A Review of Sludge-to-Energy Recovery Methods
Abstract
:1. Introduction
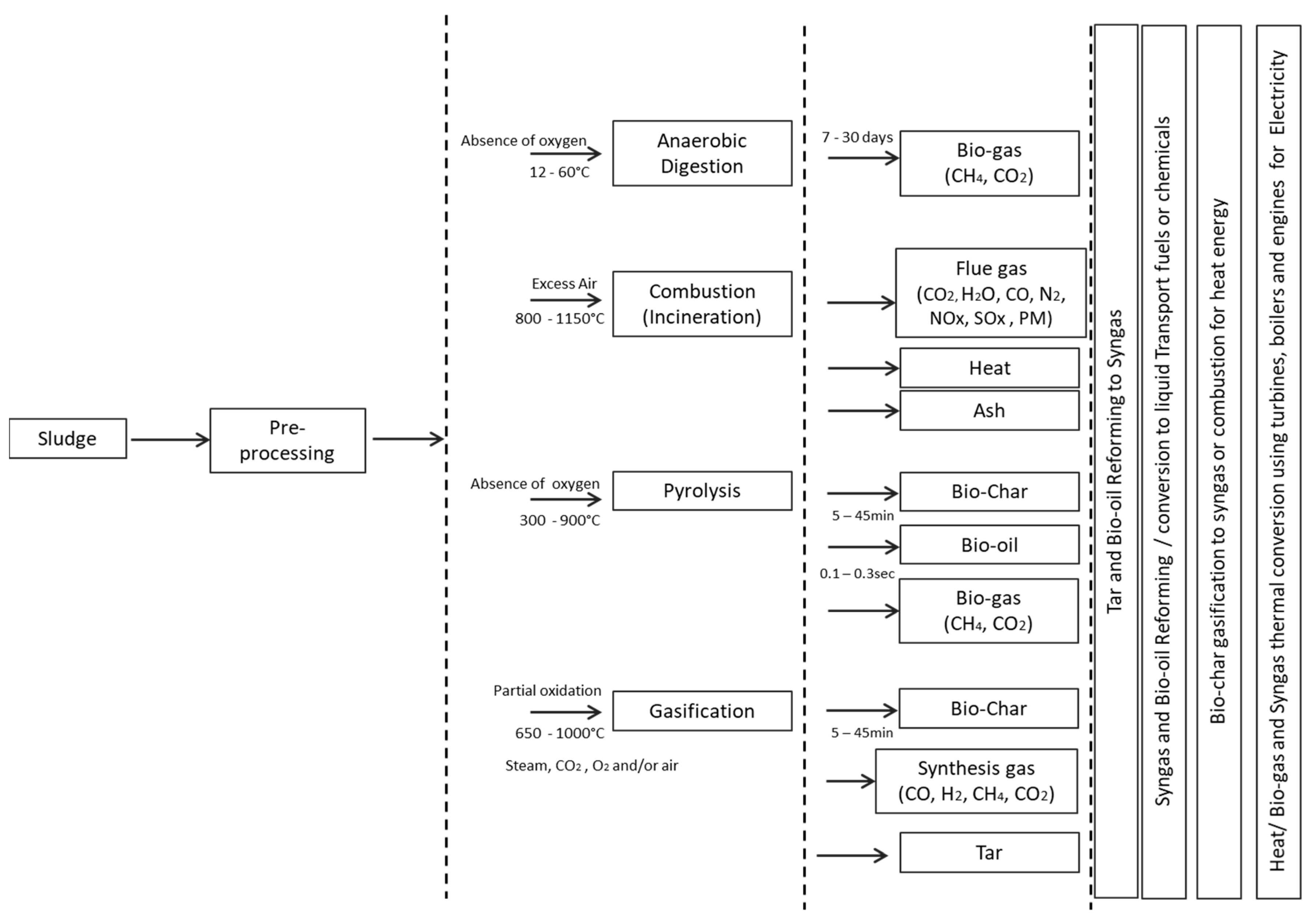
2. Sludge-to-Energy Recovery Methods
2.1. Pre-Processing of Sludge
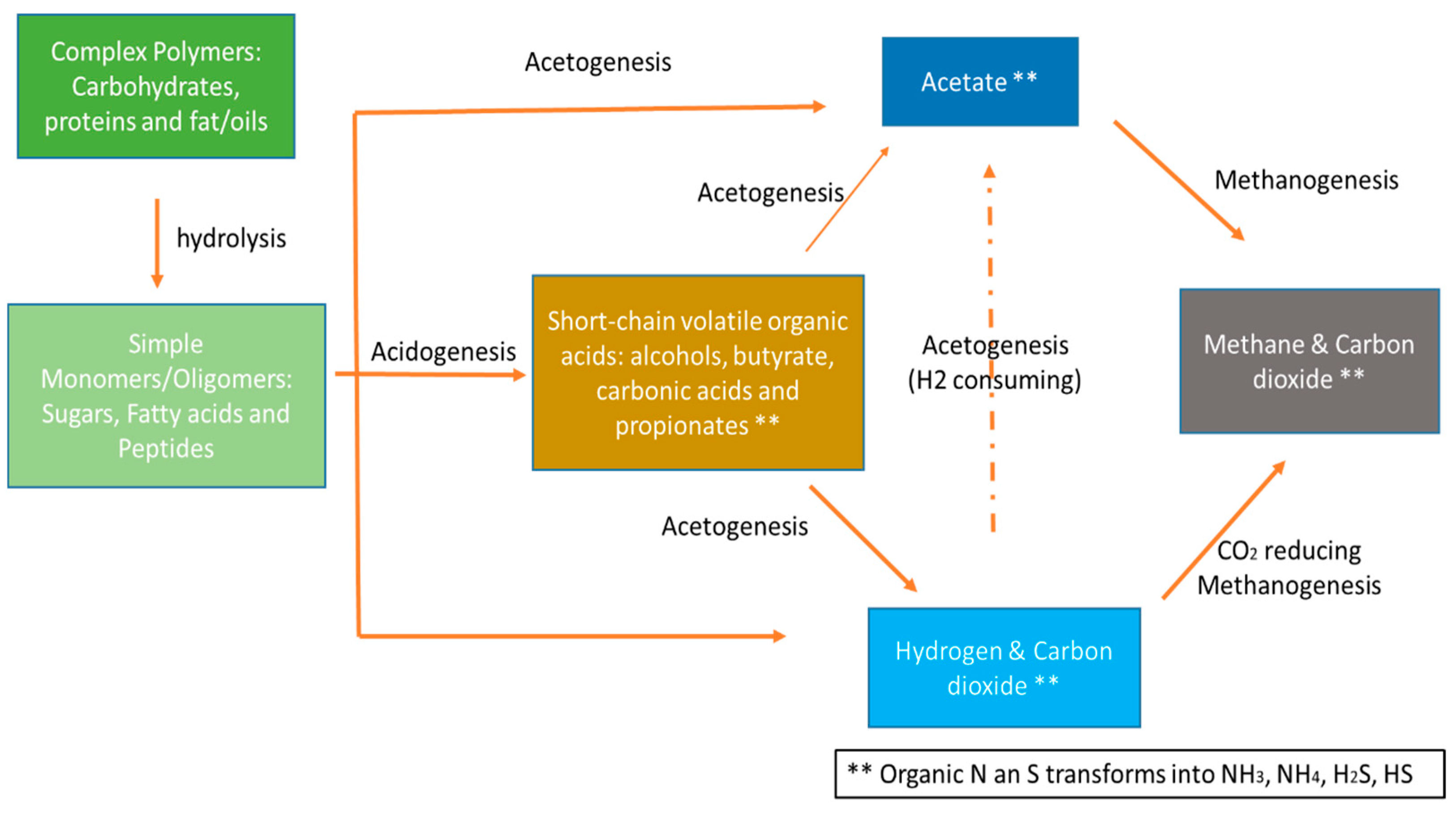
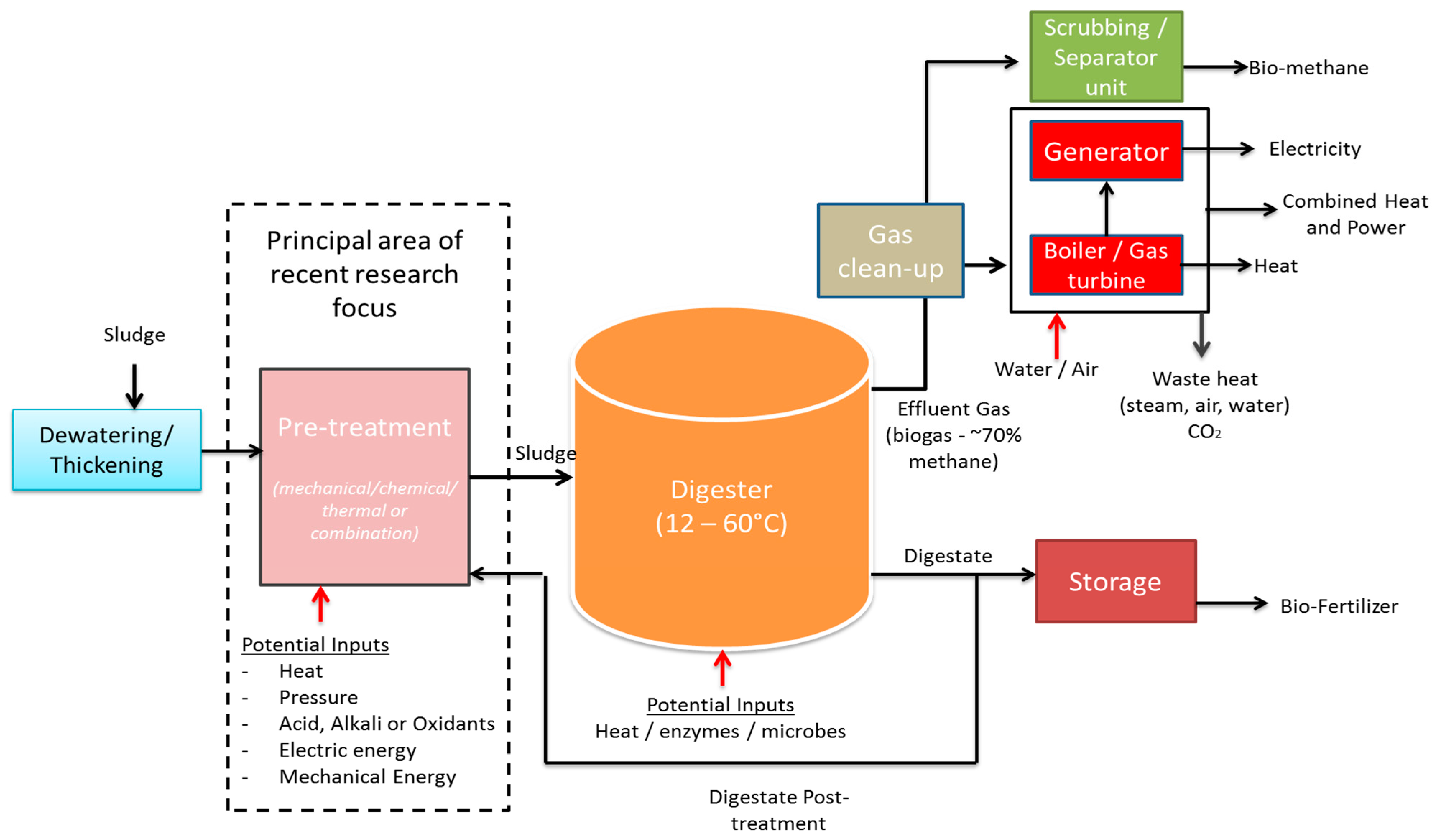
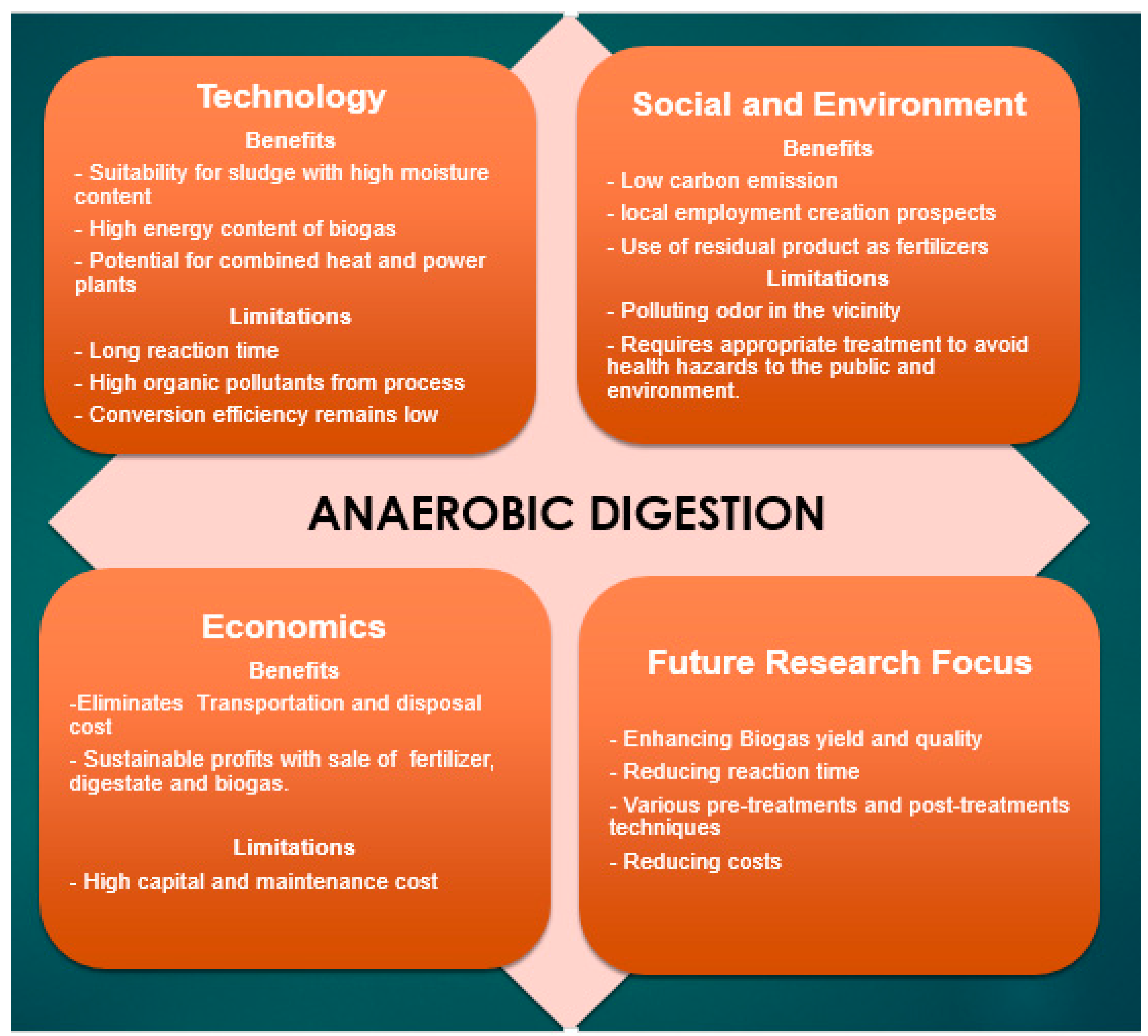
2.2. Anaerobic Digestion
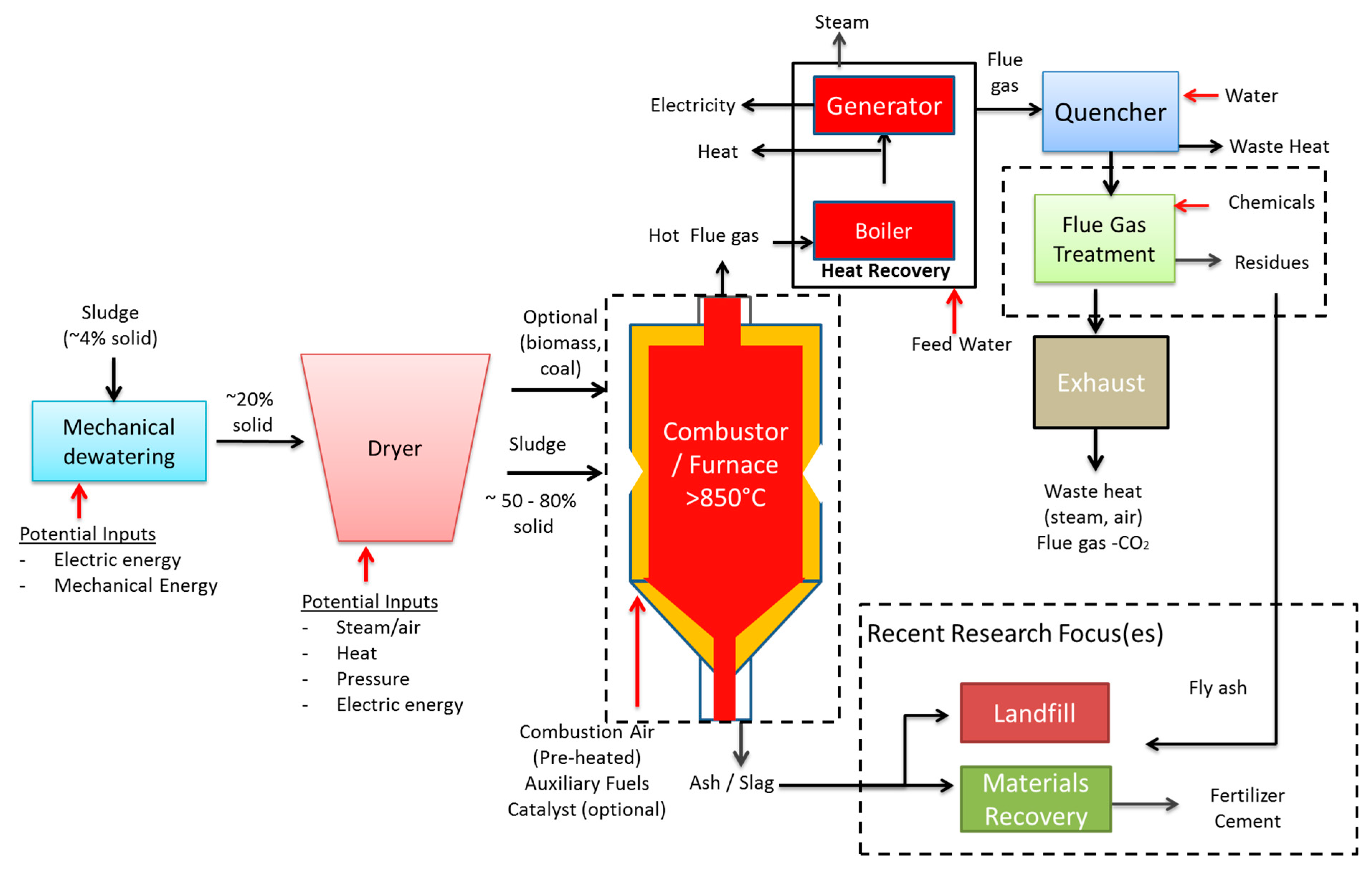
2.3. Combustion
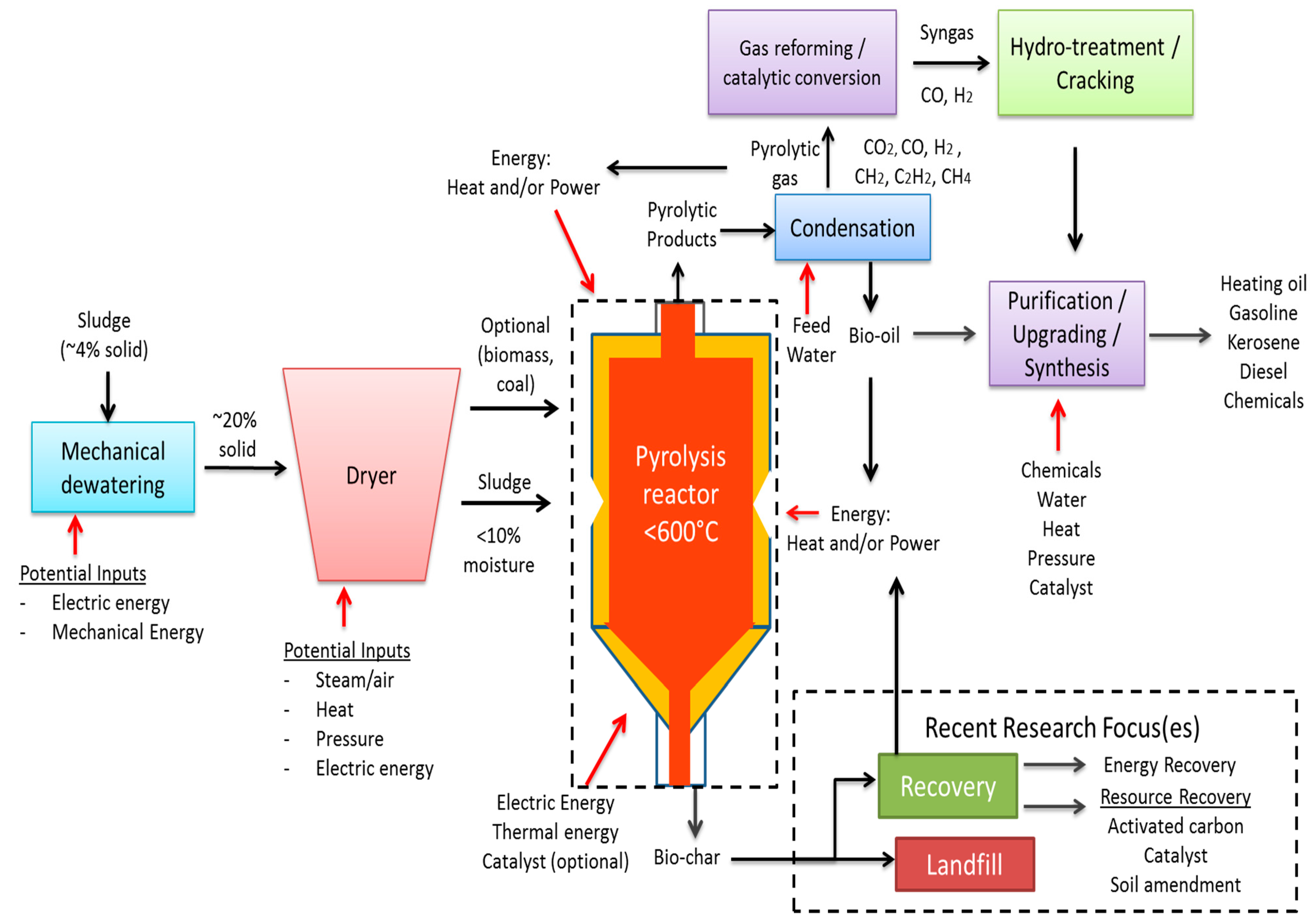
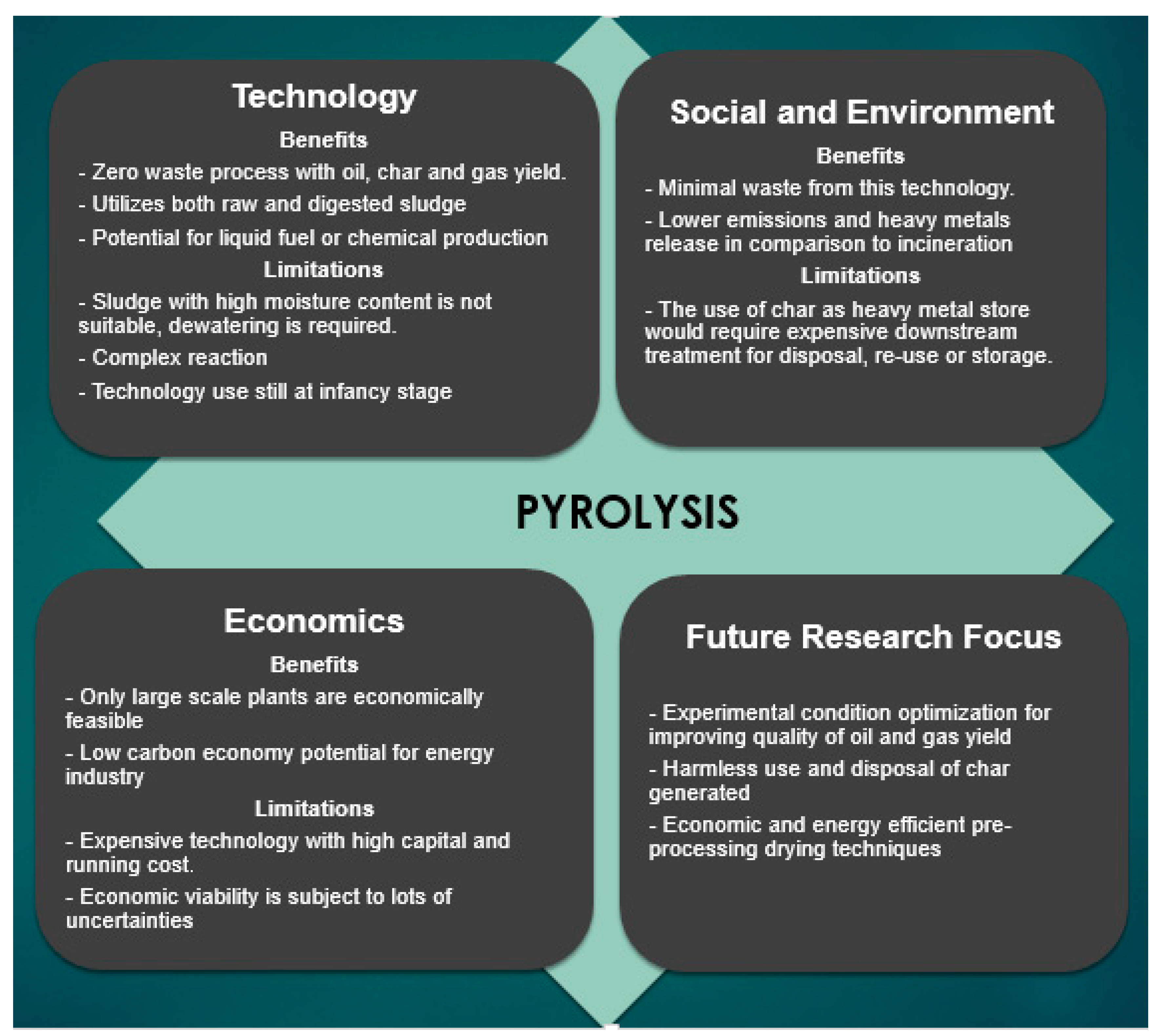
2.4. Pyrolysis
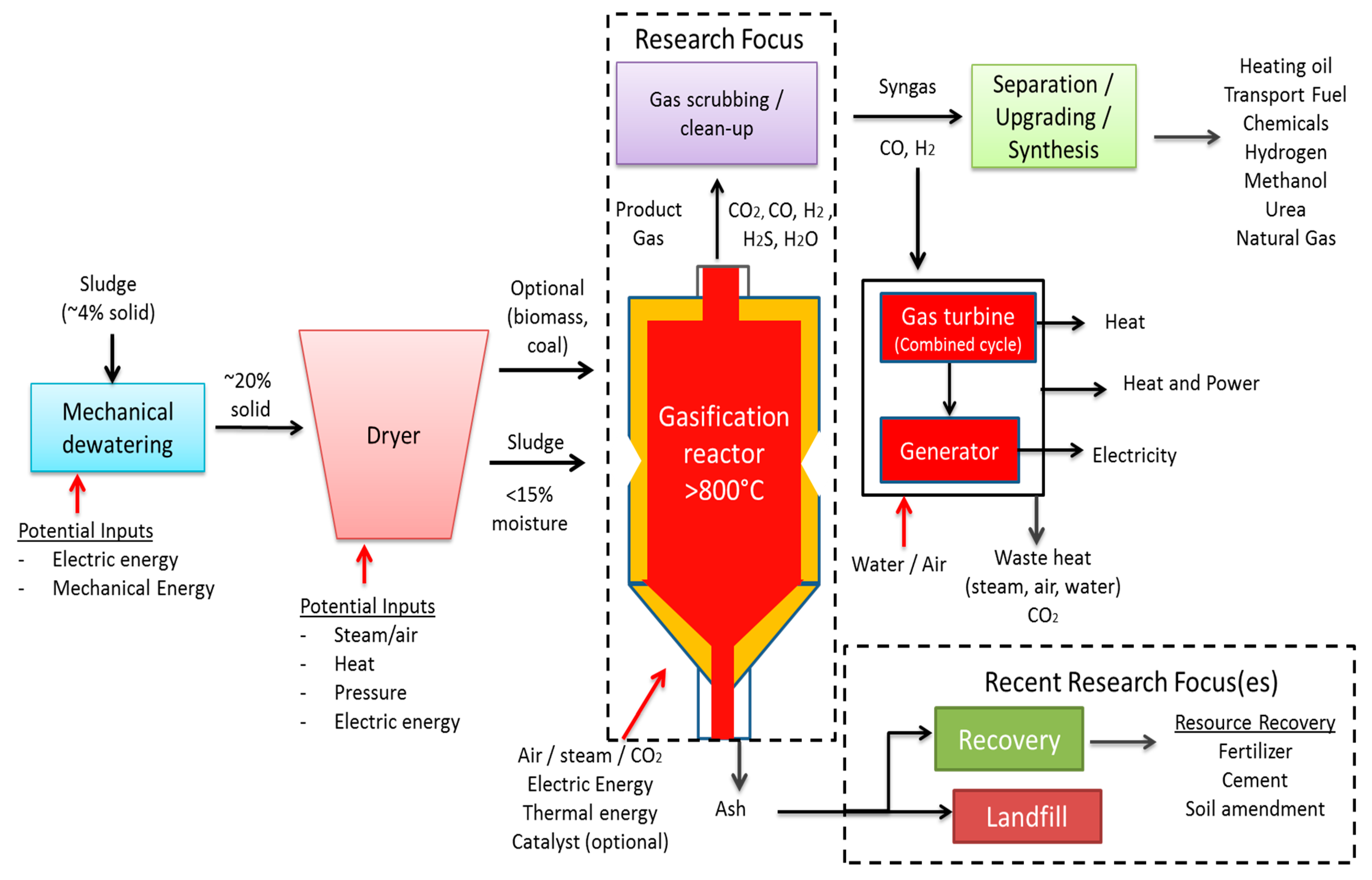
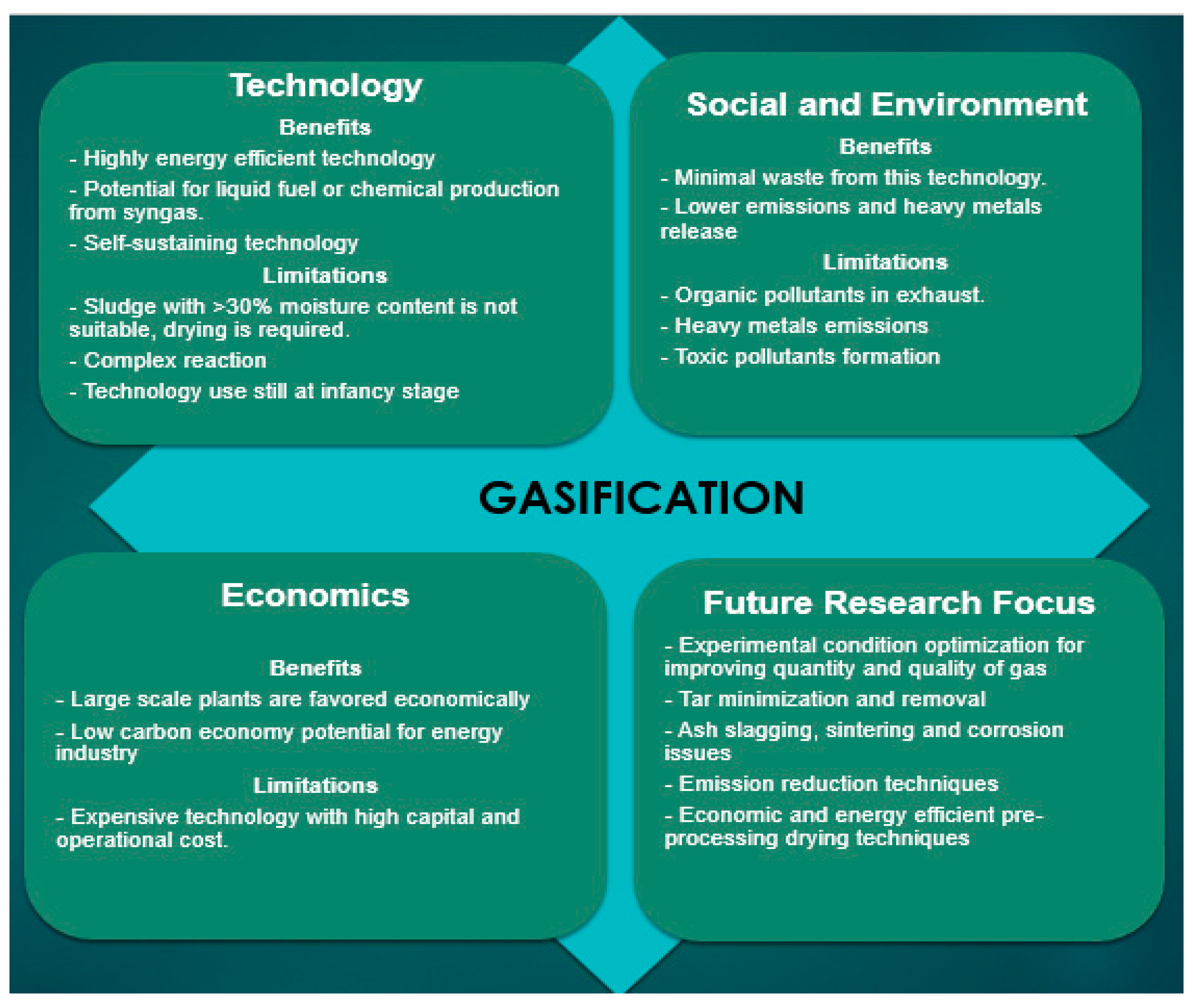
2.5. Gasification
3. Conclusions and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Seiple, T.E.; Coleman, A.M.; Skaggs, R.L. Municipal wastewater sludge as a sustainable bioresource in the United States. J. Environ. Manag. 2017, 197, 673–680. [Google Scholar] [CrossRef] [PubMed]
- Syed-Hassan, S.S.A.; Wang, Y.; Hu, S.; Su, S.; Xiang, J. Thermochemical processing of sewage sludge to energy and fuel: Fundamentals, challenges and considerations. Renew. Sustain. Energy Rev. 2017, 80, 888–913. [Google Scholar] [CrossRef]
- Cieślik, B.M.; Namieśnik, J.; Konieczka, P. Review of sewage sludge management: Standards, regulations and analytical methods. J. Clean. Prod. 2015, 90, 1–15. [Google Scholar] [CrossRef]
- Magdziarz, A.; Dalai, A.K.; Koziński, J.A. Chemical composition, character and reactivity of renewable fuel ashes. Fuel 2016, 176, 135–145. [Google Scholar] [CrossRef]
- Harrison, E.Z.; Oakes, S.R.; Hysell, M.; Hay, A. Organic chemicals in sewage sludges. Sci. Total Environ. 2006, 367, 481–497. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mulchandani, A.; Westerhoff, P. Recovery opportunities for metals and energy from sewage sludges. Bioresour. Technol. 2016, 215, 215–226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chan, W.P.; Wang, J.-Y. Comprehensive characterisation of sewage sludge for thermochemical conversion processes—Based on Singapore survey. Waste Manag. 2016, 54, 131–142. [Google Scholar] [CrossRef] [PubMed]
- Vaxelaire, J.; Cézac, P. Moisture distribution in activated sludges: A review. Water Res. 2004, 38, 2215–2230. [Google Scholar] [CrossRef] [PubMed]
- Winkler, M.K.H.; Bennenbroek, M.H.; Horstink, F.H.; van Loosdrecht, M.C.M.; van de Pol, G.J. The biodrying concept: An innovative technology creating energy from sewage sludge. Bioresour. Technol. 2013, 147, 124–129. [Google Scholar] [CrossRef] [PubMed]
- Mills, N.; Pearce, P.; Farrow, J.; Thorpe, R.B.; Kirkby, N.F. Environmental & economic life cycle assessment of current & future sewage sludge to energy technologies. Waste Manag. 2014, 34, 185–195. [Google Scholar] [CrossRef]
- Lee, I.-S.; Parameswaran, P.; Rittmann, B.E. Effects of solids retention time on methanogenesis in anaerobic digestion of thickened mixed sludge. Bioresour. Technol. 2011, 102, 10266–10272. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Chen, W.; Hong, J. Life-cycle environmental and economic assessment of sewage sludge treatment in China. J. Clean. Prod. 2014, 67, 79–87. [Google Scholar] [CrossRef]
- Włodarczyk-Makuła, M. Persistence of two-, three- and four-ring of PAHs in sewage sludge deposited in different light conditions. Desalin. Water Treat. 2016, 57, 1184–1199. [Google Scholar] [CrossRef]
- Ding, H.H.; Chang, S.; Liu, Y. Biological hydrolysis pretreatment on secondary sludge: Enhancement of anaerobic digestion and mechanism study. Bioresour. Technol. 2017, 244, 989–995. [Google Scholar] [CrossRef] [PubMed]
- Tsai, W.-T. An Analysis of the Use of Biosludge as an Energy Source and Its Environmental Benefits in Taiwan. Energies 2012, 5, 3064–3073. [Google Scholar] [CrossRef] [Green Version]
- Raheem, A.; Sikarwar, V.S.; He, J.; Dastyar, W.; Dionysiou, D.D.; Wang, W.; Zhao, M. Opportunities and challenges in sustainable treatment and resource reuse of sewage sludge: A review. Chem. Eng. J. 2018, 337, 616–641. [Google Scholar] [CrossRef]
- Ruffino, B.; Campo, G.; Cerutti, A.; Zanetti, M.C.; Scibilia, G.; Lorenzi, E.; Genon, G. Enhancement of waste activated sludge (WAS) anaerobic digestion by means of pre- and intermediate treatments. In Proceedings of the International Conference on Sustainable Solid Waste Management, Limassol, Cyprus, 21–24 June 2017. [Google Scholar]
- Nielsen, H.B.; Thygesen, A.; Thomsen, A.B.; Schmidt, J.E. Anaerobic digestion of waste activated sludge—Comparison of thermal pretreatments with thermal inter-stage treatments. J. Chem. Technol. Biotechnol. 2011, 86, 238–245. [Google Scholar] [CrossRef]
- Valo, A.; Carrère, H.; Delgenès, J.P. Thermal, chemical and thermo-chemical pre-treatment of waste activated sludge for anaerobic digestion. J. Chem. Technol. Biotechnol. 2004, 79, 1197–1203. [Google Scholar] [CrossRef]
- Sivagurunathan, P.; Zhen, G.; Kim, S.; Saratale, G.D. A review on bio-electrochemical systems (BESs) for the syngas and value added biochemicals production. Chemosphere 2017, 177, 84–92. [Google Scholar] [CrossRef]
- Meegoda, J.N.; Li, B.; Patel, K.; Wang, L.B. A Review of the Processes, Parameters, and Optimization of Anaerobic Digestion. Int. J. Environ. Res. Public Health 2018, 15, 2224. [Google Scholar] [CrossRef]
- Roubík, H.; Mazancová, J.; Le Dinh, P.; Dinh Van, D.; Banout, J. Biogas Quality across Small-Scale Biogas Plants: A Case of Central Vietnam. Energies 2018, 11, 1794. [Google Scholar] [CrossRef]
- Pant, D.; Singh, A.; Bogaert, G.V.; Olsen, S.I.; Nigam, P.S.; Dielsa, L.; Vanbroekhoven, K. Bioelectrochemical systems (BES) for sustainable energy production and product recovery from organic wastes and industrial wastewaters. RSC Adv. 2012, 2, 1248–1263. [Google Scholar] [CrossRef]
- Jong, E.; Jungmeier, G. Chapter 1—Biorefinery Concepts in Comparison to Petrochemical Refineries. Ind. Biorefineries White Biotechnol. 2015, 3–33. [Google Scholar] [CrossRef]
- Zacharof, M.-P. The filtration characteristics of anaerobic digester effluents employing cross flow ceramic membrane microfiltration for nutrient recovery. Desalination 2014, 341, 27–37. [Google Scholar] [CrossRef]
- Gerardo, M.L.; Zacharof, M.-P.; Lovitt, R.W. Strategies for the recovery of nutrients and metals from anaerobically digested dairy farm sludge using cross-flow microfiltration. Water Res. 2013, 47, 4833–4842. [Google Scholar] [CrossRef] [PubMed]
- Zacharof, M.-P.; Mandale, S.J.; Williams, P.M.; Lovitt, R.W. Nanofiltration of treated digested agricultural wastewater for recovery of carboxylic acids. J. Clean. Prod. 2016, 112, 4749–4761. [Google Scholar] [CrossRef]
- Gottumukkala, L.D.; Haigh, K.; Collard, F.; Rensburg, E.V.; Görgens, J. Opportunities and prospects of biorefinery-based valorisation of pulp and paper sludge. Bioresour. Technol. 2016, 215, 37–49. [Google Scholar] [CrossRef] [PubMed]
- Tic, W.J.; Guziałowska-Tic, J.; Pawlak-Kruczek, H.; Woźnikowski, E.; Zadorożny, A.; Niedźwiecki, Ł.; Wnukowski, M.; Krochmalny, K.; Czerep, M.; Ostrycharczyk, M.; et al. Novel Concept of an Installation for Sustainable Thermal Utilization of Sewage Sludge. Energies 2018, 11, 748. [Google Scholar] [CrossRef]
- Aryal, N.; Kvist, T. Alternative of Biogas Injection into the Danish Gas Grid System—A Study from Demand Perspective. Chem. Eng. 2018, 2, 43. [Google Scholar] [CrossRef]
- Guo, L.; Zhang, Z.; Gao, M.; She, Z.; Zhao, Y.; Guo, Y.; Sun, J. Comparison of thermophilic bacteria and alkyl polyglucose pretreatment on two-stage anaerobic digestion with waste sludge: Biogas production potential and substrate metabolism process. Bioresour. Technol. 2018, 249, 694–703. [Google Scholar] [CrossRef]
- Devlin, D.C.; Esteves, S.R.R.; Dinsdale, R.M.; Guwy, A.J. The effect of acid pretreatment on the anaerobic digestion and dewatering of waste activated sludge. Bioresour. Technol. 2011, 102, 4076–4082. [Google Scholar] [CrossRef] [PubMed]
- Riau, V.; De la Rubia, M.A.; Pérez, M. Upgrading the temperature-phased anaerobic digestion of waste activated sludge by ultrasonic pretreatment. Chem. Eng. J. 2015, 259, 672–681. [Google Scholar] [CrossRef] [Green Version]
- Martín, M.Á.; González, I.; Serrano, A.; Siles, J.Á. Evaluation of the improvement of sonication pre-treatment in the anaerobic digestion of sewage sludge. J. Environ. Manag. 2015, 147, 330–337. [Google Scholar] [CrossRef] [PubMed]
- Houtmeyers, S.; Degrève, J.; Willems, K.; Dewil, R.; Appels, L. Comparing the influence of low power ultrasonic and microwave pre-treatments on the solubilisation and semi-continuous anaerobic digestion of waste activated sludge. Bioresour. Technol. 2014, 171, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Zhen, G.; Lu, X.; Kato, H.; Zhao, Y.; Li, Y.-Y. Overview of pretreatment strategies for enhancing sewage sludge disintegration and subsequent anaerobic digestion: Current advances, full-scale application and future perspectives. Renew. Sustain. Energy Rev. 2017, 69, 559–577. [Google Scholar] [CrossRef]
- Lin, Y.; Wang, D.; Wu, S.; Wang, C. Alkali pretreatment enhances biogas production in the anaerobic digestion of pulp and paper sludge. J. Hazard. Mater. 2009, 170, 366–373. [Google Scholar] [CrossRef] [PubMed]
- Weemaes, M.; Grootaerd, H.; Simoens, F.; Verstraete, W. Anaerobic digestion of ozonized biosolids. Water Res. 2000, 34, 2330–2336. [Google Scholar] [CrossRef]
- Takashima, M.; Tanaka, Y. Acidic thermal post-treatment for enhancing anaerobic digestion of sewage sludge. J. Environ. Chem. Eng. 2014, 2, 773–779. [Google Scholar] [CrossRef]
- Ebenezer, A.V.; Arulazhagan, P.; Adish Kumar, S.; Yeom, I.-T.; Rajesh Banu, J. Effect of deflocculation on the efficiency of low-energy microwave pretreatment and anaerobic biodegradation of waste activated sludge. Appl. Energy 2015, 145, 104–110. [Google Scholar] [CrossRef]
- Coelho, N.M.G.; Droste, R.L.; Kennedy, K.J. Evaluation of continuous mesophilic, thermophilic and temperature phased anaerobic digestion of microwaved activated sludge. Water Res. 2011, 45, 2822–2834. [Google Scholar] [CrossRef]
- Appels, L.; Houtmeyers, S.; Degrève, J.; Van Impe, J.; Dewil, R. Influence of microwave pre-treatment on sludge solubilization and pilot scale semi-continuous anaerobic digestion. Bioresour. Technol. 2013, 128, 598–603. [Google Scholar] [CrossRef] [PubMed]
- Wilson, C.A.; Tanneru, C.T.; Banjade, S.; Murthy, S.N.; Novak, J.T. Anaerobic Digestion of Raw and Thermally Hydrolyzed Wastewater Solids Under Various Operational Conditions. Water Environ. Res. 2011, 83, 815–825. [Google Scholar] [CrossRef] [PubMed]
- Pilli, S.; More, T.; Yan, S.; Tyagi, R.D.; Surampalli, R.Y. Anaerobic digestion of thermal pre-treated sludge at different solids concentrations—Computation of mass-energy balance and greenhouse gas emissions. J. Environ. Manag. 2015, 157, 250–261. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.; Liu, H.; Chen, S.; Dichtl, N.; Dai, X.; Li, N. Effects of thermal hydrolysis on organic matter solubilization and anaerobic digestion of high solid sludge. Chem. Eng. J. 2015, 264, 174–180. [Google Scholar] [CrossRef]
- Cao, Y.; Pawlowski, A. Sewage sludge-to-energy approaches based on anaerobic digestion and pyrolysis: Brief overview and energy efficiency assessment. Renew. Sustain. Energy Rev. 2012, 16, 1657–1665. [Google Scholar] [CrossRef]
- Morales-Polo, C.; Cledera-Castro, M.M.; Moratilla Soria, B.Y. Reviewing the Anaerobic Digestion of Food Waste: From Waste Generation and Anaerobic Process to Its Perspectives. Appl. Sci. 2018, 8, 1804. [Google Scholar] [CrossRef]
- Keucken, A.; Habagil, M.; Batstone, D.; Jeppsson, U.; Arnell, M. Anaerobic Co-Digestion of Sludge and Organic Food Waste—Performance, Inhibition, and Impact on the Microbial Community. Energies 2018, 11, 2325. [Google Scholar] [CrossRef]
- Guimarães, C.S.; Maia, D.R.S.; Serra, E.G. Construction of Biodigesters to Optimize the Production of Biogas from Anaerobic Co-Digestion of Food Waste and Sewage. Energies 2018, 11, 870. [Google Scholar] [CrossRef]
- Wu, M.-H.; Lin, C.-L.; Huang, W.-C.; Chen, J.-W. Characteristics of pervious concrete using incineration bottom ash in place of sandstone graded material. Constr. Build. Mater. 2016, 111, 618–624. [Google Scholar] [CrossRef]
- Ogada, T.; Werther, J. Combustion characteristics of wet sludge in a fluidized bed: Release and combustion of the volatiles. Fuel 1996, 75, 617–626. [Google Scholar] [CrossRef]
- Urciuolo, M.; Solimene, R.; Chirone, R.; Salatino, P. Fluidized bed combustion and fragmentation of wet sewage sludge. Exp. Therm. Fluid Sci. 2012, 43, 97–104. [Google Scholar] [CrossRef]
- Folgueras, M.B.; Alonso, M.; Díaz, R.M. Influence of sewage sludge treatment on pyrolysis and combustion of dry sludge. Energy 2013, 55, 426–435. [Google Scholar] [CrossRef]
- Werther, J.; Ogada, T. Sewage sludge combustion. Prog. Energy Combust. Sci. 1999, 25, 55–116. [Google Scholar] [CrossRef]
- Dennis, J.S.; Lambert, R.J.; Milne, A.J.; Scott, S.A.; Hayhurst, A.N. The kinetics of combustion of chars derived from sewage sludge. Fuel 2005, 84, 117–126. [Google Scholar] [CrossRef]
- Cui, H.; Ninomiya, Y.; Masui, M.; Mizukoshi, H.; Sakano, T.; Kanaoka, C. Fundamental Behaviors in Combustion of Raw Sewage Sludge. Energy Fuels 2006, 20, 77–83. [Google Scholar] [CrossRef]
- Rong, H.; Wang, T.; Zhou, M.; Wang, H.; Hou, H.; Xue, Y. Combustion Characteristics and Slagging during Co-Combustion of Rice Husk and Sewage Sludge Blends. Energies 2017, 10, 438. [Google Scholar] [CrossRef]
- Chen, G.-B.; Chatelier, S.; Lin, H.-T.; Wu, F.-H.; Lin, T.-H. A Study of Sewage Sludge Co-Combustion with Australian Black Coal and Shiitake Substrate. Energies 2018, 11, 3436. [Google Scholar] [CrossRef]
- Fytili, D.; Zabaniotou, A. Utilization of sewage sludge in EU application of old and new methods—A review. Renew. Sustain. Energy Rev. 2008, 12, 116–140. [Google Scholar] [CrossRef]
- Kijo-Kleczkowska, A.; Środa, K.; Otwinowski, H. Study into Combustion of Sewage Sludge as Energetic Fuel/Badania Spalania Osadów Ściekowych Jako Paliwa Energetycznego. Arch. Min. Sci. 2013, 58, 1085. [Google Scholar] [CrossRef]
- Batistella, L.; Silva, V.; Suzin, R.C.; Virmond, E.; Althoff, C.A.; Moreira, R.F.P.M.; José, H.J. Gaseous emissions from sewage sludge combustion in a moving bed combustor. Waste Manag. 2015, 46, 430–439. [Google Scholar] [CrossRef]
- Kijo-Kleczkowska, A.; Środa, K.; Kosowska-Golachowska, M.; Musiał, T.; Wolski, K. Combustion of pelleted sewage sludge with reference to coal and biomass. Fuel 2016, 170, 141–160. [Google Scholar] [CrossRef]
- Lin, Y.; Liao, Y.; Yu, Z.; Fang, S.; Ma, X. The investigation of co-combustion of sewage sludge and oil shale using thermogravimetric analysis. Thermochim. Acta 2017, 653, 71–78. [Google Scholar] [CrossRef]
- Huang, L.; Liu, J.; He, Y.; Sun, S.; Chen, J.; Sun, J.; Chang, K.; Kuo, J.; Ning, X.A. Thermodynamics and kinetics parameters of co-combustion between sewage sludge and water hyacinth in CO2/O2 atmosphere as biomass to solid biofuel. Bioresour. Technol. 2016, 218, 631–642. [Google Scholar] [CrossRef]
- Wang, Z.; Hong, C.; Xing, Y.; Li, Y.; Feng, L.; Jia, M. Combustion behaviors and kinetics of sewage sludge blended with pulverized coal: With and without catalysts. Waste Manag. 2018, 74, 288–296. [Google Scholar] [CrossRef]
- Kijo-Kleczkowska, A.; Środa, K.; Kosowska-Golachowska, M.; Musiał, T.; Wolski, K. Experimental research of sewage sludge with coal and biomass co-combustion, in pellet form. Waste Manag. 2016, 53, 165–181. [Google Scholar] [CrossRef] [PubMed]
- Deng, S.; Tan, H.; Wang, X.; Yang, F.; Cao, R.; Wang, Z.; Ruan, R. Investigation on the fast co-pyrolysis of sewage sludge with biomass and the combustion reactivity of residual char. Bioresour. Technol. 2017, 239, 302–310. [Google Scholar] [CrossRef]
- Niu, X.; Shen, L. Release and transformation of phosphorus in chemical looping combustion of sewage sludge. Chem. Eng. J. 2018, 335, 621–630. [Google Scholar] [CrossRef]
- Cheng, X.; Zhang, M.; Wang, Z.; Xu, G.; Ma, C. IR and kinetic study of sewage sludge combustion at different oxygen concentrations. Waste Manag. 2018, 74, 279–287. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wang, T.; Zhou, M.; Hou, H.; Xue, Y.; Wang, H. Rice husk and sewage sludge co-combustion ash: Leaching behavior analysis and cementitious property. Constr. Build. Mater. 2018, 163, 63–72. [Google Scholar] [CrossRef]
- Han, X.; Niu, M.; Jiang, X.; Liu, J. Combustion Characteristics of Sewage Sludge in a Fluidized Bed. Ind. Eng. Chem. Res. 2012, 51, 10565–10570. [Google Scholar] [CrossRef]
- Hao, Z.; Yang, B.; Jahng, D. Combustion characteristics of biodried sewage sludge. Waste Manag. 2018, 72, 296–305. [Google Scholar] [CrossRef]
- Hong, J.; Xu, C.; Hong, J.; Tan, X.; Chen, W. Life cycle assessment of sewage sludge co-incineration in a coal-based power station. Waste Manag. 2013, 33, 1843–1852. [Google Scholar] [CrossRef]
- Morais, J.; Barbosa, R.; Lapa, N.; Mendes, B.; Gulyurtlu, I. Environmental and socio-economic assessment of co-combustion of coal, biomass and non-hazardous wastes in a Power Plant. Resour. Conserv. Recycl. 2011, 55, 1109–1118. [Google Scholar] [CrossRef]
- Donatello, S.; Cheeseman, C.R. Recycling and recovery routes for incinerated sewage sludge ash (ISSA): A review. Waste Manag. 2013, 33, 2328–2340. [Google Scholar] [CrossRef]
- Magdziarz, A.; Wilk, M. Thermogravimetric study of biomass, sewage sludge and coal combustion. Energy Convers. Manag. 2013, 75, 425–430. [Google Scholar] [CrossRef]
- Kirubakaran, V.; Sivaramakrishnan, V.; Nalini, R.; Sekar, T.; Premalatha, M.; Subramanian, P. A review on gasification of biomass. Renew. Sustain. Energy Rev. 2009, 13, 179–186. [Google Scholar] [CrossRef]
- Ruiz, J.A.; Juárez, M.C.; Morales, M.P.; Muñoz, P.; Mendívil, M.A. Biomass gasification for electricity generation: Review of current technology barriers. Renew. Sustain. Energy Rev. 2013, 18, 174–183. [Google Scholar] [CrossRef]
- Zhang, L.; Xu, C.C.; Champagne, P. Overview of recent advances in thermo-chemical conversion of biomass. Energy Convers. Manag. 2010, 51, 969–982. [Google Scholar] [CrossRef]
- Balat, M.; Balat, M.; Kırtay, E.; Balat, H. Main routes for the thermo-conversion of biomass into fuels and chemicals. Part 1: Pyrolysis systems. Energy Convers. Manag. 2009, 50, 3147–3157. [Google Scholar] [CrossRef]
- Rollinson, A.N.; Oladejo, J.M. ‘Patented blunderings’, efficiency awareness, and self-sustainability claims in the pyrolysis energy from waste sector. Resour. Conserv. Recycl. 2019, 141, 233–342. [Google Scholar] [CrossRef]
- Alvarez, J.; Amutio, M.; Lopez, G.; Bilbao, J.; Olazar, M. Fast co-pyrolysis of sewage sludge and lignocellulosic biomass in a conical spouted bed reactor. Fuel 2015, 159, 810–818. [Google Scholar] [CrossRef]
- Font, R.; Fullana, A.; Conesa, J.A.; Llavador, F. Analysis of the pyrolysis and combustion of different sewage sludges by TG. J. Anal. Appl. Pyrolysis 2001, 58–59, 927–941. [Google Scholar] [CrossRef]
- Fonts, I.; Gea, G.; Azuara, M.; Ábrego, J.; Arauzo, J. Sewage sludge pyrolysis for liquid production: A review. Renew. Sustain. Energy Rev. 2012, 16, 2781–2805. [Google Scholar] [CrossRef]
- Xu, W.Y.; Wu, D. Comprehensive utilization of the pyrolysis products from sewage sludge. Environ. Technol. 2015, 36, 1731–1744. [Google Scholar] [CrossRef] [PubMed]
- Yi, S.; Jang, Y.-C.; An, A.K. Potential for energy recovery and greenhouse gas reduction through waste-to-energy technologies. J. Clean. Prod. 2018, 176, 503–511. [Google Scholar] [CrossRef]
- Alvarez, J.; Lopez, G.; Amutio, M.; Artetxe, M.; Barbarias, I.; Arregi, A.; Bilbao, J.; Olazar, M. Characterization of the bio-oil obtained by fast pyrolysis of sewage sludge in a conical spouted bed reactor. Fuel Process. Technol. 2016, 149, 169–175. [Google Scholar] [CrossRef]
- Lehto, J.; Oasmaa, A.; Solantausta, Y.; Kytö, M.; Chiaramonti, D. Review of fuel oil quality and combustion of fast pyrolysis bio-oils from lignocellulosic biomass. Appl. Energy 2014, 116, 178–190. [Google Scholar] [CrossRef]
- Álvarez, E.A.; Mochón, M.C.; Sánchez, J.C.J.; Rodríguez, M.T. Heavy metal extractable forms in sludge from wastewater treatment plants. Chemosphere 2002, 47, 765–775. [Google Scholar] [CrossRef]
- Shen, L.; Zhang, D.-K. An experimental study of oil recovery from sewage sludge by low-temperature pyrolysis in a fluidised-bed. Fuel 2003, 82, 465–472. [Google Scholar] [CrossRef]
- Pedroza, M.M.; Sousa, J.F.; Vieira, G.E.G.; Bezerra, M.B.D. Characterization of the products from the pyrolysis of sewage sludge in 1kg/h rotating cylinder reactor. J. Anal. Appl. Pyrolysis 2014, 105, 108–115. [Google Scholar] [CrossRef]
- Park, H.J.; Heo, H.S.; Park, Y.-K.; Yim, J.-H.; Jeon, J.-K.; Park, J.; Ryu, C.; Kim, S.-S. Clean bio-oil production from fast pyrolysis of sewage sludge: Effects of reaction conditions and metal oxide catalysts. Bioresour. Technol. 2010, 101, S83–S85. [Google Scholar] [CrossRef] [PubMed]
- Domínguez, A.; Menéndez, J.A.; Pis, J.J. Hydrogen rich fuel gas production from the pyrolysis of wet sewage sludge at high temperature. J. Anal. Appl. Pyrolysis 2006, 77, 127–132. [Google Scholar] [CrossRef]
- Xie, Q.; Peng, P.; Liu, S.; Min, M.; Cheng, Y.; Wan, Y.; Li, Y.; Lin, X.; Liu, Y.; Chen, P.; et al. Fast microwave-assisted catalytic pyrolysis of sewage sludge for bio-oil production. Bioresour. Technol. 2014, 172, 162–168. [Google Scholar] [CrossRef] [PubMed]
- Lin, K.-H.; Lai, N.; Zeng, J.-Y.; Chiang, H.-L. Temperature influence on product distribution and characteristics of derived residue and oil in wet sludge pyrolysis using microwave heating. Sci. Total Environ. 2017, 584–585, 1248–1255. [Google Scholar] [CrossRef] [PubMed]
- Gao, N.; Li, J.; Qi, B.; Li, A.; Duan, Y.; Wang, Z. Thermal analysis and products distribution of dried sewage sludge pyrolysis. J. Anal. Appl. Pyrolysis 2014, 105, 43–48. [Google Scholar] [CrossRef]
- Jin, J.; Li, Y.; Zhang, J.; Wu, S.; Cao, Y.; Liang, P.; Zhang, J.; Wong, M.H.; Wang, M.; Shan, S.; et al. Influence of pyrolysis temperature on properties and environmental safety of heavy metals in biochars derived from municipal sewage sludge. J. Hazard. Mater. 2016, 320, 417–426. [Google Scholar] [CrossRef] [PubMed]
- Gao, N.; Quan, C.; Liu, B.; Li, Z.; Wu, C.; Li, A. Continuous Pyrolysis of Sewage Sludge in a Screw-Feeding Reactor: Products Characterization and Ecological Risk Assessment of Heavy Metals. Energy Fuels 2017, 31, 5063–5072. [Google Scholar] [CrossRef]
- Zhou, J.; Liu, S.; Zhou, N.; Fan, L.; Zhang, Y.; Peng, P.; Anderson, E.; Ding, K.; Wang, Y.; Liu, Y.; et al. Development and application of a continuous fast microwave pyrolysis system for sewage sludge utilization. Bioresour. Technol. 2018, 256, 295–301. [Google Scholar] [CrossRef]
- Zhuang, L.; Zhou, S.; Wang, Y.; Liu, Z.; Xu, R. Cost-effective production of Bacillus thuringiensis biopesticides by solid-state fermentation using wastewater sludge: Effects of heavy metals. Bioresour. Technol. 2011, 102, 4820–4826. [Google Scholar] [CrossRef]
- Longo, S.; Katsou, E.; Malamis, S.; Frison, N.; Renzi, D.; Fatone, F. Recovery of volatile fatty acids from fermentation of sewage sludge in municipal wastewater treatment plants. Bioresour. Technol. 2015, 175, 436–444. [Google Scholar] [CrossRef]
- Chen, H.; Chen, D.; Hong, L. Influences of activation agent impregnated sewage sludge pyrolysis on emission characteristics of volatile combustion and De-NOx performance of activated char. Appl. Energy 2015, 156, 767–775. [Google Scholar] [CrossRef]
- Chen, T.; Zhang, Y.; Wang, H.; Lu, W.; Zhou, Z.; Zhang, Y.; Ren, L. Influence of pyrolysis temperature on characteristics and heavy metal adsorptive performance of biochar derived from municipal sewage sludge. Bioresour. Technol. 2014, 164, 47–54. [Google Scholar] [CrossRef]
- Park, E.-S.; Kang, B.-S.; Kim, J.-S. Recovery of Oils with High Caloric Value and Low Contaminant Content by Pyrolysis of Digested and Dried Sewage Sludge Containing Polymer Flocculants. Energy Fuels 2008, 22, 1335–1340. [Google Scholar] [CrossRef]
- Pokorna, E.; Postelmans, N.; Jenicek, P.; Schreurs, S.; Carleer, R.; Yperman, J. Study of bio-oils and solids from flash pyrolysis of sewage sludges. Fuel 2009, 88, 1344–1350. [Google Scholar] [CrossRef] [Green Version]
- Lee, Y.-E.; Kim, I.-T.; Yoo, Y.-S. Stabilization of High-Organic-Content Water Treatment Sludge by Pyrolysis. Energies 2018, 11, 3292. [Google Scholar] [CrossRef]
- Kim, Y.; Parker, W. A technical and economic evaluation of the pyrolysis of sewage sludge for the production of bio-oil. Bioresour. Technol. 2008, 99, 1409–1416. [Google Scholar] [CrossRef] [PubMed]
- Ischia, M.; Maschio, R.D.; Grigiante, M.; Baratieri, M. Clay–sewage sludge co-pyrolysis. A TG–MS and Py–GC study on potential advantages afforded by the presence of clay in the pyrolysis of wastewater sewage sludge. Waste Manag. 2011, 31, 71–77. [Google Scholar] [CrossRef]
- Fonts, I.; Juan, A.; Gea, G.; Murillo, M.B.; Sánchez, J.L. Sewage Sludge Pyrolysis in Fluidized Bed, 1: Influence of Operational Conditions on the Product Distribution. Ind. Eng. Chem. Res. 2008, 47, 5376–5385. [Google Scholar] [CrossRef]
- Ma, R.; Sun, S.; Geng, H.; Fang, L.; Zhang, P.; Zhang, X. Study on the characteristics of microwave pyrolysis of high-ash sludge, including the products, yields, and energy recovery efficiencies. Energy 2018, 144, 515–525. [Google Scholar] [CrossRef]
- Huang, Y.-F.; Shih, C.-H.; Chiueh, P.-T.; Lo, S.-L. Microwave co-pyrolysis of sewage sludge and rice straw. Energy 2015, 87, 638–644. [Google Scholar] [CrossRef]
- Chen, G.-B.; Li, J.-W.; Lin, H.-T.; Wu, F.-H.; Chao, Y.-C. A Study of the Production and Combustion Characteristics of Pyrolytic Oil from Sewage Sludge Using the Taguchi Method. Energies 2018, 11, 2260. [Google Scholar] [CrossRef]
- Roche, E.; de Andrés, J.M.; Narros, A.; Rodríguez, M.E. Air and air-steam gasification of sewage sludge. The influence of dolomite and throughput in tar production and composition. Fuel 2014, 115, 54–61. [Google Scholar] [CrossRef]
- Damartzis, T.; Zabaniotou, A. Thermochemical conversion of biomass to second generation biofuels through integrated process design—A review. Renew. Sustain. Energy Rev. 2011, 15, 366–378. [Google Scholar] [CrossRef]
- Ferrasse, J.H.; Seyssiecq, I.; Roche, N. Thermal Gasification: A Feasible Solution for Sewage Sludge Valorisation? Chem. Eng. Technol. 2003, 26, 941–945. [Google Scholar] [CrossRef]
- Sikarwar, V.S.; Zhao, M.; Clough, P.; Yao, J.; Zhong, X.; Memon, M.Z.; Shah, N.; Anthony, E.J.; Fennell, P.S. An overview of advances in biomass gasification. Energy Environ. Sci. 2016, 9, 2939–2977. [Google Scholar] [CrossRef] [Green Version]
- Werle, S. Gasification of a Dried Sewage Sludge in a Laboratory Scale Fixed Bed Reactor. Energies 2015, 8, 8562–8572. [Google Scholar] [CrossRef] [Green Version]
- Dogru, M.; Midilli, A.; Howarth, C.R. Gasification of sewage sludge using a throated downdraft gasifier and uncertainty analysis. Fuel Process. Technol. 2002, 75, 55–82. [Google Scholar] [CrossRef]
- Midilli, A.; Dogru, M.; Howarth, C.R.; Ling, M.J.; Ayhan, T. Combustible gas production from sewage sludge with a downdraft gasifier. Energy Convers. Manag. 2001, 42, 157–172. [Google Scholar] [CrossRef]
- McKendry, P. Energy production from biomass (part 3): Gasification technologies. Bioresour. Technol. 2002, 83, 55–63. [Google Scholar] [CrossRef]
- Nilsson, S.; Gómez-Barea, A.; Cano, D.F. Gasification reactivity of char from dried sewage sludge in a fluidized bed. Fuel 2012, 92, 346–353. [Google Scholar] [CrossRef]
- Mun, T.-Y.; Kang, B.-S.; Kim, J.-S. Production of a Producer Gas with High Heating Values and Less Tar from Dried Sewage Sludge through Air Gasification Using a Two-Stage Gasifier and Activated Carbon. Energy Fuels 2009, 23, 3268–3276. [Google Scholar] [CrossRef]
- De Andrés, J.M.; Narros, A.; Rodríguez, M.E. Air-steam gasification of sewage sludge in a bubbling bed reactor: Effect of alumina as a primary catalyst. Fuel Process. Technol. 2011, 92, 433–440. [Google Scholar] [CrossRef] [Green Version]
- Arjharn, W.; Hinsui, T.; Liplap, P.; Raghavan, G.S.V. Evaluation of an Energy Production System from Sewage Sludge Using a Pilot-Scale Downdraft Gasifier. Energy Fuels 2013, 27, 229–236. [Google Scholar] [CrossRef]
- Seggiani, M.; Puccini, M.; Raggio, G.; Vitolo, S. Effect of sewage sludge content on gas quality and solid residues produced by cogasification in an updraft gasifier. Waste Manag. 2012, 32, 1826–1834. [Google Scholar] [CrossRef]
- Choi, Y.-K.; Ko, J.-H.; Kim, J.-S. A new type three-stage gasification of dried sewage sludge: Effects of equivalence ratio, weight ratio of activated carbon to feed, and feed rate on gas composition and tar, NH3, and H2S removal and results of approximately 5 h gasification. Energy 2017, 118, 139–146. [Google Scholar] [CrossRef]
- Mun, T.-Y.; Kim, J.-O.; Kim, J.-W.; Kim, J.-S. Influence of operation conditions and additives on the development of producer gas and tar reduction in air gasification of construction woody wastes using a two-stage gasifier. Bioresour. Technol. 2011, 102, 7196–7203. [Google Scholar] [CrossRef]
- Manyà, J.J.; Aznar, M.; Sánchez, J.L.; Arauzo, J.; Murillo, M.B. Further Experiments on Sewage Sludge Air Gasification: Influence of the Nonstationary Period on the Overall Results. Industrial & Engineering Chemistry Research 2006, 45, 7313–7320. [Google Scholar] [CrossRef]
- Nipattummakul, N.; Ahmed, I.I.; Kerdsuwan, S.; Gupta, A.K. Hydrogen and syngas production from sewage sludge via steam gasification. Int. J. Hydrogen Energy 2010, 35, 11738–11745. [Google Scholar] [CrossRef]
- Mun, T.-Y.; Kim, J.-W.; Kim, J.-S. Air gasification of dried sewage sludge in a two-stage gasifier: Part 1. The effects and reusability of additives on the removal of tar and hydrogen production. Int. J. Hydrogen Energy 2013, 38, 5226–5234. [Google Scholar] [CrossRef]
- García, G.; Monzón, A.; Bimbela, F.; Sánchez, J.L.; Ábrego, J. Desulfurization and Catalytic Gas Cleaning in Fluidized-Bed Co-gasification of Sewage Sludge–Coal Blends. Energy Fuels 2013, 27, 2846–2856. [Google Scholar] [CrossRef]
- De Andrés, J.M.; Narros, A.; Rodríguez, M.E. Behaviour of dolomite, olivine and alumina as primary catalysts in air–steam gasification of sewage sludge. Fuel 2011, 90, 521–527. [Google Scholar] [CrossRef] [Green Version]
- Choi, Y.-K.; Ko, J.-H.; Kim, J.-S. Gasification of dried sewage sludge using an innovative three-stage gasifier: Clean and H2-rich gas production using condensers as the only secondary tar removal apparatus. Fuel 2018, 216, 810–817. [Google Scholar] [CrossRef]
- Chen, S.; Sun, Z.; Zhang, Q.; Hu, J.; Xiang, W. Steam gasification of sewage sludge with CaO as CO2 sorbent for hydrogen-rich syngas production. Biomass Bioenergy 2017, 107, 52–62. [Google Scholar] [CrossRef]
- Cao, J.-P.; Huang, X.; Zhao, X.-Y.; Wang, B.-S.; Meesuk, S.; Sato, K.; Wei, X.-Y.; Takarada, T. Low-temperature catalytic gasification of sewage sludge-derived volatiles to produce clean H2-rich syngas over a nickel loaded on lignite char. Int. J. Hydrogen Energy 2014, 39, 9193–9199. [Google Scholar] [CrossRef]
- Lee, K.-W.; Lee, W.C.; Lee, H.J.; Dong, J.I. Gasification characteristics of sewage sludge combined with wood biomass. J. Mater. Cycles Waste Manag. 2014, 16, 642–649. [Google Scholar] [CrossRef]
- Aznar, M.; Manyà, J.J.; García, G.; Sánchez, J.L.; Murillo, M.B. Influence of Freeboard Temperature, Fluidization Velocity, and Particle Size on Tar Production and Composition during the Air Gasification of Sewage Sludge. Energy Fuels 2008, 22, 2840–2850. [Google Scholar] [CrossRef]
- Manyà, J.J.; Sánchez, J.L.; Gonzalo, A.; Arauzo, J. Air Gasification of Dried Sewage Sludge in a Fluidized Bed: Effect of the Operating Conditions and In-Bed Use of Alumina. Energy Fuels 2005, 19, 629–636. [Google Scholar] [CrossRef]
- Lee, U.; Dong, J.; Chung, J.N. Experimental investigation of sewage sludge solid waste conversion to syngas using high temperature steam gasification. Energy Convers. Manag. 2018, 158, 430–436. [Google Scholar] [CrossRef]
- Freda, C.; Cornacchia, G.; Romanelli, A.; Valerio, V.; Grieco, M. Sewage sludge gasification in a bench scale rotary kiln. Fuel 2018, 212, 88–94. [Google Scholar] [CrossRef]
- Campoy, M.; Gómez-Barea, A.; Ollero, P.; Nilsson, S. Gasification of wastes in a pilot fluidized bed gasifier. Fuel Process. Technol. 2014, 121, 63–69. [Google Scholar] [CrossRef]
- Reed, G.P.; Paterson, N.P.; Zhuo, Y.; Dugwell, D.R.; Kandiyoti, R. Trace Element Distribution in Sewage Sludge Gasification: Source and Temperature Effects. Energy Fuels 2005, 19, 298–304. [Google Scholar] [CrossRef]
- Sharma, A.K. Experimental investigations on a 20 kWe, solid biomass gasification system. Biomass Bioenergy 2011, 35, 421–428. [Google Scholar] [CrossRef]
- Peng, L.; Wang, Y.; Lei, Z.; Cheng, G. Co-gasification of wet sewage sludge and forestry waste in situ steam agent. Bioresour. Technol. 2012, 114, 698–702. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Liu, H.; Li, W.; Xu, J.; Liang, Q. Behavior of Phosphorus during Co-gasification of Sewage Sludge and Coal. Energy Fuels 2012, 26, 2830–2836. [Google Scholar] [CrossRef]
- Manara, P.; Zabaniotou, A. Towards sewage sludge based biofuels via thermochemical conversion—A review. Renew. Sustain. Energy Rev. 2012, 16, 2566–2582. [Google Scholar] [CrossRef]
- Kistler, R.C.; Widmer, F.; Brunner, P.H. Behavior of chromium, nickel, copper, zinc, cadmium, mercury, and lead during the pyrolysis of sewage sludge. Environ. Sci. Technol. 1987, 21, 704–708. [Google Scholar] [CrossRef] [PubMed]
- Elled, A.-L.; Åmand, L.-E.; Leckner, B.; Andersson, B.-Å. The fate of trace elements in fluidised bed combustion of sewage sludge and wood. Fuel 2007, 86, 843–852. [Google Scholar] [CrossRef]
- Shen, Y.; Yoshikawa, K. Recent progresses in catalytic tar elimination during biomass gasification or pyrolysis—A review. Renew. Sustain. Energy Rev. 2013, 21, 371–392. [Google Scholar] [CrossRef]
- Campoy, M.; Gómez-Barea, A.; Fuentes-Cano, D.; Ollero, P. Tar Reduction by Primary Measures in an Autothermal Air-Blown Fluidized Bed Biomass Gasifier. Ind. Eng. Chem. Res. 2010, 49, 11294–11301. [Google Scholar] [CrossRef]
- Smoliński, A.; Howaniec, N.; Bąk, A. Utilization of Energy Crops and Sewage Sludge in the Process of Co-Gasification for Sustainable Hydrogen Production. Energies 2018, 11, 809. [Google Scholar] [CrossRef]

| Author(s) | Fuel Types Investigated | Reactor Type Used | Treatment Methods | Observations |
|---|---|---|---|---|
| Devlin et al. [32] | Thickened waste activated sludge and inoculum sludge (15–20 days old) | Full scale, semi-continuous (12 days) anaerobic digester at 15 days hydraulic retention time (HRT) at 35 °C | Acid pre-treatment—HCl for attaining pH 6–1 (chemical) | Optimal acid dosing for attaining pH of 2 obtained same biogas yield in 13 digestion days versus 21 days required for untreated sludge. 14.3% increase in methane yield. |
| Lin et al. [37] | Pulp and Paper sludge | Batch reactor at 42 days HRT at 37 °C | Alkali pre-treatment—4 g/8 g/16 g NaOH/100 g total solid sludge (chemical) | 54–88% increase in methane production with 8 g NaOH being the optimal amount of pre-treatment chemical. Alkaline pre-treatment can be more suitable for soluble chemical oxygen demands (COD) degradation (83–93%) |
| Weemaes et al. [38] | Sewage Sludge (7 days old) | Batch reactor at 30 days HRT at 33 °C | Ozonation at 10–50 mg O3/l gas for up to 3 h 38 min to get 0.05 g/0.1 g/0.2 g O3/g COD | Partial oxidation was promoted by the ozonation reaction which increased methane production by a factor of 1.8. Digestion rate accelerated by a factor of 2.2. Optimal ozone dose of 0.1 g O3/g COD. |
| Valo et al. [19] | Waste activated sludge | Continuous reactor at 20 days HRT at 35 °C | Alkali pre-treatment: pH 10–12 (KOH) | 21.4% increase in soluble COD with increase in pH from 10 to 12 |
| Valo et al. [19] | Waste activated sludge | Continuous reactor at 20 days HRT at 35 °C | Thermo-chemical pre-treatment—130 °C and 170 °C for 30–60 min, pH 10–12 (KOH) | 21.3% increase in soluble COD at 130 °C, further 32% increase in SCOD at 170 °C at pH 10. SCOD reaches 83% for pH 12 sludge at 170 °C. 72–78% increase in biogas yield, 36.4% increase in COD removal was achieved. |
| Takashima and Tanaka [39] | Anaerobically digested sludge | Batch reactor at 20 days HRT at 35 °C | Thermo-chemical post-treatment—25 °C 100 °C and 180 °C for 1 h, pH 2/4/6 (HCl) | Methane production increased from 0.11 g COD-CH4 to 0.18, 0.25 and 0.32 g COD-CH4 at 25, 100 and 180 °C. Volatile soluble solid destruction also increased by 6.7, 11.3 and 26% respectively. |
| Takashima and Tanaka [39] | Anaerobically digested sludge | Continuous reactor at 20 days HRT at 35 °C | Thermo-chemical post-treatment—170 °C for 1 h, pH 5–6 (HCl and H2SO4) | Methane production increased by 14–21%. Volatile soluble solid destruction also increased by a factor of 2–2.5. Optimal dose of 3.3mmol HCl or 1.8 mmol H2SO4 per day. |
| Nielsen et al. [18] | Waste activated sludge and inoculum sludge | Batch reactor for 40 days or two-steps of 19–21 days each in inter-stage at 37 °C | Thermal pre- and inter-stage treatment at moderate temperature: water bath at 80 °C | Pre-treatment: total volatile solid increased from 2 to 29%. Inter-stage: total volatile solid increased from 2 to 27%. |
| Nielsen et al. [18] | Waste activated sludge and inoculum sludge | Batch reactor for 40 days or two-steps of 19–21 days each in inter-stage at 37 °C | Thermal pre- and inter-stage treatment at high temperature at 130–170 °C | Pre-treatment: total volatile solid increased from 2 to 17% at 130 °C and 44% at 170 °C. Methane yield increased by 13% and 9% respectively. Inter-stage: total volatile solid increased from 2 to 25% and 57% at 130 °C and 170 °C respectively with 9 and 29% increase in methane yield. |
| Nielsen et al. [18] | Waste activated sludge and inoculum sludge | Batch reactor for 40 days or two-steps of 19–21 days each in inter-stage at 37 °C | Thermochemical pre- and inter-stage treatment at 170 °C, pH 10 using KOH | Pre-treatment: total volatile solid increased from 2 to 68% at 170 °C. Methane yield increased by 2%. Inter-stage: total volatile solid increased from 2 to 74% and 28% increase in methane yield. |
| Riau et al. [33] | Waste activated sludge and inoculum sludge | Two batch reactors for 20 days solid retention time (SRT) at 55 °C (thermophilic) and 35 °C (mesophilic) | Mechanical pre- and inter-stage treatment using sonication at 24 KHz, 400 W at 5–10 min | Total methane production increased by 42%, volatile solid removal by 13% and SCOD elimination was increased by 22%. |
| Martín et al. [34] | Sewage Sludge | Batch reactor at 35 °C for 10 days | Mechanical pre-treatment using sonication at 150 W at 0–60 min | 38–91% soluble COD fraction. 64–95% increase in methane yield with optimal enhancement at 45 min sonication. |
| Houtmeyers et al. [35] | Thickened sludge (500 g) | Semi-continuous reactor at 20 days HRT for 67 days at 37 °C | Mechanical pre-treatment using ultrasonic pre-treatment at 100 W at 8 min | 1741% increase in soluble COD fraction. 24% increase in Sludge removal. 27% increase in biogas yield |
| Houtmeyers et al. [35] | Thickened sludge (500 g) | Semi-continuous reactor at 20 days HRT for 67 days at 37 °C | Physiochemical pre-treatment using microwave radiation at 800 W at 2.45 ghz for 1 min | 117% increase in soluble COD fraction. 45% increase in Sludge removal. 20% increase in biogas yield |
| Ebenezer et al. [40] | Activated Sludge | Batch reactor at 35 °C for 35 days | Physiochemical pre-treatment using microwave radiation at 900 W at 2.45 ghz for 30–300 s (40–96 °C) | 950% increase in soluble COD fraction. 47% increase in biodegradability. Increase in biogas yield. |
| Coelho et al. [41] | Thickened sludge | 10 semi-continuous reactors for 20 days at 55 °C (thermophilic) and 35 °C (mesophilic) | Physiochemical pre-treatment using microwave radiation at 1250 W at 2.45 ghz for 6 min | 63–207% increase in biogas production. 50% increase in volatile solid removal. |
| Appels et al. [42] | Activated Sludge | Semi-continuous reactor at 20 days HRT—42 days at 37 °C | Physiochemical pre-treatment using microwave radiation at 800 W at 2.45 ghz for 3.5 min | 214% increase in soluble COD fraction. 50% Increase in biogas yield. 67% increase in solids removal. |
| Wilson et al. [43] | Mixed sludge | Semi-continuous reactor at 15–20 days SRT at 37–42 °C | Thermal hydrolysis pre-treatment at 150–170 °C, 4.8–7.9 bar. | 56–62% reduction in volatile solid in digestate. 24–59% increase in biogas production |
| Pilli et al. [44] | Primary, Secondary and mixed sludge | Semi-continuous reactor at 30 days SRT at 35 °C | Thermal pre-treatment using hydrolyser at 134–140 °C, 3.4 bar for 30 min. | 12.6% reduction in volatile solid in digestate. 40.2% increase in methane production |
| Xue et al. [45] | dewatered sludge (high solid sludge) | Batch reactor at 28 days SRT at 37 °C | Thermal pre-treatment using low temperature hydrolysis at 60–90 °C, for 1–72 h | 557–1678% increase in SCOD, negligible change in biogas yield. Optimal SRT of 18–20 days |
| Xue et al. [45] | dewatered sludge (high solid sludge) | batch reactor at 28 days SRT at 37 °C | Thermal pre-treatment using high temperature hydrolysis at 120–180 °C, for 15–180 min | 582–1087% increase in SCOD, 6.3–16.5% increase in biogas yield. SRT reduction to 12–14 days |
| Chemical (Acid or Alkali Treatment) | Chemical (Ozonation) | Ultrasonication | Microwave Irradiation | Thermal Treatment | |
|---|---|---|---|---|---|
| Pro(s) | Methane yield increase | Methane yield increase | Methane yield increase | Methane yield increase | methane yield increase |
| Simple and easy to integrate | Flexible operation | Low cost pre-treatment method | Fast and uniform heating process | Reduce sludge volume | |
| Low cost pre-treatment method | Reduced sludge volume | Easy maintenance | Easy to control | Improves dewater-ability | |
| Con(s) | High cost of chemicals | High energy requirements | High energy requirements | High energy requirements | High energy requirements |
| Special reactor requirements | Mineralization potential of cellular matter | Unsuitable for lignocellulosic biomass | Scale up challenges | High capital cost | |
| Toxicity of some alkali like Na+ | - | - | - | Potential for ammonia generation |
| Author(s) | Fuel Types Investigated | Reactor Type Used | Observations |
|---|---|---|---|
| Hao et al. [72] | Thermally dried (TD) and bio-dried (BD) sewage sludge (50–450 µm) | Horizontal tube furnace for isothermal combustion at 600–1000 °C (air, 10 min) | Removal of ~26% more moisture in BDSS resulted in higher LHV (37.9%), higher combustion index and prevents incomplete combustion. ~20% and 15.7% less nitrogenous gas and CO emission in the flue gas. Up to 26.5% less NO but increase in SO2 emitted in BDSS in comparison to TDSS. |
| Niu and Shen [68] | Dewatered Sewage sludge (200–450 µm) | Quartz fluidized bed for chemical looping combustion at 850–950 °C (5 vol% O2 in N2 and reduced by 8 vol% CO, 15 min) | High Phosphorus (66.8–69.3%) availability in fly ash from sewage sludge processing with potential to use as fertilizer. However, The high P content influences the activity of the hematite oxygen carrier in the reaction. Ca and Fe in sludge ash promoted the reduction reactivity of the hematite. |
| Cheng et al. [69] | Air dried Municipal sewage sludge (80–120 µm) | Thermogravimetric analyzer (TGA) at 800 °C, 40 °C/min (100 mL/min N2 with 0–20% O2) | Main difference in the reaction in inert atmosphere and slightly oxygenated atmosphere is in the char and ash combustion zone. ~10 vol% O2 was adequate for complete combustion of char particles, although at higher temperature range in comparison to 15 and 20 vol%. |
| Chen et al. [70] | Rice husk and sewage sludge | Muffle furnace at 600 & 750 °C for sewage sludge and rice husk/sewage sludge ash | High Fe and heavy metals in sewage sludge ash. In the rice husk/sewage sludge blend, heavy metals were stabilized, reducing emission and retention in ash. Mechanical properties of the ash derived from co-combustion has potential use as cement material substitute. |
| Kijo-Kleczkowska, et al. [62] | Sewage sludge; brown and hard coal; pine and straw (as pellets) | Bench reactor at 800–900 °C in air | Visually detected reaction time—Coal (403–700 s) > Sewage Sludge (200 s) > Biomass (80–100 s). Volatiles / Char combustion time—Coal (9–20; 77–88%); Sludge (23/73%); biomass (26–27; 68–70%) of reaction time. However, the volatiles oxidation is the most important stage for biomass and sludge combustion. Optimisation of reactor temperature and air flow velocity for different particle size such that at 7.5 mm, sludge requires 800–800 °C and 2.8 m/s to minimise ignition time and surface temperature of fuel while ensuring burnout. This could be used to match fuels that can be co-blended together i.e., brown coal or willow with 800–850 °C and 2.8 m/s optimal condition. |
| Batistella et al. [61] | Dewatered sewage sludge | Pilot scale Rotary dryer (128–354 °C) with Moving bed combustor at 700–800 °C (Inlet); 950–1150 °C (post combustion) | CO emission was negligible from the combustor, however the dehydration process at lower temperature emits CO. This was reduced by controlling and optimizing the oxygen content (from exhaust gas) and temperature of the dryer. The NOx emissions remained below standard limits by optimising excess air, air/fuel feed rate and maintaining low reaction temperature. The high temperature in the post combustion chamber aided decomposition of PAH in flue gas. Low calcium content of fuel resulted in higher SOx emission and high particulate matter. |
| Lin et al. [63] | Activated sewage sludge and dewatered oil shale blends at 10, 30, 50, 70, 90 wt% sludge (<178 µm) | Thermogravimetric analyzer (TGA) at 1000 °C, 10–30 °C/min (80 mL/min air) | Blending with sewage sludge resulted in easier ignition properties due to its higher volatile content. Ash content increased due to higher ash of oil shale. Blends with 10 wt% sludge resulted in combustion promotion by reducing activation energy. |
| Huang et al. [64] | Sewage sludge and water hyacinth blends at 10, 20, 30 and 40 wt% water hyacinth | TGA at 1000 °C, 10–40 °C/min (50 mL/min CO2/O2) | Drying and combustion reaction zone of sewage sludge was improved by blending with water hyacinth, enhancing reactivity. |
| Wang et al. [65] | Sewage sludge and bituminous coal blends at 5–50 wt% (<75 µm). | TGA at 900 °C, 20 °C/min (80 mL/min N2/O2). 5% catalyst (CaO, CeO2, MnO2 and Fe2O3) | The blending of sewage sludge with coal at high fractions (>10 wt%) deteriorated the combustion characteristics of the blends in comparison to coal. The use of catalysts, particularly Ce- and Fe-based, drastically improved the ignition and combustion properties of the blends. |
| Kijo-Kleczkowska et al. [66] | Sewage sludge blended with lignite, hard coal and willow at 50:50 wt% (as pellets) | Bench reactor at 800–900 °C in air | Volatiles / Char combustion time—Sludge + lignite (24; 73%); Sludge + hard coal (13/84%); sludge + willow (28; 67%) of reaction time. Blending with sludge reduces ignition time especially in coal samples, extends the reaction time for biomass blend and maximum surface temperature. |
| Deng et al. [67] | Sewage sludge and wheat straw blends at 20–80 wt% biomass (<200 µm) | TGA at 1000 °C, 20 °C/min (100 mL/min N2/O2). | The addition of wheat straw to sewage sludge improves the char combustion reactivity and heat released due to changes in physiochemical properties such as higher surface area and pore volume of the pyrolytic char. |
| Author(s) | Fuel types Investigated | Reactor Type Used | Observations |
|---|---|---|---|
| Alvarez et al. [87] | Sewage sludge (0.5–3 mm) (Anaerobic digested and thermally dried before use) | Conical spouted bed reactor at 450, 500 and 600 °C with <100 ms residence time (fast pyrolysis) | Bio-oil yields was 44.8, 48.5 and 45.4 wt% at 450, 500 and 600 °C. Liquid yield was maximised at 500 °C due to low volatile residence time and lack of secondary cracking reactions. Char and gas were highest at 450 °C and 600 °C respectively. The fuel properties differ from hydrocarbon and biomass derived oils, hence more suitable for chemical production. |
| Jin et al. [97] | Sewage sludge | Homemade pyrolysis and carbonization furnace (400–600 °C) for 60 min | Biochar yield decreased from 60.6 to 53.1% with increasing temperatures. High concentration of heavy metals retained in biochar. Biochar has lower surface area in comparison to other adsorbents due to pore blockages by tar. Decrease in the amount of bioavailable heavy metals while oxidative and residual fractions increased which indicates lower levels of ecological risks with biochar disposal or usage |
| Gao et al. [96] | Dried sewage sludge | Horizontal tubular furnace reactor (2 L/min N2 at 450–650 °C. slow and fast pyrolysis at 8 and 100 °C/min) | Fast Pyrolysis—Char yield reduced from 47.07 to 29.96% with increasing temperature. Tar yield increased to maximum (46.14%) at 550 °C and then decreased with further increase in temperature. Gas yield increases with increase in temperature. Slow Pyrolysis—Decrease in char (33.24–53.6%) and tar (32.18–38.28%) and increase in gases (14.24–28.64%) due to secondary cracking reactions. |
| Lin et al. [95] | Wet sludge (84.9 ± 1.2 wt% water) | Multimode microwave reactor (900 W, 200 mL/min N2, 400–800 °C for 30 min) | Gas phase: 15–29%; Liquid phase: 14–20%; and Solid fraction: 57–69%. The maximum yield and heating value (HV) of bio-oil was obtained at 600 °C and the HV 2.7 times of the dried sludge cake. Microwave processing is promising for sludge. |
| Park et al. [104] | Digested and dried sewage sludge | Fluidized bed reactor (3 kW, 200 mL/min N2, 446–720 °C for 30 min) | Maximum of 54 wt% of bio-oil was obtained with HV of about 2 times that of the dried sewage sludge HV of gas and char ranged from 5–30 and 5–10 MJ/Kg. The bio-oil has negligible hazardous metals but has very high N content. |
| Pokorna et al. [105] | Thickened excess activated sludge (AKP), dewatered digested sludge (DIG), and dried excessive activated sludge (OLDA) | Semi-continuous lab scale flash pyrolysis reactor at 500 °C | Bio-oil yields varies between 39.2–57.5%. The water content of bio-oil varies between 10.3–17% based on the sludge type. HV ranged between 23.9–29.0 MJ/kg for bio-oil and 5.2–10.6 MJ/kg for char. |
| Kim and Parker [107] | Primary sewage sludge, thickened waste activated sludge and digested sludge bio-solids | Cylindrical batch pyrolysis reactor at 250–500° C with zeolite as catalyst and acid pre-treatment. | Calorific value of 32–42MJ/kg and 7–23MJ/kg for bio-oil and char respectively. Oil yield ranges between 4–42% with maximum at 500 °C for primary sludge. Other types of sludge generated maximum oil at ~400 °C. Char yield varies between 33–87% with maximum at 250 °C. The use of zeolite reduces char yield but does not impact the oil yield considerably. Pre-treatment did not enhance the bio-oil yield. Most economically viable option is the use of primary sludge at 500 °C. |
| Zhou et al. [99] | Sewage sludge (78% water content) | Benchtop batch and continuous microwave reactor (1000 W and 2.45 GHz at 450–600 °C) with HZSM-5 catalyst | Bio-oil yield ranges from 16.47–39 wt% with maximum at 550 °C. Char yield decreased from 62.26–32.98 wt% with increasing temperature. The continuous reactor increased bio-oil yields by ~16%. The use of HZSM-5 decreased the bio-oil generated in both reactors. |
| Fonts et al. [109] | Digested and dried sewage sludge | Lab scale fluidized bed reactor at 450–650 °C, 3.5–5.5 L/min and feed rate of 3–6 g/min for 75 min | Solid, liquid and gas yields are between 46.1–63.0%, 23.4–40.7% and 7.3–27.9% respectively with maximum yield at 450, 550 and 650, respectively. The feed rate and N2 flow rate does not affect the liquid yield, low feed rate and N2 flow rate enhances solid yield. |
| Ischia et al. [108] | Sewage Sludge | TGA at 1000 °C, 10 °C/min (80 mL/min He). Clay catalyst. | 52 wt% mass reduction up to 600 °C with evolved gases mostly hydrocarbons. With clay as catalyst, concentration of CO reduced with higher CO2. |
| Pedroza et al. [91] | Dried sewage sludge | Continuous feed rotating cylinder reactor (500–600 °C, 50 mL/min Nitrogen, 4–22 g/min mass) | High temperature increases gas fraction with 23.9% gas yield at 600 °C with 56% synthesis gas concentration. Decrease in char with increasing temperature with lowest of 52.7% at 600 °C. Bio-oil yield was maximum at 500 °C with 10.5%. |
| Gao et al. [98] | Dried sewage sludge | Continuous feed screw reactor (500–800 °C; 6–46 min residence time; Nitrogen, 4–100 g/min mass) | Char yield reduces by ~9 wt% while gas yield increases by ~9 wt% with 40 min increase in residence time. Bio-oil yield increased by ~3% with 17min increase but decreased afterwards. Optimal residence time for char conversion and bio-oil maximization was determined to be 23 min. Gas and char yield increases and decreases with increase in temperature respectively while bio-oil increases till 700 °C then decreased drastically at 800 °C. The ecological risk of char obtained remained moderate in comparison to the fuel ash. |
| Ma et al. [110] | Dewatered high ash sludge (88.10wt% moisture) | Microwave reactor at 0.7–1 kW with 10 mL/min Nitrogen (SiC, graphite and residue absorbent) | Biogas (10.74–16.93%), bio-oil (2.98–3.52%) and biochar (78.27–84.30%) yield with various absorbents. Pyrolysis with SiC and graphite gave the highest biogas and bio-oil yield respectively. Biochar and biogas yield reduced and increased with increasing input power. |
| Xie et al. [94] | Sewage sludge | Microwave oven of 750 W at 2.45 GHz with HZSM-5 catalyst from 450–600 °C | Maximum yield of bio-oil was achieved at 550 °C. The oil and gas yield are maximum without the use of catalyst while char yield increased with catalyst loading. However, the quality of bio-oil (lowest oxygen- and nitrogen-containing compounds) was improved at catalyst to feed ratio of 2:1. |
| Huang et al. [111] | Dry sewage sludge cake and rice straw | Single mode Microwave at 2.45 GHz | The use of rice straw enhanced microwave absorption and efficiency of the reaction. The need for further study is required for establishing feasibility of microwave co-pyrolysis. |
| Chen et al. [112] | Dried Digested Sewage Sludge | Fixed bed Tubular furnace, 700 mL/min N2, 450 °C, 60 min | Maximum pyrolytic oil yield of 10.19 wt%. The heating rate, temperature, residence time and nitrogen flow rate affects the yield. |
| Author(s) | Fuel Types Investigated | Reactor Type Used | Observations |
|---|---|---|---|
| Choi et al. [133] | Digested sewage sludge | Three stage gasifier (auger (650 °C), fluidized bed (815 °C) and tar cracking (815 °C) reactors connected serially), gasifying agent—pre-heated air, Catalyst—activated carbon, ER—0.3 | The use of activated carbon increase syngas yield by 12%, while reducing tar, hydrogen sulphide and ammonia content in the syngas. In addition, the carbon conversion and tar removal efficiency increased by 10 and 26% respectively. The use of Fe- and Ni-impregnated activated carbon reduced the hydrogen sulphide and ammonia respectively. Increasing the gasification time (~8 h) effectively removed tar in output. |
| Roche et al. [113] | Dried Sludge | Laboratory scale fluidized bed gasifier (800 °C), gasifying agent—air + steam, ER—0.3, Catalyst—dolomite, feed rate—110—322 kg/h m2 | Different feed rate influenced the quality and composition of the tar generated. Higher feed rate decreased syngas yield while increasing tar content while lower feed rate maximized H2 and CO in product. The use of dolomite enhanced tar removal efficiency to ~71%. 20–36% increase in H2 yield with dolomite. |
| Nipattummakul et al. [131] | Solar dried sewage sludge | Laboratory scale fixed bed gasifier (700–1000 °C), gasifying agent—steam (3 g/min) | Char gasification, H2 and CO content of the producer gas increased with increasing temperature. Energy conversion efficiency was maximum at 1000 °C. |
| Manyà et al. [138] | Digested sewage sludge | Laboratory scale fluidized bed gasifier (750–850 °C), gasifying agent—air, ER—0.25–0.35, alumina bed | The heating value and cold gas efficiency increased with increasing temperature, attributed to steam reforming and cracking of tar. The gas yield increased with increasing ER. H2 content of syngas increases with the alumina bed. |
| Chen et al. [134] | Sewage sludge | Laboratory scale fixed bed gasifier (650–850 °C), gasifying agent—steam (0.2 g/min), Catalyst—calcium oxide(CaO), Ni- and Fe-impregnated CaO | Without the CaO sorbents, hydrogen fraction and overall syngas yield increases with increase temperature. The use of CaO at lower temperatures increased the H2 fraction by enhancing tar cracking, however CaO with higher temperatures, the H2 fraction reduced while CO2 increased. Though carbon conversion efficiency and cold gas efficiency increased with CaO use, it had negligible influence on char gasification but on tar cracking. The impregnation of metals improved methane reforming and char conversion. |
| Lee et al. [139] | Dried sewage sludge | Laboratory scale gasifier (1000 °C), gasifying agent—steam (2.5–20 g/min) | Higher reaction temperature improved water gas shift reaction rates, hence CO2 and H2 generation rates increased at the expense of CO and CH4 with time. Increase in steam flow rate increased the syngas generation rates at lower residence time. Carbon conversion is enhanced with higher steam flow rate. |
| Freda et al. [140] | Oven-dried sewage Sludge | Bench scale rotary Kiln gasifier, 750—850 °C, ER 0.05–0.24 | Producer gas of higher heating value was obtained at temperatures of 800–850 °C and ER of 0.15–0.24. Maximum cold gas efficiency of 67% was obtained at 850 °C with ER of 0.16 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oladejo, J.; Shi, K.; Luo, X.; Yang, G.; Wu, T. A Review of Sludge-to-Energy Recovery Methods. Energies 2019, 12, 60. https://doi.org/10.3390/en12010060
Oladejo J, Shi K, Luo X, Yang G, Wu T. A Review of Sludge-to-Energy Recovery Methods. Energies. 2019; 12(1):60. https://doi.org/10.3390/en12010060
Chicago/Turabian StyleOladejo, Jumoke, Kaiqi Shi, Xiang Luo, Gang Yang, and Tao Wu. 2019. "A Review of Sludge-to-Energy Recovery Methods" Energies 12, no. 1: 60. https://doi.org/10.3390/en12010060





