.J. Metabolic specialization in itaconic acid production: A tale of two fungi. Curr. Opin. Biotechnol. 2019, 62, 153–159. [Google Scholar] [CrossRef]
- Zambanini, T.; Hosseinpour Tehrani, H.; Geiser, E.; Merker, D.; Schleese, S.; Krabbe, J.; Buescher, J.M.; Meurer, G.; Wierckx, N.; Blank, L.M. Efficient itaconic acid production from glycerol with Ustilago vetiveriae TZ1. Biotechnol. Biofuels 2017, 10, 131. [Google Scholar] [CrossRef]
- Haskins, R.; Thorn, J.; Boothroyd, B. Biochemistry of the Ustilaginales: XI. Metabolic products of Ustilago zeae in submerged culture. Can. J. Microbiol. 1955, 1, 749–756. [Google Scholar] [CrossRef]
- Guevarra, E.D.; Tabuchi, T. Production of 2-hydroxyparaconic and itatartaric acids by Ustilago cynodontis and simple recovery process of the acids. Agric. Biol. Chem. 1990, 54, 2359–2365. [Google Scholar] [CrossRef][Green Version]
- Lewis, T.; Nichols, P.D.; McMeekin, T.A. Evaluation of extraction methods for recovery of fatty acids from lipid-producing microheterotrophs. J. Microbiol. Methods 2000, 43, 107–116. [Google Scholar] [CrossRef]
- Klose, J.; Kronstad, J.W. The multifunctional beta-oxidation enzyme is required for full symptom development by the biotrophic maize pathogen Ustilago maydis. Eukaryot. Cell 2006, 5, 2047–2061. [Google Scholar] [CrossRef]
- Sommer, R. Yeast extracts: Production, properties and components. Food Aust. 1998, 50, 181–183. [Google Scholar]
- Zavala-Moreno, A.; Arreguin-Espinosa, R.; Pardo, J.P.; Romero-Aguilar, L.; Guerra-Sánchez, G. Nitrogen source affects glycolipid production and lipid accumulation in the phytopathogen fungus Ustilago maydis. Adv. Microbiol. 2014, 4, 934. [Google Scholar] [CrossRef]
- Gibson, D.M.; King, B.C.; Hayes, M.L.; Bergstrom, G.C. Plant pathogens as a source of diverse enzymes for lignocellulose digestion. Curr. Opin. Microbiol. 2011, 14, 264–270. [Google Scholar] [CrossRef]
- Geiser, E.; Reindl, M.; Blank, L.M.; Feldbrügge, M.; Wierckx, N.; Schipper, K. Activating intrinsic carbohydrate-active enzymes of the smut fungus Ustilago maydis for the degradation of plant cell wall components. Appl. Environ. Microbiol. 2016, 82, 5174–5185. [Google Scholar] [CrossRef]
- Couturier, M.; Navarro, D.; Olivé, C.; Chevret, D.; Haon, M.; Favel, A.; Lesage-Meessen, L.; Henrissat, B.; Coutinho, P.M.; Berrin, J.-G. Post-genomic analyses of fungal lignocellulosic biomass degradation reveal the unexpected potential of the plant pathogen Ustilago maydis. BMC Genom. 2012, 13, 57. [Google Scholar] [CrossRef]
- Gyamerah, M. Factors affecting the growth form of Aspergillus terreus NRRL 1960 in relation to itaconic acid fermentation. Appl. Microbiol. Biotechnol. 1995, 44, 356–361. [Google Scholar] [CrossRef]
- Benito, B.; Garciadeblás, B.; Pérez-Martín, J.; Rodríguez-Navarro, A. Growth at high pH and sodium and potassium tolerance in media above the cytoplasmic pH depend on ENA ATPases in Ustilago maydis. Eukaryot. Cell 2009, 8, 821–829. [Google Scholar] [CrossRef]
- Stratford, M.; Nebe-von-Caron, G.; Steels, H.; Novodvorska, M.; Ueckert, J.; Archer, D.B. Weak-acid preservatives: pH and proton movements in the yeast Saccharomyces cerevisiae. Int. J. Food Microbiol. 2013, 161, 164–171. [Google Scholar] [CrossRef]
- Lambert, R.; Stratford, M. Weak-acid preservatives: Modelling microbial inhibition and response. J. Appl. Microbiol. 1999, 86, 157–164. [Google Scholar] [CrossRef]
- Larsson, S.; Palmqvist, E.; Hahn-Hägerdal, B.; Tengborg, C.; Stenberg, K.; Zacchi, G.; Nilvebrant, N.-O. The generation of fermentation inhibitors during dilute acid hydrolysis of softwood. Enzym. Microb. Technol. 1999, 24, 151–159. [Google Scholar] [CrossRef]
- Maiorella, B.; Blanch, H.W.; Wilke, C.R. By-product inhibition effects on ethanolic fermentation by Saccharomyces cerevisiae. Biotechnol. Bioeng. 1983, 25, 103–121. [Google Scholar] [CrossRef]
- Szengyel, Z.; Zacchi, G. Effect of acetic acid and furfural on cellulase production of Trichoderma reesei RUT C30. Appl. Biochem. Biotechnol. 2000, 89, 31–42. [Google Scholar] [CrossRef]
- Modig, T.; Liden, G.; Taherzadeh, M.J. Inhibition effects of furfural on alcohol dehydrogenase, aldehyde dehydrogenase and pyruvate dehydrogenase. Biochem. J. 2002, 363, 769–776. [Google Scholar] [CrossRef]
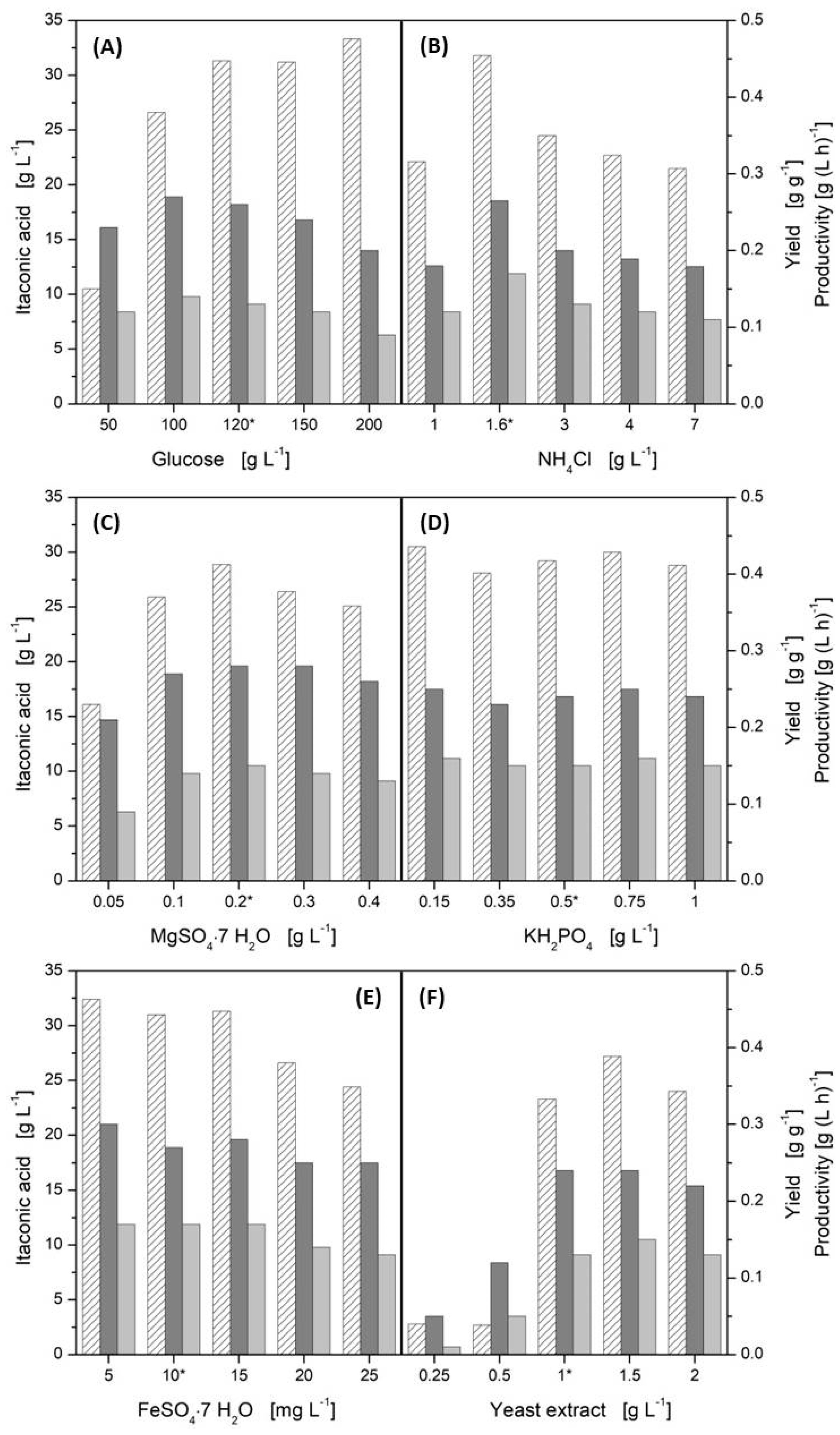

| Monosaccharide | Productivity [g (L·h)−1] | YP/S [w/w] |
|---|---|---|
| Glucose | 0.16 | 0.24 |
| Mannose | 0.09 | 0.22 |
| Fructose | 0.09 | 0.17 |
| Arabinose | 0.04 | 0.06 |
| Xylose | 0.02 | 0.04 |
| Galactose | <0.01 | <0.01 |
| Rhamnose | - | - |
| pH-Value [-] | Itaconic Acid [g·L−1] | Productivity [g (L·h)−1] | YP/S [w/w] |
|---|---|---|---|
| 5.5 | 23.7 | 0.15 | 0.25 |
| 6.0 | 31.7 | 0.23 | 0.34 |
| 6.5 | 18.6 | 0.12 | 0.19 |
| 7.0 | 15.9 | 0.10 | 0.16 |
| Aeration [vvm] | Itaconic Acid [g·L−1] | Productivity [g·(L·h)−1] | YP/S [w/w] | CDW [g·L−1] |
|---|---|---|---|---|
| 0.1 | 29.8 | 0.22 | 0.30 | 15.7 |
| 0.5 | 26.1 | 0.16 | 0.26 | 17.5 |
| 1.0 | 23.6 | 0.12 | 0.24 | 21.3 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krull, S.; Lünsmann, M.; Prüße, U.; Kuenz, A. Ustilago Rabenhorstiana—An Alternative Natural Itaconic Acid Producer. Fermentation 2020, 6, 4. https://doi.org/10.3390/fermentation6010004
Krull S, Lünsmann M, Prüße U, Kuenz A. Ustilago Rabenhorstiana—An Alternative Natural Itaconic Acid Producer. Fermentation. 2020; 6(1):4. https://doi.org/10.3390/fermentation6010004
Chicago/Turabian StyleKrull, Susan, Malin Lünsmann, Ulf Prüße, and Anja Kuenz. 2020. "Ustilago Rabenhorstiana—An Alternative Natural Itaconic Acid Producer" Fermentation 6, no. 1: 4. https://doi.org/10.3390/fermentation6010004
APA StyleKrull, S., Lünsmann, M., Prüße, U., & Kuenz, A. (2020). Ustilago Rabenhorstiana—An Alternative Natural Itaconic Acid Producer. Fermentation, 6(1), 4. https://doi.org/10.3390/fermentation6010004