Memory Under Stress: How Post Traumatic Stress Disorder Affects Working Memory in Adults: A Scoping Review
Abstract
1. Introduction
- (a)
- Intrusive re-experiencing (e.g., flashbacks or nightmares);
- (b)
- Avoidance of trauma-related stimuli;
- (c)
- Negative alterations in cognition and mood (e.g., memory impairments, emotional numbing);
- (d)
- Hyperarousal and heightened reactivity (e.g., irritability, sleep disturbances).
2. Materials and Methods
2.1. Literature Searches
2.2. Selection Criteria
2.3. Data Extraction
2.4. Data Analysis
3. Results
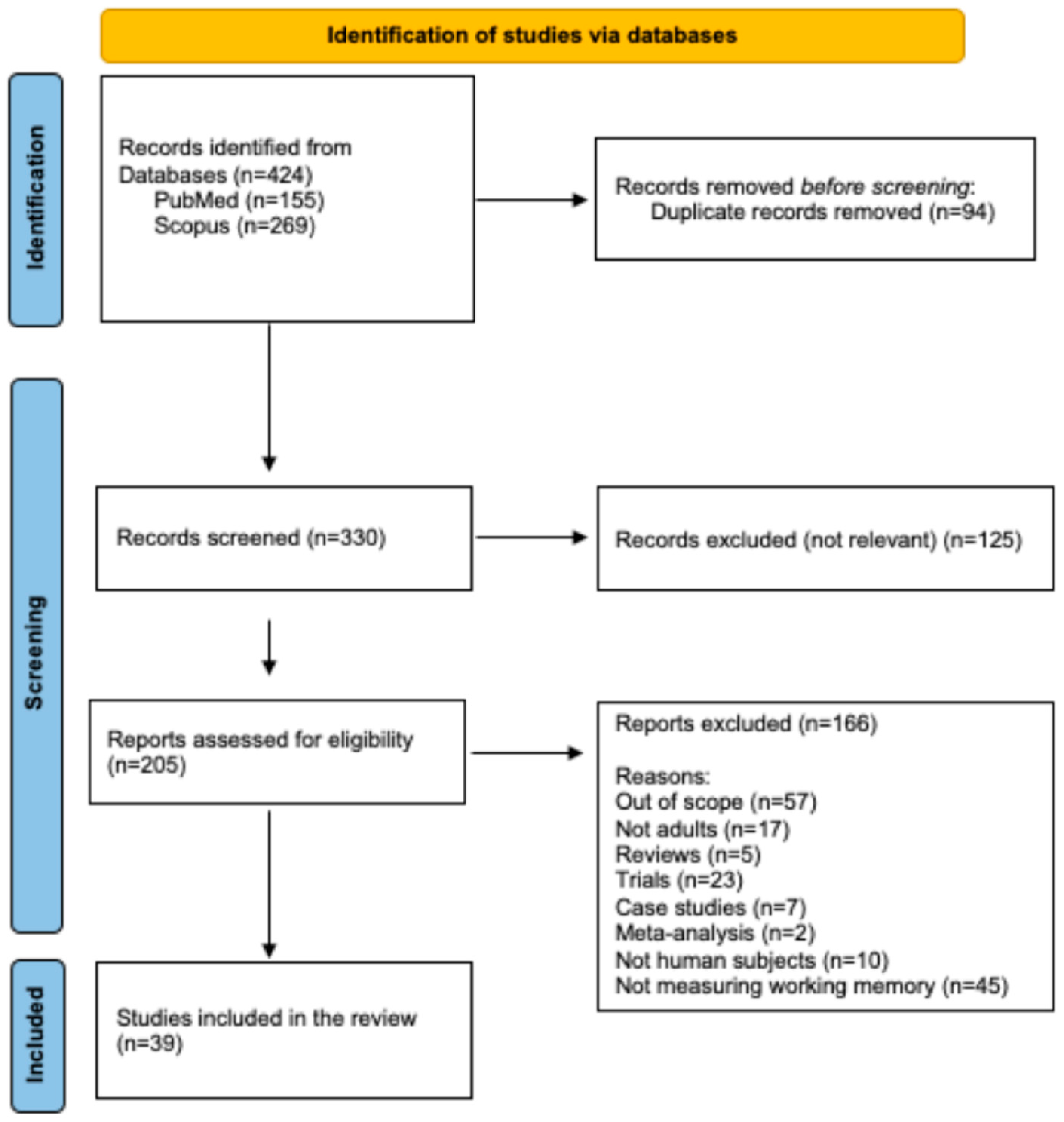
3.1. Database Searches
3.2. Study Characteristics
3.3. Participants’ Profile
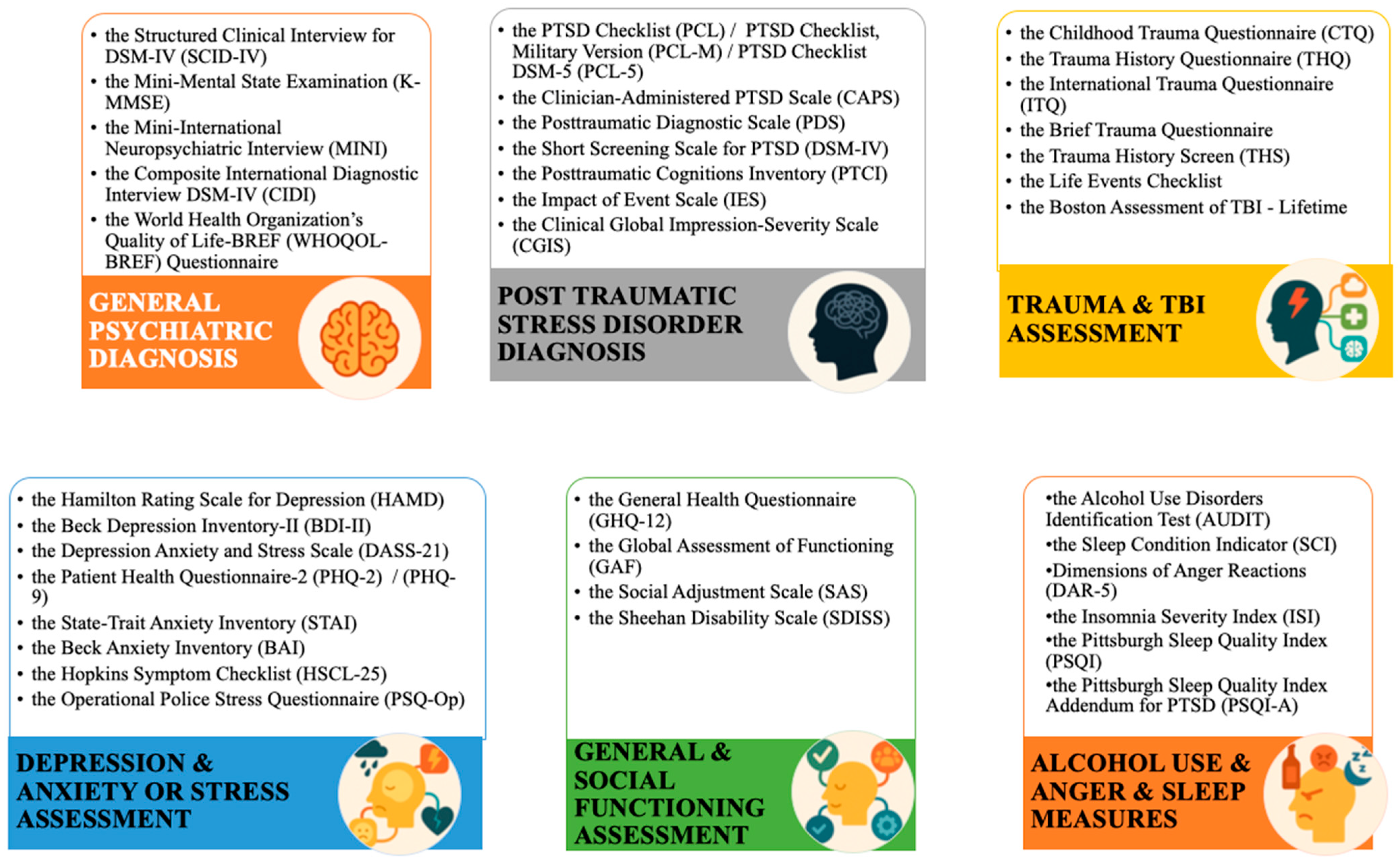
3.4. Trauma Characteristics and Assessment Tools
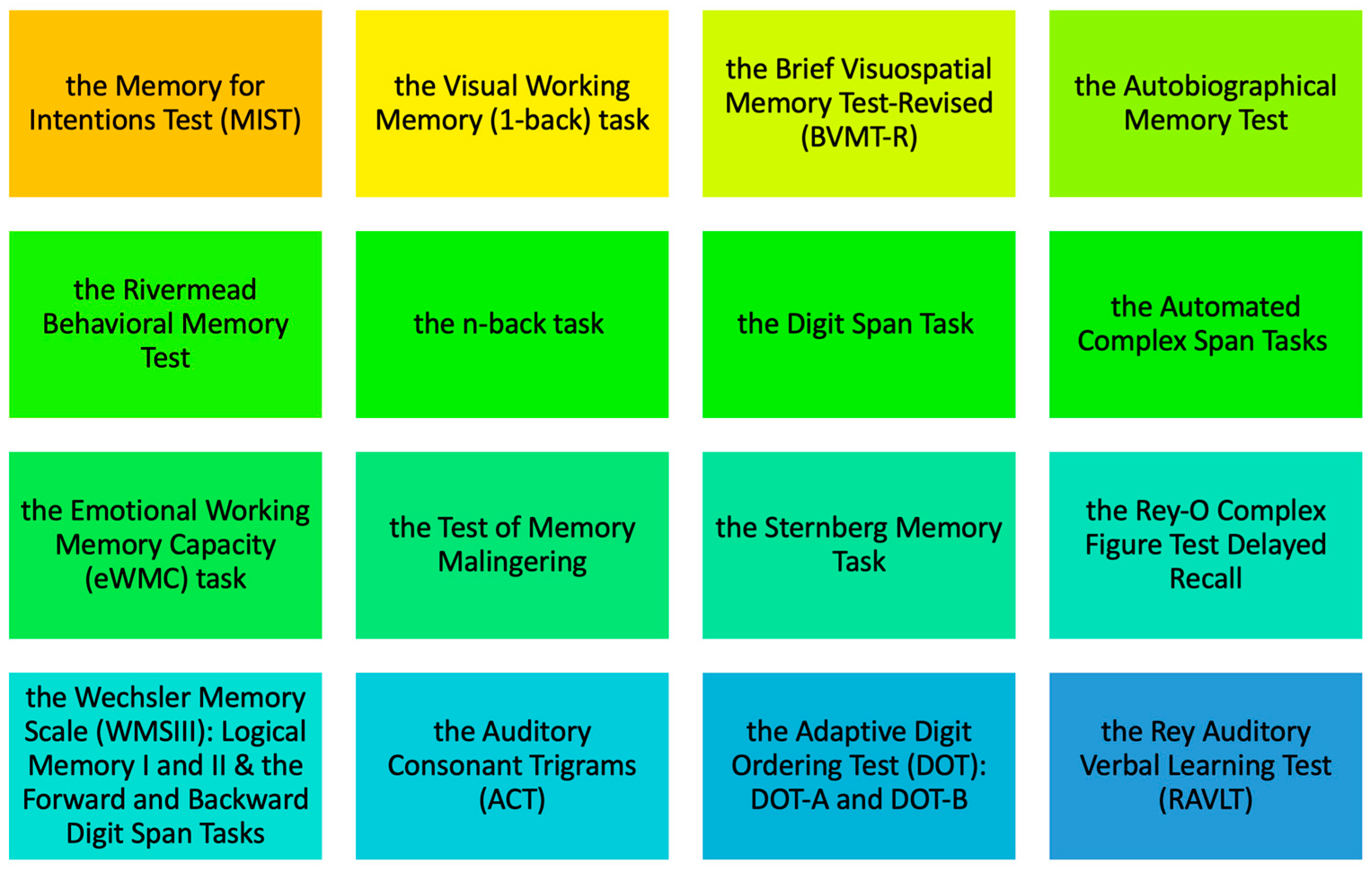
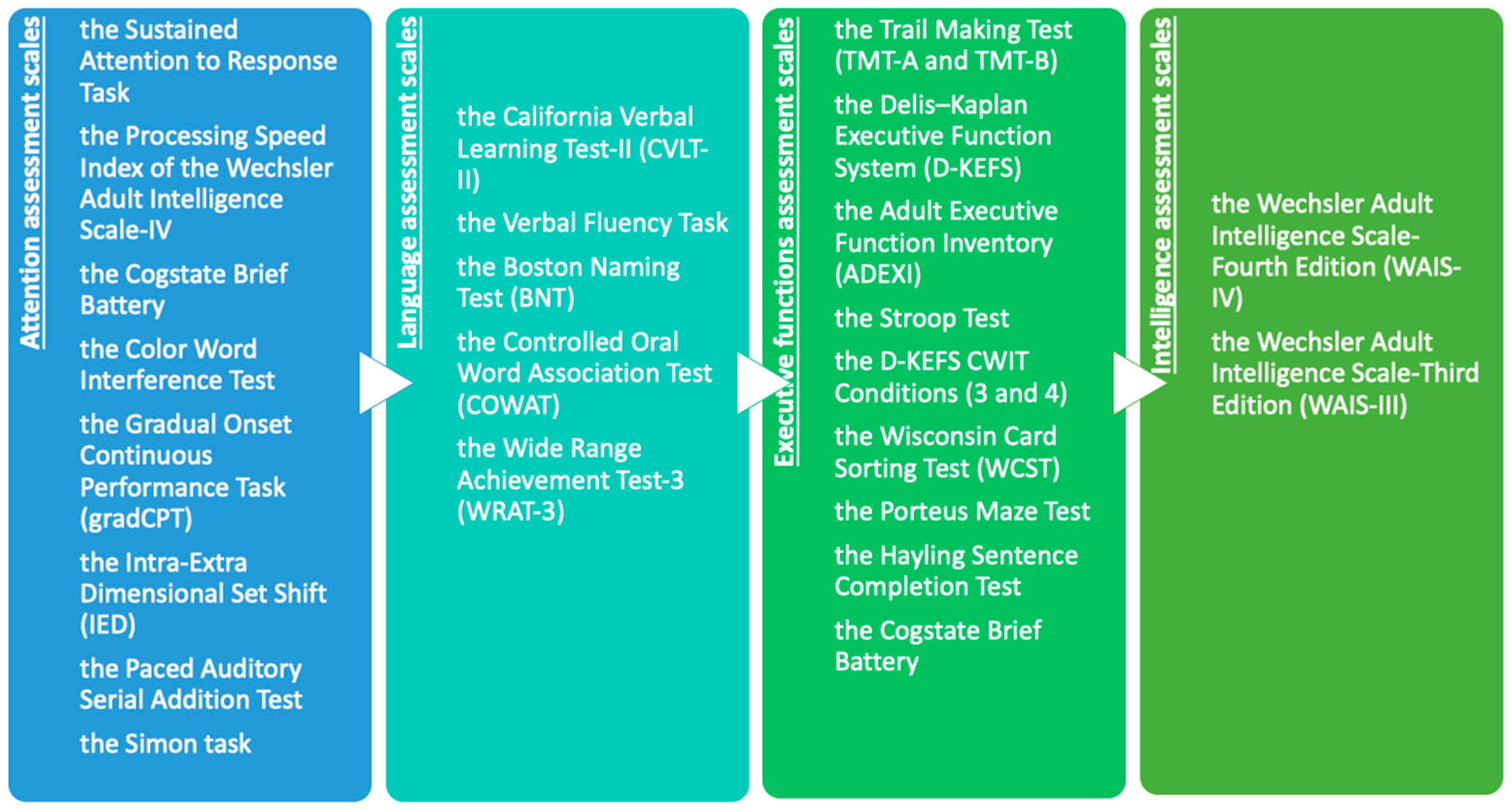
3.5. Neuropsychological Assessment Tools
4. Discussion
4.1. PTSD and WM Impairments
4.2. Neural Mechanisms and Functional Connectivity Alterations in PTSD
4.3. Impact of Trauma Type and Severity on WM in PTSD
4.4. Performance Patterns in WM Tasks in PTSD
4.5. Recovery Potential and Prognostic Indicators for WM in PTSD
4.6. Gender, Age, and Occupational Variations in PTSD-Related Cognitive Deficits
4.7. Strengths and Limitations of the Study
4.8. Practical Implications and Future Directions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PTSD | Post-Traumatic Stress Disorder |
| WM | Working Memory |
| DSM-5 | Diagnostic and Statistical Manual of Mental Disorders, 5th Edition |
| ICD-11 | International Classification of Diseases, 11th Revision |
| PRISMA-ScR | Preferred Reporting Items for Systematic Reviews and Meta-Analyses extension for Scoping Reviews |
| APOE | Apolipoprotein E (APOE ε4 allele) |
| BDNF | Brain-Derived Neurotrophic Factor (BDNF Val66Met polymorphism) |
| rsFC | Resting-State Functional Connectivity |
| IFG | Inferior Frontal Gyrus |
| STG | Superior Temporal Gyrus |
| DLPFC | Dorsolateral Prefrontal Cortex |
| vlPFC | Ventrolateral Prefrontal Cortex |
| dlPFC | Dorsolateral Prefrontal Cortex (αναφέρεται επίσης με μικρά) |
| CPTSD | Complex Post-Traumatic Stress Disorder |
| DSO | Disturbances in Self-Organization |
| ERP | Event-Related Potential |
| PM | Prospective Memory |
| SWM | Social Working Memory |
| DMN | Default Mode Network |
| DMPFC | Dorsomedial Prefrontal Cortex |
| HC/HCs | Healthy Controls |
| CBT | Cognitive Behavioral Therapy |
| IQ | Intelligence Quotient |
| TBI/mTBI | Traumatic Brain Injury/mild Traumatic Brain Injury |
| ACC | Anterior Cingulate Cortex |
| IPL | Inferior Parietal Lobule |
| SWA | Slow-Wave Activity |
| REM | Rapid Eye Movement (sleep phase) |
| PET | Positron Emission Tomography |
| sLORETA | standardized Low-Resolution Brain Electromagnetic Tomography |
| WMC | Working Memory Capacity |
| NTC | Non-Traumatized Controls |
| ASD | Acute Stress Disorder |
| CMI | Chronic Multisymptom Illness |
Appendix A
References
- American Psychiatric Association (Ed.). (2013). Diagnostic and statistical manual of mental disorders: DSM-5 (5th ed.). American Psychiatric Association. [Google Scholar]
- Baddeley, A. D., & Hitch, G. (1974). Working memory. In G. H. Bower (Ed.), Psychology of learning and motivation (Vol. 8, pp. 47–89). Academic Press. [Google Scholar] [CrossRef]
- Ben-Zion, Z., Korem, N., Fine, N. B., Katz, S., Siddhanta, M., Funaro, M. C., Duek, O., Spiller, T. R., Danböck, S. K., Levy, I., & Harpaz-Rotem, I. (2023). Structural neuroimaging of hippocampus and amygdala subregions in posttraumatic stress disorder: A scoping review. Biological Psychiatry Global Open Science, 4(1), 120–134. [Google Scholar] [CrossRef]
- Biscoe, N., New, E., & Murphy, D. (2024). Complex PTSD symptom clusters and executive function in UK Armed Forces veterans: A cross-sectional study. BMC Psychology, 12(1), 209. [Google Scholar] [CrossRef]
- Bisson Desrochers, A., Rouleau, I., Angehrn, A., Vasiliadis, H.-M., Saumier, D., & Brunet, A. (2021). Trauma on duty: Cognitive functioning in police officers with and without posttraumatic stress disorder (PTSD). European Journal of Psychotraumatology, 12(1), 1959117. [Google Scholar] [CrossRef]
- Bremner, J. D. (2006). Traumatic stress: Effects on the brain. Dialogues in Clinical Neuroscience, 8(4), 445–461. [Google Scholar] [CrossRef] [PubMed]
- Center for Substance Abuse Treatment (US). (2014). Exhibit 1.3-4, DSM-5 diagnostic criteria for PTSD [Text]. Substance Abuse and Mental Health Services Administration (US). Available online: https://www.ncbi.nlm.nih.gov/books/NBK207191/ (accessed on 7 December 2025).
- DeGutis, J., Esterman, M., McCulloch, B., Rosenblatt, A., Milberg, W., & McGlinchey, R. (2015). Posttraumatic psychological symptoms are associated with reduced inhibitory control, not general executive dysfunction. Journal of the International Neuropsychological Society, 21(5), 342–352. [Google Scholar] [CrossRef] [PubMed]
- Drigas, A. S., & Papoutsi, C. (2018). A new layered model on emotional intelligence. Behavioral Sciences, 8(5), 45. [Google Scholar] [CrossRef]
- Ehlers, A., & Clark, D. M. (2000). A cognitive model of posttraumatic stress disorder. Behaviour Research and Therapy, 38(4), 319–345. [Google Scholar] [CrossRef]
- Flaks, M. K., Malta, S. M., Almeida, P. P., Bueno, O. F. A., Pupo, M. C., Andreoli, S. B., Mello, M. F., Lacerda, A. L. T., Mari, J. J., & Bressan, R. A. (2014). Attentional and executive functions are differentially affected by post-traumatic stress disorder and trauma. Journal of Psychiatric Research, 48(1), 32–39. [Google Scholar] [CrossRef] [PubMed]
- Flanagan, J. C., Hand, A., Jarnecke, A. M., Moran-Santa Maria, M. M., Brady, K. T., & Joseph, J. E. (2018). Effects of oxytocin on working memory and executive control system connectivity in posttraumatic stress disorder. Experimental and Clinical Psychopharmacology, 26(4), 391–402. [Google Scholar] [CrossRef]
- Galletly, C., Clark, C. R., McFarlane, A. C., & Weber, D. L. (2001). Working memory in posttraumatic stress disorder—An event-related potential study. Journal of Traumatic Stress, 14(2), 295–309. [Google Scholar] [CrossRef]
- Goldstein, E. B. (2011). Cognitive psychology: Connecting mind, research, and everyday experience (3rd ed.). Wadsworth, Cengage Learning. [Google Scholar]
- Haaland, K. Y., Sadek, J. R., Keller, J. E., & Castillo, D. T. (2016). Neurocognitive correlates of successful treatment of PTSD in female veterans. Journal of the International Neuropsychological Society: JINS, 22(6), 643–651. [Google Scholar] [CrossRef]
- Havelka Mestrovic, A., Tudor, L., Nedic Erjavec, G., Nikolac Perkovic, M., Svob Strac, D., Kovacic Petrovic, Z., & Pivac, N. (2020). The impact of BDNF Val66Met on cognitive skills in veterans with posttraumatic stress disorder. Neuroscience Letters, 735, 135235. [Google Scholar] [CrossRef]
- Hinojosa, C. A., George, G. C., & Ben-Zion, Z. (2024). Neuroimaging of posttraumatic stress disorder in adults and youth: Progress over the last decade on three leading questions of the field. Molecular Psychiatry, 29(10), 3223–3244. [Google Scholar] [CrossRef]
- Honzel, N., Justus, T., & Swick, D. (2014). Posttraumatic stress disorder is associated with limited executive resources in a working memory task. Cognitive, Affective & Behavioral Neuroscience, 14(2), 792–804. [Google Scholar] [CrossRef]
- International Classification of Diseases [ICD]. (n.d.). Available online: https://www.who.int/standards/classifications/classification-of-diseases (accessed on 4 August 2025).
- Jelinek, L., Moritz, S., Randjbar, S., Sommerfeldt, D., Püschel, K., & Seifert, D. (2008). Does the evocation of traumatic memories confound subsequent working memory performance in posttraumatic stress disorder (PTSD)? Depress Anxiety, 25(2), 175–179. [Google Scholar] [CrossRef] [PubMed]
- Katsarava, M., Schilcher-Freier, J., & Gaschler, R. (2025). Trauma type affects the perceived severity of symptoms and intensity of the recommended intervention in laypeople’s perspective on PTSD. Discover Mental Health, 5(1), 75. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.-W., Park, J.-I., & Yang, J.-C. (2024). Brain morphological changes and functional neuroanatomy related to cognitive and emotional distractors during working memory maintenance in post-traumatic stress disorder. Brain Research Bulletin, 211, 110946. [Google Scholar] [CrossRef]
- Koso, M., & Hansen, S. (2006). Executive function and memory in posttraumatic stress disorder: A study of Bosnian war veterans. European Psychiatry: The Journal of the Association of European Psychiatrists, 21(3), 167–173. [Google Scholar] [CrossRef]
- LaGoy, A. D., Kaskie, R., Connaboy, C., Germain, A., & Ferrarelli, F. (2021). Overnight sleep parameter increases in frontoparietal areas predict working memory improvements in healthy participants but not in individuals with posttraumatic stress disorder. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging, 6(11), 1110–1117. [Google Scholar] [CrossRef]
- Lawn, R. B., Jha, S. C., Liu, J., Sampson, L., Murchland, A. R., Sumner, J. A., Roberts, A. L., Disner, S. G., Grodstein, F., Kang, J. H., Kubzansky, L. D., Chibnik, L. B., & Koenen, K. C. (2022). The association of posttraumatic stress disorder, depression, and head injury with mid-life cognitive function in civilian women. Depression and Anxiety, 39(3), 220–232. [Google Scholar] [CrossRef]
- Lawrence, K. A., Rippey, C. S., Welikson, B., Pietrzak, R. H., & Adams, T. G. (2023). Interactive association of posttraumatic stress disorder, apolipoprotein ε4 genotype, and age on cognitive functioning. International Journal of Geriatric Psychiatry, 38(2), e5888. [Google Scholar] [CrossRef]
- Mathew, A. S., Lotfi, S., Bennett, K. P., Larsen, S. E., Dean, C., Larson, C. L., & Lee, H.-J. (2022). Association between spatial working memory and Re-experiencing symptoms in PTSD. Journal of Behavior Therapy and Experimental Psychiatry, 75, 101714. [Google Scholar] [CrossRef]
- McDermott, T. J., Badura-Brack, A. S., Becker, K. M., Ryan, T. J., Khanna, M. M., Heinrichs-Graham, E., & Wilson, T. W. (2016). Male veterans with PTSD exhibit aberrant neural dynamics during working memory processing: An MEG study. Journal of Psychiatry & Neuroscience: JPN, 41(4), 251–260. [Google Scholar] [CrossRef]
- Moores, K. A., Clark, C. R., McFarlane, A. C., Brown, G. C., Puce, A., & Taylor, D. J. (2008). Abnormal recruitment of working memory updating networks during maintenance of trauma-neutral information in post-traumatic stress disorder. Psychiatry Research, 163(2), 156–170. [Google Scholar] [CrossRef] [PubMed]
- Narita-Ohtaki, R., Hori, H., Itoh, M., Lin, M., Niwa, M., Ino, K., Imai, R., Ogawa, S., Sekiguchi, A., Matsui, M., Kunugi, H., Kamo, T., & Kim, Y. (2018). Cognitive function in Japanese women with posttraumatic stress disorder: Association with exercise habits. Journal of Affective Disorders, 236, 306–312. [Google Scholar] [CrossRef]
- Nejati, V., Salehinejad, M. A., & Sabayee, A. (2018). Impaired working memory updating affects memory for emotional and non-emotional materials the same way: Evidence from post-traumatic stress disorder (PTSD). Cognitive Processing, 19(1), 53–62. [Google Scholar] [CrossRef] [PubMed]
- Pankey, B. S., Riedel, M. C., Cowan, I., Bartley, J. E., Pintos Lobo, R., Hill-Bowen, L. D., Salo, T., Musser, E. D., Sutherland, M. T., & Laird, A. R. (2022). Extended functional connectivity of convergent structural alterations among individuals with PTSD: A neuroimaging meta-analysis. Behavioral and Brain Functions, 18(1), 9. [Google Scholar] [CrossRef]
- Popescu, M., Popescu, E.-A., DeGraba, T. J., Fernandez-Fidalgo, D. J., Riedy, G., & Hughes, J. D. (2019). Post-traumatic stress disorder is associated with altered modulation of prefrontal alpha band oscillations during working memory. Clinical Neurophysiology: Official Journal of the International Federation of Clinical Neurophysiology, 130(10), 1869–1881. [Google Scholar] [CrossRef]
- Roberts, A. L., Liu, J., Lawn, R. B., Jha, S. C., Sumner, J. A., Kang, J. H., Rimm, E. B., Grodstein, F., Kubzansky, L. D., Chibnik, L. B., & Koenen, K. C. (2022). Association of posttraumatic stress disorder with accelerated cognitive decline in middle-aged women. JAMA Network Open, 5(6), e2217698. [Google Scholar] [CrossRef]
- Runyan, A., Philippi, C. L., Pessin, S., Velez, C. S., Wade, B. S. C., Marie Drennon, A., Cooper, D. B., Kennedy, J. E., Bowles, A. O., Lewis, J. D., Reid, M. W., York, G. E., Newsome, M. R., Wilde, E. A., & Tate, D. F. (2022). Comparing resting-state connectivity of working memory networks in U.S. Service members with mild traumatic brain injury and posttraumatic stress disorder. Brain Research, 1796, 148099. [Google Scholar] [CrossRef]
- Schindler, L., Stalder, T., Kirschbaum, C., Plessow, F., Schönfeld, S., Hoyer, J., Trautmann, S., & Steudte-Schmiedgen, S. (2020). Cognitive functioning in posttraumatic stress disorder before and after cognitive-behavioral therapy. Journal of Anxiety Disorders, 74, 102265. [Google Scholar] [CrossRef]
- Schweizer, S., & Dalgleish, T. (2011). Emotional working memory capacity in posttraumatic stress disorder (PTSD). Behaviour Research and Therapy, 49(8), 498–504. [Google Scholar] [CrossRef] [PubMed]
- Schweizer, S., & Dalgleish, T. (2016). The impact of affective contexts on working memory capacity in healthy populations and in individuals with PTSD. Emotion, 16(1), 16–23. [Google Scholar] [CrossRef]
- Scott, J. C., Woods, S. P., Wrocklage, K. M., Schweinsburg, B. C., Southwick, S. M., & Krystal, J. H. (2016). Prospective memory in posttraumatic stress disorder. Journal of the International Neuropsychological Society: JINS, 22(7), 724–734. [Google Scholar] [CrossRef]
- Shaw, M. E., Moores, K. A., Clark, R. C., McFarlane, A. C., Strother, S. C., Bryant, R. A., Brown, G. C., & Taylor, J. D. (2009). Functional connectivity reveals inefficient working memory systems in post-traumatic stress disorder. Psychiatry Research, 172(3), 235–241. [Google Scholar] [CrossRef]
- Sippel, L. M., Holtzheimer, P. E., Huckins, J. F., Collier, E., Feilong, M., Wheatley, T., & Meyer, M. L. (2021). Neurocognitive mechanisms of poor social connection in posttraumatic stress disorder: Evidence for abnormalities in social working memory. Depression and Anxiety, 38(6), 615–625. [Google Scholar] [CrossRef]
- Stangor, C., & Walinga, J. (2014). 9.1 Memories as types and stages. Available online: https://opentextbc.ca/introductiontopsychology/chapter/8-1-memories-as-types-and-stages/ (accessed on 1 January 2020).
- Steudte-Schmiedgen, S., Stalder, T., Kirschbaum, C., Weber, F., Hoyer, J., & Plessow, F. (2014). Trauma exposure is associated with increased context-dependent adjustments of cognitive control in patients with posttraumatic stress disorder and healthy controls. Cognitive, Affective & Behavioral Neuroscience, 14(4), 1310–1319. [Google Scholar] [CrossRef]
- Stricker, N. H., Keller, J. E., Castillo, D. T., & Haaland, K. Y. (2015). The neurocognitive performance of female veterans with posttraumatic stress disorder. Journal of Traumatic Stress, 28(2), 102–109. [Google Scholar] [CrossRef] [PubMed]
- Sumner, J. A., Hagan, K., Grodstein, F., Roberts, A. L., Harel, B., & Koenen, K. C. (2017). Posttraumatic stress disorder symptoms and cognitive function in a large cohort of middle-aged women. Depression and Anxiety, 34(4), 356–366. [Google Scholar] [CrossRef]
- Swain, T. L., Keeping, C. A., Lewitzka, S., & Takarangi, M. K. T. (2023). I forgot that I forgot: PTSD symptom severity in a general population correlates with everyday diary-recorded prospective memory failures. Memory & Cognition, 51(6), 1331–1345. [Google Scholar] [CrossRef]
- Swick, D., Cayton, J., Ashley, V., & Turken, A. U. (2017). Dissociation between working memory performance and proactive interference control in post-traumatic stress disorder. Neuropsychologia, 96, 111–121. [Google Scholar] [CrossRef] [PubMed]
- Tian, F., Yennu, A., Smith-Osborne, A., Gonzalez-Lima, F., North, C. S., & Liu, H. (2014). Prefrontal responses to digit span memory phases in patients with post-traumatic stress disorder (PTSD): A functional near infrared spectroscopy study. NeuroImage: Clinical, 4, 808–819. [Google Scholar] [CrossRef]
- Toomey, R., Alpern, R. E., Reda, D. J., Baker, D. G., Vasterling, J. J., Blanchard, M. S., & Eisen, S. A. (2021). A cohort study of neuropsychological functioning in spouses of U.S. Gulf War veterans. Life Sciences, 284, 119894. [Google Scholar] [CrossRef]
- Tricco, A. C., Lillie, E., Zarin, W., O’Brien, K. K., Colquhoun, H., Levac, D., Moher, D., Peters, M. D. J., Horsley, T., Weeks, L., Hempel, S., Akl, E. A., Chang, C., McGowan, J., Stewart, L., Hartling, L., Aldcroft, A., Wilson, M. G., Garritty, C., … Straus, S. E. (2018). PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Annals of Internal Medicine, 169(7), 467–473. [Google Scholar] [CrossRef]
- Veltmeyer, M. D., Clark, C. R., McFarlane, A. C., Moores, K. A., Bryant, R. A., & Gordon, E. (2009). Working memory function in post-traumatic stress disorder: An event-related potential study. Clinical Neurophysiology: Official Journal of the International Federation of Clinical Neurophysiology, 120(6), 1096–1106. [Google Scholar] [CrossRef]
- Weber, D. L., Clark, C. R., McFarlane, A. C., Moores, K. A., Morris, P., & Egan, G. F. (2005). Abnormal frontal and parietal activity during working memory updating in post-traumatic stress disorder. Psychiatry Research, 140(1), 27–44. [Google Scholar] [CrossRef]
- Yehuda, R., Harvey, P. D., Buchsbaum, M., Tischler, L., & Schmeidler, J. (2007). Enhanced effects of cortisol administration on episodic and working memory in aging veterans with PTSD. Neuropsychopharmacology: Official Publication of the American College of Neuropsychopharmacology, 32(12), 2581–2591. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J., Xiong, K., Qiu, M., Zhang, Y., Xie, B., Wang, J., Li, M., Chen, H., Zhang, Y., & Zhang, J. (2013). Negative emotional distraction on neural circuits for working memory in patients with posttraumatic stress disorder. Brain Research, 1531, 94–101. [Google Scholar] [CrossRef] [PubMed]

| Database | Search Terms/Query | Filters Applied |
|---|---|---|
| PubMed | (post traumatic stress disorder OR PTSD) AND (working memory) AND (adults) |
|
| Scopus | TITLE-ABS-KEY (((post AND traumatic AND stress AND disorder) OR PTSD) AND working AND memory AND adults) AND (LIMIT-TO (PUBSTAGE, “final”)) AND (LIMIT-TO (DOCTYPE, “ar”)) AND (LIMIT-TO (LANGUAGE, “English”)) AND (LIMIT-TO (EXACTKEYWORD, “Adult”) OR LIMIT-TO (EXACTKEYWORD, “Human”)) |
|
| Category | Subcategory | Number of Studies (n) | Percentage (%) |
|---|---|---|---|
| PTSD Sample Size | 1–100 participants | 32 | 82.1% |
| 101–250 participants | 1 | 2.6% | |
| 251–500 participants | 2 | 5.1% | |
| >500 participants | 4 | 10.3% | |
| Age Reporting | Age range reported | 26 | 66.7% |
| Age range not reported | 13 | 33.3% | |
| Control Group | Matched healthy controls | 31 | 79.5% |
| No control group | 8 | 20.5% | |
| Control Group Type | Exposed to trauma (no PTSD diagnosis) | 3 | 7.7% of total |
| Non-PTSD clinical group (e.g., orthopedic injury) | 1 | 2.6% of total |
| Author, (Year) | Study Origin | Study Type | Patients N | Patients’ Age | Controls | Controls N | Controls’ Age | Type of Trauma/Stress | Main Findings Related To WM |
|---|---|---|---|---|---|---|---|---|---|
| Toomey et al. (2021) | USA | L | 470 | Ν/A | yes | 524 | N/A | chronic | Spouses experiencing mental health conditions such as PTSD and anxiety exhibited notable impairments in attention, WM, and general memory functioning. These cognitive difficulties appeared to be relatively independent of their partners’ deployment status. In contrast, individuals diagnosed with chronic multisymptomatic illness (CMI) demonstrated reduced motor speed, along with a general trend toward diminished verbal memory and attention/WM capacities. |
| Kim et al. (2024) | Republic of Korea | C | 22 (12 M/10 F) | Mean Age: 34.2 ± 13.3 years | yes | 22 (12 M/10 F) | Mean Age: 33.6 ± 9.9 years | chronic | Relative to healthy controls (HCs), individuals diagnosed with PTSD exhibited reduced gray matter volume (GMV) in the inferior frontal gyrus (IFG). Moreover, they demonstrated lower accuracy and prolonged reaction times on a face recognition task involving trauma-related distractors. In addition, functional imaging revealed heightened activation in the superior temporal gyrus (STG) during trials with trauma-related distractors. This evidence shows that altered neural activation patterns in PTSD may underlie deficits in cognitive control over ambiguous stimuli and impaired regulation of emotional distraction. |
| Narita-Ohtaki et al. (2018) | Japan | C | 42 F | Age Range: 21–59 years | yes | 66 F | Age Range: 20–64 years | chronic | Compared to healthy controls, individuals with PTSD exhibited lower performance across all assessed cognitive domains, including immediate memory, visuospatial construction, language, attention, and delayed memory, as well as the overall score on the Repeatable Battery for the Assessment of Neuropsychological Status (RBANS). Among these domains, memory impairments were the most pronounced. PTSD patients who engaged in regular physical exercise demonstrated better performance in delayed memory tasks compared to their non-exercising counterparts—a difference that remained statistically significant even after adjusting for potential confounding factors in a multiple regression analysis. |
| Runyan et al. (2022) | USA | C | 127 (110 M/17 F) | Age Range: 18–59 years | yes | 57 (46 M/11 F) | Age Range: 18–59 years | chronic | The results indicated reduced resting-state functional connectivity (rsFC) between white matter (WM) regions of the ventrolateral prefrontal cortex (vlPFC), lateral premotor cortex, and dorsolateral prefrontal cortex (dlPFC), and areas within the dorsal attention and somatomotor networks in both the mild traumatic brain injury (mTBI) and PTSD groups, relative to healthy controls. Compared to individuals with mTBI, those with PTSD exhibited further reductions in rsFC between the lateral premotor WM seed region and the middle occipital gyrus, as well as between the dlPFC WM seed region and the paracentral lobule. Only connectivity involving the vlPFC was correlated with WM performance across participants. |
| Biscoe et al. (2024) | UK | C | 428 | Ν/A | no | chronic | Both PTSD and complex PTSD (CPTSD) were associated with greater impairments in inhibitory control and WM. All PTSD symptom clusters, as well as the disturbances in self-organization (DSO) clusters characteristic of CPTSD, were linked to deficits in both inhibition and WM. Furthermore, consistent evidence indicates that sleep deprivation is also closely associated with WM impairments. | ||
| Swick et al. (2017) | USA | C | 29 M | no significant differences | yes | 29 M | no significant differences | chronic | No significant differences were observed in the degree of WM impairment between verbal and visual stimuli among patients. Combat veterans diagnosed with PTSD demonstrated reduced accuracy on a recent probes WM task in comparison to a mixed control group consisting of both combat-exposed and non-exposed veterans. Importantly, the observed WM deficit in accuracy was consistent across stimulus types, with comparable performance decrements for both verbal and visual conditions. |
| Flanagan et al. (2018) | USA | C | 16 (9 M/7 F) | Ν/A | yes | 18 (7 M/11 F) | N/A | chronic | The findings further suggest that administration of a single intranasal dose of oxytocin, compared to placebo, was associated with enhanced performance in the most cognitively demanding WM task condition among participants with PTSD. Oxytocin modulated functional connectivity between the left dorsolateral prefrontal cortex (DLPFC) and the anterior cingulate cortex during the n-back task, resulting in increased connectivity. These results offer novel evidence supporting the potential of oxytocin to ameliorate executive functioning deficits in individuals with PTSD. |
| Nejati et al. (2018) | Iran | C | 30 M | Mean Age: 46.62 years | yes | 30 M | Mean Age: 46.62 years | chronic | emotional Crucially, WM performance did not significantly differ between emotional and non-emotional stimuli in terms of either accuracy or reaction time. Individuals with PTSD exhibited impaired WM across both stimulus types. The results imply that WM deficits in PTSD are likely driven by a more general underlying dysfunction, which we propose reflects impaired WM updating mechanisms. Specifically, in PTSD, updating processes may be substantially disrupted, resulting in the accumulation of irrelevant information within WM, ultimately overloading its capacity and hindering the encoding of new material. |
| Lawrence et al. (2023) | USA | C | 1244 (98 F/1146 M) | Average Age: 61.8 years | no | chronic | A significant interaction between APOE ε4 status, PTSD, and age was associated with poorer performance in learning and WM, but not in attention or psychomotor functioning. In contrast, the PTSD-by-age interaction was specifically linked to deficits in attention and psychomotor abilities. The results imply that PTSD may represent a critical target for mitigating age-related cognitive decline, particularly in learning and WM domains, among individuals carrying the APOE ε4 allele. | ||
| McDermott et al. (2016) | USA | C | 27 M | Ν/A | yes | 24 M | N/A | chronic | Our core findings indicate that PTSD is associated with physiological alterations during WM processing, with pronounced effects observed in the right supramarginal gyrus and inferior frontal regions. PTSD-related differences emerged during the early encoding phase, where patients exhibited enhanced alpha oscillatory activity in the right inferior frontal gyrus (IFG) compared to healthy controls. As encoding progressed, these differences extended to the right supramarginal and temporal cortices, and persisted throughout the maintenance phase, with sustained involvement of the right IFG. |
| Haaland et al. (2016) | UK | L | 42 F | Ν/A | No | N/A | N/A | chronic | Stronger pre-treatment learning and memory abilities predicted a more favorable response to PTSD treatment. Our findings further indicate that symptom reduction following a multi-component intervention was associated with improvements in cognitive flexibility, specifically in measures labeled inhibition/switching, even after controlling for changes in depressive symptoms. In contrast, changes in PTSD symptom severity were not significantly related to alterations in learning, memory, or WM performance. |
| Popescu et al. (2019) | USA | C | 35 | Ν/A | no | N/A | N/A | chronic | Our principal finding demonstrates that greater severity of PTSD symptoms is negatively correlated with load-related increases in alpha-band power during the retention phase of WM, and is further associated with an elevated rate of false positive responses. These results suggest that enhanced alpha oscillatory amplitude during the retention interval may serve as a neurophysiological mechanism for filtering or “forgetting” weakly activated, irrelevant, or outdated information. Conversely, an impaired ability to appropriately amplify alpha activity in task-relevant cortical regions may reflect deficient pattern separation processes, potentially contributing to fear generalization and the re-experiencing of intrusive traumatic memories. |
| Sumner et al. (2017) | USA | L | 14,029 F | Average Age: 25–42 years | no | N/A | N/A | chronic | Among middle-aged women, elevated PTSD symptom severity was associated with poorer performance on measures of psychomotor speed, attention, learning, and WM. Women with a history of trauma and probable depression, as well as those with probable PTSD in the absence of depression, exhibited lower cognitive performance across composite domains when compared to women without trauma exposure or probable depression. The results imply that PTSD, even in the absence of comorbid depression, may be independently associated with cognitive impairments. |
| Scott et al. (2016) | USA | C | 40 | Ν/A | yes | 38 | Ν/A | chronic/acute | Veterans diagnosed with PTSD demonstrated moderately lower performance on time-based prospective memory (PM) tasks compared to controls, with the majority of errors classified as PM failures. Reduced time-based PM performance was specifically associated with the hyperarousal symptom cluster of PTSD. Within the PTSD group, time-based PM was also related to neuropsychological indices of retrospective memory and executive functioning. However, PTSD remained a significant predictor of impaired PM performance even after accounting for age, retrospective memory, and executive function, indicating an independent contribution of PTSD to PM deficits. |
| Lawn et al. (2022) | USA | L | 10,681 F | Mean age: 64.9 years | no | N/A | N/A | chronic | n this sample of middle-aged civilian women, both PTSD and depression were associated with poorer performance on tasks assessing psychomotor speed, attention, learning, and WM. A history of head injury was also related to PTSD and depression, and when multiple head injuries were reported, this was further associated with greater deficits in learning and WM. This indicates that head injury, PTSD, and depression may jointly contribute to cognitive impairments, particularly in domains involving memory and attentional processing. |
| Schweizer and Dalgleish (2016) | UK | L | 11 M/16 F | Median age: 46 years | yes | 12 M/19 F (exp.1) 14 M/45 F (exp.2) | Median age: 23 years (exp.1) Median age: 45 years (exp.2) | chronic | Experiments 1 and 2 confirmed the hypothesis that WM capacity (WMC) is reduced in the presence of emotional, as opposed to neutral, distractors across both student and community samples. Experiment 3 extended these findings to a clinical population: among motor vehicle accident survivors, those with a history of PTSD exhibited selectively diminished WMC when exposed to trauma-related emotional distraction, compared to survivors without PTSD. |
| Havelka Mestrovic et al. (2020) | Croatia | C | 199 M | Median Age: 42 years | yes | 116 M | Median Age: 39 years | chronic | Our findings confirmed the presence of WM difficulties and attentional deficits in individuals with PTSD. Among veterans diagnosed with PTSD, carriers of the BDNF Val66Met A allele exhibited lower scores in short-term visual memory tasks, potentially reflecting reduced efficiency in processing visual and spatial information relative to GG homozygotes. Moreover, the impact of the BDNF Val66Met polymorphism on cognitive and executive functioning was moderated by age. Importantly, PTSD diagnosis emerged as a highly significant predictor of both diminished executive performance and poorer visual WM. |
| Stricker et al. (2015) | USA | C | 56 F | Ν/A | yes | 53 F | Ν/A | chronic | In our sample of women with PTSD, executive functions—including WM and response inhibition/switching—were poorer compared to healthy controls. Consistent with prior research, greater dorsolateral prefrontal cortex (DLPFC) activation has been linked to more effective suppression of unwanted memories in healthy individuals, potentially serving as a compensatory mechanism in PTSD. As hypothesized, verbal learning and memory were positively associated with executive function performance, independent of processing speed, PTSD symptom severity, and depressive symptoms. Additionally, intelligence quotient (IQ) was related to verbal learning and memory outcomes within the PTSD group. |
| Bisson Desrochers et al. (2021) | Canada | C | 31 | Age Range: 18–65 years | yes | 30 | Age Range: 18–65 years | chronic | Police officers diagnosed with PTSD demonstrated lower cognitive performance compared to healthy controls, particularly in the domains of executive functioning, verbal learning and memory, and lexical access. In individuals with PTSD, memory impairments appear to stem primarily from subtle deficits in the encoding of verbal information, which in turn compromise both learning and retrieval processes. Within the PTSD group, higher levels of intrusion symptoms were associated with reduced executive function, attention, and WM. Furthermore, increased intrusion and avoidance symptom severity was linked to slower information processing speed. |
| Weber et al. (2005) | Australia | C | 10 (7 M/3 F) | Mean Age: 50.8 ± 4.6 years | yes | 10 (7 M/3 F) | Mean Age: 47.4 ± 3.3 years | chronic | High-resolution event-related potential (ERP) topography and standardized low-resolution brain electromagnetic tomography (sLORETA) source analysis corroborated previous PET findings of abnormal fronto-parietal activity during WM updating in individuals with PTSD. These abnormalities involved attentional control, WM processes, and interactions with medial temporal regions typically engaged during episodic memory tasks. WM-related activity was found to be negatively associated with intrusion and avoidance symptoms, but positively associated with hyperarousal. These deficits were observed even for trauma-neutral stimuli, suggesting that individuals with PTSD experience pervasive difficulties in attending to and integrating novel information into WM |
| Moores et al. (2008) | Australia | C | 13 (8 M/5 F) | Mean Age: 44.23 ± 9.18 years | yes | 12 (7 M/5 F) | Mean Age: 40.41 ± 10.93 years | chronic | The findings underscore that individuals with PTSD exhibit abnormal recruitment of brain regions typically involved in WM updating—such as the dorsolateral prefrontal cortex (DLPFC) and inferior parietal lobule (IPL)—even during WM maintenance operations. In contrast, other regions consistently implicated in the neurobiology of PTSD, including the anterior cingulate cortex (ACC), hippocampus, and brainstem, displayed atypically reduced activation during WM updating. These results suggest that PTSD patients must engage broader cortical areas, including WM updating networks, to perform even basic WM maintenance tasks—possibly reflecting the cognitive symptoms commonly reported in PTSD, such as difficulties in concentration and memory. |
| Mathew et al. (2022) | USA | C | 76 | Ν/A | no | N/A | N/A | chronic | The data revealed a significant association between visuospatial WM functioning and the re-experiencing symptom cluster of PTSD. Specifically, the visuospatial WM index accounted for a substantial proportion of the variance in re-experiencing symptoms, suggesting that visuospatial WM capacity serves as a meaningful cognitive correlate of this symptom dimension. Additionally, our findings indicate that repeated trauma exposure was positively associated with poorer visuospatial memory performance, further implicating this domain as sensitive to cumulative traumatic load. |
| Roberts et al. (2022) | USA | L | 12,270 F | Age Range: 50–71 years | no | N/A | N/A | chronic | In this large-scale longitudinal cohort study of trauma-exposed women aged 50 to 71, elevated PTSD symptomatology was associated with greater declines in cognitive functioning over time. Specifically, women with a high number of PTSD symptoms demonstrated greater reductions in learning, WM, psychomotor speed, and attentional capacity compared to those with no symptoms. Even among women with moderate PTSD symptom levels, cognitive decline in learning and WM remained significant. Predictive modeling indicated that women with high PTSD symptom burden experienced approximately twice the rate of decline in learning and WM relative to asymptomatic counterparts. |
| Flaks et al. (2014) | Brazil | C | 81 (PTSD+) 70 (PTSD−) | Age Range: 18–60 years | yes | 50 | Age Range: 18–60 years | chronic | With regard to visual attentional processing, individuals with PTSD (PTSD+ group) demonstrated poorer performance compared to healthy controls (HC) on the forward spatial span task. Furthermore, the PTSD+ group showed deficits in executive functioning, processing speed, and inhibitory control. These results suggest that individuals with PTSD exhibit marked impairments in sustaining attention over time, particularly when rapid processing and inhibition of concurrent stimuli are required. Such cognitive difficulties may reflect underlying neurobiological alterations associated with PTSD, potentially disrupting concentration, information processing speed, selective attention, resistance to distraction, and inhibitory control. |
| Schweizer and Dalgleish (2011) | UK | C | 39 | Age Range: 17–65 years | yes | 21 | Age Range: 17–65 years | chronic | The results indicated that individuals with a current or past history of PTSD performed worse on the emotional WM capacity (eWMC) task compared to controls when processing valence-neutral content. Group differences were most pronounced in trials involving 5- or 6-sentence sequences, while performance on both shorter (4-sentence) and more complex (7-sentence) trials appeared less sensitive to PTSD-related differences. This indicates that trauma-exposed individuals with any history of PTSD exhibit heightened WM impairments in emotionally salient contexts, particularly under moderate cognitive load, compared to individuals without PTSD history. |
| Koso and Hansen (2006) | Bosnia, Sweden | C | 20 M | mid-40s | yes | 20 M | mid-40s | chronic | Our findings provide further evidence that PTSD is associated with impaired sustained attention, as individuals with PTSD demonstrated difficulty maintaining vigilance over time. Even during relatively simple visual scanning tasks, the PTSD group exhibited slower initiation and production of well-defined verbal responses, alongside notable impairments in inhibiting automatic or habitual responses. Additionally, deficits in WM were observed, as reflected by lower performance on the WAIS digit span task. |
| Shaw et al. (2009) | Australia, Canada | C | 13 (8 M/5 F) | Age Range: 30–55 years | yes | 12 (7 M/5 F) | Age Range: 28–59 yrs | chronic | In healthy controls, updating and maintenance processes within WM engaged partially overlapping but functionally distinct neural networks. In contrast, individuals with PTSD predominantly recruited a single fronto-parietal network for both processes. This pattern suggests a reduced capacity to flexibly modulate neural activity in response to differing task demands, potentially reflecting impaired cognitive control and neural efficiency in PTSD. |
| Zhang et al. (2013) | China | C | 20 | Age Range: 18–40 years | yes | 20 | Age Range: 20–38 years | chronic | In the presence of negative, as opposed to neutral, distractors, individuals with PTSD exhibited heightened activation in emotion-related brain regions, accompanied by reduced activation in regions associated with WM. Compared to healthy controls, the PTSD group appeared more vulnerable to the interfering effects of negative emotional stimuli, as evidenced by both diminished performance on WM tasks and attenuated engagement of WM-related neural networks. |
| Sippel et al. (2021) | USA | C | 31 | Age Range: 18–55 years | yes | 21 | Age Range: 18–55 years | chronic | PTSD may be associated with impaired social WM (SWM) abilities, particularly under conditions of increased information-processing demands. Hyperactivation of the default mode network (DMN), and specifically the dorsomedial prefrontal cortex (DMPFC), may serve both as a compensatory mechanism and a contributing factor to difficulties in social connectedness. The DMN—especially its dorsomedial subsystem—has been shown to preferentially retrieve socially painful memories. In our findings, individuals with PTSD exhibited hyperactivity in this region during high-load SWM tasks (i.e., four-load trials), highlighting the possible interference of trauma-related social content with goal-directed cognitive processes. |
| Jelinek et al. (2008) | Germany | C | 15 | Ν/A | yes | 18 | Ν/A | chronic/ acute | Results from the ANOVA analysis indicated that performance on the WM task remained stable following the evocation of traumatic memories. No significant changes were observed in either trauma-exposed individuals with PTSD or those without the disorder. These findings offer preliminary evidence that neurocognitive impairment in PTSD may not be a direct consequence of transient psychological activation following trauma recall, suggesting a more stable, underlying cognitive dysfunction. |
| LaGoy et al. (2021) | USA | C | 33 | Ν/A | yes | 46 | Ν/A | chronic | Contrary to initial expectations, increases in slow-wave activity (SWA) were inversely associated with improvements in WM among participants with PTSD. Higher SWA levels were also linked to more severe sleep disturbances in this group. Specifically, greater SWA in frontoparietal regions predicted diminished gains—or even declines—in WM performance. In addition, decreased REM-related theta activity was associated with improved WM performance in the PTSD group, a pattern opposite to that observed in healthy controls. This indicates that REM sleep may serve a different functional role in PTSD, and that non-REM SWA could be related to both daytime cognitive inefficiency and nocturnal behavioral symptoms in affected individuals. |
| Honzel et al. (2014) | USA | C | 18 (17 M/1 F) | Mean Age: 33.5 ± 7.2 years | yes | 16 (15 M/1 F) | Mean Age: 36.4 ± 8.6 years | chronic | The results support a dissociation between interference resolution and dual-task performance mechanisms during WM processing. While individuals with PTSD exhibited frontal scalp activation patterns comparable to healthy controls under single-task conditions, they failed to demonstrate the expected shift in scalp topography during dual-task conditions. These findings suggest reduced cortical recruitment—particularly within the frontal and parietal regions—during WM updating when cognitive demands are elevated by a concurrent task, indicating possible impairments in cognitive flexibility and resource allocation in PTSD. |
| Tian et al. (2014) | USA | C | 16 M | Age Range: 21–56 years | yes | 16 M | Age Range: 21–56 years | chronic | In the present study, statistical analyses of task-evoked hemodynamic responses revealed significant abnormalities in prefrontal activation among veterans with PTSD compared to healthy controls. Control participants displayed relatively symmetrical activation across both hemispheres during all three phases of the WM task. In contrast, veterans with PTSD exhibited left-lateralized activation in the dorsolateral prefrontal cortex (DLPFC) during the encoding and maintenance phases. More critically, PTSD was associated with marked deactivations during the retrieval phase, particularly in the right prefrontal cortex, suggesting disrupted neural engagement during memory access. |
| Steudte-Schmiedgen et al. (2014) | USA, Germany | C | 24 | Ν/A | yes | 26 (TC) 30 (HC) | Ν/A | chronic/acute | The central finding of this study was that both individuals with PTSD and trauma-exposed controls (TC) exhibited heightened conflict-driven cognitive control adjustments. This pattern coincided with evidence of altered long-term cortisol secretion in both groups. The increased conflict adaptation observed in these trauma-exposed individuals—who were characterized by lower basal cortisol levels—aligns with previous findings showing less flexible cognitive control under conditions of experimentally elevated salivary cortisol following acute stress. Furthermore, in the TC group, a diminished ability to maintain information in WM was associated with more severe intrusion symptoms, indicating a link between intrusive re-experiencing and WM maintenance deficits. |
| Veltmeyer et al. (2009) | Australia | C | 34 (17 M/17 F) | Average Age: 40.67 ± 11.14 years | yes | 136 (68 M/68 F) | Average Age: 40.23 ± 11.33 years | chronic | Patients with PTSD exhibited reduced event-related potential (ERP) amplitudes compared to healthy controls. They also demonstrated longer reaction times, greater reaction time variability, and lower accuracy in target detection. Subgroup analyses revealed that unmedicated PTSD patients exhibited delayed reaction times, while only those receiving selective serotonin reuptake inhibitors (SSRIs) were more likely to miss targets. Importantly, despite exhibiting similar levels of symptom severity, medicated patients displayed impaired WM functioning. Interestingly, reduced ERP amplitudes were associated with fewer clinical symptoms, whereas poorer task performance correlated with greater symptom severity, suggesting a possible dissociation between neural reactivity and behavioral outcomes in PTSD. |
| Galletly et al. (2001) | Australia | C | 18 (10 M/8 F) | Mean Age: 40.6 years | yes | 18 (8 M/10 F) | Mean Age: 39.4 years | chronic | The absence of late positive activity over frontal regions during target stimulus processing in individuals with PTSD may reflect persistent deficits in the executive control of attentional and evaluative processes. The presence of such abnormalities during a relatively simple task suggests that individuals with PTSD may be at a significant disadvantage when navigating and responding to more complex sensory environments. Consistent with these neural findings, the PTSD group demonstrated lower accuracy in target detection. Taken together, these results point to impairments in both the short-term storage and executive processing components of WM, aligning with theoretical models of PTSD that emphasize the role of hyperarousal and dysfunction within frontal-subcortical circuits. |
| DeGutis et al. (2015) | USA | C | 18 M | Ν/A | yes | 19 M | Ν/A | chronic | Within a cohort of trauma-exposed veterans, PTSD symptoms were found to be specifically associated with deficits in inhibitory control, rather than with generalized executive dysfunction. The overlapping symptomatology between PTSD and depression suggests that these inhibitory control deficits may reflect shared psychological distress common to both disorders, rather than being uniquely tied to core PTSD features such as re-experiencing. These findings further support the growing theoretical perspective that impaired inhibitory control constitutes a fundamental cognitive mechanism underlying post-traumatic symptomatology, possibly mediated by shared neural substrates. |
| Schindler et al. (2020) | Germany | C | 58 (4 M/54 F) | Age Range: 18–65 years | yes | 39 TC (7 M/32 F) 45 HC (7 M/38 F) | Age Range: 18–65 years | chronic | Patients with PTSD demonstrated greater conflict adaptation compared to the non-traumatized control (NTC) group, along with reduced autobiographical memory specificity relative to both control groups. The latter finding appears to be a particularly robust cognitive correlate of PTSD. Further analyses examining the predictive value of baseline cognitive functioning for treatment outcomes revealed that performance on the backward digit span task predicted reductions in depressive symptoms following cognitive-behavioral therapy (CBT); however, no other significant predictors were identified. |
| Yehuda et al. (2007) | USA | C | 13 M | Mean Age: 63 years Age Range: 52–81 years | yes | 17 M | Mean Age: 63 years Age Range: 52–81 years | chronic | The results indicated that cortisol administration enhanced episodic memory performance across both PTSD and control groups. However, improvements in WM performance were observed only in individuals with PTSD. This preferential enhancement of WM in the PTSD group may reflect an interaction between PTSD symptomatology and age-related factors, as previous findings in younger cohorts showed cortisol-related impairments on similar WM tasks. These results suggest that the cognitive effects of cortisol may be modulated by both psychiatric status and aging, particularly in relation to WM processes. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ganis, O.; Tsiakiri, A.; Christidi, F.; Katsikidou, M.; Arvaniti, A.; Samakouri, M. Memory Under Stress: How Post Traumatic Stress Disorder Affects Working Memory in Adults: A Scoping Review. Int. J. Cogn. Sci. 2025, 1, 4. https://doi.org/10.3390/ijcs1010004
Ganis O, Tsiakiri A, Christidi F, Katsikidou M, Arvaniti A, Samakouri M. Memory Under Stress: How Post Traumatic Stress Disorder Affects Working Memory in Adults: A Scoping Review. International Journal of Cognitive Sciences. 2025; 1(1):4. https://doi.org/10.3390/ijcs1010004
Chicago/Turabian StyleGanis, Olga, Anna Tsiakiri, Foteini Christidi, Magdalini Katsikidou, Aikaterini Arvaniti, and Maria Samakouri. 2025. "Memory Under Stress: How Post Traumatic Stress Disorder Affects Working Memory in Adults: A Scoping Review" International Journal of Cognitive Sciences 1, no. 1: 4. https://doi.org/10.3390/ijcs1010004
APA StyleGanis, O., Tsiakiri, A., Christidi, F., Katsikidou, M., Arvaniti, A., & Samakouri, M. (2025). Memory Under Stress: How Post Traumatic Stress Disorder Affects Working Memory in Adults: A Scoping Review. International Journal of Cognitive Sciences, 1(1), 4. https://doi.org/10.3390/ijcs1010004








