A Comprehensive Review of Bioactive Peptides from Marine Fungi and Their Biological Significance
Abstract
:1. Introduction
2. Bioactive Peptides in Particular Fungal Genera
2.1. Acremonium
2.2. Ascotricha
2.3. Aspergillus
2.4. Asteromyces
2.5. Ceratodictyon
2.6. Clonostachys
2.7. Emericella
2.8. Exserohilum
2.9. Microsporum
2.10. Metarrhizium
2.11. Penicillium
2.12. Scytalidium
2.13. Simplicillium
2.14. Stachylidium
2.15. Talaromyces
2.16. Trichoderma
2.17. Zygosporium
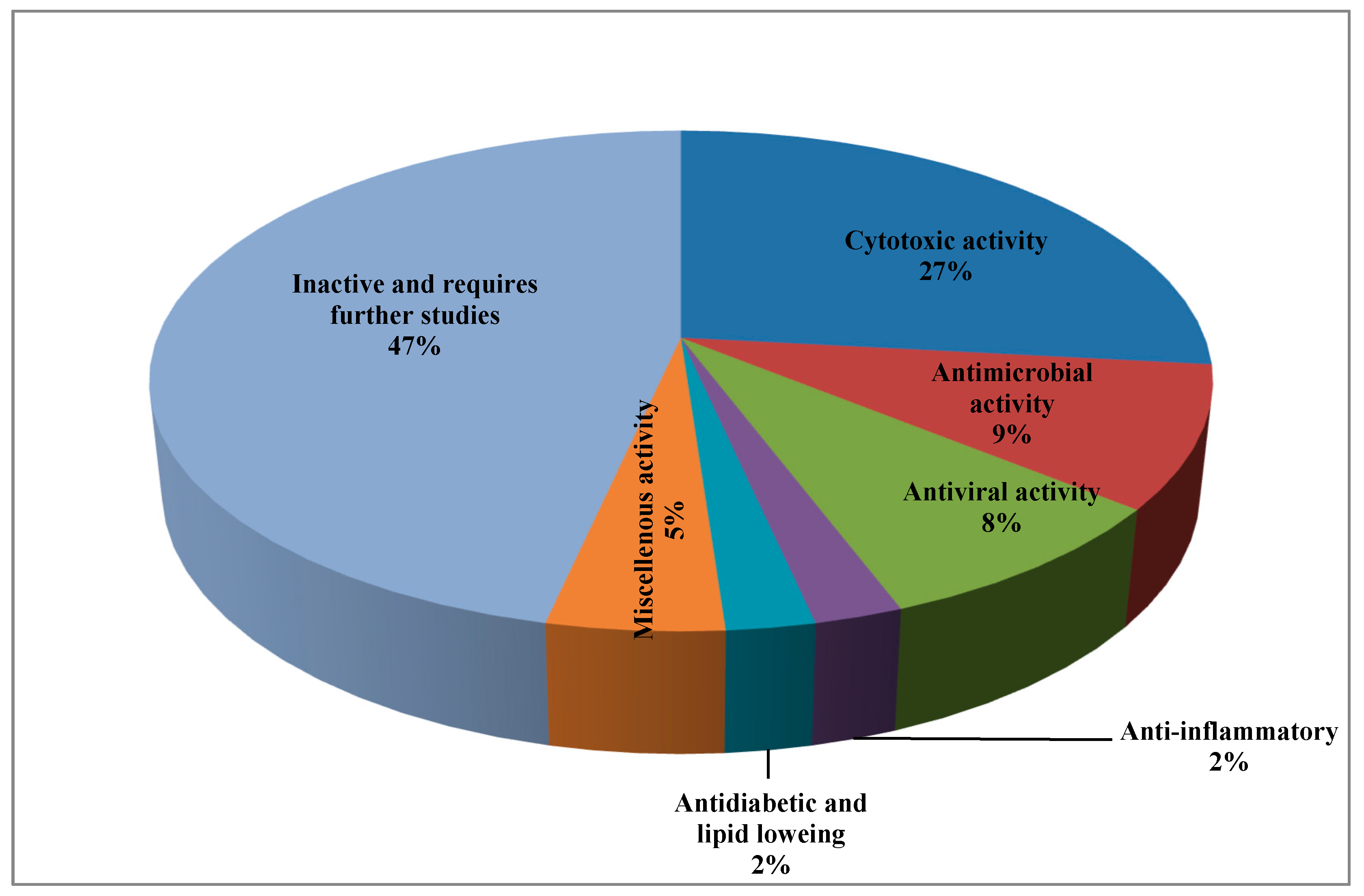
3. Discussion and General Perspectives
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jin, L.; Quan, C.; Hou, X.; Fan, S. Potential pharmacological resources: Natural bioactive compounds from marine-derived fungi. Mar. Drugs 2016, 14, 76. [Google Scholar] [CrossRef] [PubMed]
- Rateb, M.E.; Ebel, R. Secondary metabolites of fungi from marine habitats. Nat. Prod. Rep. 2011, 28, 290–344. [Google Scholar] [CrossRef] [PubMed]
- Schueffler, A.; Anke, T. Fungal natural products in research and development. Nat. Prod. Rep. 2014, 31, 1425–1448. [Google Scholar] [CrossRef] [PubMed]
- Blunt, J.W.; Copp, B.R.; Keyzers, R.A.; Munro, M.H.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2015, 32, 116–211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blunt, J.W.; Copp, B.R.; Keyzers, R.A.; Munro, M.H.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2017, 34, 235–294. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, H.K.; Lee, H.H.; Seo, C.H.; Park, Y. Antimicrobial and immunomodulatory properties and applications of marine-derived proteins and peptides. Mar. Drugs 2019, 17, 350. [Google Scholar] [CrossRef] [PubMed]
- Saleem, M.; Ali, M.S.; Hussain, S.; Jabbar, A.; Ashraf, M.; Lee, Y.S. Marine natural products of fungal origin. Nat. Prod. Rep. 2007, 24, 1142–1152. [Google Scholar] [CrossRef]
- Sable, R.; Parajuli, P.; Jois, S. Peptides, peptidomimetics, and polypeptides from marine sources: A wealth of natural sources for pharmaceutical applications. Mar. Drugs 2017, 15, 124. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Wahab, N.M.; Harwoko, H.; Müller, W.E.; Hamacher, A.; Kassack, M.U.; Fouad, M.A.; Kamel, M.S.; Lin, W.; Ebrahim, W.; Liu, Z. Cyclic heptapeptides from the soil-derived fungus Clonostachys rosea. Bioorg. Med. Chem. 2019, 27, 3954–3959. [Google Scholar] [CrossRef]
- Liang, X.; Nong, X.-H.; Huang, Z.-H.; Qi, S.-H. Antifungal and antiviral cyclic peptides from the deep-sea-derived fungus Simplicillium obclavatum EIODSF 020. J. Agric. Food Chem. 2017, 65, 5114–5121. [Google Scholar] [CrossRef]
- Capon, R.J.; Skene, C.; Stewart, M.; Ford, J.; Richard, A.; Williams, L.; Lacey, E.; Gill, J.H.; Heiland, K.; Friedel, T. Aspergillicins A–E: Five novel depsipeptides from the marine-derived fungus Aspergillus carneus. Org. Biomol. Chem. 2003, 1, 1856–1862. [Google Scholar] [CrossRef]
- Chen, Z.; Song, Y.; Chen, Y.; Huang, H.; Zhang, W.; Ju, J. Cyclic heptapeptides, cordyheptapeptides C–E, from the marine-derived fungus Acremonium persicinum SCSIO 115 and their cytotoxic activities. J. Nat. Prod. 2012, 75, 1215–1219. [Google Scholar] [CrossRef]
- Boot, C.M.; Tenney, K.; Valeriote, F.A.; Crews, P. Highly N-methylated linear peptides produced by an atypical sponge-derived Acremonium sp. J. Nat. Prod. 2006, 69, 83–92. [Google Scholar] [CrossRef]
- Boot, C.M.; Amagata, T.; Tenney, K.; Compton, J.E.; Pietraszkiewicz, H.; Valeriote, F.A.; Crews, P. Four classes of structurally unusual peptides from two marine-derived fungi: Structures and bioactivities. Tetrahedron. 2007, 63, 9903–9914. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.-R.; Li, D.-Y.; Wang, P.-L.; Hua, H.-M.; Wu, X.; Li, Z.-L. A new 3, 4-seco-lanostane triterpenoid from a marine-derived fungus Ascotricha sp. ZJ-M-5. Acta Pharm. Sin. 2013, 48, 89–93. [Google Scholar]
- Uchoa, P.K.S.; Pimenta, A.T.; Braz-Filho, R.; de Oliveira, M.d.C.F.; Saraiva, N.N.; Rodrigues, B.S.; Pfenning, L.H.; Abreu, L.M.; Wilke, D.V.; Florêncio, K.G. New cytotoxic furan from the marine sediment-derived fungi Aspergillus niger. Nat. Prod. Rep. 2017, 31, 2599–2603. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, X.-M.; Feng, Y.; Wang, B.-G. Phenethyl-α-pyrone derivatives and cyclodipeptides from a marine algous endophytic fungus Aspergillus niger EN–13. Nat. Prod. Rep. 2010, 24, 1036–1043. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Zhu, H.; Hong, K.; Wang, Y.; Liu, P.; Wang, X.; Peng, X.; Zhu, W. Novel cyclic hexapeptides from marine-derived fungus, Aspergillus sclerotiorum PT06-1. Org. Lett. 2009, 11, 5262–5265. [Google Scholar] [CrossRef] [PubMed]
- Prompanya, C.; Fernandes, C.; Cravo, S.; Pinto, M.; Dethoup, T.; Silva, A.; Kijjoa, A. A new cyclic hexapeptide and a new isocoumarin derivative from the marine sponge-associated fungus Aspergillus similanensis KUFA 0013. Mar. Drugs 2015, 13, 1432–1450. [Google Scholar] [CrossRef] [PubMed]
- You, M.; Liao, L.; Hong, S.; Park, W.; Kwon, D.; Lee, J.; Noh, M.; Oh, D.-C.; Oh, K.-B.; Shin, J. Lumazine peptides from the marine-derived fungus Aspergillus terreus. Mar. Drugs 2015, 13, 1290–1303. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.-W.; Lin, Y.; Lu, Y.-J.; Zhou, X.-F.; Liu, Y.-H. Peptides and polyketides isolated from the marine sponge-derived fungus Aspergillus terreus SCSIO 41008. Chin. J. Nat. Med. 2019, 17, 149–154. [Google Scholar] [CrossRef]
- He, F.; Bao, J.; Zhang, X.-Y.; Tu, Z.-C.; Shi, Y.-M.; Qi, S.-H. Asperterrestide A, a cytotoxic cyclic tetrapeptide from the marine-derived fungus Aspergillus terreus SCSGAF0162. J. Nat. Prod. 2013, 76, 1182–1186. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.-C.; Bao, H.-Y.; Liu, Y.-Y.; Nie, Y.-Y.; Yang, J.-M.; Hong, P.-Z.; Zhang, Y. Depsidone Derivatives and a cyclopeptide produced by marine fungus Aspergillus unguis under chemical induction and by its plasma induced mutant. Molecules 2018, 23, 2245. [Google Scholar] [CrossRef] [PubMed]
- Capon, R.J.; Ratnayake, R.; Stewart, M.; Lacey, E.; Tennant, S.; Gill, J.H. Aspergillazines A–E: Novel heterocyclic dipeptides from an Australian strain of Aspergillus unilateralis. Org. Biomol. Chem. 2005, 3, 123–129. [Google Scholar] [CrossRef]
- Peng, J.; Gao, H.; Zhang, X.; Wang, S.; Wu, C.; Gu, Q.; Guo, P.; Zhu, T.; Li, D. Psychrophilins E–H and versicotide C, cyclic peptides from the marine-derived fungus Aspergillus versicolor ZLN-60. J. Nat. Prod. 2014, 77, 2218–2223. [Google Scholar] [CrossRef] [PubMed]
- Hou, X.-M.; Zhang, Y.-H.; Hai, Y.; Zheng, J.-Y.; Gu, Y.-C.; Wang, C.-Y.; Shao, C.-L. Aspersymmetide A, a new centrosymmetric cyclohexapeptide from the marine-derived fungus Aspergillus versicolor. Mar. Drugs 2017, 15, 363. [Google Scholar] [CrossRef] [PubMed]
- Fremlin, L.J.; Piggott, A.M.; Lacey, E.; Capon, R.J. Cottoquinazoline A and cotteslosins A and B, metabolites from an Australian marine-derived strain of Aspergillus versicolor. J. Nat. Prod. 2009, 72, 666–670. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Gu, B.; Yang, L.; Yang, F.; Lin, H. New anti-inflammatory cyclopeptides from a sponge-derived fungus Aspergillus violaceofuscus. Front. Chem. 2018, 6, 226. [Google Scholar] [CrossRef] [PubMed]
- Ebada, S.S.; Fischer, T.; Hamacher, A.; Du, F.-Y.; Roth, Y.O.; Kassack, M.U.; Wang, B.-G.; Roth, E.H. Psychrophilin E, a new cyclotripeptide, from co-fermentation of two marine alga-derived fungi of the genus Aspergillus. Nat. Prod. Res. 2014, 28, 776–781. [Google Scholar] [CrossRef]
- Ma, X.; Nong, X.-H.; Ren, Z.; Wang, J.; Liang, X.; Wang, L.; Qi, S.-H. Antiviral peptides from marine gorgonian-derived fungus Aspergillus sp. SCSIO 41501. Tetrahedron Lett. 2017, 58, 1151–1155. [Google Scholar] [CrossRef]
- Zhou, X.; Fang, P.; Tang, J.; Wu, Z.; Li, X.; Li, S.; Wang, Y.; Liu, G.; He, Z.; Gou, D. A novel cyclic dipeptide from deep marine-derived fungus Aspergillus sp. SCSIOW2. Nat. Prod. Res. 2016, 30, 52–57. [Google Scholar] [CrossRef]
- Zheng, C.J.; Wu, L.Y.; Li, X.B.; Song, X.M.; Niu, Z.G.; Song, X.P.; Chen, G.Y.; Wang, C.Y. Structure and Absolute Configuration of Aspergilumamide A, a novel lumazine peptide from the mangrove-derived fungus Aspergillus sp. Helv. Chim. Acta. 2015, 98, 368–373. [Google Scholar] [CrossRef]
- Gulder, T.; Hong, H.; Correa, J.; Egereva, E.; Wiese, J.; Imhoff, J.; Gross, H. Isolation, structure elucidation and total synthesis of lajollamide A from the marine fungus Asteromyces cruciatus. Mar. Drugs 2012, 10, 2912–2935. [Google Scholar] [CrossRef]
- Komatsu, K.; Shigemori, H.; Kobayashi, J.i. Dictyonamides A and B, new peptides from marine-derived fungus. J. Org. Chem. 2001, 66, 6189–6192. [Google Scholar] [CrossRef]
- Adachi, K.; Kanoh, K.; Wisespongp, P.; Nishijima, M.; Shizuri, Y. Clonostachysins A and B, new anti-dinoflagellate cyclic peptides from a marine-derived fungus. J. Antibiot. 2005, 58, 145. [Google Scholar] [CrossRef]
- Malmstrøm, J. Unguisins A and B: New cyclic peptides from the marine-derived fungus Emericella unguis. J. Nat. Prod. 1999, 62, 787–789. [Google Scholar] [CrossRef]
- Oh, D.-C.; Kauffman, C.A.; Jensen, P.R.; Fenical, W. Induced production of emericellamides A and B from the marine-derived fungus Emericella sp. in competing co-culture. J. Nat. Prod. 2007, 70, 515–520. [Google Scholar] [CrossRef] [PubMed]
- Tan, R.X.; Jensen, P.R.; Williams, P.G.; Fenical, W. Isolation and structure assignments of rostratins A− D, cytotoxic disulfides produced by the marine-derived fungus Exserohilum r ostratum. J. Nat. Prod. 2004, 67, 1374–1382. [Google Scholar] [CrossRef] [PubMed]
- Gu, W.; Cueto, M.; Jensen, P.R.; Fenical, W.; Silverman, R.B. Microsporins A and B: New histone deacetylase inhibitors from the marine-derived fungus Microsporum cf. gypseum and the solid-phase synthesis of microsporin A. Tetrahedron 2007, 63, 6535–6541. [Google Scholar] [CrossRef]
- Wang, N.; Cui, C.-B.; Li, C.-W. A new cyclic dipeptide penicimutide: The activated production of cyclic dipeptides by introduction of neomycin-resistance in the marine-derived fungus Penicillium purpurogenum G59. Arch. Pharm. Res. 2016, 39, 762–770. [Google Scholar] [CrossRef] [PubMed]
- Meyer, S.W.; Mordhorst, T.F.; Lee, C.; Jensen, P.R.; Fenical, W.; Köck, M. Penilumamide, a novel lumazine peptide isolated from the marine-derived fungus, Penicillium sp. CNL-338. Org. Biomol. Chem. 2010, 8, 2158–2163. [Google Scholar] [CrossRef] [PubMed]
- Hong, R.; Yang, Y. Antitumor metabolites from marine sediment derived Penicillium sp. WF-06. World Notes Antibiots 2011, 2. [Google Scholar]
- Scopel, M.; Abraham, W.-R.; Henriques, A.T.; Macedo, A.J. Dipeptide cis-cyclo (Leucyl-Tyrosyl) produced by sponge associated Penicillium sp. F37 inhibits biofilm formation of the pathogenic Staphylococcus epidermidis. Bioorg. Med. Chem. Lett. 2013, 23, 624–626. [Google Scholar] [CrossRef]
- Rowley, D.C.; Kelly, S.; Jensen, P.; Fenical, W. Synthesis and structure–activity relationships of the halovirs, antiviral natural products from a marine-derived fungus. Bioorg. Med. Chem. 2004, 12, 4929–4936. [Google Scholar] [CrossRef] [PubMed]
- Rowley, D.C.; Kelly, S.; Kauffman, C.A.; Jensen, P.R.; Fenical, W. Halovirs A–E, new antiviral agents from a marine-derived fungus of the genus Scytalidium. Bioorg. Med. Chem. 2003, 11, 4263–4274. [Google Scholar] [CrossRef]
- Liang, X.; Zhang, X.-Y.; Nong, X.-H.; Wang, J.; Huang, Z.-H.; Qi, S.-H. Eight linear peptides from the deep-sea-derived fungus Simplicillium obclavatum EIODSF 020. Tetrahedron. 2016, 72, 3092–3097. [Google Scholar] [CrossRef]
- El Maddah, F.; Kehraus, S.; Nazir, M.; Almeida, C.; König, G.M. Insights into the biosynthetic origin of 3-(3-furyl) alanine in Stachylidium sp. 293 K04 tetrapeptides. J. Nat. Prod. 2016, 79, 2838–2845. [Google Scholar] [CrossRef] [PubMed]
- Almeida, C.; Maddah, F.E.; Kehraus, S.; Schnakenburg, G.; König, G.M. Endolides A and B, vasopressin and serotonin-receptor interacting N-methylated peptides from the sponge-derived fungus Stachylidium sp. Organic letters. 2016, 18, 528–531. [Google Scholar] [CrossRef]
- Dewapriya, P.; Khalil, Z.G.; Prasad, P.; Salim, A.A.; Cruz-Morales, P.; Marcellin, E.; Capon, R.J. Talaropeptides AD: Structure and biosynthesis of extensively N-methylated linear peptides from an Australian marine tunicate-derived Talaromyces sp. Front. Chem. 2018, 6, 394. [Google Scholar] [CrossRef] [PubMed]
- Van Bohemen, A.-I.; Zalouk-Vergnoux, A.; Poirier, L.; Phuong, N.N.; Inguimbert, N.; Salah, K.B.H.; Ruiz, N.; Pouchus, Y.F. Development and validation of LC–MS methods for peptaibol quantification in fungal extracts according to their lengths. J. Chromatogr. B 2016, 1009, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Herbel, V.; Wink, M. Mode of action and membrane specificity of the antimicrobial peptide snakin-2. PeerJ 2016, 4, e1987. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Herbel, V.; Schäfer, H.; Wink, M. Recombinant production of snakin-2 (an antimicrobial peptide from tomato) in E. coli and analysis of its bioactivity. Molecules 2015, 20, 14889–14901. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Schäfer, H.; Reichling, J.; Wink, M. Bactericidal properties of the antimicrobial peptide Ib-AMP4 from Impatiens balsamina produced as a recombinant fusion-protein in Escherichia coli. Biotechnol. J. 2013, 8, 1213–1220. [Google Scholar]
- Torres-García, C.; Pulido, D.; Albericio, F.; Royo, M.; Nicolás, E. Triazene as a powerful tool for solid-phase derivatization of phenylalanine containing peptides: Zygosporamide analogues as a proof of concept. J. Org. Chem. 2014, 79, 11409–11415. [Google Scholar] [CrossRef] [PubMed]
- Oh, D.-C.; Jensen, P.R.; Fenical, W. Zygosporamide, a cytotoxic cyclic depsipeptide from the marine-derived fungus Zygosporium masonii. Tetrahedron Lett. 2006, 47, 8625–8628. [Google Scholar] [CrossRef]
- Fosgerau, K.; Hoffmann, T. Peptide therapeutics: Current status and future directions. Drug Discov. Today. 2015, 20, 122–128. [Google Scholar] [CrossRef]

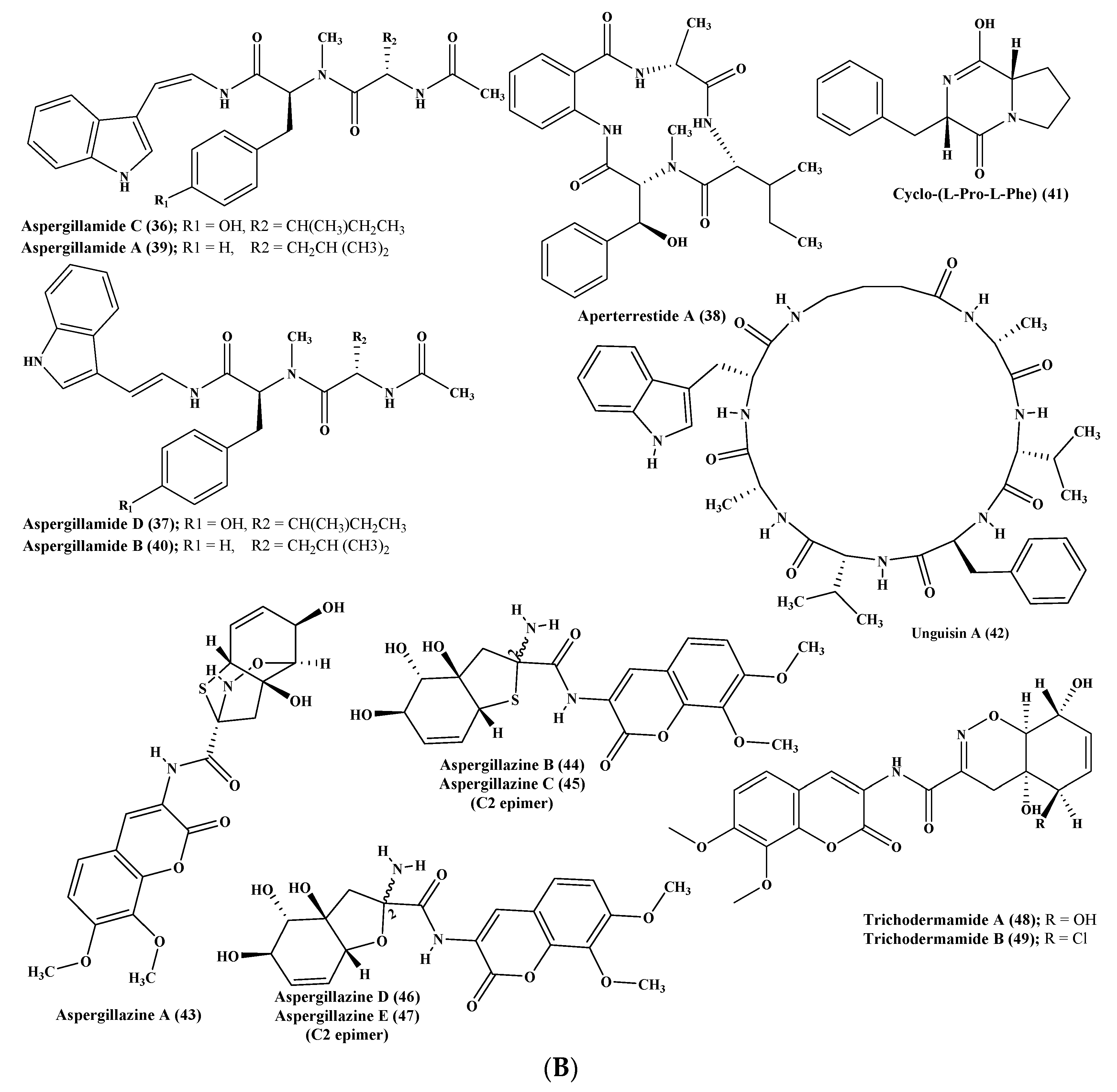
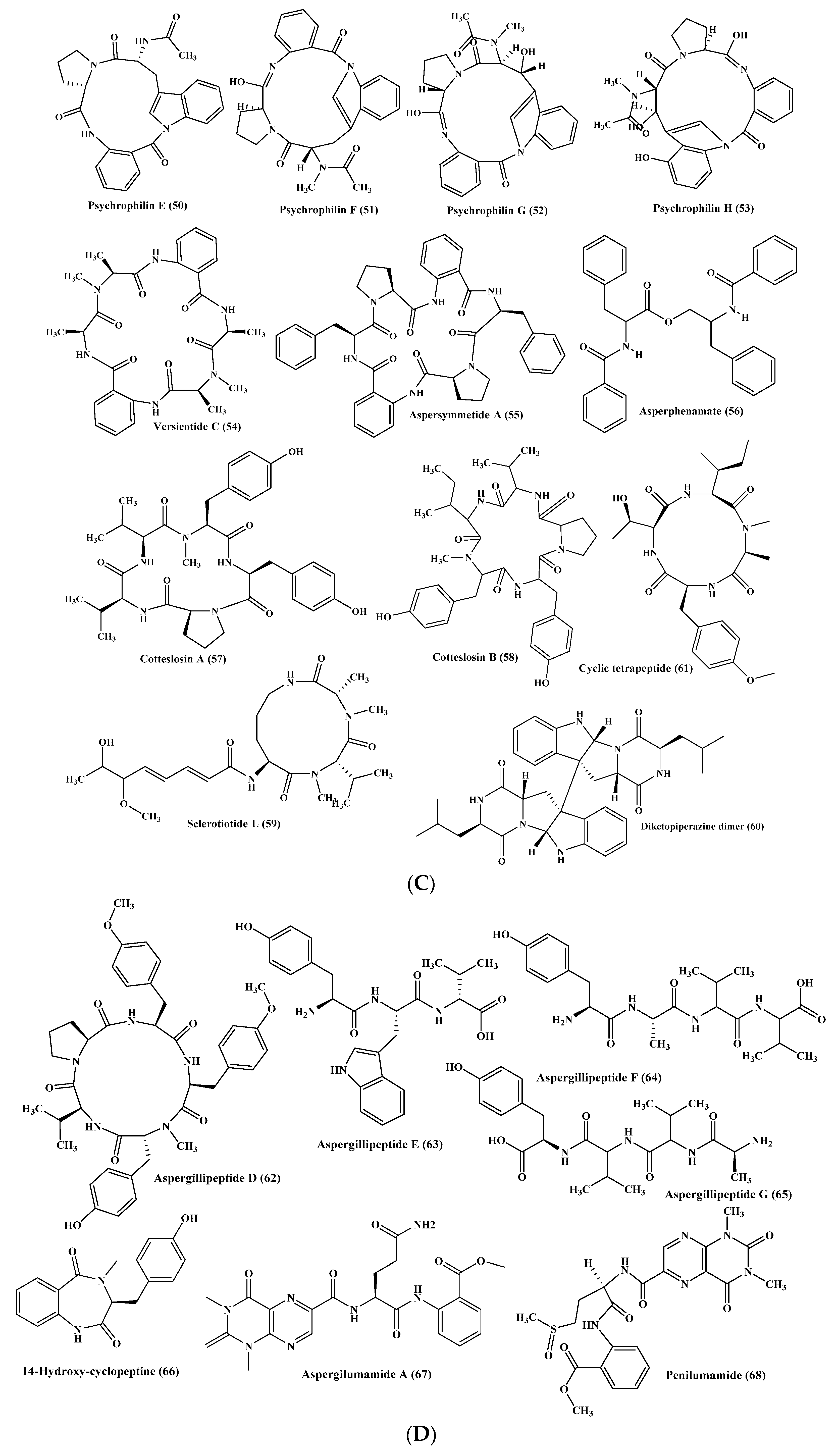
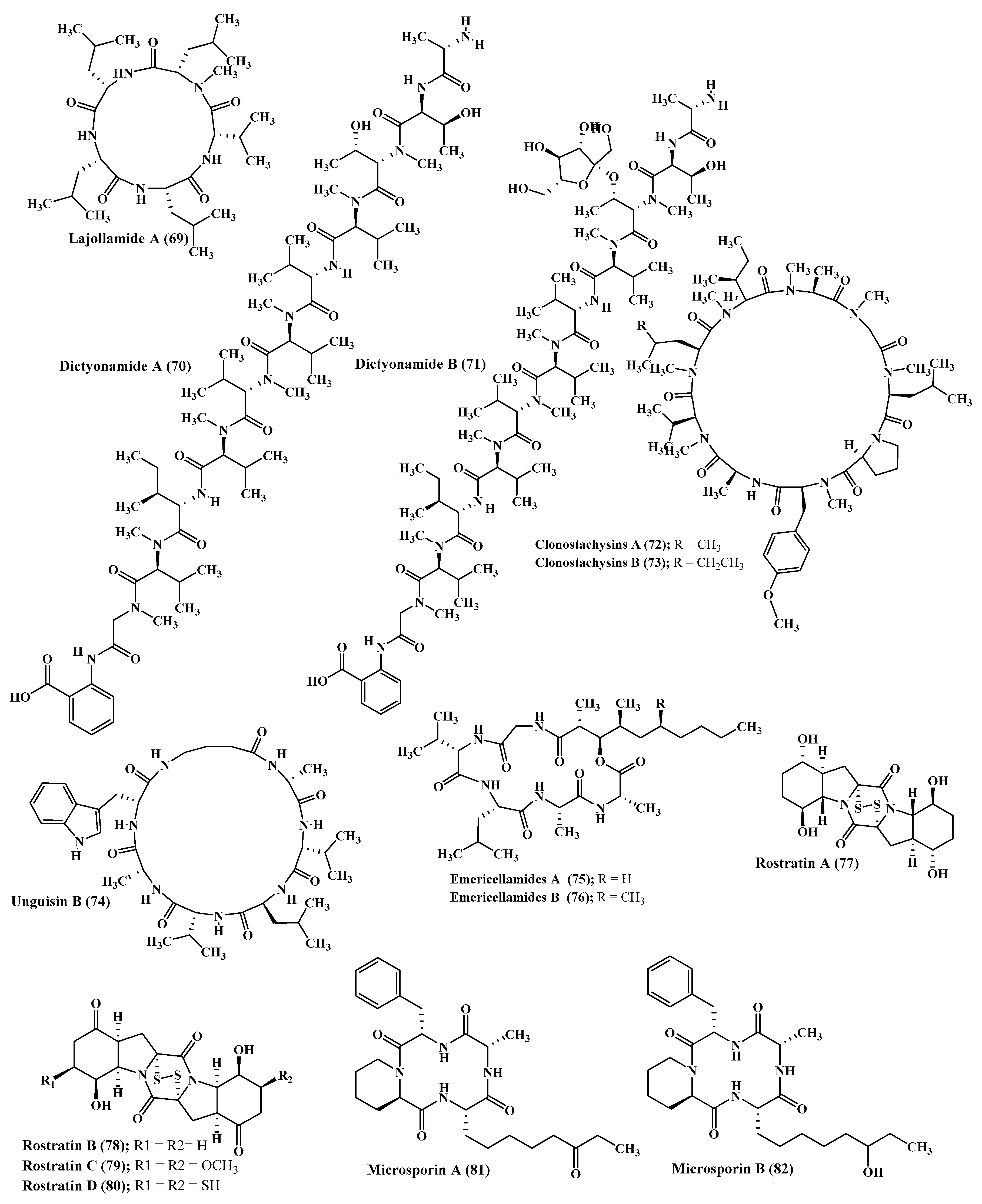
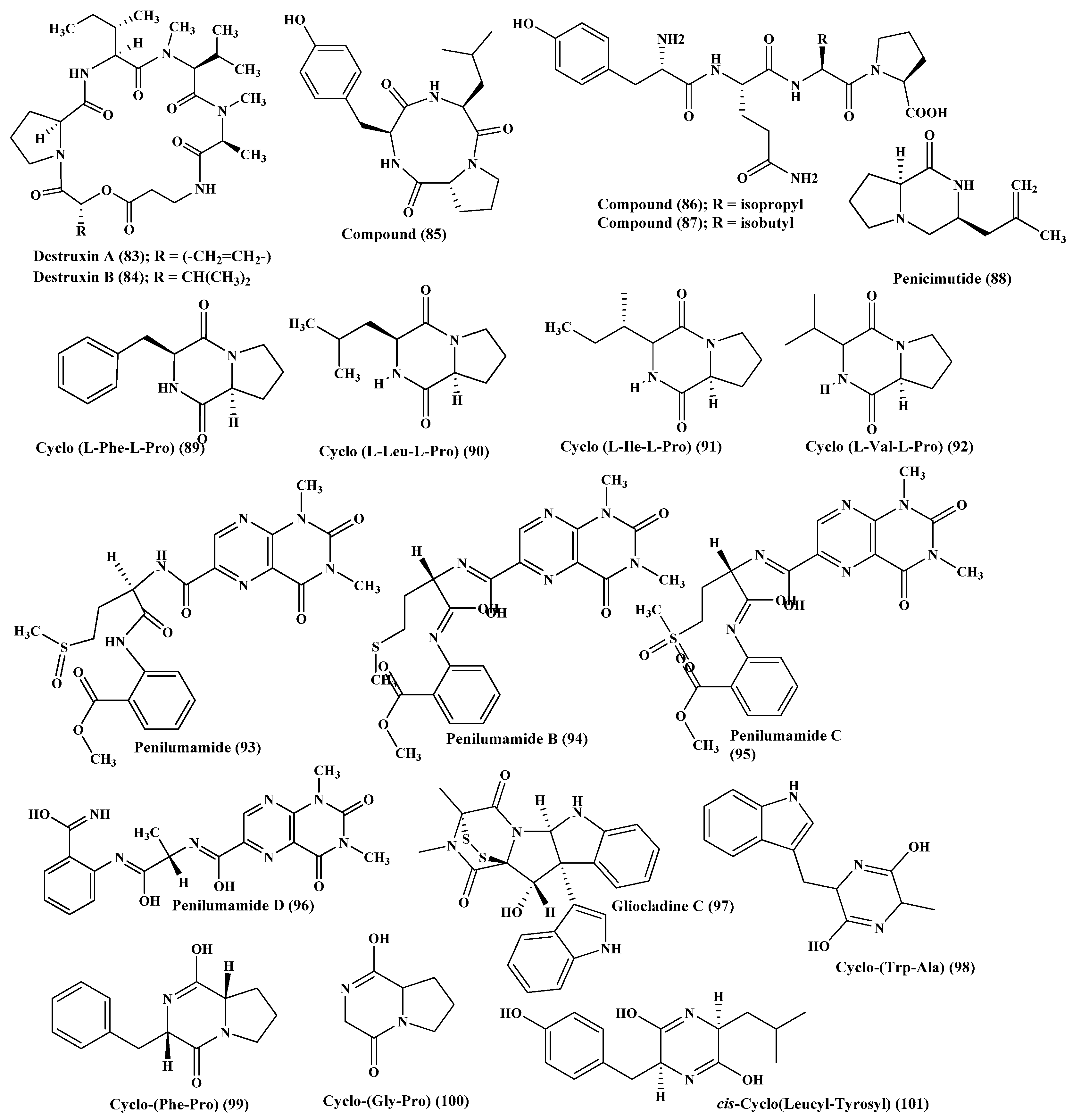
| Compound | Genus | Biological Activity | Reference |
|---|---|---|---|
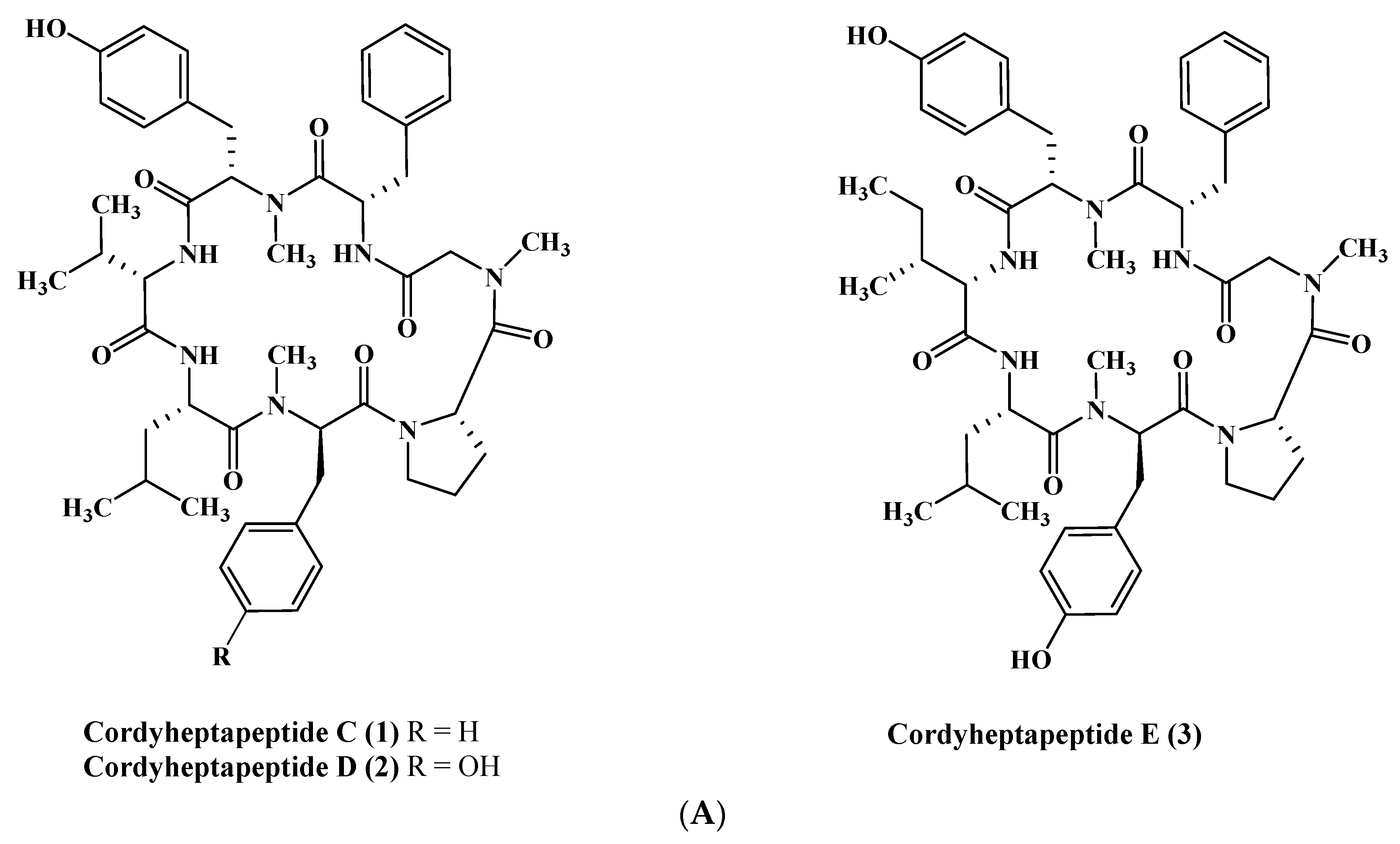
| Cordyheptapeptide C (1) | Acremonium |
| [12] |
| Cordyheptapeptide E (3) | Acremonium |
| [12] |
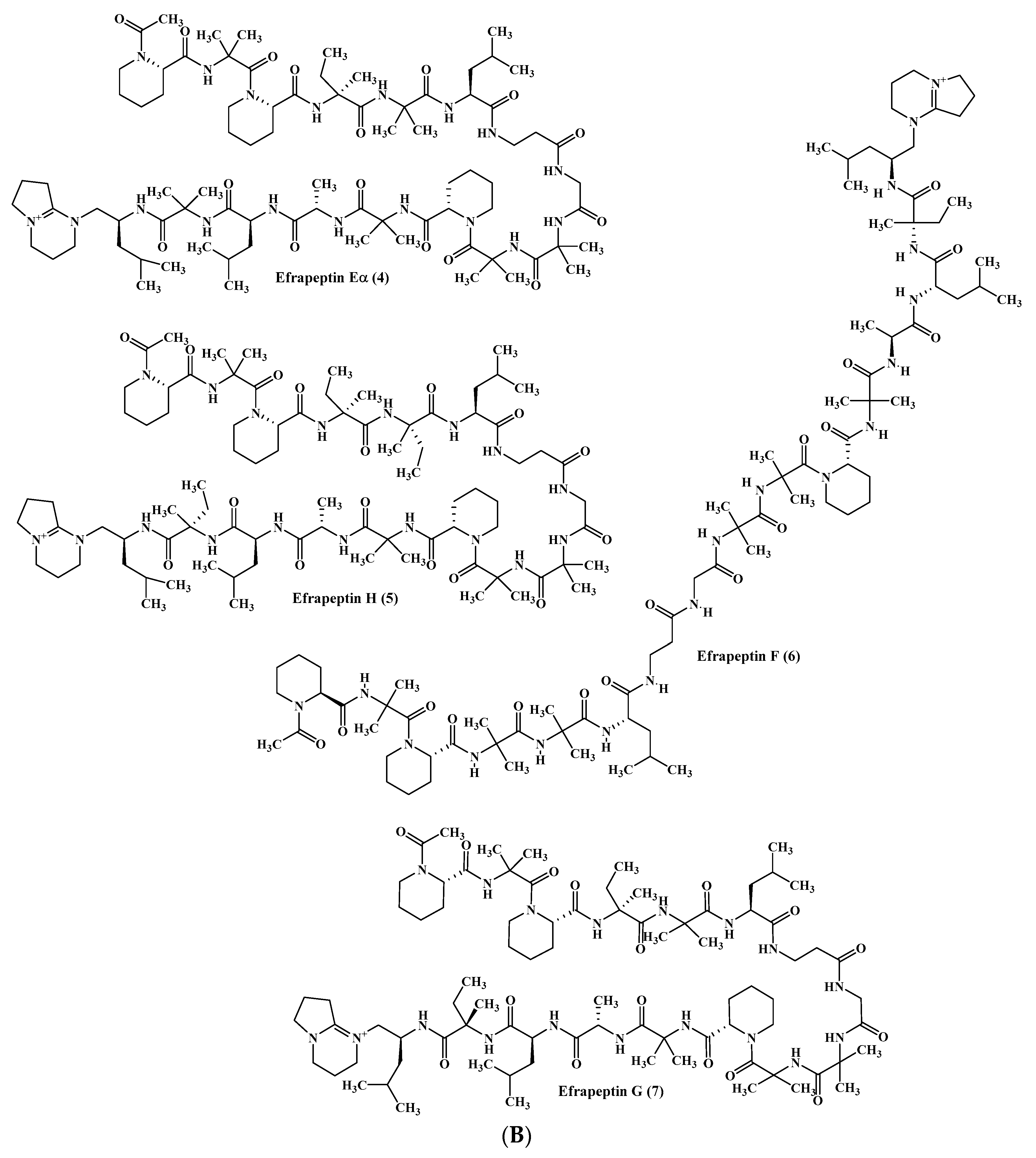
| Efrapeptin Eα (4) | Acremonium |
| [13,14] |
| Efrapeptin F (6) | Acremonium |
| [13,14] |
| Efrapeptin G (7) | Acremonium |
| [13,14] |
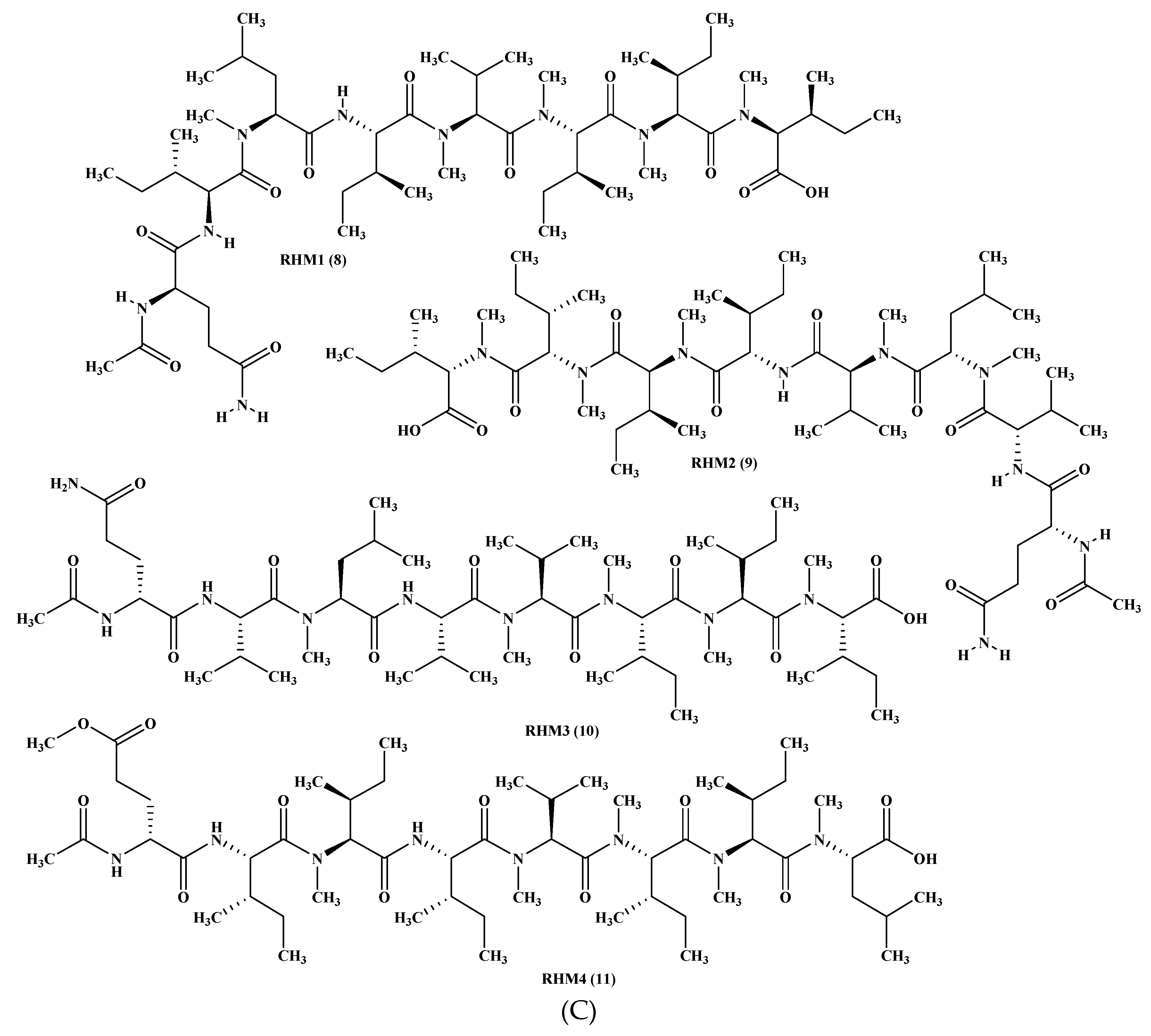
| RHM1 (8) | Acremonium |
| [13,14] |
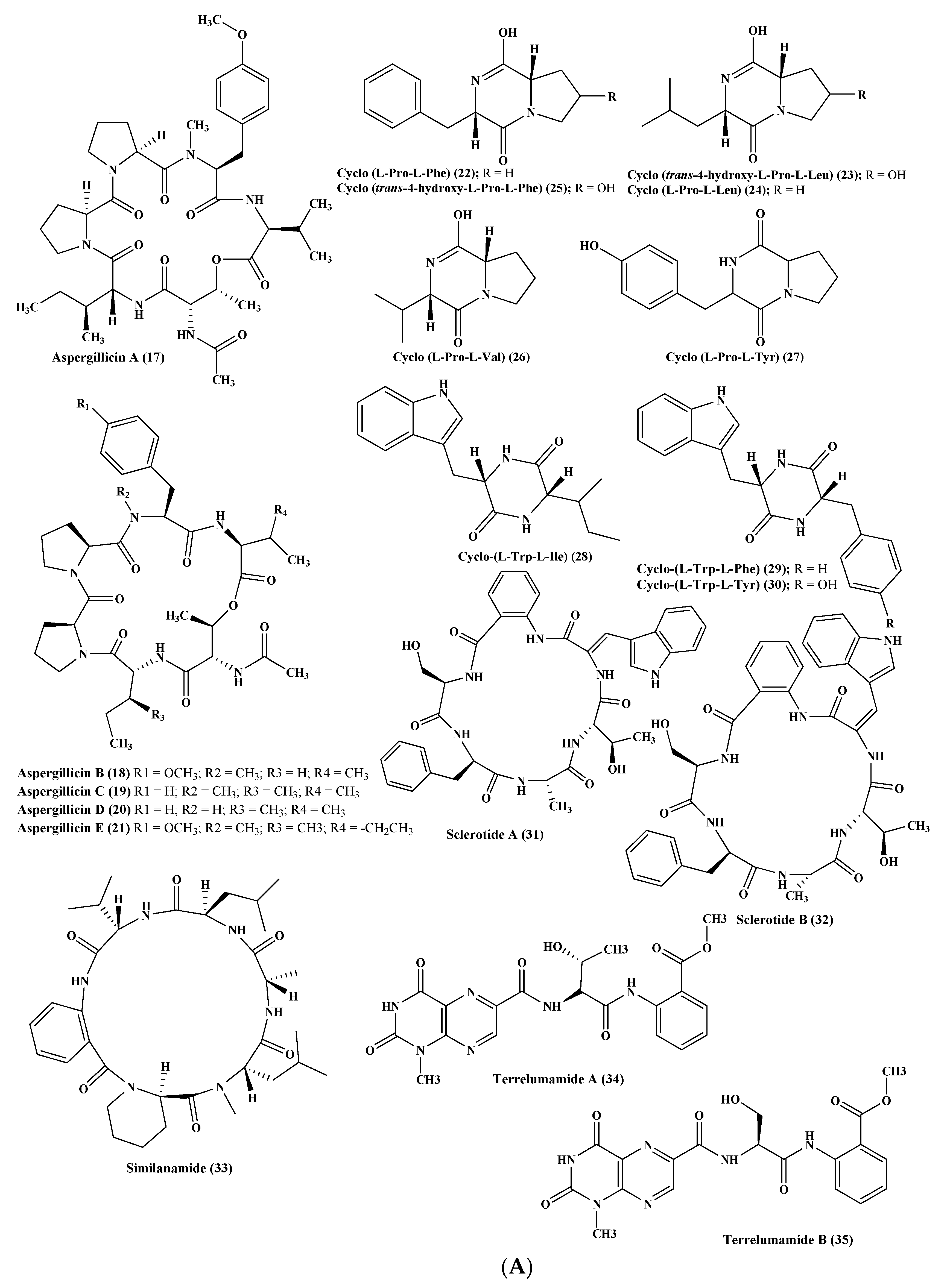
| Aspergillicins A–E (17–21) | Aspergillus |
| [11] |
| Cyclo-(L-Trp-L-Tyr) (30) | Aspergillus |
| [17] |
| Sclerotide A (31) | Aspergillus |
| [18] |
| Sclerotide B (32) | Aspergillus |
| [18] |
| Similanamide (33) | Aspergillus |
| [19] |
| Terrelumamide A (34) | Aspergillus |
| [20,21,22] |
| Terrelumamide B (35) | Aspergillus |
| [20,21,22] |
| Compound (38) | Aspergillus |
| [20,21,22]. |
| Psychrophilin E (50) | Aspergillus |
| [29] |
| Psychrophilin G (52) | Aspergillus |
| [25] |
| Aspersymmetide A (55) | Aspergillus |
| [26] |
| Cotteslosin A (57) | Aspergillus |
| [27] |
| Diketopiperazine dimer (60) | Aspergillus |
| [28] |
| Cyclic tetrapeptide (61) | Aspergillus |
| [28] |
| Aspergillipeptid D (62) | Aspergillus |
| [30] |
| Aspergillipeptide E (63) | Aspergillus |
| [30] |
| 14-Hydroxy-cyclopeptine (66) | Aspergillus |
| [31] |
| Lajollamide A (69) | Asteromyces |
| [33] |
| Dictyonamide A (70) | Ceratodictyon |
| [34] |
| Clonostachysin A (72) | Clonostachys |
| [35] |
| Clonostachysin B (73) | Clonostachys |
| [35] |
| Unguisin A (42) | Emericella |
| [37] |
| Emericellamide B (76) | Emericella |
| [37] |
| Rostratins A–D (77–80) | Exserohilum |
| [38] |
| Microsporin A (81) | Microsporum |
| [39] |
| Microsporin B (82) | Microsporum |
| [39] |
| Compound (85) | Penicillium |
| [39] |
| Compounds (86–87) | Penicillium |
| [39] |
| Penicimutide (88) | Penicillium |
| [40] |
| Gliocladine C (97) | Penicillium |
| [42] |
| cis-Cyclo (Leucyl-Tyrosyl) (101) | Penicillium |
| [43] |
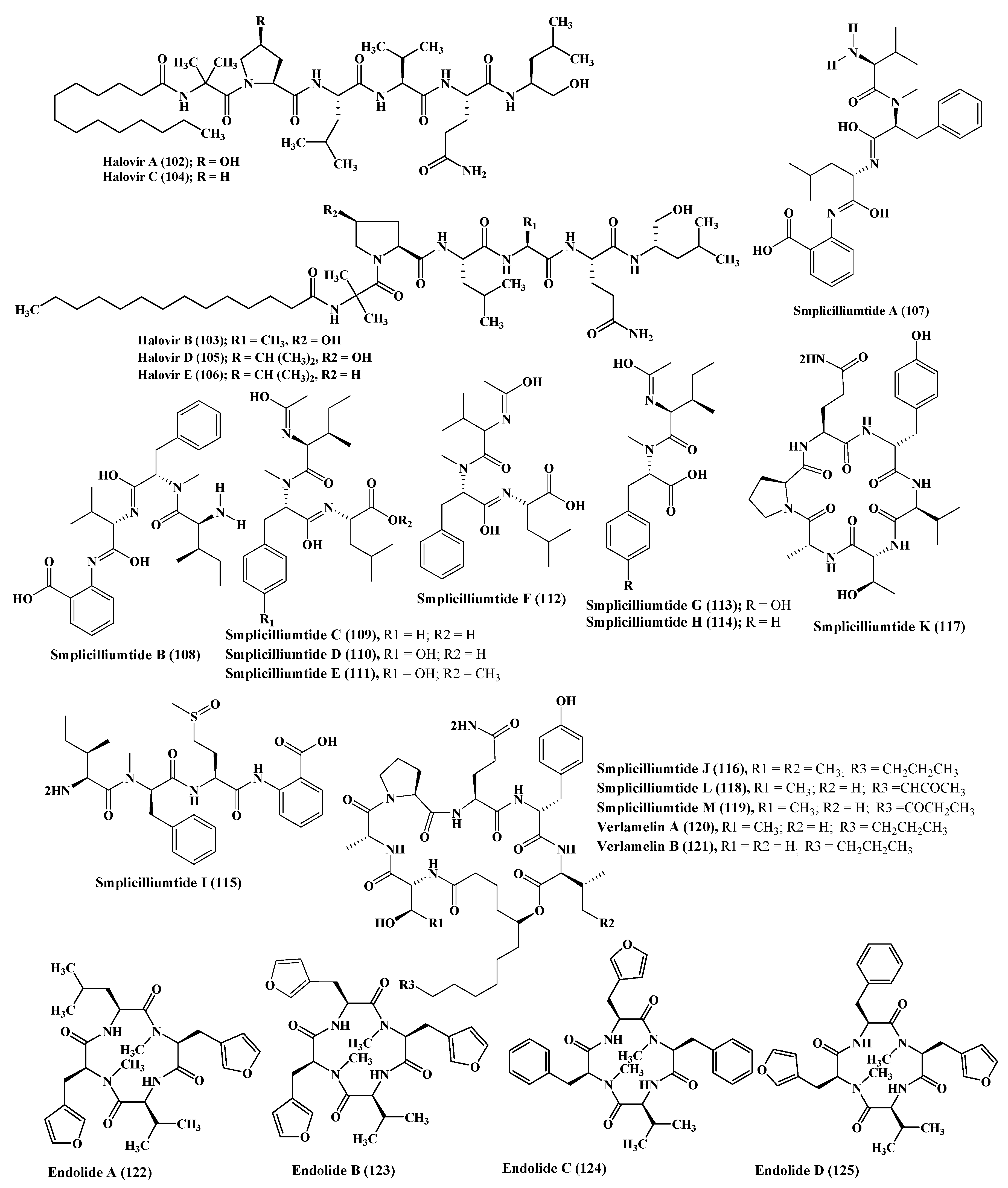
| Halovir A (102) | Scytalidium |
| [44,45] |
| Halovirs B–E (103–106) | Scytalidium |
| [44,45] |
| Simplicilliumtide A (107) | Simplicillium |
| [10,46] |
| Simplicilliumtide D (110) | Simplicillium |
| [10,46] |
| Simplicilliumtides E (111), G (113), and H (114) | Simplicillium |
| [10,46] |
| Simplicilliumtide J (116) | Simplicillium |
| [10,46] |
| Endolide A (122) | Stachylidium |
| [48] |
| Endolide B (123) | Stachylidium |
| [48] |
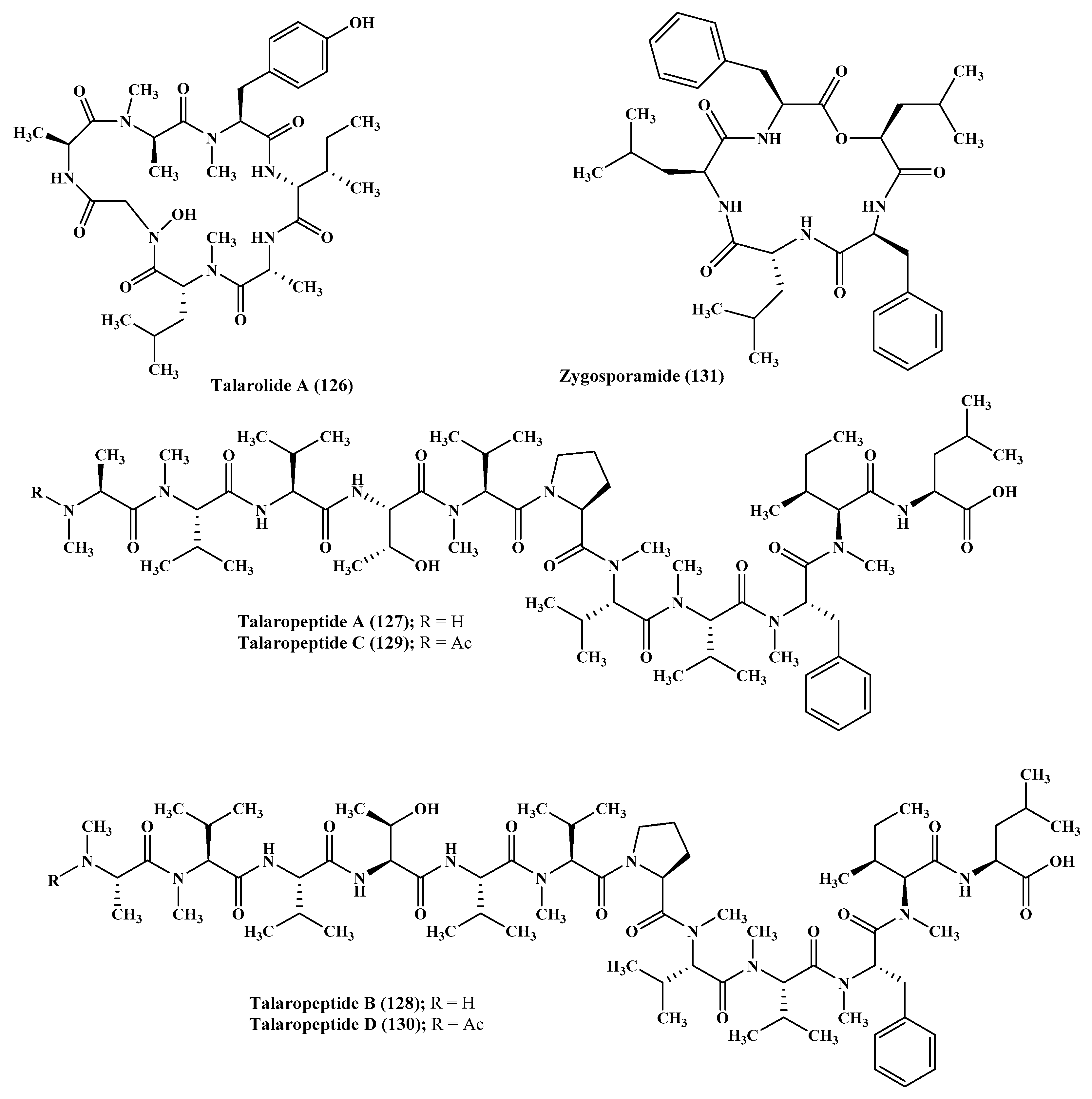
| Talaropeptide A (127) | Talaromyces |
| [49] |
| Talaropeptide B (128) | Talaromyces |
| [49] |
| Zygosporamide (131) | Zygosporium |
| [54,55] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Youssef, F.S.; Ashour, M.L.; Singab, A.N.B.; Wink, M. A Comprehensive Review of Bioactive Peptides from Marine Fungi and Their Biological Significance. Mar. Drugs 2019, 17, 559. https://doi.org/10.3390/md17100559
Youssef FS, Ashour ML, Singab ANB, Wink M. A Comprehensive Review of Bioactive Peptides from Marine Fungi and Their Biological Significance. Marine Drugs. 2019; 17(10):559. https://doi.org/10.3390/md17100559
Chicago/Turabian StyleYoussef, Fadia S., Mohamed L. Ashour, Abdel Nasser B. Singab, and Michael Wink. 2019. "A Comprehensive Review of Bioactive Peptides from Marine Fungi and Their Biological Significance" Marine Drugs 17, no. 10: 559. https://doi.org/10.3390/md17100559









