The Importance of Mineralogical Knowledge in the Sustainability of Artisanal Gold Mining: A Mid-South Peru Case
Abstract
:1. Introduction
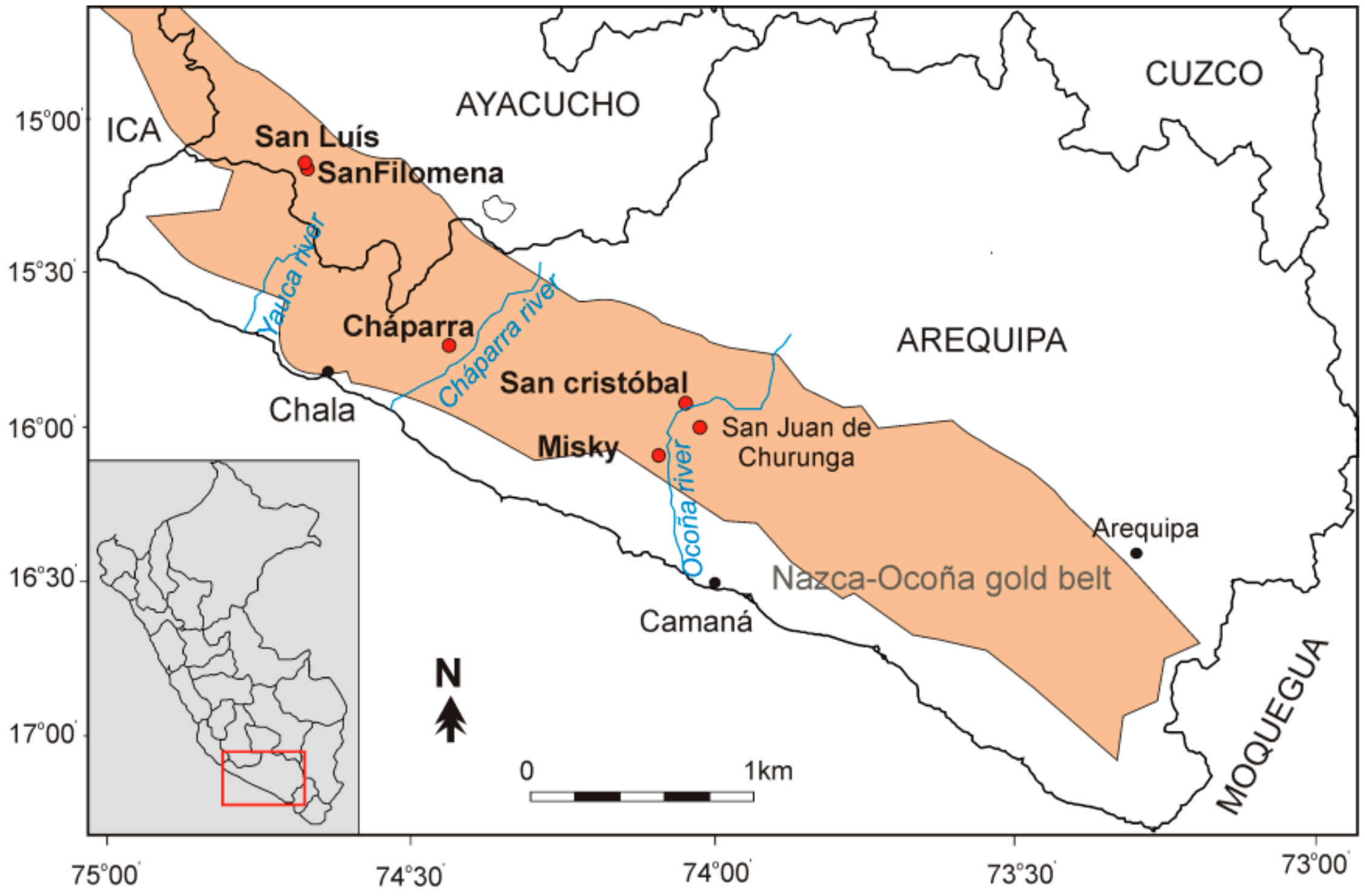
2. Mining Sites
2.1. General Characteristics
2.2. Geology
2.2.1. Geological Setting
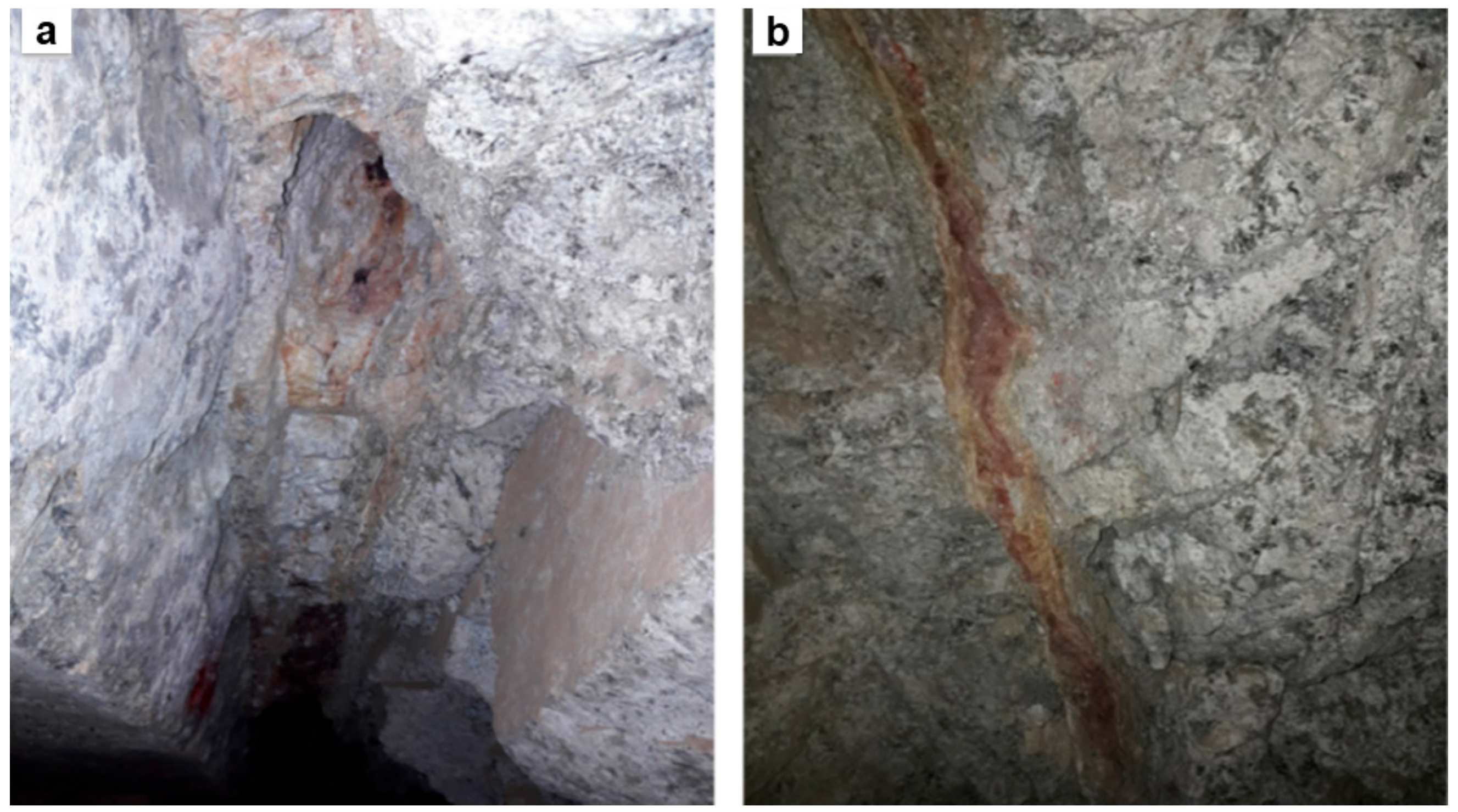
2.2.2. Gold Mineralization
2.3. Mineral Processing
3. Materials and Methods
4. Results
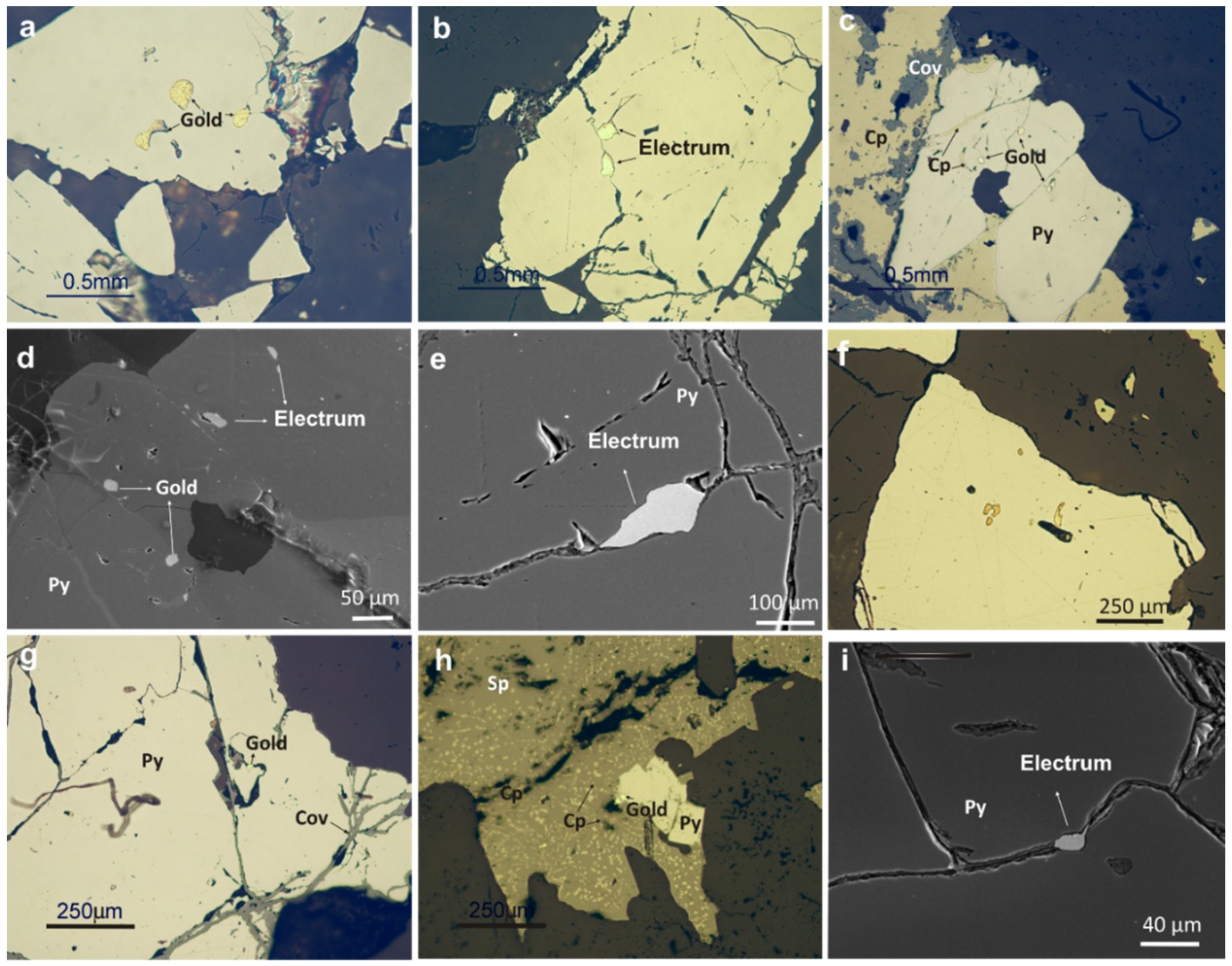
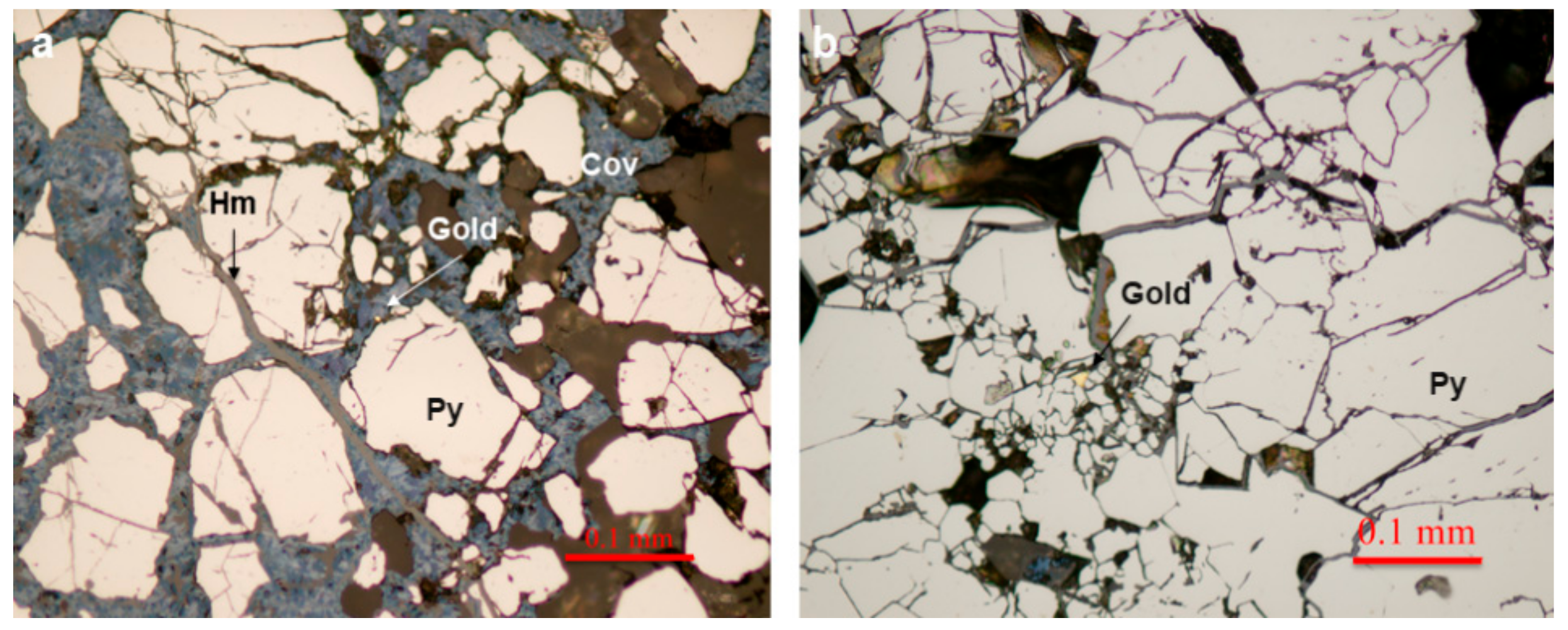
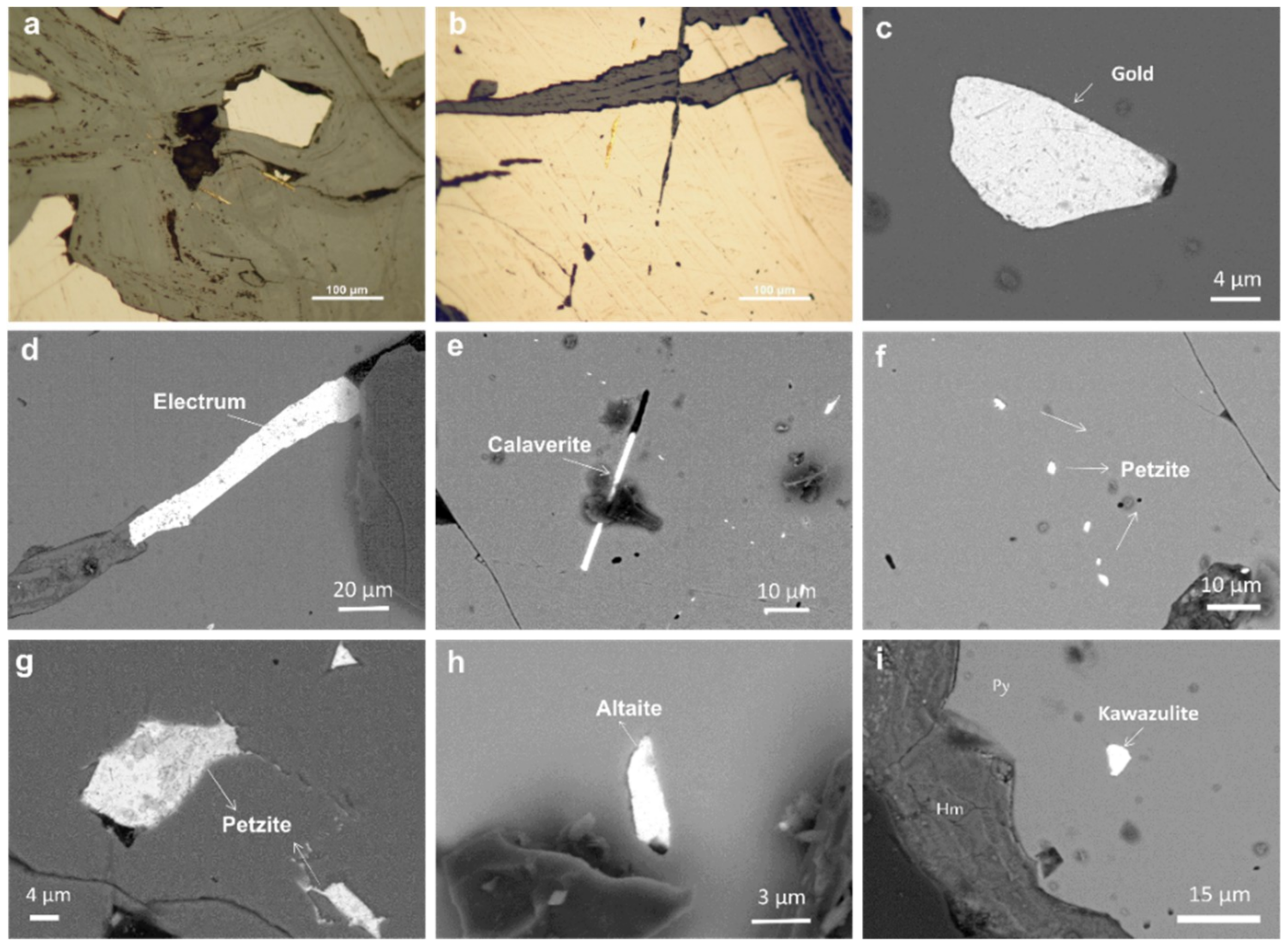
4.1. Host Vein Mineralogy
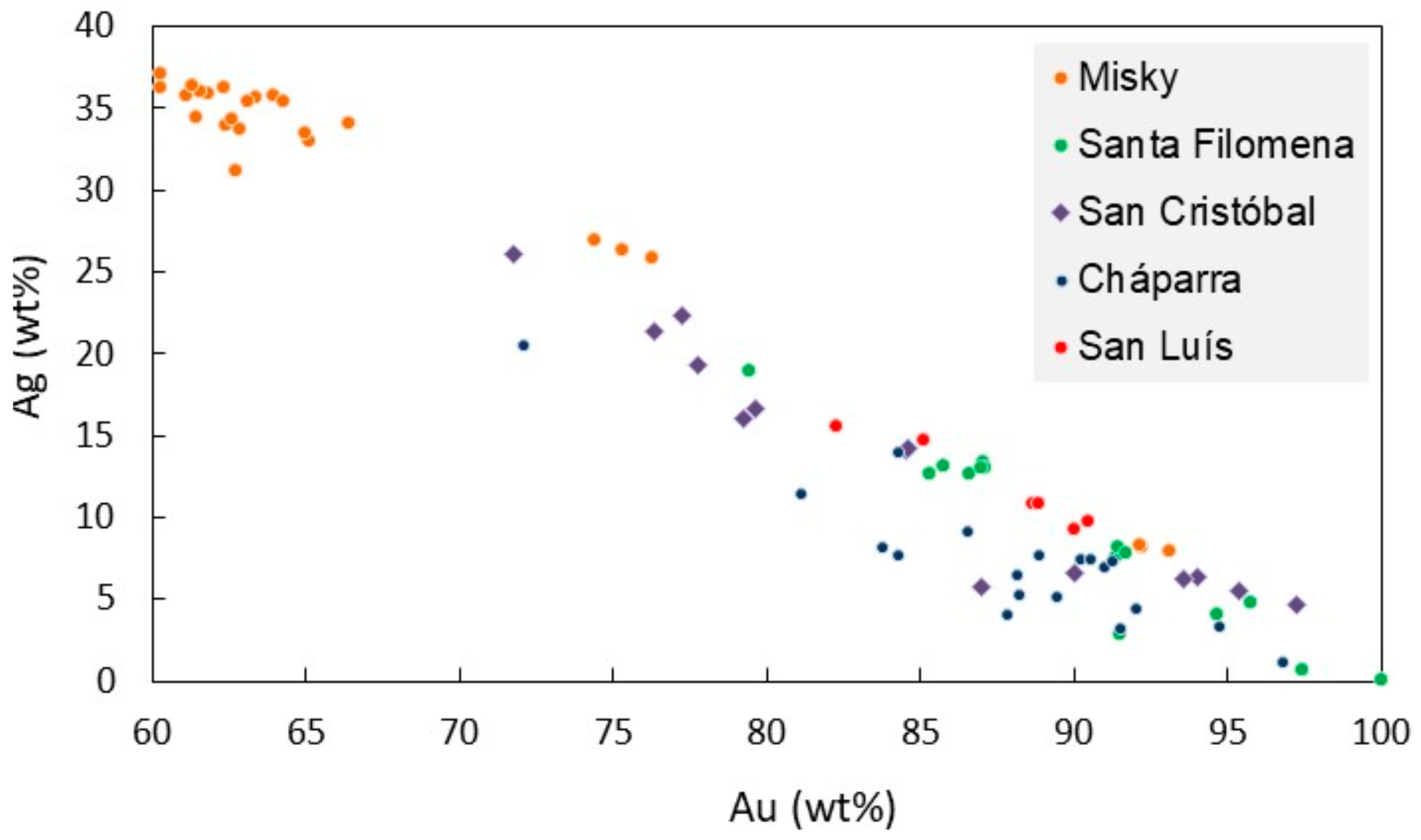
4.2. Gold Mineralogy
4.3. Geochemistry of Ores
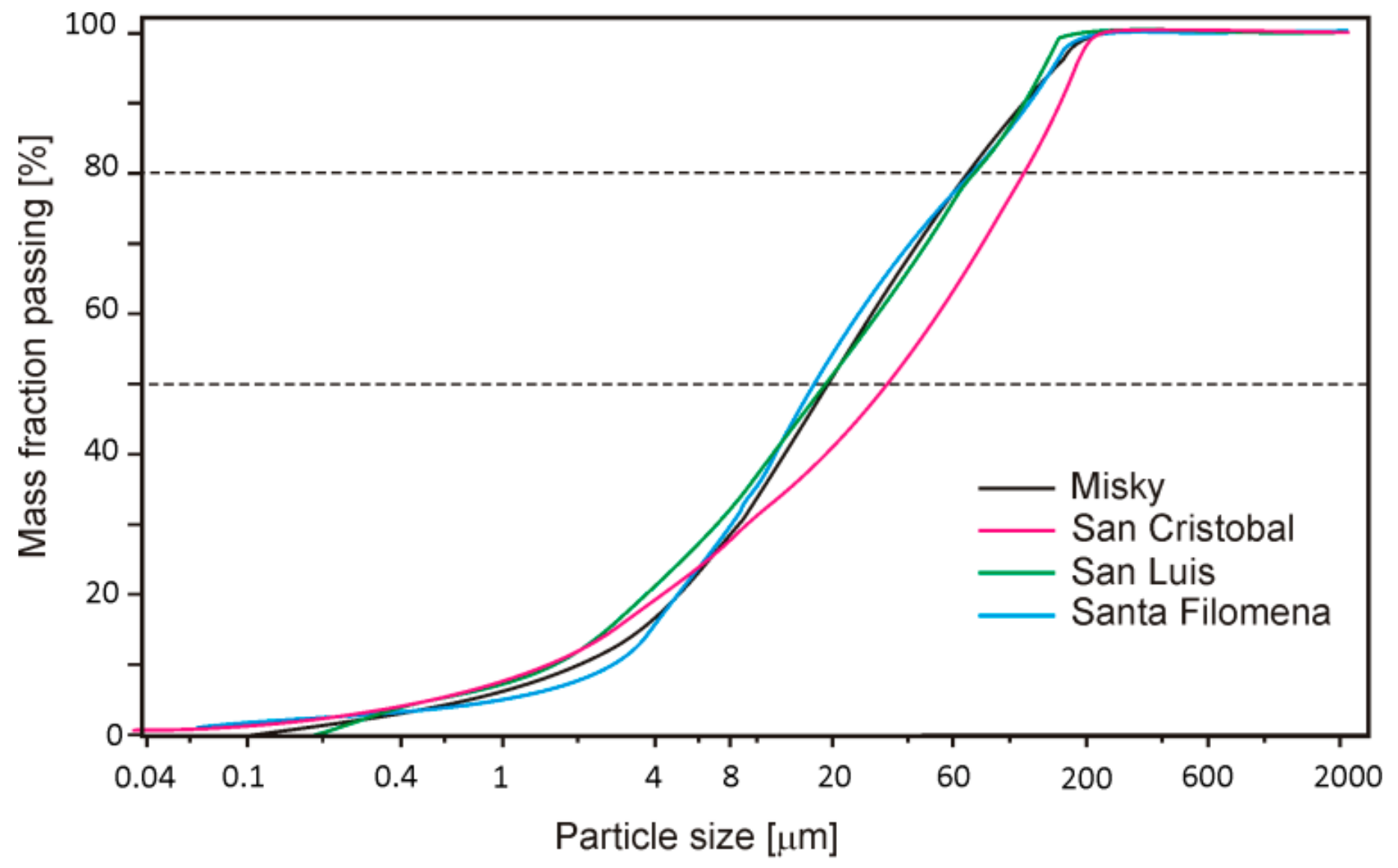
4.4. Tailings
5. Discussion
5.1. Gold Recovery
5.1.1. Processing by Amalgamation
5.1.2. Processing of Refractory Gold Ores
5.2. Clean Processing Methods for Gold Artisanal Mining
5.3. Final Recommendations
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Veiga, M.M.; Baker, R. Protocols for Environmental and Health Assessment of Mercury Released by Artisanal and Small-Scale Gold Miners; United Nations: New York, NY, USA, 2004; p. 289. [Google Scholar]
- Seccatore, J.; Veiga, M.; Origliasso, C.; Marin, T.; De Tomi, G. An estimation of the artisanal small-scale production of gold in the world. Sci. Total Environ. 2014, 496, 662–667. [Google Scholar] [CrossRef] [PubMed]
- IGF (Intergovernmental Forum on Mining, Minerals, Metals and Sustainable Development). Global Trends in Artisanal and Small-Scale Mining (ASM): A Review of Key Numbers and Issues; The International Institute for Sustainable Development: Winnipeg, MB, Canada, 2017. [Google Scholar]
- UNIDO (United Nations Industrial Development Organisation). Global Impacts of Mercury Supply and Demand in Small-Scale Gold Mining: Global Mercury Project; UNEP: Nairobi, Kenya, 2007. [Google Scholar]
- Corbett, T.; O’Faircheallaigh, C.; Regan, A. ‘Designated areas’ and the regulation of artisanal and small-scale mining. Land Use Policy 2017, 68, 393–401. [Google Scholar] [CrossRef]
- World Bank Group. Economy Profile of Peru. Doing Business 2019; World Bank: Washington, DC, USA, 2018; Available online: https://openknowledge.worldbank.org/handle/10986/30811 (accessed on 20 February 2019).
- Cortés-McPherson, D. Expansion of small-scale gold mining in Madre de Dios: “capital interests” and the emergence of a new elite of entrepreneurs in the Peruvian Amazon. Extr. Ind. Soc. 2019. [Google Scholar] [CrossRef]
- Valdivia, S.M.; Ugaya, C.M. Life Cycle Inventories of Gold Artisanal and Small-Scale Mining Activities in Peru: Toward Indicators for South America. J. Ind. Ecol. 2011, 15, 922–936. [Google Scholar] [CrossRef]
- Hilson, G.; Maconachie, R. Formalising artisanal and small-scale mining: Insights, contestations and clarifications. Area 2017, 49, 443–451. [Google Scholar] [CrossRef]
- UNEP. Analysis of Formalization Approaches in the Artisanal and Small-Scale Gold Mining Sector Based on Experiences in Ecuador, Mongolia, Peru, Tanzania and Uganda. Peru Case Study; Report; UNEP: Nairobi, Kenya, 2012; 33p. [Google Scholar]
- Veiga, M.M.; Angeloci, G.; Ñiquen, W.; Seccatore, J. Reducing mercury pollution by training Peruvian artisanal gold miners. J. Clean. Prod. 2015, 94, 268–277. [Google Scholar] [CrossRef]
- Sousa, R.N.; Veiga, M.M.; Klein, B.; Telmer, K.; Gunson, A.J.; Bernaudat, L. Strategies for reducing the environmental impact of reprocessing mercury-contaminated tailings in the artisanal and small-scale gold mining sector: Insights from Tapajos River Basin, Brazil. J. Clean. Prod. 2010, 18, 1757–1766. [Google Scholar] [CrossRef]
- Stocklin-Weinberg, R.; Veiga, M.M.; Marshall, B.G. Training artisanal miners: A proposed framework with performance evaluation indicators. Sci. Total Environ. 2019, 660, 1533–1541. [Google Scholar] [CrossRef]
- Keovilignavong, O. Mining governance dilemma and impacts: A case of gold mining in Phu-Hae, Lao PDR. Resour. Policy 2019, 61, 141–150. [Google Scholar] [CrossRef]
- Hinton, J.J.; Veiga, M.M.; Veiga, A.T.C. Clean artisanal gold mining: A utopian approach? J. Clean. Prod. 2003, 11, 99–115. [Google Scholar] [CrossRef]
- Labonne, B. Who is afraid of artisanal and small-scale mining (ASM)? Extr. Ind. Soc. 2014, 1, 121–123. [Google Scholar] [CrossRef]
- Laurence, D. Establishing a sustainable mining operation: An overview. J. Clean. Prod. 2011, 19, 278–284. [Google Scholar] [CrossRef]
- McMahon, G.; Evia, J.L.; Pascó-Font, A.; Sánchez, J.M. An Environmental Study of Artisanal, Small, and Medium Mining in Bolivia, Chile, and Peru; Technical Paper No. 429; The World Bank: Washington, DC, USA, 1999. [Google Scholar]
- Loredo, J.; Soto, J.; Ordonez, A.; Alvarez, R. Mercury and arsenic pollution associated to artisanal gold mining in Huanca (Ayacucho Department, Peru). Fresenius Environ. Bull. 2009, 18, 391–398. [Google Scholar]
- Cordy, P.; Veiga, M.M.; Salih, I.; Al-Saadi, S.; Console, S.; Garcia, O.; Mesa, L.A.; Velásquez-López, P.C.; Roeser, M. Mercury contamination from artisanal gold mining in Antioquia, Colombia: The world’s highest per capita mercury pollution. Sci. Total Environ. 2011, 410, 154–160. [Google Scholar] [CrossRef] [PubMed]
- Gibb, H.; O’Leary, K.G. Mercury exposure and health impacts among individuals in the artisanal and small-scale gold mining community: A comprehensive review. Environ. Health Perspect. 2014, 122, 667–672. [Google Scholar] [CrossRef] [PubMed]
- Zolnikov, T.R.; Ramírez Ortiz, D. A systematic review on the management and treatment of mercury in artisanal gold mining. Sci. Total Environ. 2018, 633, 816–824. [Google Scholar] [CrossRef] [PubMed]
- Esdaile, L.; Chalker, J.M. The Mercury Problem in Artisanal and Small-Scale Gold Mining. Chem. Eur. J. 2018, 24, 6905–6916. [Google Scholar] [CrossRef]
- Seccatore, J.; de Tomi, G.; Veiga, M. Efficiency as a road to sustainability in small scale mining. Mater. Sci. Forum 2015, 805, 395–402. [Google Scholar] [CrossRef]
- Kuramoto, J.R. Artisanal and Informal Mining in Peru; Report; International Institute for Environment and Development: Washington, DC, USA, 2001. [Google Scholar]
- Harris, D.D. The Mineralogy of gold and its relevance to gold recoveries. Miner. Deposita 1990, 25, S3–S7. [Google Scholar] [CrossRef]
- Goodall, W.R.; Scales, P.J.; Butcher, A.R. The use of QEMSCAN and diagnostic leaching in the characterisation of visible gold in complex ores. Miner. Eng. 2015, 18, 877–886. [Google Scholar] [CrossRef]
- Chryssoulis, S.L.; McMullen, J. Mineralogical investigation of gold ores. Dev. Miner. Process. 2005, 15, 21–71. [Google Scholar]
- Gorain, B.K.; Kondos, P.D.; Lakshmanan, V.I. Innovations in gold and silver processing. In Innovative Process Development in Metallurgical Industry; Springer: Cham, Switzerland, 2016; pp. 393–428. [Google Scholar]
- Sykora, S.; Cooke, D.R.; Meffre, S.; Stephanov, A.S.; Gardner, K.; Scott, R.; Selley, D.; Harris, A.C. Evolution of pyrite trace element compositions from porphyry-style and epithermal conditions at the Lihir gold deposit: Implications for ore genesis and mineral processing. Econ. Geol. 2018, 113, 193–208. [Google Scholar] [CrossRef]
- Coetzee, L.L.; Theron, S.J.; Martin, G.J.; Van der Merwe, J.D.; Stanek, T.A. Modern gold deportments and its application to industry. Miner. Eng. 2011, 24, 565–575. [Google Scholar] [CrossRef]
- Cook, N.J.; Chryssoulis, S.L. Concentrations of invisible gold in the common sulphides. Can. Miner. 1990, 28, 1–16. [Google Scholar]
- Vaughan, J.P. The process mineralogy of gold: The classification of ore types. JOM 2004, 56, 46–48. [Google Scholar] [CrossRef]
- Petruk, W. Applied Mineralogy in the Mining Industry; Elsevier: Amsterdam, The Netherlands, 2000. [Google Scholar]
- Childs, J. From ‘criminals of the earth’ to ‘stewards of the environment’: The social and environmental justice of Fair Trade gold. Geoforum 2014, 57, 129–137. [Google Scholar] [CrossRef]
- Fritz, M.M.; Maxson, P.A.; Baumgartner, R.J. The mercury supply chain, stakeholders and their responsibilities in the quest for mercury-free gold. Resour. Policy 2016, 50, 177–192. [Google Scholar] [CrossRef] [Green Version]
- Cobbing, E.J.; Pitcher, W.S.; Taylor, W.P. Segments and super-units in the Coastal Batholith of Peru. The J. Geol. 1977, 85, 625–631. [Google Scholar] [CrossRef]
- Cobbing, E.J. The segmented coastal Batholith of Peru: Its relationship to volcanicity and metallogenesis. Earth Sci. Rev. 1982, 18, 241–251. [Google Scholar] [CrossRef]
- Vargas, A.R. Estudio geológico-minero de la faja aurífera Nazca-Ocoña; Technical Report; Ingemmet: Lima, Peru, 1978; p. 179. [Google Scholar]
- Vidal, C.E. Metallogenesis associated with the Coastal Batholith of Peru: A review. In Magmatism at a plate edge. The Peruvian Andes; Blckie & Son Ltd.: Glasgow, UK, 1985; pp. 243–249. [Google Scholar]
- Steinmüller, K. Depósitos Metálicos en el Perú. Su Metalogénia, Sus Modelos, su Exploración y el Medio Ambiente; Ingemmet: Lima, Peru, 1999; p. 171. [Google Scholar]
- Acosta, J.; Quispe, J.; Rivera, R.; Valencia, M.; Chirif, H.; Huanacuni, D.; Rodríguez, I.; Villarreal, E.; Paico, D.; Santisteban, A. Mapa Metalogenético del Oro en el Perú; INGEMET: Lima, Peru, 2010. [Google Scholar]
- Sillitoe, R.H.; Thompson, J.F. Intrusion–Related Vein Gold Deposits: Types, Tectono-Magmatic Settings and Difficulties of Distinction from Orogenic Gold Deposits. Resour. Geol. 1998, 48, 237–250. [Google Scholar] [CrossRef]
- Cardozo, M.; Cedillo, E. Geologic-metallogenetic evolution of the Peruvian Andes. In Stratabound ore Deposits in the Andes; Springer: Berlin/Heidelberg, Germany, 1990; pp. 37–60. [Google Scholar]
- Acosta, J. Características metalogénicas de los yacimientos asociados a los arcos magmáticos mesozoicos y cenozoicos del sur del Perú (Latitudes 14°–16°S); INGEMET: Lima, Peru, Unpublished Technical Report; 2006. [Google Scholar]
- Palacios, S.; Alfonso, P.; mata-Perelló, J.M. Caracterización del Yacimiento de Oro de Misky, Sur del Perú. Macla 2011, 15, 159–160. [Google Scholar]
- Dominy, S.; O’Connor, L.; Glass, H.; Purevgerel, S.; Xie, Y. Towards Representative Metallurgical Sampling and Gold Recovery Testwork Programmes. Minerals 2018, 8, 193. [Google Scholar] [CrossRef]
- Whitehouse, A.E.; Posey, H.H.; Gillis, T.D.; Long, M.B.; Mulyana, A.A.S. Mercury discharges from small scale gold mines in North Sulawesi, Indonesia: Managing a change from mercury to cyanide. In Proceedings of the 7th International Conference on Acid Rock Drainage (ICARD), St. Louis, MO, USA, 26–30 March 2006; Volume 26, pp. 2354–2368. [Google Scholar]
- Hylander, L.D.; Plath, D.; Miranda, C.R.; Lücke, S.; Öhlander, J.; Rivera, A.T.F. Comparison of Different Gold Recovery Methods with Regard to Pollution Control and Efficiency. Clean Soil Air Water 2007, 35, 52–61. [Google Scholar] [CrossRef]
- Velásquez-López, P.C.; Veiga, M.M.; Klein, B.; Shandro, J.A.; Hall, K. Cyanidation of mercury-rich tailings in artisanal and small-scale gold mining: Identifying strategies to manage environmental risks in Southern Ecuador. J. Clean. Prod. 2011, 19, 1125–1133. [Google Scholar] [CrossRef]
- Krisnayanti, B.D.; Anderson, C.W.; Utomo, W.H.; Feng, X.; Handayanto, E.; Mudarisna, N.; Ikram, H.; Khususiah. Assessment of environmental mercury discharge at a four-year-old artisanal gold mining area on Lombok Island, Indonesia. J. Environ. Monit. 2012, 14, 2598–2607. [Google Scholar] [CrossRef] [PubMed]
- Veiga, M.M.; Angeloci, G.; Hitch, M.; Colón, P.; Velásquez-López, P.C. Processing centres in artisanal gold mining. J. Clean. Prod. 2014, 64, 535–544. [Google Scholar] [CrossRef]
- La Brooy, S.R.; Linge, H.G.; Walker, G.S. Review of gold extraction from ores. Miner. Eng. 1994, 7, 1213–1241. [Google Scholar] [CrossRef]
- Kyle, J.H.; Breuer, P.L.; Bunney, K.G.; Pleysier, R. Review of trace toxic elements (Pb, Cd, Hg, As, Sb, Bi, Se, Te) and their deportment in gold processing: Part II: Deportment in gold ore processing by cyanidation. Hydrometallurgy 2012, 111, 10–21. [Google Scholar] [CrossRef]
- González-Anaya, J.A.; Nava-Alonso, F.; Pecina-Treviño, E.T. Gold recovery optimization of a refractory concentrate by ultrafine grinding—A laboratory study. Min. Metall. Explor. 2011, 28, 94–101. [Google Scholar] [CrossRef]
- Dyer, L.G.; Sauber, M.; Dixon, D.G.; Asselin, E. On the refractory nature of precious metal tellurides. Hydrometallurgy 2017, 169, 488–495. [Google Scholar] [CrossRef]
- Lorenzen, L. Some guidelines to the design of a diagnostic leaching experiment. Miner. Eng. 1995, 8, 247–256. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, Y.; Richmond, W.; Wang, H.P. Processing technologies for gold-telluride ores. Int. J. Min. Met. Mater. 2010, 17, 1–10. [Google Scholar] [CrossRef]
- Jayasekera, S.; Jayasekera, I.M.; Ritchie, J. Avraamides. Prospects for the direct leaching of gold tellurides—Recent developments. In Proceedings of the World Gold ’91, Cairns, Parkville, Australia, 21–25 April 1991; pp. 181–183. [Google Scholar]
- Venter, D.; Chryssoulis, S.L.; Mulpeter, T. Using mineralogy to optimize gold recovery by direct cyanidation. JOM 2004, 56, 53–56. [Google Scholar] [CrossRef]
- Azizi, A.; Petre, C.F.; Olsen, C.; Larachi, F. Electrochemical behavior of gold cyanidation in the presence of a sulphide-rich industrial ore versus its major constitutive sulphide minerals. Hydrometallurgy 2010, 101, 108–119. [Google Scholar] [CrossRef]
- Kim, R.; Ghahreman, A. The effect of ore mineralogy on the electrochemical gold dissolution behavior in various cyanide and oxygen concentrations; Effect of sulfidic ores containing heavy metals. Hydrometallurgy 2019, 184, 75–87. [Google Scholar] [CrossRef]
- Malloch, K.R.; Craw, D. Comparison of contrasting gold mine processing residues in a temperate rain forest, New Zealand. Appl. Geochem. 2017, 84, 61–75. [Google Scholar] [CrossRef]
- Velásquez-López, P.C.; Veiga, M.M.; Hall, K. Mercury balance in amalgamation in artisanal and small-scale gold mining: Identifying strategies for reducing environmental pollution in Portovelo-Zaruma, Ecuador. J. Clean. Prod. 2010, 18, 226–232. [Google Scholar] [CrossRef]
- Drace, K.; Kiefer, A.M.; Veiga, M.M.; Williams, M.K.; Ascari, B.; Knapper, K.A.; Logan, K.M.; Breslin, V.M.; Skidmore, A.; Bolt, D.A.; et al. Mercury-free, small-scale artisanal gold mining in Mozambique: Utilization of magnets to isolate gold at clean tech mine. J. Clean. Prod. 2012, 32, 88–95. [Google Scholar] [CrossRef]
- Davies, G.R. A toxic free future: Is there a role for alternatives to mercury in small-scale gold mining? Futures 2014, 62, 113–119. [Google Scholar] [CrossRef]
- Aylmore, M.G. Alternative lixiviants to cyanide for leaching gold ores. In Gold Ore Processing; Elsevier: Amsterdam, The Netherlands, 2016; pp. 447–484. [Google Scholar]
- Appel, P.W.; Na-Oy, L. The borax method of gold extraction for small-scale miners. J. Health Pollut. 2012, 2, 5–10. [Google Scholar] [CrossRef]
- Appel, P.W.U.; Na-Oy, L.D. Mercury-free gold extraction using borax for small-scale gold miners. J. Environ. Protect. 2014, 5, 493. [Google Scholar] [CrossRef]
- Steckling, N.; Bose-O’Reilly, S.; Shoko, D.; Muschack, S.; Schierl, R. Testing local conditions for the introduction of a mercury-free gold extraction method using borax in Zimbabwe. J. Health Pollut. 2014, 4, 54–61. [Google Scholar] [CrossRef]
- Riederer, A.; Caravanos, J. 1 BORAX-Summary of Health Risks Associated with Using Borax in Artisanal and Small-Scale Gold Mining; Blacksmith Institute: New York, NY, USA, 2013. [Google Scholar]
- Anderson, C.; Moreno, F.; Meech, J. A field demonstration of gold phytoextraction technology. Miner. Eng. 2005, 18, 385–392. [Google Scholar] [CrossRef]
- Wilson-Corral, V.; Anderson, C.; Rodriguez-Lopez, M.; Arenas-Vargas, M.; Lopez-Perez, J. Phytoextraction of gold and copper from mine tailings with Helianthus annuus L. and Kalanchoe serrata L. Miner. Eng. 2011, 24, 1488–1494. [Google Scholar] [CrossRef]
- Sheoran, V.; Sheoran, A.S.; Poonia, P. Phytomining of gold: A review. J. Geochem. Explor. 2013, 128, 42–50. [Google Scholar] [CrossRef]
- Hidayati, N.; Juhaeti, T.; Syarif, F. Mercury and cyanide contaminations in gold mine environment and possible solution of cleaning up by using phytoextraction. HAYATI J. Biosci. 2009, 16, 88–94. [Google Scholar] [CrossRef]
- Handayanto, E.; Muddarisna, N.; Krisnayanti, B.D. Induced phytoextraction of mercury and gold from cyanidation tailings of small-scale gold mining area of West Lombok, Indonesia. Adv. Environ. Biol. 2014, 1277–1285. [Google Scholar]
- Alcantara, H.J.P.; Doronila, A.I.; Kolev, S.D. Phytoextraction potential of Manihot esculenta Crantz.(cassava) grown in mercury-and gold-containing biosolids and mine tailings. Miner. Eng. 2010, 114, 57–63. [Google Scholar] [CrossRef]
- Moreno, F.N.; Anderson, C.W.; Stewart, R.B.; Robinson, B.H.; Nomura, R.; Ghomshei, M.; Meech, J.A. Effect of thioligands on plant-Hg accumulation and volatilisation from mercury-contaminated mine tailings. Plant Soil 2005, 275, 233. [Google Scholar] [CrossRef]
- Whitehead, J.A.; Lawrance, G.A.; McCluskey, A. ‘Green’ leaching: Recyclable and selective leaching of gold-bearing ore in an ionic liquid. Green Chem. 2004, 6, 313–315. [Google Scholar] [CrossRef]
- Jenkin, G.R.; Al-Bassam, A.Z.; Harris, R.C.; Abbott, A.P.; Smith, D.J.; Holwell, D.A.; Chapman, R.J.; Stanley, C.J. The application of deep eutectic solvent ionic liquids for environmentally-friendly dissolution and recovery of precious metals. Miner. Eng. 2016, 87, 18–24. [Google Scholar] [CrossRef] [Green Version]
- Amankwah, R.K.; Styles, M.T.; Nartey, R.S.; Al-Hassan, S. The application of direct smelting of gold concentrates as an alternative to mercury amalgamation in small-scale gold mining operations in Ghana. Int. J. Environ. Pollut. 2010, 41, 304–315. [Google Scholar] [CrossRef]
- Nam, K.; Jung, B.; An, J.; Ha, T.; Tran, T.; Kim, M. Use of chloride-hipochlorite leachants to recover gold from tailing. Miner. Porcess. 2008, 86, 131–140. [Google Scholar] [CrossRef]
- Radulescu, R.; Filcenco-Olteanu, A.; Panturu, E.; Grigoras, L. New hidrometallurgical process for gold recovery. Chimie si Ingeneria Mediului 2008, 53, 135–139. [Google Scholar]
- Drouin, A.; Lemieux, D.; Lalancette, J.M.; Chouinard, C. Demonstration campaign results on a cyanide-free process for gold extraction from a refractory pyrite concentrate. In Proceedings of the SME Annual Conference and Expo 2017: Creating Value in a Cyclical Environment, Denver, CO, USA, 19–22 February 2017; pp. 172–176. [Google Scholar]
- UNEP. Minamata Convention on Mercury: Text and Annexes; UNEP: Geneva, Switzerland, 2013. [Google Scholar]




| Department | Location | Material | Vein/Mine | Coordinates (UTM) |
|---|---|---|---|---|
| Arequipa | Misky | Ore/ROM | Charpera | 693,031/8,233,564 |
| Misky | Ore | Nivel 950 | 693,176/8,233,423 | |
| Misky | Ore | Soledad | 693,890/8,233,267 | |
| Misky | Ore | 7 bocas | 693,663/8,233,808 | |
| Misky | Ore | Posta | 693,795/8,233,681 | |
| San Martín | Tailings | 692,507/8,235,121 | ||
| San Cristóbal | ROM | 718,850/8,265,624 | ||
| San Cristóbal | Tailings | 719,055/8,265,415 | ||
| Ayacucho | Cháparra | Ore | Macdesa | 619,113/8,263,400 |
| Cháparra | Ore | Aurex | 622,896/8,260,510 | |
| Cháparra | Ore | Alto Perú | 626,568/8,258,890 | |
| San Luis | Ore | Clider/principal | 576,757/8,304,716 | |
| San Luis | ROM | |||
| San Luis | Tailings | 576,488/8,304,588 | ||
| Santa Filomena | Ore | ANA/Anampa | 577,608/8,300,549 | |
| Santa Filomena | ROM | |||
| Santa Filomena | Tailings | 577,608/8,300,550 |
| Pyrite | Tetrahedrite | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| wt. % | Mk17 | Mk14a | Mk14b | Mk106 | SC1 | SC78a | SC78b | SC68 | Ch1 | Ch4a | Ch4b |
| S | 53.74 | 54.21 | 53.94 | 53.80 | 53.04 | 53.57 | 53.51 | 53.58 | 54.16 | 29.79 | 24.3 |
| Fe | 45.80 | 45.97 | 45.08 | 46.57 | 45.22 | 45.41 | 45.62 | 45.99 | 45.62 | 15.44 | 6.97 |
| Cu | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.03 | 32.35 | 38.23 |
| Zn | - | - | - | 0.10 | - | - | - | - | - | 0.16 | 0.36 |
| Te | 0.00 | 0.00 | 0.00 | 0.00 | 0.04 | 0.00 | 0.00 | 0.02 | 0.00 | - | - |
| Se | 0.01 | 0.03 | 0.00 | 0.00 | 0.00 | 0.02 | 0.02 | 0.00 | 0.09 | 0.00 | 0.00 |
| Ag | 0.00 | 0.00 | 0.21 | 0.05 | 0.00 | 0.00 | 0.08 | 0.13 | 0.05 | 0.19 | 0.35 |
| Au | 0.00 | 0.10 | 0.58 | 0.61 | 0.04 | 0.05 | 0.00 | 0.21 | 0.00 | 0.00 | 0.58 |
| As | - | - | - | 0.00 | - | - | - | - | 0.01 | 0.00 | 0.32 |
| Sb | 0.01 | 0.00 | 0.01 | 0.08 | 0.00 | 0.02 | 0.00 | 0.00 | 0.02 | 13.77 | 20.03 |
| Pb | 0.09 | 0.15 | 0.11 | 0.13 | 0.37 | 0.17 | 0.11 | 0.10 | 0.05 | 0.02 | 0.10 |
| Total | 99.65 | 100.46 | 99.93 | 99.40 | 101.48 | 99.25 | 99.34 | 100.02 | 100.00 | 99.02 | 97.01 |
| Area | Location | Sample | Type | Au | Ag | Te | Zn | As | Hg |
|---|---|---|---|---|---|---|---|---|---|
| Misky | Posta | MK-35 | ore | 134 | 45 | - | 98 | - | - |
| Misky | - | Average | ROM | 38 | 57 | - | 192 | - | - |
| San Cristóbal | - | SRC2 | ROM | 59 | 143 | - | 3560 | - | - |
| San Cristóbal | - | SRC3 | ROM | 33 | 117 | - | 5590 | 519 | 9 |
| San Cristóbal | - | QU2 | ROM | 28 | 138 | - | 4920 | 428 | 6 |
| Cháparra | San Martín | R-3a | Ore | 111 | 7 | - | 18 | 226 | <1 |
| Cháparra | Gallinazo | R-7b | Ore | 27 | 1 | - | 6 | 12 | <1 |
| Cháparra | Comuna | R-9a | Ore | 25 | 8 | - | 331 | 10,900 | <1 |
| Cháparra | Alto Perú | R-11 | Ore | 3 | 1 | - | 9 | 236 | <1 |
| Cháparra | 600-Aurex | R-12a | Ore | 18 | 0 | - | 2 | 126 | <1 |
| San Luis | Clider | SL-1 | Ore | 1 | - | - | - | - | - |
| San Luis | Main vein | SL-2m | Ore | 29 | - | - | - | - | - |
| San Luis | Main vein | SL-11 | Ore | 60 | - | 80 | - | - | - |
| Santa Filomena | Anampa | FDM-3 | ore | 23 | - | 10 | - | - | - |
| Area | Sample | Process | Au | Ag | Zn | As | Hg |
|---|---|---|---|---|---|---|---|
| San Cristóbal | SRC1 | A | 19 | 176 | 2.620 | 434 | 917 |
| San Cristóbal | SRC4 | A | 56 | 207 | >10.000 | 973 | 902 |
| San Cristóbal | QU1 | C | 11 | 175 | 8.640 | 472 | 228 |
| San Cristóbal | QU3 | C | 1 | 64 | 3.110 | 291 | 589 |
| Cháparra | Ch-1 | A | 27 | 4 | 11 | 404 | 68 |
| Cháparra | Ch-2 | A | 35 | 11 | 1.780 | 727 | 49 |
| Cháparra | Ch-3 | A | 8 | 2 | 11 | 411 | 117 |
| Cháparra | Ch-4 | A | 16 | 4 | 1.510 | 370 | 324 |
| Cháparra | Ch-5 | A | 5 | 7 | 19 | 209 | 252 |
| San Luis | L-6 | A | 52 | 10 | 14 | 48 | < 1 |
| San Luis | L-7 | A | 40 | 8 | 11 | 44 | < 1 |
| San Luis | L-2 | A | 35 | 13 | |||
| San Luis | PLO4 | C | 1 | ||||
| Santa Filomena | SFE | C | 16 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alfonso, P.; Anticoi, H.; Yubero, T.; Bascompta, M.; Henao, L.; Garcia-Valles, M.; Palacios, S.; Yáñez, J. The Importance of Mineralogical Knowledge in the Sustainability of Artisanal Gold Mining: A Mid-South Peru Case. Minerals 2019, 9, 345. https://doi.org/10.3390/min9060345
Alfonso P, Anticoi H, Yubero T, Bascompta M, Henao L, Garcia-Valles M, Palacios S, Yáñez J. The Importance of Mineralogical Knowledge in the Sustainability of Artisanal Gold Mining: A Mid-South Peru Case. Minerals. 2019; 9(6):345. https://doi.org/10.3390/min9060345
Chicago/Turabian StyleAlfonso, Pura, Hernan Anticoi, Teresa Yubero, Marc Bascompta, Laura Henao, Maite Garcia-Valles, Silvia Palacios, and Juan Yáñez. 2019. "The Importance of Mineralogical Knowledge in the Sustainability of Artisanal Gold Mining: A Mid-South Peru Case" Minerals 9, no. 6: 345. https://doi.org/10.3390/min9060345
APA StyleAlfonso, P., Anticoi, H., Yubero, T., Bascompta, M., Henao, L., Garcia-Valles, M., Palacios, S., & Yáñez, J. (2019). The Importance of Mineralogical Knowledge in the Sustainability of Artisanal Gold Mining: A Mid-South Peru Case. Minerals, 9(6), 345. https://doi.org/10.3390/min9060345









