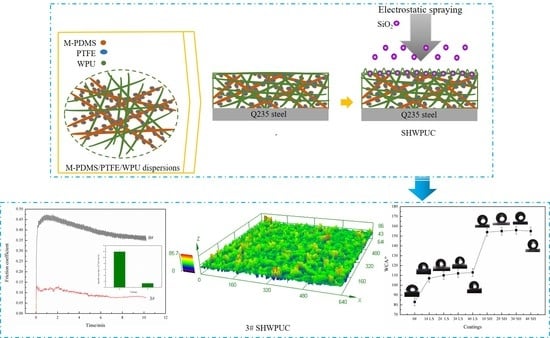
Fabrication and Properties of Superhydrophobic Waterborne Polyurethane Composites with Micro-Rough Surface Structure Using Electrostatic Spraying
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Materials
2.2. Preparation of the LSWPUCs
2.3. Preparation of the SHWPUCs
2.4. Measurements
2.4.1. WCA Test
2.4.2. Adhesion Test
2.4.3. Corrosion Resistance Test
2.4.4. Wear Resistance Test
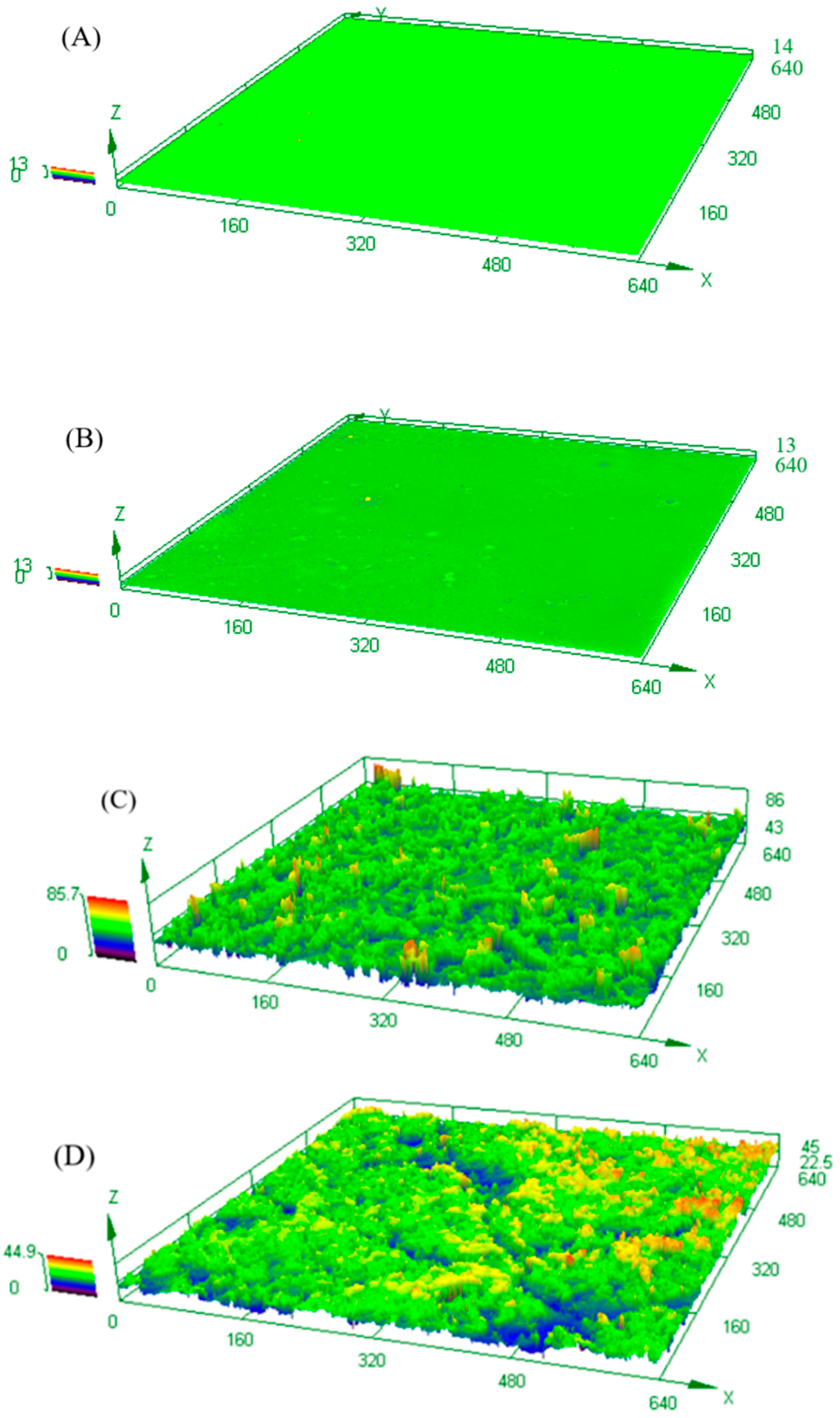
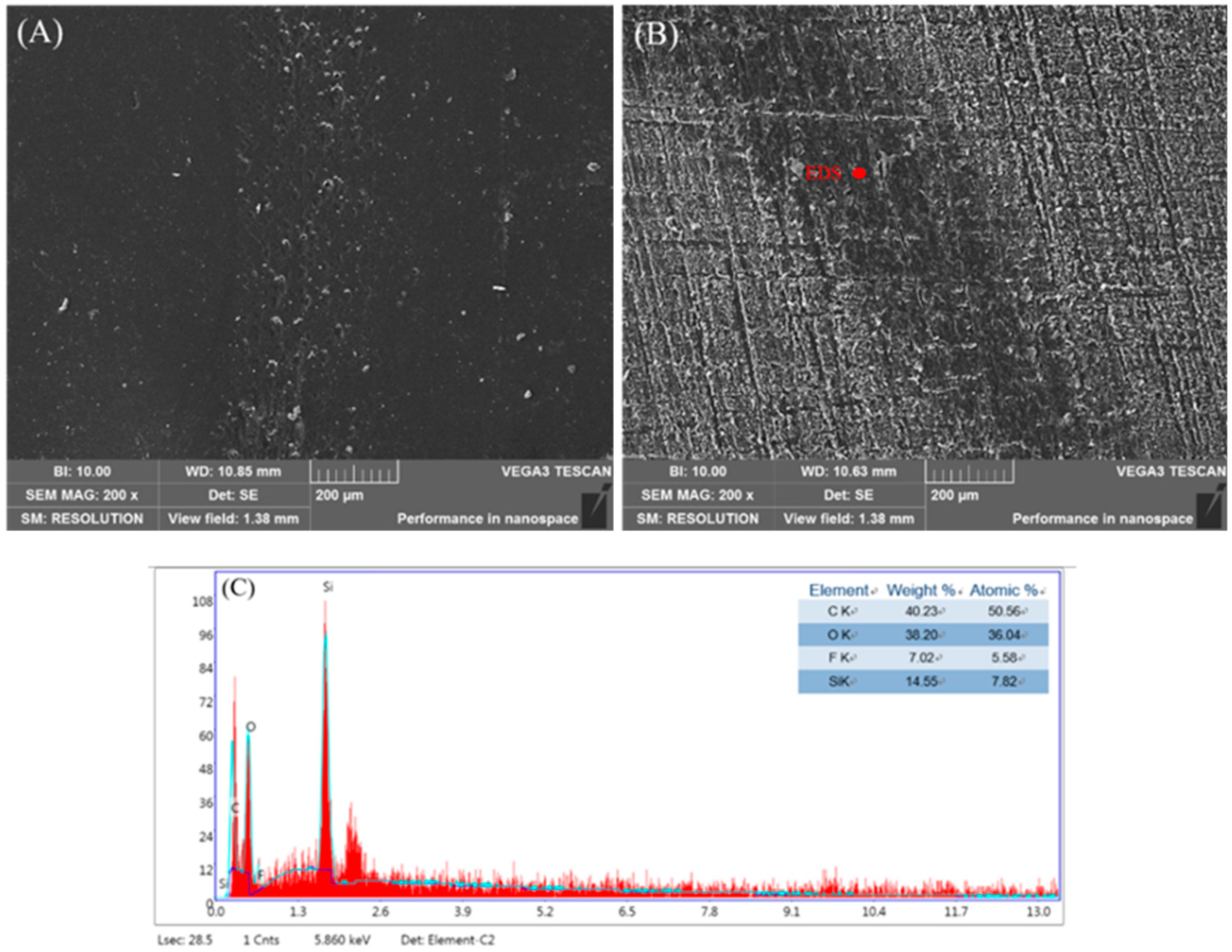
2.4.5. Morphology Analysis
3. Results and Discussion
3.1. Hydrophobicity of WPU Composites
3.2. Adhesion of the SHWPUCs
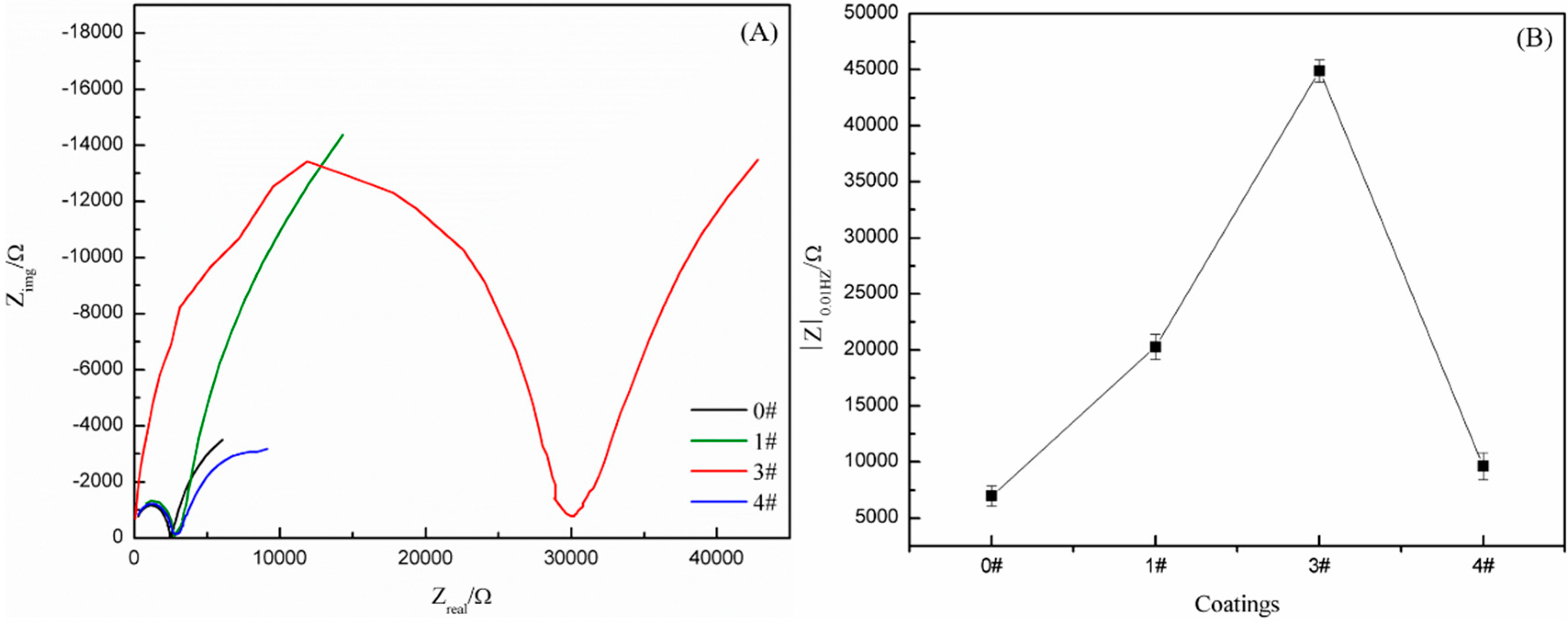
3.3. Corrosion Resistance of the SHWPUCs
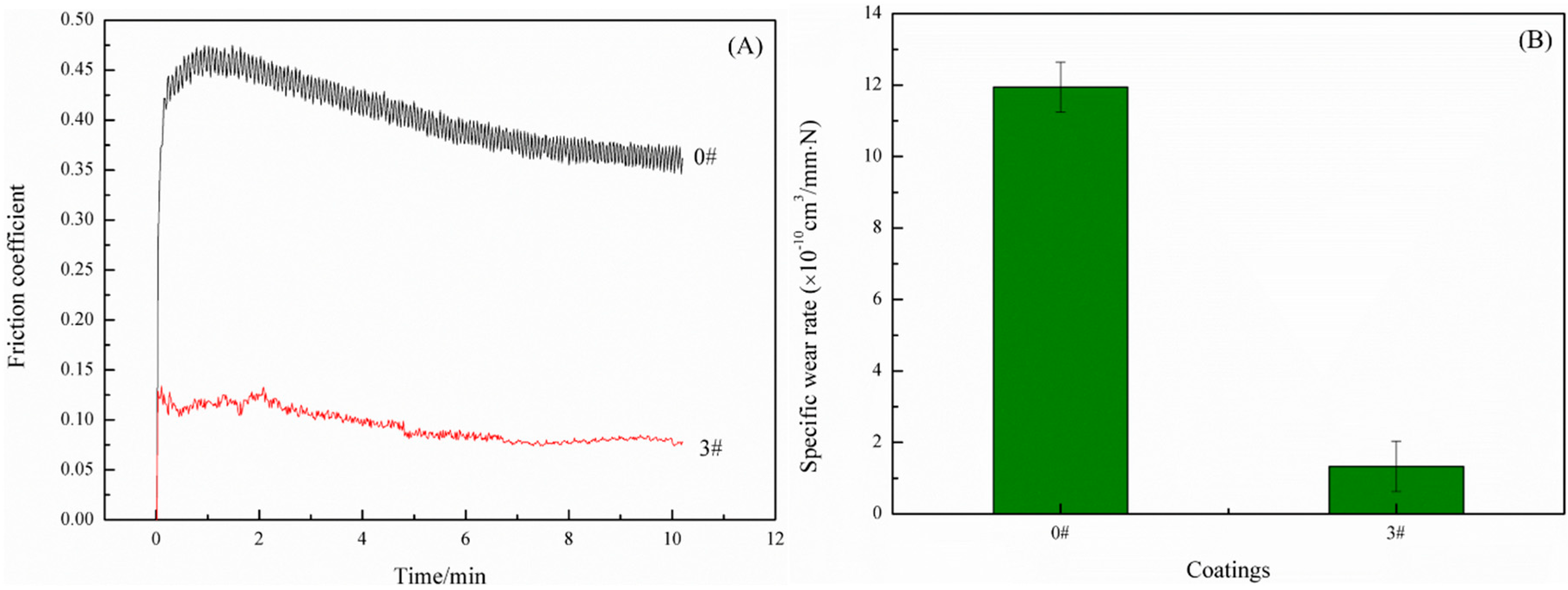
3.4. Wear Resistance of the SHWPUCs
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Shang, Y.; Si, Y.; Raza, A.; Yang, L.; Mao, X.; Ding, B.; Yu, J. An in situ polymerization approach for the synthesis of superhydrophobic and superoleophilic nanofibrous membranes for oil-water separation. Nanoscale 2012, 4, 7847–7854. [Google Scholar] [CrossRef] [PubMed]
- Yokoi, N.; Manabe, K.; Tenjimbayashi, M.; Shiratori, S. Optically transparent superhydrophobic surfaces with enhanced mechanical abrasion resistance enabled by mesh structure. ACS Appl. Mater. Inter. 2015, 7, 4809–4816. [Google Scholar] [CrossRef] [PubMed]
- Mukhopadhyay, A.; Midha, V.K. A review on designing the waterproof-breathable fabrics Part I: Fundamental principles and designing aspects of breathable fabrics. J. Ind. Text. 2008, 37, 225–262. [Google Scholar] [CrossRef]
- Solouki Bonab, V.; Manas-Zloczower, I. Chemorheology of thermoplastic polyurethane and thermoplastic polyurethane/carbon nanotube composite systems. Polymers 2016, 99, 513–520. [Google Scholar] [CrossRef]
- Yang, P.; Wang, Y.; Lu, L.; Yu, X.; Liu, L. Surface hydrophobic modification of polyurethanes by diaryl carbene chemistry: Synthesis and characterization. Appl. Surf. Sci. 2018, 435, 346–351. [Google Scholar] [CrossRef]
- Arukula, R.; Narayan, R.; Sreedhar, B.; Rao, C.R.K. High corrosion resistant–redox active–moisture curable–conducting polyurethanes. Prog. Org. Coat. 2016, 94, 79–89. [Google Scholar] [CrossRef]
- Yun, S.; Im, H.; Kim, J. The effect of different hard segments in polyurethane on the electrical conductivity of polyurethane grafted multi-walled carbon nanotube/polyurethane nanocomposites. Synth. Met. 2011, 161, 1361–1367. [Google Scholar] [CrossRef]
- Wang, F.F.; Feng, L.J.; Li, G.Z. Properties of waterborne polyurethane conductive coating with low MWCNTs content by electrostatic spraying. Polymers 2018, 10, 1406. [Google Scholar] [CrossRef]
- Huang, A.; Peng, X.; Turng, L. In-situ fibrillated polytetrafluoroethylene (PTFE) in thermoplastic polyurethane (TPU) via melt blending: Effect on rheological behavior, mechanical properties, and microcellular foamability. Polymers 2018, 134, 263–274. [Google Scholar] [CrossRef]
- Wang, X.C.; Jing, X.; Peng, Y.Y.; Ma, Z.K.; Liu, C.T.; Turng, L.S.; Shen, C.Y. The effect of nanoclay on the crystallization behavior, microcellular structure, and mechanical properties of thermoplastic polyurethane nanocomposite foams. Polym. Eng. Sci. 2016, 56, 319–327. [Google Scholar] [CrossRef]
- Hu, B.; Deng, J.; Zheng, H.; Yu, S.; Gao, C. Synthesis of chiral oligomer-grafted biodegradable polyurethanes and their chiral-dependent influence on bone marrow stem cell behaviors. Macromol. Rapid Commun. 2016, 37, 1331–1336. [Google Scholar] [CrossRef] [PubMed]
- Honarkar, H. Waterborne polyurethanes: A Review. J. Disper. Sci. Technol. 2018, 39, 507–516. [Google Scholar] [CrossRef]
- Zhang, Y.; Shao, L.; Dong, D.; Wang, Y. Enhancement of water and organic solvent resistances of a waterborne polyurethane film by incorporating liquid polysulfide. RSC Adv. 2016, 6, 17163–17171. [Google Scholar] [CrossRef]
- Tong, Y.; Bohm, S.; Song, M. The capability of graphene on improving the electrical conductivity and anti-corrosion properties of Polyurethane coatings. Appl. Surf. Sci. 2017, 424, 72–81. [Google Scholar] [CrossRef]
- Sung, Y.H.; Kim, Y.D.; Choi, H.J.; Shin, R.; Kang, S.; Lee, H. Fabrication of superhydrophobic surfaces with nano-in-microstructures using UV-nanoimprint lithography and thermal shrinkage films. Appl. Surf. Sci. 2015, 349, 169–173. [Google Scholar] [CrossRef]
- Xi, J.; Jiang, L. Biomimic superhydrophobic surface with high adhesive forces. Ind. Eng. Chem. Res. 2008, 47, 6354–6357. [Google Scholar] [CrossRef]
- Iijima, M.; Hayakawa, M.; Tatami, J.; Nakamura, K.; Nagashima, S. Design of nanoscale structured composite particles through mechanical process for fabricating a powder layer with rapid drying properties. Chem. Eng. Sci. 2019, 203, 113–121. [Google Scholar] [CrossRef]
- Simpson, J.T.; Hunter, S.R.; Aytug, T. Superhydrophobic materials and coatings: a review. Rep. Prog. Phys. 2015, 78, 086501. [Google Scholar] [CrossRef]
- Feng, S.; Zhong, Z.; Wang, Y.; Xing, W.; Drioli, E. Progress and perspectives in PTFE membrane: Preparation, modification, and applications. J. Membr. Sci. 2018, 549, 332–349. [Google Scholar] [CrossRef]
- Qiang, F.; Hu, L.L.; Gong, L.X.; Zhao, L.; Li, S.N.; Tang, L.C. Facile synthesis of super-hydrophobic, electrically conductive and mechanically flexible functionalized graphene nanoribbon/polyurethane sponge for efficient oil/water separation at static and dynamic states. Chem. Eng. J. 2018, 334, 2154–2166. [Google Scholar] [CrossRef]
- Koti Reddy, C.; Shailaja, D. Improving hydrophobicity of polyurethane by PTFE incorporation. J. Appl. Polym. Sci. 2015, 132, 42779. [Google Scholar] [CrossRef]
- Huang, C.; Cheng, Q. Learning from nacre: Constructing polymer nanocomposites. Compos. Sci. Technol. 2017, 150, 141–166. [Google Scholar] [CrossRef]
- Liang, Y.; Ju, J.; Deng, N.; Zhou, X.; Yan, J.; Kang, W.; Cheng, B. Super-hydrophobic self-cleaning bead-like SiO2@PTFE nanofiber membranes for waterproof-breathable applications. Appl. Surf. Sci. 2018, 442, 54–64. [Google Scholar] [CrossRef]
- Abenojar, J.; Tutor, J.; Ballesteros, Y.; del Real, J.C.; Martínez, M.A. Erosion-wear, mechanical and thermal properties of silica filled epoxy nanocomposites. Compos. Part B Eng. 2017, 120, 42–53. [Google Scholar] [CrossRef]
- Shin, M.S.; Lee, Y.L.; Rahman, M.M.; Kim, H.D. Synthesis and properties of waterborne fluorinated polyurethane-acrylate using a solvent-/emulsifier-free method. Polymers 2013, 54, 4873–4882. [Google Scholar] [CrossRef]
- Krol, P.; Krol, B.; Stagraczynski, R.; Skrzypiec, K. Waterborne cationomer polyurethane coatings with improved hydrophobic properties. J. Appl. Polym. Sci. 2013, 127, 2508–2519. [Google Scholar] [CrossRef]
- Serkis, M.; Spirkova, M.; Hodan, J.; Kredatusova, J. Nanocomposites made from thermoplastic waterborne polyurethane and colloidal silica. The influence of nanosilica type and amount on the functional properties. Prog. Org. Coat. 2016, 101, 342–349. [Google Scholar] [CrossRef]
- Zhang, S.; Yu, A.; Liu, S.; Zhao, J.; Jiang, J.; Liu, X. Effect of silica nanoparticles on structure and properties of waterborne UV-curable polyurethane nanocomposites. Polym. Bull. 2012, 68, 1469–1482. [Google Scholar] [CrossRef]
- Wang, F.F.; Feng, L.J.; Lu, M. Mechanical Properties of Multi-Walled Carbon Nanotube/Waterborne Polyurethane Conductive Coatings Prepared by Electrostatic Spraying. Polymers 2019, 11, 714. [Google Scholar] [CrossRef]
- Roach, P.; Shirtcliffe, N.J.; Newton, M.I. Progress in superhydrophobic surface development. Soft Matter 2008, 4, 224–240. [Google Scholar] [CrossRef]




| Samples | #1 | #2 | #3 | #4 |
|---|---|---|---|---|
| WPU (g) | 6 | 8 | 8 | 6 |
| PTFE (g) | 0.1 | 0.2 | 0.3 | 0.3 |
| M-PDMS (g) | 0.2 | 0.2 | 0.4 | 0.6 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, F.; Feng, L.; Li, G.; Zhai, Z.; Ma, H.; Deng, B.; Zhang, S. Fabrication and Properties of Superhydrophobic Waterborne Polyurethane Composites with Micro-Rough Surface Structure Using Electrostatic Spraying. Polymers 2019, 11, 1748. https://doi.org/10.3390/polym11111748
Wang F, Feng L, Li G, Zhai Z, Ma H, Deng B, Zhang S. Fabrication and Properties of Superhydrophobic Waterborne Polyurethane Composites with Micro-Rough Surface Structure Using Electrostatic Spraying. Polymers. 2019; 11(11):1748. https://doi.org/10.3390/polym11111748
Chicago/Turabian StyleWang, Fangfang, Lajun Feng, Guangzhao Li, Zhe Zhai, Huini Ma, Bo Deng, and Shengchao Zhang. 2019. "Fabrication and Properties of Superhydrophobic Waterborne Polyurethane Composites with Micro-Rough Surface Structure Using Electrostatic Spraying" Polymers 11, no. 11: 1748. https://doi.org/10.3390/polym11111748
APA StyleWang, F., Feng, L., Li, G., Zhai, Z., Ma, H., Deng, B., & Zhang, S. (2019). Fabrication and Properties of Superhydrophobic Waterborne Polyurethane Composites with Micro-Rough Surface Structure Using Electrostatic Spraying. Polymers, 11(11), 1748. https://doi.org/10.3390/polym11111748