Optimization of an Ultrasound-Assisted Extraction for Simultaneous Determination of Antioxidants in Sesame with Response Surface Methodology
Abstract
1. Introduction
2. Experimental
2.1. Reagents and Standards
2.2. Preparation of Standard Solutions
2.3. Samples
2.4. Extraction Process
2.5. Optimization of the Extraction Process Parameters
2.5.1. Single Factor Experiment
2.5.2. Plackett–Burman Design
2.5.3. Box–Behnken Design
2.6. HPLC-MS/MS Conditions
2.7. Peak Identification
2.8. Statistical Analysis
3. Results and Discussion
3.1. Optimization of Extraction Conditions by Single Factor Experiments
3.1.1. Effect of Methanol Volume Fraction
3.1.2. Effect of Extraction Time
3.1.3. Effect of Liquid-Solid Ratio
3.1.4. Effect of Ultrasonic Power
3.1.5. Effect of Ultrasonic Temperature
3.1.6. Effect of Extraction Times
3.2. Selecting Important Parameters via Plackett–Burman Design
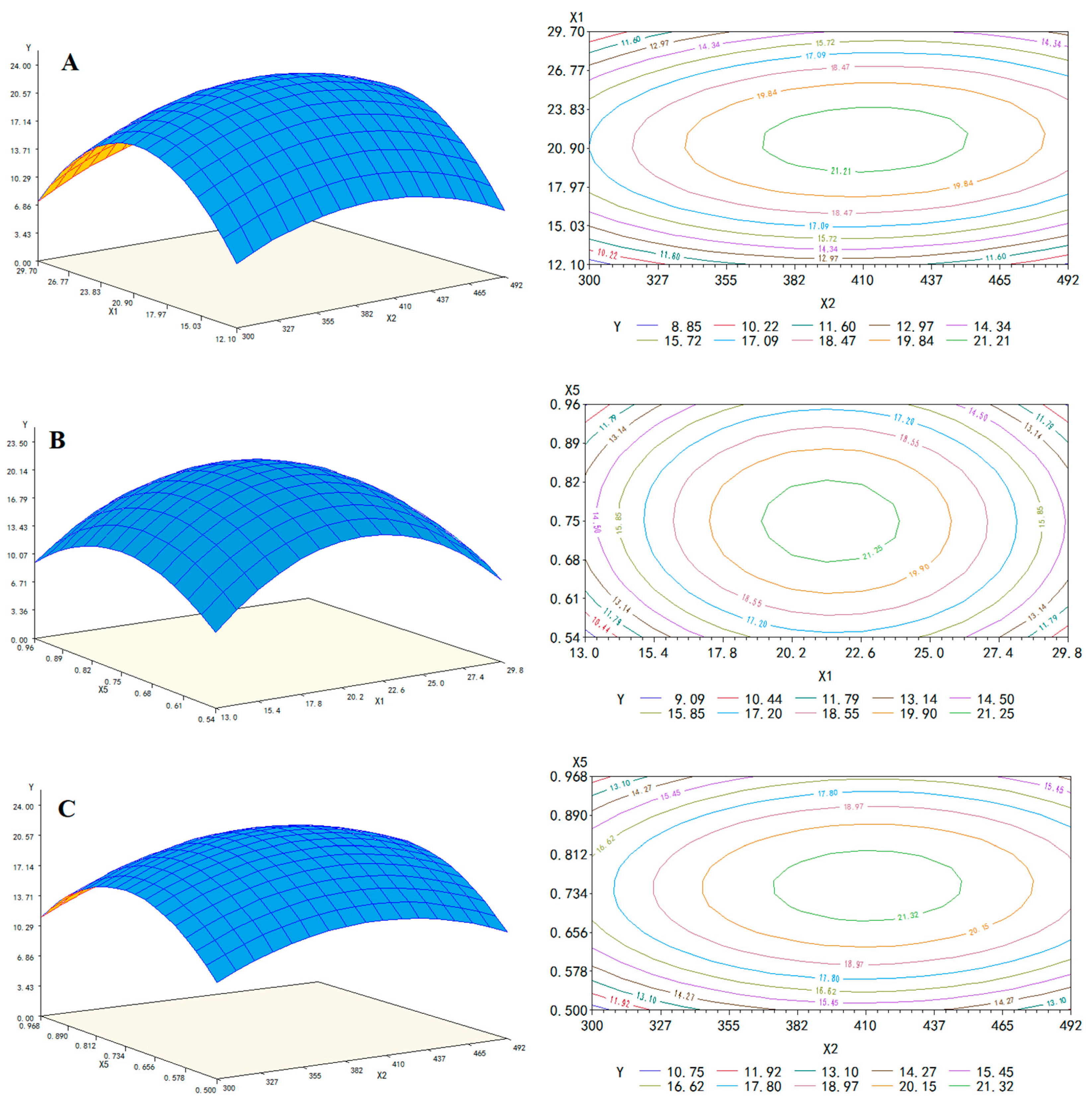
3.3. Optimizing Important Parameters by Box-Behnken Design and Response Surface Methodology
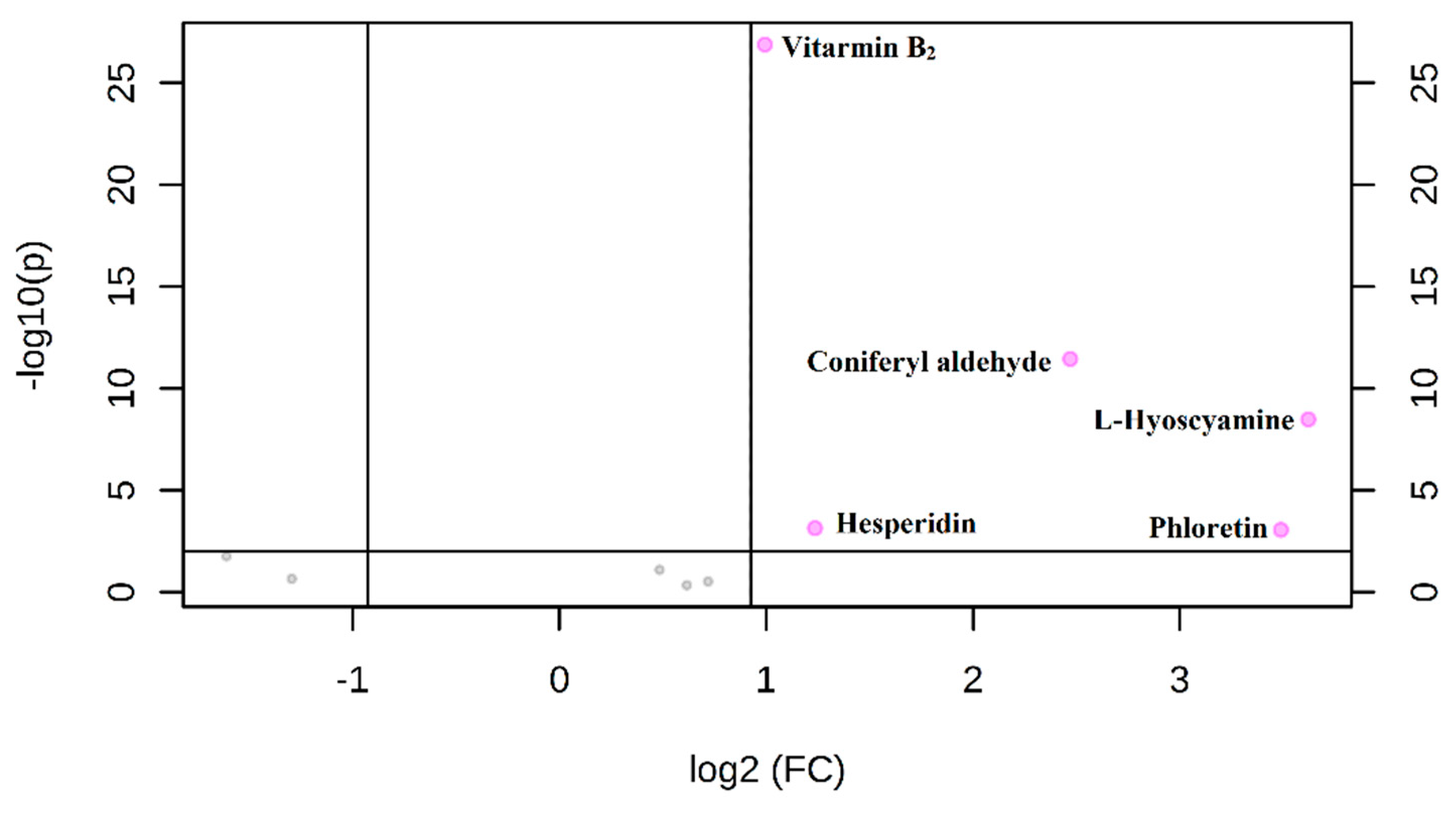
3.4. Method Validation
3.4.1. Linearity, Limit of Detection, and Limit of Quantification
3.4.2. Matrix Effect (ME)
3.4.3. Precision
3.4.4. Recovery
3.5. Application to Sesame Seeds Samples
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hu, Q.; Xu, J.; Chen, S.; Yang, F. Antioxidant activity of extracts of black sesame seed (Sesamum inicum L.) by supercritical carbon dioxide extraction. J. Agric. Food Chem. 2004, 52, 943–947. [Google Scholar] [CrossRef]
- Miyake, Y.; Fukumoto, S.; Okada, M.; Sakaide, K.; Nakamura, Y.; Osawa, T. Antioxidative catechol lignans converted from sesamin and sesaminoltriglucocide by culturing with Aspergillus. J. Agric. Food Chem. 2005, 53, 22–27. [Google Scholar] [CrossRef]
- Kiso, Y. Antioxidative roles of sesamin, a functional lignan in sesame seed, and its effect on lipid-and alcohol-metabolism in the liver: A DNA microarray study. Biofactors 2004, 21, 191–196. [Google Scholar] [CrossRef]
- Wang, D.D.; Zhang, L.X.; Huang, X.R.; Wang, X.; Yang, R.N.; Mao, J.; Wang, X.F.; Wang, X.P.; Zhang, Q.; Li, P.W. Identification of nutritional components in black sesame determined by widely targeted metabolomics and traditional Chinese medicines. Molecules 2018, 23, 1180. [Google Scholar] [CrossRef]
- Kuntic, V.; Filipovic, I.; Vujic, Z. Effects of rutin and hesperidin and their Al(III) and Cu(II) Complexes on in Vitro Plasma Coagulation Assays. Molecules 2011, 16, 1378–1388. [Google Scholar] [CrossRef]
- Suarez, J.; Herrera, M.D.; Marhuenda, E. In vitro scavenger and antioxidant properties of hesperidin and neohesperidindihydrochalcone. Phytomedicine 1998, 5, 469–473. [Google Scholar] [CrossRef]
- Nandakumar, N.; Balasubramanian, M.P. Hesperidin a citrus bioflavonoid modulates hepatic biotransformation enzymes and enhances intrinsic antioxidants in experimental breast cancer rats challenged with 7, 12-dimethylbenz (a) anthracene. J. Exp. Ther. Oncol. 2012, 9, 321–335. [Google Scholar]
- Lee, H.J.; Im, A.R.; Kim, S.M.; Kang, H.S.; Lee, J.D.; Chae, S. The flavonoid hesperidin exerts anti-photoaging effect by downregulating matrix metalloproteinase (MMP)-9 expression via mitogen activated protein kinase (MAPK)-dependent signaling pathways. BMC Complement. Altern. Med. 2018, 18, 39. [Google Scholar] [CrossRef]
- Frelin, O.; Huang, L.L.; Hasnain, G.; Jeffryes, J.G.; Ziemak, M.J.; Rocca, J.R.; Wang, B.; Rice, J.; Roje, S.; Yurgel, S.N.; et al. A directed-overflow and damage-control N-glycosidase in riboflavin biosynthesis. Biochem. J. 2015, 466, 137–145. [Google Scholar] [CrossRef]
- Sykes, M.; Croucher, J.; Smith, R.A. Proficiency testing has improved the quality of data of total vitamin B2 analysis in liquid dietary supplement. Anal. Bioanal. Chem. 2011, 400, 305–310. [Google Scholar] [CrossRef]
- Araki, A.; Yoshimura, Y.; Sakurai, T.; Umegaki, H.; Kamada, C.; Iimuro, S.; Ohashi, Y.; Ito, H. Low intakes of carotene, vitamin B-2, pantothenate and calcium predict cognitive decline among elderly patients with diabetes mellitus: The Japanese Elderly Diabetes Intervention Trial. Geriatr. Gerontol. Int. 2017, 17, 1168–1175. [Google Scholar] [CrossRef]
- Gunther, L.; Beck, R.; Xiong, G.M.; Potschka, H.; Jahn, K.; Bartenstein, P.; Brandt, T.; Dutia, M.; Dieterich, M.; Strupp, M.; et al. N-Acetyl-L-Leucine Accelerates Vestibular Compensation after Unilateral Labyrinthectomy by Action in the Cerebellum and Thalamus. PLoS ONE 2015, 10, e0120891. [Google Scholar] [CrossRef]
- Xing, Z.; Gao, S.; Duan, Y.; Han, H.B.; Li, L.; Yang, Y.; Li, Q.S. Delivery of DNAzyme targeting aurora kinase A to inhibit the proliferation and migration of human prostate cancer. Int. J. Nanomed. 2015, 10, 5715–5727. [Google Scholar]
- Cavdar, H.K.; Yanik, D.K.; Gogus, F.; Fadiloglu, S. A novel modified lipid: Enzymatic esterification of 2-monoacylglycerol with N-acetyl-l-leucine. J. Food Sci. 2018, 83, 597–604. [Google Scholar] [CrossRef]
- Hänninen, T.; Kontturi, E.; Vuorinen, T. Distribution of lignin and its coniferyl alcohol and coniferyl aldehyde groups in Piceaabies and Pinus sylvestris as observed by Raman imaging. Phytochemistry 2011, 72, 1889–1895. [Google Scholar] [CrossRef]
- Jeong, Y.J.; Jung, M.G.; Son, Y.; Jang, J.H.; Lee, Y.J.; Kim, S.H.; Ko, Y.G.; Lee, Y.S.; Lee, H.J. Coniferyl aldehyde attenuates radiation enteropathy by inhibiting cell death and promoting endothelial cell function. PLoS ONE 2015, 10, e0128552. [Google Scholar] [CrossRef]
- Shin, S.; Kum, H.; Ryu, D.; Kim, M.; Jung, E.; Park, D. Protective effects of a new phloretin derivative against UVB-induced damage in skin cell model and human volunteers. Int. J. Mol. Sci. 2014, 15, 18919–18940. [Google Scholar] [CrossRef]
- Barreca, D.; Bellocco, E.; Lagana, G.; Ginestra, G.; Bisignano, C. Biochemical and antimicrobial activity of phloretin and its glycosilated derivatives present in apple and kumquat. Food Chem. 2014, 6, 292–297. [Google Scholar] [CrossRef]
- Liu, S.Y.; Yuan, Y.J.; Zhou, Y.J.; Zhao, M.; Chen, Y.N.; Cheng, J.Q.; Lu, Y.R.; Liu, J.P. Phloretin attenuates hyperuricemia-induced endothelial dysfunction through co-inhibiting inflammation and GLUT9-mediated uric acid uptake. J. Cell. Mol. Med. 2017, 21, 2553–2562. [Google Scholar] [CrossRef]
- Lynch, C.R.; Khandekar, S.; Lynch, S.M.; DiSario, J.A. Sublingual L-hyoscyamine for duodenal antimotility during ERCP: A prospective randomized double-blinded study. Gastrointest. Endosc. 2007, 66, 748–752. [Google Scholar] [CrossRef]
- Lummis, S.C.R.; Beene, D.L.; Lee, L.W.; Lester, H.A.; Broadhurst, R.W.; Dougherty, D.A. Cis-trans isomerization at a proline opens the pore of a neurotransmitter-gated ion channel. Nature 2005, 438, 248–252. [Google Scholar] [CrossRef]
- Sturaro, G.; Cigolini, G.; Menilli, L.; Cola, F.; Di, L.R.; Tasso, A.; Conconi, M.T.; Miolo, G. Antiproliferative activity of 8-methoxypsoralen on DU145 prostate cancer cells under UVA and blue light. Photochem. Photobiol. Sci. 2017, 16, 1182–1193. [Google Scholar] [CrossRef]
- Sung, J.H.; Gim, S.A.; Koh, P.O. Ferulic acid attenuates the cerebral ischemic injury-induced decrease in Peroxiredoxin-2 and thioredoxin expression. Neurosci. Lett. 2014, 566, 88–92. [Google Scholar] [CrossRef]
- Grigonis, D.; Venskutonis, P.R.; Sivik, B.; Sandahl, M.; Eskilsson, C.S. Comparison of different extraction techniques for isolation of antioxidants from sweet grass(Hierochloeodorata). J. Supercrit. Fluids 2005, 33, 223–233. [Google Scholar] [CrossRef]
- Zheng, Y.; Xu, X.L.; Yuan, F.; Yao, M.Y.; Ji, S.L.; Huang, Z.Q.; Zhang, F. Simultaneous Analysis of Simple Coumarins and Furocoumarines in Cigarettes by Solid-Phase Extraction with Gas Chromatography-Mass Spectrometry. J. AOAC Int. 2017, 100, 1559–1564. [Google Scholar] [CrossRef]
- Huang, W.; Xue, A.; Niu, H.; Jia, Z.; Wang, J.W. Optimised ultrasonic-assisted extraction of flavonoids from Folium eucommiae and evaluation of antioxidant activity in multi-test systems in vitro. Food Chem. 2009, 114, 1147–1154. [Google Scholar] [CrossRef]
- Larrauri, J.A.; Ruperez, P.; Saura, F. Calixto Effect of drying temperature on the stability of polyphenols and antioxidant activity of red grape pomace peels. J. Agric. Food Chem. 1997, 45, 1390–1393. [Google Scholar] [CrossRef]
- Mugwagwa, L.R.; Chimphango, A.F.A. Box-Behnken design based multi-objective optimisation of sequential extraction of pectin and anthocyanins from mango peels. Carbohydr. Polym. 2019, 219, 29–38. [Google Scholar] [CrossRef]
- Wang, W.J.; Ma, X.B.; Xu, Y.T.; Cao, Y.Q.; Jiang, Z.M.; Ding, T.; Ye, X.Q.; Liu, D.H. Ultrasound-assisted heating extraction of pectin from grapefruit peel: Optimization and comparison with the conventional method. Food Chem. 2015, 178, 106–114. [Google Scholar] [CrossRef]
- Ravisankar, S.; Abegaz, K.; Awika, J.M. Structural profile of soluble and bound phenolic compounds in teff (Eragrostistef) reveals abundance of distinctly different flavones in white and brown varieties. Food Chem. 2018, 263, 265–274. [Google Scholar] [CrossRef]
- Borges, P.R.S.; Tavares, E.G.; Guimarāes, I.C.; de Paulo Rocha, R.; Araujo, A.B.S.; Nunes, E.E.; de Barros Vilas Boas, E.V. Obtaining a protocol for extraction of phenolics from açaí fruit pulp through Plackett–Burman design and response surface methodology. Food Chem. 2016, 210, 189–199. [Google Scholar] [CrossRef]
- Dhanya, G.; Swetha, S.; Nampoothiri, K.M.; Rajeev, K.S.; Ashok, P. Response surface methodology for the optimization of alpha amylase production by Bacillus amyloliquefaciens. Bioresour. Technol. 2008, 11, 4597–4602. [Google Scholar]
- Sueli, R.; Gustavo, A.S.P. Ultrasound extraction of phenolic compounds from coconut (Cocos nucifera) shell powder. J. Food Eng. 2007, 80, 869–872. [Google Scholar]
- Pascacio-Villafan, C.; Lapointe, S.; Williams, T.; Sivinski, J.; Niedz, R.; Aluja, R. Mixture-Amount design and response surface modeling to assess the effects of flavonoids and phenolic acids on developmental performance of anastrephaludens. J. Chem. Ecol. 2014, 40, 297–306. [Google Scholar] [CrossRef][Green Version]
- Rambla-Alegre, M.; Esteve-Romero, J.; Carda-Broch, S. Is it really necessary to validate an analytical method or not? That is the question. J. Chromatogr. A 2012, 1232, 101–109. [Google Scholar] [CrossRef]
- Dong, H.; Xiao, K.J. Modified QuEChERS combined with ultra high performance liquid chromatography tandem mass spectrometry to determine seven biogenic amines in Chinese traditional condiment soy sauce. Food Chem. 2017, 229, 502–508. [Google Scholar] [CrossRef]
- de Sousa, F.A.; Costa, A.I.G.; de Queiroz, M.E.L.R.; Teofilo, R.F.; Neves, A.A.; de Pinho, G.P. Evaluation of matrix effect on the GC response of eleven pesticides by PCA. Food Chem. 2012, 135, 179–185. [Google Scholar] [CrossRef]
- Wu, R.; Ma, F.; Zhang, L.X.; Li, P.W.; Li, G.M.; Zhang, Q.; Zhang, W.; Wang, X.P. Simultaneous determination of phenolic compounds in sesame oil using LC-MS/MS combined with magnetic carboxylated multi-walled carbon nanotubes. Food Chem. 2016, 204, 334–342. [Google Scholar] [CrossRef]
- Kiba, N.; Oyama, Y.; Furusawa, M. Development of a high-performance liquid chromatographic system with enzyme reactors for the determination of N-acetyl branched-chain amino acids. Talanta 1995, 42, 449–453. [Google Scholar] [CrossRef]
- Dos Santos, W.D.; Ferrarese, M.; Ferrarese Filho, O. High performance liquid chromatography method for the determination of cinnamyl alcohol dehydrogenase activity in soybean roots. Plant Physiol. Biochem. 2006, 44, 511–515. [Google Scholar] [CrossRef]
- Wang, L.B.; Mi, L.; Feng, T.; Liu, X.Y.; Zhang, S.Y. Pharmacokinetic studies of phloretin in beagle dogs plasma using LC–MS/MS. Acta Chromatogr. 2017, 29, 443–447. [Google Scholar] [CrossRef]
- Kokotkiewicz, A.; Migas, P.; Stefanowicz, J.; Luczkiewicz, M.; Krauze-Baranowska, M. Densitometric TLC analysis for the control of tropane and steroidal alkaloids in Lyciumbarbarum. Food Chem. 2017, 221, 535–540. [Google Scholar] [CrossRef]
| Name | Retention Time/min | Parent Ion | Product Ion | Collision Energy/eV | Ion Polarity | Tube Lens/V |
|---|---|---|---|---|---|---|
| d-Homoproline | 0.83 | 130.132 | 84.370 > 56.427 | 14/32 | + | 86 |
| Vitarmin B2 | 3.81 | 377.066 | 243.233 > 172.283 | 22/36 | + | 123 |
| l-Hyoscyamine | 3.88 | 290.100 | 124.303 > 93.400 | 22/31 | + | 98 |
| N-Acetyl-l-leucine | 4.21 | 174.110 | 86.398 > 128.294 | 16/7 | + | 86 |
| Hesperidin | 4.28 | 611.160 | 303.232 > 177.324 | 21/34 | + | 142 |
| Ferulic acid | 4.48 | 193.020 | 134.060 | 16 | − | 62 |
| Coniferyl aldehyde | 4.86 | 179.065 | 147.226 > 91.311 | 26/13 | + | 88 |
| Phloretin | 5.26 | 275.026 | 107.282 > 77.372 | 20/46 | + | 103 |
| 5-Methoxypsoralen | 5.80 | 217.100 | 202.156 > 89.343 | 21/46 | + | 94 |
| 8-Methoxypsoralen | 6.06 | 217.100 | 202.156 > 174.183 | 20/29 | + | 92 |
| Analytes | Linear Range (μg/kg) | Linear Equation | R2 Value | LOD (μg/kg) | LOQ (μg/kg) |
|---|---|---|---|---|---|
| d-Homoproline | 0.1–2000 | y = 43990x − 711749 | 0.9995 | 0.05 | 0.16 |
| Vitarmin B2 | 0.1–1800 | y =15838x − 408323 | 0.9993 | 0.03 | 0.09 |
| l-Hyoscyamine | 0.1–1500 | y = 91957.5x + 12035 | 0.9996 | 0.01 | 0.04 |
| N-Acetyl-l-leucine | 1.0–1800 | y = 4045.59x − 173347 | 0.9993 | 0.11 | 0.34 |
| Hesperidin | 1.0–2000 | y = 3213.93x − 44406.7 | 0.9998 | 0.02 | 0.07 |
| Ferulic acid | 50.0–2000 | y = 15.2178x − 3105.91 | 0.9998 | 0.02 | 0.06 |
| Coniferyl aldehyde | 10.0–1800 | y = 5845.91x − 143573 | 0.9993 | 0.08 | 0.25 |
| Phloretin | 0.5–1500 | y = 4079.1x − 25758 | 0.9996 | 0.07 | 0.21 |
| 5-Methoxypsoralen | 0.5–1800 | y = 6620.08x − 61279 | 0.9997 | 0.08 | 0.24 |
| 8-Methoxypsoralen | 0.5–2000 | y = 35504.8x − 951479 | 0.9990 | 0.08 | 0.25 |
| Analytes | Intra-Day Precision (RSD %, n = 3) | Inter-Day Precision (RSD %, n = 5) | Recovery (%, n = 3) | Matrix Effect (%, n = 3) |
|---|---|---|---|---|
| - | 50/200/500 μg/kg | 50/200/500 μg/kg | 50/200/500 μg/kg | 50/200/500 μg/kg |
| d-Homoproline | 3.6/1.9/4.6 | 6.7/3.9/4.1 | 117.7/114.5/118.3 | 8.2/9.4/11.2 |
| Vitamin B2 | 2.2/7.7/3.6 | 8.1/9.2/10.4 | 71.1/78.6/73.5 | −13.8/−9.2/−10.3 |
| l-Hyoscyamine | 1.9/3.2/4.4 | 5.6/3.3/8.2 | 80.9/89.1/104.8 | −6.4/−5.1/−3.8 |
| N-Acetyl-l-leucine | 5.8/8.7/9.6 | 6.3/7.1/8.4 | 100.8/109.1/90.2 | −4.5/−3.9/−8.1 |
| Hesperidin | 5.4/4.9/3.7 | 5.5/4.9/9.4 | 82.4/79.3/89.5 | −10.8/−7.4/−6.9 |
| Ferulic acid | 4.3/2.8/5.9 | 7.6/5.9/8.7 | 95.9/103.4/109.1 | 9.1/6.3/8.5 |
| Coniferyl aldehyde | 3.8/5.7/7.9 | 8.6/4.2/9.6 | 99.6/106.3/110.4 | 6.8/5.8/9.4 |
| Phloretin | 6.7/7.8/8.4 | 6.5/9.4/12.9 | 97.7/98.6/105.3 | −5.4/−4.6/−5.2 |
| 5-Methoxypsoralen | 3.9/5.8/7.4 | 8.8/5.9/6.3 | 107.5/110.8/115.1 | 8.6/9.0/10.6 |
| 8-Methoxypsoralen | 4.8/3.3/8.0 | 7.1/6.4/4.9 | 89.6/96.2/107.4 | 5.5/4.9/8.7 |
| Analytes | Black Sesame Seeds/(mg/kg) | White Sesame Seeds/(mg/kg) |
|---|---|---|
| Vitamin B2 | 0.10 ± 0.01 | 0.05 ± 0.01 |
| l-Hyoscyamine | 0.53 ± 0.08 | 0.04 ± 0.01 |
| Hesperidin | 0.13 ± 0.01 | 0.06 ± 0.01 |
| Coniferyl aldehyde | 1.99 ± 0.56 | 0.41 ± 0.15 |
| Phloretin | 5.05 ± 0.75 | 0.47 ± 0.09 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, D.; Zhang, L.; Xu, Y.; Qi, X.; Wang, X.; Wang, X.; Zhang, Q.; Li, P. Optimization of an Ultrasound-Assisted Extraction for Simultaneous Determination of Antioxidants in Sesame with Response Surface Methodology. Antioxidants 2019, 8, 321. https://doi.org/10.3390/antiox8080321
Wang D, Zhang L, Xu Y, Qi X, Wang X, Wang X, Zhang Q, Li P. Optimization of an Ultrasound-Assisted Extraction for Simultaneous Determination of Antioxidants in Sesame with Response Surface Methodology. Antioxidants. 2019; 8(8):321. https://doi.org/10.3390/antiox8080321
Chicago/Turabian StyleWang, Dandan, Liangxiao Zhang, Yueqing Xu, Xin Qi, Xuefang Wang, Xiupin Wang, Qi Zhang, and Peiwu Li. 2019. "Optimization of an Ultrasound-Assisted Extraction for Simultaneous Determination of Antioxidants in Sesame with Response Surface Methodology" Antioxidants 8, no. 8: 321. https://doi.org/10.3390/antiox8080321
APA StyleWang, D., Zhang, L., Xu, Y., Qi, X., Wang, X., Wang, X., Zhang, Q., & Li, P. (2019). Optimization of an Ultrasound-Assisted Extraction for Simultaneous Determination of Antioxidants in Sesame with Response Surface Methodology. Antioxidants, 8(8), 321. https://doi.org/10.3390/antiox8080321








