Deep Margin Elevation: Current Evidence and a Critical Approach to Clinical Protocols—A Narrative Review
Abstract
1. Introduction
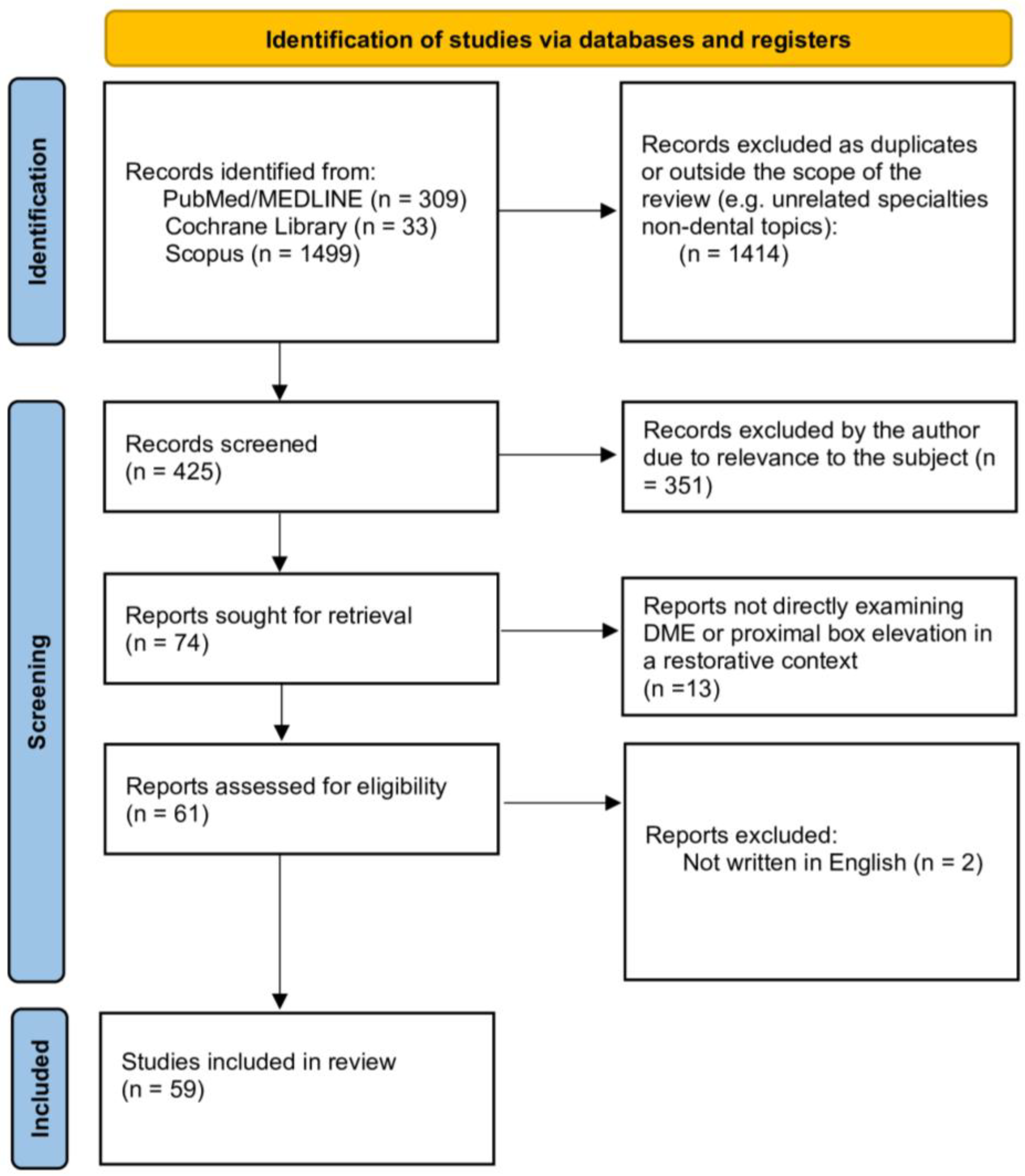
2. Materials and Methods
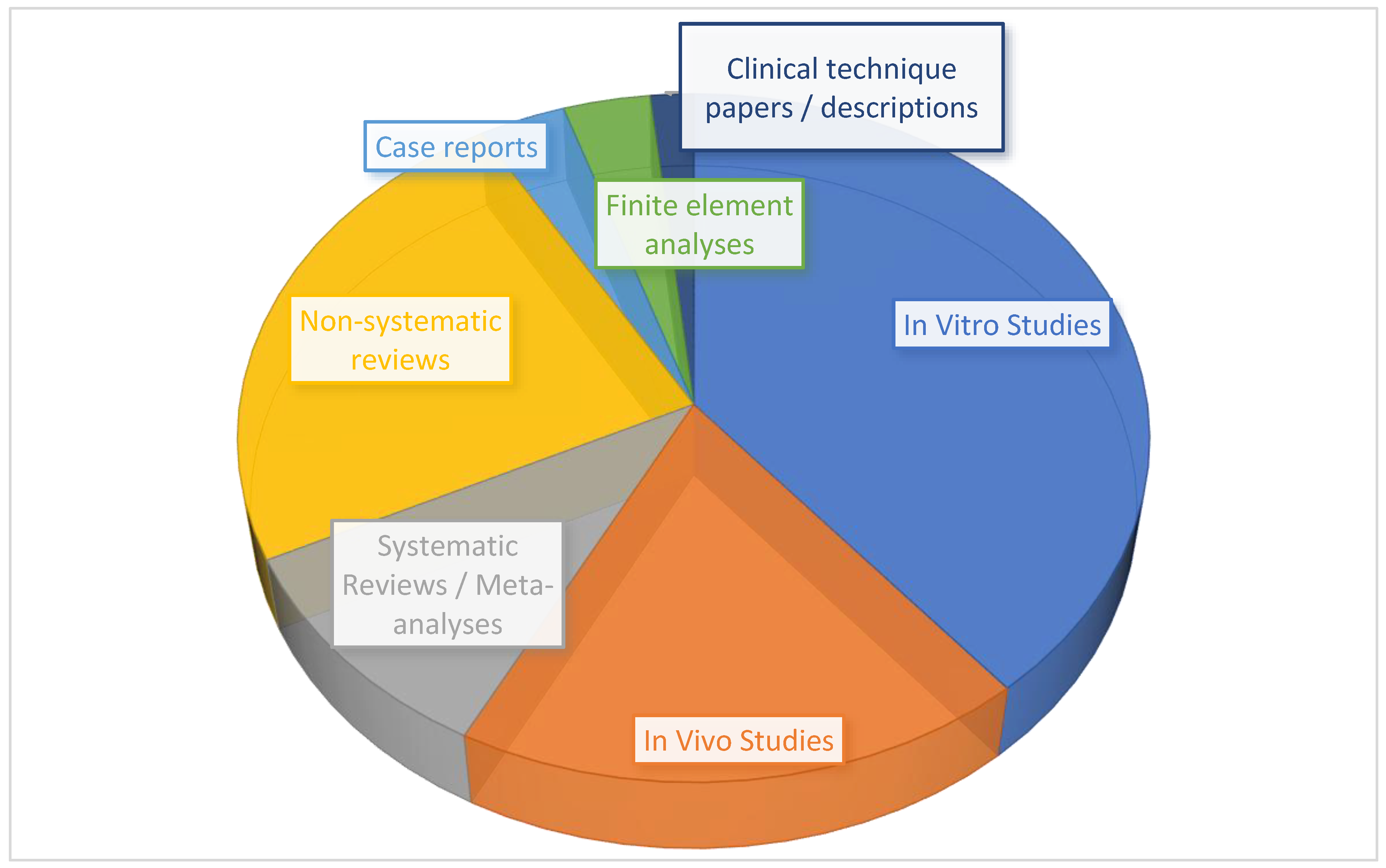
3. Results
3.1. Evolution of DME
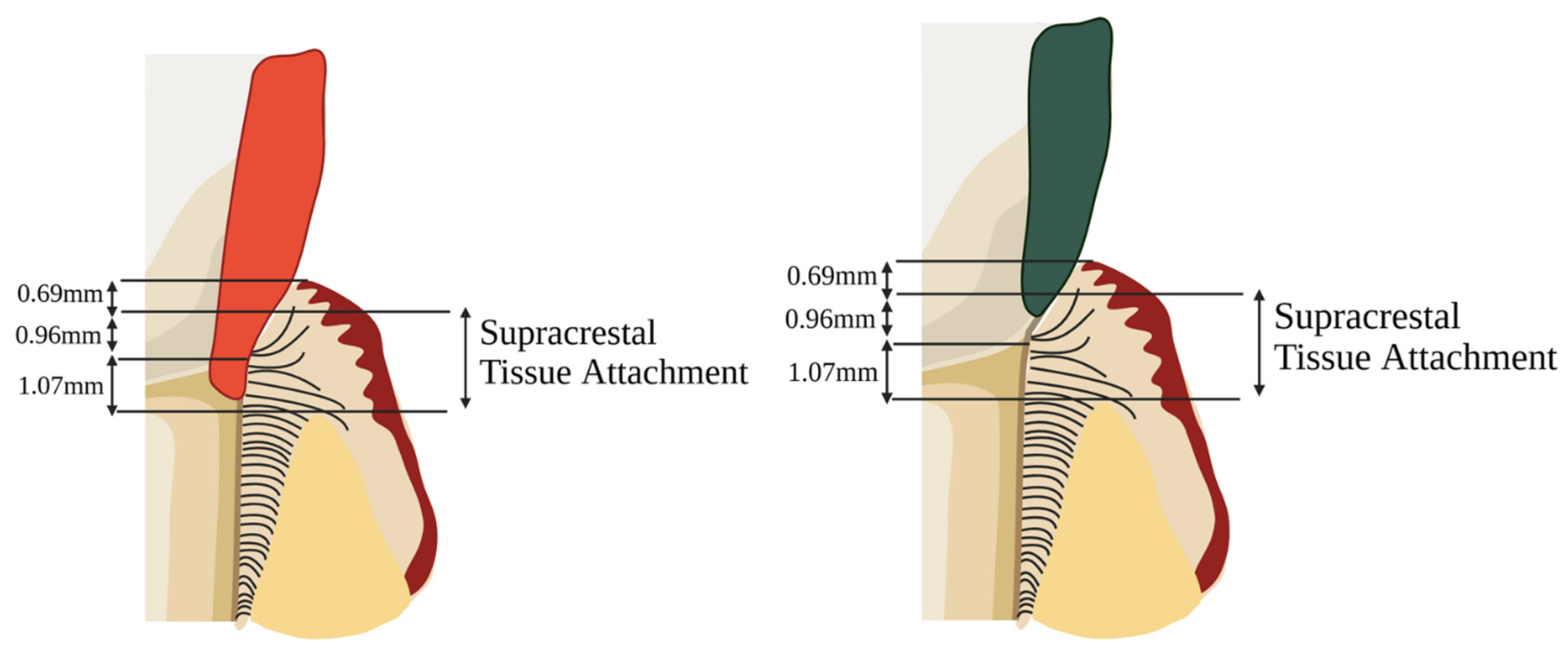
3.2. Indications for DME
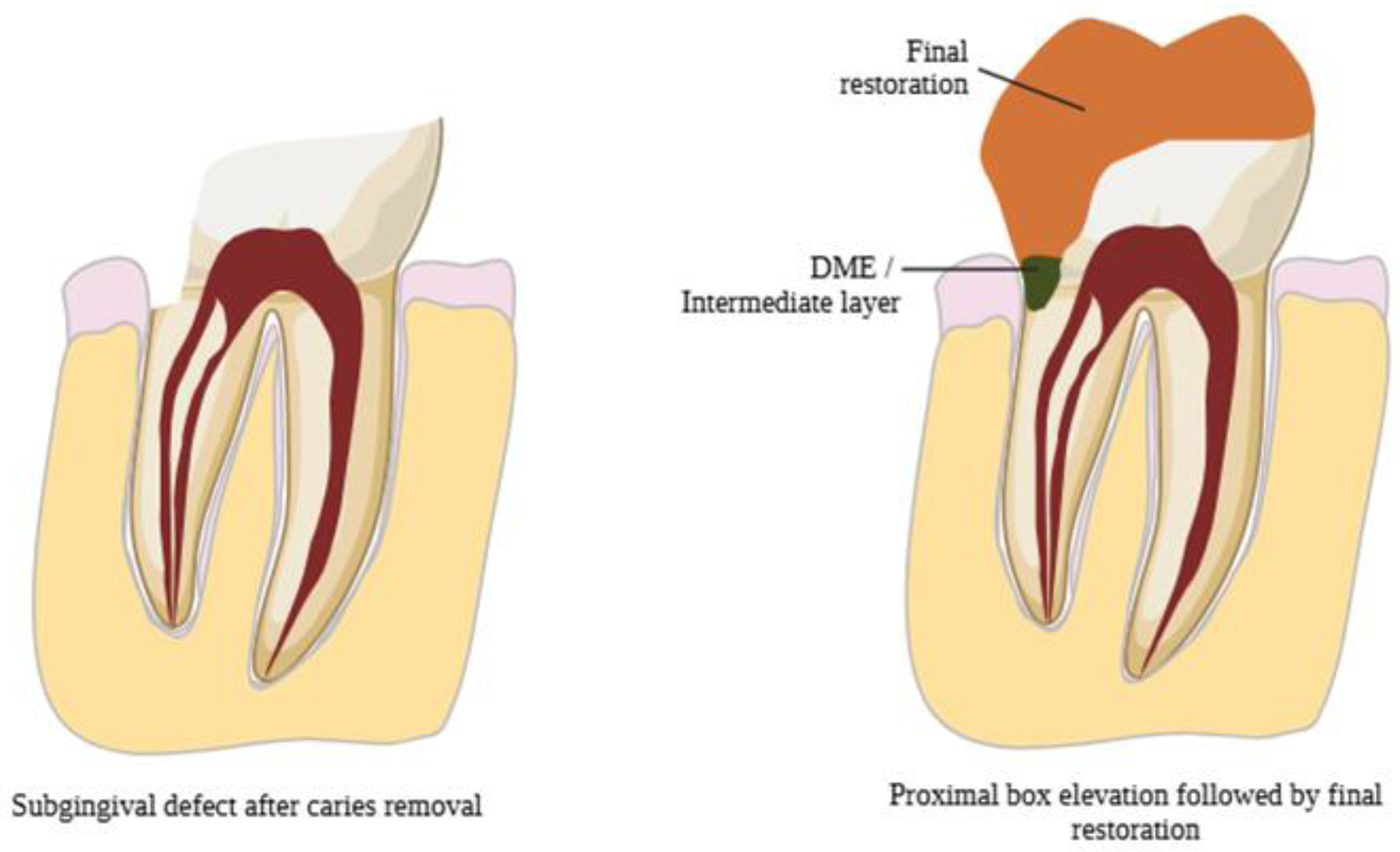
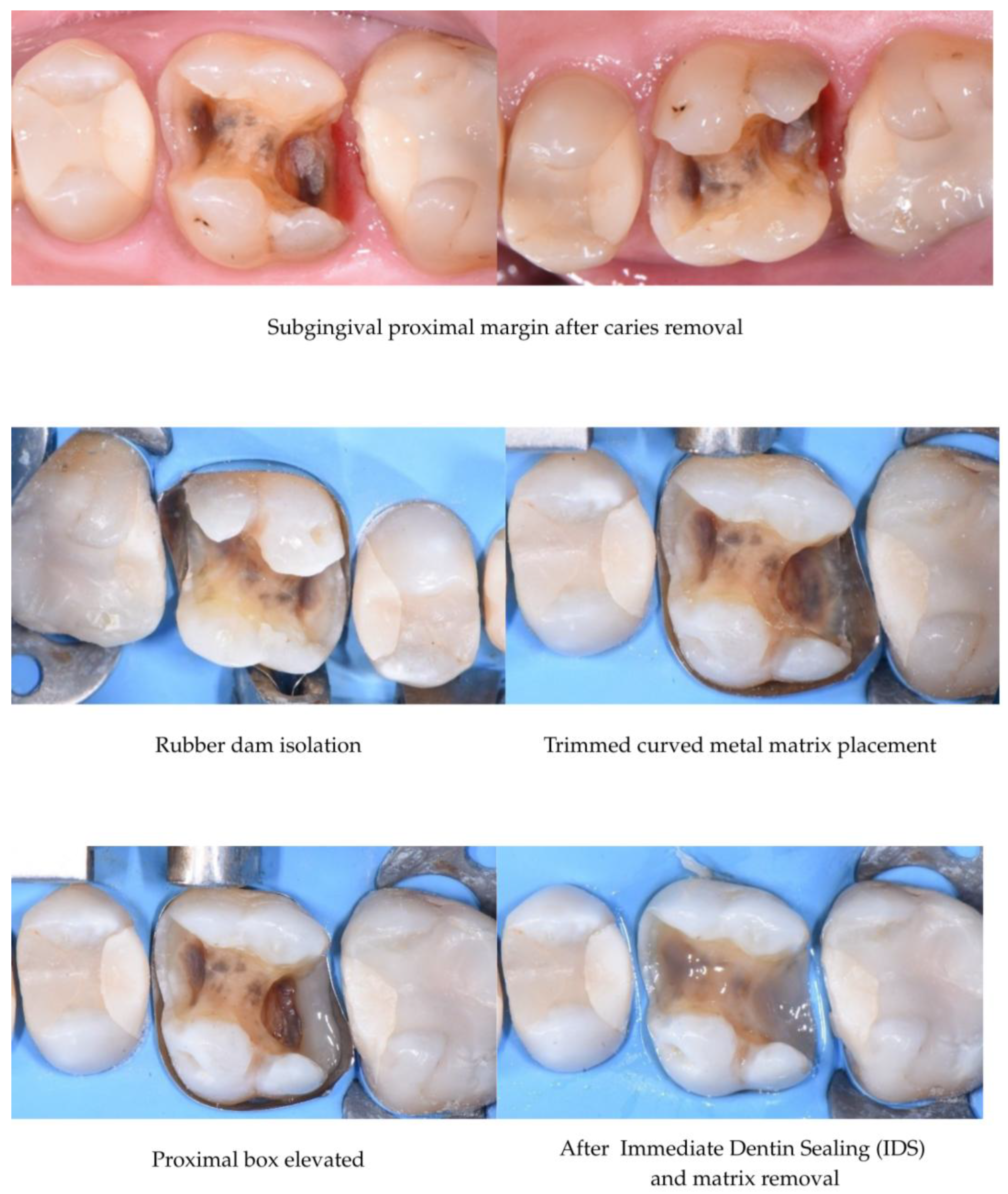
3.3. DME Technique
3.4. Microleakage & Marginal Adaptation
3.5. Bond Strength & Layering
3.6. Periodontal Response
3.7. Failure Rate
4. Discussion
4.1. Adhesive Strategies and Material Selection for Deep Margins
4.2. Long-Term Clinical Performance and Failures
4.3. DME Compared to Alternative Margin Management Techniques
4.4. Case Selection, Classifications, and Clinical Guidelines
4.5. Future Research Directions
4.6. Summary and Clinical Recommendations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| DME | Deep Margin Elevation |
| STA | Supracrestal Tissue Attachment |
| IDS | Immediate Dentin Sealing |
| PTFE | Polytetrafluoroethylene |
| GIC | Glass Ionomer Cement |
| μTBS | Micro Tensile Bond Strength |
| BoP | Bleeding on Probing |
| PI | Plaque Index |
| PPD | Probing Pocket Depth |
| GI | Gingival Index |
| SCL | Surgical Crown Lengthening |
| RMGIC | Resin Modified Glass Ionomer |
| OE | Orthodontic Extrusion |
References
- Sadeghnezhad, P.; Sarraf Shirazi, A.; Borouziniat, A.; Majidinia, S.; Soltaninezhad, P.; Nejat, A.H. Enhancing subgingival margin restoration: A comprehensive review and meta-analysis of deep margin elevation’s impact on microleakage. Evid. Based Dent. 2024, 25, 212. [Google Scholar] [CrossRef]
- Aldakheel, M.; Aldosary, K.; Alnafissah, S.; Alaamer, R.; Alqahtani, A.; Almuhtab, N. Deep margin elevation: Current concepts and clinical considerations—A review. Medicina 2022, 58, 1482. [Google Scholar] [CrossRef]
- Palma, P.J.; Neto, M.A.; Messias, A.; Amaro, A.M. Microtensile Bond Strength of Composite Restorations: Direct vs. Semi-Direct Technique Using the Same Adhesive System. J. Compos. Sci. 2025, 9, 203. [Google Scholar] [CrossRef]
- Alam, M.N.; Ibraheem, W.; Ramalingam, K.; Sethuraman, S.; Basheer, S.N.; Peeran, S.W. Identification, evaluation, and correction of supracrestal tissue attachment (previously biologic width) violation: A case presentation with literature review. Cureus 2024, 16, e58128. [Google Scholar] [CrossRef]
- Felemban, M.F.; Khattak, O.; Alsharari, T.; Alzahrani, A.H.; Ganji, K.K.; Iqbal, A. Relationship between deep marginal elevation and periodontal parameters: A systematic review. Medicina 2023, 59, 1948. [Google Scholar] [CrossRef]
- Fichera, G.; Mazzitelli, C.; Picciariello, V.; Maravic, T.; Josic, U.; Mazzoni, A.; Breschi, L. Structurally compromised teeth. Part II: A novel approach to peripheral build-up procedures. J. Esthet. Restor. Dent. 2023, 36, 20–31. [Google Scholar] [CrossRef]
- Lee, B.; Shin, J.; Jeong, T.; Park, S.; Lee, E. Combined treatment of surgical extrusion and crown lengthening procedure for severe crown-root fracture of a growing patient: A case report. BMC Oral Health 2024, 24, 1498. [Google Scholar] [CrossRef]
- Uravić Crljenica, M.; Perasso, R.; Imelio, M.; Viganoni, C.; Pozzan, L. A systematic and comprehensive protocol for rapid orthodontic extrusion. J. Esthet. Restor. Dent. 2024, 36, 838–844. [Google Scholar] [CrossRef]
- Tu, K.-W.; Kuo, C.-H.; Hung, C.-C.; Yan, D.-Y.; Mau, J.L.P. Strategic sequencing of orthodontic treatment and periodontal regenerative surgery: A literature review. J. Dent. Sci. 2025, 20, 1391–1397. [Google Scholar] [CrossRef]
- Feu, D. Orthodontic treatment of periodontal patients: Challenges and solutions, from planning to retention. Dent. Press J. Orthod. 2020, 25, 79–116. [Google Scholar] [CrossRef]
- Bazos, P.; Magne, P. Bio-emulation: Biomimetically emulating nature utilizing a histoanatomic approach; visual synthesis. Int. J. Esthet. Dent. 2014, 9, 330–352. [Google Scholar]
- Magne, P.B. Biomimetic Restorative Dentistry; Quintessence: Batavia, IL, USA, 2022; Volume 1, pp. 360–363. [Google Scholar]
- Dietschi, D.; Spreafico, R. Current clinical concepts for adhesive cementation of tooth-colored posterior restorations. Pract. Periodontics Aesthet. Dent. 1998, 10, 47–54. [Google Scholar]
- Magne, P.; Spreafico, R. Deep Margin Elevation: A Paradigm Shift. Am. J. Esthet. Dent. 2012, 2, 86–96. Available online: https://s3.amazonaws.com/kajabi-storefronts-production/sites/24049/themes/419830/downloads/uzJurSGFQvGCHAzU40tF_Deep_Margin_Elevation.pdf (accessed on 28 April 2025).
- Taylor, A.; Burns, L. Deep margin elevation in restorative dentistry: A scoping review. J. Dent. 2024, 146, 105066. [Google Scholar] [CrossRef]
- Kwon, T.; Lamster, I.B.; Levin, L. Current concepts in the management of periodontitis. Int. Dent. J. 2021, 71, 462–476. [Google Scholar] [CrossRef]
- Chun, E.P.; de Andrade, G.S.; Grassi, E.D.A.; Garaicoa, J.; Garaicoa-Pazmino, C. Impact of deep margin elevation procedures upon periodontal parameters: A systematic review. Eur. J. Prosthodont. Restor. Dent. 2023, 31, 10–21. [Google Scholar] [CrossRef]
- University of Derby. Literature Reviews: Systematic Searching at Various Levels. Available online: https://libguides.derby.ac.uk/literature-reviews/prisma-lr (accessed on 28 April 2025).
- Mugri, M.H.; Sayed, M.E.; Nedumgottil, B.M.; Bhandi, S.; Raj, A.T.; Testarelli, L.; Khurshid, Z.; Jain, S.; Patil, S. Treatment prognosis of restored teeth with crown lengthening vs. deep margin elevation: A systematic review. Materials 2021, 14, 6733. [Google Scholar] [CrossRef]
- Loguercio, A.D.; Alessandra, R.; Mazzocco, K.C.; Dias, A.L.; Busato, A.L.; Singer, J.D.M.; Rosa, P. Microleakage in class II composite resin restorations: Total bonding and open sandwich technique. J. Adhes. Dent. 2002, 4, 137–144. [Google Scholar]
- Spreafico, R.; Marchesi, G.; Turco, G.; Frassetto, A.; Di Lenarda, R.; Mazzoni, A.; Cadenaro, M.; Breschi, L. Evaluation of the in vitro effects of cervical marginal relocation using composite resins on the marginal quality of CAD/CAM crowns. J. Adhes. Dent. 2016, 18, 355–362. [Google Scholar] [CrossRef]
- Welbury, R.R.; Murray, J.J. A clinical trial of the glass-ionomer cement-composite resin “sandwich” technique in Class II cavities in permanent premolar and molar teeth. Quintessence Int. 1990, 6, 507–512. [Google Scholar]
- Andersson-Wenckert, I.E.; van Dijken, J.W.; Kieri, C. Durability of extensive Class II open-sandwich restorations with a resin-modified glass ionomer cement after 6 years. Am. J. Dent. 2004, 17, 43–50. [Google Scholar] [PubMed]
- Da Silva Gonçalves, D.; Cura, M.; Ceballos, L.; Fuentes, M.V. Influence of proximal box elevation on bond strength of composite inlays. Clin. Oral Investig. 2017, 21, 247–254. [Google Scholar] [CrossRef]
- Ferrari, M.; Koken, S.; Grandini, S.; Ferrari Cagidiaco, E.; Joda, T.; Discepoli, N. Influence of cervical margin relocation (CMR) on periodontal health: 12-month results of a controlled trial. J. Dent. 2018, 69, 70–76. [Google Scholar] [CrossRef]
- Ismail, E.H.; Ghazal, S.S.; Alshehri, R.D.; Albisher, H.N.; Albishri, R.S.; Balhaddad, A.A. Navigating the practical-knowledge gap in deep margin elevation: A step towards a structured case selection—A review. Saudi Dent. J. 2024, 36, 674–681. [Google Scholar] [CrossRef]
- Frese, C.; Wolff, D.; Staehle, H.J. Proximal box elevation with resin composite and the dogma of biological width: Clinical R2-technique and critical review. Oper. Dent. 2014, 39, 22–31. [Google Scholar] [CrossRef]
- Samartzi, T.K.; Papalexopoulos, D.; Ntovas, P.; Rahiotis, C.; Blatz, M.B. Deep margin elevation: A literature review. Dent. J. 2022, 10, 48. [Google Scholar] [CrossRef]
- Alizadeh Oskoee, P.; Dibazar, S. Deep margin elevation; indications and periodontal considerations. J. Adv. Periodontol. Implant Dent. 2024, 16, 91–93. [Google Scholar] [CrossRef]
- Eggmann, F.; Ayub, J.M.; Conejo, J.; Blatz, M.B. Deep margin elevation—Present status and future directions. J. Esthet. Restor. Dent. 2023, 35, 26–47. [Google Scholar] [CrossRef]
- Daghrery, A.; Jabarti, E.; Baras, B.H.; Mitwalli, H.; Al Moaleem, M.M.; Khojah, M.Z.; Khayat, W.; Albar, N.H. Impact of thermal aging on marginal adaptation in lithium disilicate CAD/CAM crowns with deep proximal box elevation. Med. Sci. Monit. 2025, 31, e947191. [Google Scholar] [CrossRef]
- Balci, Ş.N.; Tekçe, N.; Tuncer, S.; Demirci, M. The effect of different deep margin elevation methods on the fracture strength of CAD-CAM restorations. Am. J. Dent. 2024, 37, 115–120. [Google Scholar]
- Ismail, H.S.; Ali, A.I.; Elawsya, M.E. Influence of curing mode and aging on the bonding performance of universal adhesives in coronal and root dentin. BMC Oral Health 2024, 24, 1188. [Google Scholar] [CrossRef]
- Baldi, A.; Rossi, T.; Comba, A.; Monticone, L.; Paolone, G.; Sannino, I.; Vichi, A.; Goracci, C.; Scotti, N. Three-Dimensional Internal Voids and Marginal Adaptation in Deep Margin Elevation Technique: Efficiency of Highly Filled Flowable Composites. J. Adhes. Dent. 2024, 26, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Francois, P.; Attal, J.P.; Fasham, T.; Troizier-Cheyne, M.; Gouze, H.; Abdel-Gawad, S.; Le Goff, S.; Dursun, E.; Ceinos, R. Flexural Properties, Wear Resistance, and Microstructural Analysis of Highly Filled Flowable Resin Composites. Oper. Dent. 2024, 49, 597–607. [Google Scholar] [CrossRef]
- Bresser, R.A.; Carvalho, M.A.; Naves, L.Z.; Melma, H.; Cune, M.S.; Gresnigt, M.M.M. Biomechanical Behavior of Molars Restored with Direct and Indirect Restorations in Combination with Deep Margin Elevation. J. Mech. Behav. Biomed. Mater. 2024, 152, 106459. [Google Scholar] [CrossRef]
- Reddy, K.H.; Priya, B.D.; Malini, D.L.; Mohan, T.M.; Bollineni, S.; Gandhodi, H.C. Deep margin elevation in class II cavities: A comparative evaluation of microleakage and interface integrity using confocal laser microscopy and scanning electron microscopy. J. Conserv. Dent. Endod. 2024, 27, 529–534. [Google Scholar] [CrossRef]
- Baldi, A.; Comba, A.; Rossi, T.; Monticone, L.; Berutti, E.; Scotti, N. Effect of flowable viscosities on deep margin elevation: A microCT study. Dent. Mater. 2023, 39 (Suppl. 1), e69–e70. [Google Scholar] [CrossRef]
- Vichitgomen, J.; Srisawasdi, S. Deep margin elevation with resin composite and resin-modified glass-ionomer on marginal sealing of CAD-CAM ceramic inlays: An in vitro study. Am. J. Dent. 2021, 34, 327–332. [Google Scholar]
- Zhang, H.; Li, H.; Cong, Q.; Zhang, Z.; Du, A.; Wang, Y. Effect of proximal box elevation on fracture resistance and microleakage of premolars restored with ceramic endocrowns. PLoS ONE 2021, 16, e0252269. [Google Scholar] [CrossRef]
- Juloski, J.; Köken, S.; Ferrari, M. No correlation between two methodological approaches applied to evaluate cervical margin relocation. Dent. Mater. J. 2020, 39, 624–632. [Google Scholar] [CrossRef]
- Jawaed, N.U.; Abidi, S.Y.; Qazi, F.U.; Ahmed, S. An in-vitro evaluation of microleakage at the cervical margin between two different Class II restorative techniques using dye penetration method. J. Coll. Physicians Surg. Pak. 2016, 26, 748–752. [Google Scholar]
- Frankenberger, R.; Hehn, J.; Hajtó, J.; Krämer, N.; Naumann, M.; Koch, A.; Roggendorf, M.J. Effect of proximal box elevation with resin composite on marginal quality of ceramic inlays in vitro. Clin. Oral Investig. 2013, 17, 177–183. [Google Scholar] [CrossRef]
- De Mattos Pimenta Vidal, C.; Pavan, S.; Briso, A.L.; Bedran-Russo, A.K. Effects of three restorative techniques in the bond strength and nanoleakage at gingival wall of Class II restorations subjected to simulated aging. Clin. Oral Investig. 2013, 17, 627–633. [Google Scholar] [CrossRef]
- Lefever, D.; Gregor, L.; Bortolotto, T.; Krejci, I. Supragingival Relocation of Subgingivally Located Margins for Adhesive Inlays/Onlays with Different Materials. J. Adhes. Dent. 2012, 14, 561–567. [Google Scholar] [CrossRef]
- Roggendorf, M.J.; Krämer, N.; Dippold, C.; Vosen, V.E.; Naumann, M.; Jablonski-Momeni, A.; Frankenberger, R. Effect of proximal box elevation with resin composite on marginal quality of resin composite inlays in vitro. J. Dent. 2012, 40, 1068–1073. [Google Scholar] [CrossRef] [PubMed]
- De Goes, M.F.; Giannini, M.; Di Hipólito, V.; Carrilho, M.R.; Daronch, M.; Rueggeberg, F.A. Microtensile Bond Strength of Adhesive Systems to Dentin with or without Application of an Intermediate Flowable Resin Layer. Braz. Dent. J. 2008, 19, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Stockton, L.W.; Tsang, S.T. Microleakage of Class II posterior composite restorations with gingival margins placed entirely within dentin. J. Can. Dent. Assoc. 2007, 73, 255. [Google Scholar]
- Hausdörfer, T.; Lechte, C.; Kanzow, P.; Rödig, T.; Wiegand, A. Periodontal health in teeth treated with deep-margin-elevation and CAD/CAM partial lithium disilicate restorations—A prospective controlled trial. Clin. Oral Investig. 2024, 28, 670. [Google Scholar] [CrossRef]
- Adson, O.; Sarıkaya, T.Y.; Şükran, B. Margin Elevation for Posterior Indirect Restorations: 6-Month Clinical Outcomes. Int. Dent. J. 2024, 74 (Suppl. 1), S176. [Google Scholar] [CrossRef]
- Gözetici-Çil, B.; Öztürk-Bozkurt, F.; Genç-Çalışkan, G.; Yılmaz, B.; Aksaka, N.; Özcan, M. Clinical Performance of Posterior Indirect Resin Composite Restorations with the Proximal Box Elevation Technique: A Prospective Clinical Trial up to 3 Years. J. Adhes. Dent. 2024, 26, 19–30. [Google Scholar] [CrossRef]
- Aziz, A.M.; Suliman, S.; Sulaiman, T.A.; Abdulmajeed, A. Clinical and Radiographical Evaluation of CAD-CAM Crowns with and without Deep Margin Elevation: 10-Year Results. J. Prosthet. Dent. 2024, in press. [Google Scholar] [CrossRef]
- Cieplik, F.; Hiller, K.A.; Buchalla, W.; Federlin, M.; Scholz, K.J. Randomized clinical split-mouth study on a novel self-adhesive bulk-fill restorative vs. a conventional bulk-fill composite for restoration of class II cavities—Results after three years. J. Dent. 2022, 125, 104275. [Google Scholar] [CrossRef] [PubMed]
- Muscholl, C.; Zamorska, N.; Schoilew, K.; Sekundo, C.; Meller, C.; Büsch, C.; Wolff, D.; Frese, C. Retrospective Clinical Evaluation of Subgingival Composite Resin Restorations with Deep-Margin Elevation. J. Adhes. Dent. 2022, 24, 335–344. [Google Scholar] [CrossRef] [PubMed]
- Bertoldi, C.; Monari, E.; Cortellini, P.; Generali, L.; Lucchi, A.; Spinato, S.; Zaffe, D. Clinical and histological reaction of periodontal tissues to subgingival resin composite restorations. Clin. Oral Investig. 2020, 24, 1001–1011. [Google Scholar] [CrossRef]
- Bresser, R.A.; Gerdolle, D.; van den Heijkant, I.A.; Sluiter-Pouwels, L.M.A.; Cune, M.S.; Gresnigt, M.M.M. Up to 12 years clinical evaluation of 197 partial indirect restorations with deep margin elevation in the posterior region. J. Dent. 2019, 91, 103227. [Google Scholar] [CrossRef]
- Oppermann, R.V.; Gomes, S.C.; Cavagni, J.; Cayana, E.G.; Conceição, E.N. Response to proximal restorations placed either subgingivally or following crown lengthening in patients with no history of periodontal disease. Int. J. Periodontics Restor. Dent. 2016, 36, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Ismail, H.S.; Ali, A.I.; Mehesen, R.E.; Juloski, J.; Garcia-Godoy, F.; Mahmoud, S.H. Deep proximal margin rebuilding with direct esthetic restorations: A systematic review of marginal adaptation and bond strength. Restor. Dent. Endod. 2022, 47, e15. [Google Scholar] [CrossRef]
- Plotino, G.; Abella Sans, F.; Duggal, M.S.; Grande, N.M.; Krastl, G.; Nagendrababu, V.; Gambarini, G. Present Status and Future Directions: Surgical Extrusion, Intentional Replantation and Tooth Autotransplantation. Int. Endod. J. 2022, 55 (Suppl. 3), 827–842. [Google Scholar] [CrossRef]
- Juloski, J.; Köken, S.; Ferrari, M. Cervical Margin Relocation in Indirect Adhesive Restorations: A Literature Review. J. Prosthodont. Res. 2018, 62, 273–280. [Google Scholar] [CrossRef]
- Sarfati, A.; Tirlet, G. Deep Margin Elevation versus Crown Lengthening: Biologic Width Revisited. Int. J. Esthet. Dent. 2018, 13, 334–356. [Google Scholar] [PubMed]
- Dablanca-Blanco, A.B.; Blanco-Carrión, J.; Martín-Biedma, B.; Varela-Patiño, P.; Bello-Castro, A.; Castelo-Baz, P. Management of Large Class II Lesions in Molars: How to Restore and When to Perform Surgical Crown Lengthening? Restor. Dent. Endod. 2017, 42, 240–252. [Google Scholar] [CrossRef]
- Veneziani, M. Adhesive Restorations in the Posterior Area with Subgingival Cervical Margins: New Classification and Differentiated Treatment Approach. Eur. J. Esthet. Dent. 2010, 5, 50–76. [Google Scholar]
- Padbury, A., Jr.; Eber, R.; Wang, H.L. Interactions between the gingiva and the margin of restorations. J. Clin. Periodontol. 2003, 30, 379–385. [Google Scholar] [CrossRef]
- Butt, A. Cervical margin relocation and indirect restorations: Case report and literature review. Dent. Update 2021, 48, 93–97. [Google Scholar] [CrossRef]
- Ghezzi, C.; Brambilla, G.; Conti, A.; Dosoli, R.; Ceroni, F.; Ferrantino, L. Cervical margin relocation: Case series and new classification system. Int. J. Esthet. Dent. 2019, 14, 272–284. [Google Scholar] [PubMed]
- Wu, F.; Su, X.; Shi, Y.; Bai, J.; Feng, J.; Sun, X.; Wang, X.; Wang, H.; Wen, J.; Kang, J. Comparison of the Biomechanical Effects of the Post-Core Crown, Endocrown and Inlay Crown after Deep Margin Elevation and Its Clinical Significance. BMC Oral Health 2024, 24, 990. [Google Scholar] [CrossRef]
- Mahmoudi Yamchi, F.; Abbasi, M.; Atri, F.; Ahmadi, E. Influence of Deep Margin Elevation Technique with Two Restorative Materials on Stress Distribution of e.max Endocrown Restorations: A Finite Element Analysis. Int. J. Dent. 2024, 2024, 6753069. [Google Scholar] [CrossRef]
- Magne, P.; Mori Ubaldini, A.L. Thermal and bioactive optimization of a unidose 3-step etch-and-rinse dentin adhesive. J. Prosthet. Dent. 2020, 124, 487.e1–487.e7. [Google Scholar] [CrossRef]
- Kuper, N.K.; Opdam, N.J.; Bronkhorst, E.M.; Huysmans, M.C. The influence of approximal restoration extension on the development of secondary caries. J. Dent. 2012, 40, 241–247. [Google Scholar] [CrossRef]
- Amesti-Garaizabal, A.; Agustín-Panadero, R.; Verdejo-Solá, B.; Fons-Font, A.; Fernández-Estevan, L.; Montiel-Company, J.; Solá-Ruíz, M.F. Fracture resistance of partial indirect restorations made with CAD/CAM technology: A systematic review and meta-analysis. J. Clin. Med. 2019, 8, 1932. [Google Scholar] [CrossRef]
- Ölçer Us, Y.; Aydınoğlu, A.; Erşahan, Ş.; Erdem Hepşenoğlu, Y.; Sağır, K.; Üşümez, A. A Comparison of the Effects of Incremental and Snowplow Techniques on the Mechanical Properties of Composite Restorations. Aust. Dent. J. 2024, 69, 40–48. [Google Scholar] [CrossRef]
- Guo, J.; Wang, Z.; Li, X.; Sun, C.; Gao, E.; Li, H. A Comparison of the Fracture Resistances of Endodontically Treated Mandibular Premolars Restored with Endocrowns and Glass Fiber Post-Core Retained Conventional Crowns. J. Adv. Prosthodont. 2016, 8, 489–493. [Google Scholar] [CrossRef] [PubMed]
- Van Dijken, J.W.; Sjöström, S.; Wing, K. The Effect of Different Types of Composite Resin Fillings on Marginal Gingiva. J. Clin. Periodontol. 1987, 14, 185–189. [Google Scholar] [CrossRef] [PubMed]
- Reichardt, E.; Krug, R.; Bornstein, M.M.; Tomasch, J.; Verna, C.; Krastl, G. Orthodontic Forced Eruption of Permanent Anterior Teeth with Subgingival Fractures: A Systematic Review. Int. J. Environ. Res. Public Health 2021, 18, 12580. [Google Scholar] [CrossRef] [PubMed]
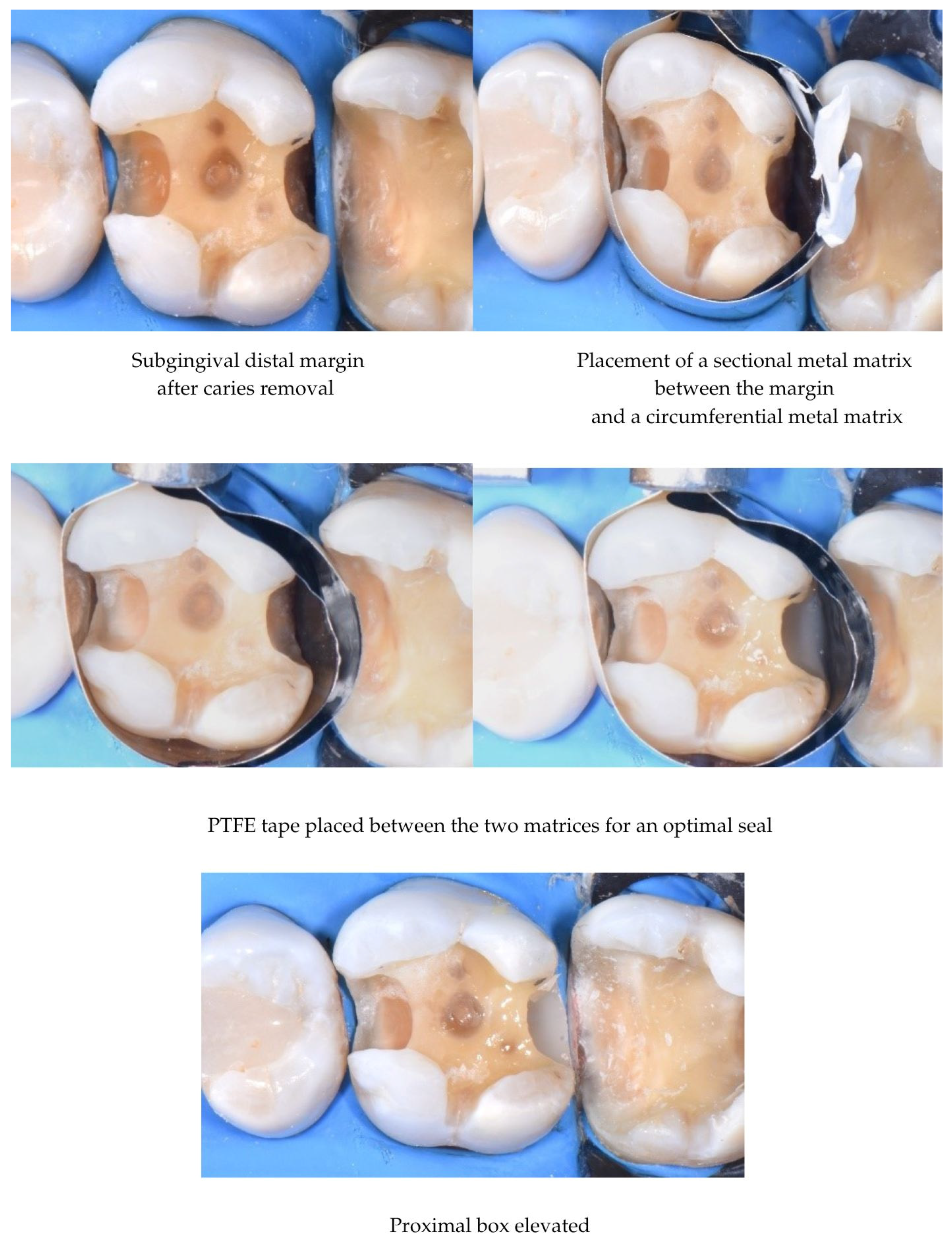
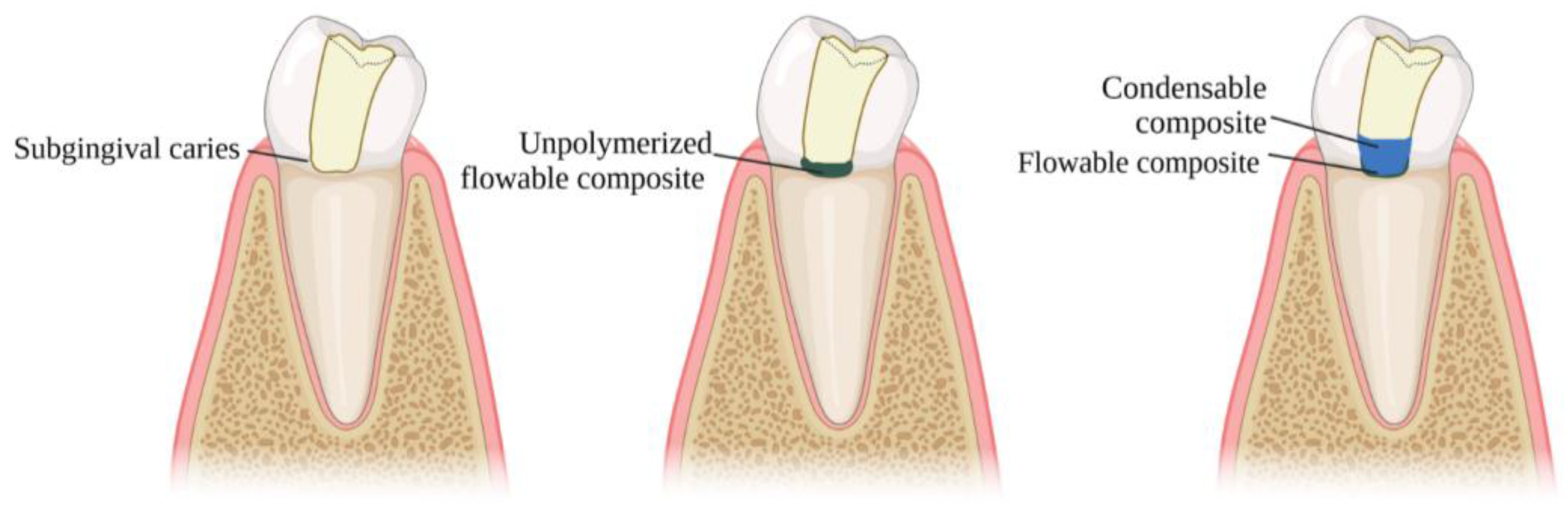
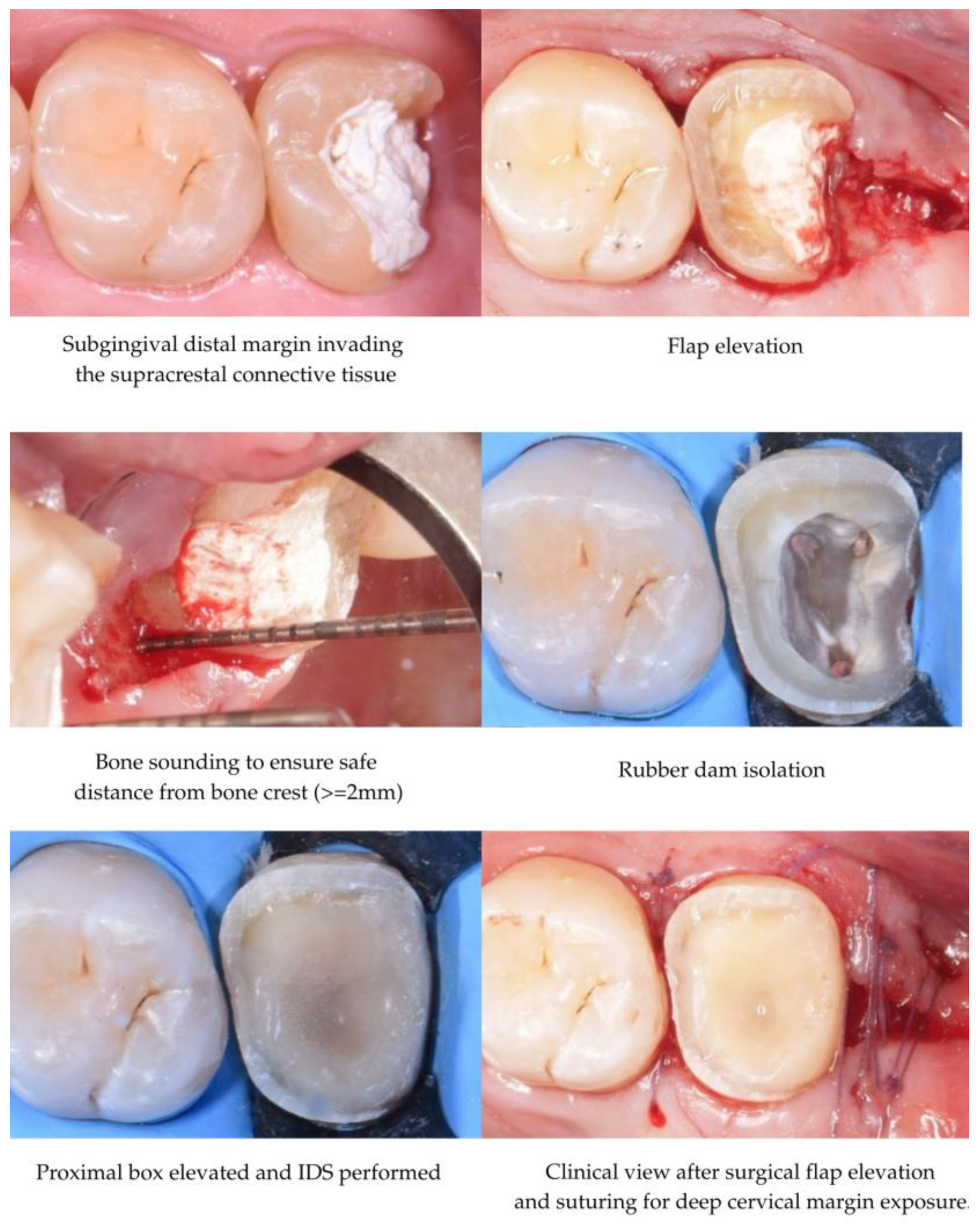
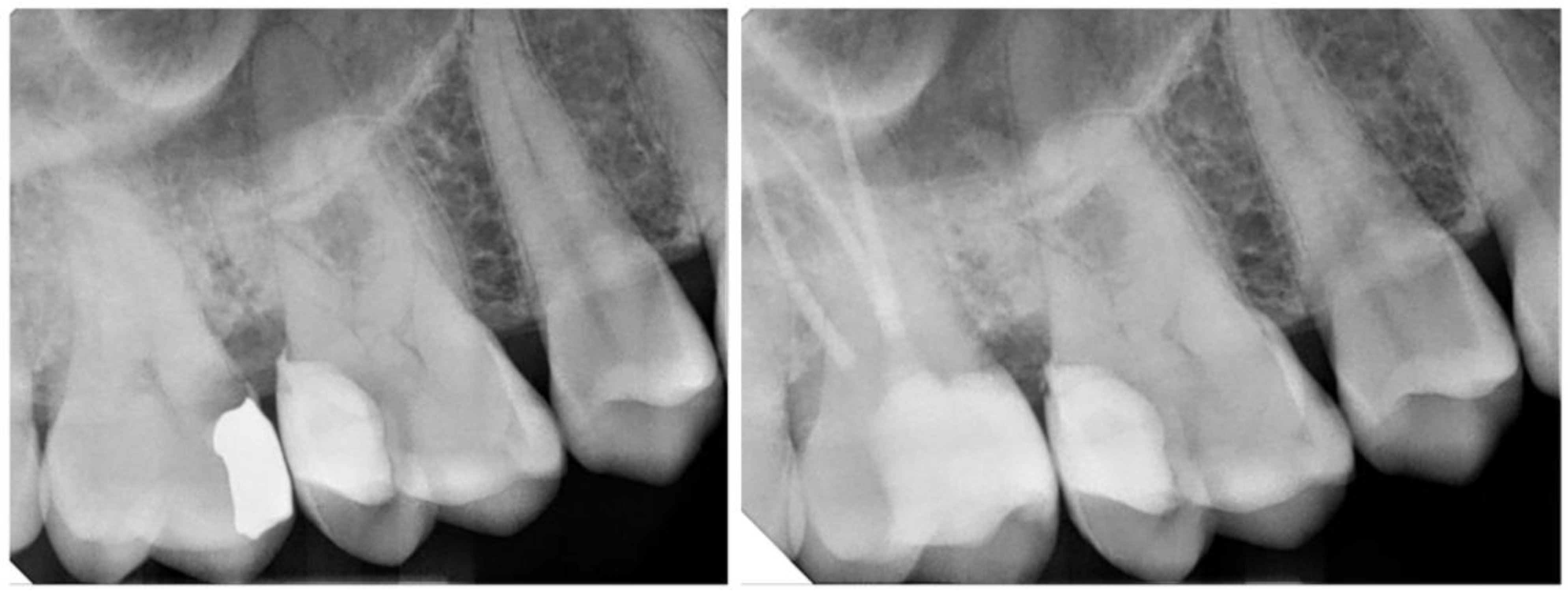
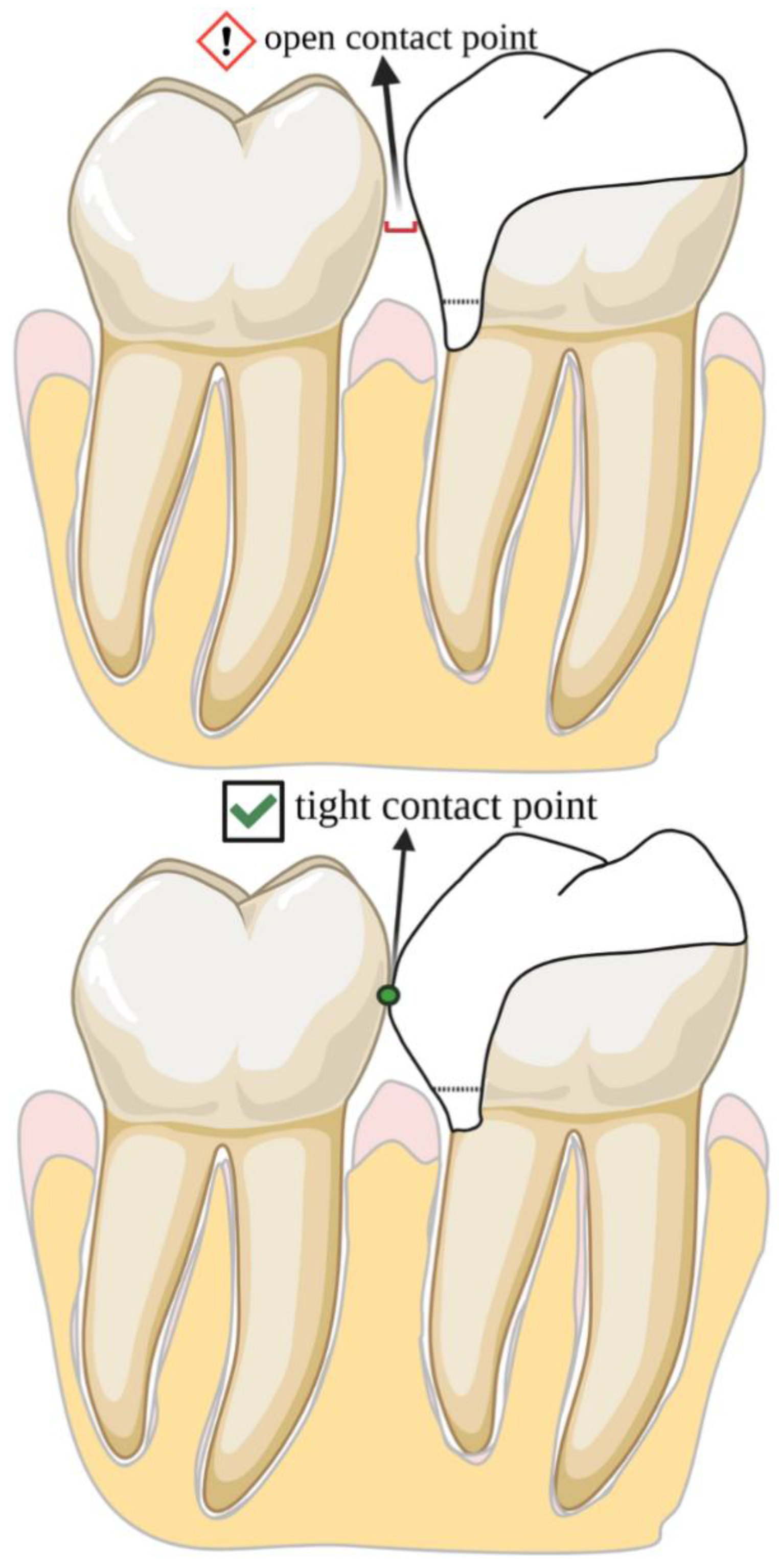
| Author(s), Year | Study Type | Materials/Methods | Aging Simulation/Means of Evaluation | Groups Compared | Key Findings |
|---|---|---|---|---|---|
| 1. Palma et al., 2025 [3] | In vitro study | Two-step self-etch adhesive system | Storage at 37 °C in distilled water for 1 month | Direct vs. semi-direct composite restoration | Semi-direct showed better or equal bond strength |
| 2. Daghrery et al., 2025 [31] | In vitro study | Lithium disilicate CAD/CAM crowns + composite DME | Thermal aging, SEM analysis | DME vs. no DME | Marginal gaps increased after aging |
| 3. Balci et al., 2024 [32] | In vitro study | DME with bulk-fill, conventional and fiber-reinforced composites | Fracture strength test, thermocycling (10,000 cycles) | Various composites for DME | Fiber-reinforced showed highest fracture resistance |
| 4. Ismail et al., 2024 [33] | In vitro study | Universal adhesives, different curing systems | Thermocycling & water storage (6 months), μTBS testing | Light-cure vs. self-cure | Light-curing better; aging reduced performance |
| 5. Baldi et al., 2024 [34] | In vitro micro-CT | Highly filled vs. conventional flowable composites for DME | Micro-CT, thermocycling (5000 cycles) | Flowable types | Highly filled flowables showed better adaptation, fewer voids |
| 6. François et al., 2024 [35] | In vitro study | Highly filled flowable composites | Flexural strength, wear resistance, SEM (no thermal aging) | 5 materials tested | Material selection critical for DME base layer |
| 7. Bresser et al., 2024 [36] | In vitro study | Direct composite, lithium disilicate onlay, endocrown (all w/DME) | Mechanical testing, thermocycling, cyclic loading | Three types of restoration | Endocrowns with DME may offer superior biomechanics |
| 8. Reddy et al., 2024 [37] | In vitro study | Flowable vs. incremental DME technique | SEM analysis | Bulk-fill flowable vs. incremental | Incremental technique had less microleakage, better margins |
| 9. Baldi et al., 2023 [38] | In vitro study | Low, medium and high viscosity flowable composites | MicroCT comparison | Flowable viscosity levels | Medium-viscosity had best adaptation and minimized voids |
| 10. Fichera et al., 2023 [6] | In vitro study | Composite resins for peripheral build-ups | Not applicable | Novel vs. traditional peripheral build-up | Novel method improved seal and strength |
| 11. Vichitgomen et al., 2021 [39] | In vitro study | Composite vs. RMGIC for proximal box elevation | Dye penetration, thermocycling (10,000 cycles) | Composite DME vs. RMGIC vs. control | Composite showed better marginal sealing |
| 12. Zhang et al., 2021 [40] | In vitro study | Lithium disilicate endocrown with DME | Microleakage, fracture resistance, thermocycling (10,000 cycles) | DME vs. no DME | DME improved seal, similar fracture resistance |
| 13. Juloski et al., 2020 [41] | In vitro study | Resin composite DME with CAD/CAM inlays | No aging simulation | SEM vs. dye penetration methods | No correlation between methods |
| 14. Da Silva Gonçalves et al., 2017 [24] | In vitro study | Adper Single Bond 2 adhesive | Immediate bond strength test | Inlays with vs. without DME | DME improved bond strength significantly |
| 15. Jawaed et al., 2016 [42] | In vitro study | Not specified | Dye penetration | DME vs. no DME | Adhesive system important for cervical margin sealing |
| 16. Spreafico et al., 2016 [21] | In vitro study | Composite for cervical margin relocation under CAD/CAM crowns | Microscopic marginal quality assessment | Composite relocation vs. control | Composite improved marginal quality |
| 17. Frankenberger et al., 2013 [43] | In vitro study | Composite-based DME, ceramic inlay | Thermocycling (5000 cycles), SEM | Inlays with vs. without DME | DME improved marginal quality in dentin |
| 18. de Mattos Pimenta Vidal et al., 2013 [44] | In vitro study | Composite, RMGIC, open sandwich technique | Thermocycling, μTBS, nanoleakage | Direct resin, open sandwich, indirect inlay | Open sandwich had less nanoleakage; indirect inlay stronger |
| 19. Lefever et al., 2012 [45] | In vitro study | Various DME materials with self-etch adhesive | SEM marginal adaptation (no aging) | Flowable vs. packable for different inlays | DME effective, but performance material-dependent |
| 20. Roggendorf et al., 2012 [46] | In vitro study | Ceramic inlay over composite DME | Thermocycling (5000 cycles), SEM | With vs. without DME | DME enhanced marginal adaptation |
| 21. De Goes et al., 2008 [47] | In vitro study | Intermediate flowable resin layer | μTBS test (no thermal aging) | With vs. without flowable layer | Flowable layer improved bond strength in some systems |
| 22. Stockton et al., 2007 [48] | In vitro study | Composite resin, bonding agent not specified | Dye penetration | Not applicable | Difficulty sealing dentin margins; bonding system quality is crucial |
| 23. Loguercio et al., 2002 [20] | In vitro study | Class II restorations, various bonding techniques | Dye penetration | Total-etch bonding vs. open sandwich | Sandwich technique reduced microleakage |
| Author, Year | Type of Study | Materials/Methods | Aging Simulation/Means of Evaluation | Groups Compared | Key Findings |
|---|---|---|---|---|---|
| 1. Hausdörfer et al., 2024 [49] | Prospective clinical trial | DME with composite, CAD/CAM lithium disilicate | Periodontal assessment after 12 months | Lithium disilicate indirect partial coverage restoration with DME vs. without DME | No significant differences in periodontal health between DME and non-DME groups |
| 2. Adson et al., 2024 [50] | Clinical trial | Composite-based margin elevation under indirect restorations | 6-month clinical follow-up | DME vs. non-DME groups in indirect restorations | DME is clinically acceptable in short-term; preserves tooth structure |
| 3. Gözetici-Çil et al., 2024 [51] | Prospective clinical trial | Indirect resin composite & proximal box elevation | 6-month intervals, FDI criteria, no artificial aging | Posterior indirect composite restorations with DME | DME shows predictable outcomes in posterior restorations up to 3 years |
| 4. Aziz et al., 2024 [52] | Long-term clinical & radiographic study | DME under CAD/CAM ceramic crowns | Clinical and radiographic evaluation over 10 years | CAD-CAM crowns with vs. without DME | DME is a viable long-term option for subgingival margins in full crowns |
| 5. Cieplik et al., 2022 [53] | 3-year randomized clinical test | Self-adhesive bulk fill vs. Filtek Bulk Fill with Scotchbond Universal | Clinical evaluation (USPHS); 3-year recall | Novel self-adhesive bulk fill vs. conventional bulk fill composite + adhesive | Self-adhesive bulk fills perform acceptably, but conventional systems offer better marginal aesthetics |
| 6. Muscholl et al., 2022 [54] | Retrospective clinical study | Composite-based DME | Retrospective analysis, up to 5 years clinical follow-up | Subgingival Class II composite restorations with DME | DME with composite performs reliably in the long term |
| 7. Bertoldi et al., 2020 [55] | Clinical & histological Study | Composite-based DME | Clinical evaluation | Subgingival composite vs. supragingival margins | Subgingival restorations caused a mild inflammatory response but no attachment loss when finished and polished |
| 8. Bresser et al., 2019 [56] | Long-term clinical evaluation | Indirect restorations with DME; composite for DME & ceramic/composite onlay | Clinical follow-up; USPHS criteria | Single group | DME with indirect restorations can yield high longevity over 12 years |
| 9. Ferrari et al., 2018 [25] | Controlled clinical study | Evaluation of periodontal parameters around restorations with and without cervical margin relocation | 12 month follow-up | Teeth with cervical margin relocation (CMR) vs. control (no CMR) | No significant detrimental effects on periodontal health from CMR over 12 months |
| 10. Oppermann et al., 2016 [57] | Clinical trial | Composite-based restoration in both groups | Periodontal assessment over 12 months | Subgingival restoration vs. crown lengthening + supragingival margin | Subgingival restorations showed slightly more inflammation but no significant attachment loss |
| 11. Andersson et al., 2004 [23] | Clinical study | Open-sandwich restorations using resin-modified glass ionomer cement in Class II cavities | 6-years follow-up | N/A | Supports longevity of RMGIC in open-sandwich technique |
| Authors | Type of Study | Materials/ Methods | Aging Simulation/Means of Evaluation | Groups Compared | Key Findings |
|---|---|---|---|---|---|
| 1. Sadeghnezhad et al., 2024 [1] | Systematic Review | Adhesives/composites for DME | N/A | DME vs. non-DME | DME reduces microleakage compared to non-DME |
| 2. Ismail et al., 2024 [26] | Literature Review | Clinical protocols and case selection | Literature review | N/A | Highlights the need for clear guidelines and careful case selection |
| 3. Alizadeh et al., 2024 [29] | Review & Clinical Commentary | Indications and periodontal considerations | N/A | N/A | Emphasizes respecting periodontal tissues during DME |
| 4. Alam et al., 2024 [4] | Case Report & Review | Surgical and restorative materials | N/A | N/A | Proper management avoids biologic width violation |
| 5. Chun et al., 2023 [17] | Systematic Review | DME procedures and periodontal parameters | N/A | Various DME materials vs. controls | DME generally safe for periodontal health; sometimes improves it |
| 6. Eggman et al., 2023 [30] | Systematic Review | Adhesive systems for subgingival margins | N/A | N/A | DME is a conservative alternative to surgery |
| 7. Felemban et al., 2023 [5] | Systematic Review | Various DME materials | Clinical data | DME vs. no DME (periodontal health) | No adverse effects on periodontium when properly performed |
| 8. Samartzi et al., 2022 [28] | Literature Review | Review of clinical and lab studies | N/A | N/A | DME improves restorative success, alternative to crown lengthening |
| 9. Ismail et al., 2022 [58] | Systematic Review | Bonding agents in DME | Thermocycling, μTBS, SEM | Various DME protocols | Satisfactory adaptation and bonding with proper adhesive technique |
| 10. Aldakheel et al., 2022 [2] | Narrative Review | Adhesives/composites for DME | N/A | Conceptual analysis | DME is effective with proper case selection |
| 11. Plotino et al., 2022 [59] | Literature Review | Surgical extrusion, replantation, transplantation | Literature-based review | Surgical extrusion vs. replantation vs. transplantation | Surgical extrusion can be viable when DME or crown lengthening is not |
| 12. Juloski et al., 2018 [60] | Narrative Review | Composite DME with adhesive indirect restorations | Thermocycling and fatigue (in included studies) | Various DME protocols and materials | DME generally effective if executed properly |
| 13. Sarfati et al., 2018 [61] | Narrative Review | DME and supracrestal tissue attachment | Literature analysis | DME vs. crown lengthening | DME avoids surgery if tissue management is respected |
| 14. Dablanca-Blanco et al., 2017 [62] | Clinical Guideline & Review | Class II direct/indirect restorations with/without crown lengthening | Review with clinical criteria | Restorative vs. surgical margin control | DME feasible if isolation and tissue health are adequate |
| 15. Mugri et al., 2021 [19] | Systematic Review | DME or crown lengthening | N/A | Crown lengthening vs. DME | DME is conservative and effective vs. crown lengthening |
| 16. Frese et al., 2014 [27] | Clinical Technique Description & Critical Literature Review | Presentation of the R2-technique for proximal box elevation | N/A | N/A | Supports resin composite PBE to preserve biological width and periodontal health |
| 17. Bazos & Magne, 2014 [11] | Review | Biomimetic restorative materials | N/A | Only conceptual | Biomimetic approaches improve esthetics and longevity |
| 18. Magne & Spreafico, 2012 [14] | Narrative Review | Composite, 4th gen bonding agent | N/A | N/A | Introduced the DME concept as a restorative alternative to crown lengthening |
| 19. Veneziani et al., 2010 [63] | Narrative Review with Protocol | Subgingival margin classification (Class I–IV) | Clinical examples | Subgingival margin classes | Classification system guides decision-making |
| 20. Padbury et al., 2003 [64] | Narrative Review | N/A | N/A | N/A | Margins violating STA (biologic width) cause inflammation and attachment loss |
| Authors | Type of Study | Materials/Methods | Aging Simulation/Means of Evaluation | Groups Compared | Key Findings |
|---|---|---|---|---|---|
| 1. Butt et al., 2021 [65] | Case report & Narrative Review | Composite DME & ceramic onlay | Clinical follow-up (no artificial aging) | Single case (DME with indirect ceramic onlay) & literature context | DME is a viable solution when isolation is achievable and biological width is respected |
| 2. Ghezzi et al., 2019 [66] | Case series and classification proposal | Composite-based DME | Clinical follow-up | Clinical cases with DME | Proposed a new classification system for DME based on margin location and tissue type |
| Authors | Type of Study | Materials/Methods | Aging Simulation/Means of Evaluation | Groups Compared | Key Findings |
|---|---|---|---|---|---|
| 1. Wu et al., 2024 [67] | Finite element analysis | Biomechanical comparison of restorative designs after DME | 3D modeling and stress distribution analysis | Endocrown, inlay crown, and post-core crown after DME | Endocrowns after DME may be biomechanically more favorable than traditional post-core designs |
| 2. Mahmoudi Yamchi et al., 2024 [68] | Finite element analysis | DME with composite vs. resin-modified glass ionomer under e.max endocrowns | 3D finite element stress distribution analysis | DME with composite vs. RMGIC | Biomechanically, supports the use of composite over RMGIC as DME base under endocrowns biomechanically |
| 3. Dietschi & Spreafico, 1998 [13] | Clinical technique paper | Adhesive cementation with viscous, densely filled resin cements; layering approach | N/A | N/A | One of the earliest descriptions of a stratified adhesive cementation approach; highlights clinical limitations of earlier adhesive systems and provisionalization methods |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karageorgiou, A.; Fostiropoulou, M.; Antoniadou, M.; Pappa, E. Deep Margin Elevation: Current Evidence and a Critical Approach to Clinical Protocols—A Narrative Review. Adhesives 2025, 1, 10. https://doi.org/10.3390/adhesives1030010
Karageorgiou A, Fostiropoulou M, Antoniadou M, Pappa E. Deep Margin Elevation: Current Evidence and a Critical Approach to Clinical Protocols—A Narrative Review. Adhesives. 2025; 1(3):10. https://doi.org/10.3390/adhesives1030010
Chicago/Turabian StyleKarageorgiou, Athanasios, Maria Fostiropoulou, Maria Antoniadou, and Eftychia Pappa. 2025. "Deep Margin Elevation: Current Evidence and a Critical Approach to Clinical Protocols—A Narrative Review" Adhesives 1, no. 3: 10. https://doi.org/10.3390/adhesives1030010
APA StyleKarageorgiou, A., Fostiropoulou, M., Antoniadou, M., & Pappa, E. (2025). Deep Margin Elevation: Current Evidence and a Critical Approach to Clinical Protocols—A Narrative Review. Adhesives, 1(3), 10. https://doi.org/10.3390/adhesives1030010







