Technology Landscape Review of In-Sensor Photonic Intelligence: From Optical Sensors to Smart Devices
Abstract
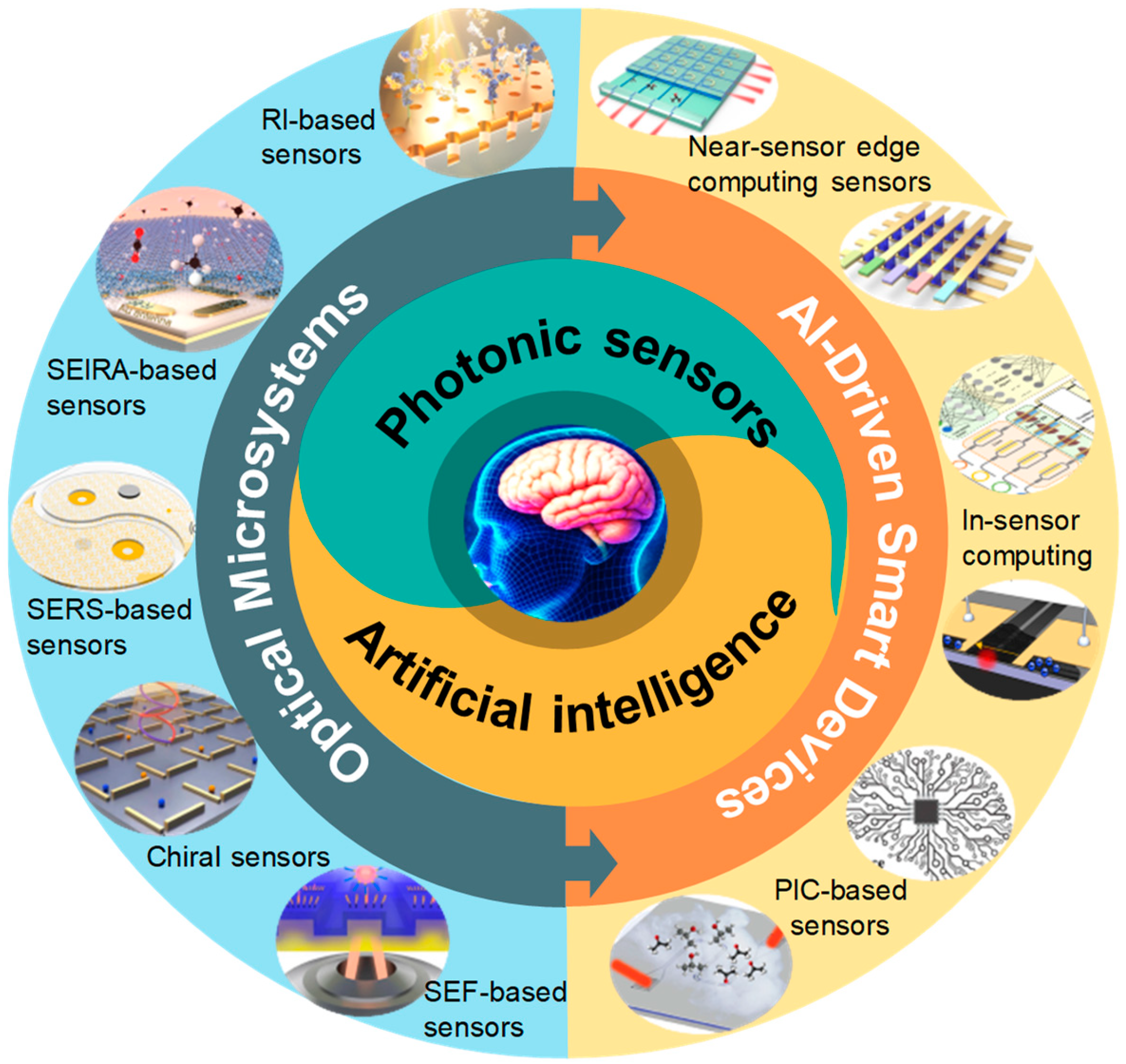
1. Introduction
2. Basic Sensing Technologies
2.1. Mechanism of RI Sensing
2.2. Sensing Mechanism of Surface-Enhanced Infrared Absorption (SEIRA) Spectroscopy
2.3. Sensing Mechanism of Surface-Enhanced Raman Spectroscopy (SERS)
2.4. Sensing Mechanism of Surface Plasmon-Enhanced Chiral Spectroscopy
2.5. Sensing Mechanism of Surface-Enhanced Fluorescence (SEF) Spectroscopy
3. Applications of Optical Sensors
3.1. Refractive Index Sensing

3.2. Surface-Enhanced Infrared Absorption Spectroscopy

3.3. Surface-Enhanced Raman Spectroscopy
3.4. Surface Plasmon-Enhanced Chiral Spectroscopy

3.5. Surface-Enhanced Fluorescence Spectroscopy

4. Photonic Integrated Circuits and In-Sensor Computing
4.1. Photonic Integrated Circuits (PICs)

4.2. In-Sensor Computing


5. Challenges, Conclusions and Outlook
5.1. Challenges
5.2. Conclusions and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AI | Artificial Intelligence |
| PIC | Photonic integrated circuits |
| SEIRA | Surface-enhanced infrared absorption |
| RI | Refractive index |
| SERS | Surface-enhanced Raman spectroscopy |
| SEF | Surface-enhanced fluorescence |
| CD | Circular dichroism |
| ELISA | Enzyme-linked immunosorbent assays |
| PSA | prostate-specific antigen |
| LSPR | Localized surface plasmon resonance |
References
- Zhou, H.; Li, D.; Lv, Q.; Lee, C. Integrative plasmonics: Optical multi-effects and acousto-electric-thermal fusion for biosensing, energy conversion, and photonic circuits. Chem. Soc. Rev. 2025, 54, 5342–5432. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Liu, W.; Chen, H.; Wang, C.; Tan, Q.; Mi, R.; Wang, R.; Zhou, Y.; Xing, E.; Tang, J.; et al. High-precision ethanol concentration microsensor with global spectra aided by the multi-layer perceptron. Opt. Express 2024, 32, 42983. [Google Scholar] [CrossRef]
- Xiao, Z.; Liu, W.; Xu, S.; Zhou, J.; Ren, Z.; Lee, C. Recent Progress in Silicon-Based Photonic Integrated Circuits and Emerging Applications. Adv. Opt. Mater. 2023, 11, 2301028. [Google Scholar] [CrossRef]
- Zhuge, Y.; Ren, Z.; Xiao, Z.; Zhang, Z.; Liu, X.; Liu, W.; Xu, S.; Ho, C.P.; Li, N.; Lee, C. Photonic Bayesian Neural Networks: Leveraging Programmable Noise for Robust and Uncertainty-Aware Computing. Adv. Sci. 2025, 12, e2500525. [Google Scholar] [CrossRef]
- Zhou, J.; Liu, X.; Zhou, H.; Xu, S.; Xie, J.; Xu, C.; Liu, W.; Zhang, Z.; Lee, C. Artificial-Intelligence-Enhanced Mid-infrared Lab-on-a-Chip for Mixture Spectroscopy Analysis. Laser Photonics Rev. 2024, 19, 2400754. [Google Scholar] [CrossRef]
- Lialiou, P.; Maglogiannis, I. Students’ Burnout Symptoms Detection Using Smartwatch Wearable Devices: A Systematic Literature Review. AI Sens. 2025, 1, 2. [Google Scholar] [CrossRef]
- Zhou, H.; Xu, L.; Ren, Z.; Zhu, J.; Lee, C. Machine learning-augmented surface-enhanced spectroscopy toward next-generation molecular diagnostics. Nanoscale Adv. 2023, 5, 538–570. [Google Scholar] [CrossRef]
- Wang, C.; He, T.; Zhou, H.; Zhang, Z.; Lee, C. Artificial intelligence enhanced sensors—Enabling technologies to next-generation healthcare and biomedical platform. Bioelectron. Med. 2023, 9, 17. [Google Scholar] [CrossRef]
- Zhou, H.; Li, D.; He, X.; Hui, X.; Guo, H.; Hu, C.; Mu, X.; Wang, Z.L. Bionic Ultra-Sensitive Self-Powered Electromechanical Sensor for Muscle-Triggered Communication Application. Adv. Sci. 2021, 8, e2101020. [Google Scholar] [CrossRef]
- Nguyen, T.Q.; Prima, O.D.A.; Irfan, S.A.; Purnomo, H.D.; Tanone, R. CORE-ReID V2: Advancing the Domain Adaptation for Object Re-Identification with Optimized Training and Ensemble Fusion. AI Sens. 2025, 1, 4. [Google Scholar] [CrossRef]
- Rabinovitch, A.; Baruch, E.B.; Siton, M.; Avital, N.; Yeari, M.; Malka, D. Efficient Detection of Mind Wandering During Reading Aloud Using Blinks, Pitch Frequency, and Reading Rate. AI 2025, 6, 83. [Google Scholar] [CrossRef]
- Leng, T.; Li, L.; Lee, C. Journal Editorial: Welcome to the New Era of AI-Enabled Sensing. AI Sens. 2025, 1, 1. [Google Scholar] [CrossRef]
- Yi, X.; Tian, X.; Zhou, L.; Li, L.; Nguyen, L.; Minasian, R. Integrated Microresonator-Based Microwave Photonic Sensors Assisted by Machine Learning. J. Light. Technol. 2024, 42, 4271–4280. [Google Scholar] [CrossRef]
- Tao, Z.; You, J.; Ouyang, H.; Yan, Q.; Du, S.; Zhang, J.; Jiang, T. Silicon photonic convolution operator exploiting on-chip nonlinear activation function. Opt. Lett. 2025, 50, 582–585. [Google Scholar] [CrossRef]
- Wu, N.; Sun, Y.; Hu, J.; Yang, C.; Bai, Z.; Wang, F.; Cui, X.; He, S.; Li, Y.; Zhang, C.; et al. Intelligent nanophotonics: When machine learning sheds light. eLight 2025, 5, 5. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, X.; Zhou, H.; Xu, S.; Lee, C. Advances in Machine-Learning Enhanced Nanosensors: From Cloud Artificial Intelligence Toward Future Edge Computing at Chip Level. Small Struct. 2023, 5, 2300325. [Google Scholar] [CrossRef]
- Dong, B.; Aggarwal, S.; Zhou, W.; Ali, U.E.; Farmakidis, N.; Lee, J.S.; He, Y.; Li, X.; Kwong, D.-L.; Wright, C.D.; et al. Higher-dimensional processing using a photonic tensor core with continuous-time data. Nat. Photonics 2023, 17, 1080–1088. [Google Scholar] [CrossRef]
- Sun, Z.; He, T.; Ren, Z.; Wang, C.; Liu, X.; Zhang, Z.; Zhou, J.; Guo, X.; Yang, Y.; Lee, C. Moving Toward Human-Like Perception and Sensation Systems—From Integrated Intelligent Systems to Decentralized Smart Devices. SmartSys 2025, 1, e4. [Google Scholar] [CrossRef]
- Janneh, M.; De Marcellis, A.; Palange, E.; Rizza, C.; Ciattoni, A.; Mengali, S. Modelling of Nanoantenna-Based Optical Sensors for High-Sensitivity High-Resolution Infrared Spectroscopy of Chemical Compounds. Sensors 2015, 319, 109–113. [Google Scholar]
- Lowry, T.W.; Prommapan, P.; Rainer, Q.; Van Winkle, D.; Lenhert, S. Lipid Multilayer Grating Arrays Integrated by Nanointaglio for Vapor Sensing by an Optical Nose. Sensors 2015, 15, 20863–20872. [Google Scholar] [CrossRef]
- Swargiary, K.; Jitpratak, P.; Pathak, A.K.; Viphavakit, C. Low-Cost ZnO Spray-Coated Optical Fiber Sensor for Detecting VOC Biomarkers of Diabetes. Sensors 2023, 23, 7916. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Yang, C.; Hu, D.; Li, D.; Hui, X.; Zhang, F.; Chen, M.; Mu, X. Terahertz biosensing based on bi-layer metamaterial absorbers toward ultra-high sensitivity and simple fabrication. Appl. Phys. Lett. 2019, 115, 143507. [Google Scholar] [CrossRef]
- Zhou, H.; Yang, C.; Hu, D.; Dou, S.; Hui, X.; Zhang, F.; Chen, C.; Chen, M.; Yang, Y.; Mu, X. Integrating a Microwave Resonator and a Microchannel with an Immunochromatographic Strip for Stable and Quantitative Biodetection. ACS Appl. Mater. Interfaces 2019, 11, 14630–14639. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Hu, D.; Yang, C.; Chen, C.; Ji, J.; Chen, M.; Chen, Y.; Yang, Y.; Mu, X. Multi-Band Sensing for Dielectric Property of Chemicals Using Metamaterial Integrated Microfluidic Sensor. Sci. Rep. 2018, 8, 14801. [Google Scholar] [CrossRef]
- Walden, S.L.; Poudel, P.; Zou, C.; Tanaka, K.; Paul, P.; Szeghalmi, A.; Siefke, T.; Pertsch, T.; Schacher, F.H.; Staude, I. Two-Color Spatially Resolved Tuning of Polymer-Coated Metasurfaces. ACS Nano 2024, 18, 5079–5088. [Google Scholar] [CrossRef]
- Luo, M.; Zhou, Y.; Zhao, X.; Guo, Z.; Li, Y.; Wang, Q.; Liu, J.; Luo, W.; Shi, Y.; Liu, A.Q.; et al. High-sensitivity optical sensors empowered by quasi-bound states in the continuum in a hybrid metal-dielectric metasurface. ACS Nano 2024, 18, 6477–6486. [Google Scholar] [CrossRef]
- Yang, Y.M.; Kravchenko, I.I.; Briggs, D.P.; Valentine, J. All-dielectric metasurface analogue of electromagnetically induced transparency. Nat. Commun. 2014, 5, 5753. [Google Scholar] [CrossRef]
- Spacková, B.; Wrobel, P.; Bocková, M.; Homola, J. Optical Biosensors Based on Plasmonic Nanostructures: A Review. Proc. IEEE 2016, 104, 2380–2408. [Google Scholar] [CrossRef]
- Otte, M.A.; Sepúlveda, B.; Ni, W.H.; Juste, J.P.; Liz-Marzán, L.M.; Lechuga, L.M. Identification of the Optimal Spectral Region for Plasmonic and Nanoplasmonic Sensing. ACS Nano 2010, 4, 349–357. [Google Scholar] [CrossRef]
- Zhou, H.; Li, D.; Ren, Z.; Xu, C.; Wang, L.-F.; Lee, C. Surface plasmons-phonons for mid-infrared hyperspectral imaging. Sci. Adv. 2024, 10, eado3179. [Google Scholar] [CrossRef]
- Li, D.; Zhou, H.; Ren, Z.; Lee, C. Advances in MEMS, Optical MEMS, and Nanophotonics Technologies for Volatile Organic Compound Detection and Applications. Small Sci. 2024, 5, 202400250. [Google Scholar] [CrossRef]
- Li, D.; Yadav, A.; Zhou, H.; Roy, K.; Thanasekaran, P.; Lee, C. Advances and Applications of Metal-Organic Frameworks (MOFs) in Emerging Technologies: A Comprehensive Review. Glob. Chall. 2024, 8, 2300244. [Google Scholar] [CrossRef] [PubMed]
- Miao, X.L.; Luk, T.S.; Liu, P.Q. Liquid-Metal-Based Nanophotonic Structures for High-Performance SEIRA Sensing. Adv. Mater. 2022, 34, 2107950. [Google Scholar] [CrossRef]
- Wagner, M.; Seifert, A.; Liz-Marzan, L.M. Towards multi-molecular surface-enhanced infrared absorption using metal plasmonics. Nanoscale Horiz. 2022, 7, 1259–1278. [Google Scholar] [CrossRef]
- Kozuch, J.; Ataka, K.; Heberle, J. Surface-enhanced infrared absorption spectroscopy. Nat. Rev. Methods Primers 2023, 3, 70. [Google Scholar] [CrossRef]
- Chen, C.; Liu, W.; Tian, S.; Hong, T. Novel Surface-Enhanced Raman Spectroscopy Techniques for DNA, Protein and Drug Detection. Sensors 2019, 19, 1712. [Google Scholar] [CrossRef]
- Mondol, A.S.; Patel, M.D.; Ruger, J.; Stiebing, C.; Kleiber, A.; Henkel, T.; Popp, J.; Schie, I.W. Application of High-Throughput Screening Raman Spectroscopy (HTS-RS) for Label-Free Identification and Molecular Characterization of Pollen. Sensors 2019, 19, 4428. [Google Scholar] [CrossRef]
- Park, S.; Lee, J.; Khan, S.; Wahab, A.; Kim, M. Machine Learning-Based Heavy Metal Ion Detection Using Surface-Enhanced Raman Spectroscopy. Sensors 2022, 22, 596. [Google Scholar] [CrossRef]
- Xu, X.; Zhang, W.-Y.; Ma, X.-Y.; Qin, X.; Jiang, T.-W.; Li, H.; Zhang, Y.; Jiang, K.; Cai, W.-B. Toward hyphenated in situ infrared and Raman spectroscopies in interfacial electrochemistry. Anal. Chem. 2025, 97, 1047–1053. [Google Scholar] [CrossRef]
- Martens, R.R.; Gozdzialski, L.; Newman, E.; Gill, C.; Wallace, B.; Hore, D.K. Optimized machine learning approaches to combine surface-enhanced Raman scattering and infrared data for trace detection of xylazine in illicit opioids. Analyst 2025, 150, 700–711. [Google Scholar] [CrossRef]
- Kang, H.; Lee, J.; Moon, J.; Lee, T.; Kim, J.; Jeong, Y.; Lim, E.K.; Jung, J.; Jung, Y.; Lee, S.J.; et al. Multiplex Detection of Foodborne Pathogens using 3D Nanostructure Swab and Deep Learning-Based Classification of Raman Spectra. Small 2024, 20, e2308317. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Sun, L.; Zhang, Q. Multicomponent chiral plasmonic hybrid nanomaterials: Recent advances in synthesis and applications. Nanoscale Adv. 2024, 6, 318–336. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Ren, Y.; He, M.; Qi, H. Nanoparticle manipulation based on chiral plasmon effects. Phys. Chem. Chem. Phys. 2024, 26, 17860–17868. [Google Scholar] [CrossRef] [PubMed]
- Biswas, A.; Cencillo-Abad, P.; Shabbir, M.W.; Karmakar, M.; Chanda, D. Tunable plasmonic superchiral light for ultrasensitive detection of chiral molecules. Sci. Adv. 2024, 10, eadk2560. [Google Scholar] [CrossRef]
- He, P.; Li, W.; Ji, H.; Chang, H.; Yu, Y. Supercritical-lens light-sheet fluorescence microscopy with sub-diffraction-limit axial resolution, enhanced FOV, and chip-scale illumination. Opt. Express 2025, 33, 10563–10573. [Google Scholar] [CrossRef]
- Zhang, S.; Fan, D.; Yan, Q.; Lu, Y.; Wu, D.; Fu, B.; Zhao, M. Single-molecule fluorescence imaging of photocatalytic nanomaterials. J. Mater. Chem. A 2024, 12, 19627–19662. [Google Scholar] [CrossRef]
- Xiong, Y.; Huang, Q.; Canady, T.D.; Barya, P.; Liu, S.; Arogundade, O.H.; Race, C.M.; Che, C.; Wang, X.; Zhou, L.; et al. Photonic crystal enhanced fluorescence emission and blinking suppression for single quantum dot digital resolution biosensing. Nat. Commun. 2022, 13, 4647. [Google Scholar] [CrossRef]
- Fernández-Sánchez, C.; McNeil, C.J.; Rawson, K.; Nilsson, O.; Leung, H.Y.; Gnanapragasam, V. One-step immunostrip test for the simultaneous detection of free and total prostate specific antigen in serum. J. Immunol. Methods 2005, 307, 1–12. [Google Scholar] [CrossRef]
- Mikolajczyk, S.D.; Catalona, W.J.; Evans, C.L.; Linton, H.J.; Millar, L.S.; Marker, K.M.; Katir, D.; Amirkhan, A.; Rittenhouse, H.G. Proenzyme forms of prostate-specific antigen in serum improve the detection of prostate cancer. Clin. Chem. 2004, 50, 1017–1025. [Google Scholar] [CrossRef]
- Khan, Y.; Li, A.; Chang, L.; Li, L.; Guo, L. Gold nano disks arrays for localized surface plasmon resonance based detection of PSA cancer marker. Sens. Actuators B Chem. 2018, 255, 1298–1307. [Google Scholar] [CrossRef]
- Zhang, Y.-n.; Wang, M.; Zhu, N.; Han, B.; Liu, Y. Optical fiber hydrogen sensor based on self-assembled PDMS/Pd-WO3 microbottle resonator. Sens. Actuators B 2023, 375, 132866. [Google Scholar] [CrossRef]
- Théry, C.; Ostrowski, M.; Segura, E. Membrane vesicles as conveyors of immune responses. Nat. Rev. Immunol. 2009, 9, 581–593. [Google Scholar] [CrossRef] [PubMed]
- Colombo, M.; Raposo, G.; Théry, C. Biogenesis, Secretion, and Intercellular Interactions of Exosomes and Other Extracellular Vesicles. Annu. Rev. Cell Dev. Biol. 2014, 30, 255–289. [Google Scholar] [CrossRef]
- Simpson, R.J.; Lim, J.W.E.; Moritz, R.L.; Mathivanan, S. Exosomes: Proteomic insights and diagnostic potential. Expert Rev. Proteom. 2009, 6, 267–283. [Google Scholar] [CrossRef] [PubMed]
- Shao, H.L.; Im, H.; Castro, C.M.; Breakefield, X.; Weissleder, R.; Lee, H.H. New Technologies for Analysis of Extracellular Vesicles. Chem. Rev. 2018, 118, 1917–1950. [Google Scholar] [CrossRef]
- El Andaloussi, S.; Maeger, I.; Breakefield, X.O.; Wood, M.J.A. Extracellular vesicles: Biology and emerging therapeutic opportunities. Nat. Rev. Drug Discov. 2013, 12, 348–358. [Google Scholar] [CrossRef]
- Wang, C.Y.; Huang, C.H.; Gao, Z.Q.; Shen, J.L.; He, J.C.; MacLachlan, A.; Ma, C.; Chang, Y.; Yang, W.; Cai, Y.X.; et al. Nanoplasmonic Sandwich Immunoassay for Tumor-Derived Exosome Detection and Exosomal PD-L1 Profiling. Acs Sens. 2021, 6, 3308–3319. [Google Scholar] [CrossRef]
- Feng, H.T.; Min, S.Y.; Huang, Y.Q.; Gan, Z.F.; Liang, C.W.; Li, W.D.; Chen, Y. Concentric gradient nanoplasmonic sensors for detecting tumor-derived extracellular vesicles. Sens. Actuators B-Chem. 2024, 400, 134899. [Google Scholar] [CrossRef]
- Wang, Y.D.; Mao, Z.H.; Chen, Q.; Koh, K.; Hu, X.J.; Chen, H.X. Rapid and sensitive detection of PD-L1 exosomes using Cu-TCPP 2D MOF as a SPR sensitizer. Biosens. Bioelectron. 2022, 201, 113954. [Google Scholar] [CrossRef]
- Théry, C.; Amigorena, S.; Raposo, G.; Clayton, A. Isolation and characterization of exosomes from cell culture supernatants and biological fluids. Curr. Protoc. Cell Biol. 2006, 30, 3–22. [Google Scholar] [CrossRef]
- Im, H.; Shao, H.; Park, Y.I.; Peterson, V.M.; Castro, C.M.; Weissleder, R.; Lee, H. Label-free detection and molecular profiling of exosomes with a nano-plasmonic sensor. Nat. Biotechnol. 2014, 32, 490-U219. [Google Scholar] [CrossRef] [PubMed]
- Lim, C.Z.J.; Zhang, Y.; Chen, Y.; Zhao, H.T.; Stephenson, M.C.; Ho, N.R.Y.; Chen, Y.; Chung, J.; Reilhac, A.; Loh, T.P.; et al. Subtyping of circulating exosome-bound amyloid beta reflects brain plaque deposition. Nat. Commun. 2019, 10, 11. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.H.; Iketani, S.; Guo, Y.C.; Chan, J.F.W.; Wang, M.; Liu, L.Y.; Luo, Y.; Chu, H.; Huang, Y.M.; Nair, M.S.; et al. Striking antibody evasion manifested by the Omicron variant of SARS-CoV-2. Nature 2022, 602, 676–681. [Google Scholar] [CrossRef]
- Zhu, F.C.; Zhuang, C.L.; Chu, K.; Zhang, L.; Zhao, H.; Huang, S.J.; Su, Y.Y.; Lin, H.Y.; Yang, C.L.; Jiang, H.M.; et al. Safety and immunogenicity of a live-attenuated influenza virus vector-based intranasal SARS-CoV-2 vaccine in adults: Randomised, double-blind, placebo-controlled, phase 1 and 2 trials. Lancet Respir. Med. 2022, 10, 749–760. [Google Scholar] [CrossRef]
- Asghari, A.; Wang, C.; Yoo, K.M.; Rostamian, A.; Xu, X.C.; Shin, J.D.; Dalir, H.; Chen, R.T. Fast, accurate, point-of-care COVID-19 pandemic diagnosis enabled through advanced lab-on-chip optical biosensors: Opportunities and challenges. Appl. Phys. Rev. 2021, 8, 031313. [Google Scholar] [CrossRef]
- Tan, X.T.; Krel, M.; Dolgov, E.; Park, S.; Li, X.Z.; Wu, W.S.; Sun, Y.L.; Zhang, J.; Oo, M.K.K.; Perlin, D.S.; et al. Rapid and quantitative detection of SARS-CoV-2 specific IgG for convalescent serum evaluation. Biosens. Bioelectron. 2020, 169, 112572. [Google Scholar] [CrossRef]
- Ogata, A.F.; Maley, A.M.; Wu, C.; Gilboa, T.; Norman, M.; Lazarovits, R.; Mao, C.P.; Newton, G.; Chang, M.; Nguyen, K.; et al. Ultra-Sensitive Serial Profiling of SARS-CoV-2 Antigens and Antibodies in Plasma to Understand Disease Progression in COVID-19 Patients with Severe Disease. Clin. Chem. 2020, 66, 1562–1572. [Google Scholar] [CrossRef]
- Li, F.J.; Hong, J.P.; Guan, C.H.; Chen, K.Y.; Xie, Y.N.; Wu, Q.; Chen, J.J.; Deng, B.C.; Shen, J.Q.; Liu, X.Y.; et al. Affinity Exploration of SARS-CoV-2 RBD Variants to mAb-Functionalized Plasmonic Metasurfaces for Label-Free Immunoassay Boosting. ACS Nano 2023, 17, 3383–3393. [Google Scholar] [CrossRef]
- Yang, Y.J.; Murray, J.; Haverstick, J.; Tripp, R.A.; Zhao, Y.P. Silver nanotriangle array based LSPR sensor for rapid coronavirus detection. Sens. Actuators B-Chem. 2022, 359, 131604. [Google Scholar] [CrossRef]
- Funari, R.; Fukuyama, H.; Shen, A.Q. Nanoplasmonic multiplex biosensing for COVID-19 vaccines. Biosens. Bioelectron. 2022, 208, 114193. [Google Scholar] [CrossRef]
- Huang, L.P.; Ding, L.F.; Zhou, J.; Chen, S.L.; Chen, F.; Zhao, C.; Xu, J.Q.; Hu, W.J.; Ji, J.S.; Xu, H.; et al. One-step rapid quantification of SARS-CoV-2 virus particles via low-cost nanoplasmonic sensors in generic microplate reader and point-of-care device. Biosens. Bioelectron. 2021, 171, 112685. [Google Scholar] [CrossRef] [PubMed]
- Funari, R.; Chu, K.Y.; Shen, A.Q. Detection of antibodies against SARS-CoV-2 spike protein by gold nanospikes in an opto-microfluidic chip. Biosens. Bioelectron. 2020, 169, 112578. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.Y.; Huang, C.H.; Lu, S.H.; Kuo, I.T.; Chau, L.K. Direct detection of orchid viruses using nanorod-based fiber optic particle plasmon resonance immunosensor. Biosens. Bioelectron. 2014, 51, 371–378. [Google Scholar] [CrossRef] [PubMed]
- Zheng, S.; Kim, D.K.; Park, T.J.; Lee, S.J.; Lee, S.Y. Label-free optical diagnosis of hepatitis B virus with genetically engineered fusion proteins. Talanta 2010, 82, 803–809. [Google Scholar] [CrossRef]
- Yanik, A.A.; Huang, M.; Kamohara, O.; Artar, A.; Geisbert, T.W.; Connor, J.H.; Altug, H. An Optofluidic Nanoplasmonic Biosensor for Direct Detection of Live Viruses from Biological Media. Nano Lett. 2010, 10, 4962–4969. [Google Scholar] [CrossRef]
- Grosman, A.; Duanis-Assaf, T.; Mazurski, N.; Zektzer, R.; Frydendahl, C.; Stern, L.; Reches, M.; Levy, U. On-chip multivariant COVID 19 photonic sensor based on silicon nitride double-microring resonators. Nanophotonics 2023, 12, 2831–2839. [Google Scholar] [CrossRef]
- Yesilkoy, F.; Arvelo, E.R.; Jahani, Y.; Liu, M.; Tittl, A.; Cevher, V.; Kivshar, Y.; Altug, H. Ultrasensitive hyperspectral imaging and biodetection enabled by dielectric metasurfaces. Nat. Photonics 2019, 13, 390–396. [Google Scholar] [CrossRef]
- Jahani, Y.; Arvelo, E.R.; Yesilkoy, F.; Koshelev, K.; Cianciaruso, C.; De Palma, M.; Kivshar, Y.; Altug, H. Imaging-based spectrometer-less optofluidic biosensors based on dielectric metasurfaces for detecting extracellular vesicles. Nat. Commun. 2021, 12, 3246. [Google Scholar] [CrossRef]
- Khan, S.A.; Khan, N.Z.; Xie, Y.N.; Abbas, M.T.; Rauf, M.; Mehmood, I.; Runowski, M.; Agathopoulos, S.; Zhu, J.F. Optical Sensing by Metamaterials and Metasurfaces: From Physics to Biomolecule Detection. Adv. Opt. Mater. 2022, 10, 2200500. [Google Scholar] [CrossRef]
- Min, S.; Li, S.; Zhu, Z.; Liu, Y.; Liang, C.; Cai, J.; Han, F.; Li, Y.; Cai, W.; Cheng, X.; et al. Ultrasensitive Molecular Detection by Imaging of Centimeter-Scale Metasurfaces with a Deterministic Gradient Geometry. Adv. Mater. 2021, 33, e2100270. [Google Scholar] [CrossRef]
- Wang, Z.; Chen, J.; Khan, S.A.; Li, F.; Shen, J.; Duan, Q.; Liu, X.; Zhu, J. Plasmonic Metasurfaces for Medical Diagnosis Applications: A Review. Sensors 2021, 22, 133. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.X.; Li, G.H.; Ye, X.Y.; Zhou, B.; Zhou, J.H.; Cai, J.X. Ultrasensitive Molecular Detection at Subpicomolar Concentrations by the Diffraction Pattern Imaging with Plasmonic Metasurfaces and Convex Holographic Gratings. Adv. Sci. 2022, 9, e2201682. [Google Scholar] [CrossRef] [PubMed]
- Ansaryan, S.; Liu, Y.C.; Li, X.; Economou, A.M.; Eberhardt, C.S.; Jandus, C.; Altug, H. High-throughput spatiotemporal monitoring of single-cell secretions via plasmonic microwell arrays. Nat. Biomed. Eng. 2023, 7, 943–958. [Google Scholar] [CrossRef]
- Shrivastav, A.M.; Mishra, S.K.; Gupta, B.D. Fiber optic SPR sensor for the detection of melamine using molecular imprinting. Sens. Actuators B-Chem. 2015, 212, 404–410. [Google Scholar] [CrossRef]
- Zhou, W.C.; Li, K.W.; Wei, Y.L.; Hao, P.; Chi, M.B.; Liu, Y.S.; Wu, Y.H. Ultrasensitive label-free optical microfiber coupler biosensor for detection of cardiac troponin I based on interference turning point effect. Biosens. Bioelectron. 2018, 106, 99–104. [Google Scholar] [CrossRef]
- Huang, Y.Y.; Chen, P.W.; Liang, H.; Xiao, A.X.; Zeng, S.K.; Guan, B.O. Nucleic acid hybridization on a plasmonic nanointerface of optical microfiber enables ultrahigh-sensitive detection and potential photothermal therapy. Biosens. Bioelectron. 2020, 156, 112147. [Google Scholar] [CrossRef]
- Chen, P.W.; Huang, Y.Y.; Bo, Y.; Liang, H.; Xiao, A.X.; Guan, B.O. 3D nanointerface enhanced optical microfiber for real-time detection and sizing of single nanoparticles. Chem. Eng. J. 2021, 407, 127143. [Google Scholar] [CrossRef]
- Li, H.T.; Huang, Y.Y.; Hou, G.H.; Xiao, A.X.; Chen, P.W.; Liang, H.; Huang, Y.G.; Zhao, X.T.; Liang, L.L.; Feng, X.H.; et al. Single-molecule detection of biomarker and localized cellular photothermal therapy using an optical microfiber with nanointerface. Sci. Adv. 2019, 5, eaax4659. [Google Scholar] [CrossRef]
- Huang, Y.Y.; Chen, P.W.; Zhou, L.Y.; Zheng, J.Y.; Wu, H.T.; Liang, J.X.; Xiao, A.X.; Li, J.; Guan, B.O. Plasmonic Coupling on an Optical Microfiber Surface: Enabling Single-Molecule and Noninvasive Dopamine Detection. Adv. Mater. 2023, 35, e2304116. [Google Scholar] [CrossRef]
- Raghunandhan, R.; Chen, L.H.; Long, H.Y.; Leam, L.L.; So, P.L.; Ning, X.; Chan, C.C. Chitosan/PAA based fiber-optic interferometric sensor for heavy metal ions detection. Sens. Actuators B-Chem. 2016, 233, 31–38. [Google Scholar] [CrossRef]
- Mariani, S.; Strambini, L.M.; Barillaro, G. Electrical Double Layer-Induced Ion Surface Accumulation for Ultrasensitive Refractive Index Sensing with Nanostructured Porous Silicon Interferometers. Acs Sens. 2018, 3, 595–605. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.J.; Lu, P.; Culp, J.T.; Ohodnicki, P.R. Metal-Organic Framework Thin Film Coated Optical Fiber Sensors: A Novel Waveguide-Based Chemical Sensing Platform. Acs Sens. 2018, 3, 386–394. [Google Scholar] [CrossRef] [PubMed]
- Ribaut, C.; Voisin, V.; Malachovská, V.; Dubois, V.; Mégret, P.; Wattiez, R.; Caucheteur, C. Small biomolecule immunosensing with plasmonic optical fiber grating sensor. Biosens. Bioelectron. 2016, 77, 315–322. [Google Scholar] [CrossRef] [PubMed]
- Thakkar, P.; Fleury, C.; Stocker, G.; Dubois, F.; Dao, T.D.; Jannesari, R.; Saeidi, P.; Pühringer, G.; Ostermann, T.; Grille, T.; et al. Coupled Strip-Array Waveguides for Integrated Mid-IR Gas Sensing. Photonics 2023, 10, 55. [Google Scholar] [CrossRef]
- Magno, G.; Yam, V.; Dagens, B. Integration of Plasmonic Structures in Photonic Waveguides Enables Novel Electromagnetic Functionalities in Photonic Circuits. Appl. Sci. 2023, 13, 12551. [Google Scholar] [CrossRef]
- Gupta, R.; Barman, K.; Lee, L.Y.; Chauhan, A.; Huang, J.J. Surface acoustic wave actuated plasmonic signal amplification in a plasmonic waveguide. Discov. Nano 2024, 19, 10. [Google Scholar] [CrossRef]
- Yan, Y.; Feng, H.; Wang, C.; Ren, W. On-chip photothermal gas sensor based on a lithium niobate rib waveguide. Sens. Actuators B 2024, 405, 135392. [Google Scholar] [CrossRef]
- Adato, R.; Altug, H. In-situ ultra-sensitive infrared absorption spectroscopy of biomolecule interactions in real time with plasmonic nanoantennas. Nat. Commun. 2013, 4, 2154. [Google Scholar] [CrossRef]
- Ataka, K.; Kottke, T.; Heberle, J. Thinner, Smaller, Faster: IR Techniques To Probe the Functionality of Biological and Biomimetic Systems. Angew. Chem.-Int. Ed. 2010, 49, 5416–5424. [Google Scholar] [CrossRef]
- Neubrech, F.; Huck, C.; Weber, K.; Pucci, A.; Giessen, H. Surface-Enhanced Infrared Spectroscopy Using Resonant Nanoantennas. Chem. Rev. 2017, 117, 5110–5145. [Google Scholar] [CrossRef]
- Zhou, H.; Li, D.X.; Hui, X.D.; Mu, X.J. Infrared metamaterial for surface-enhanced infrared absorption spectroscopy: Pushing the frontier of ultrasensitive on-chip sensing. Int. J. Optomechatron. 2021, 15, 97–119. [Google Scholar] [CrossRef]
- Li, D.X.; Xu, C.; Xie, J.S.; Lee, C. Research Progress in Surface-Enhanced Infrared Absorption Spectroscopy: From Performance Optimization, Sensing Applications, to System Integration. Nanomaterials 2023, 13, 2377. [Google Scholar] [CrossRef] [PubMed]
- Hartstein, A.; Kirtley, J.R.; Tsang, J.C. Enhancement of the Infrared-Absorption from Molecular Monolayers with Thin Metal Overlayers. Phys. Rev. Lett. 1980, 45, 201–204. [Google Scholar] [CrossRef]
- Aroca, R.F.; Ross, D.J.; Domingo, C. Surface-enhanced infrared spectroscopy. Appl. Spectrosc. 2004, 58, 324A–338A. [Google Scholar] [CrossRef]
- Ataka, K.; Heberle, J. Biochemical applications of surface-enhanced infrared absorption spectroscopy. Anal. Bioanal. Chem. 2007, 388, 47–54. [Google Scholar] [CrossRef]
- Zheludev, N.I. The Road Ahead for Metamaterials. Science 2010, 328, 582–583. [Google Scholar] [CrossRef]
- Halas, N.J.; Lal, S.; Chang, W.S.; Link, S.; Nordlander, P. Plasmons in Strongly Coupled Metallic Nanostructures. Chem. Rev. 2011, 111, 3913–3961. [Google Scholar] [CrossRef]
- Brolo, A.G. Plasmonics for future biosensors. Nat. Photonics 2012, 6, 709–713. [Google Scholar] [CrossRef]
- Yang, X.X.; Sun, Z.P.; Low, T.; Hu, H.; Guo, X.D.; de Abajo, F.J.G.; Avouris, P.; Dai, Q. Nanomaterial-Based Plasmon-Enhanced Infrared Spectroscopy. Adv. Mater. 2018, 30, 1704896. [Google Scholar] [CrossRef]
- Yang, K.; Yao, X.; Liu, B.W.; Ren, B. Metallic Plasmonic Array Structures: Principles, Fabrications, Properties, and Applications. Adv. Mater. 2021, 33, 2007988. [Google Scholar] [CrossRef]
- Chen, K.; Adato, R.; Altug, H. Dual-band perfect absorber for multispectral plasmon-enhanced infrared spectroscopy. ACS Nano 2012, 6, 7998–8006. [Google Scholar] [CrossRef] [PubMed]
- Cerjan, B.; Yang, X.; Nordlander, P.; Halas, N.J. Asymmetric Aluminum Antennas for Self-Calibrating Surface Enhanced Infrared Absorption Spectroscopy. ACS Photonics 2016, 3, 354–360. [Google Scholar] [CrossRef]
- Cubukcu, E.; Zhang, S.; Park, Y.-S.; Bartal, G.; Zhang, X. Split ring resonator sensors for infrared detection of single molecular monolayers. Appl. Phys. Lett. 2009, 95, 043113. [Google Scholar] [CrossRef]
- Gottheim, S.; Zhang, H.; Govorov, A.O.; Halas, N.J. Fractal nanoparticle plasmonics: The Cayley tree. ACS Nano 2015, 9, 3284–3292. [Google Scholar] [CrossRef] [PubMed]
- Aslan, E.; Aslan, E.; Wang, R.; Hong, M.K.; Erramilli, S.; Turkmen, M.; Saracoglu, O.G.; Dal Negro, L. Multispectral Cesaro-Type Fractal Plasmonic Nanoantennas. ACS Photonics 2016, 3, 2102–2111. [Google Scholar] [CrossRef]
- Kudyshev, Z.A.; Kildishev, A.V.; Shalaev, V.M.; Boltasseva, A. Machine-learning-assisted metasurface design for high-efficiency thermal emitter optimization. Appl. Phys. Rev. 2020, 7, 021407. [Google Scholar] [CrossRef]
- Hwang, I.; Yu, J.; Lee, J.; Choi, J.H.; Choi, D.G.; Jeon, S.; Lee, J.; Jung, J.Y. Plasmon-Enhanced Infrared Spectroscopy Based on Metamaterial Absorbers with Dielectric Nanopedestals. ACS Photonics 2018, 5, 3492–3498. [Google Scholar] [CrossRef]
- Cetin, A.E.; Etezadi, D.; Altug, H. Accessible Nearfields by Nanoantennas on Nanopedestals for Ultrasensitive Vibrational Spectroscopy. Adv. Opt. Mater. 2014, 2, 866–872. [Google Scholar] [CrossRef]
- Jung, Y.; Hwang, I.; Yu, J.; Lee, J.; Choi, J.H.; Jeong, J.H.; Jung, J.Y.; Lee, J. Fano Metamaterials on Nanopedestals for Plasmon-Enhanced Infrared Spectroscopy. Sci. Rep. 2019, 9, 7834. [Google Scholar] [CrossRef]
- Miao, X.; Yan, L.; Wu, Y.; Liu, P.Q. High-sensitivity nanophotonic sensors with passive trapping of analyte molecules in hot spots. Light. Sci. Appl. 2021, 10, 5. [Google Scholar] [CrossRef]
- Rodrigo, D.; Tittl, A.; Ait-Bouziad, N.; John-Herpin, A.; Limaj, O.; Kelly, C.; Yoo, D.; Wittenberg, N.J.; Oh, S.H.; Lashuel, H.A.; et al. Resolving molecule-specific information in dynamic lipid membrane processes with multi-resonant infrared metasurfaces. Nat. Commun. 2018, 9, 2160. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Zhu, H.; Liu, F.; Zhu, A.Y.; Reed, J.C.; Yi, F.; Cubukcu, E. Silicon-on-Glass Graphene-Functionalized Leaky Cavity Mode Nanophotonic Biosensor. ACS Photonics 2014, 1, 221–227. [Google Scholar] [CrossRef]
- John-Herpin, A.; Tittl, A.; Altug, H. Quantifying the Limits of Detection of Surface-Enhanced Infrared Spectroscopy with Grating Order-Coupled Nanogap Antennas. ACS Photonics 2018, 5, 4117–4124. [Google Scholar] [CrossRef] [PubMed]
- Etezadi, D.; Warner, J.B.; Lashuel, H.A.; Altug, H. Real-Time In Situ Secondary Structure Analysis of Protein Monolayer with Mid-Infrared Plasmonic Nanoantennas. ACS Sens. 2018, 3, 1109–1117. [Google Scholar] [CrossRef]
- Etezadi, D.; Warner, J.B.; Ruggeri, F.S.; Dietler, G.; Lashuel, H.A.; Altug, H. Nanoplasmonic mid-infrared biosensor for in vitro protein secondary structure detection. Light. Sci. Appl. 2017, 6, e17029. [Google Scholar] [CrossRef]
- Hui, X.; Yang, C.; Li, D.; He, X.; Huang, H.; Zhou, H.; Chen, M.; Lee, C.; Mu, X. Infrared Plasmonic Biosensor with Tetrahedral DNA Nanostructure as Carriers for Label-Free and Ultrasensitive Detection of miR-155. Adv. Sci. 2021, 8, e2100583. [Google Scholar] [CrossRef]
- John-Herpin, A.; Kavungal, D.; von Mucke, L.; Altug, H. Infrared Metasurface Augmented by Deep Learning for Monitoring Dynamics between All Major Classes of Biomolecules. Adv. Mater. 2021, 33, 2006054. [Google Scholar] [CrossRef]
- Li, D.; Zhou, H.; Chen, Z.; Ren, Z.; Xu, C.; He, X.; Liu, T.; Chen, X.; Huang, H.; Lee, C.; et al. Ultrasensitive Molecular Fingerprint Retrieval Using Strongly Detuned Overcoupled Plasmonic Nanoantennas. Adv. Mater. 2023, 35, e2301787. [Google Scholar] [CrossRef]
- Ren, Z.; Zhang, Z.; Wei, J.; Dong, B.; Lee, C. Wavelength-multiplexed hook nanoantennas for machine learning enabled mid-infrared spectroscopy. Nat. Commun. 2022, 13, 3859. [Google Scholar] [CrossRef]
- Kavungal, D.; Magalhães, P.; Kumar, S.T.; Kolla, R.; Lashuel, H.A.; Altug, H.J.S.A. Artificial intelligence–coupled plasmonic infrared sensor for detection of structural protein biomarkers in neurodegenerative diseases. Sci. Adv. 2023, 9, eadg9644. [Google Scholar] [CrossRef]
- Zhou, H.; Ren, Z.; Li, D.; Xu, C.; Mu, X.; Lee, C. Dynamic construction of refractive index-dependent vibrations using surface plasmon-phonon polaritons. Nat. Commun. 2023, 14, 7316. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Li, D.; Ren, Z.; Mu, X.; Lee, C. Loss-induced phase transition in mid-infrared plasmonic metamaterials for ultrasensitive vibrational spectroscopy. InfoMat 2022, 4, e12349. [Google Scholar] [CrossRef]
- Wei, J.; Li, Y.; Chang, Y.; Hasan, D.M.N.; Dong, B.; Ma, Y.; Qiu, C.W.; Lee, C. Ultrasensitive Transmissive Infrared Spectroscopy via Loss Engineering of Metallic Nanoantennas for Compact Devices. ACS Appl. Mater. Inter. 2019, 11, 47270–47278. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Ren, Z.; Dong, B.; Liu, X.; Wang, C.; Tian, Y.; Lee, C. Nanometer-Scale Heterogeneous Interfacial Sapphire Wafer Bonding for Enabling Plasmonic-Enhanced Nanofluidic Mid-Infrared Spectroscopy. ACS Nano 2020, 14, 12159–12172. [Google Scholar] [CrossRef]
- Zeng, K.; Wu, C.; Guo, X.; Guan, F.; Duan, Y.; Zhang, L.L.; Yang, X.; Liu, N.; Dai, Q.; Zhang, S. Synthesized complex-frequency excitation for ultrasensitive molecular sensing. eLight 2024, 4, 1. [Google Scholar] [CrossRef]
- Chang, Y.; Hasan, D.; Dong, B.; Wei, J.; Ma, Y.; Zhou, G.; Ang, K.W.; Lee, C. All-Dielectric Surface-Enhanced Infrared Absorption-Based Gas Sensor Using Guided Resonance. ACS Appl. Mater. Interfaces 2018, 10, 38272–38279. [Google Scholar] [CrossRef] [PubMed]
- Chong, X.Y.; Zhang, Y.J.; Li, E.W.; Kim, K.J.; Ohodnicki, P.R.; Chang, C.H.; Wang, A.X. Surface-Enhanced Infrared Absorption: Pushing the Frontier for On-Chip Gas Sensing. Acs Sens. 2018, 3, 230–238. [Google Scholar] [CrossRef]
- Hasan, D.; Lee, C. Hybrid Metamaterial Absorber Platform for Sensing of CO2 Gas at Mid-IR. Adv. Sci. 2018, 5, 1700581. [Google Scholar] [CrossRef]
- Bareza, N.; Gopalan, K.K.; Alani, R.; Paulillo, B.; Pruneri, V. Mid-infrared Gas Sensing Using Graphene Plasmons Tuned by Reversible Chemical Doping. ACS Photonics 2020, 7, 879–884. [Google Scholar] [CrossRef]
- Bareza, N., Jr.; Paulillo, B.; Slipchenko, T.M.; Autore, M.; Dolado, I.; Liu, S.; Edgar, J.H.; Vélez, S.; Martín-Moreno, L.; Hillenbrand, R.; et al. Phonon-Enhanced Mid-Infrared CO2 Gas Sensing Using Boron Nitride Nanoresonators. ACS Photonics 2022, 9, 34–42. [Google Scholar] [CrossRef]
- Zhou, H.; Hui, X.; Li, D.; Hu, D.; Chen, X.; He, X.; Gao, L.; Huang, H.; Lee, C.; Mu, X. Metal-Organic Framework-Surface-Enhanced Infrared Absorption Platform Enables Simultaneous On-Chip Sensing of Greenhouse Gases. Adv. Sci. 2020, 7, 2001173. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Ren, Z.; Xu, C.; Xu, L.; Lee, C. MOF/Polymer-Integrated Multi-Hotspot Mid-Infrared Nanoantennas for Sensitive Detection of CO(2) Gas. Nano-Micro Lett. 2022, 14, 207. [Google Scholar] [CrossRef] [PubMed]
- Hasan, D.; Ho, C.P.; Lee, C. Realization of Fractal-Inspired Thermoresponsive Quasi-3D Plasmonic Metasurfaces with EOT-Like Transmission for Volumetric and Multispectral Detection in the Mid-IR Region. ACS Omega 2016, 1, 818–831. [Google Scholar] [CrossRef] [PubMed]
- Li, D.X.; Zhou, H.; Hui, X.D.; He, X.M.; Huang, H.; Zhang, J.J.; Mu, X.J.; Lee, C.K.; Yang, Y. Multifunctional Chemical Sensing Platform Based on Dual-Resonant Infrared Plasmonic Perfect Absorber for On-Chip Detection of Poly(ethyl cyanoacrylate). Adv. Sci. 2021, 8, 12. [Google Scholar] [CrossRef]
- Rodrigo, D.; Tittl, A.; John-Herpin, A.; Limaj, O.; Altug, H. Self-Similar Multiresonant Nanoantenna Arrays for Sensing from Near- to Mid-Infrared. ACS Photonics 2018, 5, 4903–4911. [Google Scholar] [CrossRef]
- Richter, F.U.; Sinev, I.; Zhou, S.; Leitis, A.; Oh, S.H.; Tseng, M.L.; Kivshar, Y.; Altug, H. Gradient High-Q Dielectric Metasurfaces for Broadband Sensing and Control of Vibrational Light-Matter Coupling. Adv. Mater. 2024, 36, e2314279. [Google Scholar] [CrossRef]
- Jangid, P.; Richter, F.U.; Tseng, M.L.; Sinev, I.; Kruk, S.; Altug, H.; Kivshar, Y. Spectral Tuning of High-Harmonic Generation with Resonance-Gradient Metasurfaces. Adv. Mater. 2023, 36, e2307494. [Google Scholar] [CrossRef]
- Liu, S.D.; Leong, E.S.; Li, G.C.; Hou, Y.; Deng, J.; Teng, J.H.; Ong, H.C.; Lei, D.Y. Polarization-Independent Multiple Fano Resonances in Plasmonic Nonamers for Multimode-Matching Enhanced Multiband Second-Harmonic Generation. ACS Nano 2016, 10, 1442–1453. [Google Scholar] [CrossRef]
- Tittl, A.; Leitis, A.; Liu, M.; Yesilkoy, F.; Choi, D.Y.; Neshev, D.N.; Kivshar, Y.S.; Altug, H. Imaging-based molecular barcoding with pixelated dielectric metasurfaces. Science 2018, 360, 1105–1109. [Google Scholar] [CrossRef]
- Aigner, A.; Tittl, A.; Wang, J.; Weber, T.; Kivshar, Y.; Maier, S.A.; Ren, H. Plasmonic bound states in the continuum to tailor light-matter coupling. Sci. Adv. 2022, 8, eadd4816. [Google Scholar] [CrossRef]
- Leitis, A.; Tittl, A.; Liu, M.; Lee, B.H.; Gu, M.B.; Kivshar, Y.S.; Altug, H. Angle-multiplexed all-dielectric metasurfaces for broadband molecular fingerprint retrieval. Sci. Adv. 2019, 5, eaaw2871. [Google Scholar] [CrossRef] [PubMed]
- Najem, M.; Carcenac, F.; Taliercio, T.; Gonzalez-Posada, F. Aluminum Bowties for Plasmonic-Enhanced Infrared Sensing. Adv. Opt. Mater. 2022, 10, 2201025. [Google Scholar] [CrossRef]
- Paggi, L.; Fabas, A.; El Ouazzani, H.; Hugonin, J.P.; Fayard, N.; Bardou, N.; Dupuis, C.; Greffet, J.J.; Bouchon, P. Over-coupled resonator for broadband surface enhanced infrared absorption (SEIRA). Nat. Commun. 2023, 14, 4814. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; You, E.M.; Lin, J.D.; Wang, J.H.; Luo, S.H.; Zhou, R.Y.; Zhang, C.J.; Yao, J.L.; Li, H.Y.; Li, G.; et al. Resolving nanostructure and chemistry of solid-electrolyte interphase on lithium anodes by depth-sensitive plasmon-enhanced Raman spectroscopy. Nat. Commun. 2023, 14, 3536. [Google Scholar] [CrossRef]
- Leong, S.X.; Leong, Y.X.; Tan, E.X.; Sim, H.Y.F.; Koh, C.S.L.; Lee, Y.H.; Chong, C.; Ng, L.S.; Chen, J.R.T.; Pang, D.W.C.; et al. Noninvasive and Point-of-Care Surface-Enhanced Raman Scattering (SERS)-Based Breathalyzer for Mass Screening of Coronavirus Disease 2019 (COVID-19) under 5 min. ACS Nano 2022, 16, 2629–2639. [Google Scholar] [CrossRef]
- Leong, Y.X.; Lee, Y.H.; Koh, C.S.L.; Phan-Quang, G.C.; Han, X.; Phang, I.Y.; Ling, X.Y. Surface-Enhanced Raman Scattering (SERS) Taster: A Machine-Learning-Driven Multireceptor Platform for Multiplex Profiling of Wine Flavors. Nano Lett. 2021, 21, 2642–2649. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, C.; Wang, J.; Luo, X.; Xie, L.; Zhan, S.; Kim, J.; Wang, X.; Liu, X.; Ying, Y. Wearable plasmonic-metasurface sensor for noninvasive and universal molecular fingerprint detection on biointerfaces. Sci. Adv. 2021, 7, eabe4553. [Google Scholar] [CrossRef]
- Kim, N.; Thomas, M.R.; Bergholt, M.S.; Pence, I.J.; Seong, H.; Charchar, P.; Todorova, N.; Nagelkerke, A.; Belessiotis-Richards, A.; Payne, D.J.; et al. Surface enhanced Raman scattering artificial nose for high dimensionality fingerprinting. Nat. Commun. 2020, 11, 207. [Google Scholar] [CrossRef]
- Son, W.K.; Choi, Y.S.; Han, Y.W.; Shin, D.W.; Min, K.; Shin, J.; Lee, M.J.; Son, H.; Jeong, D.H.; Kwak, S.Y. In vivo surface-enhanced Raman scattering nanosensor for the real-time monitoring of multiple stress signalling molecules in plants. Nat. Nanotechnol. 2023, 18, 205–216. [Google Scholar] [CrossRef]
- Zhao, Y.; Askarpour, A.N.; Sun, L.; Shi, J.; Li, X.; Alu, A. Chirality detection of enantiomers using twisted optical metamaterials. Nat. Commun. 2017, 8, 14180. [Google Scholar] [CrossRef]
- Hendry, E.; Carpy, T.; Johnston, J.; Popland, M.; Mikhaylovskiy, R.V.; Lapthorn, A.J.; Kelly, S.M.; Barron, L.D.; Gadegaard, N.; Kadodwala, M. Ultrasensitive detection and characterization of biomolecules using superchiral fields. Nat. Nanotechnol. 2010, 5, 783–787. [Google Scholar] [CrossRef] [PubMed]
- Kakkar, T.; Keijzer, C.; Rodier, M.; Bukharova, T.; Taliansky, M.; Love, A.J.; Milner, J.J.; Karimullah, A.S.; Barron, L.D.; Gadegaard, N.; et al. Superchiral near fields detect virus structure. Light. Sci. Appl. 2020, 9, 195. [Google Scholar] [CrossRef] [PubMed]
- Hajji, M.; Cariello, M.; Gilroy, C.; Kartau, M.; Syme, C.D.; Karimullah, A.; Gadegaard, N.; Malfait, A.; Woisel, P.; Cooke, G.; et al. Chiral Quantum Metamaterial for Hypersensitive Biomolecule Detection. ACS Nano 2021, 15, 19905–19916. [Google Scholar] [CrossRef]
- Tabouillot, V.; Kumar, R.; Lalaguna, P.L.; Hajji, M.; Clarke, R.; Karimullah, A.S.; Thomson, A.R.; Sutherland, A.; Gadegaard, N.; Hashiyada, S.; et al. Near-Field Probing of Optical Superchirality with Plasmonic Polarized Luminescence for Enhanced Bio-Detection. ACS Photonics 2022, 9, 3617–3624. [Google Scholar] [CrossRef]
- Mohammadi, E.; Tavakoli, A.; Dehkhoda, P.; Jahani, Y.; Tsakmakidis, K.L.; Tittl, A.; Altug, H. Accessible Superchiral Near-Fields Driven by Tailored Electric and Magnetic Resonances in All-Dielectric Nanostructures. ACS Photonics 2019, 6, 1939–1946. [Google Scholar] [CrossRef]
- Kim, R.M.; Huh, J.H.; Yoo, S.; Kim, T.G.; Kim, C.; Kim, H.; Han, J.H.; Cho, N.H.; Lim, Y.C.; Im, S.W.; et al. Enantioselective sensing by collective circular dichroism. Nature 2022, 612, 470–476. [Google Scholar] [CrossRef]
- Keiderling, T.A. Protein and peptide secondary structure and conformational determination with vibrational circular dichroism. Curr. Opin. Chem. Biol. 2002, 6, 682–688. [Google Scholar] [CrossRef]
- Vázquez-Guardado, A.; Chanda, D. Superchiral Light Generation on Degenerate Achiral Surfaces. Phys. Rev. Lett. 2018, 120, 137601. [Google Scholar] [CrossRef]
- Xu, C.; Ren, Z.; Zhou, H.; Zhou, J.; Li, D.; Lee, C. Near-Field Coupling Induced Less Chiral Responses in Chiral Metamaterials for Surface-Enhanced Vibrational Circular Dichroism. Adv. Funct. Mater. 2023, 34, 2314482. [Google Scholar] [CrossRef]
- Xu, C.; Ren, Z.; Zhou, H.; Zhou, J.; Ho, C.P.; Wang, N.; Lee, C. Expanding chiral metamaterials for retrieving fingerprints via vibrational circular dichroism. Light. Sci. Appl. 2023, 12, 154. [Google Scholar] [CrossRef]
- Kurouski, D. Advances of Vibrational Circular Dichroism (VCD) in bioanalytical chemistry. A review. Anal. Chim. Acta 2017, 990, 54–66. [Google Scholar] [CrossRef] [PubMed]
- Janesko, B.G.; Scuseria, G.E. Surface enhanced Raman optical activity of molecules on orientationally averaged substrates: Theory of electromagnetic effects. J. Chem. Phys. 2006, 125, 124704. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.T.; Zhang, Z.L.; Wang, P.J.; Li, Q.; Ma, F.C.; Xu, H.X. Remotely excited Raman optical activity using chiral plasmon propagation in Ag nanowires. Light. Sci. Appl. 2013, 2, e112. [Google Scholar] [CrossRef]
- Pour, S.O.; Rocks, L.; Faulds, K.; Graham, D.; Parchansky, V.; Bour, P.; Blanch, E.W. Through-space transfer of chiral information mediated by a plasmonic nanomaterial. Nat. Chem. 2015, 7, 591–596. [Google Scholar] [CrossRef]
- Ma, W.; Cheng, F.; Liu, Y. Deep-Learning-Enabled On-Demand Design of Chiral Metamaterials. ACS Nano 2018, 12, 6326–6334. [Google Scholar] [CrossRef]
- Choi, W.J.; Cheng, G.; Huang, Z.Y.; Zhang, S.; Norris, T.B.; Kotov, N.A. Terahertz circular dichroism spectroscopy of biomaterials enabled by kirigami polarization modulators. Nat. Mater. 2019, 18, 820–826. [Google Scholar] [CrossRef]
- McDonnell, C.; Deng, J.H.; Sideris, S.; Ellenbogen, T.; Li, G.X. Functional THz emitters based on Pancharatnam-Berry phase nonlinear metasurfaces. Nat. Commun. 2021, 12, 30. [Google Scholar] [CrossRef]
- Choi, W.J.; Yano, K.; Cha, M.; Colombari, F.M.; Kim, J.Y.; Wang, Y.C.; Lee, S.H.; Sun, K.; Kruger, J.M.; De Moura, A.F.; et al. Chiral phonons in microcrystals and nanofibrils of biomolecules. Nat. Photonics 2022, 16, 366–373. [Google Scholar] [CrossRef]
- Kim, M.; Tsukruk, V.V. Spectroscopy finds chiral phonons. Nat. Photonics 2022, 16, 337–338. [Google Scholar] [CrossRef]
- Probst, P.T.; Mayer, M.; Gupta, V.; Steiner, A.M.; Zhou, Z.; Auernhammer, G.K.; König, T.A.F.; Fery, A. Mechano-tunable chiral metasurfaces via colloidal assembly. Nat. Mater. 2021, 20, 1024–1028. [Google Scholar] [CrossRef]
- Drexhage, K.; Kuhn, H.; Schäfer, F. Variation of the fluorescence decay time of a molecule in front of a mirror. Ber. Bunsenges. Phys. Chem. 1968, 72, 329. [Google Scholar] [CrossRef]
- Lakowicz, J.R. Radiative decay engineering: Biophysical and biomedical applications. Anal. Biochem. 2001, 298, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Geddes, C.D.; Gryczynski, I.; Malicka, J.; Gryczynski, Z.; Lakowicz, J.R. Metal-enhanced fluorescence: Potential applications in HTS. Comb. Chem. High Throughput Screen. 2003, 6, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Aslan, K.; Lakowicz, J.R.; Szmacinski, H.; Geddes, C.D. Metal-enhanced fluorescence solution-based sensing platform. J. Fluoresc. 2004, 14, 677–679. [Google Scholar] [CrossRef]
- Aslan, K.; Gryczynski, I.; Malicka, J.; Matveeva, E.; Lakowicz, J.R.; Geddes, C.D. Metal-enhanced fluorescence: An emerging tool in biotechnology. Curr. Opin. Biotechnol. 2005, 16, 55–62. [Google Scholar] [CrossRef]
- Lakowicz, J.R.; Geddes, C.D.; Gryczynski, I.; Malicka, J.; Gryczynski, Z.; Aslan, K.; Lukomska, J.; Matveeva, E.; Zhang, J.A.; Badugu, R.; et al. Advances in surface-enhanced fluorescence. J. Fluoresc. 2004, 14, 425–441. [Google Scholar] [CrossRef]
- Aslan, K.; Lakowicz, J.R.; Geddes, C.D. Metal-enhanced fluorescence using anisotropic silver nanostructures: Critical progress to date. Anal. Bioanal. Chem. 2005, 382, 926–933. [Google Scholar] [CrossRef]
- Schalkhammer, T.; Aussenegg, F.R.; Leitner, A.; Brunner, H.; Hawa, G.; Lobmaier, C.; Pittner, F. Detection of fluorophore-labelled antibodies by surface-enhanced fluorescence on metal nanoislands. In Proceedings of the Conference on Biomedical Sensing, Imaging, and Tracking Technologies II, San Jose, CA, USA, 11–13 February 1997; pp. 129–136. [Google Scholar]
- Lakowicz, J.R.; Shen, B.; Gryczynski, Z.; D’Auria, S.; Gryczynski, I. Intrinsic fluorescence from DNA can be enhanced by metallic particles. Biochem. Biophys. Res. Commun. 2001, 286, 875–879. [Google Scholar] [CrossRef]
- Matveeva, E.G.; Gryczynski, Z.; Lakowicz, J.R. Myoglobin immunoassay based on metal particle-enhanced fluorescence. J. Immunol. Methods 2005, 302, 26–35. [Google Scholar] [CrossRef]
- Liz-Marzán, L.M. Tailoring surface plasmons through the morphology and assembly of metal nanoparticles. Langmuir 2006, 22, 32–41. [Google Scholar] [CrossRef]
- Xia, Y.N.; Xiong, Y.J.; Lim, B.; Skrabalak, S.E. Shape-Controlled Synthesis of Metal Nanocrystals: Simple Chemistry Meets Complex Physics? Angew. Chem.-Int. Ed. 2009, 48, 60–103. [Google Scholar] [CrossRef] [PubMed]
- Tang, F.; He, F.; Cheng, H.C.; Li, L.D. Self-Assembly of Conjugated Polymer-Ag@SiO2 Hybrid Fluorescent Nanoparticles for Application to Cellular Imaging. Langmuir 2010, 26, 11774–11778. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Qiang, W.B.; Vuki, M.; Xu, D.K.; Chen, H.Y. Fluorescence Enhancement of Silver Nanoparticle Hybrid Probes and Ultrasensitive Detection of IgE. Anal. Chem. 2011, 83, 8945–8952. [Google Scholar] [CrossRef] [PubMed]
- Kühn, S.; Håkanson, U.; Rogobete, L.; Sandoghdar, V. Enhancement of single-molecule fluorescence using a gold nanoparticle as an optical nanoantenna. Phys. Rev. Lett. 2006, 97, 017402. [Google Scholar] [CrossRef]
- Taminiau, T.H.; Stefani, F.D.; Segerink, F.B.; Van Hulst, N.F. Optical antennas direct single-molecule emission. Nat. Photonics 2008, 2, 234–237. [Google Scholar] [CrossRef]
- Kinkhabwala, A.; Yu, Z.F.; Fan, S.H.; Avlasevich, Y.; Müllen, K.; Moerner, W.E. Large single-molecule fluorescence enhancements produced by a bowtie nanoantenna. Nat. Photonics 2009, 3, 654–657. [Google Scholar] [CrossRef]
- Fromm, D.P.; Sundaramurthy, A.; Schuck, P.J.; Kino, G.; Moerner, W.E. Gap-dependent optical coupling of single “Bowtie” nanoantennas resonant in the visible. Nano Lett. 2004, 4, 957–961. [Google Scholar] [CrossRef]
- Punj, D.; Mivelle, M.; Moparthi, S.B.; van Zanten, T.S.; Rigneault, H.; van Hulst, N.F.; García-Parajó, M.F.; Wenger, J. A plasmonic ‘antenna-in-box’ platform for enhanced single-molecule analysis at micromolar concentrations. Nat. Nanotechnol. 2013, 8, 512–516. [Google Scholar] [CrossRef]
- Bardhan, R.; Grady, N.K.; Cole, J.R.; Joshi, A.; Halas, N.J. Fluorescence Enhancement by Au Nanostructures: Nanoshells and Nanorods. ACS Nano 2009, 3, 744–752. [Google Scholar] [CrossRef]
- Khatua, S.; Paulo, P.M.R.; Yuan, H.F.; Gupta, A.; Zijlstra, P.; Orrit, M. Resonant Plasmonic Enhancement of Single-Molecule Fluorescence by Individual Gold Nanorods. ACS Nano 2014, 8, 4440–4449. [Google Scholar] [CrossRef]
- Ray, K.; Badugu, R.; Lakowicz, J.R. Sulforhodamine adsorbed Langmuir-Blodgett layers on silver island films: Effect of probe distance on the metal-enhanced fluorescence. J. Phys. Chem. C 2007, 111, 7091–7097. [Google Scholar] [CrossRef] [PubMed]
- Luan, J.; Seth, A.; Gupta, R.; Wang, Z.; Rathi, P.; Cao, S.; Gholami Derami, H.; Tang, R.; Xu, B.; Achilefu, S.; et al. Ultrabright fluorescent nanoscale labels for the femtomolar detection of analytes with standard bioassays. Nat. Biomed. Eng. 2020, 4, 518–530. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Liu, M.; Xing, W.; Wang, F.; Zhang, H.; Wei, X.; Schmitthenner, H.; Xie, X.; Xia, X.; Yang, J. A near-infrared fluorescence-enhancing plasmonic biosensing microarray identifies soluble PD-L1 and ICAM-1 as predictive checkpoint biomarkers for cancer immunotherapy. Biosens. Bioelectron. 2023, 240, 115633. [Google Scholar] [CrossRef]
- Chowdhury, M.H.; Ray, K.; Gray, S.K.; Pond, J.; Lakowicz, J.R. Aluminum Nanoparticles as Substrates for Metal-Enhanced Fluorescence in the Ultraviolet for the Label-Free Detection of Biomolecules. Anal. Chem. 2009, 81, 1397–1403. [Google Scholar] [CrossRef]
- Chen, Z.; Li, H.; Jia, W.C.; Liu, X.H.; Li, Z.M.; Wen, F.; Zheng, N.; Jiang, J.D.; Xu, D.K. Bivalent Aptasensor Based on Silver-Enhanced Fluorescence Polarization for Rapid Detection of Lactoferrin in Milk. Anal. Chem. 2017, 89, 5901–5909. [Google Scholar] [CrossRef]
- Li, C.Y.; Duan, S.; Yi, J.; Wang, C.; Radjenovic, P.M.; Tian, Z.Q.; Li, J.F. Real-time detection of single-molecule reaction by plasmon-enhanced spectroscopy. Sci. Adv. 2020, 6, eaba6012. [Google Scholar] [CrossRef]
- Acuna, G.P.; Möller, F.M.; Holzmeister, P.; Beater, S.; Lalkens, B.; Tinnefeld, P. Fluorescence Enhancement at Docking Sites of DNA-Directed Self-Assembled Nanoantennas. Science 2012, 338, 506–510. [Google Scholar] [CrossRef]
- Lu, H.P.; Xie, X.S. Single-molecule spectral fluctuations at room temperature. Nature 1997, 385, 143–146. [Google Scholar] [CrossRef]
- Tenopala-Carmona, F.; Fronk, S.; Bazan, G.C.; Samuel, I.D.W.; Penedo, J.C. Real-time observation of conformational switching in single conjugated polymer chains. Sci. Adv. 2018, 4, eaao5786. [Google Scholar] [CrossRef]
- Abdulhalim, I.; Karabchevsky, A.; Patzig, C.; Rauschenbach, B.; Fuhrmann, B.; Eltzov, E.; Marks, R.; Xu, J.; Zhang, F.; Lakhtakia, A. Surface-enhanced fluorescence from metal sculptured thin films with application to biosensing in water. Appl. Phys. Lett. 2009, 94, 063106. [Google Scholar] [CrossRef]
- Hu, R.B.; Yang, Y.; Liu, Y.; Liao, T.; Liu, Y.Y.; Tang, J.H.; Wang, G.H.; Wang, G.X.; Liang, Y.Y.; Yuan, J.; et al. Multiplexed evaluation of immunity against SARS-CoV-2 variants using surface enhanced fluorescence from a nanostructured plasmonic chip. J. Nanobiotechnol. 2022, 20, 533. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.L.; Zhao, X.; Ouyang, J.; Na, N.; Mao, J.P. Single-Molecule Evaluation of the SARS-CoV-2 Nucleocapsid Protein Using Gold Particle-in-a-Frame Nanostructures Enhanced Fluorescent Assay. Anal. Chem. 2023, 95, 5267–5274. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Zhang, C.; Zheng, J.Y.; Yao, J.; Zhao, Y.S. Optical modulation based on direct photon-plasmon coupling in organic/metal nanowire heterojunctions. Adv. Mater. 2012, 24, 5681–5686. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhang, Q.; Yip, J.N.; Xiong, Q.; Sum, T.C. Wavelength tunable single nanowire lasers based on surface plasmon polariton enhanced Burstein-Moss effect. Nano Lett. 2013, 13, 5336–5343. [Google Scholar] [CrossRef]
- Xu, Q.; Schmidt, B.; Pradhan, S.; Lipson, M. Micrometre-scale silicon electro-optic modulator. Nature 2005, 435, 325–327. [Google Scholar] [CrossRef]
- Melikyan, A.; Alloatti, L.; Muslija, A.; Hillerkuss, D.; Schindler, P.C.; Li, J.; Palmer, R.; Korn, D.; Muehlbrandt, S.; Van Thourhout, D.; et al. High-speed plasmonic phase modulators. Nat. Photonics 2014, 8, 229–233. [Google Scholar] [CrossRef]
- Neutens, P.; Van Dorpe, P.; De Vlaminck, I.; Lagae, L.; Borghs, G. Electrical detection of confined gap plasmons in metal–insulator–metal waveguides. Nat. Photonics 2009, 3, 283–286. [Google Scholar] [CrossRef]
- Ming, T.; Zhao, L.; Xiao, M.; Wang, J. Resonance-coupling-based plasmonic switches. Small 2010, 6, 2514–2519. [Google Scholar] [CrossRef]
- Sorger, V.J.; Oulton, R.F.; Ma, R.-M.; Zhang, X. Toward integrated plasmonic circuits. MRS Bull. 2012, 37, 728–738. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, Z.; Zhou, J.; Liu, W.; Zhou, G.; Lee, C. Artificial Intelligence-Enhanced Waveguide “Photonic Nose”—Augmented Sensing Platform for VOC Gases in Mid-Infrared. Small 2024, 20, e2400035. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, Z.; Zhou, J.; Liu, W.; Zhou, G.; Lee, C. Development of Photonic In-Sensor Computing Based on a Mid-Infrared Silicon Waveguide Platform. ACS Nano 2024, 18, 22938–22948. [Google Scholar] [CrossRef]
- Ren, Z.; Zhang, Z.; Zhuge, Y.; Xiao, Z.; Xu, S.; Zhou, J.; Lee, C. Near-Sensor Edge Computing System Enabled by a CMOS Compatible Photonic Integrated Circuit Platform Using Bilayer AlN/Si Waveguides. Nanomicro Lett. 2025, 17, 261. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, H.; Li, D.; Lee, C. Technology Landscape Review of In-Sensor Photonic Intelligence: From Optical Sensors to Smart Devices. AI Sens. 2025, 1, 5. https://doi.org/10.3390/aisens1010005
Zhou H, Li D, Lee C. Technology Landscape Review of In-Sensor Photonic Intelligence: From Optical Sensors to Smart Devices. AI Sensors. 2025; 1(1):5. https://doi.org/10.3390/aisens1010005
Chicago/Turabian StyleZhou, Hong, Dongxiao Li, and Chengkuo Lee. 2025. "Technology Landscape Review of In-Sensor Photonic Intelligence: From Optical Sensors to Smart Devices" AI Sensors 1, no. 1: 5. https://doi.org/10.3390/aisens1010005
APA StyleZhou, H., Li, D., & Lee, C. (2025). Technology Landscape Review of In-Sensor Photonic Intelligence: From Optical Sensors to Smart Devices. AI Sensors, 1(1), 5. https://doi.org/10.3390/aisens1010005







