Preclinical and Clinical Feasibility Studies as the First Step Before Forthcoming Intravesical Instillation of [211At]At-anti-CA-IX Antibody (ATO-101™) Study in Patients with Non-Muscle-Invasive Bladder Cancer Unresponsive to Standard of Care
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Radiochemistry
Labeling of Girentuximab with At-211 ([211At]At-anti-CA-IX Antibody (ATO-101™))
2.2. Preclinical Study
2.2.1. Biodistribution of [211At]At-anti-CA-IX Antibody (ATO-101™) in Healthy Mice
2.2.2. Toxicity Evaluation of [211At]At-anti-CA-IX Antibody (ATO-101™) in Healthy Mice
2.2.3. Measurement of Affinity Constant of [211At]At-anti-CA-IX Antibody in RT-112 Cells
2.2.4. Labeling of Girentuximab with Lutetium-177
2.2.5. Analysis of Compared Cell Toxicity Using [177Lu]Lu-Girentuximab and [211At]At-anti-CA-IX Antibody (ATO-101™)
2.2.6. Clinical Proof of Concept of Intravesical PET Imaging in Patients: PERTINENCE Study Procedure Using [89Zr]Zr-Girentuximab
2.2.7. PET/CT Imaging
2.2.8. Quantitative 89Zr Activity Analysis in Blood
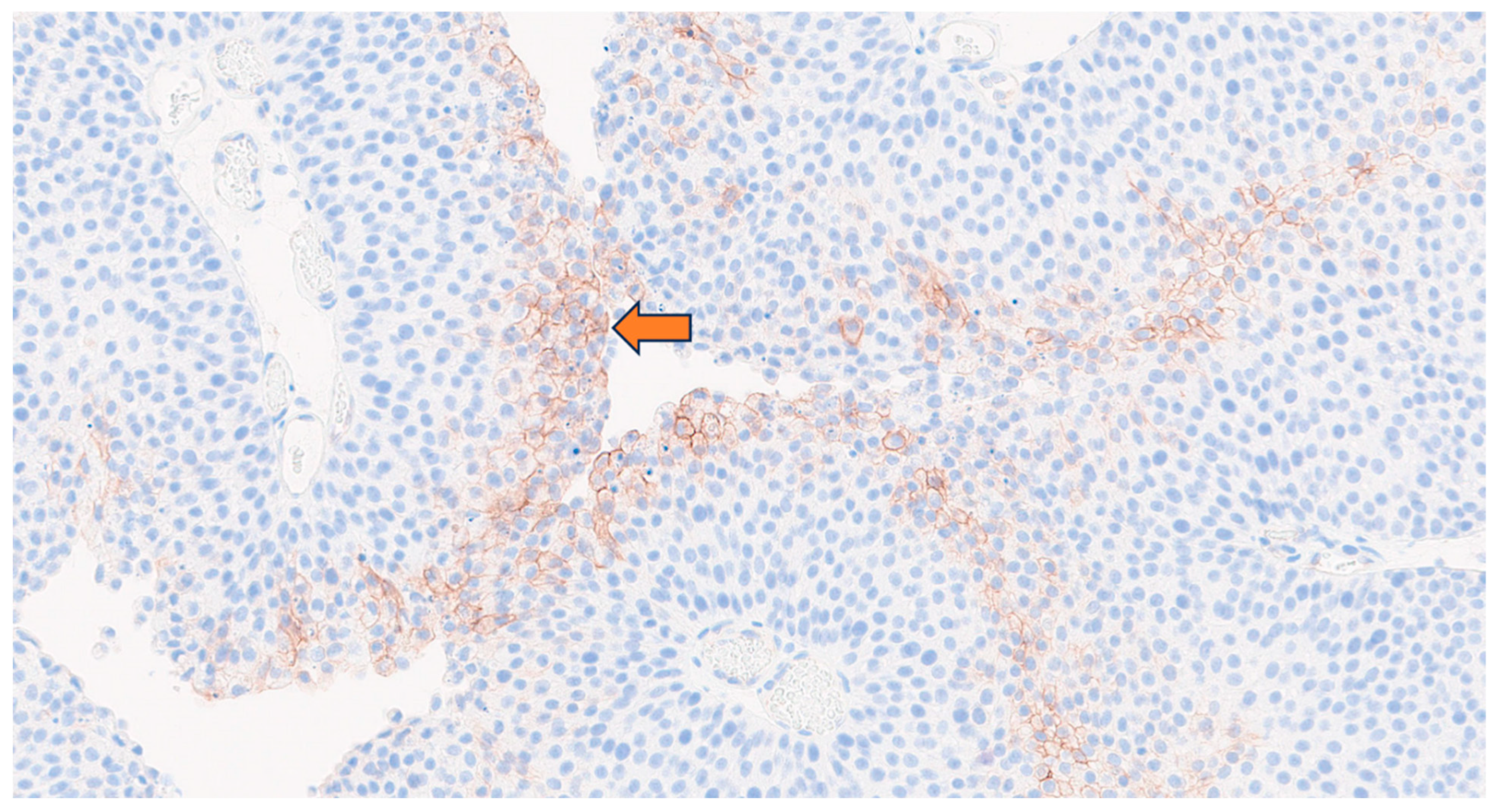
2.2.9. Immunohistochemistry for CA-IX Expression
2.2.10. Dosimetry Estimation
3. Results
3.1. Measurement of Affinity Constant of [211At]At-anti-CA-IX Antibody in RT112 Cells
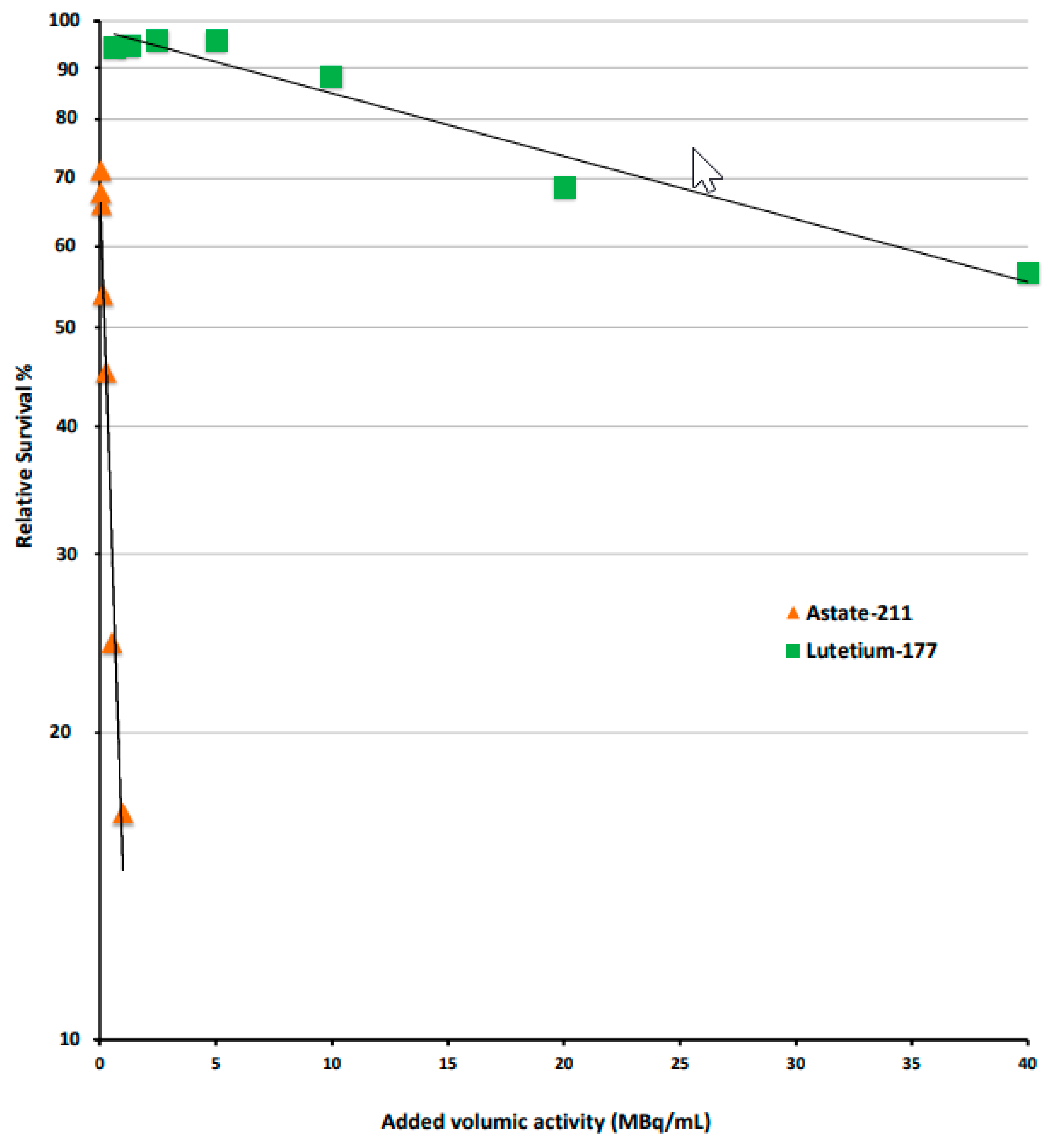
3.2. Compared Cytotoxicity in RT112 Cells
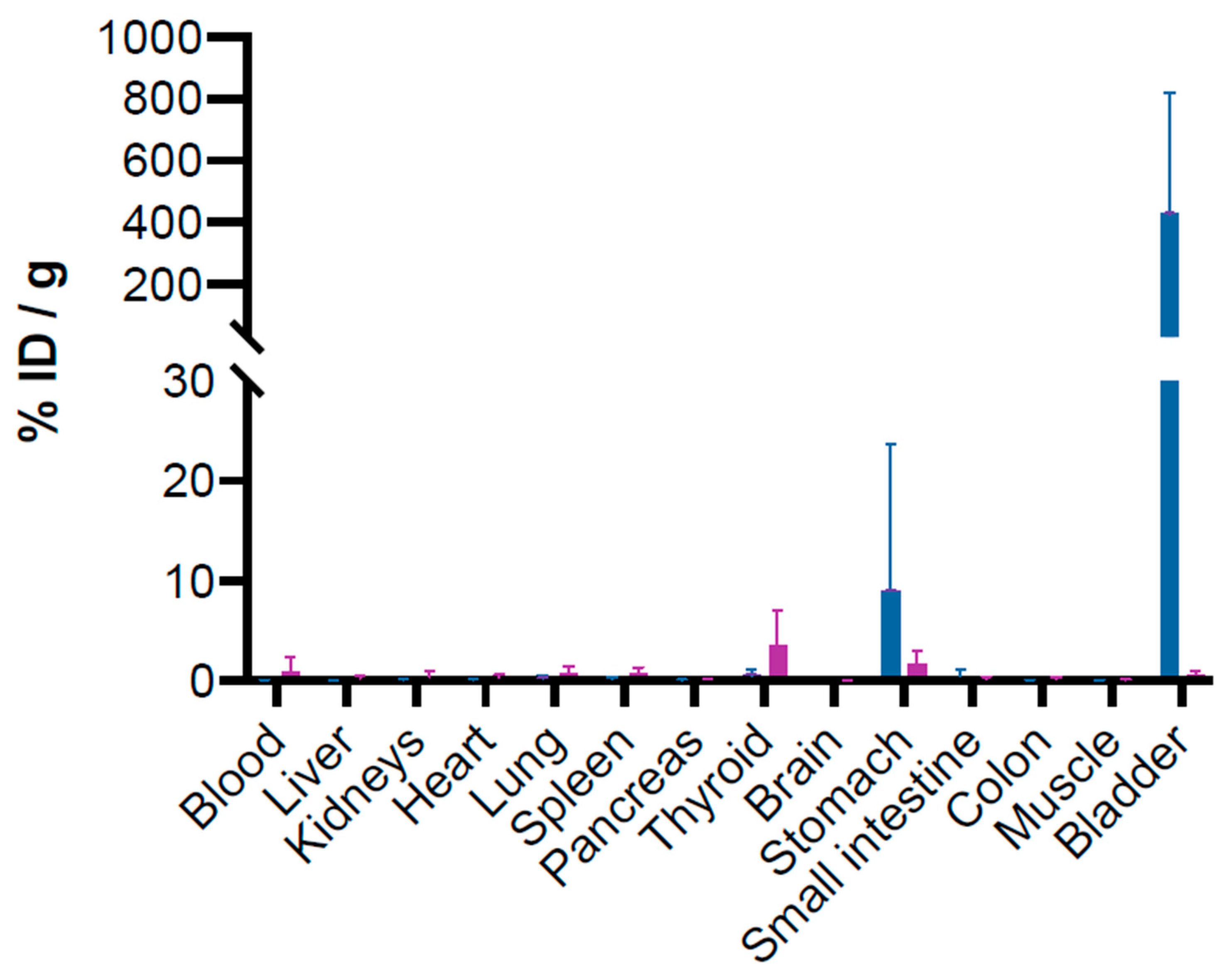
3.3. Biodistribution of [211At]At-anti-CA-IX Antibody (ATO-101™) in Healthy Mice
3.4. Cytotoxicity Evaluation in Healthy Mice
3.5. Clinical Proof of Concept of Intravesical Therapy in Patients: PERTINENCE Study Procedure Using [89Zr]Zr-Girentuximab
3.6. Dosimetry Estimation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Artigas, C.; Mileva, M.; Flamen, P.; Karfis, I. Targeted Radionuclide Therapy: An Emerging Field in Solid Tumours. Curr. Opin. Oncol. 2021, 33, 493–499. [Google Scholar] [CrossRef] [PubMed]
- Strosberg, J.; El-Haddad, G.; Wolin, E.; Hendifar, A.; Yao, J.; Chasen, B.; Mittra, E.; Kunz, P.L.; Kulke, M.H.; Jacene, H.; et al. Phase 3 Trial of 177Lu-Dotatate for Midgut Neuroendocrine Tumors. N. Engl. J. Med. 2017, 376, 125–135. [Google Scholar] [CrossRef] [PubMed]
- Hennrich, U.; Eder, M. [177Lu]Lu-PSMA-617 (PluvictoTM): The First FDA-Approved Radiotherapeutical for Treatment of Prostate Cancer. Pharmaceuticals 2022, 15, 1292. [Google Scholar] [CrossRef]
- Zacherl, M.J.; Gildehaus, F.J.; Mittlmeier, L.; Böning, G.; Gosewisch, A.; Wenter, V.; Unterrainer, M.; Schmidt-Hegemann, N.; Belka, C.; Kretschmer, A.; et al. First Clinical Results for PSMA-Targeted α-Therapy Using 225Ac-PSMA-I&T in Advanced-mCRPC Patients. J. Nucl. Med. 2021, 62, 669–674. [Google Scholar] [CrossRef]
- Rosenblat, T.L.; McDevitt, M.R.; Carrasquillo, J.A.; Pandit-Taskar, N.; Frattini, M.G.; Maslak, P.G.; Park, J.H.; Douer, D.; Cicic, D.; Larson, S.M.; et al. Treatment of Patients with Acute Myeloid Leukemia with the Targeted Alpha-Particle Nanogenerator Actinium-225-Lintuzumab. Clin. Cancer Res. 2022, 28, 2030–2037. [Google Scholar] [CrossRef] [PubMed]
- Eychenne, R.; Chérel, M.; Haddad, F.; Guérard, F.; Gestin, J.-F. Overview of the Most Promising Radionuclides for Targeted Alpha Therapy: The “Hopeful Eight”. Pharmaceutics 2021, 13, 906. [Google Scholar] [CrossRef]
- Chatal, J.-F.; Kraeber-Bodéré, F.; Chérel, M.; Haddad, F. Alphatherapy, the New Impetus to Targeted Radionuclide Therapy? Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 1362–1363. [Google Scholar] [CrossRef]
- Zalutsky, M.R.; Reardon, D.A.; Akabani, G.; Coleman, R.E.; Friedman, A.H.; Friedman, H.S.; McLendon, R.E.; Wong, T.Z.; Bigner, D.D. Clinical Experience with α-Particle Emitting 211At: Treatment of Recurrent Brain Tumor Patients with 211At-Labeled Chimeric Antitenascin Monoclonal Antibody 81C6. J. Nucl. Med. 2007, 49, 30–38. [Google Scholar] [CrossRef]
- Kamat, A.M.; Hahn, N.M.; Efstathiou, J.A.; Lerner, S.P.; Malmström, P.-U.; Choi, W.; Guo, C.C.; Lotan, Y.; Kassouf, W. Bladder Cancer. Lancet 2016, 388, 2796–2810. [Google Scholar] [CrossRef]
- Van Rhijn, B.W.G.; Burger, M.; Lotan, Y.; Solsona, E.; Stief, C.G.; Sylvester, R.J.; Witjes, J.A.; Zlotta, A.R. Recurrence and Progression of Disease in Non–Muscle-Invasive Bladder Cancer: From Epidemiology to Treatment Strategy. Eur. Urol. 2009, 56, 430–442. [Google Scholar] [CrossRef]
- Neuzillet, Y.; Pradère, B.; Xylinas, E.; Allory, Y.; Audenet, F.; Loriot, Y.; Masson-Lecomte, A.; Roumiguié, M.; Seisen, T.; Traxer, O.; et al. French AFU Cancer Committee Guidelines—Update 2022–2024: Non-Muscle-Invasive Bladder Cancer (NMIBC). Prog. Urol. 2022, 32, 1102–1140. [Google Scholar] [CrossRef] [PubMed]
- Steinberg, R.L.; Thomas, L.J.; Nepple, K.G. Intravesical and Alternative Bladder-Preservation Therapies in the Management of Non–Muscle-Invasive Bladder Cancer Unresponsive to Bacillus Calmette-Guérin. Urol. Oncol. Semin. Orig. Investig. 2016, 34, 279–289. [Google Scholar] [CrossRef] [PubMed]
- Autenrieth, M.E.; Seidl, C.; Bruchertseifer, F.; Horn, T.; Kurtz, F.; Feuerecker, B.; D’Alessandria, C.; Pfob, C.; Nekolla, S.; Apostolidis, C.; et al. Treatment of Carcinoma in Situ of the Urinary Bladder with an Alpha-Emitter Immunoconjugate Targeting the Epidermal Growth Factor Receptor: A Pilot Study. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 1364–1371. [Google Scholar] [CrossRef] [PubMed]
- Pfost, B.; Seidl, C.; Autenrieth, M.; Saur, D.; Bruchertseifer, F.; Morgenstern, A.; Schwaiger, M.; Senekowitsch-Schmidtke, R. Intravesical α-Radioimmunotherapy with 213Bi-Anti-EGFR-mAb Defeats Human Bladder Carcinoma in Xenografted Nude Mice. J. Nucl. Med. 2009, 50, 1700–1708. [Google Scholar] [CrossRef]
- Klatte, T.; Belldegrun, A.S.; Pantuck, A.J. The Role of Carbonic Anhydrase IX as a Molecular Marker for Transitional Cell Carcinoma of the Bladder. BJU Int. 2008, 101, 45–48. [Google Scholar] [CrossRef]
- Klatte, T.; Seligson, D.B.; Rao, J.Y.; Yu, H.; De Martino, M.; Kawaoka, K.; Wong, S.G.; Belldegrun, A.S.; Pantuck, A.J. Carbonic Anhydrase IX in Bladder Cancer: A Diagnostic, Prognostic, and Therapeutic Molecular Marker. Cancer 2009, 115, 1448–1458. [Google Scholar] [CrossRef]
- Turner, K.J.; Crew, J.P.; Wykoff, C.C.; Watson, P.H.; Poulsom, R.; Pastorek, J.; Ratcliffe, P.J.; Cranston, D.; Harris, A.L. The Hypoxia-Inducible Genes VEGF and CA9 Are Differentially Regulated in Superficial vs Invasive Bladder Cancer. Br. J. Cancer 2002, 86, 1276–1282. [Google Scholar] [CrossRef]
- Sherwood, B.T.; Colquhoun, A.J.; Richardson, D.; Bowman, K.J.; O’Byrne, K.J.; Kockelbergh, R.C.; Symonds, R.P.; Mellon, J.K.; Jones, G.D.D. Carbonic Anhydrase IX Expression and Outcome after Radiotherapy for Muscle-Invasive Bladder Cancer. Clin. Oncol. 2007, 19, 777–783. [Google Scholar] [CrossRef]
- Morgan, K.A.; Wichmann, C.W.; Osellame, L.D.; Cao, Z.; Guo, N.; Scott, A.M.; Donnelly, P.S. Tumor Targeted Alpha Particle Therapy with an Actinium-225 Labelled Antibody for Carbonic Anhydrase IX. Chem. Sci. 2024, 15, 3372–3381. [Google Scholar] [CrossRef]
- Pettenati, C.; Ingersoll, M.A. Mechanisms of BCG Immunotherapy and Its Outlook for Bladder Cancer. Nat. Rev. Urol. 2018, 15, 615–625. [Google Scholar] [CrossRef]
- Perrin, J.; Capitao, M.; Allard, M.; Chouin, N.; Gouard, S.; Marionneau-Lambot, S.; Louvet, C.; Donnadieu, E.; Bruchertseifer, F.; Morgenstern, A.; et al. Targeted Alpha Particle Therapy Remodels the Tumor Microenvironment and Improves Efficacy of Immunotherapy. Int. J. Radiat. Oncol. 2022, 112, 790–801. [Google Scholar] [CrossRef]
- Gorin, J.-B.; Ménager, J.; Gouard, S.; Maurel, C.; Guilloux, Y.; Faivre-Chauvet, A.; Morgenstern, A.; Bruchertseifer, F.; Chérel, M.; Davodeau, F.; et al. Antitumor Immunity Induced after α Irradiation. Neoplasia 2014, 16, 319–328. [Google Scholar] [CrossRef] [PubMed]
- Maingueneau, C.; Berdal, M.; Eychenne, R.; Gaschet, J.; Chérel, M.; Gestin, J.; Guérard, F. 211At and 125I-Labeling of (Hetero)Aryliodonium Ylides: Astatine Wins Again. Chem. Eur. J. 2022, 28, e202104169. [Google Scholar] [CrossRef]
- Guérard, F.; Navarro, L.; Lee, Y.-S.; Roumesy, A.; Alliot, C.; Chérel, M.; Brechbiel, M.W.; Gestin, J.-F. Bifunctional Aryliodonium Salts for Highly Efficient Radioiodination and Astatination of Antibodies. Bioorg. Med. Chem. 2017, 25, 5975–5980. [Google Scholar] [CrossRef]
- Valentin, J. Basic Anatomical and Physiological Data for Use in Radiological Protection: Reference Values. Ann. ICRP 2002, 32, 1–277. [Google Scholar]
- Bolch, W.E.; Jokisch, D.; Zankl, M.; Eckerman, K.F.; Fell, T.; Manger, R.; Endo, A.; Hunt, J.; Kim, K.P.; Petoussi-Henss, N. ICRP Publication 133: The ICRP Computational Framework for Internal Dose Assessment for Reference Adults: Specific Absorbed Fractions. Ann. ICRP 2016, 45, 5–73. [Google Scholar] [CrossRef]
- Andersson, M.; Johansson, L.; Eckerman, K.; Mattsson, S. IDAC-Dose 2.1, an Internal Dosimetry Program for Diagnostic Nuclear Medicine Based on the ICRP Adult Reference Voxel Phantoms. EJNMMI Res. 2017, 7, 88. [Google Scholar] [CrossRef]
- Ziegler, J.F.; Ziegler, M.D.; Biersack, J.P. SRIM—The Stopping and Range of Ions in Matter (2010). Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 2010, 268, 1818–1823. [Google Scholar] [CrossRef]
- Cao, W.; Chen, G.; Wu, L.; Yu, K.N.; Sun, M.; Yang, M.; Jiang, Y.; Jiang, Y.; Xu, Y.; Peng, S.; et al. Ionizing Radiation Triggers the Antitumor Immunity by Inducing Gasdermin E-Mediated Pyroptosis in Tumor Cells. Int. J. Radiat. Oncol. 2023, 115, 440–452. [Google Scholar] [CrossRef]
- Kisbenedek, L.; Szeldeli, P.; Biró, G.; Balogh, F. Vesicoureteral Reflux Following Transurethral Resection of Bladder Tumours Atthe Ureteral Orifice. Eur. Urol. 1982, 8, 9–10. [Google Scholar] [CrossRef]
- Keir, M.J.; Lambert, H.J.; Coulthard, M.G. Maximizing the Sensitivity of the Indirect Radionuclide Cystogram: A Retrospective Audit. Pediatr. Nephrol. 2013, 28, 2137–2141. [Google Scholar] [CrossRef] [PubMed]
- Kim, L.H.C.; Patel, M.I. Transurethral Resection of Bladder Tumour (TURBT). Transl. Androl. Urol. 2020, 9, 3056–3072. [Google Scholar] [CrossRef] [PubMed]
- Barker, H.; Aaltonen, M.; Pan, P.; Vähätupa, M.; Kaipiainen, P.; May, U.; Prince, S.; Uusitalo-Järvinen, H.; Waheed, A.; Pastoreková, S.; et al. Role of Carbonic Anhydrases in Skin Wound Healing. Exp. Mol. Med. 2017, 49, e334. [Google Scholar] [CrossRef] [PubMed]
- Meredith, R.; Wessels, B.; Knox, S. Risks to Normal Tissues From Radionuclide Therapy. Semin. Nucl. Med. 2008, 38, 347–357. [Google Scholar] [CrossRef]
- Albertsson, P.; Bäck, T.; Bergmark, K.; Hallqvist, A.; Johansson, M.; Aneheim, E.; Lindegren, S.; Timperanza, C.; Smerud, K.; Palm, S. Astatine-211 Based Radionuclide Therapy: Current Clinical Trial Landscape. Front. Med. 2023, 9, 1076210. [Google Scholar] [CrossRef]


| Patient | Sex | Age (Years) | Initial TCC Diagnosis | Treatments Received | TURB Pre-Imaging | 89Zr-TLX250 Imaging | TURB Post-Imaging | FFPE Samples | Biopsy Results | CA-IX—EP161 Cell Marque | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Positive Cells % | Intensity (1+, 2+, 3+) | ||||||||||
| 1 | M | 80 | Oct 2020 | BCG × 6: Apr–May 2021 | Nov 2021 | Dec 2021 | Feb 2022 | TURB pre-imaging | Non-invasive papillary urothelial cancer, pTaG3 | 10 | 2+ |
| Biopsy post-imaging | Non-invasive papillary urothelial cancer, pTaG3 | 20 | 2+ | ||||||||
| Biopsy post-imaging | Non-invasive papillary urothelial cancer, pTaG3 | 10 | 2+ | ||||||||
| 2 | M | 72 | Jun 2021 | BCG × 6: Aug–Sept 2021 | Dec 2021 | Jan 2022 | Feb 2022 | TURB pre-imaging | Non-invasive papillary urothelial cancer, pTaG3 | 3 | 2+ |
| Biopsy post-imaging | Inflammatory bladder wall without tumor | 0 | Not applicable | ||||||||
| 3 | F | 71 | May 2004 | BCG × 6: Jul–Aug 2005 Mitomycin x 6: Nov–Dec 2006 BCG × 6: Nov–Dec 2005 BCG × 3: Apr 2007 + Oct 2007 | Nov 2021 | Feb 2022 | - | TURB pre-imaging | Non-invasive papillary urothelial cancer, pTaG3 | 2 | 2+ |
| 4 | M | 69 | Feb 2019 | Mitomycin × 6: Apr–Jun 2019 BCG × 6: Aug–Sept 2000 | Jan 2022 | Mar 2022 | - | TURB pre-imaging | Non-invasive papillary urothelial cancer, pTaG3 | 0 | Not applicable |
| 5 | M | 73 | Nov 2016 | BCG × 6: Apr–May 2016 BCG × 6: Mar–Apr 2019 BCG × 3: Aug 2020+ Janv 2021 | Jan 2022 | Mar 2022 | - | TURB pre-imaging | Non-invasive papillary urothelial cancer, pTaG3 | 1 | 2+ |
| 6 | F | 77 | Aug 2021 | BCG × 6: Nov–Dec 2021 | Jul 2022 | Aug 2022 | Sept 2022 | TURB pre-imaging | Non-invasive papillary urothelial cancer, pTaG3 | 5 | 2+ |
| Biopsy post-imaging | Inflammatory and scarred bladder wall without tumor | 10 | 2+ | ||||||||
| Radionuclide | Residence [h] Time | Absorbed Dose per Unit of Activity Administered [mSv/MBq] | |
|---|---|---|---|
| Male | Female | ||
| 211At | 1.820 | 0.158 | 0.195 |
| 211Po | 1.819 | 0.662 | 0.827 |
| Total | 0.820 | 1.022 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rousseau, C.; Baumgartner, P.; Heymann, M.-F.; Taupin, M.; Geffroy, M.; Chatal, J.-F.; Gautier, G.; Allam, N.; Gaschet, J.; Eychenne, R.; et al. Preclinical and Clinical Feasibility Studies as the First Step Before Forthcoming Intravesical Instillation of [211At]At-anti-CA-IX Antibody (ATO-101™) Study in Patients with Non-Muscle-Invasive Bladder Cancer Unresponsive to Standard of Care. Cancers 2025, 17, 1190. https://doi.org/10.3390/cancers17071190
Rousseau C, Baumgartner P, Heymann M-F, Taupin M, Geffroy M, Chatal J-F, Gautier G, Allam N, Gaschet J, Eychenne R, et al. Preclinical and Clinical Feasibility Studies as the First Step Before Forthcoming Intravesical Instillation of [211At]At-anti-CA-IX Antibody (ATO-101™) Study in Patients with Non-Muscle-Invasive Bladder Cancer Unresponsive to Standard of Care. Cancers. 2025; 17(7):1190. https://doi.org/10.3390/cancers17071190
Chicago/Turabian StyleRousseau, Caroline, Pierre Baumgartner, Marie-Françoise Heymann, Manon Taupin, Maïwenn Geffroy, Jean-François Chatal, Gaëlle Gautier, Nadia Allam, Joëlle Gaschet, Romain Eychenne, and et al. 2025. "Preclinical and Clinical Feasibility Studies as the First Step Before Forthcoming Intravesical Instillation of [211At]At-anti-CA-IX Antibody (ATO-101™) Study in Patients with Non-Muscle-Invasive Bladder Cancer Unresponsive to Standard of Care" Cancers 17, no. 7: 1190. https://doi.org/10.3390/cancers17071190
APA StyleRousseau, C., Baumgartner, P., Heymann, M.-F., Taupin, M., Geffroy, M., Chatal, J.-F., Gautier, G., Allam, N., Gaschet, J., Eychenne, R., Guérard, F., Gestin, J.-F., Varmenot, N., & Chérel, M. (2025). Preclinical and Clinical Feasibility Studies as the First Step Before Forthcoming Intravesical Instillation of [211At]At-anti-CA-IX Antibody (ATO-101™) Study in Patients with Non-Muscle-Invasive Bladder Cancer Unresponsive to Standard of Care. Cancers, 17(7), 1190. https://doi.org/10.3390/cancers17071190






