1. Introduction
H. pylori is a gram negative, spiral-shaped human pathogen infecting an estimated 50% of the global population. There is great disparity in the prevalence of infection between developed and developing nations. The average prevalence in developed countries in those <40 years old is 20% whereas developing countries have a prevalence rate of 80%–90% [
1]. This makes measures for control most relevant for regions, such as South and Far East Asia, Africa and Latin America.
H. pylori is able to survive the acidic environment of the stomach and to adhere to the gastric mucosa, colonizing the mucosal lining of the stomach. An estimated 10
4–10
7 H. pylori colony forming units (CFU) per g of gastric mucus can be found in infected persons [
2].
H. pylori is associated with a number of gastrointestinal diseases, such as peptic ulcer disease and gastric cancer [
3]. Infection by
H. pylori may lead to an inflammatory response, increased secretion of gastric acid, and type-B gastritis. There is evidence of a relationship between the level of gastric colonization by
H. pylori and the probability of symptoms/onset of disease [
4]. In the National Health and Nutrition Examination Survey III,
H pylori was not associated with all-cause mortality.
H pylori was strongly positively related to gastric cancer mortality. There was an inverse association of
H pylori status with stroke mortality, pointing to possible protective effects [
5].
The management of
H. pylori infections is still a matter of discussion. The fourth edition of the Maastricht Consensus Report provides diagnostic guidelines and therapeutic strategies for
H. pylori infection [
6]. In dyspeptic patients, a test-and-treat strategy is proposed. Therapeutic options for
H. pylori-infection aiming at complete eradication include various combinations of proton pump inhibitors combined with two to three antibiotics. This complex approach has inherently high risks of side effects and non-compliance. Furthermore, this push towards eradication has been challenged for developing countries like India where high prevalence, increasing resistance, diversity of strains, together with high risk of recurring infections would make test-and-treat potentially more harmful than beneficial at the community level [
7]. The Maastricht Consensus Report also does not state that eradication treatment is indicated for asymptomatic individuals with
H. pylori.
Therefore, there remains a therapeutic gap for persons infected by
H. pylori, but still without clinically relevant pathology [
8].
The efficacy of using probiotics in the prevention and treatment of various gastrointestinal diseases has been summarized in a recent review, confirming the potential for beneficial health effects [
9]. These benefits could apply to the treatment of infections by
H. pylori as well. Of special interest in that context is the increasing evidence that not just living probiotics but also dead cells or even cell fractions seem to be sufficient to modify biological responses. Adams reports several cases of heat killed probiotic strains exerting positive influences, including reduction of cholesterol, attenuation of allergic response and pain modulation. [
10].
The aim of the development of a specific
Lactobacillus is to close the therapeutic gap in asymptomatic infection and to provide new preventive options to patients by lowering the risk for gastric ulcer or carcinoma, thereby avoiding severe adverse effects and treatment expenses. In a previous placebo-controlled proof-of-concept
in vivo study we tested
Lactobacillus reuteri strain DSMZ17648 (Pylopass™/Lonza) [
11]. This specific strain was found by screening hundreds of
Lactobacilli strains of a large culture collection (Organobalance GmbH, Berlin, Germany). This strain was tested for antibiotic resistance and no resistance was identified. A significant reduction in
13C-urea breath test (UBT) indicated reduction of bacteria load after active treatment with freeze-dried cells, while no effects were found for placebo control in asymptomatic humans after the two-week supplementation period.
Lactobacillus reuteri is found in both human breast milk as well as the microflora of the gastrointestinal tract. Strains of
L. reuteri have been shown to confer health benefits in a variety of cases, including infant colic, gastrointestinal disorders in children and feeding intolerance in pre-term infants [
12]. Inhibitory effects of
L. reuteri (ATCC 55730) on
H. pylori have been reported as well [
13].
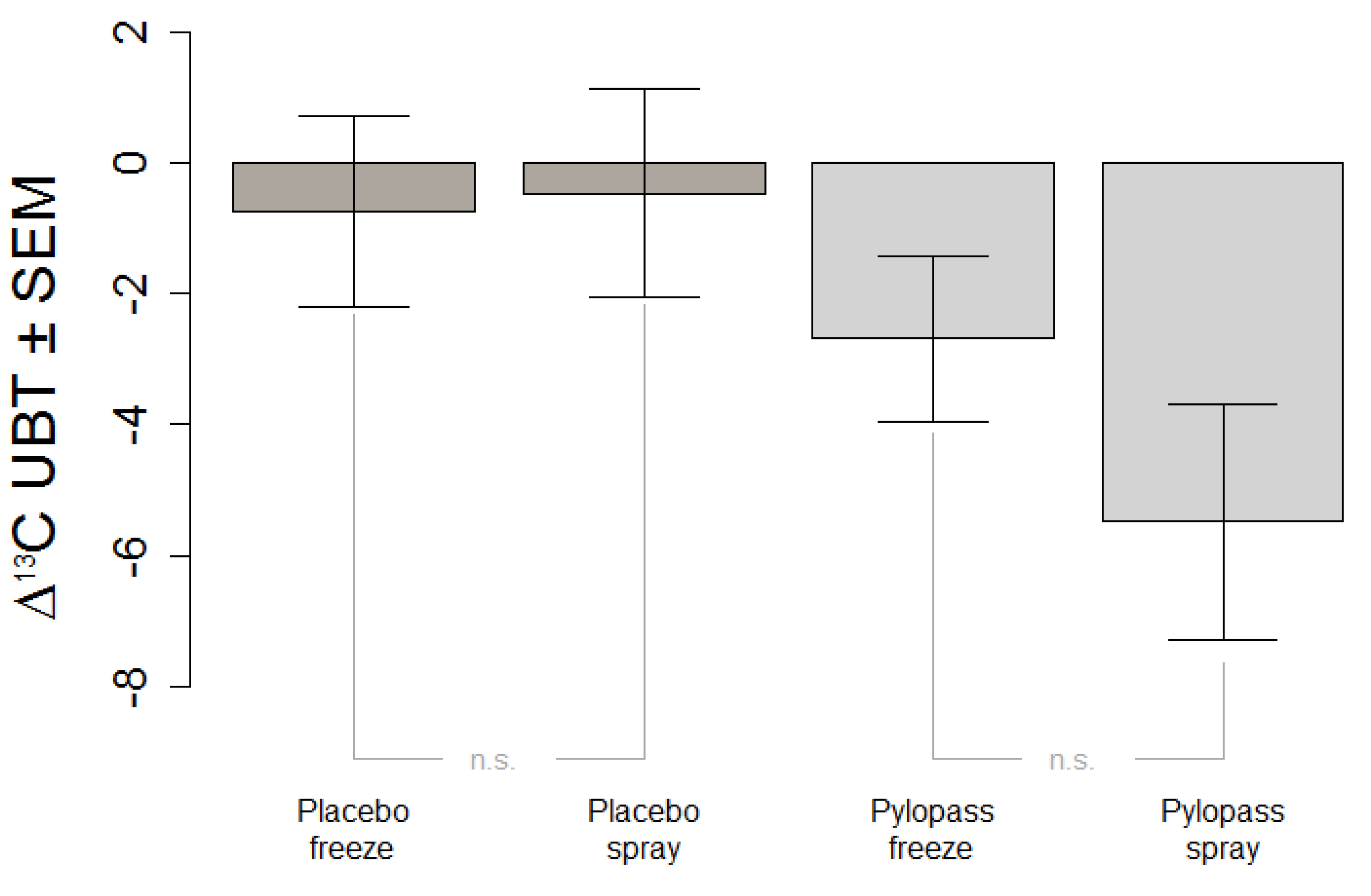
The primary objective of this study was to replicate in an independent sample previous findings of the impact of two weeks of Pylopass™ supplementation on H. pylori load as measured by 13C-UBT. The secondary objective of this study was to evaluate whether a change in the manufacturing process of L. reuteri DSMZ17648 from freeze-drying to spray-drying impacts the effectiveness of the supplement. Spray-drying does have obvious advantages over freeze-drying not only with regard to production cost but also with regard to storage stability of the product.
2. Experimental Section
The study was approved by the local ethics advisory committee (Charité, Berlin, Germany). The study was conducted according to the Declaration of Helsinki and is registered at ISRCTN (International Standard Randomised Controlled Trial Number ISRCTN70607306).
2.1. Study Population
Sample size was estimated in a power calculation as 20 subjects; allowing for potential drop-outs this number was increased by 10%. The study population included 22
H. pylori positive subjects (5 male, 17 female, mean age 47 ± 16). Subjects were included if they had reached the age of 18, had documented informed consent, a positive finding in the antibody-based screening test (Diagnostik Nord, Schwerin, Germany), and a positive
H. pylori finding in the
13C urea breath test (Helicobacter Test INFAI
®, (INFAI GmbH, Köln, Germany), δ ≥ 12‰). Exclusion criteria were intake of any medication interfering with the action of the lactobacilli, previous surgical procedures affecting stomach or small intestine with potential interference with the study, e.g., gastrectomy or gastric bypass, diabetes type 1 or 2, familiar lipid metabolism diseases, liver disease, kidney insufficiency, autoimmune disease, organ transplantation, weight changes >3 kg over the last three months, eradication therapy, lactose intolerance, oral intake of antibiotics <3 months ago, intake of PPIs or H2 antagonists, pregnancy or lactation, alcohol or drug abuse, psychiatric diseases or participation at other clinical trials at the same time. None of the participants met the criteria set forth by the Maastricht Guidelines for eradication therapy [
6].
2.2. Study Supplements
The test product (active ingredient) consisted of spray-dried dead cells of the Lactobacillus reuteri strain DSMZ17648, prepared as solid tablets (Quimifarma S.L., Yuncos (Toledo), Spain) for oral application. Each tablet contained 5 × 109 cells and the daily dosage of 4 tablets translates into 2 × 1010 cells. Supplement and placebo tablets were identical in weight (250 mg), size, color and flavor.
2.3. Study Protocol
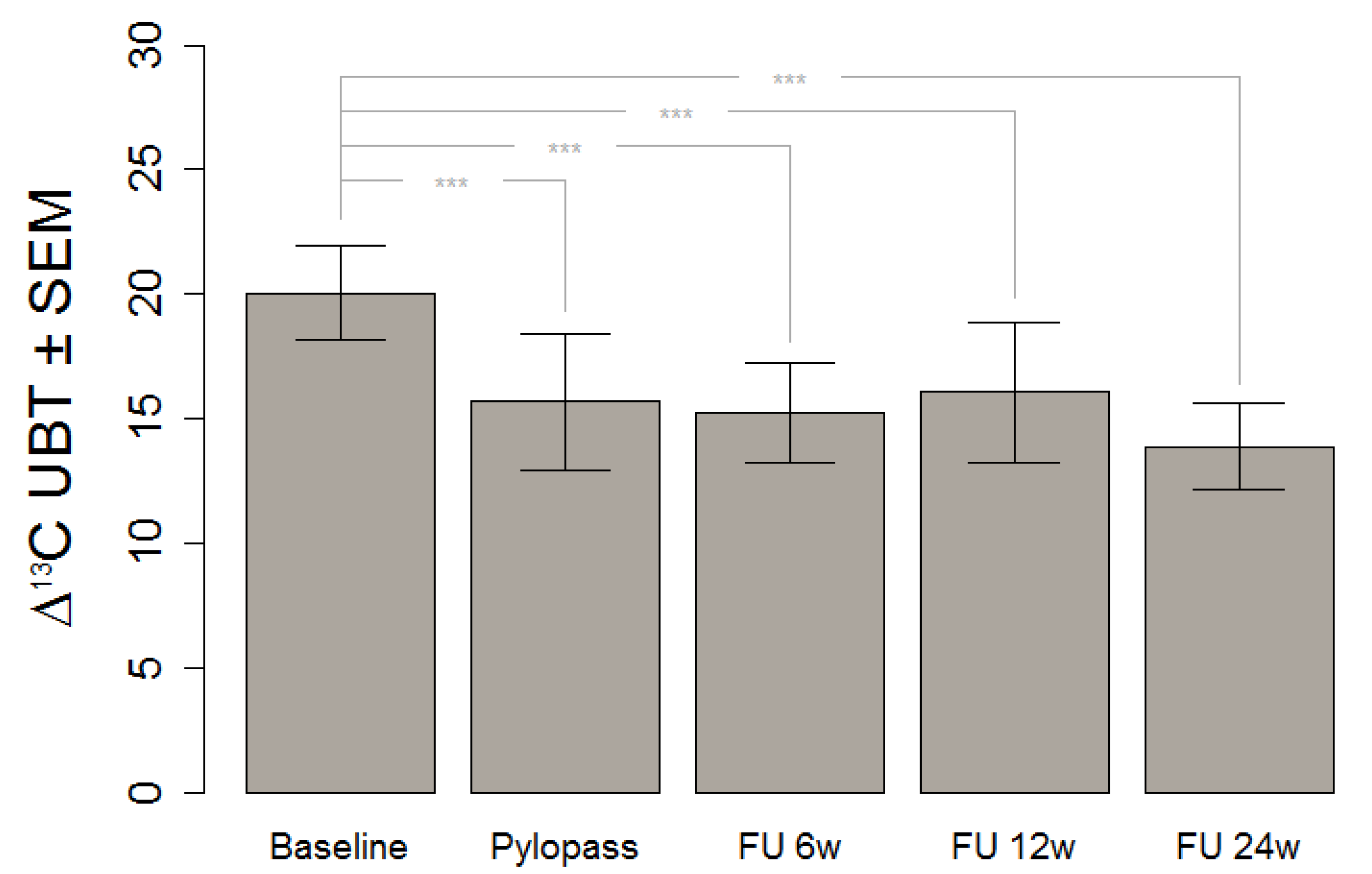
Supplement and placebo were given in a single-blinded, crossover design. All tests were done at the clinical research center of the Charité in Berlin-Buch. Following a first urea breath test, there were 14 days of placebo-run in. After a second breath test, the supplement was given for 14 days, followed by a third breath test. To test mid-term effects, follow-up measures were taken at 6, 12, and 24 weeks after the end of supplementation.
Subjects were instructed to take two tablets after breakfast as well as after their evening meal. During the supplementation period participants were instructed not to initiate any lifestyle or dietary changes.
Subjects were asked to answer a study-specific questionnaire to document well-being, any potential side effects, smoking, alcohol use, nutrition and medication.
To test for physiological changes relevant in terms of safety, fasting blood samples were taken before and after the supplementation period to determine levels of cholesterol, triglycerides, glucose, gamma-GT, GPT (ALAT), GOT (ASAT), creatinine, erythrocytes, hematocrit, hemoglobin, MCV (mean corpuscular volume), MCHC (mean corpuscular hemoglobin concentration), leukocytes, thrombocytes, total bilirubin, uric acid, urea, CRP (C-reactive protein), and the coagulation parameters INR (international normalized ratio) and PTT (partial thromboplastin time).
2.4. Outcome Measurements
Detection of
H. pylori infection in the screening phase was performed with an antibody-based quick test (Diagnostik Nord, Schwerin, Germany). Quantification of colonization for confirmation of eligibility and to verify effects of DSMZ17648 was accomplished by a breath test, as this diagnostic approach is best suited to detection of intraindividual changes [
14]. Helicobacter Test INFAI
® (INFAI GmbH, Köln, Germany) is a breath test for direct non-invasive semi-quantitative detection of the bacterium
H. pylori [
15]. The test is based on the urease activity of
H. pylori. Patients ingest 75 mg of the
13C urea isotope. In the presence of
H. pylori,
13C urea is hydrolyzed to ammonium and
13C-labelled carbon dioxide, which is detectable by mass spectroscopy in the breath. As there is a small amount of naturally occurring
13C present in the exhalation air even in the absence of urease activity, breath samples are taken before and 30 min after the ingestion of
13C urea. An infection with
H. pylori is regarded as proven if the difference in
13C/
12C of 0-min-value and 30-min-value exceeds 4‰. If there is no difference, the test is negative, indicating no infection with
H. pylori. There is a quantitative relation between urease activity and amount of
13C in breath in fasted subjects, that indirectly relates to the level of colonization by
H. pylori, making it well suited to detect reduction or eradication of the bacteria [
15,
16]. In this study, subjects were included if their breath test value was >12‰, indicating a moderately high colonization level of
H. pylori. Specificity (98.5%) and sensitivity (97.9%) of Helicobacter Test INFAI
® are comparable to traditional invasive diagnostic methods (endoscopy or biopsy) [
17].
2.5. Statistics
All data were entered into a dedicated trial database. Statistical analysis was conducted using R (Version 3.0.1, R Foundation for Statistical Computing, Vienna, Austria) [
18]. We computed differences in
13C Urea Breath Test (UBT) values against initial measurements: ∆active =
13C UBT active −
13C UBT initial, ∆placebo =
13C UBT placebo −
13C UBT initial. Additionally, the absolute test values between the various study time-points were compared:
13C UBT initial,
13C UBT active (after 14 days active treatment),
13C UBT placebo (after 14 days placebo treatment).
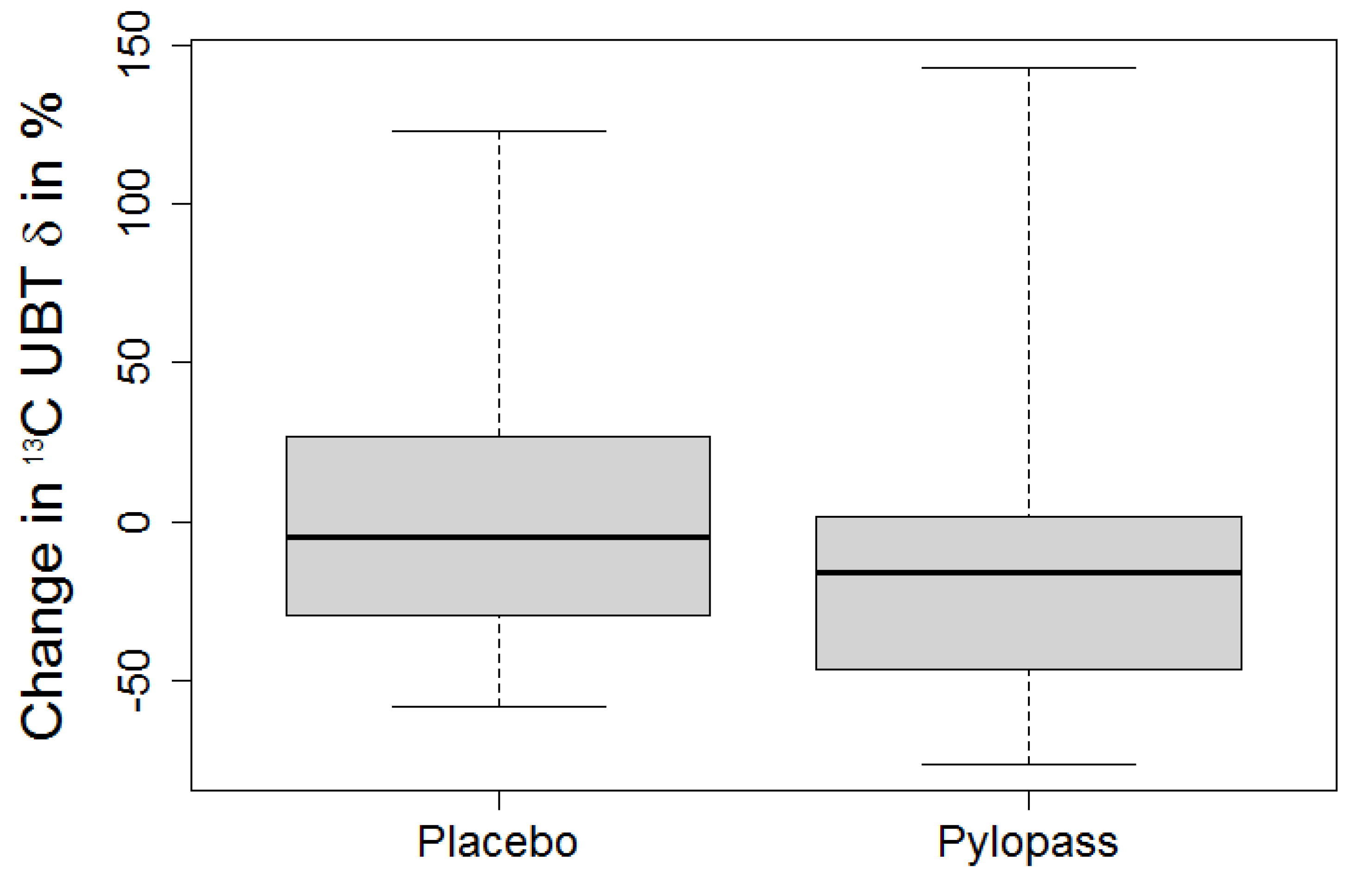
Due to the selection criteria (13C UBT > 12‰ at baseline), deviation from normal distribution was assumed and differences were tested by Skillings-Mack test for multiple repeated measurements. If significant, pairwise Wilcoxon tests were applied to distinguish placebo and treatment effects. An error level of 5% was set as threshold for significance. Results are reported as median and 1st/3rd quartile.
4. Discussion
Despite the causal link between
H. pylori infections and gastrointestinal pathology, only a minority of infected subjects actually develops disease [
19]. For those with acute symptoms, eradication in a test-and treat approach is usually recommended as the standard procedure. Yet on the population level, this may neither be necessary nor achievable, especially in regions with high prevalence and poorly developed health systems. Given the interaction between infections with
H. pylori and mental stress in gastrointestinal diseases [
20], short-term reduction may be another viable option during stressful life events, such as, e.g., exams, excessive work load, or other emotional distress.
This in vivo study shows a significant decrease of H. pylori stomach colonization after Lactobacillus reuteri DSMZ17648 supplementation in asymptomatic subjects with detectable H. pylori infection. It replicates our previous findings in an independent general population sample free of overt gastrointestinal diseases or alarm symptoms. Primary objective of this study was the reduction of H. pylori as measured by 13C urease breath test (Helicobacter Test INFAI®) after a 14 day supplementation period of L. reuteri DSMZ17648 (Pylopass™) at a daily dose of 2 × 1010 non-viable spray-dried cells.
This finding gives a strong foundation for the assumption that consumption of DSMZ17648 might exert a preventive effect of secondary diseases and related symptoms due to H. pylori infection. It is of special relevance that the preventive effect is carried over well after the supplementation itself, at least for the six month period covered by our follow-up.
Probiotics are defined as live microorganisms which when administered in adequate amounts confer a health benefit to the host (FAO/WHO) [
21]. In the context of
H. pylori infection, probiotics are administered along with eradication therapy to ease side-effects. Medeiros
et al. [
22] studied the impact of
Lactobacillus acidopholus on side effects associated with a common triple regimen eradication therapy but found no measurable effect. However, there are some studies where probiotics are used alone in the treatment of
H. pylori, based on a broad range of cultures and outcomes. Francavilla
et al. supplemented a different strain of
Lactobacillus reuteri (ATCC 55730) for four weeks and found a significant reduction in
H. pylori load [
13]. As they had included patients showing some gastrointestinal symptoms, they could verify a reduction in symptom scores during treatment. A study applying
L. casei for three weeks reported a non-significant suppressive effect [
23]. Fourteen
H. pylori-positive subjects receiving drinks with 10
8 colony-forming units/mL
L. casei thrice daily during meals for three weeks were compared to six untreated
H. pylori-positive subjects as controls. Urease activity decreased in nine of the 14 (64%) subjects with
L. casei supplementation and in two of the six (33%) controls (
p = 0.22). A slight, but nonsignificant trend towards a suppressive effect of
L. casei on
H. pylori in vivo may exist.
In vitro experiments showed that viable
L. casei are required for
H. pylori growth inhibition.
Reduction of
H. pylori with
L. brevis administration has also been reported [
24]. Twenty-two
H. pylori-positive dyspeptic patients randomly (ratio 1:1) received high oral doses of lyophilisated
L. brevis (CD2) or placebo nine times a day for three weeks.
L. brevis (CD2) treatment did not eradicate
H. pylori. However, a reduction in the UBT delta values occurred, suggesting a decrease in intragastric bacterial load.
In the case of
L. acidophilus (
johnsonii) La1 the whey-based supernatant was found to induce a marked decrease in
H. pylori breath test analyses, both in combination with Omeprazol and alone [
25]. While these studies showed some promising results, further research is needed to determine the efficacy of such methodology in a general population.
Potential mechanisms by which probiotics may influence
H. pylori may include strengthening of the non-immunological barriers representing a first line of defense against pathogenic bacteria by producing antimicrobial substances, or stabilization of the gut mucosal barrier. Other mechanisms could be sequestration of
H. pylori, competitive binding to adhesion receptors for
H. pylori [
26,
27], binding to surface structures of
H. pylori, thus preventing adhesion to the mucosa. Antimicrobial substances have been implicated in the inhibition of
H. pylori by lactic acid bacteria. Short chain fatty acids (SCFAs, e.g., formic, acetic, propionic, butyric and lactic acid) have an important role in decreasing the pH, and are produced during the catabolism of carbohydrates. Such antimicrobial activity could either be due to a direct effect on
H. pylori but also a secondary effect of the inhibition of its urease activity [
28]. Current research suggested that living cells may not be mandatory for health effects, as dead cells, cell fractions, or supernatant may be as efficient in some cases. For the specific strain of
L. reuteri studied in this project, supplementation of both freeze-dried and spray-dried cells has been shown to reduce
H. pylori. The most plausible explanation is that structures on the cell surface of this selected strain are responsible for the therapeutic effect and were unaffected by the different drying processes.
DSMZ17648 specifically co-aggregates
H. pylori under
in vitro conditions and in artificial gastric juice, without interfering with other bacteria of the commensal intestinal flora [
29]. This specific binding may mask surface structures of
H. pylori and interfere with
Helicobacter motility. The aggregated pathogens presumably no longer adhere to the gastric mucosa and are cleared from the stomach. An additional mode of action may be competition for specific binding proteins [
30].
The present study should be regarded as a pilot trial, as sample size was limited. Larger clinical studies should be conducted in the future to validate these results. Establishment of predictors for response to DSMZ17648 as well as more precise estimation of effect size will require larger samples. Although most other human trials [
22,
23,
24,
25] have used the 13C urea breath test to study the effects of probiotics on
H. pylori colonization, the outcome of
13C Urea Breath Test reflects
H. pylori colonization density in a semi-quantitative way only, making intraindividual changes in test scores only a proxy for intervention effects. Deviation from fasting conditions may influence test outcomes as well [
31].