Age-Related Macular Degeneration Screening—What Is Next?
Abstract
Introduction
Risk factors
Pathogenesis
Diagnosis and classification
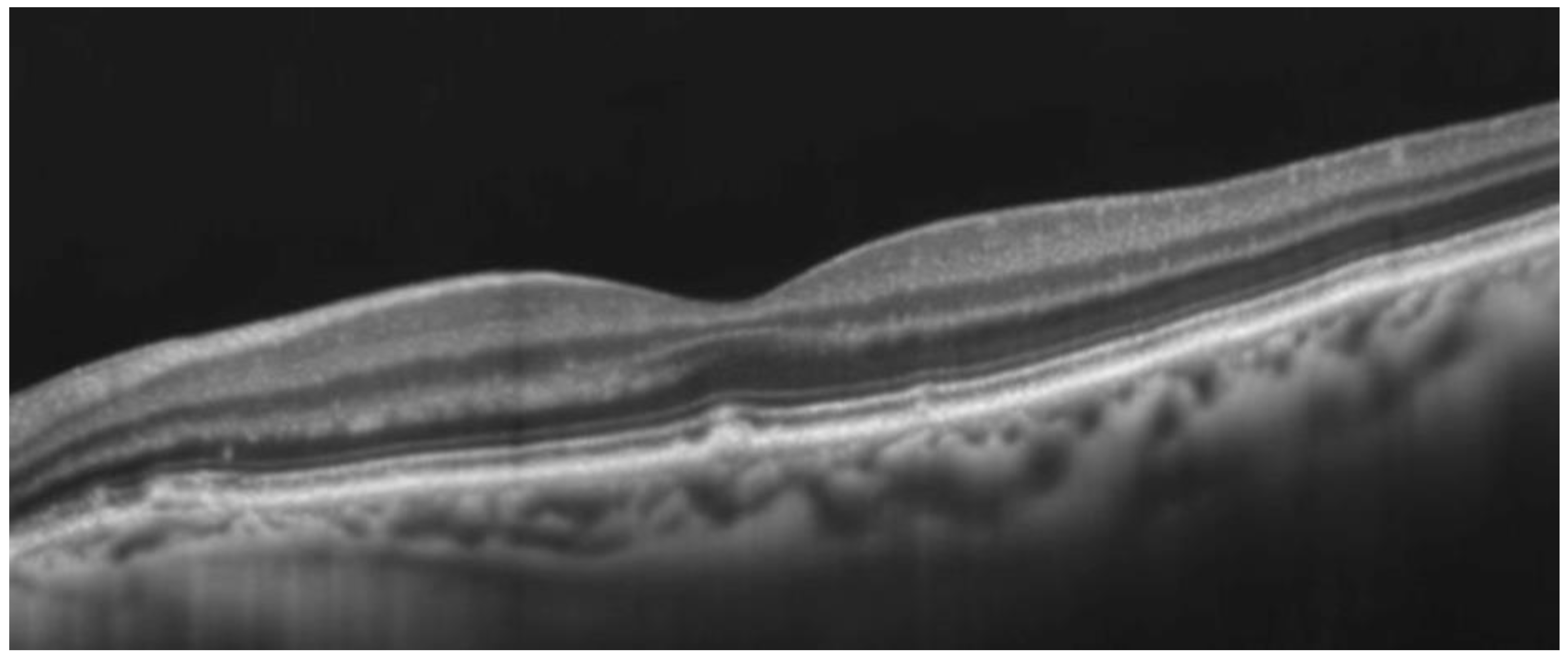
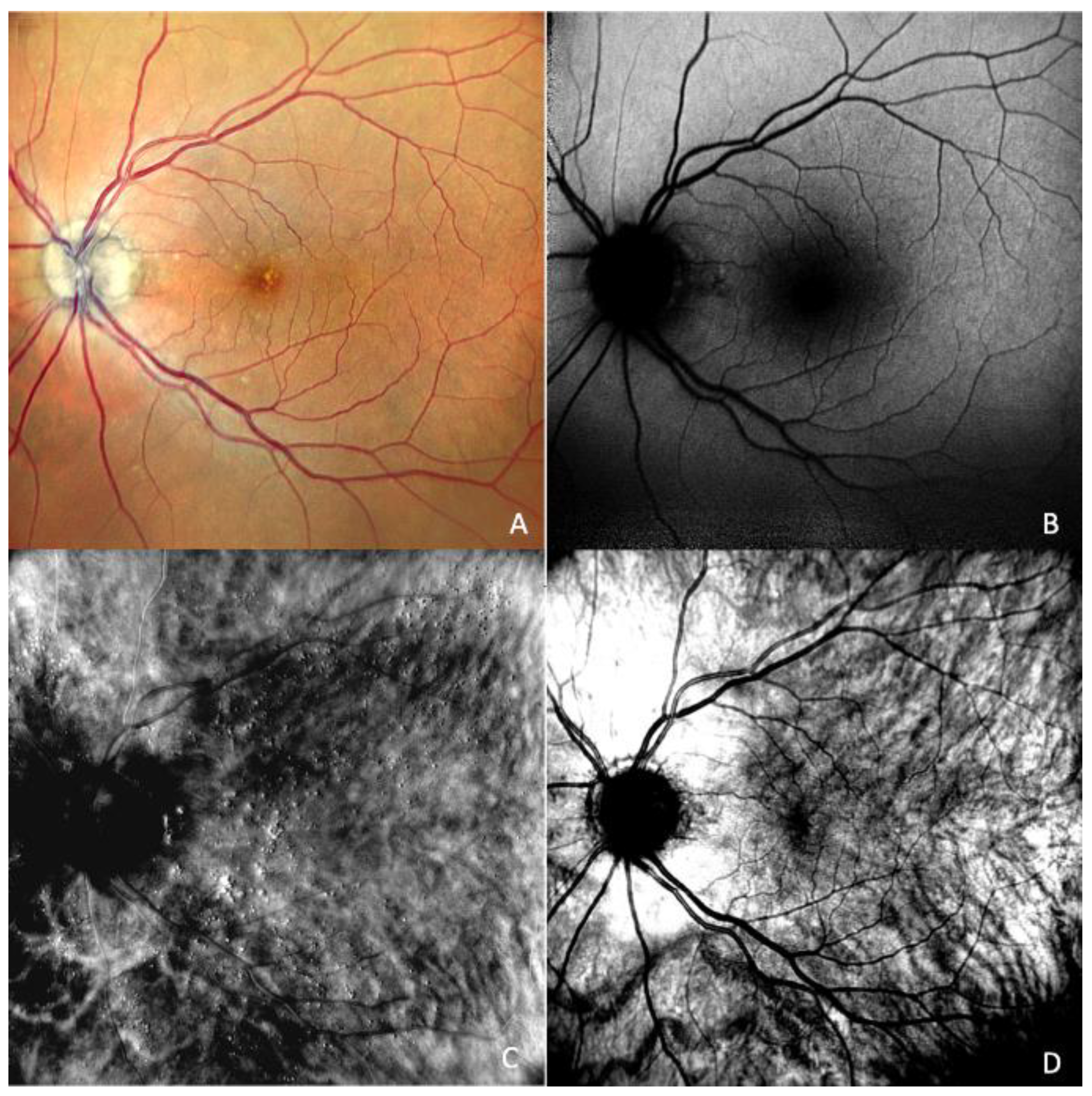
Multimodality
Machine learning and artificial intelligence in AMD
Screening for AMD
Prevention and treatment
Conclusion
References
- Mitchell, P.; Liew, G.; Gopinath, B.; Wong, T.Y. Age-related macular degeneration. Lancet 2018, 392, 1147–1159. [Google Scholar] [CrossRef] [PubMed]
- Wong, W.L.; Su, X.; Li, X.; Cheung, C.M.; Klein, R.; Cheng, C.Y.; Wong, T.Y. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: a systematic review and meta-analysis. Lancet Glob Health 2014, 2, e106–e116, Epub 2014 Jan 3. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.; Hamada, S.; Membrey, W.L.; Chong, V. Screening for age-related macular degeneration using nonstereo digital fundus photographs. Eye (Lond) 2006, 20, 471–475. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Moreno, J.M.; Arias, L.; Abraldes, M.J.; Montero, J.; Udaondo, P.; RAMDEBURS study group. Economic burden of age-related macular degeneration in routine clinical practice: the RAMDEBURS study. Int Ophthalmol. 2021, 41, 3427–3436, Epub 2021 Jun 10. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lambert, N.G.; ElShelmani, H.; Singh, M.K.; Mansergh, F.C.; Wride, M.A.; Padilla, M.; Keegan, D.; Hogg, R.E.; Ambati, B.K. Risk factors and biomarkers of age-related macular degeneration. Prog Retin Eye Res. 2016, 54, 64–102, Epub 2016 May 6. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Saunier, V.; Merle, B.M.J.; Delyfer, M.N.; Cougnard-Grégoire, A.; Rougier, M.B.; Amouyel, P.; Lambert, J.C.; Dartigues, J.F.; Korobelnik, J.F.; Delcourt, C. Incidence of and Risk Factors Associated With Age-Related Macular Degeneration: Four-Year Follow-up From the ALIENOR Study. JAMA Ophthalmol. 2018, 136, 473–481. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chakravarthy, U.; Wong, T.Y.; Fletcher, A.; Piault, E.; Evans, C.; Zlateva, G.; Buggage, R.; Pleil, A.; Mitchell, P. Clinical risk factors for age-related macular degeneration: a systematic review and meta-analysis. BMC Ophthalmol. 2010, 10, 31. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kawasaki, R.; Wang, J.J.; Aung, T.; Tan, D.T.; Mitchell, P.; Sandar, M.; Saw, S.M.; Wong, T.Y.; Singapore Malay Eye Study Group. Prevalence of age-related macular degeneration in a Malay population: the Singapore Malay Eye Study. Ophthalmology 2008, 115, 1735–1741, Epub 2008 Apr 25. [Google Scholar] [CrossRef] [PubMed]
- Rudnicka, A.R.; Jarrar, Z.; Wormald, R.; Cook, D.G.; Fletcher, A.; Owen, C.G. Age and gender variations in age-related macular degeneration prevalence in populations of European ancestry: a meta-analysis. Ophthalmology 2012, 119, 571–580, Epub 2011 Dec 15. [Google Scholar] [CrossRef] [PubMed]
- Fritsche, L.G.; Igl, W.; Bailey, J.N.; Grassmann, F.; Sengupta, S.; Bragg-Gresham, J.L.; Burdon, K.P.; Hebbring, S.J.; Wen, C.; Gorski, M.; Kim, I.K.; Cho, D.; Zack, D.; Souied, E.; Scholl, H.P.; Bala, E.; Lee, K.E.; Hunter, D.J.; Sardell, R.J.; Mitchell, P.; Merriam, J.E.; Cipriani, V.; Hoffman, J.D.; Schick, T.; Lechanteur, Y.T.; Guymer, R.H.; Johnson, M.P.; Jiang, Y.; Stanton, C.M.; Buitendijk, G.H.; Zhan, X.; Kwong, A.M.; Boleda, A.; Brooks, M.; Gieser, L.; Ratnapriya, R.; Branham, K.E.; Foerster, J.R.; Heckenlively, J.R.; Othman, M.I.; Vote, B.J.; Liang, H.H.; Souzeau, E.; McAllister, I.L.; Isaacs, T.; Hall, J.; Lake, S.; Mackey, D.A.; Constable, I.J.; Craig, J.E.; Kitchner, T.E.; Yang, Z.; Su, Z.; Luo, H.; Chen, D.; Ouyang, H.; Flagg, K.; Lin, D.; Mao, G.; Ferreyra, H.; Stark, K.; von Strachwitz, C.N.; Wolf, A.; Brandl, C.; Rudolph, G.; Olden, M.; Morrison, M.A.; Morgan, D.J.; Schu, M.; Ahn, J.; Silvestri, G.; Tsironi, E.E.; Park, K.H.; Farrer, L.A.; Orlin, A.; Brucker, A.; Li, M.; Curcio, C.A.; Mohand-Saïd, S.; Sahel, J.A.; Audo, I.; Benchaboune, M.; Cree, A.J.; Rennie, C.A.; Goverdhan, S.V.; Grunin, M.; Hagbi-Levi, S.; Campochiaro, P.; Katsanis, N.; Holz, F.G.; Blond, F.; Blanché, H.; Deleuze, J.F.; Igo, R.P., Jr.; Truitt, B.; Peachey, N.S.; Meuer, S.M.; Myers, C.E.; Moore, E.L.; Klein, R.; Hauser, M.A.; Postel, E.A.; Courtenay, M.D.; Schwartz, S.G.; Kovach, J.L.; Scott, W.K.; Liew, G.; Tan, A.G.; Gopinath, B.; Merriam, J.C.; Smith, R.T.; Khan, J.C.; Shahid, H.; Moore, A.T.; McGrath, J.A.; Laux, R.; Brantley, M.A., Jr.; Agarwal, A.; Ersoy, L.; Caramoy, A.; Langmann, T.; Saksens, N.T.; de Jong, E.K.; Hoyng, C.B.; Cain, M.S.; Richardson, A.J.; Martin, T.M.; Blangero, J.; Weeks, D.E.; Dhillon, B.; van Duijn, C.M.; Doheny, K.F.; Romm, J.; Klaver, C.C.; Hayward, C.; Gorin, M.B.; Klein, M.L.; Baird, P.N.; den Hollander, A.I.; Fauser, S.; Yates, J.R.; Allikmets, R.; Wang, J.J.; Schaumberg, D.A.; Klein, B.E.; Hagstrom, S.A.; Chowers, I.; Lotery, A.J.; Léveillard, T.; Zhang, K.; Brilliant, M.H.; Hewitt, A.W.; Swaroop, A.; Chew, E.Y.; Pericak-Vance, M.A.; DeAngelis, M.; Stambolian, D.; Haines, J.L.; Iyengar, S.K.; Weber, B.H.; Abecasis, G.R.; Heid, I.M. A large genome-wide association study of age-related macular degeneration highlights contributions of rare and common variants. Nat Genet. 2016, 48, 134–143, Epub 2015 Dec 21. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Schultz, N.M.; Bhardwaj, S.; Barclay, C.; Gaspar, L.; Schwartz, J. Global Burden of Dry Age-Related Macular Degeneration: A Targeted Literature Review. Clin Ther. 2021, 43, 1792–1818, Epub 2021 Sep 20. [Google Scholar] [CrossRef] [PubMed]
- Age-Related Eye Disease Study Research Group. A randomized, placebo-controlled, clinical trial of high-dose supplementation with vitamins C and E, beta carotene, and zinc for age-related macular degeneration and vision loss: AREDS report no. 8. Arch Ophthalmol. 2001, 119, 1417–1436, Erratum in Arch Ophthalmol. 2008, 126, 1251. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Age-Related Eye Disease Study 2 (AREDS2) Research Group; Chew, E.Y.; Clemons, T.E.; Sangiovanni, J.P.; Danis, R.P.; Ferris, F.L., 3rd; Elman, M.J.; Antoszyk, A.N.; Ruby, A.J.; Orth, D.; Bressler, S.B.; Fish, G.E.; Hubbard, G.B.; Klein, M.L.; Chandra, S.R.; Blodi, B.A.; Domalpally, A.; Friberg, T.; Wong, W.T.; Rosenfeld, P.J.; Agrón, E.; Toth, C.A.; Bernstein, P.S.; Sperduto, R.D. Secondary analyses of the effects of lutein/zeaxanthin on age-related macular degeneration progression: AREDS2 report No. 3. JAMA Ophthalmol. 2014, 132, 142–149. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sarks, S.; Cherepanoff, S.; Killingsworth, M.; Sarks, J. Relationship of Basal laminar deposit and membranous debris to the clinical presentation of early age-related macular degeneration. Invest Ophthalmol Vis Sci. 2007, 48, 968–977. [Google Scholar] [CrossRef] [PubMed]
- Flores, R.; Carneiro, Â.; Vieira, M.; Tenreiro, S.; Seabra, M.C. Age-Related Macular Degeneration: Pathophysiology, Management, and Future Perspectives. Ophthalmologica 2021, 244, 495–511, Epub 2021 Jun 15. [Google Scholar] [CrossRef] [PubMed]
- Laíns, I.; Wang, J.; Providência, J.; Mach, S.; Gil, P.; Gil, J.; Marques, M.; Armstrong, G.; Garas, S.; Barreto, P.; Kim, I.K.; Vavvas, D.G.; Miller, J.W.; Husain, D.; Silva, R.; Miller, J.B. Choroidal Changes Associated With Subretinal Drusenoid Deposits in Age-related Macular Degeneration Using Swept-source Optical Coherence Tomography. Am J Ophthalmol. 2017, 180, 55–63, Epub 2017 Jun 1. [Google Scholar] [CrossRef] [PubMed]
- McLeod, D.S.; Grebe, R.; Bhutto, I.; Merges, C.; Baba, T.; Lutty, G.A. Relationship between RPE and choriocapillaris in age-related macular degeneration. Invest Ophthalmol Vis Sci. 2009, 50, 4982–4991, Epub 2009 Apr 8. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sadda, S.R.; Guymer, R.; Holz, F.G.; Schmitz-Valckenberg, S.; Curcio, C.A.; Bird, A.C.; Blodi, B.A.; Bottoni, F.; Chakravarthy, U.; Chew, E.Y.; Csaky, K.; Danis, R.P.; Fleckenstein, M.; Freund, K.B.; Grunwald, J.; Hoyng, C.B.; Jaffe, G.J.; Liakopoulos, S.; Monés, J.M.; Pauleikhoff, D.; Rosenfeld, P.J.; Sarraf, D.; Spaide, R.F.; Tadayoni, R.; Tufail, A.; Wolf, S.; Staurenghi, G. Consensus Definition for Atrophy Associated with Age-Related Macular Degeneration on OCT: Classification of Atrophy Report 3. Ophthalmology 2018, 125, 537–548, Epub 2017 Nov 2. Erratum in Ophthalmology 2019, 126, 177. [Google Scholar] [CrossRef] [PubMed]
- Penn, J.S.; Madan, A.; Caldwell, R.B.; Bartoli, M.; Caldwell, R.W.; Hartnett, M.E. Vascular endothelial growth factor in eye disease. Prog Retin Eye Res. 2008, 27, 331–371, Epub 2008 May 28. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kliffen, M.; Sharma, H.S.; Mooy, C.M.; Kerkvliet, S.; de Jong, P.T. Increased expression of angiogenic growth factors in age-related maculopathy. Br J Ophthalmol. 1997, 81, 154–162. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Spaide, R.F.; Jaffe, G.J.; Sarraf, D.; Freund, K.B.; Sadda, S.R.; Staurenghi, G.; Waheed, N.K.; Chakravarthy, U.; Rosenfeld, P.J.; Holz, F.G.; Souied, E.H.; Cohen, S.Y.; Querques, G.; Ohno-Matsui, K.; Boyer, D.; Gaudric, A.; Blodi, B.; Baumal, C.R.; Li, X.; Coscas, G.J.; Brucker, A.; Singerman, L.; Luthert, P.; Schmitz-Valckenberg, S.; Schmidt-Erfurth, U.; Grossniklaus, H.E.; Wilson, D.J.; Guymer, R.; Yannuzzi, L.A.; Chew, E.Y.; Csaky, K.; Monés, J.M.; Pauleikhoff, D.; Tadayoni, R.; Fujimoto, J. Consensus Nomenclature for Reporting Neovascular Age-Related Macular Degeneration Data: Consensus on Neovascular Age-Related Macular Degeneration Nomenclature Study Group. Ophthalmology 2020, 127, 616–636, Epub 2019 Nov 14. Erratum in Ophthalmology 2020, 127, 1434–1435. [Google Scholar] [CrossRef] [PubMed]
- Forshaw, T.R.J.; Minör, Å.S.; Subhi, Y.; Sørensen, T.L. Peripheral Retinal Lesions in Eyes with Age-Related Macular Degeneration Using Ultra-Widefield Imaging: A Systematic Review with Meta-analyses. Ophthalmol Retina 2019, 3, 734–743, Epub 2019 Apr 18. [Google Scholar] [CrossRef] [PubMed]
- Domalpally, A.; Danis, R.; Agrón, E.; Blodi, B.; Clemons, T.; Chew, E.; Age-Related Eye Disease Study 2 Research Group. Evaluation of Geographic Atrophy from Color Photographs and Fundus Autofluorescence Images: Age-Related Eye Disease Study 2 Report Number 11. Ophthalmology 2016, 123, 2401–2407, Epub 2016 Jul 19. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- National Institute for Health and Care Excellence (NICE). Age-related macular degeneration: diagnosis and management. NICE Guideline, no. 82, Jan. 2018. Available online: https://www.ncbi.nlm.nih.gov/books/NBK536479/ (accessed on 5 March 2023).
- Bjerager, J.; Schneider, M.; Potapenko, I.; van Dijk, E.H.C.; Faber, C.; Grauslund, J.; Pfau, K.; Huemer, J.; Muttuvelu, D.V.; Rasmussen, M.L.R.; Sabaner, M.C.; Subhi, Y. Diagnostic Accuracy of the Amsler Grid Test for Detecting Neovascular Age-Related Macular Degeneration: A Systematic Review and Meta-analysis. JAMA Ophthalmol. 2023; Epub ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Ferris, F.L., 3rd; Wilkinson, C.P.; Bird, A.; Chakravarthy, U.; Chew, E.; Csaky, K.; Sadda, S.R.; Beckman Initiative for Macular Research Classification Committee. Clinical classification of age-related macular degeneration. Ophthalmology 2013, 120, 844–851, Epub 2013 Jan 16. [Google Scholar] [CrossRef] [PubMed]
- Davis, M.D.; Gangnon, R.E.; Lee, L.Y.; Hubbard, L.D.; Klein, B.E.; Klein, R.; Ferris, F.L.; Bressler, S.B.; Milton, R.C.; Age-Related Eye Disease Study Group. The Age-Related Eye Disease Study severity scale for age-related macular degeneration: AREDS Report No. 17. Arch Ophthalmol. 2005, 123, 1484–1498, Erratum in Arch Ophthalmol. 2006, 124, 289–290. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lei, J.; Balasubramanian, S.; Abdelfattah, N.S.; Nittala, M.G.; Sadda, S.R. Proposal of a simple optical coherence tomography-based scoring system for progression of age-related macular degeneration. Graefes Arch Clin Exp Ophthalmol. 2017, 255, 1551–1558, Epub 2017 May 22. [Google Scholar] [CrossRef] [PubMed]
- Holz, F.G.; Sadda, S.R.; Staurenghi, G.; Lindner, M.; Bird, A.C.; Blodi, B.A.; Bottoni, F.; Chakravarthy, U.; Chew, E.Y.; Csaky, K.; Curcio, C.A.; Danis, R.; Fleckenstein, M.; Freund, K.B.; Grunwald, J.; Guymer, R.; Hoyng, C.B.; Jaffe, G.J.; Liakopoulos, S.; Monés, J.M.; Oishi, A.; Pauleikhoff, D.; Rosenfeld, P.J.; Sarraf, D.; Spaide, R.F.; Tadayoni, R.; Tufail, A.; Wolf, S.; Schmitz-Valckenberg, S.; CAM group. Imaging Protocols in Clinical Studies in Advanced Age-Related Macular Degeneration: Recommendations from Classification of Atrophy Consensus Meetings. Ophthalmology 2017, 124, 464–478, Epub 2017 Jan 18. [Google Scholar] [CrossRef] [PubMed]
- Cicinelli, M.V.; Rabiolo, A.; Sacconi, R.; Carnevali, A.; Querques, L.; Bandello, F.; Querques, G. Optical coherence tomography angiography in dry age-related macular degeneration. Surv Ophthalmol. 2018, 63, 236–244, Epub 2017 Jun 23. [Google Scholar] [CrossRef] [PubMed]
- Gualino, V.; et al. OPTICAL COHERENCE TOMOGRAPHY, FLUORESCEIN ANGIOGRAPHY, AND DIAGNOSIS OF CHOROIDAL NEOVASCULARIZATION IN AGE-RELATED MACULAR DEGENERATION. Retina 2019, 39, 1664–1671. [Google Scholar] [CrossRef] [PubMed]
- Pilotto, E.; Guidolin, F.; Convento, E.; Spedicato, L.; Vujosevic, S.; Cavarzeran, F.; Midena, E. Fundus autofluorescence and microperimetry in progressing geographic atrophy secondary to age-related macular degeneration. Br J Ophthalmol 2013, 97, 622–626, Epub 2013 Feb 14. [Google Scholar] [CrossRef] [PubMed]
- Yung, M.; Klufas, M.A.; Sarraf, D. Clinical applications of fundus autofluorescence in retinal disease. Int J Retina Vitreous 2016, 2, 12. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ueda-Arakawa, N.; Ooto, S.; Tsujikawa, A.; Yamashiro, K.; Oishi, A.; Yoshimura, N. SENSITIVITY AND SPECIFICITY OF DETECTING RETICULAR PSEUDODRUSEN IN MULTIMODAL IMAGING IN JAPANESE PATIENTS. Retina 2013, 33, 490–497. [Google Scholar] [CrossRef] [PubMed]
- Diniz, B.; Ribeiro, R.M.; Rodger, D.C.; Maia, M.; Sadda, S. Drusen detection by confocal aperture-modulated infrared scanning laser ophthalmoscopy. Br J Ophthalmol. 2013, 97, 285–290, Epub 2012 Dec 21. [Google Scholar] [CrossRef] [PubMed]
- Acton, J.H.; Cubbidge, R.P.; King, H.; Galsworthy, P.; Gibson, J.M. Drusen detection in retro-mode imaging by a scanning laser ophthalmoscope. Acta Ophthalmol. 2011, 89, e404–e411, Epub 2011 Feb 18. [Google Scholar] [CrossRef] [PubMed]
- Corradetti, G.; Corvi, F.; Sadda, S.R. Subretinal Drusenoid Deposits Revealed by Color SLO and Retro-Mode Imaging. Ophthalmology 2021, 128, 409. [Google Scholar] [CrossRef] [PubMed]
- Cozzi, M.; Monteduro, D.; Parrulli, S.; Corvi, F.; Zicarelli, F.; Corradetti, G.; Sadda, S.R.; Staurenghi, G. Sensitivity and Specificity of Multimodal Imaging in Characterizing Drusen. Ophthalmol Retina 2020, 4, 987–995, Epub 2020 Apr 23. [Google Scholar] [CrossRef] [PubMed]
- Shin, Y.U.; Lee, B.R. Retro-mode Imaging for retinal pigment epithelium alterations in central serous chorioretinopathy. Am J Ophthalmol. 2012, 154, 155–163.e4, Epub 2012 Apr 13. [Google Scholar] [CrossRef] [PubMed]
- Elsharkawy, M.; Elrazzaz, M.; Ghazal, M.; Alhalabi, M.; Soliman, A.; Mahmoud, A.; El-Daydamony, E.; Atwan, A.; Thanos, A.; Sandhu, H.S.; Giridharan, G.; El-Baz, A. Role of Optical Coherence Tomography Imaging in Predicting Progression of Age-Related Macular Disease: A Survey. Diagnostics (Basel) 2021, 11, 2313. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Loo, J.; et al. Joint multimodal deep learning-based automatic segmentation of ICGA and OCT images for assessment of PCV biomarkers. Ophthalmology Science 2023, 100292. [Google Scholar] [CrossRef] [PubMed]
- Lad, E.; Sleiman, K.; Banks, D.L.; Hariharan, S.; Clemons, T.; Herrmann, R.; Dauletbekov, D.; Giani, A.; Chong, V.; Chew, E.Y.; Toth, C.A. Machine learning OCT predictors of progression from intermediate age-related macular degeneration to geographic atrophy and vision loss. Ophthalmol Sci. 2022, 2, 100160. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Karnon, J.; Czoski-Murray, C.; Smith, K.; Brand, C.; Chakravarthy, U.; Davis, S.; Bansback, N.; Beverley, C.; Bird, A.; Harding, S.; Chisholm, I.; Yang, Y.C. A preliminary model-based assessment of the cost-utility of a screening programme for early age-related macular degeneration. Health Technol Assess. 2008, 12, iii–iv, ix–124. [Google Scholar] [CrossRef] [PubMed]
- Tamura, H.; Akune, Y.; Hiratsuka, Y.; Kawasaki, R.; Kido, A.; Miyake, M.; Goto, R.; Yamada, M. Real-world effectiveness of screening programs for age-related macular degeneration: amended Japanese specific health checkups and augmented screening programs with OCT or AI. Jpn J Ophthalmol. 2022, 66, 19–32, Epub 2022 Jan 7. [Google Scholar] [CrossRef] [PubMed]
- Chan, C.K.; Gangwani, R.A.; McGhee, S.M.; Lian, J.; Wong, D.S. Cost-Effectiveness of Screening for Intermediate Age-Related Macular Degeneration during Diabetic Retinopathy Screening. Ophthalmology 2015, 122, 2278–2285, Epub 2015 Aug 24. [Google Scholar] [CrossRef] [PubMed]
- Brady, C.J.; Garg, S. Telemedicine for Age-Related Macular Degeneration. Telemed J E Health 2020, 26, 565–568, Epub 2020 Mar 25. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- García-Layana, A.; et al. A Screening Tool for Self-Evaluation of Risk for Age-Related Macular Degeneration: Validation in a Spanish Population. Trans. Vis. Sci. Tech. 2022, 11, 23. [Google Scholar] [CrossRef] [PubMed]
- Comparison of Age-related Macular Degeneration Treatments Trials (CATT) Research Group; Martin, D.F.; Maguire, M.G.; Fine, S.L.; Ying, G.S.; Jaffe, G.J.; Grunwald, J.E.; Toth, C.; Redford, M.; Ferris, F.L., 3rd. Ranibizumab and bevacizumab for treatment of neovascular age-related macular degeneration: two-year results. Ophthalmology 2012, 119, 1388–1398, Epub 2012 May 1. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Heier, J.S.; Brown, D.M.; Chong, V.; Korobelnik, J.F.; Kaiser, P.K.; Nguyen, Q.D.; Kirchhof, B.; Ho, A.; Ogura, Y.; Yancopoulos, G.D.; Stahl, N.; Vitti, R.; Berliner, A.J.; Soo, Y.; Anderesi, M.; Groetzbach, G.; Sommerauer, B.; Sandbrink, R.; Simader, C.; Schmidt-Erfurth, U.; VIEW 1 and VIEW 2 Study Groups. Intravitreal aflibercept (VEGF trap-eye) in wet age-related macular degeneration. Ophthalmology 2012, 119, 2537–2548, Epub 2012 Oct 17. Erratum in Ophthalmology 2013, 120, 209–210. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.P.; Jhaveri, C.; Wykoff, C.C.; Gale, R.P.; Staurenghi, G.; Iida, T.; Koh, A.; B, G.; Gedif, K.; Singer, M. Efficacy Outcomes of Brolucizumab Versus Aflibercept in Neovascular Age-Related Macular Degeneration Patients with Early Residual Fluid. Ophthalmol Retina 2022, 6, 377–386, Epub 2021 Dec 27. [Google Scholar] [CrossRef] [PubMed]
- Flaxel, C.J.; Adelman, R.A.; Bailey, S.T.; Fawzi, A.; Lim, J.I.; Vemulakonda, G.A.; Ying, G.S. Age-Related Macular Degeneration Preferred Practice Pattern®. Ophthalmology 2020, 127, P1–P65, Epub 2019 Sep 25. Erratum in Ophthalmology 2020, 127, 1279. [Google Scholar] [CrossRef] [PubMed]
- Berkowitz, S.T.; Patel, S. Value of Anti-Vascular Endothelial Growth Factor Gene Therapy for Neovascular Age-Related Macular Degeneration. Ophthalmol Retina 2021, 5, 357–364, Epub 2020 Aug 17. [Google Scholar] [CrossRef] [PubMed]
- Abidi, M.; Karrer, E.; Csaky, K.; Handa, J.T. A Clinical and Preclinical Assessment of Clinical Trials for Dry Age-Related Macular Degeneration. Ophthalmol Sci. 2022, 2, 100213. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chichagova, V.; Hallam, D.; Collin, J.; Zerti, D.; Dorgau, B.; Felemban, M.; Lako, M.; Steel, D.H. Cellular regeneration strategies for macular degeneration: past, present and future. Eye (Lond) 2018, 32, 946–971, Epub 2018 Mar 5. [Google Scholar] [CrossRef] [PubMed]
© 2023 by the author. The materials published in RJMP are protected by copyright. No part of this publication may be reproduced, copied or transmitted in any form or purpose. The manuscripts sent to the RJMP become the property of the publication and the authors declare on their own responsibility that the materials sent are original and have not been sent to other publishing houses.
Share and Cite
Ranetti, A.E.; Stanca, H.T. Age-Related Macular Degeneration Screening—What Is Next? Rom. J. Prev. Med. 2023, 2, 47-55. https://doi.org/10.3390/rjpm2010047
Ranetti AE, Stanca HT. Age-Related Macular Degeneration Screening—What Is Next? Romanian Journal of Preventive Medicine. 2023; 2(1):47-55. https://doi.org/10.3390/rjpm2010047
Chicago/Turabian StyleRanetti, Antonia Elena, and Horia Tudor Stanca. 2023. "Age-Related Macular Degeneration Screening—What Is Next?" Romanian Journal of Preventive Medicine 2, no. 1: 47-55. https://doi.org/10.3390/rjpm2010047
APA StyleRanetti, A. E., & Stanca, H. T. (2023). Age-Related Macular Degeneration Screening—What Is Next? Romanian Journal of Preventive Medicine, 2(1), 47-55. https://doi.org/10.3390/rjpm2010047




