Environmental Heavy Metal Contamination in Southern Brazilian Mangroves: Biomonitoring Using Crassostrea rhizophorae and Laguncularia racemosa as Green Health Indicators
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Methodology
2.2.1. Sediment Collection and Analysis
2.2.2. Collection and Preparation of Crassostrea rhizophorae Samples
2.2.3. Collection and Preparation of Laguncularia racemosa Samples
2.2.4. Heavy Metal Analysis in Biological Samples and Sediments
2.2.5. Statistical Analyses
3. Results
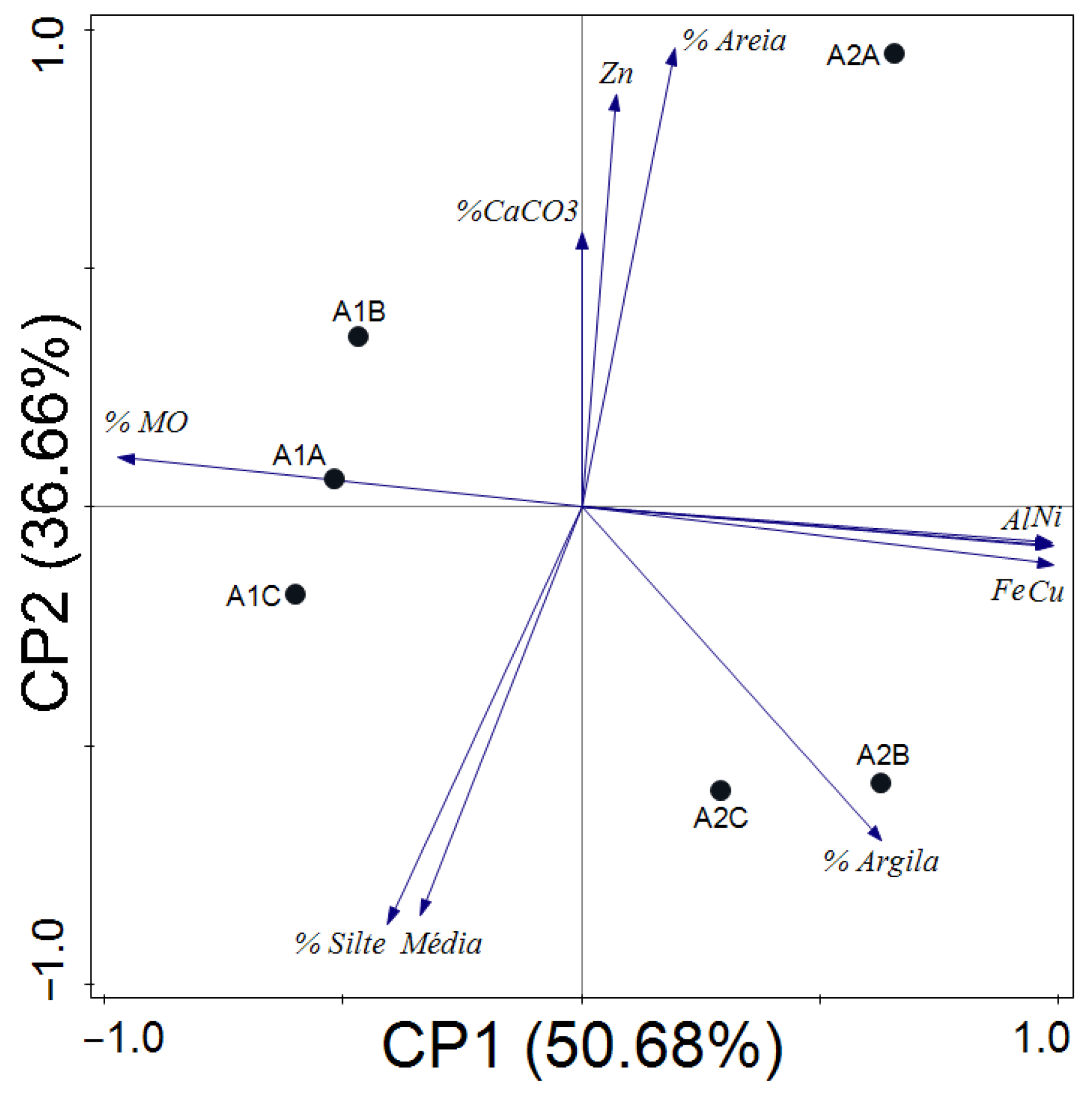
3.1. Sediments
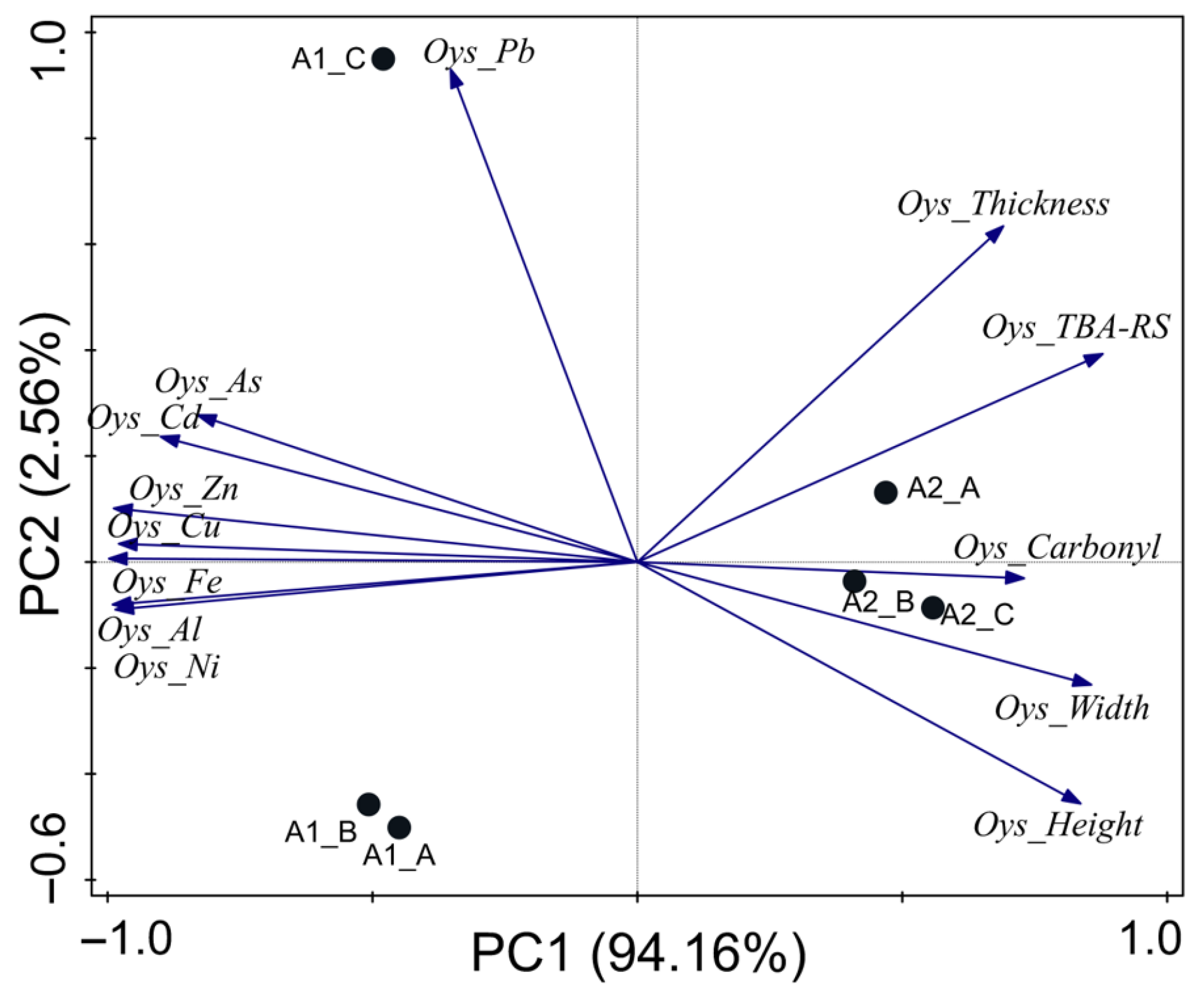
3.2. Crassostrea rhizophorae
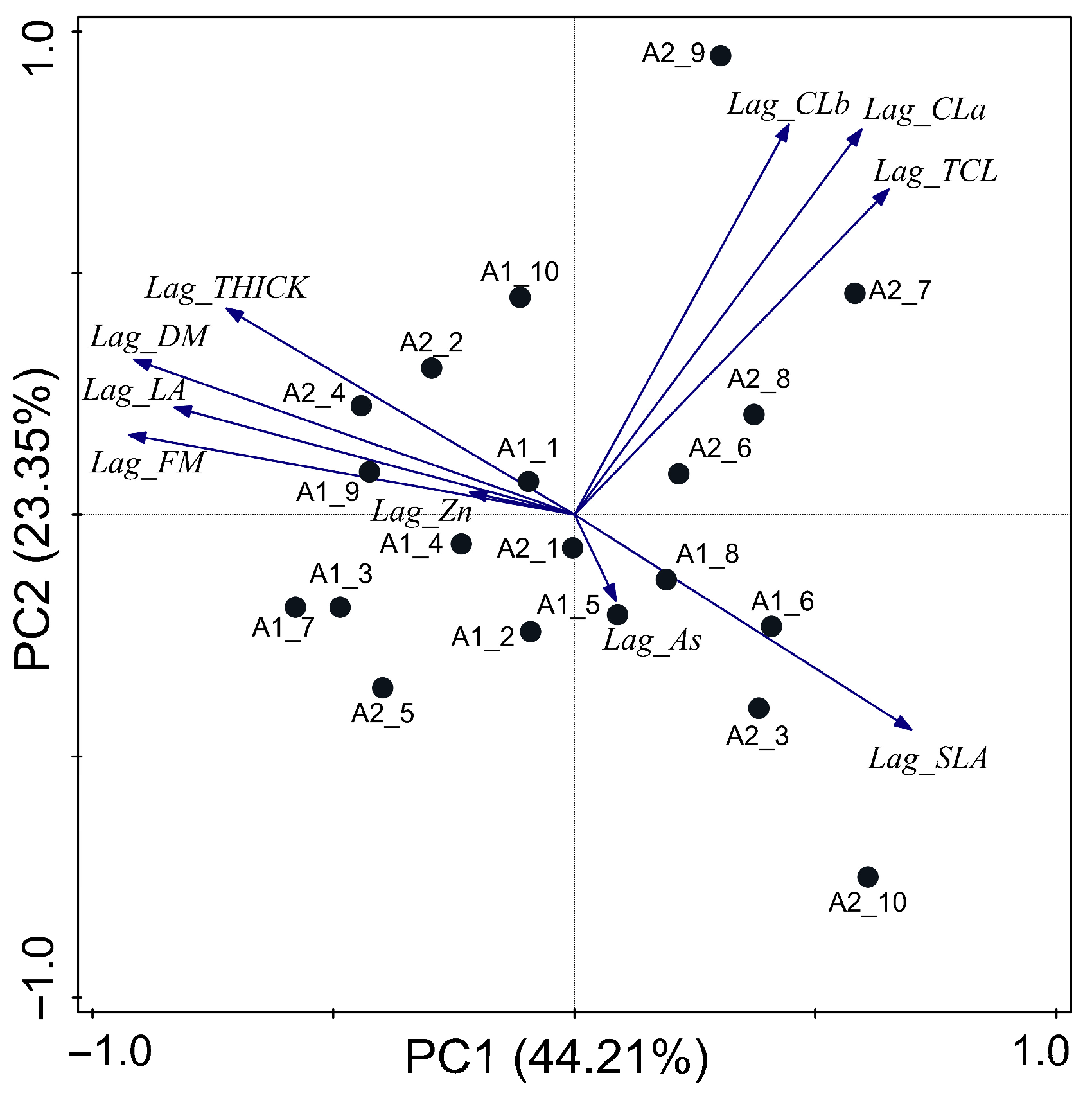
3.3. Laguncularia racemosa
4. Discussion
4.1. Sediments
4.2. Crassostrea rhizophorae
4.3. Laguncularia racemosa
4.4. Human Health Implications
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Giri, C.; Ochieng, E.; Tieszen, L.L.; Zhu, Z.; Singh, A.; Loveland, T.; Masek, J.; Duke, N. Status and distribution of mangrove forests of the world using earth observation satellite data. Glob. Ecol. Biogeogr. 2011, 20, 154–159. [Google Scholar] [CrossRef]
- Duke, N.; Nagelkerken, I.; Agardy, T.; Wells, S.; Van Lavieren, H.; Huxham, M. The Importance of Mangroves to People: A Call to Action; United Nations Environment Programme (UNEP): Nairobi, Kenya, 2014. [Google Scholar] [CrossRef]
- Madi, A.P.L.M.; Boeger, M.R.T.; Larcher, L.; Pelozo, A.; Sereneski, C.; Reissmann, C.B.; Padial, A.A. Estrutura do componente de regeneração natural e arbóreo de dois manguezais no estado do paraná. Cienc. Florest. 2016, 26, 159–170. [Google Scholar] [CrossRef]
- Lee, S.Y.; Primavera, J.H.; Dahdouh-Guebas, F.; McKee, K.; Bosire, J.O.; Cannicci, S.; Diele, K.; Fromard, F.; Koedam, N.; Marchand, C.; et al. Ecological role and services of tropical mangrove ecosystems: A reassessment. Glob. Ecol. Biogeogr. 2014, 23, 726–743. [Google Scholar] [CrossRef]
- Gomes, M.T.U.; Lorenzen, P.; Wandscheer, C.B. Carbono azul: Manguezais, um potencial sumidouro de carbono para a América Latina e Caribe. Rev. Estud. Av. 2024, 40, 149–182. [Google Scholar] [CrossRef]
- Akram, H.; Hussain, S.; Mazumdar, P.; Chua, K.O.; Butt, T.E.; Harikrishna, J.A. Mangrove Health: A Review of Functions, Threats, and Challenges Associated with Mangrove Management Practices. Forests 2023, 14, 1698. [Google Scholar] [CrossRef]
- Holguin, G.; Vazquez, P.; Bashan, Y. The role of sediment microorganisms in the productivity, conservation, and rehabilitation of mangrove ecosystems: An overview. Biol. Fertil. Soils 2001, 33, 265–278. [Google Scholar] [CrossRef]
- Godoy, M.D.; de Lacerda, L.D. Mangroves Response to Climate Change: A Review of Recent Findings on Mangrove Extension and Distribution. An. Acad. Bras. Cienc. 2015, 87, 651–667. [Google Scholar] [CrossRef]
- Santos, T.O.; Andrade, K.D.; Santos, H.V.S.; Castaneda, D.A.F.G.; Santana, M.B.S.; Holanda, F.S.R.; Santos, M.J.C. Caracterização estrutural de bosques de mangue: Estuário do São Francisco. Sci. Plena 2012, 84b, 1–7. Available online: https://www.scientiaplena.org.br/sp/article/view/1010 (accessed on 28 August 2025).
- Amaral, É.B.M.; Santos, C.V. Poluição e invasão de mangues em zonas litorâneas. Unisanta Biosci. 2022, 11, 117–123. [Google Scholar]
- Alves, R.J.M.; Pinheiro, D.d.S.; Teixeira, J.M.J.; Alves, R.J.M.; Tavares-Martins, A.C.C. Expansão urbana e percepção ambiental: Uma análise sobre a relação homem-manguezal na cidade de Marapanim, Pará. Mundo Amaz. 2023, 14, 154–179. [Google Scholar] [CrossRef]
- Vieira, C.V.; Filho, N.O.H.; Bonetti, C.V.D.H.C.; Bonetti, J. Caracterização morfosedimentar e setorização do complexo estuarino da baía da babitonga/sc. Bol. Parana. Geociencias 2008, 62, 12783. [Google Scholar] [CrossRef]
- Herbst, D.F.; Gerhardinger, L.C.; Vila-Nova, D.A.; de Carvalho, F.G.; Hanazaki, N. Integrated and deliberative multidimensional assessment of a subtropical coastal-marine ecosystem (Babitonga bay, Brazil). Ocean Coast. Manag. 2020, 196, 105279. [Google Scholar] [CrossRef]
- Tureck, C.R.; Haak, L.; da Cunha, S.M.B.; Destefani, A.; Vieira, C.V.; Kim, B.S.M.; Trevizani, T.H.; Ferreira, P.A.d.L.; Figueira, R. Qualidade de sedimentos na Baía Babitonga e sua implicação na reabertura do Canal do Linguado (SC), Brasil. Acta Biológica Catarin. 2024, 11, 46–61. [Google Scholar] [CrossRef]
- Kilca, R.V.; Mel, J.C.F., Jr.; Esemann-Quadros, K.; Larcher, L.; Pfuetzenreuter, A. Os manguezais e marismas da Baía Babitonga: Uma síntese. Rev. CEPSUL-Biodiversidade Conserv. Mar. 2019, 8, eb2019002. [Google Scholar] [CrossRef]
- Matias, L.; Silva, M.D. Monitoramento e análise da vegetação de manguezal no litoral sul de Alagoas. J. Environ. Anal. Prog. 2017, 2, 312–319. [Google Scholar] [CrossRef]
- Fedorova, D.G.; Karpova, G.V.; Ukenov, B.S. The Accumulation of Heavy Metals in the Leaves of Crataegus Sanguinea Pall. (Redhaw Hawthorn) in the Urban Environment (On the Example of Orenburg). IOP Conf. Ser. Earth Environ. Sci. 2021, 670, 012030. [Google Scholar] [CrossRef]
- Acosta-Dacal, A.; Hernández-Marrero, M.E.; Rial-Berriel, C.; Díaz-Díaz, R.; Bernal-Suárez, M.d.M.; Zumbado, M.; Henríquez-Hernández, L.A.; Boada, L.D.; Luzardo, O.P. Comparative study of organic contaminants in agricultural soils at the archipelagos of the Macaronesia. Environ. Pollut. 2022, 301, 118979. [Google Scholar] [CrossRef]
- Badamasi, H. Biomonitoring of Air Pollution Using Plants. J. Environ. Sci. 2017, 2, 27–39. [Google Scholar]
- Mulgrew, A.; Williams, P. Biomonitoring of Air Quality Using Plants; Monitoring and Assessment Research Centre: London, UK, 2000. [Google Scholar]
- Elturk, M.; Abdullah, R.; Zakaria, R.M.; Abu Bakar, N.K. Heavy metal contamination in mangrove sediments in Klang estuary, Malaysia: Implication of risk assessment. Estuarine. Coast. Shelf Sci. 2019, 226, 106266. [Google Scholar] [CrossRef]
- Maurya, P.; Kumari, R. Toxic metals distribution, seasonal variations and environmental risk assessment in surficial sediment and mangrove plants (A. marina), Gulf of Kachchh (India). J. Hazard. Mater. 2021, 413, 125345. [Google Scholar] [CrossRef]
- Xu, S.; Lin, C.; Qiu, P.; Song, Y.; Yang, W.; Xu, G.; Feng, X.; Yang, Q.; Yang, X.; Niu, A. Tungsten- and cobalt-dominated heavy metal contamination of mangrove sediments in Shenzhen, China. Mar. Pollut. Bull. 2015, 100, 562–566. [Google Scholar] [CrossRef] [PubMed]
- Robin, S.L.; Alfaro, A.C.; Gututuauva, K.; Marchand, C. Dynamics of major and trace elements during leaf litter decomposition in semi-arid mangrove subject to urban runoff. Mar. Pollut. Bull. 2025, 211, 117475. [Google Scholar] [CrossRef]
- Usman, A.R.; Alkredaa, R.S.; Al-Wabel, M. Heavy metal contamination in sediments and mangroves from the coast of Red Sea: Avicennia marina as potential metal bioaccumulator. Ecotoxicol. Environ. Saf. 2013, 97, 263–270. [Google Scholar] [CrossRef]
- Santos, T.A.; Bonfim, T.M.; Silva, F.S.; Silva, A.G.; de Ferreira Bandeira, M.L.S.; de Jesus, R.M.; Nascimento, L.D. Determinação de metais em um sistema estuarino: Laguncularia Racemosa como um potencial indicador de contaminação. Rev. Bras. De Ciências Ambient. 2018, 49, 51–65. [Google Scholar] [CrossRef]
- Silva, C.A.R.; Rainbow, P.S.; Smith, B.D. Biomonitoring of trace metal contamination in mangrove-lined Brazilian coastal systems using the oyster Crassostrea rhizophorae: Comparative study of regions affected by oil, salt pond and shrimp farming activities. Hydrobiologia 2003, 501, 199–206. [Google Scholar] [CrossRef]
- Senez-Mello, T.M.; Crapez, M.A.C.; e Silva, C.A.R.; Silva, E.T.; Fonseca, E.M. Heavy metals bioconcentration in Crassostrea rhizophorae: A site-to-site transplant experiment at the Potengi estuary, Rio Grande do Norte, Brazil. Sci. Rep. 2020, 10, 246. [Google Scholar] [CrossRef]
- Ruthes, A.M.; Silva, M.M.; Melo Júnior, J.C.F. Leaf changes in Avicennia schaueriana following a massive herbivory event by Hyblaea puera (Lepidoptera) in South Brazil. Braz. J. Dev. 2021, 7, 47275–47286. [Google Scholar]
- CATARINA, Fundação do Meio Ambiente de Santa. Atlas Ambiental da Região de Joinville: Complexo Hídrico da Baía da Babitonga; FATMA: Florianópolis, Brazil, 2003. [Google Scholar]
- Vieira, C.V.; Filho, N.O.H. Paisagem marinha da Baía da Babitonga, nordeste do estado de Santa Catarina. Rev. Bras. Geogr. Física 2017, 10, 1017–1034. [Google Scholar]
- Truccolo, E.C.; Schettini, C.A.F. Marés astronômicas na baía Babitonga, SC. Notas Técnicas Facimar 1999, 3, 57–66. [Google Scholar] [CrossRef]
- Silva, L.F. Alterações morfodinâmicas no Canal do Linguado pela remoção do dique que o separa da Baía da Babitonga (SC). Ph.D. thesis, Universidade Federal do Rio Grande do Sul, Alegre, Brazil, 2011. [Google Scholar]
- Cremer, M.J. O estuário da Baía da Babitonga. In Diagnóstico Ambiental da baía da Babitonga; UNIVILLE: Joinville, France, 2006; pp. 15–19. [Google Scholar]
- Kilca, R.V.; Alberti, L.F.; Souza, A.M.; Wolf, L. Estrutura de uma floresta de mangue na Baía da Babitonga, São Francisco do Sul, SC. Ciência E Nat. 2011, 33, 57–72. [Google Scholar]
- Júnior, J.C.F.M.; Silva, M.M.; Boeger, M.R.T.; Souza, T.F.; Vieira, C.V. Patrimônio natural das restingas da baía Babitonga, Santa Catarina, Brasil. Rev. CEPSUL-Biodiversidade Conserv. Mar. 2018, 7, eb2018002. [Google Scholar] [CrossRef]
- Suguio, K. Introdução a Sedimentologia; EDUSP: São Paulo, Brazil, 1973. [Google Scholar]
- Galehouse, J.S. Sedimentation analysis. In Procedures in Sedimentary Petrology; Wiley-Interscience: New York, NY, USA, 1971. [Google Scholar]
- Folk, R.L.; Ward, W.C. Brazos river bar: A Study in the Significance of Grain-Size Parameters. J. Sediment. Res. 1957, 27, 3–26. [Google Scholar] [CrossRef]
- Camargo, M.G. SysGran: Um sistema de código aberto para análises granulométricas do sedimento. Rev. Bras. Geo-Ciências 2006, 36, 371–378. [Google Scholar] [CrossRef]
- Aslam, S.; Chan, M.W.H.; Siddiqui, G.; Boczkaj, G.; Kazmi, S.J.H.; Kazmi, M.R. A comprehensive assessment of environmental pollution by means of heavy metal analysis for oysters’ reefs at Hab River Delta, Balochistan, Pakistan. Mar. Pollut. Bull. 2020, 153, 110970. [Google Scholar] [CrossRef] [PubMed]
- Azevedo, J.A.M.; Barros, A.B.; de Miranda, P.R.B.; da Costa, J.G.; Nascimento, V.X. Biomonitoring of Heavy Metals (Fe, Zn, Cu, Mn, Cd and Cr) in Oysters: Crassostrea rhizophorae of Mangrove Areas of Alagoas (Brazil). Braz. Arch. Biol. Technol. 2019, 62, e19180211. [Google Scholar] [CrossRef]
- Ohkawa, H.; Ohishi, N.; Yagi, K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979, 95, 351–358. [Google Scholar] [CrossRef] [PubMed]
- Reznick, A.Z.; Packer, L. Oxidative damage to proteins: Spectrophotometric method for carbonyl assay. Methods Enzymol. 1994, 233, 357–363. [Google Scholar] [CrossRef]
- Filardi, F.L.R.; Barros, F.D.; Baumgratz, J.F.A.; Bicudo, C.E.; Cavalcanti, T.B.; Coelho, M.A.N.; Costa, A.F.; Goldenberg, R.; Labiak, P.H.; Lanna, J.M. Brazil Flora Group, Brazilian Flora 2020: Innovation and collaboration to meet Target 1 of the Global Strategy for Plant Con-servation (GSPC). Rodriguésia 2018, 69, 1513–1527. [Google Scholar]
- Schaeffer-Novelli, Y.; Adaime, R.R.; Cintron-Molero, G.; de Camargo, T.M. Variability of Mangrove Ecosystems along the Brazilian Coast. Estuaries 1990, 13, 204–218. [Google Scholar] [CrossRef]
- Bartz, M.C.; Júnior, J.C.F.d.M.; Larcher, L. Variação morfológica de Laguncularia racemosa (L.) C. F. Gaertn. (Combretaceae) em áreas de manguezal e de transição entre manguezal e floresta de restinga. Biotemas 2014, 28, 21–29. [Google Scholar] [CrossRef][Green Version]
- Bernini, E.; Rezende, C.E. Litterfall in a mangrove in Southeast Brazil. Pan-Am. J. Aquat. Sci. 2010, 5, 508–519. [Google Scholar][Green Version]
- Sobrado, M.A. Leaf characteristics and gas exchange of the mangrove Laguncularia racemosa as affected by salinity. Photosynthetica 2005, 43, 217–221. [Google Scholar] [CrossRef]
- Lee, J.A.; Hendry, G.A.F.; Grime, J.P. Methods in Comparative Plant Ecology: A Laboratory Manual. J. Ecol. 1993, 81, 832. [Google Scholar] [CrossRef]
- Martin, D.; Kopp, J. Method 200.7 Determination of Metals and Trace Elements in Water and Wastes by Inductively Coupled Plasma-atomic Emission Spectrometry Revision 4.4. USEPA-ICP Users Group; U.S. Environmental Protection Agency: Cincinnati, OH, USA, 1994. [Google Scholar]
- Filho, P.J.S.; Böhm, E.M.; Böhm, G.M.; Montenegro, G.O.; Silveira, L.A.; Betemps, G.R. Determination of hydrocarbons transported by urban runoff in sediments of São Gonçalo Channel (Pelotas—RS, Brazil). Mar. Pollut. Bull. 2017, 114, 1088–1095. [Google Scholar] [CrossRef]
- Uysal, K.; Köse, E.; Bülbül, M.; Dönmez, M.; Erdoğan, Y.; Koyun, M.; Ömeroğlu, Ç.; Özmal, F. The comparison of heavy metal accumulation ratios of some fish species in Enne Dame Lake (Kütahya/Turkey). Environ. Monit. Assess. 2008, 157, 355–362. [Google Scholar] [CrossRef]
- Gotelli, N.J.; Ellison, A.M. A Primer of Ecological Statistics; Sinauer Associates, Inc.: Sunderland, MA, USA, 2004. [Google Scholar]
- Vieira, S. Bioestatística: Tópicos Avançados, 5th ed.; Editora Guanabara Koogan: Rio de Janeiro, Brazil, 2023. [Google Scholar]
- Bourgeron, P.; Legendre, P.; Legendre, L. Numerical Ecology. Arctic, Antarct. Alp. Res. 2000, 32, 218. [Google Scholar] [CrossRef]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences, 2nd ed.; Lawrence Erlbaum Associates: Hillsdale, NJ, USA, 1988. [Google Scholar]
- El-Sharkawy, M.; Alotaibi, M.O.; Li, J.; Du, D.; Mahmoud, E. Heavy Metal Pollution in Coastal Environments: Ecological Implications and Management Strategies: A Review. Sustainability 2025, 17, 701. [Google Scholar] [CrossRef]
- Kim, B.S.M.; Angeli, J.L.F.; Ferreira, P.A.L.; De Mahiques, M.M.; Figueira, R.C.L. A multivariate approach and sediment quality index evaluation applied to Baixada Santista, Southeastern Brazil. Mar. Pollut. Bull. 2019, 143, 72–80. [Google Scholar] [CrossRef]
- Angeli, J.L.F.; Kim, B.S.M.; Paladino, Í.M.; Nagai, R.H.; Martins, C.C.; de Mahiques, M.M.; Figueira, R.C.L. Statistical assessment of background levels for metal contamination from a subtropical estuarine system in the SW Atlantic (Paranaguá Estuarine System, Brazil). J. Sediment. Environ. 2020, 5, 137–150. [Google Scholar] [CrossRef]
- Kim, B.S.M.; Bícego, M.C.; Taniguchi, S.; Siegle, E.; Oliveira, R.; Alcántara-Carrió, J.; Figueira, R.C.L. Organic and inorganic contamination in sediments from Araçá Bay, São Sebastião, Brazil. Ocean Coast. Manag. 2018, 164, 42–51. [Google Scholar] [CrossRef]
- Kim, B.S.M.; Angeli, J.L.F.; Ferreira, P.A.L.; de Mahiques, M.M.; Figueira, R.C.L. Critical evaluation of different methods to calculate the Geoaccumulation Index for environmental studies: A new approach for Baixada Santista—Southeastern Brazil. Mar. Pollut. Bull. 2018, 127, 548–552. [Google Scholar] [CrossRef]
- Jeng, A.S.; Bergseth, H. Chemical and Mineralogical Properties of Norwegian Alum Shale Soils, with Special Emphasis on Heavy Metal Content and Availability. Acta Agric. Scand. Sect. B-Soil Plant Sci. 1992, 42, 88–93. [Google Scholar] [CrossRef]
- Tessier, A.; Campbell, P.G.C.; Bisson, M. Sequential extraction procedure for the speciation of particulate trace metals. Anal. Chem. 1979, 51, 844–851. [Google Scholar] [CrossRef]
- Du Laing, G.; Rinklebe, J.; Vandecasteele, B.; Meers, E.; Tack, F. Trace metal behaviour in estuarine and riverine floodplain soils and sediments: A review. Sci. Total. Environ. 2009, 407, 3972–3985. [Google Scholar] [CrossRef] [PubMed]
- Förstner, U. Sediment criteria development. In Sediments and Environmental Geochemistry; Springer: Berlin/Heidelberg, Germany, 1990. [Google Scholar]
- Luoma, S.N.; Rainbow, P.S. Metal Contamination in Aquatic Environments: Science and Lateral Management; Cambridge University Press: Cambridge, UK, 2008. [Google Scholar]
- Bai, J.; Cui, B.; Chen, B.; Zhang, K.; Deng, W.; Gao, H.; Xiao, R. Spatial distribution and ecological risk assessment of heavy metals in surface sediments from a typical plateau lake wetland, China. Ecol. Model. 2011, 222, 301–306. [Google Scholar] [CrossRef]
- Oladimeji, T.; Oyedemi, M.; Emetere, M.; Agboola, O.; Adeoye, J.; Odunlami, O. Review on the impact of heavy metals from industrial wastewater effluent and removal technologies. Heliyon 2024, 10, e40370. [Google Scholar] [CrossRef]
- Yap, C.K.; Saleem, M.; Tan, W.S.; Syazwan, W.M.; Azrizal-Wahid, N.; Nulit, R.; Ibrahim, M.H.; Mustafa, M.; Rahman, M.A.A.; Edward, F.B.; et al. Ecological–Health Risk Assessments of Copper in the Sediments: A Review and Synthesis. Pollutants 2022, 2, 269–288. [Google Scholar] [CrossRef]
- Che, K.; Zhu, X.; Tang, G.; Zhao, M.; Pan, J. In Situ Electroplating of Ir@Carbon Cloth as High-Performance Selective Oxygen Evolution Reaction Catalyst for Direct Electrolytic Recovery of Lead. Catalysts 2023, 13, 322. [Google Scholar] [CrossRef]
- Bari, G.A. Electrodeposition of Nickel. In Modern Electroplating; John Wiley & Sons: New York, NY, USA, 2010; pp. 79–111. [Google Scholar]
- Iglesias, I.; Venâncio, S.; Pinho, J.L.; Avilez-Valente, P.; Vieira, J.M.P. Two Models Solutions for the Douro Estuary: Flood Risk Assessment and Breakwater Effects. Estuaries Coasts 2019, 42, 348–364. [Google Scholar] [CrossRef]
- Ke, Y.; Ou, C.; Guo, X.; Liu, S.; Yao, C.; Shi, B.; Que, H. Heavy Metal Accumulation in Oysters from an Aquaculture Area in the Luoyangjiang River Estuary. Toxics 2024, 12, 645. [Google Scholar] [CrossRef]
- Kanhai, L.D.K.; Gobin, J.F.; Beckles, D.M.; Lauckner, B.; Mohammed, A. Metals in sediments and mangrove oysters (Crassostrea rhizophorae) from the Caroni Swamp, Trinidad. Environ. Monit. Assess. 2013, 186, 1961–1976. [Google Scholar] [CrossRef] [PubMed]
- Rebelo, M.d.F.; Amaral, M.C.R.D.; Pfeiffer, W.C. High Zn and Cd accumulation in the oyster Crassostrea rhizophorae, and its relevance as a sentinel species. Mar. Pollut. Bull. 2003, 46, 1354–1358. [Google Scholar] [CrossRef] [PubMed]
- Kandziora-Ciupa, M.; Nadgórska-Socha, A.; Barczyk, G.; Ciepał, R. Bioaccumulation of heavy metals and ecophysiological responses to heavy metal stress in selected populations of Vaccinium myrtillus L. and Vaccinium vitis-idaea L. Ecotoxicology 2017, 26, 966–980. [Google Scholar] [CrossRef]
- Dang, T.T.; Vo, T.A.; Duong, M.T.; Pham, T.M.; Van Nguyen, Q.; Nguyen, T.Q.; Bui, M.Q.; Syrbu, N.N.; Van Do, M. Heavy metals in cultured oysters (Saccostrea glomerata) and clams (Meretrix lyrata) from the northern coastal area of Vietnam. Mar. Pollut. Bull. 2022, 184, 114140. [Google Scholar] [CrossRef]
- Alfonso, J.A.; Handt, H.; Mora, A.; Vásquez, Y.; Azocar, J.; Marcano, E. Temporal distribution of heavy metal concentrations in oysters Crassostrea rhizophorae from the central Venezuelan coast. Mar. Pollut. Bull. 2013, 73, 394–398. [Google Scholar] [CrossRef]
- Phillips, D.J.H.; Rainbow, P.S. Biomonitoring of trace aquatic contaminants. J. Mar. Biol. Assoc. UK 1994, 74, 263. [Google Scholar] [CrossRef]
- Wallner-Kersanach, M.; Theede, H.; Eversberg, U.; Lobo, S. Accumulation and Elimination of Trace Metals in a Transplantation Experiment with Crassostrea rhizophorae. Arch. Environ. Contam. Toxicol. 2000, 38, 40–45. [Google Scholar] [CrossRef]
- Rabaoui, L.; Cusack, M.; Saderne, V.; Krishnakumar, P.K.; Lin, Y.-J.; Shemsi, A.M.; El Zrelli, R.; Arias-Ortiz, A.; Masqué, P.; Duarte, C.M.; et al. Anthropogenic-induced acceleration of elemental burial rates in blue carbon repositories of the Arabian Gulf. Sci. Total. Environ. 2020, 719, 135177. [Google Scholar] [CrossRef]
- Defew, L.H.; Mair, J.M.; Guzman, H.M. An assessment of metal contamination in mangrove sediments and leaves from Punta Mala Bay, Pacific Panama. Mar. Pollut. Bull. 2005, 50, 547–552. [Google Scholar] [CrossRef]
- Kulkarni, V.; Awad, J.; van Leeuwen, J.; Drikas, M.; Chow, C.; Cook, D.; Medlock, A.; Trolio, R.; Amal, R. Impact of zinc on biologically mediated monochloramine decay in waters from a field based pilot scale drinking water distribution system. Chem. Eng. J. 2018, 339, 240–248. [Google Scholar] [CrossRef]
- Senez-Mello, T.M.; Crapez, M.A.C.; e Silva, C.A.R.; da Silva, E.T.; da Fonseca, E.M. Spatial Variability of Heavy Metals in Native Oysters From an Anthropogenically Impacted Estuary in Northeastern Brazil. Front. Mar. Sci. 2020, 7, 412. [Google Scholar] [CrossRef]
- Arrivabene, H.P.; Souza, I.; Có, W.L.O.; Rodella, R.A.; Wunderlin, D.A.; Milanez, C.R. Functional traits of selected mangrove species in Brazil as biological indicators of different environmental conditions. Sci. Total. Environ. 2014, 476–477, 496–504. [Google Scholar] [CrossRef]
- Esteban, R.; Barrutia, O.; Artetxe, U.; Fernández-Marín, B.; Hernández, A.; García-Plazaola, J.I. Internal and external factors affecting photosynthetic pigment composition in plants: A meta-analytical approach. New Phytol. 2015, 206, 268–280. [Google Scholar] [CrossRef]
- Silva, B.P.; Saballo, H.M.; Lobo, A.K.M.; Neto, M.C.L. The plasticity of the photosynthetic apparatus and antioxidant responses are critical for the dispersion of Rhizophora mangle along a salinity gradient. Aquat. Bot. 2022, 185, 103609. [Google Scholar] [CrossRef]
- Pascoalini, S.S.; Lopes, D.M.d.S.; Falqueto, A.R.; Tognella, M.M.P. Abordagem ecofisiológica dos manguezais: Uma revisão. Biotemas 2014, 27, 1–11. [Google Scholar] [CrossRef][Green Version]
- Cavalcante, E.d.R.; Ribeiro, V.V.; Taddei, R.R.; Castro, Í.B.; Alves, M.J. High levels of anthropogenic litter trapped in a mangrove area under the influence of different uses. Mar. Pollut. Bull. 2024, 200, 116045. [Google Scholar] [CrossRef] [PubMed]
- Inoue, M.T. Teor de clorofila de seis espécies arbóreas sob influência da poeira de cimento. Floresta 2010, 40, 17841. [Google Scholar] [CrossRef]
- Thawale, P.R.; Babu, S.S.; Wakode, R.R.; Singh, S.K.; Kumar, S.; Juwarkar, A.A. Biochemical changes in plant leaves as a biomarker of pollution due to anthropogenic activity. Environ. Monit. Assess. 2011, 177, 527–535. [Google Scholar] [CrossRef]
- Mondal, I.; De, A.; Nandi, S.; Thakur, S.; Raman, M.; Jose, F.; De, T.K. Estimation of Chlorophyll-a, TSM and salinity in mangrove dominated tropical estuarine areas of Hooghly River, North East Coast of Bay of Bengal, India using sentinel-3 data. Acta Geophys. 2023, 72, 303–322. [Google Scholar] [CrossRef]
- Wei, Z.; Yu, Y.; Yi, Y. Prediction of Future Chlorophyll-a Concentrations in Large Eutrophic Shallow Lakes Under Multiple Stressors. Earth’s Futur. 2025, 13, e2024EF005293. [Google Scholar] [CrossRef]
- Gonçalves, M.V.P.; Alva, J.C.R.; Silva, I.I.; Cruz, M.J.M.; Silva, A.C.M.; Junior, A.B.d.S.R. Biogeoquímica e conservação dos bosques de mangue da APA Tinharé-Boipeba, Baixo Sul da Bahia (Brasil): Metais-traços em sedimentos e folhas da Laguncularia racemosa (L.) C. F. Gaertn (Combretaceae). Rev. Concil. 2022, 22, 419–522. [Google Scholar] [CrossRef]
- Souza, A.K.R.; Morassuti, C.Y.; de Deus, W.B. Poluição do ambiente por metais pesados e utilização de vegetais como bioindicadores. Acta Biomed. Bras. 2018, 9, 95–106. [Google Scholar] [CrossRef]
- Kabata-Pendias, A.; Pendias, H. Trace Elements in Soils and Plants; CRC: Boca Raton, FL, USA, 1985. [Google Scholar]
- Ahmadi, A.; Moore, F.; Keshavarzi, B.; Shahimi, H.; Hooda, P.S. Bioaccumulation of selected trace elements in some aquatic organisms from the proximity of Qeshm Island ecosystems: Human health perspective. Mar. Pollut. Bull. 2022, 182, 113966. [Google Scholar] [CrossRef]
- Ozuni, E.; Andoni, E.; Castrica, M.; Balzaretti, C.M.; Brecchia, G.; Agradi, S.; Curone, G.; Di Cesare, F.; Fehri, N.E.; Luke, B.; et al. Human exposure to heavy metals and possible public health risks via consumption of mussels M. galloprovincialis from the Albanian sea cost. Chemosphere 2024, 368, 143689. [Google Scholar] [CrossRef] [PubMed]
- Vieira, K.; Delgado, J.; Lima, L.; Souza, P.; Crapez, M.; Correa, T.; Aguiar, V.; Neto, J.B.; Fonseca, E. Human health risk assessment associated with the consumption of mussels (Perna perna) and oysters (Crassostrea rhizophorae) contaminated with metals and arsenic in the estuarine channel of Vitória Bay (ES), Southeast Brazil. Mar. Pollut. Bull. 2021, 172, 112877. [Google Scholar] [CrossRef]
- Fu, L.; Lu, X.; Niu, K.; Tan, J.; Chen, J. Bioaccumulation and human health implications of essential and toxic metals in freshwater products of Northeast China. Sci. Total. Environ. 2019, 673, 768–776. [Google Scholar] [CrossRef] [PubMed]
- Chiesa, L.; Ceriani, F.; Caligara, M.; Di Candia, D.; Malandra, R.; Panseri, S.; Arioli, F. Mussels and clams from the italian fish market. is there a human exposition risk to metals and arsenic? Chemosphere 2018, 194, 644–649. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Shi, Q.; Liu, C.; Sun, Q.; Zeng, X. Effects of Endocrine-Disrupting Heavy Metals on Human Health. Toxics 2023, 11, 322. [Google Scholar] [CrossRef]
- Guo, C.; Hu, L.; Jiang, L.; Feng, H.; Hu, B.; Zeng, T.; Song, S.; Zhang, H. Toxic arsenic in marketed aquatic products from coastal cities in China: Occurrence, human dietary exposure risk, and coexposure risk with mercury and selenium. Environ. Pollut. 2021, 295, 118683. [Google Scholar] [CrossRef]
- Duarte, L.F.d.A.; Ortega, A.d.S.B.; Paço, M.d.S.; Nobre, C.R.; Sadauskas-Henrique, H.; Gusso-Choueri, P.K.; Souza, I.C.; Carvalho, M.V.; Monferrán, M.V.; Wunderlin, D.A.; et al. Coastal fallout of settleable particulate matter: Metal bioaccumulation and sublethal toxicity in estuarine bivalves with implications for human health. Mar. Pollut. Bull. 2025, 220, 118441. [Google Scholar] [CrossRef]
- Angon, P.B.; Islam, S.; Kc, S.; Das, A.; Anjum, N.; Poudel, A.; Suchi, S.A. Sources, effects and present perspectives of heavy metals contamination: Soil, plants and human food chain. Heliyon 2024, 10, e28357. [Google Scholar] [CrossRef] [PubMed]
- Teschke, R. Aluminum, Arsenic, Beryllium, Cadmium, Chromium, Cobalt, Copper, Iron, Lead, Mercury, Molybdenum, Nickel, Platinum, Thallium, Titanium, Vanadium, and Zinc: Molecular Aspects in Experimental Liver Injury. Int. J. Mol. Sci. 2022, 23, 12213. [Google Scholar] [CrossRef] [PubMed]
- Zhuzzhassarova, G.; Azarbayjani, F.; Zamaratskaia, G. Fish and Seafood Safety: Human Exposure to Toxic Metals from the Aquatic Environment and Fish in Central Asia. Int. J. Mol. Sci. 2024, 25, 1590. [Google Scholar] [CrossRef] [PubMed]
- El-Shorbagy, M.A.; Abdel-Moniem, S.M.; Ghanem, M.H.; Embaby, M.A.; Kourany, M.S.; El-Kady, A.A.; Abbas, M.M.M. Elucidating the Environmental and Health Risks of Trace Element Pollution in Red Sea Fish from Nuweiba City, Aqaba Gulf, Egypt. Biol. Trace Element Res. 2025, 203, 1618–1636. [Google Scholar] [CrossRef]
- Closset, M.; Cailliau, K.; Slaby, S.; Marin, M. Effects of Aluminium Contamination on the Nervous System of Freshwater Aquatic Vertebrates: A Review. Int. J. Mol. Sci. 2021, 23, 31. [Google Scholar] [CrossRef]
- Sohrabi, N.; Kalantari, N.; Amiri, V.; Saha, N.; Berndtsson, R.; Bhattacharya, P.; Ahmad, A. A probabilistic-deterministic analysis of human health risk related to the exposure to potentially toxic elements in groundwater of Urmia coastal aquifer (NW of Iran) with a special focus on arsenic speciation and temporal variation. Stoch. Environ. Res. Risk Assess. 2020, 35, 1509–1528. [Google Scholar] [CrossRef]
- Banaee, M.; Zeidi, A.; Mikušková, N.; Faggio, C. Assessing Metal Toxicity on Crustaceans in Aquatic Ecosystems: A Comprehensive Review. Biol. Trace Element Res. 2024, 202, 5743–5761. [Google Scholar] [CrossRef]
- Lu, H.; Zhang, Y.; Zhang, X.; Wang, R.; Guo, T.; Wang, Q.; Zhao, H.; Xing, M. New insights into zinc alleviating renal toxicity of arsenic-exposed carp (Cyprinus carpio) through YAP-TFR/ROS signaling pathway. Pestic. Biochem. Physiol. 2024, 196, 106153. [Google Scholar] [CrossRef]
- Teschke, R. Copper, Iron, Cadmium, and Arsenic, All Generated in the Universe: Elucidating Their Environmental Impact Risk on Human Health Including Clinical Liver Injury. Int. J. Mol. Sci. 2024, 25, 6662. [Google Scholar] [CrossRef]
- Singh, G.; Sharma, S. Heavy metal contamination in fish: Sources, mechanisms and consequences. Aquat. Sci. 2024, 86, 107. [Google Scholar] [CrossRef]
- Timpano, A.J.; Jones, J.W.; Beaty, B.; Hull, M.; Soucek, D.J.; Zipper, C.E. Combined effects of copper, nickel, and zinc on growth of a freshwater mussel (Villosa iris) in an environmentally relevant context. Aquat. Toxicol. 2022, 242, 106038. [Google Scholar] [CrossRef]

| Area | Sediment Type | Gravel (%) | Sand (%) | Silt (%) | Clay (%) | OM (%) | CaCO3 (%) |
|---|---|---|---|---|---|---|---|
| A1 (Espinheiros) | Medium silt | 0 | 21.37 ± 5.9 | 72.19 ± 2.9 | 6.43 ± 2.8 | 50.87 ± 5.3 | 8.85 ± 0.7 |
| A2 (Vila da Glória) | Fine to medium silt | 1.33 ± 2.3 | 24.02 ± 8.7 | 63.05 ± 7.6 | 11.60 ± 3.1 | 17.08 ± 0.6 | 8.14 ± 2.5 |
| Area | Cu (mg·kg−1) | Al (mg·kg−1) | Fe (mg·kg−1) | Zn (mg·kg−1) | Ni (mg·kg−1) |
|---|---|---|---|---|---|
| A1 (Espinheiros) | 2.17 ± 0.38 | 4149 ± 1046 | 2090 ± 472 | 452 ± 580 | 2.14 ± 0.70 |
| A2 (Vila da Glória) | 5.96 ± 1.05 | 14,478 ± 2727 | 10,407 ± 1758 | 89.8 ± 18.7 | 7.48 ± 1.26 |
| A1 | A2 | |||
|---|---|---|---|---|
| Variables | Mean (s.d.) | Mean (s.d.) | t | p-Value |
| Cu | 85.76 (±8.60) | 37.10 (±4.38) | 8.72 | 0.0009 * |
| Ni | 0.47 (±0.60) | 0.00 (±0.00) | 12.66 | 0.0002 * |
| Zn | 13,735.96 (±1713.27) | 3555.33 (±193.45) | 10.22 | 0.0005 * |
| Al | 279.57 (±57.92) | 77.26 (±15.25) | 5.85 | 0.0042 * |
| Fe | 421.68 (±44.36) | 98.26 (±17.23) | 11.76 | 0.0002 * |
| Cd | 0.38 (±0.03) | 0.26 (±0.04) | 3.74 | 0.0199 * |
| Pb | 0.06 (±0.09) | 0.01 (±0.02) | 0.76 | 0.4859 ns |
| As | 1.39 (±0.19) | 0.91 (±0.22) | 2.79 | 0.0490 ns |
| Height | 43.17 (±5.95) | 58.57 (±4.34) | −3.62 | 0.0223 * |
| Width | 35.67 (±2.15) | 45.71 (±4.68) | −3.37 | 0.0279 * |
| Thickness | 16.57 (±4.57) | 22.76 (±2.14) | −2.12 | 0.1009 |
| TBA-RS | 1.86 (±0.46) | 3.13 (±0.13) | −4.60 | 0.0099 * |
| Carbonyl | 5.15 (±1.04) | 6.86 (±1.00) | −2.04 | 0.1097 ns |
| A1 | A2 | |||
|---|---|---|---|---|
| Variables | Mean (s.d.) | Mean (s.d.) | t | p-Value |
| Fresh Mass (g) | 1.82 (±0.47) | 2.01 (±0.43) | −3.00 | 0.00 * |
| Leaf Thickness (mm) | 0.42 (±0.03) | 0.44 (±0.04) | −4.69 | 0.00 * |
| Dry Mass (g) | 0.53 (±0.15) | 0.55 (±0.12) | −0.93 | 0.35 ns |
| Leaf Area (cm2) | 24.32 (±5.93) | 24.44 (±4.31) | −0.153 | 0.89 ns |
| Specific Leaf Area (cm2/g) | 45.80 (±3.78) | 44.98 (±3.33) | 1.65 | 0.10 ns |
| Chlorophyll a | 1.55 (±0.38) | 1.79 (±0.37) | −4.48 | 0.00 * |
| Chlorophyll b | 1.71 (±0.28) | 1.94 (±0.49) | −4.16 | 0.00 * |
| Total Chlorophyll | 3.25 (±0.60) | 3.73 (±0.82) | −4.67 | 0.00 * |
| Zn | 74.34 (±13.10) | 83.64 (±8.70) | −5.91 | 0.00 * |
| As | 0.22 (±0.17) | 0.28 (±0.06) | −3.83 | 0.00 * |
| Cd | nd | nd | - | - |
| Pb | nd | nd | - | - |
| Cu | nd | nd | - | - |
| Fe | nd | nd | - | - |
| Ni | nd | nd | - | - |
| Al | nd | nd | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Melo Júnior, J.C.F.d.; Vieira, C.V.; Lorenzi, L.; Oliveira, T.M.N.d.; Gastaldi, A.B.; Moletta, A.K.; Mello, A.P.d.; Aquino, A.P.M.d.; Dalmarco, D.; Corrêa, D.R.; et al. Environmental Heavy Metal Contamination in Southern Brazilian Mangroves: Biomonitoring Using Crassostrea rhizophorae and Laguncularia racemosa as Green Health Indicators. Green Health 2025, 1, 19. https://doi.org/10.3390/greenhealth1030019
Melo Júnior JCFd, Vieira CV, Lorenzi L, Oliveira TMNd, Gastaldi AB, Moletta AK, Mello APd, Aquino APMd, Dalmarco D, Corrêa DR, et al. Environmental Heavy Metal Contamination in Southern Brazilian Mangroves: Biomonitoring Using Crassostrea rhizophorae and Laguncularia racemosa as Green Health Indicators. Green Health. 2025; 1(3):19. https://doi.org/10.3390/greenhealth1030019
Chicago/Turabian StyleMelo Júnior, João Carlos Ferreira de, Celso Voos Vieira, Luciano Lorenzi, Therezinha Maria Novais de Oliveira, Alessandra Betina Gastaldi, Aline Krein Moletta, Ana Paula de Mello, Ana Paula Marcelino de Aquino, Daiane Dalmarco, Deivid Rodrigo Corrêa, and et al. 2025. "Environmental Heavy Metal Contamination in Southern Brazilian Mangroves: Biomonitoring Using Crassostrea rhizophorae and Laguncularia racemosa as Green Health Indicators" Green Health 1, no. 3: 19. https://doi.org/10.3390/greenhealth1030019
APA StyleMelo Júnior, J. C. F. d., Vieira, C. V., Lorenzi, L., Oliveira, T. M. N. d., Gastaldi, A. B., Moletta, A. K., Mello, A. P. d., Aquino, A. P. M. d., Dalmarco, D., Corrêa, D. R., Oliveira, G. B. d., Mady, L. C., Steinhorst, L., Bartz, M. C., Ineu, M. L., Barbosa, N. T., Cavichioli, N., Oliveira, R. L. d., Lopes, S. C., & Furtado, P. R. P. (2025). Environmental Heavy Metal Contamination in Southern Brazilian Mangroves: Biomonitoring Using Crassostrea rhizophorae and Laguncularia racemosa as Green Health Indicators. Green Health, 1(3), 19. https://doi.org/10.3390/greenhealth1030019







