Transforming Spent Railroad Ties into High-Value Biochar: A Sustainable Solution for Phosphorus and Nitrate Removal in Water Treatment
Abstract
1. Introduction
- (1)
- recover creosote and generate an environmentally benign biochar from end-of-life railroad ties using pyrolysis, and
- (2)
- increase the adsorption capacity of the produced biochar for removing anionic contaminants, specifically nitrate and phosphate, through chemical modification prior to pyrolysis.
2. Materials and Methods
2.1. Biochar Production
2.2. Reagent Preparation
2.3. Proximate Analysis Method
2.3.1. Moisture Content Method
2.3.2. Volatile Matter Content
2.3.3. Ash Content
2.3.4. Fixed Carbon
2.4. Determining Particle Size Distribution
2.5. Characterization of Physicochemical Properties
2.5.1. Bulk Density Analysis
2.5.2. Elemental Analysis
2.5.3. Brunauer–Emmett–Teller (BET) Surface Area
2.5.4. Electrical Conductivity
2.5.5. Scanning Electron Microscopy (SEM)
2.5.6. Functional Group Analysis
2.5.7. Polycyclic Aromatic Hydrocarbon (PAH)
2.5.8. Adsorption Isotherm Determination
2.5.9. Biochar Modification with Magnesium
3. Results and Discussion
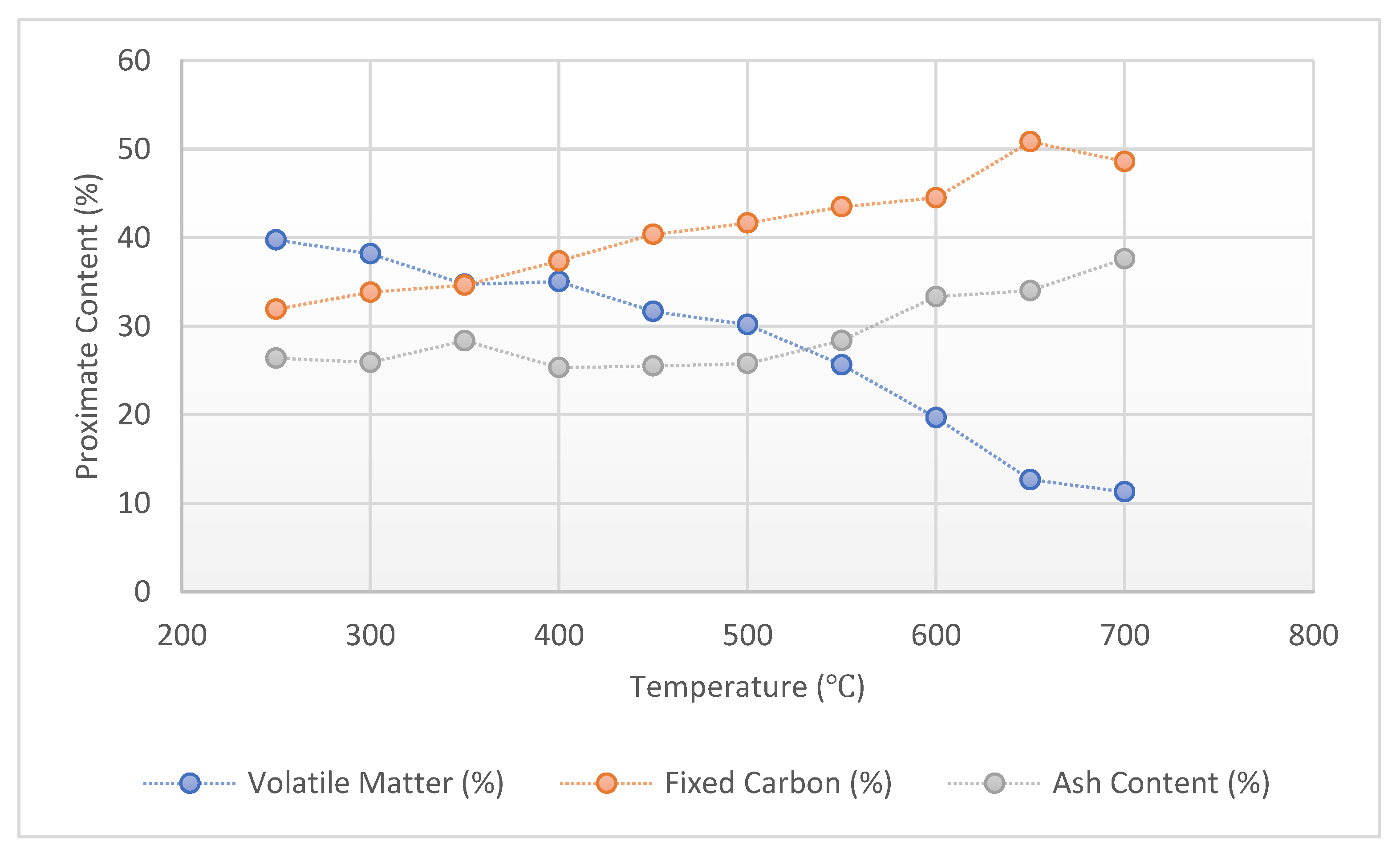
3.1. Results of Proximate Analysis
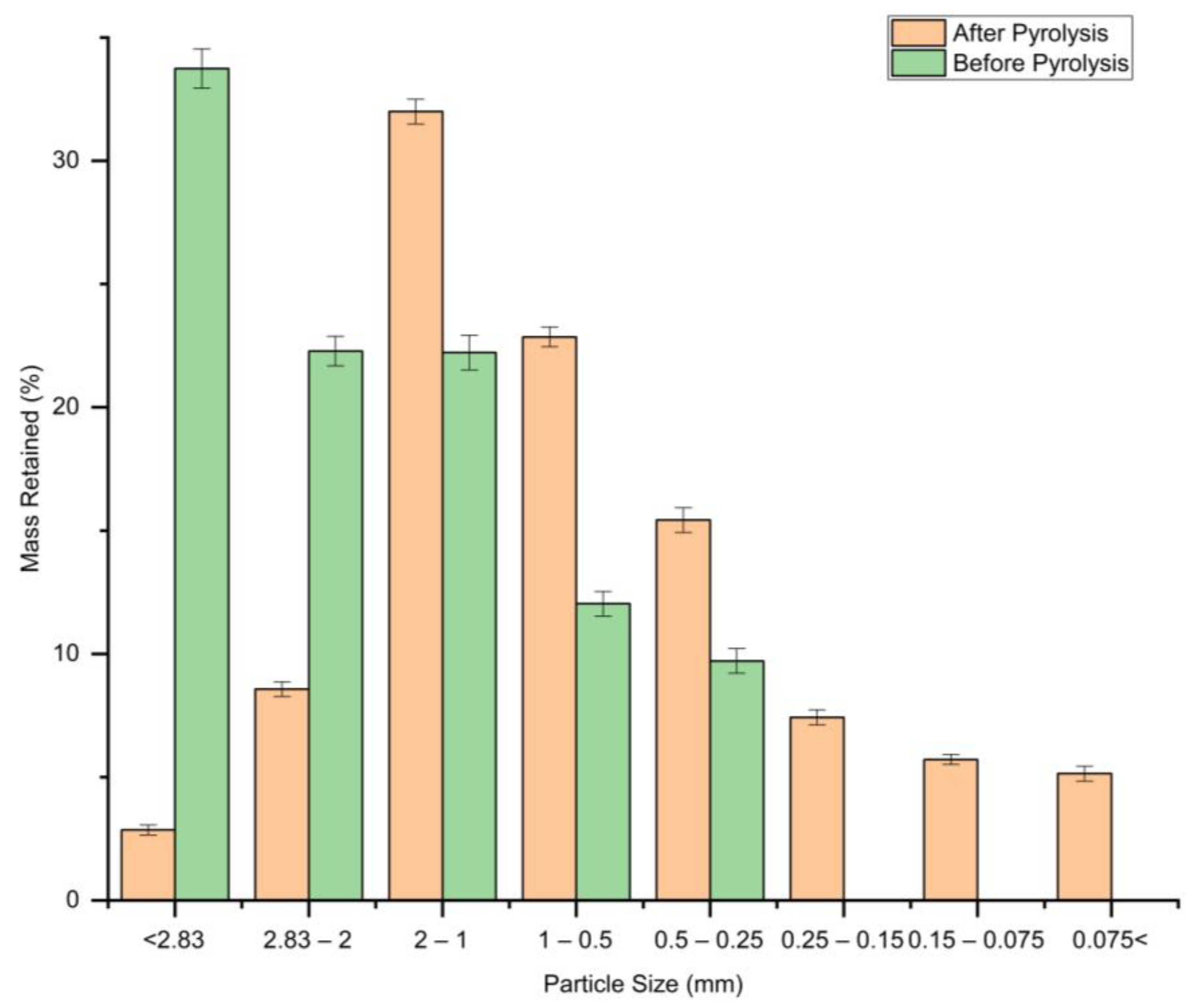
3.2. Resulting Particle Size Distribution
3.3. Resulting Physicochemical Properties
3.3.1. Resulting Moisture Content
3.3.2. Resulting Bulk Density
3.3.3. Elemental Composition
- Carbon (C)
- Nitrogen (N)
3.3.4. Surface Area
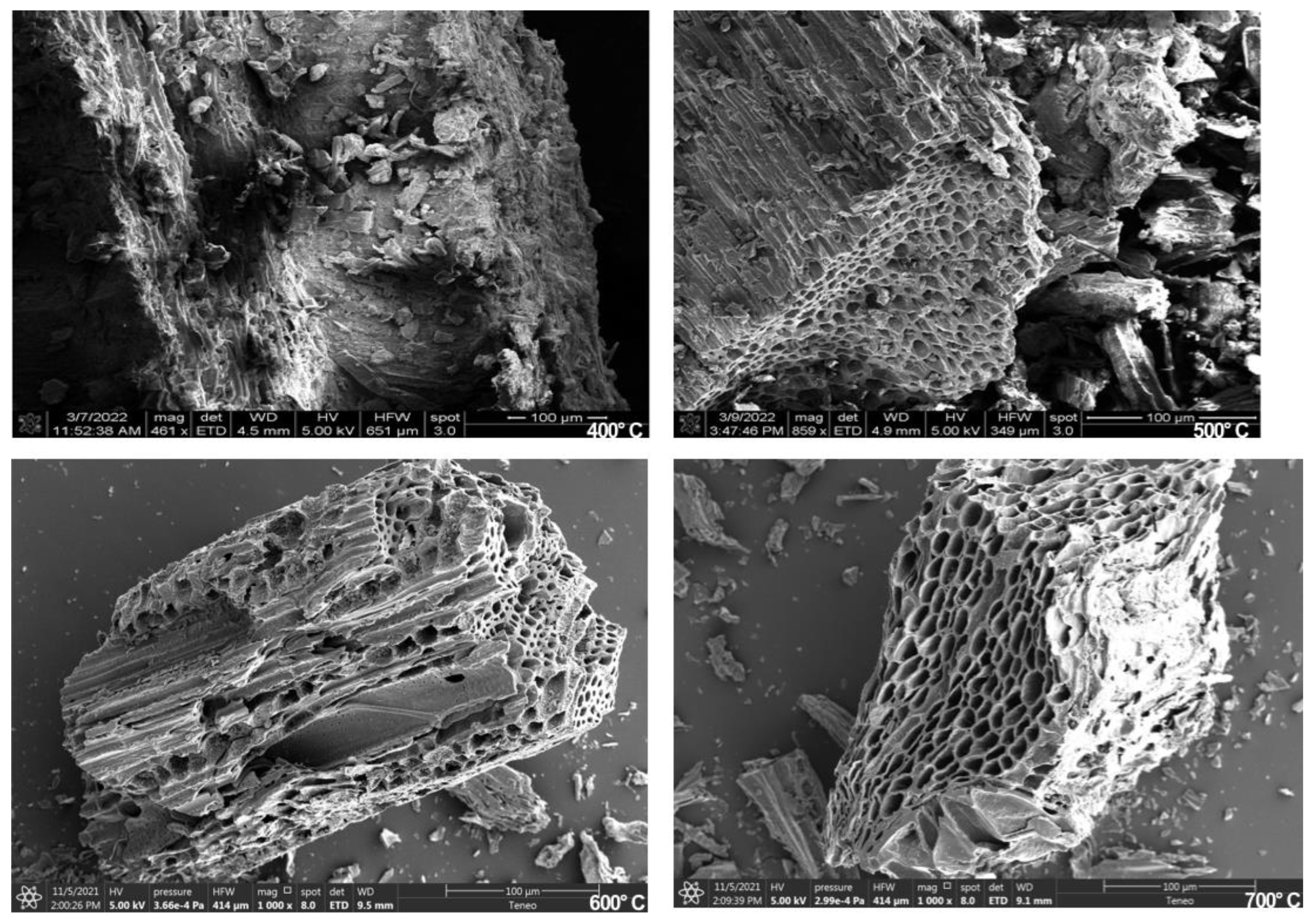
3.3.5. Scanning Electron Microscopy
3.3.6. Polycyclic Aromatic Hydrocarbon
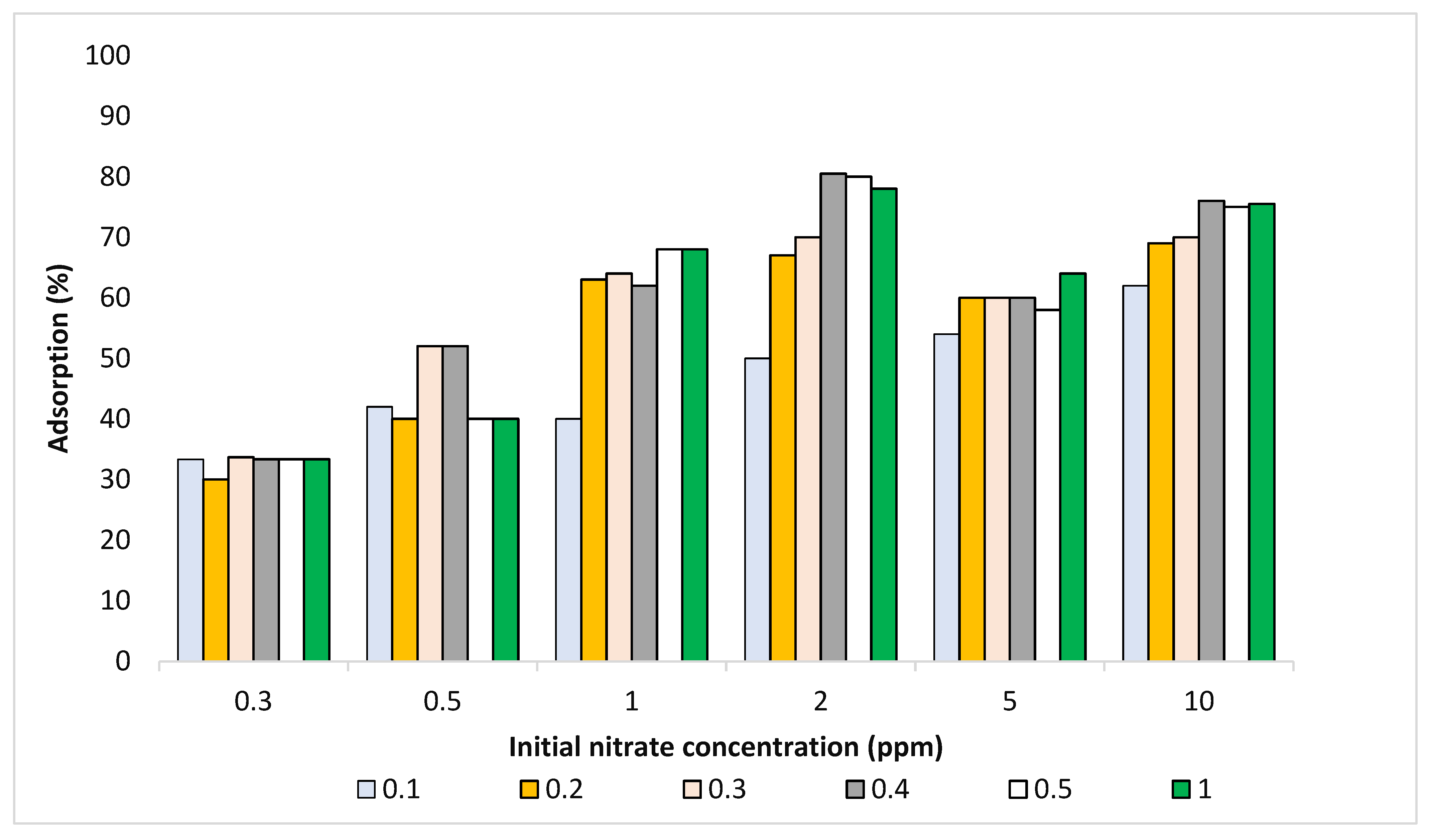
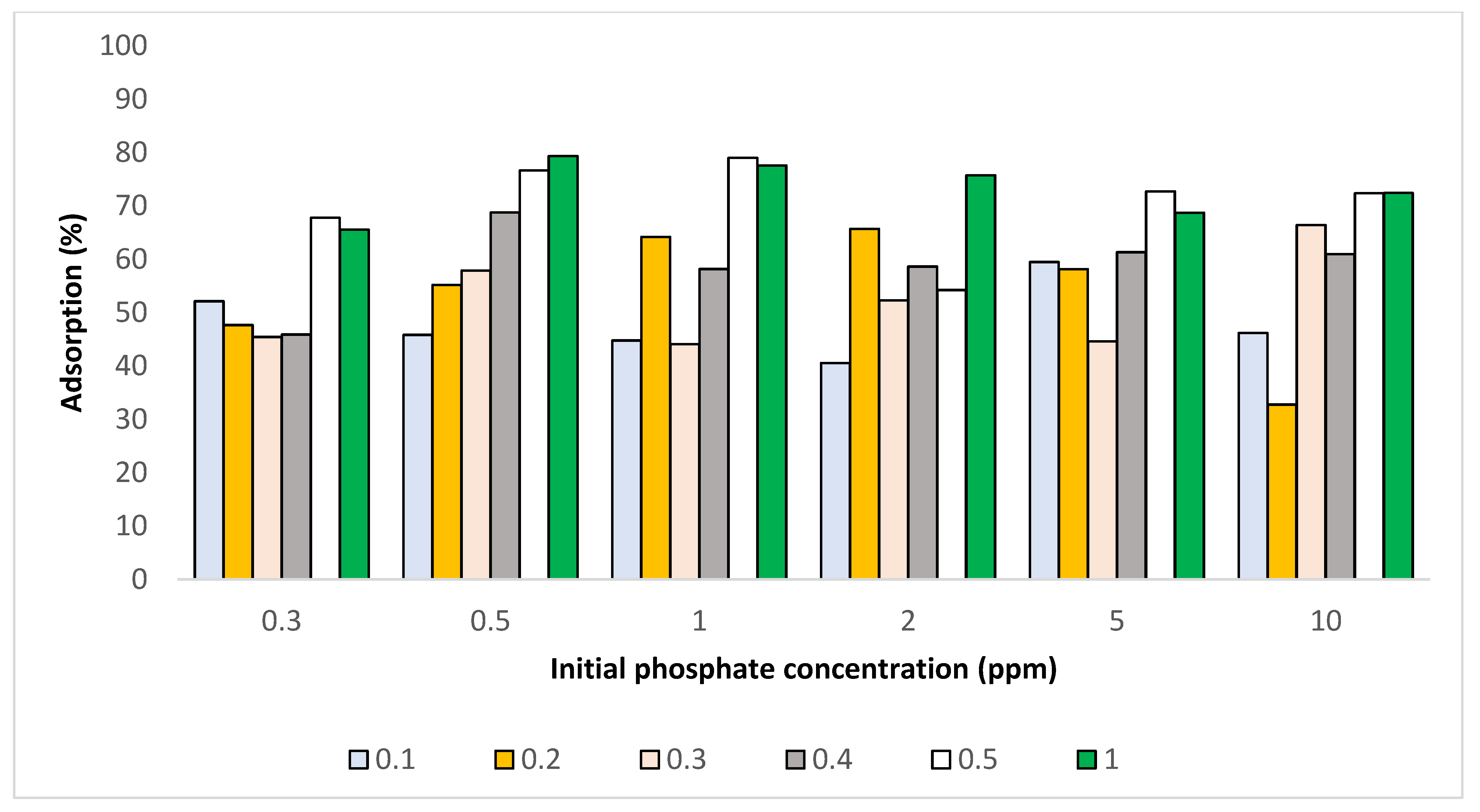
3.3.7. Resulting Adsorption Isotherms
- Langmuir and Freundlich Adsorption Isotherm
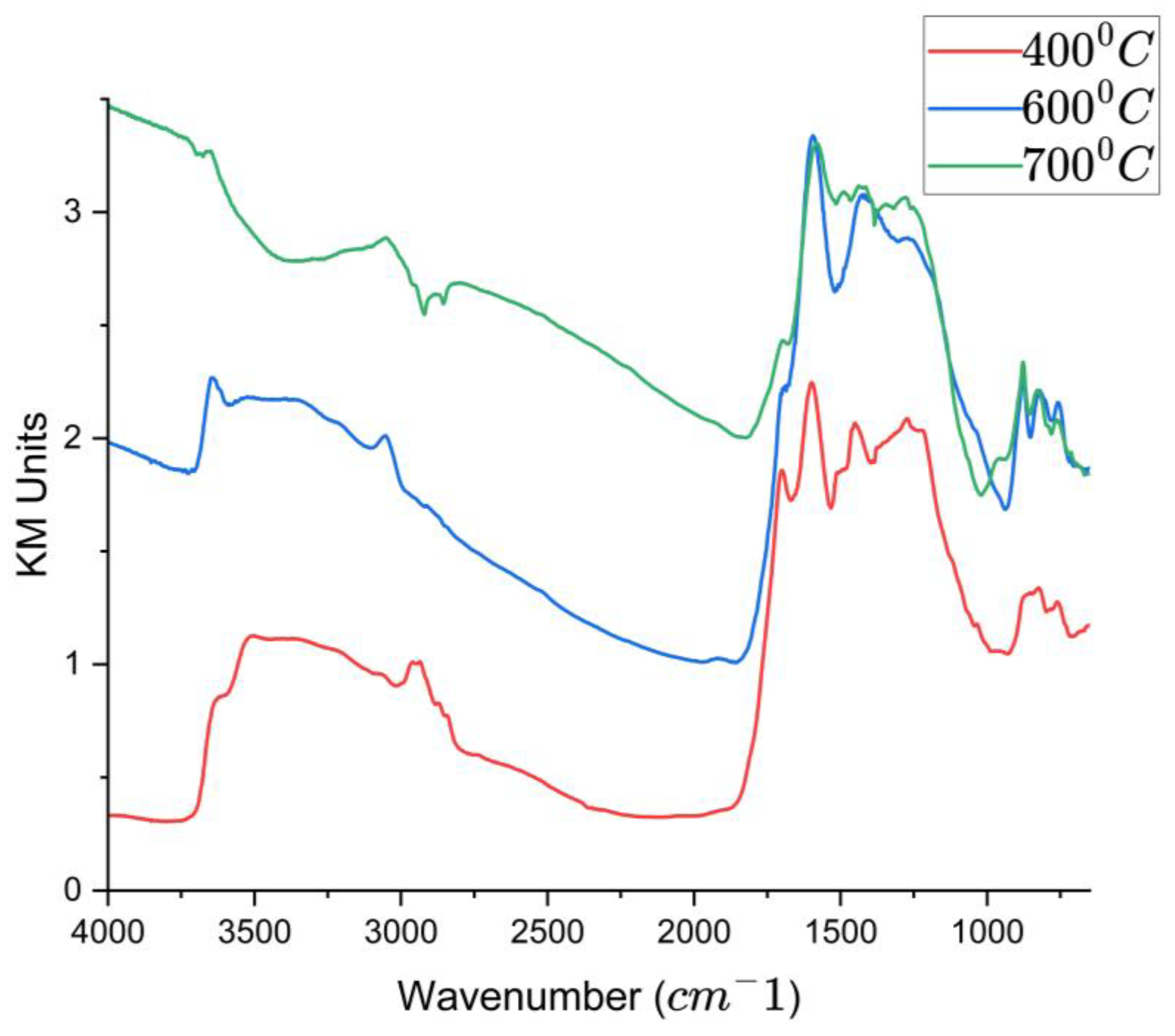
3.3.8. Functional Group
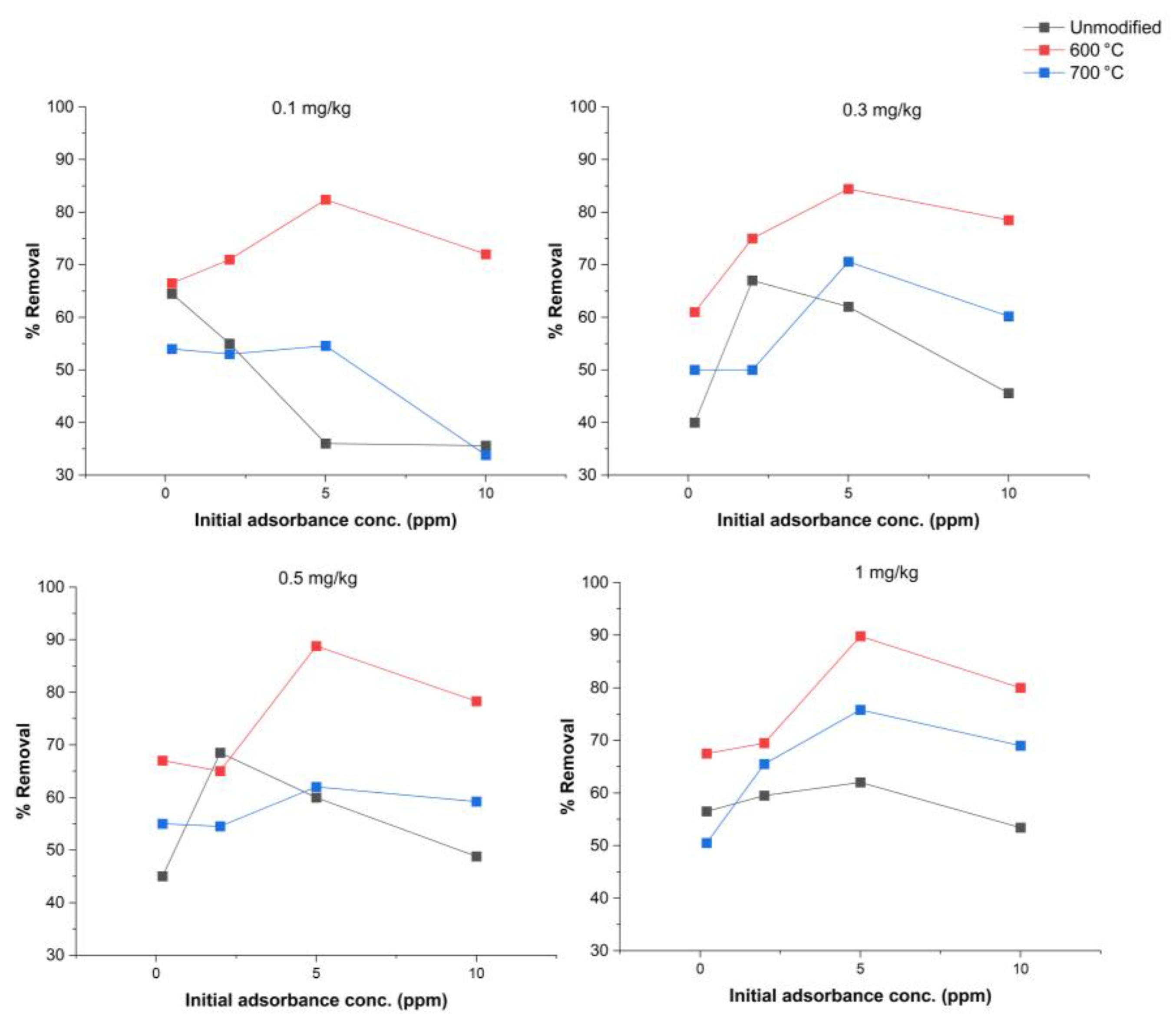
3.3.9. Modification to Alter Adsorption
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PAH | Polycyclic Aromatic Hydrocarbon |
| FTIR | Fourier Transform Infrared Spectroscopy |
| ASTM | American Society for Materials and Testing |
| SEM | Scanning Electron Microscopy |
| BET | Brunauer–Emmett–Teller |
| ICP-OES | Inductively Coupled Plasma Optical Emission Spectrometry |
| DIW | Deionized Water |
| IC | Ion Chromatography |
| IBI | International Biochar Initiative |
| AD | Anaerobically Digested |
| ppm | Parts Per Million |
| w/v | Weight per Volume |
| v/v | Volume per Volume |
| R2 | Coefficient of Determination (used in isotherm model fits) |
| NA | Not Applicable |
References
- Qiao, P.; Davalos, J.F.; Zipfel, M.G. Modeling and Optimal Design of Composite-Reinforced Wood Railroad Crosstie. Compos. Struct. 1998, 41, 87–96. [Google Scholar] [CrossRef]
- Smith, S.T. 2018 Railroad Tie Survey. J. Transp. Technol. 2019, 9, 276–286. [Google Scholar] [CrossRef][Green Version]
- Litchholt, B.; Zhang, A.; Mittelman, A.; Simon, M.C. Qualitative Lifecycle Analysis of Rail Tie Materials; Department of Transportation, Federal Railroad Administration: Washington, DC, USA, 2024.[Green Version]
- Lovett, A.H.; Dick, C.T.; Ruppert, C.; Barkan, C.P.L. Cost and Delay of Railroad Timber and Concrete Crosstie Maintenance and Replacement. Transp. Res. Rec. J. Transp. Res. Board 2015, 2476, 37–44. [Google Scholar] [CrossRef]
- Smith, S.T. 2014 Railroad Ties Survey for Railway Tie Association (RTA), Association of American Railroads (AAR), and American Short Line and Regional Railroad Association (ASLRRA). 2015. Available online: https://www.rta.org/assets/docs/RTASponsoredResearch/Environmental/ties%20survey%20report%206apr2015.pdf (accessed on 15 September 2025).
- Jung, S.-H.; Koo, W.-M.; Kim, J.-S. Fast Pyrolysis of Creosote Treated Wood Ties in a Fluidized Bed Reactor and Analytical Characterization of Product Fractions. Energy 2013, 53, 33–39. [Google Scholar] [CrossRef]
- Kim, P.; Taylor, A.; Lloyd, J.; Kim, J.-W.; Abdoulmoumine, N.; Labbé, N. Two-Step Thermochemical Process for Adding Value to Used Railroad Wood Ties and Reducing Environmental Impacts. ACS Sustain. Chem. Eng. 2017, 5, 9485–9493. [Google Scholar] [CrossRef]
- Kalina, M.; Sovova, S.; Hajzler, J.; Kubikova, L.; Trudicova, M.; Smilek, J.; Enev, V. Biochar Texture—A Parameter Influencing Physicochemical Properties, Morphology, and Agronomical Potential. Agronomy 2022, 12, 1768. [Google Scholar] [CrossRef]
- Khater, E.-S.; Bahnasawy, A.; Hamouda, R.; Sabahy, A.; Abbas, W.; Morsy, O.M. Biochar Production under Different Pyrolysis Temperatures with Different Types of Agricultural Wastes. Sci. Rep. 2024, 14, 2625. [Google Scholar] [CrossRef]
- Niedziński, T.; Łabętowicz, J.; Stępień, W.; Pęczek, T. Analysis of the Use of Biochar from Organic Waste Pyrolysis in Agriculture and Environmental Protection. J. Ecol. Eng. 2023, 24, 85–98. [Google Scholar] [CrossRef]
- Gonzalez, J.M.; Boddu, V.M.; Jackson, M.A.; Moser, B.; Ray, P. Pyrolysis of Creosote-Treated Railroad Ties to Recover Creosote and Produce Biochar. J. Anal. Appl. Pyrolysis 2020, 149, 104826. [Google Scholar] [CrossRef]
- Ahmad, M.; Rajapaksha, A.U.; Lim, J.E.; Zhang, M.; Bolan, N.; Mohan, D.; Vithanage, M.; Lee, S.S.; Ok, Y.S. Biochar as a Sorbent for Contaminant Management in Soil and Water: A Review. Chemosphere 2014, 99, 19–33. [Google Scholar] [CrossRef]
- Bolan, N.; Hoang, S.A.; Beiyuan, J.; Gupta, S.; Hou, D.; Karakoti, A.; Joseph, S.; Jung, S.; Kim, K.; Kirkham, M.B.; et al. Multifunctional Applications of Biochar Beyond Carbon Storage. Int. Mater. Rev. 2021, 67, 150–200. [Google Scholar] [CrossRef]
- Enaime, G.; Baçaoui, A.; Yaacoubi, A.; Lübken, M. Biochar for Wastewater Treatment—Conversion Technologies and Applications. Appl. Sci. 2020, 10, 3492. [Google Scholar] [CrossRef]
- Muoghalu, C.C.; Owusu, P.A.; Lebu, S.; Nakagiri, A.; Semiyaga, S.; Iorhemen, O.T.; Manga, M. Biochar as a Novel Technology for Treatment of Onsite Domestic Wastewater: A Critical Review. Front. Environ. Sci. 2023, 11, 1095920. [Google Scholar] [CrossRef]
- Schimmelpfennig, S.; Glaser, B. One Step Forward Toward Characterization: Some Important Material Properties to Distinguish Biochars. J. Environ. Qual. 2012, 41, 1001–1013. [Google Scholar] [CrossRef] [PubMed]
- Ippolito, J.A.; Cui, L.; Kammann, C.; Wrage, N.; Estavillo, J.M.; Fuertes-Mendizábal, T.; Cayuela, M.L.; Sigua, G.C.; Novak, J.M.; Spokas, K.A.; et al. Feedstock Choice, Pyrolysis Temperature and Type Influence Biochar Characteristics: A Comprehensive Meta-Data Analysis Review. Biochar 2020, 2, 421–438. [Google Scholar] [CrossRef]
- Nobaharan, K.; Bagheri Novair, S.; Asgari Lajayer, B.; van Hullebusch, E.D. Phosphorus Removal from Wastewater: The Potential Use of Biochar and the Key Controlling Factors. Water 2021, 13, 517. [Google Scholar] [CrossRef]
- Zhang, M.; Song, G.; Gelardi, D.L.; Huang, L.; Khan, E.; Mašek, O.; Parikh, S.J.; Ok, Y.S. Evaluating Biochar and Its Modifications for the Removal of Ammonium, Nitrate, and Phosphate in Water. Water Res. 2020, 186, 116303. [Google Scholar] [CrossRef]
- Mishra, B.; Remya, N.; Mohit, A. Production of Engineered Algal Biochar through Microwave Pyrolysis for Nutrient Recovery from Aqueous Solution. Environ. Qual. Manag. 2024, 33, 35–45. [Google Scholar] [CrossRef]
- Ayiania, M.; Garcia, A.; Haghighi Mood, S.; McEwen, J.-S.; Garcia-Perez, M. Novel Amorphous Carbons for the Adsorption of Phosphate: Part I. Elucidation of Chemical Structure of N-Metal-Doped Chars. ACS Omega 2022, 7, 14490–14504. [Google Scholar] [CrossRef]
- Ghassemi-Golezani, K.; Rahimzadeh, S. Biochar Modification and Application to Improve Soil Fertility and Crop Productivity. Agriculture 2022, 68, 45–61. [Google Scholar] [CrossRef]
- Gryta, A.; Skic, K.; Adamczuk, A.; Skic, A.; Marciniak, M.; Józefaciuk, G.; Boguta, P. The Importance of the Targeted Design of Biochar Physicochemical Properties in Microbial Inoculation for Improved Agricultural Productivity—A Review. Agriculture 2024, 14, 37. [Google Scholar] [CrossRef]
- Singh, B.; Camps-Arbestain, M.; Lehmann, J. Biochar: A Guide to Analytical Methods; Csiro Publishing: Victoria, Australia, 2017; ISBN 1-4863-0510-5. [Google Scholar]
- ASTM D1762-84; Standard Test Methods for Chemical Analysis of Wood Charcoal. ASTM International: West Conshohocken, PA, USA, 2013.
- ASTM D5550-06; Standard Test Method for Specific Gravity of Soil Solids by Gas Pycnometer. ASTM International: West Conshohocken, PA, USA, 2006.
- ASTM D6556-10; Standard Test Method for Carbon Black—Total and External Surface Area by Nitrogen Adsorption. ASTM International: West Conshohocken, PA, USA, 2010.
- El-Aassar, M.R.; Tamer, T.M.; El-Sayed, M.Y.; Omer, A.M.; Althobaiti, I.O.; Youssef, M.E.; Alolaimi, R.F.; El-Agammy, E.F.; Alruwaili, M.S.; Rabhy, O.O.; et al. Development of Azo Dye Immobilized Sulfonated Poly (Glycidyl Methacrylate) Polymer Composite as Novel Adsorbents for Water Treatment Applications: Methylene Blue Immobilization Isotherm, Kinetic, Thermodynamic, and Simulations Studies. Molecules 2022, 27, 8418. [Google Scholar] [CrossRef]
- Unlu, N.; Ersoz, M. Adsorption Characteristics of Heavy Metal Ions onto a Low Cost Biopolymeric Sorbent from Aqueous Solutions. J. Hazard. Mater. 2006, 136, 272–280. [Google Scholar] [CrossRef] [PubMed]
- Sebastian, S.; Mayadevi, S.; Beevi, B.S.; Mandal, S. Layered Clay-Alginate Composites for the Adsorption of Anionic Dyes: A Biocompatible Solution for Water/Wastewater Treatment. J. Water Resour. Prot. 2014, 06, 177–184. [Google Scholar] [CrossRef]
- Chatterjee, R.; Sajjadi, B.; Chen, W.-Y.; Mattern, D.L.; Hammer, N.; Raman, V.; Dorris, A. Effect of Pyrolysis Temperature on PhysicoChemical Properties and Acoustic-Based Amination of Biochar for Efficient CO2 Adsorption. Front. Energy Res. 2020, 8, 85. [Google Scholar] [CrossRef]
- Tu, P.; Zhang, G.; Wei, G.; Li, J.; Li, Y.; Deng, L.; Yuan, H. Influence of Pyrolysis Temperature on the Physicochemical Properties of Biochars Obtained from Herbaceous and Woody Plants. Bioresour. Bioprocess. 2022, 9, 131. [Google Scholar] [CrossRef]
- Ahn, K.-H.; Shin, D.-C.; Lee, Y.-E.; Jeong, Y.; Jung, J.; Kim, I.-T. Biochar Production and Demineralization Characteristics of Food Waste for Fuel Conversion. Molecules 2023, 28, 6114. [Google Scholar] [CrossRef]
- Tomczyk, A.; Sokołowska, Z.; Boguta, P. Biochar Physicochemical Properties: Pyrolysis Temperature and Feedstock Kind Effects. Rev. Environ. Sci. Biotechnol. 2020, 19, 191–215. [Google Scholar] [CrossRef]
- Tang, E.; Liao, W.; Thomas, S.C. Optimizing Biochar Particle Size for Plant Growth and Mitigation of Soil Salinization. Agronomy 2023, 13, 1394. [Google Scholar] [CrossRef]
- Liu, Z.; Niu, W.; Chu, H.; Zhou, T.; Niu, Z. Effect of the Carbonization Temperature on the Properties of Biochar Produced from the Pyrolysis of Crop Residues. BioResources 2018, 13, 3429–3446. [Google Scholar] [CrossRef]
- Wijitkosum, S. Influence of Pyrolysis Temperature and Time on Biochar Properties and Its Potential for Climate Change Mitigation. J. Hum. Earth Future 2023, 4, 472–485. [Google Scholar] [CrossRef]
- Li, X.; Liu, H.; Liu, N.; Sun, Z.; Fu, S.; Zhan, X.; Yang, J.; Zhou, R.; Zhang, H.; Zhang, J.; et al. Pyrolysis Temperature Had Effects on the Physicochemical Properties of Biochar. Plant Soil Environ. 2023, 69, 363–373. [Google Scholar] [CrossRef]
- Kujawska, J. Content of Heavy Metals in Various Biochar and Assessment Environmental Risk. J. Ecol. Eng. 2023, 24, 287–295. [Google Scholar] [CrossRef]
- Kumar, N.V.; Sawargaonkar, G.; Rani, C.S.; Pasumarthi, R.; Kale, S.B.; Prakash, T.R.; Triveni, S.; Singh, A.; Davala, M.S.; Khopade, R.; et al. Harnessing the Potential of Pigeonpea and Maize Feedstock Biochar for Carbon Sequestration, Energy Generation, and Environmental Sustainability. Bioresour. Bioprocess. 2024, 11, 5. [Google Scholar] [CrossRef]
- Wystalska, K.; Kwarciak-Kozłowska, A. The Effect of Biodegradable Waste Pyrolysis Temperatures on Selected Biochar Properties. Materials 2021, 14, 1644. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Ren, L.; Zhang, J.; Peng, H. Effects of Zeolite and Biochar Addition on Ammonia-Oxidizing Bacteria and Ammonia-Oxidizing Archaea Communities During Agricultural Waste Composting. Sustainability 2020, 12, 6336. [Google Scholar] [CrossRef]
- Mensah, R.A.; Shanmugam, V.; Narayanan, S.; Razavi, N.; Ulfberg, A.; Blanksvärd, T.; Sayahi, F.; Simonsson, P.; Reinke, B.; Försth, M.; et al. Biochar-Added Cementitious Materials—A Review on Mechanical, Thermal, and Environmental Properties. Sustainability 2021, 13, 9336. [Google Scholar] [CrossRef]
- Taha, M.R.; Mobasser, S. Adsorption of DDT and PCB by Nanomaterials from Residual Soil. PLoS ONE 2015, 10, e0144071. [Google Scholar] [CrossRef]
- Joseph, S.; Kammann, C.I.; Shepherd, J.G.; Conte, P.; Schmidt, H.-P.; Hagemann, N.; Rich, A.M.; Marjo, C.E.; Allen, J.; Munroe, P.; et al. Microstructural and Associated Chemical Changes during the Composting of a High Temperature Biochar: Mechanisms for Nitrate, Phosphate and Other Nutrient Retention and Release. Sci. Total Environ. 2018, 618, 1210–1223. [Google Scholar] [CrossRef] [PubMed]
- Sanford, J.R.; Larson, R.A.; Runge, T. Nitrate Sorption to Biochar Following Chemical Oxidation. Sci. Total Environ. 2019, 669, 938–947. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Zhang, D.; Fang, K.; Zhu, W.; Peng, Q.; Xie, Z. Enhanced Nitrate Removal by Physical Activation and Mg/Al Layered Double Hydroxide Modified Biochar Derived from Wood Waste: Adsorption Characteristics and Mechanisms. J. Environ. Chem. Eng. 2021, 9, 105184. [Google Scholar] [CrossRef]
- Feng, C.; Xing, Y.; Li, F.; Li, S.; Luo, Z. Enhancing Nitrogen Removal from Rainwater Runoff Using a Bioretention System with Magnesium-Modified Biochar as a Filler Amendment. J. Water Process Eng. 2024, 67, 106215. [Google Scholar] [CrossRef]

| Temperature (°C) | pH | Bulk Density (g/cm3) | Moisture (%) | Organic Matter (%) | Organic Carbon (%) | C (%) | N (%) |
|---|---|---|---|---|---|---|---|
| 250 | 6.04 ± 0.10 | 0.18 ± 0.00 | 1.94 ± 0.12 | 73.61 ± 3.34 | 43.42 ± 1.94 | 68 | 0.44 ± 0.06 |
| 300 | 6.00 ± 0.00 | 0.23 ± 0.03 | 2.10 ± 0.21 | 74.10 ± 4.32 | 43.08 ± 1.98 | 73 | 0.56 ± 0.03 |
| 350 | 5.67 ± 0.33 | 0.25 ± 0.00 | 2.29 ± 0.12 | 71.65 ± 2.78 | 41.66 ± 2.63 | 76 | 0.62 ± 0.02 |
| 400 | 7.57 ± 0.12 | 0.32 ± 0.00 | 2.29 ± 0.12 | 74.68 ± 6.35 | 43.42 ± 2.51 | 77 | 0.57 ± 0.03 |
| 450 | 7.10 ± 0.10 | 0.32 ± 0.00 | 2.46 ± 0.04 | 74.50 ± 5.51 | 43.31 ± 2.43 | 72 | 0.50 ± 0.06 |
| 500 | 7.00 ± 0.00 | 0.28 ± 0.03 | 2.39 ± 0.11 | 74.22 ± 4.22 | 43.15 ± 1.51 | 70 | 0.56 ± 0.07 |
| 550 | 7.10 ± 0.20 | 0.30 ± 0.04 | 2.50 ± 0.00 | 71.62 ± 4.22 | 41.64 ± 1.62 | 80 | 0.62 ± 0.05 |
| 600 | 9.57 ± 0.23 | 0.33 ± 0.00 | 2.50 ± 0.00 | 66.67 ± 4.39 | 38.76 ± 2.82 | 75 | 0.61 ± 0.03 |
| 650 | 8.99 ± 0.01 | 0.33 ± 0.00 | 2.50 ± 0.00 | 65.98 ± 4.79 | 38.36 ± 3.76 | 70 | 0.70 ± 0.03 |
| 700 | 10.27 ± 0.37 | 0.36 ± 0.02 | 2.50 ± 0.00 | 62.39 ± 4.52 | 36.28 ± 3.69 | 71 | 0.66 ± 0.02 |
| Element (ppm) | Biochar Produced at 400 °C | Biochar Produced at 600 °C | IBI Reportable Limit |
|---|---|---|---|
| Mercury—Hg | N/A | N/A | 1 |
| Boron—B | 0.14 | 0.12 | 3.15 |
| Arsenic—As | 0.03 | 0.02 | 32 |
| Chromium—Cr | 0.12 | 0.08 | 32 |
| Manganese—Mn | 1.6 | 1.46 | 32 |
| Cobalt—Co | 0.1 | 0.1 | 34 |
| Copper—Cu | 0.3 | 0.06 | 34 |
| Tin—Sn | 0.041 | 0.034 | 36 |
| Cadmium—Cd | 0.2 | 0.2 | 2 |
| Nickel—Ni | 0.7 | 0.63 | 30 |
| Selenium—Se | 0.58 | 0.61 | 36 |
| Molybdenum—Mo | 0.01 | 0.01 | 75 |
| Lead—Pb | 0.04 | 0.04 | 121 |
| Zinc—Zn | 0.65 | 0.6 | 200 |
| Iron—Fe | 52.05 | 49.9 | 1580 |
| Sodium—Na | 6.91 | 6.7 | declaration |
| Final Temperature During Pyrolysis [°C] | Surface Area (m2/g) of Biochar from 1–5 cm Pre-Pyrolysis * | Surface Area (m2/g) of Biochar from 5 to 10 mm Pre-Pyrolysis * |
|---|---|---|
| 300 | 0.613 | 0.99 |
| 400 | 0.77 | 2.6 |
| 500 | 1.9 | 79.8 |
| 600 | 373 | 431.28 |
| 700 | 378 | 454.86 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mobasser, S.; Olanrewaju, T.O.; Jafvert, C.T.; Johnston, C.; Engelberth, A.S. Transforming Spent Railroad Ties into High-Value Biochar: A Sustainable Solution for Phosphorus and Nitrate Removal in Water Treatment. Bioresour. Bioprod. 2025, 1, 5. https://doi.org/10.3390/bioresourbioprod1010005
Mobasser S, Olanrewaju TO, Jafvert CT, Johnston C, Engelberth AS. Transforming Spent Railroad Ties into High-Value Biochar: A Sustainable Solution for Phosphorus and Nitrate Removal in Water Treatment. Bioresources and Bioproducts. 2025; 1(1):5. https://doi.org/10.3390/bioresourbioprod1010005
Chicago/Turabian StyleMobasser, Shariat, Tosin O. Olanrewaju, Chad T. Jafvert, Cliff Johnston, and Abigail S. Engelberth. 2025. "Transforming Spent Railroad Ties into High-Value Biochar: A Sustainable Solution for Phosphorus and Nitrate Removal in Water Treatment" Bioresources and Bioproducts 1, no. 1: 5. https://doi.org/10.3390/bioresourbioprod1010005
APA StyleMobasser, S., Olanrewaju, T. O., Jafvert, C. T., Johnston, C., & Engelberth, A. S. (2025). Transforming Spent Railroad Ties into High-Value Biochar: A Sustainable Solution for Phosphorus and Nitrate Removal in Water Treatment. Bioresources and Bioproducts, 1(1), 5. https://doi.org/10.3390/bioresourbioprod1010005







