Bovine Lactoferrin Pre-Treatment Induces Intracellular Killing of AIEC LF82 and Reduces Bacteria-Induced DNA Damage in Differentiated Human Enterocytes
Abstract
1. Introduction
2. Results
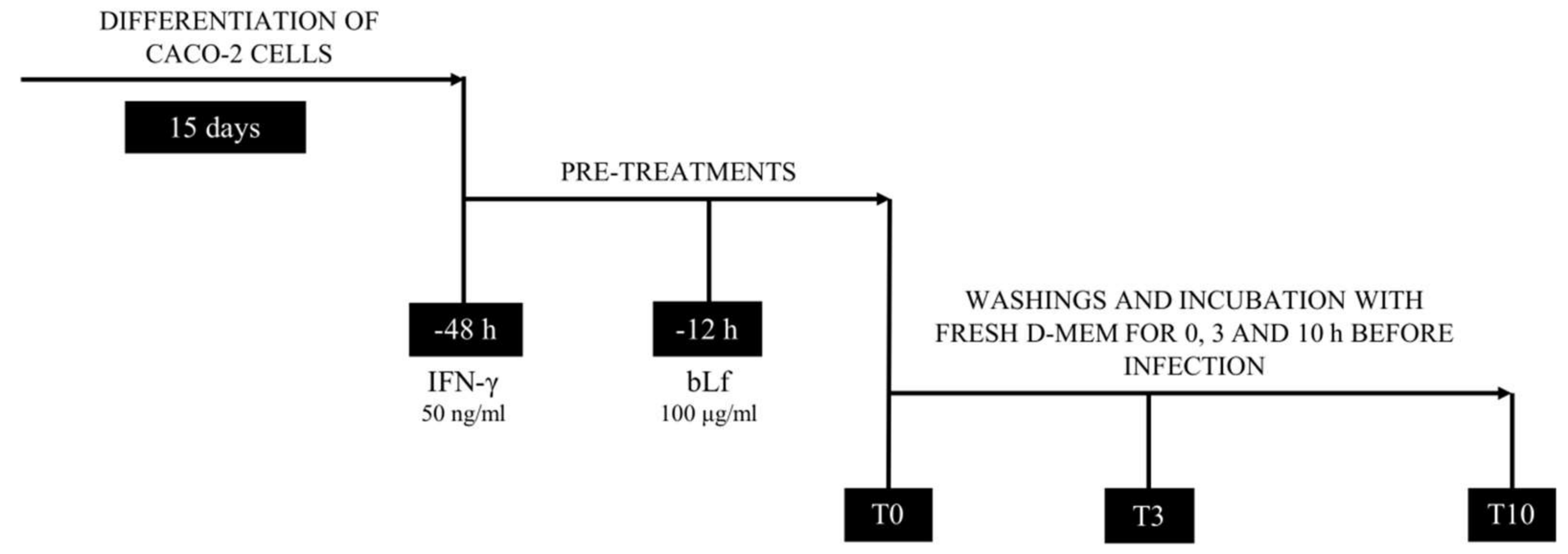
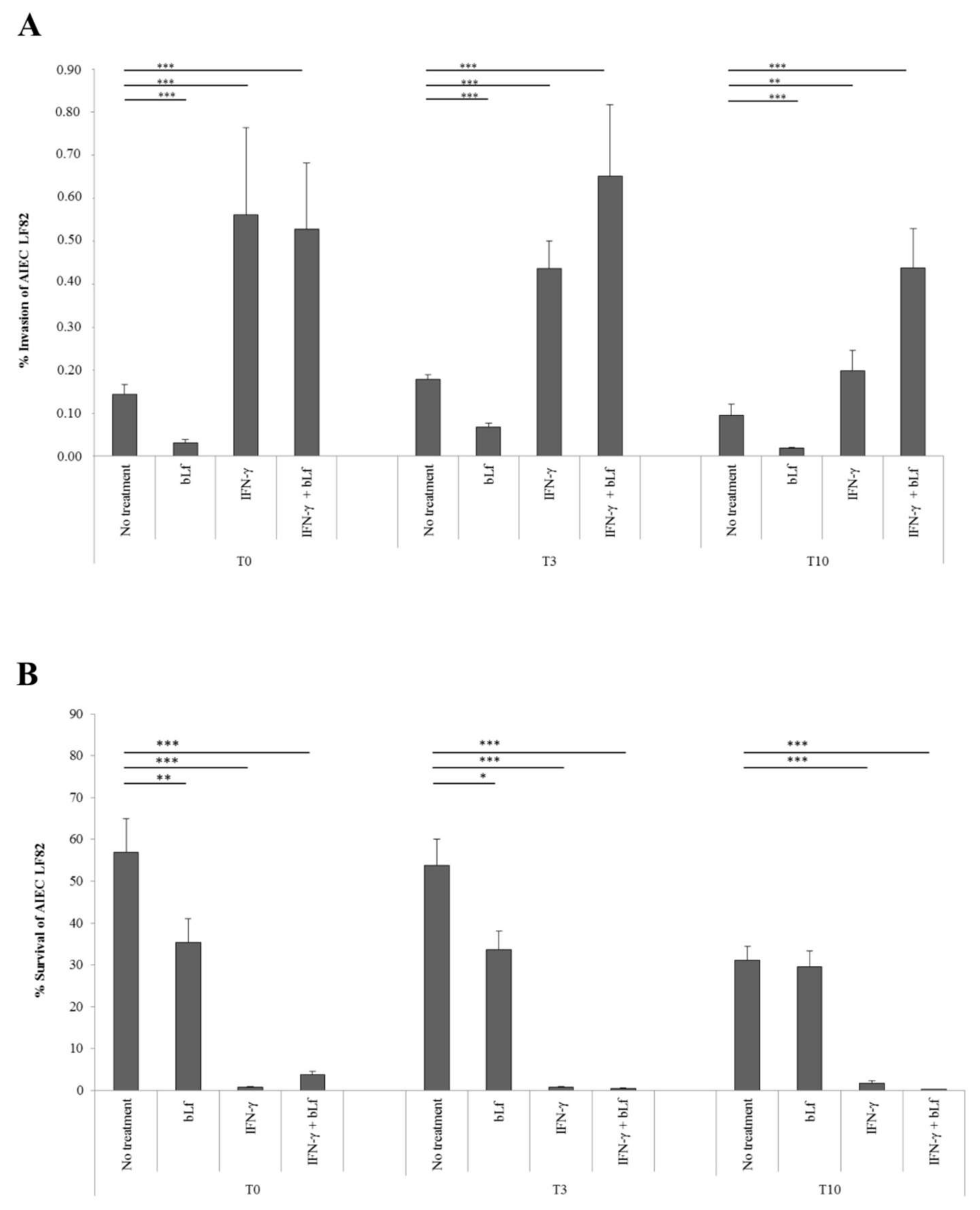
2.1. BLf and AIEC LF82 Adhesion, Invasion, and Survival into Caco-2 Cells
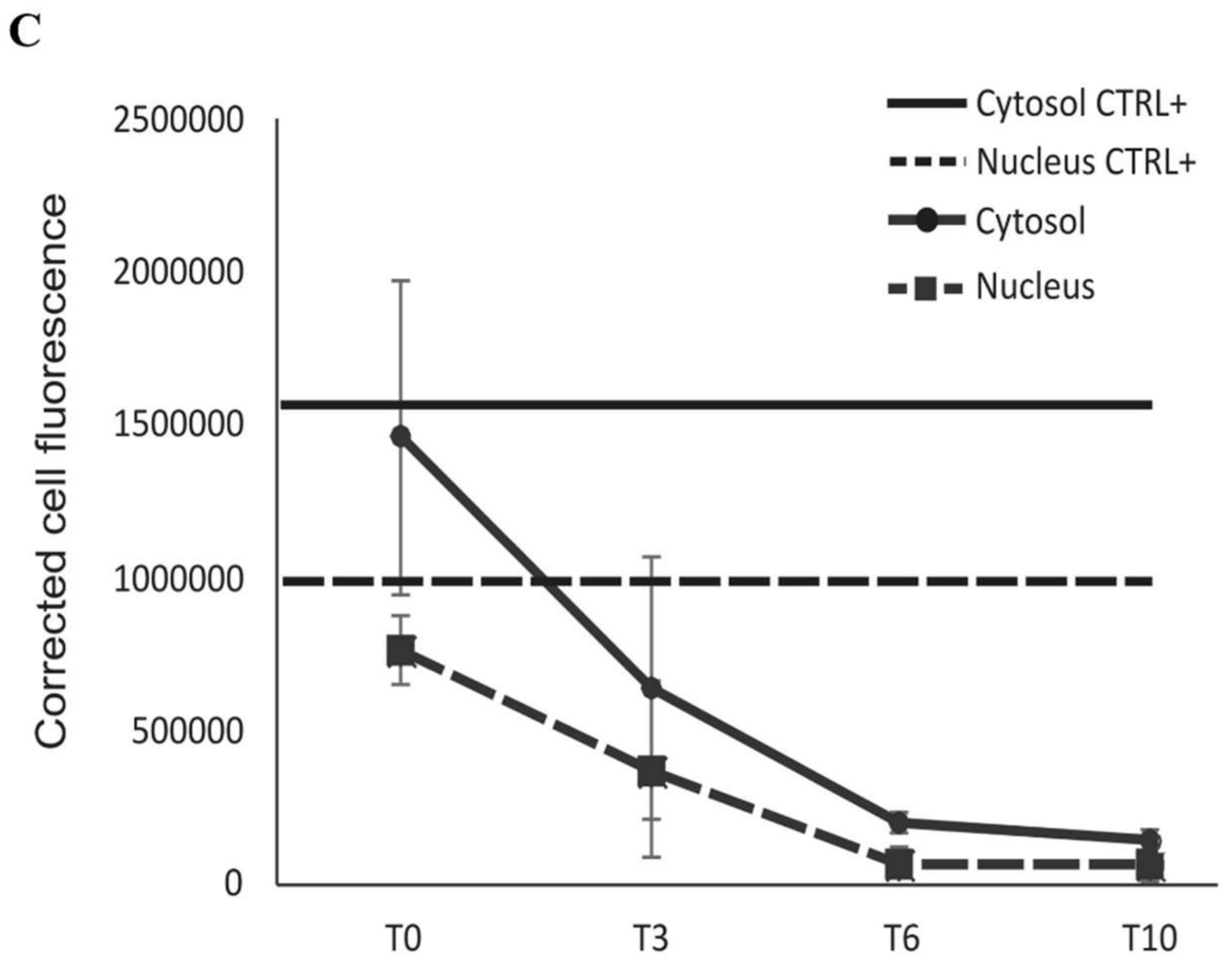
2.2. BLf Varies Its Subcellular Localization over Time
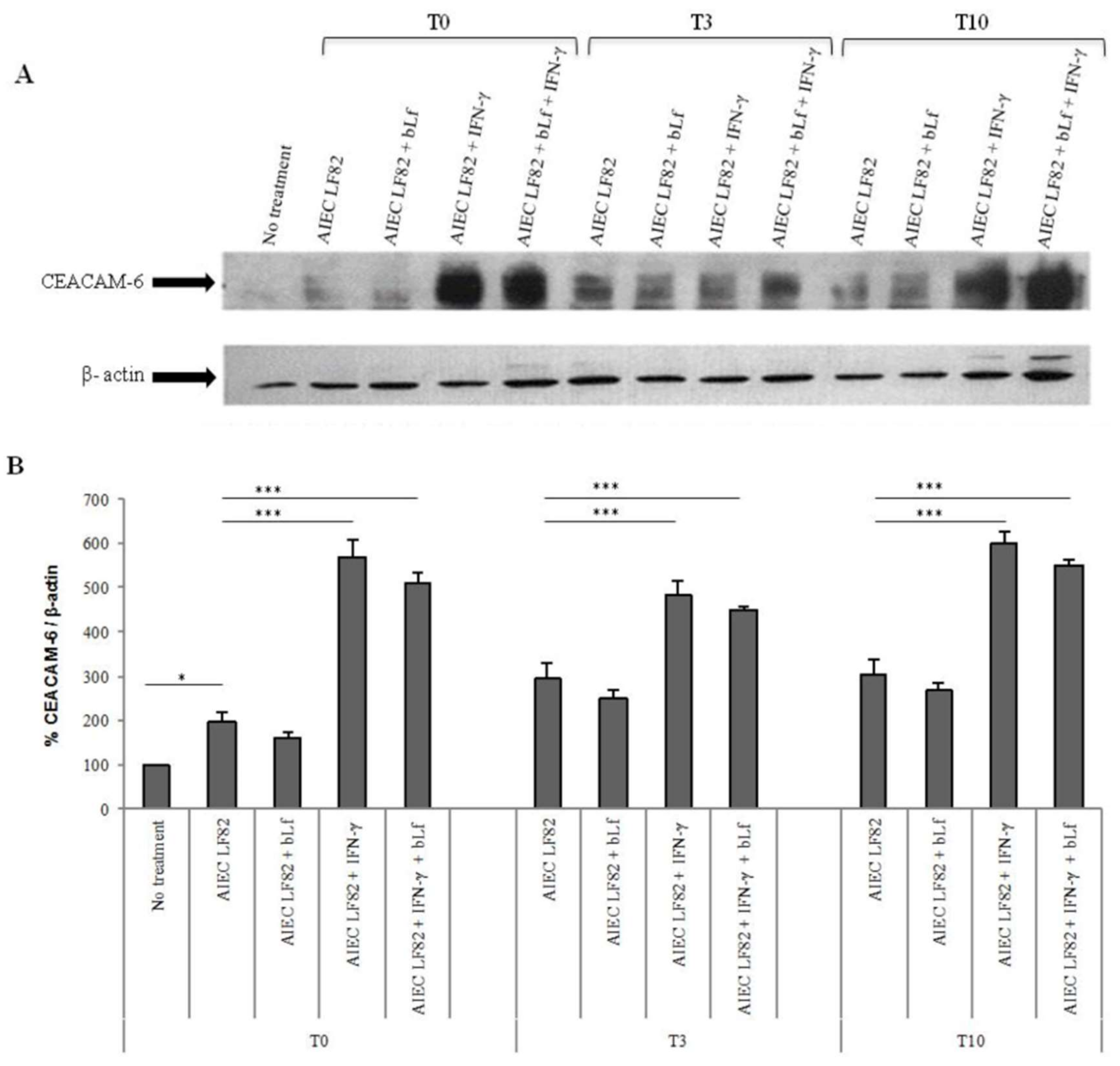
2.3. BLf Does Not Influence CEACAM-6 Expression
2.4. BLf Does Not Induce Apoptosis in LF82-Infected Caco-2 Cells
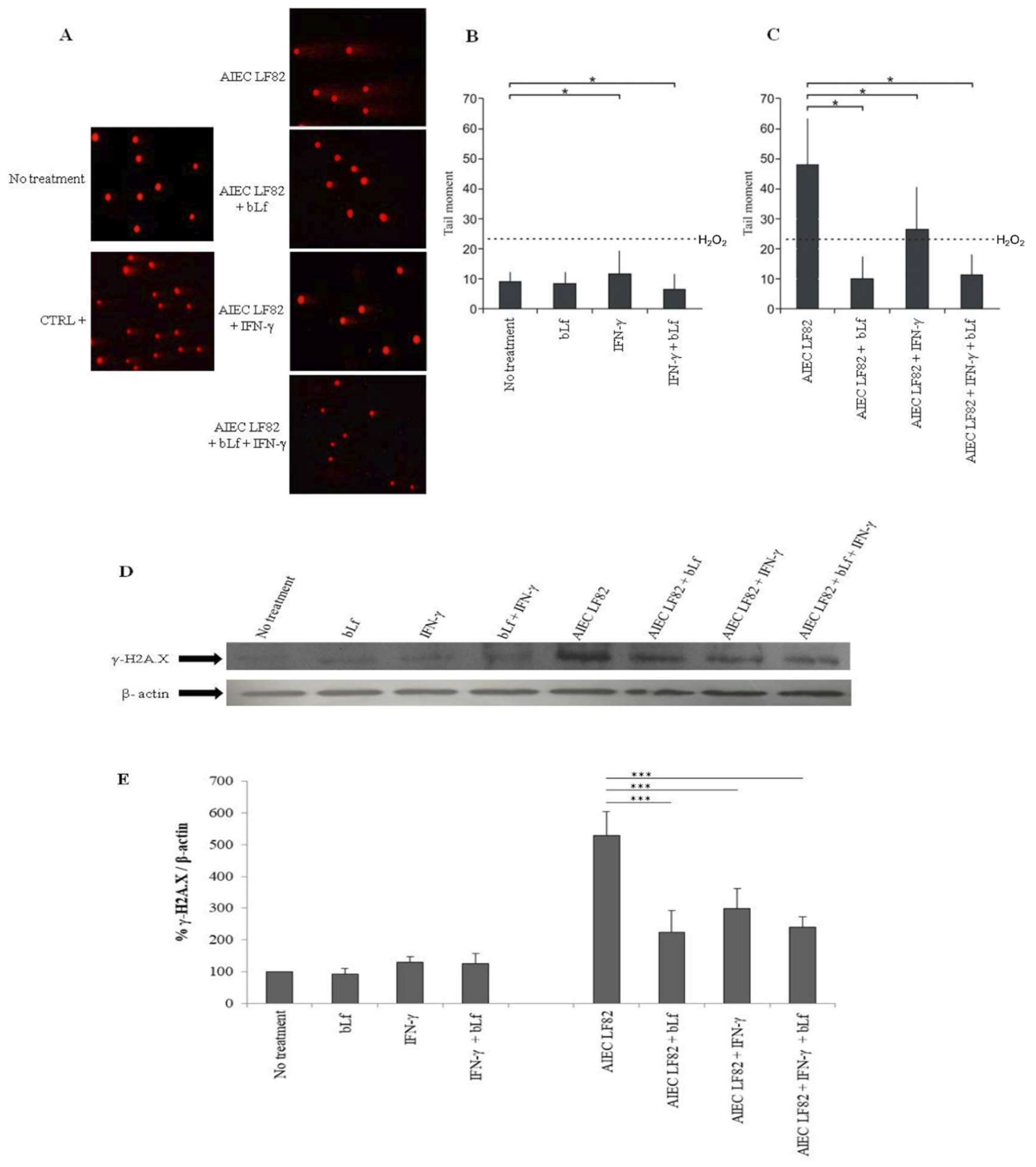
2.5. BLf Protects Caco-2 Cells from AIEC LF82-Induced DNA Damage
3. Discussion
4. Materials and Methods
4.1. Lactoferrin
4.2. Bacterial Strain
4.3. Cell Culture
4.4. Adhesion, Invasion and Survival Assays
4.5. Cell Apoptosis
4.6. Localization of Bovine Lactoferrin
4.7. Comet Assay
4.8. Western Blot Analysis
4.9. Statistical Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Darfeuille-Michaud, A.; Boudeau, J.; Bulois, P.; Neut, C.; Glasser, A.; Barnich, N.; Colombel, J.F. Hight prevalence of adherent-invasive Escherichia coli associated with ileal mucosa in Crohn’s disease. Gastroenterology 2004, 127, 412–421. [Google Scholar] [CrossRef] [PubMed]
- Shawki, A.; McCole, D.F. Mechanisms of intestinal epithelial barrier dysfunction by adherent-invasive Escherichia coli. Cell. Mol. Gastroenterol. Hepatol. 2017, 3, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Rolhion, N.; Darfeuille-Michaud, A. Adherent-Invasive Escherichia coli in Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2007, 13, 1277–1283. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, M.; Sitaraman, S.V.; Babbin, B.A.; Gerner-Smidt, P.; Ribot, E.M.; Garrett, N.; Alpern, J.A.; Akyildiz, A.; Theiss, A.L.; Nusrat, A.; et al. Invasive Escherichia coli are a feature of Crohn’s disease. Lab. Invest. 2007, 87, 1042–1105. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Medina, M.; Aldeguer, X.; Lopez-Siles, M.; Gonzalez-Huix, F.; Lopez-Oliu, C.; Dahbi, G.; Blanco, J.E.; Garcia-Gil, L.J.; Darfeuille-Michaud, A. Molecular diversity of Escherichia coli in the human gut: New ecological evidence supporting the role of adherent-invasive E. coli (AIEC) in Crohn’s disease. Inflamm. Bowel Dis. 2009, 15, 872–882. [Google Scholar] [CrossRef] [PubMed]
- Chassaing, B.; Etienne-Mesmin, L.; Bonnet, R.; Darfeuille-Michaud, A. Bile salts induce long polar fimbriae expression favouring Crohn’s disease-associated adherent-invasive Escherichia coli interaction with Peyer’s patches. Environ. Microbiol. 2013, 15, 355–371. [Google Scholar] [CrossRef] [PubMed]
- Barnich, N.; Carvalho, F.A.; Glasser, A.L.; Darcha, C.; Jantscheff, P.; Allez, M.; Peeters, H.; Bommelaer, G.; Desreumaux, P.; Colombel, J.F.; et al. CEACAM6 acts as a receptor for adherent-invasive E. coli, supporting ileal mucosa colonization in Crohn disease. J. Clin. Invest. 2007, 117, 1566–1574. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, F.A.; Barnich, N.; Sivignon, A.; Darcha, C.; Chan, C.H.; Stanners, C.P.; Darfeuille-Michaud, A. Crohn’s disease adherent-invasive Escherichia coli colonize and induce strong gut inflammation in transgenic mice expressing human CEACAM. J. Exp. Med. 2009, 206, 2179–2189. [Google Scholar] [CrossRef] [PubMed]
- Barnich, N.; Darfeuille-Michaud, A. Adherent-invasive Escherichia coli and Crohn’s disease. Curr. Opin. Gastroenterol. 2007, 23, 16–20. [Google Scholar] [CrossRef] [PubMed]
- Frioni, A.; Conte, M.P.; Cutone, A.; Longhi, C.; Musci, G.; di Patti, M.C.; Natalizi, T.; Marazzato, M.; Lepanto, M.S.; Puddu, P.; et al. Lactoferrin differently modulates the inflammatory response in epithelial models mimicking human inflammatory and infectious diseases. BioMetals 2014, 27, 843–856. [Google Scholar] [CrossRef] [PubMed]
- Glasser, A.L.; Boudeau, J.; Barnich, N.; Perruchot, M.H.; Colombel, J.F.; Darfeuille-Michaud, A. Adherent invasive Escherichia coli strains from patients with Crohn’s disease survive and replicate within macrophages without inducing host cell death. Infect. Imm. 2001, 69, 5529–5537. [Google Scholar] [CrossRef] [PubMed]
- Rahman, K.; Sasaki, M.; Nusrat, A.; Klapproth, J.M. Crohn’s disease-associated Escherichia coli survive in macrophages by suppressing NFκB signaling. Inflamm. Bowel Dis. 2014, 20, 1419–1425. [Google Scholar] [CrossRef] [PubMed]
- Berlutti, F.; Schippa, S.; Morea, C.; Sarli, S.; Perfetto, B.; Donnarumma, G.; Valenti, P. Lactoferrin down regulates pro-inflammatory cytokines up-expressed in intestinal epithelial cells infected with invasive or noninvasive Escherichia coli strains. Biochem. Cell Biol. 2006, 84, 351–357. [Google Scholar] [CrossRef] [PubMed]
- Valenti, P.; Frioni, A.; Rossi, A.; Ranucci, S.; De Fino, I.; Cutone, A.; Rosa, L.; Bragonzi, A.; Berlutti, F. Aerosolized bovine lactoferrin reduces neutrophils and pro-inflammatory cytokines in mouse models of Pseudomonas aeruginosa lung infections. Biochem. Cell Biol. 2017, 95, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Palmela, C.; Chevarin, C.; Xu, Z.; Torres, J.; Sevrin, G.; Hirten, R.; Barnich, N.; Ng, S.C.; Colombel, J.F. Adherent-invasive Escherichia coli in inflammatory bowel disease. Gut 2018, 67, 574–587. [Google Scholar] [CrossRef] [PubMed]
- Rolhion, N.; Barnich, N.; Bringer, M.A.; Glasser, A.L.; Ranc, J.; Hébuterne, X.; Hofman, P.; Darfeuille-Michaud, A. Abnormally expressed ER stress response chaperone Gp96 in CD favours adherent-invasive Escherichia coli invasion. Gut 2010, 59, 1355–1362. [Google Scholar] [CrossRef] [PubMed]
- Fahlgren, A.; Baranov, V.; Frängsmyr, L.; Zoubir, F.; Hammarström, M.L.; Hammarström, S. Interferon-gamma tempers the expression of carcinoembryonic antigen family molecules in human colon cells: A possible role in innate mucosal defence. Scand. J. Immunol. 2003, 58, 628–641. [Google Scholar] [CrossRef] [PubMed]
- Mazzarella, G.; Perna, A.; Marano, A.; Lucariello, A.; Rotondi Aufieri, V.; Melina, R.; Melina, R.; Guerra, G.; Taccone, F.S.; Iaquinto, G.; et al. Pathogenic Role of Associated Adherent-Invasive Escherichia Coli in Crohn’s disease. J. Cell. Physiol. 2017, 232, 2860–2868. [Google Scholar] [CrossRef] [PubMed]
- Brument, S.; Sivignon, A.; Dumych, T.I.; Moreau, N.; Roos, G.; Guérardel, Y.; Chalopin, T.; Deniaud, D.; Bilyy, R.O.; Darfeuille-Michaud, A.; et al. Thiazolylaminomannosides as potent antiadhesives of type 1 piliated Escherichia coli isolated from Crohn’s disease patients. J. Med. Chem. 2013, 56, 5395–5406. [Google Scholar] [CrossRef] [PubMed]
- Sivignon, A.; Yan, X.; Alvarez Dorta, D.; Bonnet, R.; Bouckaert, J.; Fleury, E.; Bernard, J.; Gouin, S.G.; Darfeuille-Michaud, A.; Barnich, N. Development of heptylmannoside-based glycoconjugate antiadhesive compounds against Adherent-Invasive Escherichia coli bacteria associated with Crohn’s disease. MBio 2015, 6, e01298–e01315. [Google Scholar] [CrossRef] [PubMed]
- Alvarez Dorta, D.; Sivignon, A.; Chalopin, T.; Dumych, T.I.; Roos, G.; Bilyy, R.O.; Deniaud, D.; Krammer, E.M.; de Ruyck, J.; Lensink, M.F. The Antiadhesive Strategy in Crohn’s Disease: Orally Active Mannosides to Decolonize Pathogenic Escherichia coli from the Gut. ChemBioChem 2016, 17, 936–952. [Google Scholar] [CrossRef] [PubMed]
- Chalopin, T.; Alvarez Dorta, D.; Sivignon, A.; Caudan, M.; Dumych, T.I.; Bilyy, R.O.; Deniaud, D.; Gouin, S.G. Second generation of thiazolylmannosides, FimH antagonists for E. coli-induced Crohn’s disease. Org. Biomol. Chem. 2016, 14, 3913–3925. [Google Scholar] [CrossRef] [PubMed]
- Valenti, P.; Antonini, G. Lactoferrin: An important host defence against microbial and viral attack. Cell. Mol. Life Sci. 2005, 62, 2576–2587. [Google Scholar] [CrossRef] [PubMed]
- Rosa, L.; Cutone, A.; Lepanto, M.S.; Paesano, R.; Valenti, P. Lactoferrin: A Natural Glycoprotein Involved in Iron and Inflammatory Homeostasis. Int. J. Mol. Sci. 2017, 18, 1985. [Google Scholar] [CrossRef] [PubMed]
- Lepanto, M.S.; Rosa, L.; Paesano, R.; Valenti, P.; Cutone, A. Lactoferrin in Aseptic and Septic Inflammation. Molecules 2019, 3, 24. [Google Scholar] [CrossRef] [PubMed]
- Ward, P.P.; Paz, E.; Conneely, O.M. Multifunctional roles of lactoferrin: A critical overview. Cell. Mol. Life Sci. 2005, 62, 2540–2548. [Google Scholar] [CrossRef] [PubMed]
- Visca, P.; Berlutti, F.; Vittorioso, P.; Dalmastri, C.; Thaller, M.C.; Valenti, P. Growth and adsorption of Streptococcus mutans 6715-13 to hydroxyapatite in the presence of lactoferrin. Med. Microbiol. Immunol. 1989, 178, 69–79. [Google Scholar] [CrossRef] [PubMed]
- Hirano, Y.; Tamura, M.; Hayashi, K. Inhibitory effect of lactoferrin on the adhesion of Prevotella nigrescens ATCC 25261 to hydroxyapatite. J. Oral Sci. 2000, 42, 125–131. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Williams, T.J.; Schneider, R.P.; Willcox, M.D. The effect of protein-coated contact lenses on the adhesion and viability of Gram negative bacteria. Curr. Eye Res. 2003, 27, 227–235. [Google Scholar] [CrossRef] [PubMed]
- Longhi, C.; Conte, M.P.; Seganti, L.; Polidoro, M.; Alfsen, A.; Valenti, P. Influence of lactoferrin on the entry process of Escherichia coli HB101 (pRI203) in HeLa cells. Med. Microbiol. Immunol. 1993, 182, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Alugupalli, K.R.; Kalfas, S. Characterization of the lactoferrin-dependent inhibition of the adhesion of Actinobacillus actinomycetemcomitans, Prevotella intermedia and Prevotella nigrescens to fibroblasts and to a reconstituted basement membrane. APMIS 1997, 105, 680–688. [Google Scholar] [CrossRef] [PubMed]
- Kawasaki, Y.; Tazume, S.; Shimizu, K.; Matsuzawa, H.; Dosako, S.; Isoda, H.; Tsukiji, M.; Fujimura, R.; Muranaka, Y.; Isihida, H. Inhibitory effects of bovine lactoferrin on the adherence of enterotoxigenic Escherichia coli to host cells. Biosci. Biotechnol. Biochem. 2000, 64, 348–354. [Google Scholar] [CrossRef] [PubMed]
- Berlutti, F.; Morea, C.; Battistoni, A.; Sarli, S.; Cipriani, P.; Superti, F.; Amendolia, M.G.; Valenti, P. Iron availability influences aggregation, biofilm, adhesion and invasion of Pseudomonas aeruginosa and Burkholderia cenocepacia. Int. J. Immunopathol. Pharmacol. 2005, 18, 661–670. [Google Scholar] [CrossRef] [PubMed]
- Sessa, R.; Di Pietro, M.; Filardo, S.; Bressan, A.; Rosa, L.; Cutone, A.; Frioni, A.; Berlutti, F.; Paesano, R.; Valenti, P. Effect of bovine lactoferrin on Chlamydia trachomatis infection and inflammation. Biochem. Cell Biol. 2017, 95, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Antonini, G.; Catania, M.R.; Greco, R.; Longhi, C.; Pisciotta, M.G.; Seganti, L.; Valenti, P. Anti-invasive activity of bovine lactoferrin towards Listeria monocytogenes. J. Food Prot. 1997, 1, 60–72. [Google Scholar]
- Ajello, M.; Greco, R.; Giansanti, F.; Massucci, M.T.; Antonini, G.; Valenti, P. Anti-invasive activity of bovine lactoferrin towards group A streptococci. Biochem. Cell Biol. 2002, 80, 119–124. [Google Scholar] [CrossRef] [PubMed]
- Willer Eda, M.; Lima Rde, L.; Giugliano, L.G. In vitro adhesion and invasion inhibition of Shigella dysenteriae, Shigella flexneri and Shigella sonnei clinical strains by human milk proteins. BMC Microbiol. 2004, 4, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Cutone, A.; Rosa, L.; Lepanto, M.S.; Scotti, M.J.; Berlutti, F.; Bonaccorsi di Patti, M.C.; Musci, G.; Valenti, P. Lactoferrin efficiently counteracts the inflammation-induced changes of the iron homeostasis system in macrophages. Front. Immunol. 2017, 15, 705. [Google Scholar] [CrossRef] [PubMed]
- Bonaccorsi di Patti, M.C.; Cutone, A.; Polticelli, F.; Rosa, L.; Lepanto, M.S.; Valenti, P.; Musci, G. The ferroportin-ceruloplasmin system and the mammalian iron homeostasis machine: Regulatory pathways and the role of lactoferrin. BioMetals 2018, 31, 399–414. [Google Scholar] [CrossRef] [PubMed]
- Lepanto, M.S.; Rosa, L.; Cutone, A.; Conte, M.P.; Paesano, R.; Valenti, P. Efficacy of Lactoferrin Oral Administration in the Treatment of Anemia and Anemia of Inflammation in Pregnant and Non-pregnant Women: An Interventional Study. Front. Immunol. 2018, 9, 2123. [Google Scholar] [CrossRef] [PubMed]
- Cutone, A.; Lepanto, M.S.; Rosa, L.; Scotti, M.J.; Rossi, A.; Ranucci, S.; De Fino, I.; Bragonzi, A.; Valenti, P.; Musci, G.; et al. Aerosolized Bovine Lactoferrin Counteracts Infection, Inflammation and Iron Dysbalance in A Cystic Fibrosis Mouse Model of Pseudomonas aeruginosa Chronic Lung Infection. Int. J. Mol. Sci. 2019, 30, 20. [Google Scholar] [CrossRef] [PubMed]
- Penco, S.; Scarfi, S.; Giovine, M.; Damonte, G.; Millo, E.; Villaggio, B.; Passalacqua, M.; Pozzolini, M.; Garrè, C.; Benatti, U. Identification of an import signal for, and the nuclear localization of, human lactoferrin. Biotechnol. Appl. Biochem. 2001, 34, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Ashida, K.; Sasaki, H.; Sasaki, Y.A.; Loönnerdal, B. Cellular internalization of lactoferrin in intestinal epithelial cells. BioMetals 2004, 17, 311–315. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, Y.A.; Lopez, V.; Lönnerdal, B. Mammalian lactoferrin receptors: Structure and function. Cell. Mol. Life Sci. 2005, 62, 2560–2575. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, Y.A.; Wong, H.; Ashida, K.Y.; Schryvers, A.B.; Loönnerdal, B. The N1 domain of human lactoferrin is required for internalization by caco-2 cells and targeting to the nucleus. Biochemistry 2008, 47, 10915–10920. [Google Scholar] [CrossRef] [PubMed]
- Paesano, R.; Natalizi, T.; Berlutti, F.; Valenti, P. Body iron delocalization: The serious drawback in iron disorders in both developing and developed countries. Pathog. Glob. Health 2012, 106, 200–216. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.W.; Lee, T.H.; Park, K.H.; Choi, S.Y.; Kim, J. Human lactoferrin suppresses TNF-α-induced intercellular adhesion molecule-1 expression via competition with NF-kB in endothelial cells. FEBS Lett. 2012, 586, 229–234. [Google Scholar] [CrossRef] [PubMed]
- Barnich, N.; Darfeuille-Michaud, A. Abnormal CEACAM6 expression in Crohn disease patients favors gut colonization and inflammation by adherent-invasive E. coli. Virulence 2010, 1, 281–282. [Google Scholar] [CrossRef] [PubMed]
- Valenti, P.; Greco, R.; Pitari, G.; Rossi, P.; Ajello, M.; Melino, G.; Antonini, G. Apoptosis of Caco-2 intestinal cells invaded by Listeria monocytogenes: Protective effect of lactoferrin. Exp. Cell Res. 1999, 250, 197–202. [Google Scholar] [CrossRef] [PubMed]
- Longhi, C.; Conte, M.P.; Ranaldi, S.; Penta, M.; Valenti, P.; Tinari, A.; Superti, F.; Seganti, L. Apoptotic death of Listeria monocytogenes-infected human macrophages induced by lactoferricin B, a bovine lactoferrin-derived peptide. Int. J. Immunopathol. Pharmacol. 2005, 18, 317–325. [Google Scholar] [CrossRef] [PubMed]
- Blais, A.; Fan, C.; Voisin, T.; Aattouri, N.; Dubarry, M.; Blachier, F.; Tome, D. Effects of lactoferrin on intestinal epithelial cell growth and differentiation: An in vivo and in vitro study. BioMetals 2014, 27, 857–874. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, D.N.; Jiang, P.; Stensballe, A.; Bendixen, E.; Sangild, P.T.; Chatterton, D.E. Bovine lactoferrin regulates cell survival, apoptosis and inflammation in intestinal epithelial cells and preterm pig intestine. J. Proteomics 2016, 139, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Tyrer, P.C.; Frizelle, F.A.; Keenan, J.I. Escherichia coli-derived outer membrane vesicles are genotoxic to human enterocyte-like cells. Infect. Agents Cancer 2014, 9, 2. [Google Scholar] [CrossRef] [PubMed]
- Kuefner, M.A.; Brand, M.; Engert, C.; Schwab, S.A.; Uder, M. Radiation Induced DNA Double-Strand Breaks in Radiology. RoFo 2015, 187, 872–878. [Google Scholar] [CrossRef] [PubMed]
- Thakur, A.; Mikkelsen, H.; Jungersen, G. Intracellular Pathogens: Host Immunity and Microbial Persistence Strategies. J. Immunol. Res. 2019, 1356540. [Google Scholar] [CrossRef] [PubMed]
- Sessa, R.; Di Pietro, M.; Filardo, S.; Bressan, A.; Mastromarino, P.; Biasucci, A.V.; Rosa, L.; Cutone, A.; Berlutti, F.; Paesano, R.; et al. Lactobacilli-lactoferrin interplay in Chlamydia trachomatis infection. Pathog. Dis. 2017, 75. [Google Scholar] [CrossRef] [PubMed]
- Valenti, P.; Rosa, L.; Capobianco, D.; Lepanto, M.S.; Schiavi, E.; Cutone, A.; Paesano, R.; Mastromarino, P. Role of lactobacilli and lactoferrin in the mucosal cervicovaginal defense. Front. Immunol. 2018, 9, 376. [Google Scholar] [CrossRef] [PubMed]
- Bretin, A.; Lucas, C.; Larabi, A.; Dalmasso, G.; Billard, E.; Barnich, N.; Bonnet, R.; Nguyen, H.T.T. AIEC infection triggers modification of gut microbiota composition in genetically predisposed mice, contributing to intestinal inflammation. Sci. Rep. 2018, 8, 12301. [Google Scholar] [CrossRef] [PubMed]
- Lashermes, A.; Boudieu, L.; Barbier, J.; Sion, B.; Gelot, A.; Barnich, N.; Ardid, D.; Carvalho, F.A. Adherent-Invasive E. coli enhances colonic hypersensitivity and P2X receptors expression during post-infectious period. Gut Microbes. 2018, 9, 26–37. [Google Scholar] [CrossRef] [PubMed]
- Cañas, M.A.; Giménez, R.; Fábrega, M.J.; Toloza, L.; Baldomà, L.; Badia, J. Outer Membrane Vesicles from the Probiotic Escherichia coli Nissle 1917 and the Commensal ECOR12 Enter Intestinal Epithelial Cells via Clathrin-Dependent Endocytosis and Elicit Differential Effects on DNA Damage. PLoS ONE 2016, 11, e0160374. [Google Scholar] [CrossRef] [PubMed]
- Bennett, R.M.; Davis, J. Lactoferrin interacts with deoxyribonucleic acid: A preferential reactivity with double-stranded DNA and dissociation of DNA-anti-DNA complexes. J. Lab. Clin. Med. 1982, 99, 127–138. [Google Scholar] [PubMed]
- Kruzel, M.L.; Actor, J.K.; Radak, Z.; Bacsi, A.; Saavedra-Molina, A.; Boldogh, I. Lactoferrin decreases LPS-induced mitochondrial dysfunction in cultured cells and in animal endotoxemia model. Innate Immun. 2010, 16, 67–79. [Google Scholar] [CrossRef] [PubMed]
- Ogasawara, Y.; Imase, M.; Oda, H.; Wakabayashi, H.; Ishii, K. Lactoferrin directly scavenges hydroxyl radicals and undergoes oxidative self-degradation: A possible role in protection against oxidative DNA damage. Int. J. Mol. Sci. 2014, 15, 1003–1013. [Google Scholar] [CrossRef] [PubMed]
- Kruzel, M.L.; Zimecki, M.; Actor, J.K. Lactoferrin in a Context of Inflammation-Induced Pathology. Front. Immunol. 2017, 8, 1438. [Google Scholar] [CrossRef] [PubMed]
- Zheng, N.; Zhang, H.; Li, S.; Wang, J.; Liu, J.; Ren, H.; Gao, Y. Lactoferrin inhibits aflatoxin B1- and aflatoxin M1-induced cytotoxicity and DNA damage in Caco-2, HEK, Hep-G2, and SK-N-SH cells. Toxicon 2018, 150, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.; Li, J.; Qin, L.; Guo, D.; Ding, H.; Deng, D. Radioprotective effect of lactoferrin in mice exposed to sublethal X-ray irradiation. Exp. Ther. Med. 2018, 16, 3143–3148. [Google Scholar] [CrossRef] [PubMed]
- Dalmasso, G.; Nguyen, H.T.T.; Faïs, T.; Massier, S.; Barnich, N.; Delmas, J.; Bonnet, R. Crohn’s Disease-Associated Adherent-Invasive Escherichia coli Manipulate Host Autophagy by Impairing SUMOylation. Cells 2019, 8, 35. [Google Scholar] [CrossRef] [PubMed]
- Rosa, L.; Cutone, A.; Lepanto, M.S.; Scotti, M.J.; Conte, M.P.; Paesano, R.; Valenti, P. Physico-chemical properties influence the functions and efficacy of commercial bovine lactoferrins. BioMetals 2018, 31, 301–312. [Google Scholar] [CrossRef] [PubMed]
- Labadie, K.; Saulnier, A.; Martin-Latil, S.; Colbère-Garapin, F. Reduced apoptosis in human intestinal cells cured of persistent poliovirus infection. J. Virol. 2007, 81, 3033–3036. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Eidet, J.R.; Pasovic, L.; Maria, R.; Jackson, C.J.; Utheim, T.P. Objective assessment of changes in nuclear morphology and cell distribution following induction of apoptosis. Diagn. Pathol. 2014, 9, 92. [Google Scholar] [CrossRef] [PubMed]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Końca, K.; Lankoff, A.; Banasik, A.; Lisowska, H.; Kuszewski, T.; Góźdź, S.; Koza, Z.; Wojcik, A. A cross platform public domain PC image analysis program for the comet assay. Mutat. Res. 2003, 534, 15–20. [Google Scholar] [CrossRef]


© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lepanto, M.S.; Rosa, L.; Cutone, A.; Scotti, M.J.; Conte, A.L.; Marazzato, M.; Zagaglia, C.; Longhi, C.; Berlutti, F.; Musci, G.; et al. Bovine Lactoferrin Pre-Treatment Induces Intracellular Killing of AIEC LF82 and Reduces Bacteria-Induced DNA Damage in Differentiated Human Enterocytes. Int. J. Mol. Sci. 2019, 20, 5666. https://doi.org/10.3390/ijms20225666
Lepanto MS, Rosa L, Cutone A, Scotti MJ, Conte AL, Marazzato M, Zagaglia C, Longhi C, Berlutti F, Musci G, et al. Bovine Lactoferrin Pre-Treatment Induces Intracellular Killing of AIEC LF82 and Reduces Bacteria-Induced DNA Damage in Differentiated Human Enterocytes. International Journal of Molecular Sciences. 2019; 20(22):5666. https://doi.org/10.3390/ijms20225666
Chicago/Turabian StyleLepanto, Maria Stefania, Luigi Rosa, Antimo Cutone, Mellani Jinnett Scotti, Antonietta Lucia Conte, Massimiliano Marazzato, Carlo Zagaglia, Catia Longhi, Francesca Berlutti, Giovanni Musci, and et al. 2019. "Bovine Lactoferrin Pre-Treatment Induces Intracellular Killing of AIEC LF82 and Reduces Bacteria-Induced DNA Damage in Differentiated Human Enterocytes" International Journal of Molecular Sciences 20, no. 22: 5666. https://doi.org/10.3390/ijms20225666
APA StyleLepanto, M. S., Rosa, L., Cutone, A., Scotti, M. J., Conte, A. L., Marazzato, M., Zagaglia, C., Longhi, C., Berlutti, F., Musci, G., Valenti, P., & Conte, M. P. (2019). Bovine Lactoferrin Pre-Treatment Induces Intracellular Killing of AIEC LF82 and Reduces Bacteria-Induced DNA Damage in Differentiated Human Enterocytes. International Journal of Molecular Sciences, 20(22), 5666. https://doi.org/10.3390/ijms20225666










