1. Introduction
Adhesives are indispensable materials in a wide range of industries, from construction and packaging to medicine and automotive. Traditionally, many adhesives have been formulated from synthetic polymers derived from fossil fuels, raising significant concerns in terms of sustainability and biodegradability. In response to this issue, current research has turned to the development of biopolymers as a promising alternative, with polysaccharides being of particular interest due to their abundance, low cost, non-toxic nature, and biodegradability (Gupta et al. (2022) [
1]).
Polysaccharides are complex carbohydrates formed by the union of multiple monosaccharides and are found abundantly in nature in sources such as plants (cellulose, starch, pectin), algae (agar, alginate), and microorganisms (xanthan, pullulan) (Chen et al. (2024) [
2]). Their diverse structure allows for a wide range of physical and chemical properties, making them versatile precursors for adhesive design. The presence of abundant hydroxyl groups in their structure facilitates the formation of hydrogen bonds and other intermolecular interactions with various surfaces, which is critical for adhesion (Chen et al. (2024) [
2]).
Despite the inherent advantages of polysaccharides, their direct application as adhesives is often limited by insufficient mechanical properties, low water resistance, and limited wetting capacity on some surfaces. To overcome these challenges, various modification strategies have been explored, including chemical crosslinking, the incorporation of plasticizers, blending with other polymers (synthetic or natural), and functionalizing their hydroxyl groups (Li et al. (2024) [
3]). The goal is to improve the bond strength, dimensional stability, and durability of polysaccharide-based adhesives. This research paper reviews recent advances in the development of polysaccharide-based adhesives, emphasizing different modification strategies and their impact on adhesive properties. Emerging applications of these biomaterials will be discussed, as well as outstanding challenges and future research directions in the pursuit of sustainable, high-performance adhesives.
Li et al. (2020) [
4] reinforces the central idea that polysaccharides offer an attractive base for adhesives due to their natural origin, biodegradability, and low cost. This review is crucial in detailing how the bond strength of these biopolymers can be optimized for different substrates such as metals, paper, and wood. The ability of polysaccharides to form hydrogen bonds and other molecular interactions is critical, but the article also underscores the need to overcome inherent limitations in terms of mechanical properties and water resistance, a common thread in biomaterials research.
On the other hand, Liu et al. (2024) [
5] brings us to a very specific and high-value field of application: regenerative medicine and wound healing. Here, polysaccharide-based bioadhesive hydrogels are of particular interest due to their biocompatibility and ability to create a moist environment conducive to healing. This work emphasizes that, beyond mere adhesion, these materials must possess additional functionalities such as controlled drug release or antimicrobial properties. This highlights a key trend: polysaccharide adhesives not only seek to replace synthetic ones, but also offer new capabilities and benefits.
The research by Wang et al. (2024) [
6] introduces an advanced modification strategy: the formation of polysaccharide-polyelectrolyte complexes. This technique is vital for improving adhesive properties, particularly in natural fiber-based materials. By combining polysaccharides with polyelectrolytes, electrostatic interactions can be generated that strengthen the polymer network and improve cohesion and adhesion, thus directly addressing the performance challenges often encountered with pure polysaccharides.
Collectively, extensive research (Uyor et al. (2024) [
7]) not only reaffirms the potential of polysaccharides as a basis for sustainable adhesives but also illustrates the growing sophistication in their design and application. Current research focuses on chemical and structural modification to overcome the limitations of native polysaccharides, enabling them to meet performance demands in applications ranging from packaging to biomedicine (Han et al. (2024) [
8]). The key to the future of polysaccharide-based adhesives lies in the ability to tailor their properties to specific needs, integrating functionality and sustainability (Ali et al. (2020) [
9]).
The search for sustainable alternatives in the field of adhesives has catapulted natural polysaccharides to the center of research. Below, we discuss the adhesive potential of specific polysaccharides such as starch, pectin, chitosan, and natural gums, comparing their properties and highlighting relevant numerical data from recent references (Joji et al. (2023) [
10], Kreitschitz et al. (2021) [
11], Lutz et al. (2022) [
12], Bashir et al. (2022) [
13]).
Starch is an abundant and inexpensive polysaccharide composed of amylose and amylopectin, which has traditionally been used as an adhesive in paper and cardboard applications. Its adhesive functionality is based on the formation of hydrogen bonds with cellulosic substrates. However, native starch has limitations such as low moisture resistance and poor mechanical properties. To overcome these, chemical and physical modifications have been explored (Suarez Da Silva (2011) [
14]).
A recent study on tannin-modified starch adhesives demonstrated a dry shear strength of up to 4.2 MPa for wood bonds, significantly outperforming native starch (2.1 MPa) and approaching the values of commercial synthetic adhesives (Qi et al. (2023) [
15]). Another work incorporating cellulose nanocrystals in starch adhesives reported a wet shear strength of 1.5 MPa on plywood, a substantial improvement over unmodified starch (0.5 MPa) (Zhang et al. (2022) [
16]). These figures demonstrate the potential of modified starch for structural applications.
Pectins are complex heteropolysaccharides present in plant cell walls, known for their gelling properties and film-forming ability. Their carboxyl-rich structure gives them an anionic character, facilitating electrostatic and hydrogen interactions with various surfaces. Adhesives based on pectin and cellulose derivatives have demonstrated tensile strengths of up to 3.8 MPa in paper bonds and good adhesion to metal substrates with lap shear strengths that can reach 1.2 MPa after optimizing the formulation with divalent ions such as calcium, which crosslink the pectin structure (Wang et al. (2023a) [
17]). Their biocompatible nature makes them attractive for biomedical applications, where adhesion strength to wet tissues is a critical factor.
Chitosan is a linear polysaccharide derived from the deacetylation of chitin, the second most abundant polysaccharide in nature. Its cationic nature (due to its amino groups) gives it unique properties, including high biocompatibility, biodegradability, and the ability to interact strongly with negatively charged surfaces. It is particularly promising for biomedical and water treatment applications. Cross-linked chitosan adhesives have demonstrated wet shear strength in porcine muscle tissue ranging from 30 to 80 kPa, depending on the cross-linking agent and concentration (Jiang et al. (2023) [
18]). For wood bonding, a chitosan-based adhesive with citric acid as a crosslinker achieved a dry shear strength of 5.1 MPa, outperforming many biomass adhesives and approaching urea-formaldehyde adhesives (Li et al. (2023) [
19]). These figures highlight the versatility of chitosan in a variety of applications.
Natural gums, such as guar gum, xanthan gum, and gum arabic, are hydrophilic polysaccharides that form viscous solutions or gels. They are commonly used as thickeners and stabilizers, but their adhesive properties are being explored, especially when modified or combined with other polymers. An adhesive based on carboxyl-modified guar gum showed a peel strength of 150 N/m on steel bonds, indicating good bond strength with metal surfaces (Kumar et al. (2022) [
20]). In another application, blends of xanthan gum and starch as paper adhesives achieved a tensile strength of 2.5 MPa, which is comparable to some synthetic adhesives for this application (Wang et al. (2023b) [
21]). These examples suggest that while gums alone may not be the strongest adhesives, their rheological properties and ability to be modified or combined make them valuable components in adhesive formulations.
Moreno Quintero et al. (2020) [
22] conducted studies on dextrin and pectin extracted from plant residues of cassava, potato and yam used in the formulation of a glue. They found that the pectin obtained is low in methoxyl and has a high degree of esterification, therefore it requires the presence of calcium ions to gel. The authors evaluated the functional and organoleptic properties of the glue samples, with the one formulated with 0.25% pectin showing the best characteristics.
The work of Mukherjee et al. (2019) [
23] describes glue capacity and biochemical properties of natural polymers from plants, specifically mucilages and gums (gum arabic from Acacia plant, pectin, seeds used include
Arabidopsis thaliana, camelina (
Camelina sativa), psyllium (
Plantago ovata), sesame (
Sesamum indicum), flax (
Linum usitatissimum), and chia (
Salvia hispanica).
Starch is a relatively inexpensive, renewable, and abundant product that has been widely used as a binder and adhesive. However, its bonding strength is not sufficient for wood. Extensive research has been conducted to improve the cohesive properties, especially water resistance, of starch-based adhesives. This review focuses on starch modification methods to enhance the properties of starch-based adhesives, allowing them to perform comparably to synthetic adhesives for wood and wood composite bonding (Gadhave et al. (2017) [
24]).
Paiva et al. (2016) [
25] investigate the use of oxidized xanthan gum and chitosan as potential natural adhesives for cork agglomerates. Xanthan gum, which is normally not water-resistant, was oxidized to improve this property and its reactivity. Tensile tests showed that the oxidized xanthan gum and chitosan provided good water resistance, unlike the unmodified xanthan gum (Hua & Chen (2023) [
26]).
Araujo et al. (2016) [
27] tested the adhesive properties of FucoPol, a bacterial polysaccharide. A FucoPol water solution was used to bond wood, glass, cardboard, and cellulose acetate film, and its shear strength was compared to that of commercial synthetic glues. The results showed that FucoPol-bonded wood joints resisted 742.2 ± 9.8 kPa of shear force. The adhesive performed comparably to commercial glues on cardboard (416.0 ± 12.9 kPa vs. 425 ± 8.9 kPa). However, it demonstrated superior performance on both glass (115.1 ± 26.2 kPa vs. 67.7–97.5 kPa) and cellulose acetate film (153.7 ± 11.3 kPa vs. 79.4–92.7 kPa).
Lin & Catchmark (2024) [
28] study polysaccharide–polyelectrolyte complexes made from carboxymethyl cellulose (CMC) and chitosan (CS) were successfully prepared using ball milling for use as adhesives in paper and wood composites. The study found that a preparation pH of 4.5 and a solid content of 9% yielded the best results, with dry wood bonding strength of 7.89 ± 0.83 MPa, with approximately 35% of that strength retained in wet conditions, and optimal adhesive loading for paper was 10 g/m
2.
Comparatively, chitosan and modified starch emerge as the polysaccharides with the greatest potential to achieve bond strengths comparable to synthetic adhesives in applications such as wood and paper, especially after modifications that improve their water resistance. Pectin stands out for its versatility and potential in biomedical and packaging applications thanks to its gelling and film-forming properties. Natural gums, while not always offering the highest bond strengths on their own, are excellent candidates for co-formulation or as rheology modifiers that improve processability and wetting. The key to the advancement of these adhesives lies in functionalization and formulation engineering, which mitigate the inherent limitations of native polysaccharides (such as low water resistance, poor mechanical properties, and temperature sensitivity). Crosslinking, incorporation of nanofillers, hybridization with other biopolymers or synthetic polymers, and pH adjustment are crucial strategies for optimizing their performance. As research continues to unravel the intricate relationships between polysaccharide structure, substrate surface chemistry, and processing conditions, the path toward fully competitive and sustainable polysaccharide adhesives becomes increasingly clear (Jiang et al. (2023) [
18] and Li et al. (2024) [
19]).
Despite significant advances in the use of biopolymers for adhesives, most studies have focused on materials such as starch, cellulose, or chitosan, which are widely available but may require complex chemical modifications. In this context, chañar brea gum (CBG), a natural polysaccharide extracted from the
Parkinsonia praecox tree, emerges as a promising and underexplored candidate. This gum not only shares the key properties of other biopolymers, such as its natural origin, biodegradability, and low cost, but its unique physicochemical characteristics, such as its structure and composition, suggest intrinsic adhesive potential that could be optimized for specific applications such as paper. This study aims to systematically investigate the performance of CBG as an adhesive for paper, focusing on the relationship between its rheological, surface, and adhesive properties to determine the optimal concentration that ensures superior performance (Bertuzzi et al. (2012) [
29]).
Chañar brea gum (CBG) is an exudate from tree
Parkinsonia precox. CBG is a water-soluble hydrocolloid which also functions as a foam former, emulsifier, stabilizer and film former (Bertuzzi et al. (2012) [
29]).
CBG contains residues of L-arabinose, D-xylose, D-glucuronic acid and 4-O-methyl D-glucuronic acid, associated with small amounts of proteins which contribute to the emulsifying properties of the gum (Cerezo et al. [
30], Castel et al. 2012 [
31], von Müller et al. 2007 [
32], Bertuzzi & Slavutsky (2019) [
33], Slavutsky et al. 2018 [
34]). CBG is highly branched and can be considered a polyarabinoglucuronoxylans (Sznaider et al. 2023 [
35]). The molecular weight varies from 2790 kDa to 192 kDa; there is a set of proteins with molecular masses ranging from 6.5 to 66 kDa (Castel et al. 2016 [
36]). By intrinsic viscosity measurements, CBG subjected to different acid hydrolysis varies from 26KDa to 1890KDa (Masuelli et al. 2018 [
37] and Bercea et al. (2024) [
38]). CBG-based films and coatings using glycerol as a plasticizer, typically exhibit amber-colored transparency with a dense, homogeneous microscopic structure (Bertuzzi & Slavutsky 2013 [
39]).
This work presents a comprehensive evaluation of the adhesive capability of chañar brea gum solutions, a biopolymer with potential for adhesive high-value-added products. The adhesive capability is a critical property in the packaging industry, as it directly determines the integrity and functionality of the final package, ensuring the protection of the product inside. The adhesive process involves the controlled glue of the surfaces of two papers films through the application of CBG, followed by dry to solidify the bond. To fully understand this adhesion behavior and its underlying mechanisms, this study employs a number of determinations that explore the fundamental adhesion properties: density, viscosity, contact angle, surface tension, mechanical testing and adhesion capability. By integrating the results obtained from all these analyses, this study seeks to evaluate and explain the “intimate relationships”—that is, the complex interconnections between the molecular structure, composition, mechanical properties, and adhesion properties of chañar brea gum (as glue). This comprehensive approach is crucial for optimizing the adhesion process and designing functional and efficient glue concentration.
2. Materials and Methods
2.1. Raw Material
Chañar brea gum was the exudate of Parkinsonia precox tree located at 32°06′36.5″ S, 65°03′56.8″ W, and was provided by “Viva el Monte”, La Travesía, Traslasierra, Córdoba, Argentina.
The exudate of CBG was dissolved in distilled water and filtered. The filtrate was precipitated with ethanol 70/30 in distilled water. The CBG precipitate was filtered again and dried for 24 h at 60 °C (Masuelli et al. (2018) [
37], Torres et al. (2020) [
40] and Torres et al. (2021) [
41]).
The paper used was A4 “Author” of 21 cm × 19 cm, and contained 80 mg fabricated from sugarcane, Tucuman, Argentina.
The tests were performed using solutions of 2.5, 5, 10, 15, 20, 25, and 30 g of CBG in 100 mL of distilled water.
The glues used were Voligoma® (Akapol S.A., Zelaya, Buenos Aires Province, Argentina), Plasticola® a polyvinylacetate, PVAc, (STA, San Martín, Buenos Aires Province, Argentina), silicone-based adhesive (STA, San Martín, Buenos Aires Province, Argentina), Plastimonte® (Viva el Monte Family Business and Yapeyú School–San Isidro, Luya-ba, Traslasierra, Córdoba, Argentina), and cornstarch glue. The cornstarch glue was prepared as follows: 100 g of cornstarch (Maizena, Unilever, Gualeguaychú, Entre Ríos, Argentina) was weighed and mixed with 250 mL of cold distilled water until fully dispersed. Then, 250 mL of water at 90 °C was added, and the mixture was stirred while heating to 105 °C until it reached a viscous consistency, 1 g NaCl added and mixed, and the glue was allowed to cool before use. Voligoma® is primarily composed of polyvinyl alcohol-co-vinyl acetate, PVA-co-VAc. Plasticola® is primarily composed of polyvinylacetate, PVAc, glue. Plastimonte® is composed of chañar brea gum in an aqueous solution.
2.2. Density
The density of the solutions was measured with a 25 cm3 pycnometer (Everglass, Valparaiso, Chile) thermostated at 25 °C.
2.3. Viscosity
The viscosity of the solutions was measured with a Brookfield DV III rheometer (AMETEK Brookfield, Middleboro, MA, USA) thermostated at 25 °C.
2.4. Surface Tension
The surface tension of the solutions was measured with a Du Noüy tensiometer (Brookfield, Middleboro, MA, USA) thermostated at 25 °C.
2.5. Contact Angle
The paper contact angle of the solutions was measured with a Micromeritics Anglometer (Norcross, GA, USA) thermostated at 25 °C.
2.6. Mechanical Test and Adhesion Strength
The mechanical properties of the materials were measured using a Brookfield CT3 (Middleboro, MS, USA) instrument, following the ASTM D882 standard (ASTM (2018) [
42]). The tests were carried out in triplicate at 25 °C and 40% relative humidity, with a constant tensile rate of 0.1 mm/min. Samples of 100 mm in length and 20 mm in width were used. The thickness of each film was determined with a micrometer. Force (F) and strain (Δl) data were recorded until the samples failed. The Tensile Strength (σ) was calculated by dividing the maximum load by the initial cross-sectional area (A). The Elongation at Break (%ϵ) was determined as the percentage change from the initial length (l = 50 mm) at failure. The Elastic Modulus (E) was obtained from the slope of the stress (σ) vs. strain (ϵ) curve in the linear elastic region (Masuelli et al. (2024) [
43], Ruano et al. (2019) [
44] and Zanon & Masuelli (2018) [
45]).
Adhesive strength was determined using paper strips. The 10 cm × 5 cm × 0.07 cm paper strips were glued on one side for up to 5 cm, leaving the other side unglued, and then folded into a “T” shape with the glued side vertical. The samples were then dried at 25 °C for 24 h. Finally, the product was packaged in a self-sealing polyethylene bag. The strips were conditioned at 25 °C for 24 h with 40% (RH). Adhesive strength was determined according to the Torres et al. (2025) [
46]. The determination was repeated three times. The maximum force (MPa) required to peel/tear the seal was recorded numerically and graphically, and the failure mode was simultaneously noted. The adhesive strength was calculated as the maximum force/film width (Das & Chowdhury (2016) [
47], and Torres et al. (2025) [
46]).
The mechanical test for different humidity and temperature was determined using Electrotech Systems equipment (MD, USA) that controlled the temperature and humidity in a closed system. The tests at different relative humidity levels were conducted at 50%, 60%, and 80% at a constant temperature of 30 °C. The tests at different temperatures were conducted at 40 °C, 50 °C, and 60 °C at a relative humidity of 40%.
3. Results & Discussion
The table presents various physicochemical properties of chañar brea gum solutions at different concentrations (% wt.), and how these properties influence the contact angle on A4 paper. The parameters in
Table 1 include concentration (c), surface tension (σ), density (ρ), viscosity (η), excess surface concentration (Γ
2(1)), work of cohesion (W
coh), contact angle (θ), spreading coefficient (s) and work of adhesion (W
adh). The greater the amount of dissolved gum, the greater the concentration of the solution.
3.1. Density (ρ)
The density of the solution tends to increase with increasing concentration of chañar brea gum (see
Figure 1). The incorporation of the polymer (CBG) into water increases the mass per unit volume of the solution, which is a typical behavior for most aqueous polymer solutions (Israelachvili (2011) [
48]).
3.2. Viscosity (η)
Figure 2 shows how the viscosity (η) of a chañar brea gum solution is affected by its concentration (c). As the concentration increases, so does the viscosity of the fluid, but not linearly.
The curve presents an exponential or nonlinear relationship between viscosity and concentration. Initially, at low concentrations (5 to 15% wt.), the increase in viscosity is relatively moderate. However, starting at a concentration of approximately 20% wt., the viscosity begins to grow much more steeply, as observed in the data points of 25 and 30% wt.
This behavior is typical of polymer solutions. At low concentrations, the polymer chains are dispersed and have sufficient space to move, resulting in a viscosity similar to that of the solvent. As the concentration increases, the chains begin to interact, intertwine, and become entangled with each other. This entanglement of the chains restricts their movement and significantly increases flow resistance, which manifests as a sharp increase in viscosity (Lide (2004) [
49]).
Analysis of this curve is crucial to understanding the behavior of chañar brea gum in various applications, such as the food, pharmaceutical, and coating industries, where it is used as a thickener, stabilizer, or suspending agent. For example, at low concentrations, CBG could be useful in formulations where only a slight texture modification is needed. At high concentrations, the strong viscosity dependence in this range indicates that small changes in concentration can have a large impact. This requires very precise control in the manufacturing processes to achieve the desired viscosity.
In summary, the data confirm that chañar brea gum is a polymer with effective thickening properties whose performance is closely linked to concentration, especially at higher concentrations, where the entanglement of macromolecules becomes the dominant factor.
From the perspective of its use as an adhesive, the relationship between viscosity and concentration of chañar brea gum is critical. Viscosity is a key factor in a material’s ability to adhere to a surface. The viscosity (η) of a fluid is directly related to its resistance to flow. In the context of an adhesive, this has several implications:
- −
Low concentrations: At low concentrations, viscosity is very low. This could result in poor adhesion because the adhesive spreads too much and fails to form a layer thick enough to bond two surfaces. This is because the cohesive forces within the adhesive are weak, making it difficult to form a cohesive film.
- −
High concentrations: As the concentration increases, viscosity increases. This is beneficial for adhesion because a more viscous fluid has greater internal cohesion, allowing it to maintain its shape and fill the pores and roughness of the surfaces being bonded. However, excessive viscosity can be detrimental. If the viscosity is too high, the gum will be difficult to apply and will not be able to flow properly to wet the surface.
The viscosity curve indicates that there is an optimal concentration point for using chañar brea gum as an adhesive. This point would be in a range where the viscosity is high enough to ensure good cohesion and wetting of surfaces, but not so high as to impede its application and flow. On
Figure 2, this could be in the range of 10 to 20% wt., where the viscosity is significant but still manageable. At concentrations above 25% wt., excessive viscosity reduces adhesion, and can lead to an excessively thick or brittle adhesive film (Brandrup et al. (1999) [
50]).
3.3. Surface Tension (σ)
As CBG is added, the surface tension progressively decreases. This decrease in surface tension with increasing concentration is a characteristic behavior of surfactants or molecules that have an affinity for the liquid-air interface, reducing the surface energy of water (Adeamson & Gast (1997) [
51]). CBG, as a polyarabinoglucuronoxylan, has hydrophilic groups and possibly some hydrophobic regions that allow it to adsorb at the interface and reduce the surface tension. CBG acts as a surface tension-reducing agent for water, which is essential for wetting, since a lower surface tension of the liquid generally facilitates spreading on a solid surface (see
Figure 3).
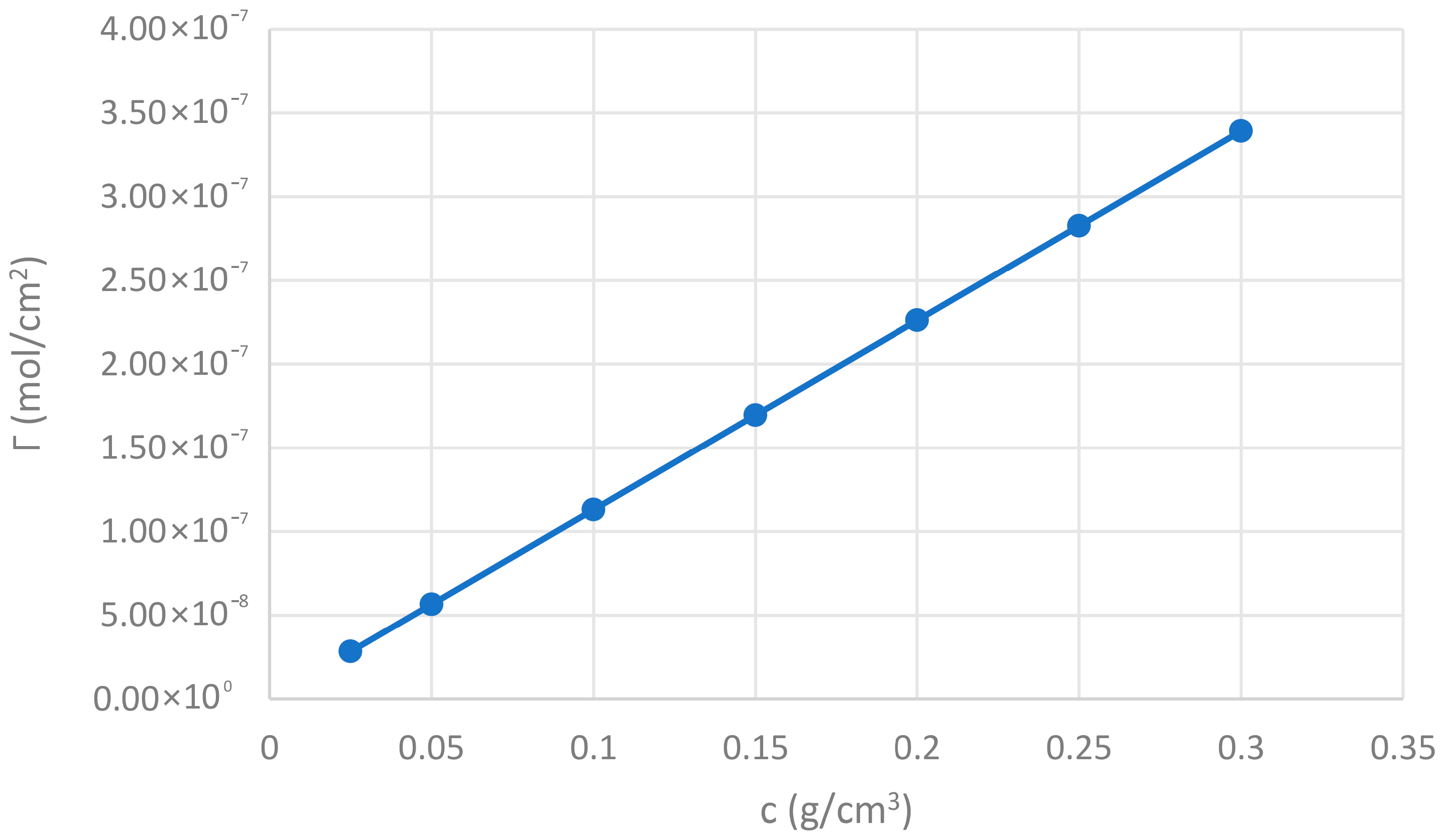
3.4. Excess Surface (Γ2(1))
The Γ
2(1) values increase in direct proportion to the gum concentration in the solution. The excess surface concentration measures the amount of solute (CBG) that accumulates at the liquid-air interface compared to the bulk of the solution (see
Figure 4). The increase in Γ
2(1) with gum concentration confirms that CBG adsorbs at the interface. This adsorption is responsible for the observed reduction in surface tension (Hiemenz & Rajagopalan (1997) [
52]).
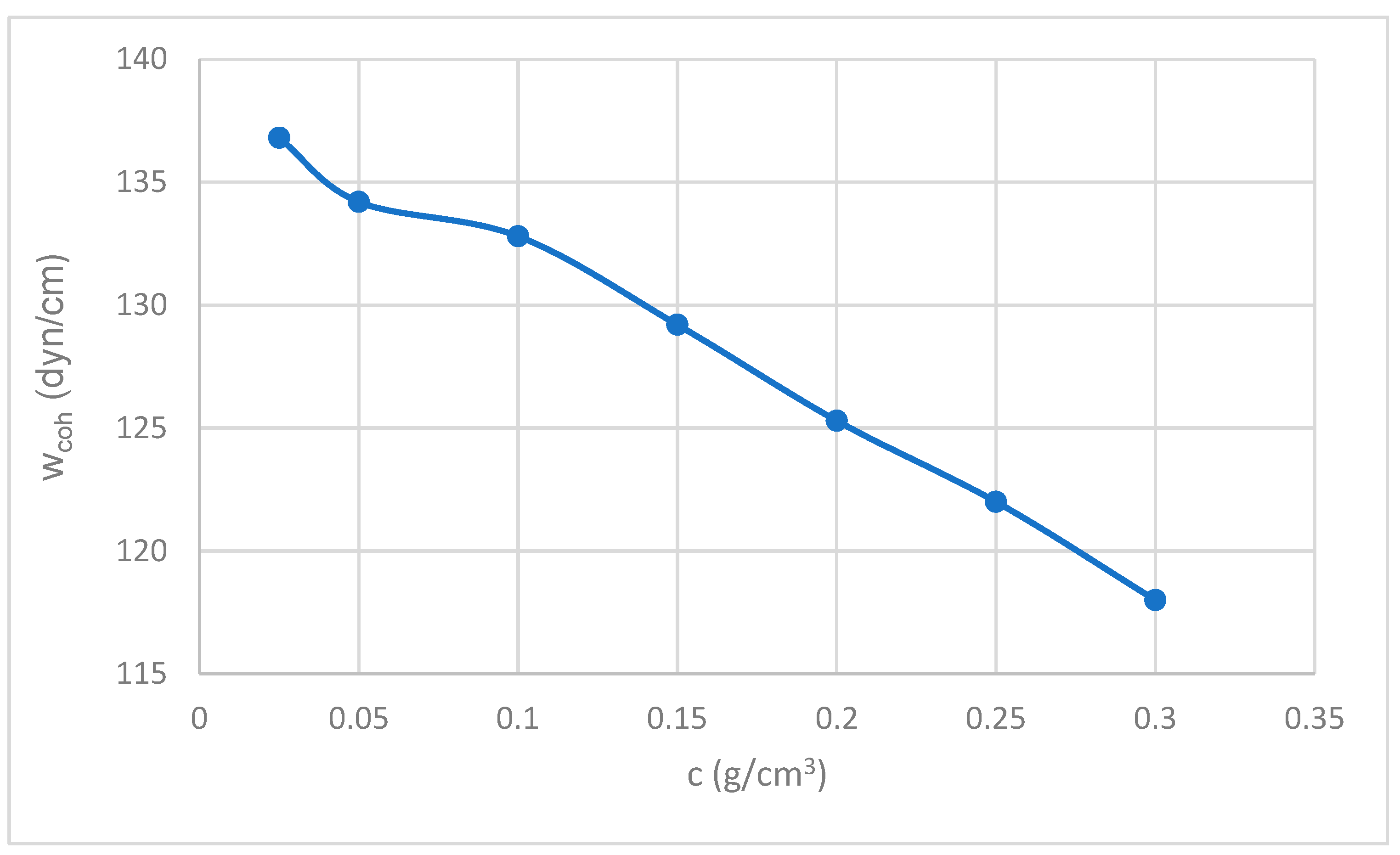
3.5. Cohesion Work (Wcoh)
The work of cohesion decreases with increasing gum concentration. The work of cohesion (W
coh = 2σ) represents the energy required to separate a column of liquid. The decrease in W
coh is consistent with the decrease in surface tension (σ). Lower cohesion indicates weaker intermolecular forces within the liquid, which is often associated with greater ease of deformation or separation of the liquid (see
Figure 5). The addition of CBG and increasing its concentration reduces the internal cohesion of the liquid, which is a direct consequence of the decrease in its surface tension (Hiemenz & Rajagopalan (1997) [
52]).
3.6. Contact Angle (θ)
The contact angle of water on A4 paper is 88°. Surprisingly, the addition of chañar brea gum increases the contact angle, ranging from 90° (2.5% wt.) to 99° (30% wt.). A contact angle greater than 90° indicates that the surface is hydrophobic or non-wettable for that liquid. An increase in the contact angle means a decrease in the wettability of the paper by the solution. This is a critical and counterintuitive result if the intention is to use the gum as a wetting agent. Although the gum reduces the surface tension of the solution (which would normally favor wetting), the paper-solution interface becomes less affine. This could be due to several factors: The gum could adsorb to the paper surface in such a way that it becomes more hydrophobic, for example, in
Figure 6, by orienting its nonpolar groups outward (Vogler (1998) [
53]).
Although viscosity increases at higher concentrations, if the gum forms a viscoelastic layer that hinders intimate contact between the liquid and the paper surface, it could increase the contact angle. However, the decrease in viscosity at higher concentrations makes this explanation less likely for the entire range.
The specific interaction between the gum (a polyarabinoglucuronoxylan) and the cellulose fibers of A4 paper might not be favorable for wetting. Despite the reduction in the surface tension of the liquid, interactions at the solid–liquid interface (σSL) could increase significantly, resulting in an increased contact angle according to Young’s equation (σSG = σSL + σL cosθ).
CBG, at the concentrations studied, does not improve the wettability of A4 paper; on the contrary, it decreases it, increasing the contact angle. This suggests that it does not act as an effective wetting agent for this substrate and conditions.
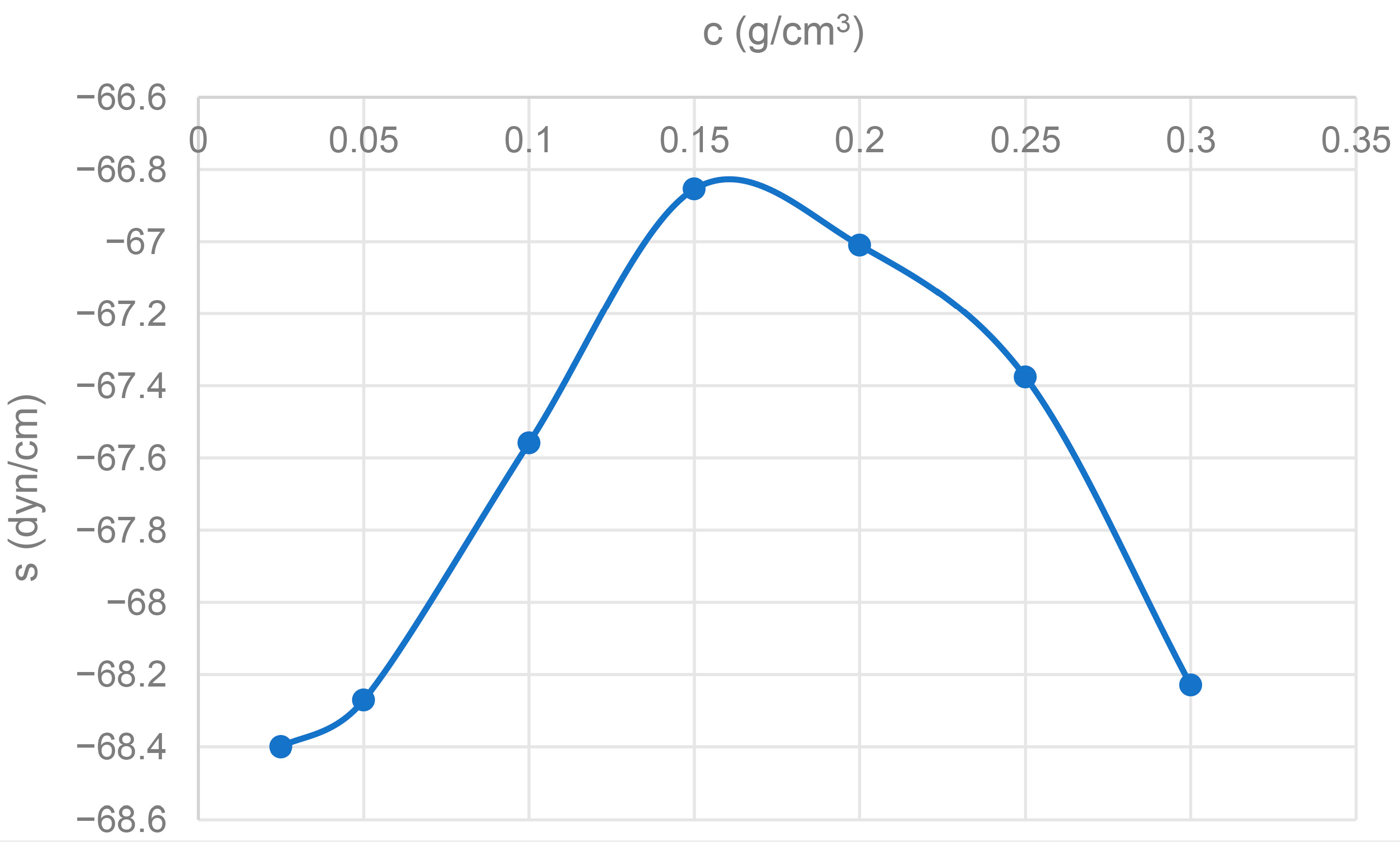
3.7. Spreading Coefficient (s)
The spreading coefficient (s) is consistently negative for all CBG solutions. A negative spreading coefficient (s < 0) indicates that the liquid will not spontaneously spread over the solid surface (Etzler (2003) [
54]). In this case, for the solution to spread, an external force or energy would be required. The persistence of a negative value and its slight variation across concentrations, despite the reduction in the solution’s surface tension, reinforces the idea of low affinity or high solid–liquid interfacial tension (σ
SL). CBG solutions do not spontaneously spread over A4 paper, which is consistent with the high contact angles observed (see
Figure 7).
3.8. Adhesion Work (Wadh)
The work of adhesion decreases with increasing gum concentration. The work of adhesion (W
adh = σ
L (1 + cosθ)) is the energy required to separate the solid–liquid interface. A decrease in W
adh means that less energy is needed to separate the solution from the paper, which is directly consistent with the increasing contact angle and decreasing wettability. Lower adhesion indicates weaker interactions between the gum solution and the paper surface. The interaction between the CBG solution and the A4 paper surface weakens as the gum concentration increases, contributing to the decrease in wettability (see
Figure 8).
Based on the above discussions, the following can be concluded:
CBG effectively acts as a surface tension-reducing agent for water. This property, along with its adsorption at the liquid-air interface (increasing Γ2(1)), is consistent with the behavior of a surfactant or a polymer with a certain amphipathic character.
The viscosity behavior is typical of a polymer in solution. Viscosity increases significantly with increasing chañar brea gum concentration. This increase is moderate at low concentrations and becomes much more pronounced at higher concentrations, indicating the intertwining of the polymer chains. The high viscosities at higher concentrations are a direct result of these interactions between polymer molecules, which makes flow more difficult, and high viscosity hinders flow and wetting. There is no evidence in the graph of a decrease in viscosity with increasing concentration.
Contrary to what might be expected from a surface tension-reducing agent, CBG does not improve the wettability of A4 paper. In contrast, CBG solutions make the paper surface more hydrophobic (higher contact angle, θ > 90°), and a decrease in the work of adhesion (Wadh) and a consistently negative spreading coefficient (s < 0) are observed.
The results suggest that, although CBG favorably modifies the surface tension of the liquid (a property of the liquid), the specific interaction between the gum and the substrate (A4 paper) does not favor wetting or spreading. It is possible that the gum adsorbs to the paper in a way that exposes hydrophobic groups, or that the cellulose in the paper is not compatible with the gum structure to promote strong adhesive interactions. Therefore, for applications requiring good wetting or spreading on cellulose surfaces such as A4 paper, CBG at these concentrations would not be a suitable additive. Its use could be more appropriate in applications where a less wettable or lower adhesion surface is desired.
3.9. Adhesive Perspective
From the perspective of its application as a glue or adhesive, the evaluation of these data is crucial to determining the suitability of chañar brea gum solutions. For a material to function effectively as an adhesive, it must meet several fundamental requirements related to surface chemistry and fluid mechanics:
Wetting: The liquid adhesive must be able to effectively wet the substrate surface. This means it must spread onto the substrate and establish intimate contact over as large an area as possible. Good wetting is characterized by a low contact angle (θ), ideally close to 0° (Israelachvili (2011) [
48]).
Spreading: Related to wetting, the adhesive should ideally spread spontaneously over the surface. This is quantified by the spreading coefficient (s), where a positive value indicates spontaneous spreading (Kwok & Neumann (1999) [
55]).
Work of Adhesion (
Wadh): Represents the energy required to separate the interface between the adhesive and the substrate. A high W
adh value indicates strong interactions between the adhesive and the substrate, which translates into a robust adhesive bond (Israelachvili (2011) [
51]).
Surface Tension (
σ): Generally, a lower surface tension of a liquid adhesive can promote wetting, as it reduces the energy of the liquid-air interface, facilitating spreading (Ebnesajjad (2010) [
56]). However, this is only part of Young’s equation.
Viscosity (
η): Viscosity influences the adhesive’s ability to flow, fill the interstices of the substrate, and form a uniform layer. Adequate viscosity is vital for the applicability and final performance of the adhesive, avoiding excessive penetration into porous substrates (known as “bond line starvation”) (Ebnesajjad (2010) [
56]).
Work of Cohesion (
Wcoh): Although W
adh focuses on the adhesive-substrate interface, the adhesive itself must possess sufficient internal cohesion (cohesive strength) to prevent fracture within the adhesive film once cured or dry. If the adhesive is weak internally, it will fail cohesively even if the adhesion to the substrate is strong (Ebnesajjad (2010) [
56]).
Data Analysis from the Adhesive Perspective
Let us analyze the data on CBG in the context of its potential as an adhesive for A4 paper:
- (a)
The contact angle of water on paper is 88°. Surprisingly, when CBG is added, the contact angle increases progressively with concentration, reaching 99° ± 4 at 30% w/v. A contact angle above 90° indicates that the surface is non-wettable for the liquid, or that the liquid “beads” onto the surface (Israelachvili (2011) [
48]). This is a highly unfavorable finding for an adhesive. A good adhesive must wet the surface to establish intimate contact and form bonds. The fact that the contact angle increases means that the CBG solution has a very low affinity or even an aversion to the A4 paper surface. Without adequate wetting, the formation of a strong adhesive bond is impossible, since sufficient surface contact is not established at the molecular level. CBG, at the concentrations tested, impairs the wettability of A4 paper, making it unsuitable for forming effective adhesive bonds on this type of substrate.
- (b)
The spreading coefficient is consistently negative for all CBG concentrations, with values around −68 dyn/cm. A negative spreading coefficient indicates that the solution will not spread spontaneously onto the paper (Kwok & Neumann (1999) [
55]). Instead, the adhesive droplet will tend to maintain a compact shape. This hampers uniform application of the adhesive and complete surface coverage, which is critical for achieving consistent bond strength across the interface. The lack of spontaneous spreading of the CBG solution onto A4 paper represents a significant limitation for its application as an adhesive, possibly requiring the application of pressure or contact-forcing methods.
- (c)
The work of adhesion decreases with increasing gum concentration, from 68.40 ± 3 dyn/cm to 49.77 ± 1 dyn/cm. The work of adhesion is a direct measure of the strength of the attractive interactions at the adhesive-substrate interface (Israelachvili (2011) [
48]). A decreasing W
adh means that the attractive forces between the gum solution and the paper weaken as more gum is added. For an adhesive, a high W
adh is sought to ensure a strong bond. This decreasing trend is therefore counterproductive to optimal adhesive performance. CBG reduces the ability of its solution to energetically adhere to A4 paper, resulting in a weaker adhesive bonding potential.
- (d)
The surface tension of the solution decreases as the CBG concentration increases. A low surface tension of the adhesive is usually desirable because it favors wetting. However, in this case, despite the reduction in σ, the contact angle increases and the work of adhesion decreases. This suggests that, although CBG reduces the internal cohesion of the liquid (W
coh also decreases), unfavorable interactions at the solid–liquid interface (σ
SL in Young’s equation) or the structure of the gum adsorbed on the paper are the dominant factors preventing wetting and adhesion (Young (1805) [
57]). Young’s equation σ
SG = σ
SL + σ
Lcosθ is fundamental to understanding wetting and the relationship between surface tensions. That is, the decrease in σ is not sufficient to overcome adhesion barriers. Although CBG reduces the surface tension of its solutions, this property does not translate into an improvement in adhesive performance on A4 paper due to other unfavorable interphase factors.
- (e)
The viscosity of chañar brea gum (CBG) solutions does not exhibit erratic behavior at low concentrations, but rather increases steadily as the concentration increases, which is typical of polymer solutions. This viscosity is crucial for the adhesive’s handling. If an adhesive is too viscous, its application becomes difficult. If its viscosity is too low, as is the case with a very dilute CBG solution, it can excessively penetrate a porous substrate such as paper, a phenomenon known as “bond line starvation”. This excessive penetration (over-wicking) results in a shortage of adhesive in the bonded zone, leading to very weak or nonexistent bonds. Therefore, adequate viscosity is vital for the adhesive’s final performance, as unstable or erratic behavior is not ideal for an adhesive (Ebnesajjad (2010) [
56]).
- (f)
Based on the analysis of wetting, spreading, work of adhesion, and viscosity data, aqueous solutions of CBG (polyarabinoglucuronoxylan) are not suitable for use as glue or adhesive on A4 paper. Although the gum reduces the surface tension of the solution (a property often desirable in adhesives), this benefit is completely offset by the unfavorable interactions at the solid–liquid interface. CBG fails to wet the paper (contact angle > 90°), does not spread spontaneously over it (negative spreading coefficient), and the interactions between the solution and the substrate are weak (low and decreasing work of adhesion). Furthermore, the high viscosity at higher CBG concentrations could compromise the formation of an effective bond line due to excessive absorption into the porous substrate. In summary, CBG’s ability to form a strong and durable adhesive bond with A4 paper is extremely limited, if not zero, under the conditions of this study. Any application as an adhesive would require significant modifications to the formulation or substrate surface to drastically improve wetting and adhesion properties.
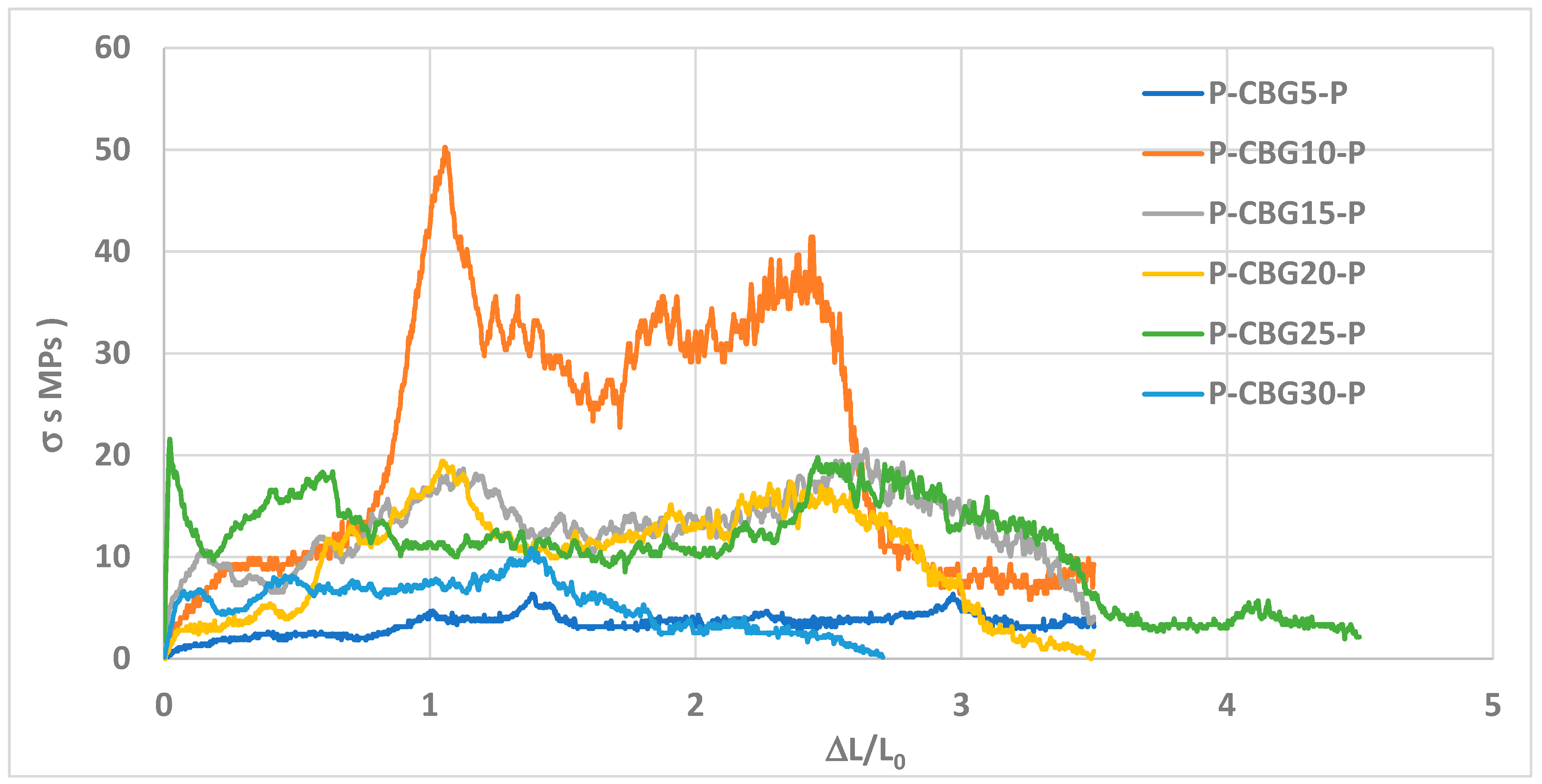
3.10. Mechanical Test
Tensile tests were performed on A4 sheets bonded with CBG. The attached table presents the results of tensile tests performed on A4 sheets of paper bonded with chañar brea gum (CBG) solutions at different concentrations (see
Figure 9 and
Table 2). Two “peaks” of adhesive strength were measured: the “1st Peak” and the “2nd Peak.” Although the units are not specified, the discussion will focus on trends and relative comparisons.
In a tensile test for an adhesive bond on paper, the force peaks typically represent:
1st Peak Adhesive Strength: Generally, this value corresponds to the maximum force the adhesive bond can withstand before primary failure sets in. This failure can be adhesive (separation between the adhesive and the substrate), cohesive (rupture within the adhesive or the substrate, in this case, the paper), or a combination. It is the most direct indicator of the initial bond strength.
2nd Peak Adhesive Strength: A second peak may indicate a more complex failure process. It could represent the force required to continue propagating an initial tear, the peel force of a secondary section of the bond, or, most likely in a paper system, the residual force required to break the paper fibers after the initial adhesive failure has occurred. Since the “2nd Peak” values are consistently lower than those of the “1st Peak,” it suggests a lower strength once the initial failure has occurred (
Table 2).
Tensile strength measures the force required to separate an adhesive from a substrate by pulling it in a perpendicular direction. For CBG, the values focus on adhesive strength, which resembles the tensile strength in a paper bonding setup. Its peak of 50.24 MPa in paper is a very high benchmark. In contrast, adhesives based on pectin and cellulose derivatives have demonstrated tensile strengths of up to 3.8 MPa in paper bonds (Wang et al. (2023a) [
17]). On the other hand, the carboxyl-modified guar gum adhesive achieved a peel strength of 150 N/m in steel bonds (Kumar et al. (2022) [
20]). Meanwhile, blends of xanthan gum and starch achieved a tensile strength of 2.5 MPa as paper adhesives (Wang et al. (2023b) [
21]). In this case, CBG is significantly superior to pectin and blends of other natural gums in its tensile strength on paper. Chañar brea gum (CBG) is notable for its exceptional adhesive strength on paper, particularly at a concentration of 10 wt%, where it outperforms all other natural polysaccharides analyzed in the data. Although Tannin-modified starch adhesives and chitosan-based adhesive with citric acid as a crosslinker have established applications in wood bonding with strengths of 4.2 MPa (Qi et al. (2023) [
15]) and 5.1 MPa (Li et al. (2024) [
19]), respectively, and pectin and cellulose derivatives shows good performance on paper and metal (Wang et al. (2023a) [
17]), the strength of CBG at 10% wt. (50.24 ± 1.19 MPa) is an order of magnitude higher. This strength could position CBG as a promising material for high-performance applications, although its behavior varies dramatically with concentration, underscoring the need for precise formulation to maximize its effectiveness.
The high adhesive strength of CBG at its optimal concentration (10% wt.) can be related to an ideal molecular crosslinking density, a distribution of polymer chains that favors optimal interaction between the gum molecules and the paper substrate. At lower concentrations (such as 5% wt.), there may be very few molecular interactions, resulting in weak adhesion. On the other hand, at very high concentrations (such as 30% wt.), the polymer chains may clump together or tangle ineffectively, which also reduces adhesion. The 10% wt. represents the equilibrium point where the CBG polymer network forms most efficiently to maximize bond strength.
So, we can state:
Adhesive Strength Trend (1st Peak): The adhesive strength (1st Peak) shows a clear dependence on the CBG concentration. Initially, it increases from 6.36 ± 0.22 MPa (5% CBG) until it reaches a significant maximum at 50.24 ± 1.19 MPa± to 10% wt. CBG (see
Table 2) and this value is surprisingly anomalous. Then, the strength decreases dramatically to 19.19 ± 0.03 MPa (20% CBG) and drops to very low values of 18.18 ± 0.04 MPa (25% CBG) and 7.85 ± 0.01MPa (30% CBG). This “peaking” behavior is common in the optimization of polymer adhesives. At low concentrations (10% wt.), the increase in the amount of polymer available to form adhesive interactions, together with a possible formation of a more robust network, contributes to a higher bond strength (Pocius (2021) [
58]). However, at very high concentrations (25–30%), the adhesive strength plummets. As mentioned above, viscosity increases dramatically at high concentrations (e.g., at 335 ± 8.2 cP for CBG 30% wt.). High viscosity hinders flow and wetting. (Kinloch (2012) [
59] and Comyn (2021) [
60]).
Our previous analyses showed that, despite the reduction in surface tension, the contact angle of the CBG solution on A4 paper increases (from 88° for water to 99° for 30% CBG), and the work of adhesion decreases. This indicates poor wetting and a low interfacial affinity between the adhesive and the substrate, which is critical for good adhesion (Adamson & Gast (1997) [
51]). At higher concentrations, this lack of affinity becomes dominant, outweighing any benefit of having more polymer available.
While direct cohesion of the dry film was not measured, a high concentration of a polymer can, in some cases, lead to brittleness or a separated phase that reduces the integrity of the adhesive film. However, the viscosity and wetting explanation seems more relevant here.
There is an optimal concentration (around 10%) where CBG exhibits its maximum adhesive strength on A4 paper. However, beyond this concentration, the bond strength decreases dramatically, which is consistent with problems of wetting, interfacial adhesion and, particularly, excessive penetration of the adhesive into the substrate due to its high viscosity.
- b.
Adhesive Strength Trend (2nd Peak): The strength of the 2nd Peak is consistently lower than that of the 1st Peak. It shows a less pronounced trend than the 1st Peak, with a maximum value of 41.438.2 ± 1.17 MPa at 10% CBG, and then a continuous decline, falling to 10.40 ± 0.01 MPa at 30% CBG. The fact that the 2nd Peak is significantly lower and follows a similar (albeit flatter) trend to the 1st Peak reinforces the idea that the 1st Peak represents the primary failure point of the bond. The 2nd Peak could be related to the force required to tear the paper fibers or overcome the strength of a partially compromised bond. The drop to very low values at high concentrations (10.40 ± 0.01 MPa at 30% CBG) suggests that, after initial failure, there is little or no residual strength left in the bond. The 2nd Peak confirms that the residual strength of the bond is lower than the initial strength, and that adhesive effectiveness is seriously compromised at high concentrations (see
Figure 9).
The tensile adhesive strength data confirm and reinforce the conclusions drawn from the contact angle and work of adhesion studies:
The previous discussion regarding contact angle (θ > 90° for CBG) and work of adhesion (decreasing Wadh) already indicated that CBG does not promote good wetting or strong interactions at the adhesive-paper interface. The results of the tensile tests, especially the drastic drop in adhesive strength at high concentrations where wetting is poorest, validate this conclusion. Although the surface tension of the solution decreases, this does not translate into an actual adhesion improvement.
The behavior and sharp increase in viscosity at high concentrations are consistent with a high level of adhesive strength. A high viscosity facilitates the penetration of the adhesive into the paper, leaving the bond line “dry” and without material to form the adhesive bridge between the surfaces (Comyn (2021) [
60]). The CBG 10% wt. sweet spot could represent an equilibrium where the polymer concentration is sufficient to form an adhesive film, and the viscosity, although decreasing, still allows for adequate film formation without excessive absorption. The consistently negative spreading coefficient predicted that the solution would not spread spontaneously. This implies that, even at the 10% wt. CBG sweet spot, the adhesive application was likely not ideally uniform, which could have further limited the maximum achievable strength.
3.10.1. Influence of Humidity and Comparisons with Other Adhesives
Table 3 shows the MPa values for the tapes bonded with CBG adhesive at different ratios, under a constant temperature of 30 °C and varying relative humidity (50%, 60%, and 80%).
Table 3 shows the maximum tensile strength (adhesive resistance) at two “peaks” for CBG concentrations of 5%, 10%, 15%, and 20% by weight, at relative humidifies of 50%, 60%, and 80%. CBG is a polysaccharide with numerous hydroxyl (-OH) groups, which gives it its adhesive properties and its sensitivity to humidity (Myllytie et al. (2009) [
61]).
For a fixed relative humidity (e.g., 60% RH, Peak 2), the strength increases significantly with concentration: 1.65 MPa (5%) → 5.97 MPa (10%) → 12.87 MPa (15%) → 11.19 MPa (20%). An increase in CBG concentration means a higher polymer density in the solution and, therefore, in the solidified adhesive layer. Upon drying, the polymer chains are more likely to interact with each other, forming a denser and stronger polymer network. The higher concentration increases the total number of intermolecular forces (including dispersion forces and hydrogen bonds) per unit volume within the adhesive layer, which translates directly into greater internal cohesion and, therefore, higher tensile strength.
At 60% and 80% RH, the strength reaches a maximum at 15% CBG (12.87 MPa and 15.40 MPa, Peak 2) and then decreases slightly or stabilizes at 20% (11.19 MPa and 12.36 MPa, Peak 2). This peak and potential decline may indicate a limit. At very high concentrations (20%), the viscosity of the adhesive solution increases dramatically. Excessive viscosity can hinder wetting (the ability of the adhesive to spread and penetrate the micro-irregularities of the paper). Poor wetting reduces the effective contact area and, therefore, the interfacial adhesion forces with the paper surface. A too thick or rigid CBG film (due to high concentration) can generate internal stresses in the bond during drying or when force is applied. If cohesion becomes much greater than adhesion, the failure mode could shift from cohesive failure (within the adhesive) to adhesive failure (at the interface), or the film itself could fracture brittlely, reducing the apparent maximum strength. Relative humidity is a critical variable for any hydrophilic polymeric adhesive like vegetable gum, since water acts as a plasticizer (da Silva Borges (2018) [
62]).
At 15% CBG (Peak 2), the strength increases significantly with humidity: 11.30 MPa (RH 50%) → 12.87 MPa (RH 60%) → 15.40 MPa (RH 80%). This is the opposite behavior expected from a pure hydrophilic polymer, where water typically plasticizes and reduces strength. The strongest hypothesis is that the paper (cellulosic substrate) is highly hygroscopic. With higher RH: 1–The paper hydrates, increasing its ductility and toughness. 2–The interface between the adhesive and the paper is strengthened due to better polymer penetration into the hydrated pores of the paper, or a stronger network of cross-linked hydrogen bonds (Gum-Water-Cellulose). 3–This increase suggests that the failure is not purely cohesive (within the adhesive), but that the measured strength is the substrate strength or the interface strength under the test conditions. If the paper becomes stronger or the interface becomes stronger due to hydration, the measured maximum strength will be higher until the paper breaks (substrate failure).
In almost all cases, the Peak 2 value is higher than the Peak 1 value (e.g., 15% CBG, RH 60%: Peak 1 = 9.43 MPa, Peak 2 = 12.87 MPa). The nature of “Peak 1” and “Peak 2” is not explicitly defined, but in a tensile test, this could mean that Peak 1 (Initial Elastic/Plastic Deformation Strength) represents the point where the first localized failure occurs, or an apparent yield strength. Peak 2 represents the maximum overall strength that the system can withstand. The fact that it is higher suggests that, after the initial reorganization or failure (Peak 1), the polymer network (cohesion) or the interface (adhesion) reorganizes under stress, or that the load is redistributed more efficiently until a global maximum is reached (Peak 2). This implies ductile rather than brittle behavior.
Table 3 shows that chañar brea gum acts as a humidity-sensitive adhesive, with concentration being the primary factor influencing the formation of the polymer network (cohesion). Relative humidity has a complex effect; at least at medium and high concentrations (15% and 20%), increased humidity not only does not weaken the adhesive to the point of failure, but it actually strengthens the bond to the paper substrate, possibly by improving the adhesion interface or enhancing the paper’s inherent strength. The 15% CBG solution at 80% RH represents the condition of maximum measured strength, representing an optimal balance between polymer network density and the beneficial effect of substrate hydration (Park et al. (2021) [
63], Tkalčec et al. (2016) [
64], Yang et al. (2025) [
65], Nechita & Roman (2020) [
66]).
This paper also includes measurements of different types of adhesives at various relative humidity levels at 30 °C, such as Voligoma
®, Plasticola
®, silicone adhesive, Plastimonte
® (see
Table 4), and cornstarch-based adhesive (Wilpiszewska & Czech (2018) [
67] and Ali et al. (2020) [
9]). The results of the mechanical tests are shown in
Table 4.
Analyzing relative humidity (RH) in
Table 4, specifically from 50% to 80%, shows that it affects the bond strength of various paper adhesives, based on their intrinsic physicochemical properties. The silicone-based adhesive, being hydrophobic, showed the greatest increase in strength (from 11.71 to 16.31). Its composition is not negatively affected by humidity. This behavior indicates that the silicone adhesive is highly resistant to ambient humidity. Furthermore, the hydration of the paper substrate could improve its own deformation capacity and strength, resulting in a higher overall bond strength without the adhesive losing cohesion.
Water-based, hydrophilic adhesives showed the expected decrease in strength, a process known as moisture-induced plasticization. For example, Voligoma (polyvinyl alcohol-co-vinyl acetate, PVA-co-VAc) showed a decrease in its strength (from 15.27 to 11.71). This is typical behavior for a hydrophilic polymer. Water penetrates and intercalates between the long chains of PVA-co-VAc, acting as a plasticizer. This reduces the strength of intermolecular interactions (Van der Waals forces), which translates directly into a loss of cohesion in the adhesive film and a lower tensile strength. Polyvinyl acetate (PVAc) exhibits similar behavior to PVA-co-VAc, although slightly less pronounced. The cornstarch-based adhesive also showed a significant decrease in performance (from 14.13 to 11.45) (Karimnejad et al. (2024) [
68] and Nizardo et al. (2024) [
69]).
Since starch is extremely hydrophilic, increased humidity causes significant plasticization of the adhesive matrix. Water is absorbed into the starch matrix, causing it to swell and weakening its internal structure, which reduces the cohesion of the dried film and decreases the bond strength.
The 15% CBG adhesive and Plastimonte® showed a significant increase in strength (from 11.30 to 15.40). The explanation suggests that, at this optimal concentration, the presence of water improves the interfacial adhesion with the cellulose of the paper. Instead of weakening the adhesive (plasticization), the water helps to achieve a superior adhesion state, shifting the failure point of the adhesive to the substrate-adhesive interface or to the substrate itself, which becomes highly hydrated and therefore better bonded.
The hydrophilic adhesives such as cornstarch, PVA-co-VAc, and PVAc are highly sensitive to ambient humidity, losing strength due to plasticization, chañar brea gum and, especially, the silicone-based adhesive, demonstrate superior performance when stability and bond strength are required in environments with high relative humidity.
3.10.2. Temperature Effects
Table 5 presents the bond strength (adhesion force) for different concentrations of Chañar Brea Gum (CBG) and a commercial cornstarch adhesive, under three temperature conditions. The adhesion mechanism for both polymers, CBG (a polysaccharide hydrocolloid, a polyarabinoglucuronoxylans) and starch (also a polysaccharide: amylose and amylopectin), is primarily based on the formation of hydrogen bonds between the abundant hydroxyl (-OH) groups of their polymeric chains and the hydroxyl groups of the cellulose fibers in the paper.
Analysis of the adhesion peaks (especially Peak 2, which usually indicates the maximum breaking strength) at the initial temperature (40 °C) reveals a critical relationship between concentration and bond strength: 1–The bond strength increases dramatically (from 1.53 MPa to 10.83 MPa at Peak 2). This occurs because a higher CBG concentration means a greater density of polymer chains available to interconnect and form hydrogen bonds with the substrate (paper). The increased material also strengthens the internal cohesion of the adhesive film. 2–The strength remains at a high level (10.83 MPa to 10.05 MPa), indicating that 10% and 15% wt. represent the most efficient concentration range, where the best balance between cohesion, adhesion, and wetting is achieved. 3–The strength decreases significantly (10.05 MPa to 7.50 MPa at Peak 2). Physicochemically, this decrease is attributed to the excessive viscosity of the 20% wt. solution. High viscosity reduces the adhesive’s ability to wet the paper surface and penetrate its capillary network, resulting in a more superficial bond and, therefore, lower overall strength (Mehrabova et al. (2025) [
70] and Elvin (2021) [
71]).
The effect of temperature on the performance of CBG at its optimal concentration (10%) reveals a trade-off between reduced viscosity and increased drying rate: 1–At 10% CBG, adhesion remains stable or even improves slightly (from 10.83 MPa to 9.92 MPa at Peak 2). The slight increase in temperature (40–50 °C) reduces viscosity, slightly improving the mobility of the polymer chains and their penetration into the substrate, which favors adhesion. 2–A significant decrease in bond strength is observed (9.92 MPa to 7.27 MPa at Peak 2) at 60 °C. The dominant factor at 60 °C is the accelerated evaporation of the solvent (water). Excessively rapid drying prevents the CBG polymer chains from fully reorganizing and interlinking before solidification (curing). The result is a more brittle adhesive film with reduced internal cohesion, leading to bond failure at lower stress levels.
The difference in performance at 40 °C is due to the fact that the optimal CBG concentration (10% wt. at 40 °C, 10.83 MPa) outperforms starch at the same temperature (1.08 MPa). This suggests that, at low temperatures, chañar brea gum, as a natural hydrocolloid, offers a higher initial affinity or a better network structure than unmodified corn starch. 2–At 60 °C, starch exhibits a significant increase in strength (from 1.08 MPa to 9.23 MPa). This phenomenon is characteristic of starch-based adhesives, where high temperatures are necessary for proper gelatinization. Heat causes the starch granules to rupture, releasing amylose and amylopectin chains, which initially reduces viscosity drastically and allows for maximum interpenetration and the formation of a highly cohesive matrix upon cooling and drying (Qi et al. (2023) [
15] and Zhang et al. (2022) [
16]).